Abstract
It has been reported that salidroside (SAL), a natural dietary isothiocyanate, exhibits neuroprotective roles in cerebral ischemia-reperfusion injury. However, to the best of our knowledge, its underlying protective mechanism remains unknown. Sirtuin 1 (SIRT1) is a class III histone deacetylase involved in a variety of cellular functions. SIRT1 has been identified as a mediator of cerebral ischemia and may induce neuroprotection by activating various intracellular downstream targets, such as forkhead box protein O3α (FOXO3α). Therefore, the present study aimed to investigate whether SAL protects human brain vascular smooth muscle cells (HBVSMC) against hypoxia/reoxygenation (H/R) injury, which is a cell model of cerebral ischemia-reperfusion injury, through regulating the SIRT1-activited signaling pathway. The present study revealed that H/R treatment significantly reduced the expression of SIRT1 protein in HBVSMCs. Additionally, pretreatment with SAL reversed the H/R-induced decrease in cellular viability, increased caspase-3 activity, the appearance of apoptotic cells and the apoptosis rate in HBVSMCs. SAL attenuated the H/R-induced decrease in the expression of SIRT1 and phosphorylated FOXO3α protein in HBVSMCs, suggesting that the protective role of SAL in H/R injury occurs via the SIRT1/FOXO3α pathway. Furthermore, sirtinol, a SIRT1-specific inhibitor, suppressed the inhibitory effects of SAL on H/R-induced cytotoxicity and apoptosis as indicated by the downregulation of cell viability and upregulation of caspase-3 activity and apoptosis rate induced by sirtinol treatment in HBVSMCs. The reversal effects of SAL on H/R-induced alternation of B-cell lymphoma (Bcl-2) and Bcl-2 associated X protein expression were also attenuated by sirtinol. These results suggest that SAL exhibits neuroprotective effects against H/R injury by activating the SIRT1/FOXO3α pathway, which may become a novel potential therapeutic target for the treatment of cerebral ischemic disease.
Keywords: salidroside, hypoxia/reoxygenation, silent information regulator 1, forkhead box protein O3α, neuroprotection
Introduction
Salidroside (SAL) is a phenylpropanoid glycoside extracted from Rhodiola rosea L., which is used in traditional Tibetan medicine and exhibits a wide range of pharmacological effects including anti-oxidative, anti-apoptotic, anti-inflammatory, anti-depressive, anti-aging and anti-fatigue effects (1–4). It has been demonstrated that SAL is neuroprotective against ischemic cerebral injury, a leading cause of disability and mortality worldwide and is therefore considered to be a major public health problem (5). It has been demonstrated that SAL reduces the infarct volume and improves neurological deficit scores in a rat model of transient focal cerebral ischemia and reperfusion (6). In addition, SAL improves learning and spatial memory during hypoxia treatment, indicating that SAL has a neuroprotective effect during hypoxia (7). Another study determined that SAL prevents the cerebral ischemic injury caused by cerebral artery occlusion and reperfusion in vivo, and neurotoxicity induced by hydrogen peroxide in vitro (8). Pretreatment with SAL also reduces the cellular damage that occurs following global cerebral ischemia/reperfusion (I/R) injury in rats (9). Although the protective effects of SAL on cerebral I/R injury have been identified, its underlying neuroprotective mechanisms remain unclear. Therefore, the present study used hypoxia/reperfusion (H/R)induced human brain vascular smooth muscle cells (HBVSMCs) as an in vitro cell model of cerebral I/R injury (10) to investigate the mechanisms underlying SAL-mediated neuroprotective activity.
Sirtuin 1 (SIRT1) is an oxidized nicotinamide adenine dinucleotide-dependent deacylase and is expressed in various tissues of the body including the heart, liver, muscle, kidney, endothelium and adipose, and its expression is particularly high in the brain (11). SIRT1 serves an essential role in a number of different processes including inflammation, cellular senescence, apoptosis, aging and stress resistance in the central nervous system (CNS). SIRT1 also serves a neuroprotective role in diseases of the CNS (12). SIRT1 has been specifically identified as a mediator of cerebral ischemia injury and may therefore be exploited as a potential target for treatments of this disease (13). A number of studies have demonstrated that SIRT1 undergoes profound changes in expression and activity, which is associated with the changes in mitochondrial function that occur following hypoxic-ischemia and reoxygenation injury (14,15). Previous studies have demonstrated that the SIRT1-mediated deacetylation and phosphorylation of downstream targets including forkhead box protein O3α (FOXO3α), which is a ubiquitously expressed mammalian forkhead transcription factor and highly expressed in the adult brain, promotes cell survival, mitochondrial function, apoptosis and inflammation in response to severe stress (16,17). A number of studies have indicated that FOXO3α serves important roles in neuronal survival in normal and disease conditions and the regulation of FOXO3α mediated by SIRT1 contributes to neuroprotection in vitro and in vivo (18–20). Further studies have determined that SIRT1-activated multiple signaling pathways, such as FOXO3α, mediate a variety of neuroprotective agents including leptin, two tetrahydroxystilbene and icariin and may attenuate ischemic injury following stroke (21) and cerebral ischemia (22,23). However, it remains unknown whether SIRT1-mediated signaling pathways serve a similar role in the neuroprotection exhibited by SAL against cerebral ischemia injury.
The present study therefore investigated whether SAL attenuates H/R injury, resulting in a neuroprotective effect, via regulation of the SIRT1/FOXO3α pathway. To the best of our knowledge, the current study is the first to investigate whether the SIRT1/FOXO3α signaling pathway contributes to the SAL-mediated prevention of H/R injury in vitro and may be a potential therapeutic target for the treatment of cerebral ischemic injury.
Materials and methods
Reagents
SAL, Sirtinol and 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reagent were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Hoechst 33342 solution, the BCA protein assay kit, radioimmunoprecipitation (RIPA) lysis buffer and BeyoECL Plus were purchased from Beyotime Institute of Biotechnology (Shanghai, China). The caspase-3 enzyme-linked immunosorbent assay (ELISA) kit was purchased from the Nanjing Jiancheng Bioengineering Research Institute (cat no. H076; Nanjing, China). The Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining kit was obtained from Sigma-Aldrich; Merck KGaA. Antibodies against SIRT1 (cat no. 9475), phosphorylated (p)-FOXO3α (cat no. 9466), B-cell lymphoma 2 (Bcl-2; cat no. 3498) and Bcl-2 associated X protein (Bax; cat no. 2772) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). GAPDH (cat no. 10494-1-AP) and secondary antibodies were obtained from ProteinTech Group, Inc. (Chicago, IL, USA).
Cell culture
Human brain vascular smooth muscle cells (HBVSMC) were purchased from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified atmosphere consisting of 5% CO2. The medium was replenished three times a week.
H/R model and drug treatment
To establish an in vitro model of I/R injury, HBVSMCs were incubated at 37°C for 4, 6 or 8 h in a hypoxic (H) chamber (94% N2, 5% CO2 and 1% O2; Biospherixhypoxia chamber) to induce oxygen-deficiency and then reoxygenated (R) by culture at 37°C in a standard incubator (5% CO2 and 20% O2) for 16 h. HBVSMCs were divided into four groups: Normal group (HBVSMCs were cultured in normal medium and standard incubator); H (4 h)/R (16 h) group; H (6 h)/R (16 h) group; H (8 h)/R (16 h) group. To determine the protective effects of SAL on H/R injury, cells were pretreated with SAL (100, 200 or 400 µM) for 30 min and then underwent hypoxia for 8 h and reoxygenation for 16 h. HBVSMCs were divided into four groups: Normal group; H (8 h)/R (16 h) group; SAL+H (8 h)/R (16 h) group; SAL (400 µM) group. To identify the role of the SIRT1/FOXO3α pathway in the SAL-induced beneficial effects against H/R injury, cells were pretreated with sirtinol (10 µM) for 30 min. Cell were then incubated with SAL (400 µM) for 30 min prior to exposure to hypoxia for 8 h followed by reoxygenation for 16 h. HBVSMCs were divided into four groups: Normal group; H (8 h)/R (16 h) group; SAL+H (8 h)/R (16 h) group; Sirtinol (10 µM) + SAL+H (8 h)/R (16 h) group. All treatments were performed in triplicate.
Cell viability assay
The viability of HBVSMCs underlying different processing conditions as described was examined using an MTT assay following the manufacturer's protocol. Briefly, cells were seeded into 96-well plates at a density of 1×104 cells/well overnight at 37°C. MTT reagent (10 µl) was added to each well and cells were incubated for a further 4 h at 37°C. Formazan was subsequently dissolved in dimethyl sulfoxide. A microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was then used to measure absorbance at 490 nm. Cell viability (%) was calculated for all groups, as a proportion of the control. Each experiment was independently performed in triplicate.
Measurement of caspase-3 activity
The activity of caspase-3 in HBVSMCs was evaluated using a commercial caspase-3 ELISA kit following the manufacturer's protocol. Briefly, cells were trypsinized in RIPA lysis buffer and centrifuged at 12,000 × g at 4°C for 10 min. The protein concentration of each group was determined using a BCA protein assay kit and equal amounts of protein were incubated with 5 µl Ac-DEVD-pNA (acetyl-Asp-Glu-Val-Asp p-nitroanilide, 0.2 mM) at 4°C for 4 h in the dark. Absorbance was measured at a wavelength of 405 nm using a microplate reader and caspase-3 activity was calculated as follows: Optical density (OD) (experimental group)/OD (control group). Each experiment was independently performed in triplicate.
Hoechst 33342 staining
HBVSMCs were seeded in 24-well culture plates at a density of 1×105 cells/well. After cells reached ~70% confluence, cells were treated as previously described, washed with cold phosphate-buffered saline (PBS) three times and fixed with 4% paraformaldehyde at 4°C for 10 min in the dark. Cells were then washed with PBS and incubated with Hoechst 33342 (1 µg/ml) for 10 min at room temperature in the dark. Following three washes with PBS, cells were observed using a fluorescence microscope (Olympus Corporation, Tokyo, Japan). To calculate the average rate of apoptosis, 5 different, random sections of each group were assessed. The apoptosis rate (%) was calculated for all groups and compared with the control group. Each experiment was performed in triplicate.
Annexin V-FITC/PI staining
The apoptosis rate of the HBVSMCs was determined using an Annexin V-FITC/PI staining kit, following the manufacturer's instructions. In brief, HBVSMCs were digested with 0.25% trypsin and washed with PBS. Subsequently, cells were harvested and resuspended in the binding buffer contained in the staining kit (106 cells/ml), prior to mixing with Annexin V-FITC (5 µl) and PI (10 µl). Following incubation for 15 min in the dark at room temperature, the apoptosis ratio was determined using flow cytometry. The experiments were repeated three times independently.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
HBVSMCs in the logarithmic phase were seeded onto 6-well plates at a density of 1×104 cells/well. Total RNA was isolated using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The quantity and purity of RNA was detected using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Inc.). First strand cDNA was reverse-transcribed from equal amount of RNA using the Prime Script RT reagent kit (Takara Bio Inc., Otsu, Japan). For RT-qPCR, the primer sequences of SIRT1 were as follows: Forward, 5′-TCATTCCTGTGAAAGTGATGACGA-3′ and reverse, 5′-CTGCCCTAGTGTCATATCATCCAA-3′. The primer sequences of the GAPDH internal control were as follows: Forward, 5′-GGCACAGTCAAGGCTGAGAATG-3′ and reverse, 5′-ATGGTGGTGAAGACGCCAGTA-3′. The PCR process was performed as follows: 50°C for 2 min, 95°C for 2 min, followed by 40 cycles at 95°C for 15 sec and 60°C for 2 min. mRNA expression was calculated and normalized using the 2-∆∆Cq method (24) and expressed as a fold of the control. The assay was performed in triplicate for each sample.
Western blot analysis
H/R-injured HBVSMC in the presence and absence of SAL or sirtinol were harvested and lysed in RIPA buffer containing protease inhibitors for 30 min at 4°C. Total proteins were collected following centrifugation at 12,000 × g for 10 min at 4°C and quantified using the BCA Protein assay kit. Equal amounts of proteins (30 µg) were separated by 12% SDS-PAGE and transferred onto polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA). Non-specific protein binding was blocked with 5% non-fat milk for 2 h at room temperature. Subsequently, the membranes were incubated with primary antibodies against SIRT1 (1:1,000), Bax (1:1,000), Bcl-2 (1:1,000), FOXO3α (1:1,000) and GAPDH (1:2,000) overnight at 4°C. Following three washes with Tris-buffered saline containing 0.05% Tween-20, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (1:5,000; cat no. LS-C146625; LifeSpan BioSciences, Seattle, WA, USA) for 2 h at room temperature. Protein bands were analyzed using the BeyoECL Plus kit, an enhanced chemiluminescence detection system. The detected protein was quantified using Image J2 software (National Institutes of Health, Bethesda, MD, USA) and expressed as a percentage compared with GAPDH expression. Each experiment was independently performed in triplicate.
Statistical analysis
Data are expressed as mean ± standard error of the mean of three independent experiments. The statistical differences among different groups were assessed using one-way analysis of variance followed by a least significant difference test. P<0.05 was considered to represent a statistically significant difference.
Results
H/R treatment reduces levels of SIRT1 protein in HBVSMCs
It has been demonstrated that SIRT1 modulates pathways involved in oxidative stress, apoptosis and inflammation to protect against ischemia/hypoxia (13). Therefore, the current study assessed the impact of H/R on the expression of SIRT1 in HBVSMCs. The present study demonstrated that the expression of SIRT1 in HBVSMCs exposed to hypoxia for 6 and 8 h followed by reoxygenation for 16 h was significantly reduced compared with the control group (P<0.05; Fig. 1A), while a shortage of oxygen (4 h) followed by reoxygenation for 16 h did not significantly affect SIRT1 expression. In addition, levels of SIRT1 mRNA were measured using RT-qPCR and it was demonstrated that H/R treatment did not significantly affect SIRT1 mRNA levels in HBVSMCs (Fig. 1B). These results indicate that the upregulation of SIRT1 protein expression may affect cerebral H/R injury. Notably, 8 h hypoxia induced the largest decline in the expression of SIRT1 protein (P<0.01), therefore the duration of hypoxia HBVSMCs were subjected to in subsequent experiments was 8 h.
Figure 1.
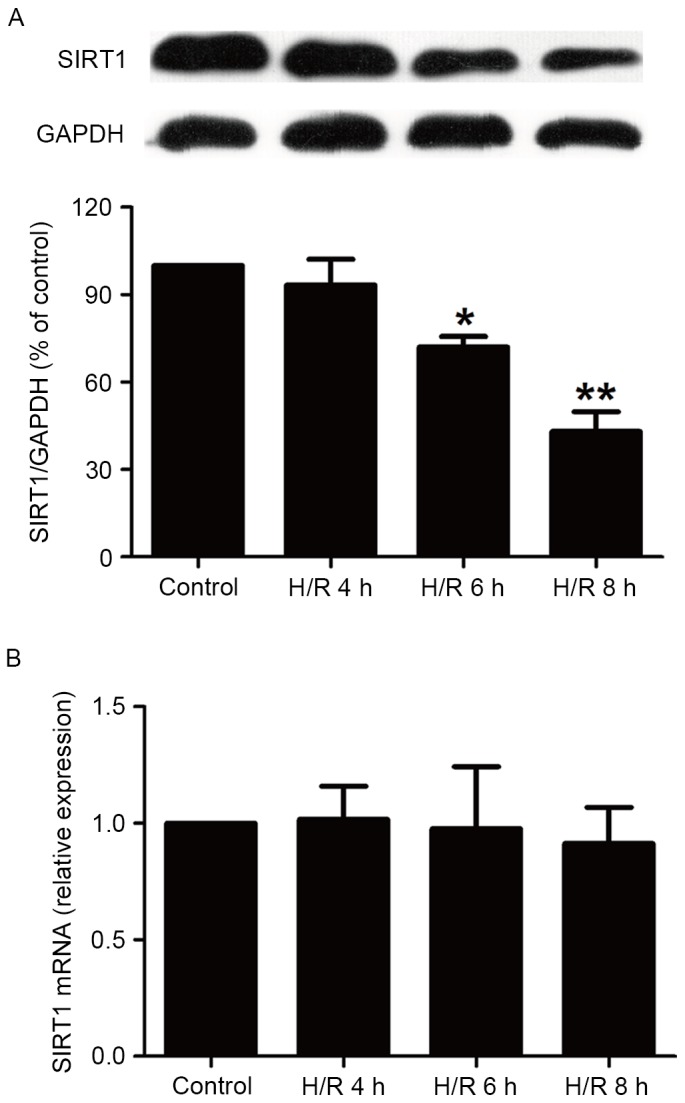
Effects of H/R treatment on the levels of SIRT1 protein and mRNA in HBVSMCs. HBVSMCs were exposed to hypoxia for 4, 6 and 8 h and then underwent reoxygenation for 16 h. (A) The expression of SIRT1 was assessed using western blot analysis and presented as a percentage of the control group. (B) SIRT1 mRNA levels were measured using reverse transcription-quantitative polymerase chain reaction. GAPDH was used as positive control. Data are presented as mean ± standard error of the mean from three independent experiments performed in triplicate. *P<0.05, **P<0.01 vs. control group. H/R, hypoxia/reoxygenation; SIRT1, sirtuin 1; HBVSMCs, human brain vascular smooth muscle cells; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
SAL attenuates H/R-induced cytotoxicity and apoptosis in HBVSMCs
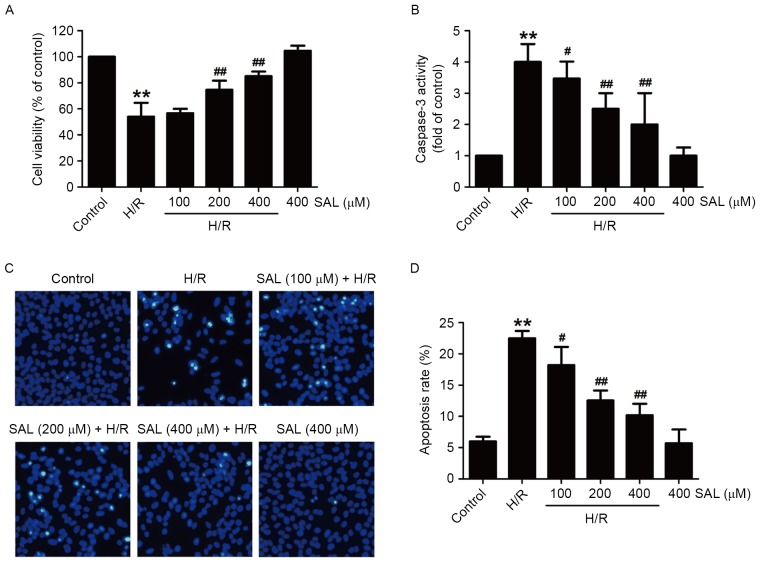
To investigate the protective effects of SAL on cerebral H/R injury, the viability of cells and apoptosis under hypoxia (8 h)/reoxygenation (16 h) treatment in the presence or absence of SAL was measured in HBVSMCs. Compared with the control group, the viability of HBVSMCs in the H/R group was significantly reduced (P<0.01). However, the downregulation of cell viability induced by H/R was significantly reversed following pretreatment with 200 or 400 µM SAL in HBVSMCs (P<0.01; Fig. 2A). In addition, pretreatment of HBVSMCs with SAL significantly reduced the H/R-induced increase in caspase-3 activity, a key regulatory factor in the process of apoptosis, in a concentration-dependent manner (P<0.05; Fig. 2B). Furthermore, following H/R treatment, HBVSMCs exhibit the phenomenon of chromatin condensation and fragmentation. Chromatin was stained brightly following H/R, however this was reversed following SAL treatment (Fig. 2C). Pretreatment with SAL also significantly reversed the upregulation of the apoptosis rate induced by H/R in a dose-dependent manner (P<0.05; Fig. 2D). Notably, the viability, caspase-3 activity and apoptosis rate of HBVSMCs treated with 400 µM SAL that did not undergo H/R were not significantly affected, suggesting that SAL alone has no effect on apoptosis. These results indicate that SAL treatment alleviates H/R-induced injuries following cerebral H/R injury.
Figure 2.
Protective effects of SAL on H/R-induced cytotoxicity and apoptosis in HBVSMC. Cells were pretreated with different concentrations of SAL (100, 200 or 400 µM) for 30 min and then exposed to hypoxia for 8 h followed by reoxygenation for 16 h. (A) The viability of HBVSMCs was detected by MTT assay and expressed as a proportion of the control. (B) Caspase-3 activity was measured using a commercialized caspase-3 assay kit and expressed as fold change of control. (C) The morphological changes of the apoptotic cells were observed using Hoechst 33324 staining (magnification, ×200) and assessed by (D) statistical analysis of the ratio of apoptotic cells to total cells. Data are presented as mean ± standard error of the mean from three independent experiments performed in triplicate. *P<0.05, **P<0.01 vs. control group; #P<0.05, ##P<0.01 vs. H/R treatment group. SAL, salidroside; H/R, hypoxia/reoxygenation; HBVSMCs, human brain vascular smooth muscle cells.
SAL prevents the H/R-induced disturbance of SIRT1 signaling pathway in HBVSMCs
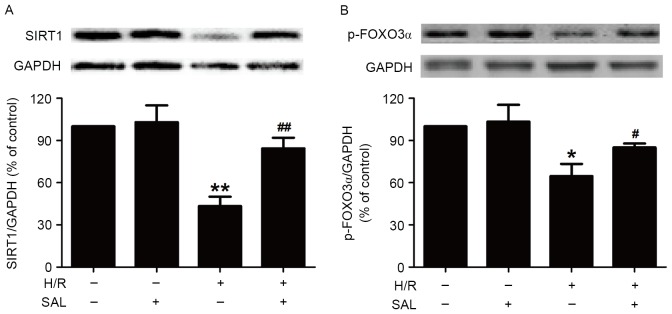
To investigate whether SAL protects HBVSMCs against H/R injury by modulating the SIRT1 signaling pathway, the present study measured the effect of SAL on the expression of SIRT1 protein in the presence or absence of H/R treatment. Pretreatment with SAL (400 µM) during H/R significantly increased the expression of SIRT1 protein in HBVSMCs compared with the H/R group (P<0.01; Fig. 3A), whereas SAL had no effect on the expression of SIRT1 in HBVSMCs that did not undergo H/R, indicating that the upregulation of SIRT1 may be involved in the protective role of SAL in H/R injury. In addition, the present study demonstrated that the downregulation of p-FOXO3α induced by H/R treatment (P<0.05) was reversed by pretreatment with SAL (400 µM) in HBVSMCs (P<0.05; Fig. 3B). SAL treatment alone had no influence on the expression of p-FOXO3α. These results suggest that the promotion of the SIRT/FOXO3α pathway may contribute to the inhibition of SAL on H/R injury.
Figure 3.
Effects of SAL on the H/R-induced decrease of SIRT1 and FOXO3α protein expression in HBVSMCs. Cells were pretreated with SAL (400 µM) for 30 min and then exposed to hypoxia for 8 h followed by reoxygenation for 16 h. The expression of (A) SIRT1 protein and (B) p-FOXO3α protein were detected using western blot analysis. GAPDH was used as positive control. Data are presented as mean ± standard error of the mean from three independent experiments performed in triplicate. *P<0.05, **P<0.01 vs. control group; #P<0.05, ##P<0.01 vs. H/R treatment group. SAL, salidroside; H/R, hypoxia/reoxygenation; SIRT1, sirtuin 1; p-FOXO3α, phosphorylated forkhead box protein O3α; HBVSMCs, human brain vascular smooth muscle cells; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Blocking the SIRT1/FOXO3α pathway attenuates the protective effect of SAL against H/R-induced cytotoxicity and apoptosis in HBVSMC
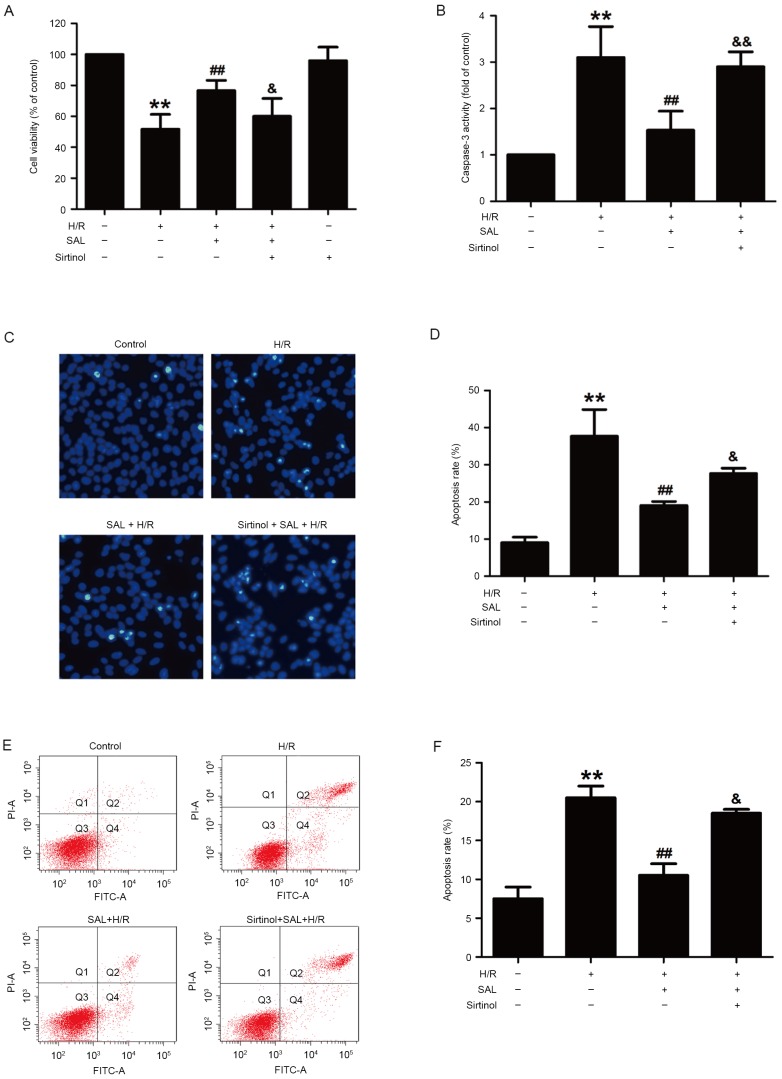
To confirm the contribution of the SIRT1/FOXO3α pathway to the SAL-induced reversal of H/R injury, sirtinol, a SIRT1-specific inhibitor, was used in subsequent experiments. The results demonstrated that pretreatment with sirtinol (10 µM) for 30 min significantly reduced the viability of HBVSMCs compared with that of the SAL and H/R co-treatment group (P<0.05), whereas sirtinol itself did not affect the viability of HBVSMCs (Fig. 4A). This indicates that SAL acted via the SIRT1/FOXO3α pathway to reverse the decrease in cell viability induced by H/R treatment. The impact of sirtinol on apoptosis was also investigated and it was demonstrated that sirtinol reversed the inhibitory effects of SAL (400 µM) on caspase-3 activity (P<0.01; Fig. 4B). This increased activity was also evident from the morphological changes induced by H/R treatment that occurred in apoptotic cells, characterized by nuclear condensation, fragmentation and bright blue fluorescence (Fig. 4C). The apoptosis rate was significantly increased following pretreatment with sirtinol compared with the H/R and SAL group (P<0.05; Fig. 4D). In addition, Annexin V-FITC/PI staining determined that sirtinol significantly inhibited the reversal effect of SAL (400 µM) on the H/R induced upregulation of the apoptosis ratio in HBVSMCs (P<0.05; Fig. 4E and F). These results indicate that the SIRT1/FOXO3α pathway mediated the SAL-induced protection against H/R-induced injury.
Figure 4.
Effects of sirtinol on the SAL-induced protection against H/R-induced cytotoxicity and apoptosis in HBVSMCs. Following pretreatment with sirtinol (10 µM) for 30 min, HBVSMCs were incubated with SAL (400 µM) for 30 min and then exposed to hypoxia for 8 h followed by reoxygenation for 16 h. (A) The viability of HBVSMCs was detected by MTT assay and expressed as a percentage of the control. (B) Caspase-3 activity was measured using a commercialized caspase-3 assay kit and expressed as a fold change compared with the control. The morphological changes in the apoptotic cells were observed by (C) Hoechst 33324 staining (magnification, ×200) and assessed by (D) statistical analysis of the ratio of apoptotic cells to total cells. (E) The apoptosis ratio of HBVSMC was detected by Annexin V-FITC/PI staining and assessed by (F) statistical analysis. Data are presented as the mean ± standard error of the mean from three independent experiments performed in triplicate. **P<0.01 vs. control group; ##P<0.01 vs. H/R group, &P<0.05, &&P<0.01 vs. SAL and H/R co-treatment group. SAL, salidroside; H/R, hypoxia/reoxygenation; HBVSMCs, human brain vascular smooth muscle cells; FITC, fluorescein isothiocyanate; PI, propidium iodide.
Inhibition of the SIRT1/FOXO3α pathway prevents the reversal effects of SAL on the H/R-induced alternations of Bax and Bcl-2 protein levels in HBVSMCs
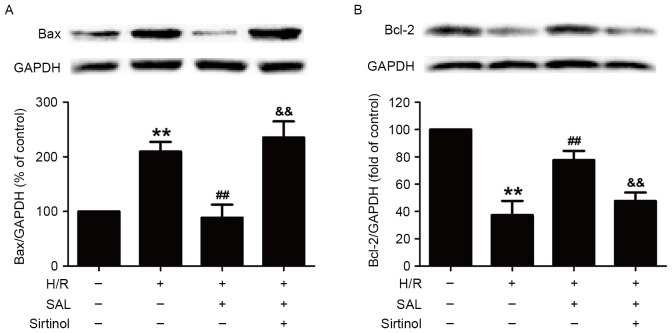
Finally, it was investigated whether the SIRT1/FOXO3α pathway was involved in the protective effect of SAL against the H/R-induced change in the expression of apoptosis-related proteins in HBVSMCs. Compared with the control group, H/R significantly increased the expression of the pro-apoptotic protein Bax (P<0.01) and this increase was reversed by pretreatment with SAL (400 µM; P<0.01; Fig. 5A). Notably, pretreatment with sirtinol (10 µM) significantly attenuated the SAL-induced downregulation of Bax expression in HBVSMCs compared with SAL and the H/R co-incubation group (P<0.01; Fig. 5A). Additionally, the significant decrease in the expression of the anti-apoptotic protein Bcl-2 induced by H/R was also significantly attenuated by SAL pre-treatment (400 µM; P<0.01; Fig. 5B). However, sirtinol reversed the SAL-induced increase in Bcl-2 expression (P<0.01; Fig. 5B). These results demonstrate that the SIRT1/FOXO3α pathway mediated the inhibitory role of SAL in H/R injury and may be involved in promoting the expression of proteins in the Bcl-2 family.
Figure 5.
Effects of sirtinol on the SAL-induced inhibitory role in H/R induced changes in Bax and Bcl-2 expression in HBVSMCs. Following pretreatment with sirtinol (10 µM) for 30 min, cells were incubated with SAL (400 µM) for 30 min and then exposed to hypoxia for 8 h followed by reoxygenation for 16 h. The expression of (A) Bax and (B) Bcl-2 was measured using western blot analysis. GAPDH was used as positive control. Data are presented as mean ± standard error of the mean from three independent experiments performed in triplicate. **P<0.01 vs. control group, ##P<0.01 vs. H/R treatment group, &&P<0.01 vs. SAL and H/R co-treatment group. SAL, salidroside; H/R, hypoxia/reoxygenation Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated X protein; HBVSMCs, human brain vascular smooth muscle cells; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
A number of studies have demonstrated that SAL exhibits neuroprotective effects following cerebral ischemic injury (6,25,26). SIRT1 regulates a variety of signaling pathways and is therefore considered to be a key mediator of cerebral ischemia (13,27). However, to the best of our knowledge, the role of the SIRT1 signaling pathway in the protection of SAL against H/R injury remains unknown. In the current study, HBVSMCs were used to establish an in vitro model of H/R injury to investigate the protective effects of SAL on H/R injury and the underlying protective mechanism of SAL during H/R. The current study indicated that the SIRT1/FOXO3 pathway is a mediator of SAL and exhibits beneficial roles in H/R-treated HBVSMCs. This suggests that SAL may be used clinically to treat patients with I/R injury and that the SIRT1/FOXO3 pathway may be a potential therapeutic target for treatments of cerebral ischemia.
Cerebral I/R-induced cytotoxicity and apoptosis serve a pivotal role in brain injury caused by I/R, in which apoptosis greatly contributes to the cell death that occurs following cerebral I/R injury (28). Caspase-3 has been identified as a pivotal mediator of apoptosis and previous studies have determined that the activity and expression of caspase-3 are upregulated in in vivo and in vitro models of ischemic stroke (29–31). Consistent with the above studies, the present study demonstrated that H/R-treated HBVSMCs, an appropriate and reproducible in vitro cell model of cerebral I/R injury, exhibited improved mimicking of the pathological conditions of I/R injury, including a decrease in cell viability and increases in caspase-3 activity and the cellular apoptosis rate. It has previously been demonstrated that SAL markedly inhibits cellular apoptosis and therefore has a neuroprotective effect (32) and the neuroprotective properties of SAL have been demonstrated in mice subjected to I/R injury, as well as in cultured nerve cells (25,26). Therefore, the present study investigated the effect of SAL on H/R injury in HBVSMCs and identified that pretreatment with SAL, which had no significant influence on cell viability and apoptosis under normal conditions, reversed H/R-induced cytotoxicity and apoptosis and increased caspase-3 activity in HBVSMCs, confirming the results of a previous study by Lai et al (33). The results of the present study clearly indicate that SAL pretreatment exhibits neuroprotective effects against H/R injury, which prompted further investigation of the underlying mechanisms.
Over the last decade, a number of studies have identified that SIRT1 serves a neuroprotective role in cerebral ischemia (15,34,35). Accumulating evidence indicates that SIRT1 is involved in a number of important physiological processes, including apoptosis, oxidative stress and the cell cycle, and therefore serves an important biological function in transcriptional regulation and signal transduction (13,36). Furthermore, intervention of the SIRT1 signaling pathway improves a variety of age-related diseases including coronary heart disease, type II diabetes and cerebral ischemia/reperfusion (37–39). Therefore, it was hypothesized that the protective functions of SAL are due to the regulation of the SIRT1 pathway that occurs following H/R injury.
In present study, it was demonstrated that H/R treatment significantly reduces the expression of SIRT1 protein in HBVSMCs, although the level of SIRT1 mRNA was unaffected by H/R. Therefore, inducing the upregulation of SIRT1 protein may contribute to H/R injury and this is consistent with the results of a previous study by Lv et al (40). The current study also assessed the effects of pretreating HBVSMCs with SAL and demonstrated that SAL markedly reversed the downregulation of SIRT1 protein expression, suggesting that the upregulation of SIRT1 may be involved in the protective effect of SAL against H/R injury. FOXO3α, which is highly expressed in the brain and heart, is involved in the regulation of apoptosis, cell survival, the cell cycle and oxidative stress (41). Transcriptional activity is regulated by SIRT1, which induces the phosphorylation of FOXO3α, regulates the activity of FOXO3α and inhibits apoptosis (42,43). Fukunaga and Shioda (44) confirmed that the expression of FOXO3α protein was inhibited in chronic cerebral ischemia. The current study demonstrated that H/R treatment decreased the expression of FOXO3α protein, while this inhibitory action was eradicated by SAL pretreatment in HBVSMCs. These results indicate that the SIRT1/FOXO3α pathway may mediate the neuroprotective effect induced by SAL. Notably, H/R downregulated the levels of p-FOXO3α more than the levels of SIRT1, which may be due to the presence of other regulatory signals contributing to the changes in FOXO3α levels following H/R treatment, such as SIRT6 (45) and the phosphatidylinositol 3-kinase/Akt signaling pathway (46), leading to a smaller decrease in levels of p-FOXO3α compared with SIRT1. In addition, the current study indicated that the inhibition of SIRT1 by sirtinol eradicated the reversal effect of SAL against the H/R-induced downregulation of cell viability and upregulation of apoptosis in HBVSMCs. This is similar to the results of a previous study, which demonstrated that sirtinol blocks the resveratrol-induced neuroprotection following cerebral ischemic damage (47). Notably, sirtinol did not affect cell viability and apoptosis in HBVSMCs that did not undergo H/R treatment, which is consistent with the results of previous studies (48,49). These results indicate that the activation of the SIRT1/FOXO3α pathway contributes to the neuroprotection induced by SAL in cells that have undergone H/R injury.
Bcl-2 family proteins are a critical checkpoint in apoptotic signal transduction cascades, which irreversibly damages cellular constituents (50). The ratio of Bcl-2/Bax protein is a determining factor in the modulation of apoptotic cell death, which is associated with the mitochondrial apoptotic pathway (51). Additionally, cerebral ischemia injury may activate the mitochondrial apoptotic pathway, as demonstrated by changes in caspase-like enzyme activation, cytochrome c release and the expression of Bcl-2 family proteins (52). In the present study, inhibition of the SIRT1/FOXO3α pathway prevented the reversal effect of SAL on the H/R-induced alternation of Bax and Bcl-2 in HBVSMCs, indicating that the SAL-activated SIRT1/FOXO3α pathway may improve mitochondrial function, thus protecting against I/R injury.
In conclusion, the current study demonstrated that pretreatment of HBVSMCs with SAL protects against H/R-induced cytotoxicity and apoptosis and that this neuroprotection depends on the activation of the SIRT1/FOXO3α pathway. These results may aid the development of novel strategies against cerebral ischemia and provide a foundation for a novel pharmacological approach to treat neuroprotective effects in patients with cerebral I/R injury or associated diseases by increasing activation of the SIRT1/FOXO3α pathway.
Acknowledgements
This study was supported by a grant from the First Affiliated Hospital of Zhengzhou University.
References
- 1.Gao J, He H, Jiang W, Chang X, Zhu L, Luo F, Zhou R, Ma C, Yan T. Salidroside ameliorates cognitive impairment in a d-galactose-induced rat model of Alzheimer's disease. Behav Brain Res. 2015;293:27–33. doi: 10.1016/j.bbr.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 2.Yang SJ, Yu HY, Kang DY, Ma ZQ, Qu R, Fu Q, Ma SP. Antidepressant-like effects of salidroside on olfactory bulbectomy-induced pro-inflammatory cytokine production and hyperactivity of HPA axis in rats. Pharmacol Biochem Behav. 2014;124:451–457. doi: 10.1016/j.pbb.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Xiao L, Li H, Zhang J, Yang F, Huang A, Deng J, Liang M, Ma F, Hu M, Huang Z. Salidroside protects Caenorhabditis elegans neurons from polyglutamine-mediated toxicity by reducing oxidative stress. Molecules. 2014;19:7757–7769. doi: 10.3390/molecules19067757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xian H, Zhao J, Zheng Y, Wang M, Huang J, Wu B, Sun C, Yang Y. MADP, a salidroside analog, protects hippocampal neurons from glutamate induced apoptosis. Life Sci. 2014;103:34–40. doi: 10.1016/j.lfs.2014.02.040. [DOI] [PubMed] [Google Scholar]
- 5.Lipp LL. Brain perfusion and oxygenation. Crit Care Nurs Clin North Am. 2014;26:389–398. doi: 10.1016/j.ccell.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Han J, Xiao Q, Lin YH, Zheng ZZ, He ZD, Hu J, Chen LD. Neuroprotective effects of salidroside on focal cerebral ischemia/reperfusion injury involve the nuclear erythroid 2-related factor 2 pathway. Neural Regen Res. 2015;10:1989–1996. doi: 10.4103/1673-5374.172317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barhwal K, Das SK, Kumar A, Hota SK, Srivastava RB. Insulin receptor A and Sirtuin 1 synergistically improve learning and spatial memory following chronic salidroside treatment during hypoxia. J Neurochem. 2015;135:332–346. doi: 10.1111/jnc.13225. [DOI] [PubMed] [Google Scholar]
- 8.Shi TY, Feng SF, Xing JH, Wu YM, Li XQ, Zhang N, Tian Z, Liu SB, Zhao MG. Neuroprotective effects of Salidroside and its analogue tyrosol galactoside against focal cerebral ischemia in vivo and H2O2-induced neurotoxicity in vitro. Neurotox Res. 2012;21:358–367. doi: 10.1007/s12640-011-9290-7. [DOI] [PubMed] [Google Scholar]
- 9.Zou YQ, Cai ZY, Mao YF, Li JB, Deng XM. Effects of salidroside-pretreatment on neuroethology of rats after global cerebral ischemia-reperfusion. Zhong Xi Yi Jie He Xue Bao. 2009;7:130–134. doi: 10.3736/jcim20090207. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 10.Kiseleva TN, Chudin AV. Experimental model of ocular ischemic diseases. Vestn Ross Akad Med Nauk. 2014:97–103. doi: 10.15690/vramn.v69i11-12.1190. (In Russian) [DOI] [PubMed] [Google Scholar]
- 11.Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: The ‘magnificent seven’, function, metabolism and longevity. Ann Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- 12.Pallàs M, Casadesús G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J, Camins A. Resveratrol and neurodegenerative diseases: Activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009;6:70–81. doi: 10.2174/156720209787466019. [DOI] [PubMed] [Google Scholar]
- 13.Meng X, Tan J, Li M, Song S, Miao Y, Zhang Q. Sirt1: Role Under the Condition of Ischemia/Hypoxia. Cell Mol Neurobiol. 2017;37:17–28. doi: 10.1007/s10571-016-0355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulose N, Raju R. Sirtuin regulation in aging and injury. Biochim Biophys Acta. 2015;1852:2442–2455. doi: 10.1016/j.bbadis.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Duan W, Li Y, Yan J, Yi W, Liang Z, Wang N, Yi D, Jin Z. New role of silent information regulator 1 in cerebral ischemia. Neurobiol Aging. 2013;34:2879–2888. doi: 10.1016/j.neurobiolaging.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Lin CH, Lin CC, Ting WJ, Pai PY, Kuo CH, Ho TJ, Kuo WW, Chang CH, Huang CY, Lin WT. Resveratrol enhanced FOXO3 phosphorylation via synergetic activation of SIRT1 and PI3K/Akt signaling to improve the effects of exercise in elderly rat hearts. Age (Dordr) 2014;36:9705. doi: 10.1007/s11357-014-9705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin W, Zhao W, Ho L, Wang J, Walsh K, Gandy S, Pasinetti GM. Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer's disease-type amyloid neuropathology and spatial memory deterioration. Ann N Y Acad Sci. 2008;1147:335–347. doi: 10.1196/annals.1427.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng K, Li Y, Long L, Li D, Jia Q, Wang Y, Shen Q, Tang Y, Wen L, Kung HF, Peng Y. Knockdown of FoxO3a induces increased neuronal apoptosis during embryonic development in zebrafish. Neurosci Lett. 2010;484:98–103. doi: 10.1016/j.neulet.2010.07.068. [DOI] [PubMed] [Google Scholar]
- 19.Mojsilovic-Petrovic J, Nedelsky N, Boccitto M, Mano I, Georgiades SN, Zhou W, Liu Y, Neve RL, Taylor JP, Driscoll M, et al. FOXO3a is broadly neuroprotective in vitro and in vivo against insults implicated in motor neuron diseases. J Neurosci. 2009;29:8236–8247. doi: 10.1523/JNEUROSCI.1805-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 21.Avraham Y, Davidi N, Porat M, Chernoguz D, Magen I, Vorobeiv L, Berry EM, Leker RR. Leptin reduces infarct size in association with enhanced expression of CB2, TRPV1, SIRT-1 and leptin receptor. Curr Neurovasc Res. 2010;7:136–143. doi: 10.2174/156720210791184943. [DOI] [PubMed] [Google Scholar]
- 22.Zhu HR, Wang ZY, Zhu XL, Wu XX, Li EG, Xu Y. Icariin protects against brain injury by enhancing SIRT1-dependent PGC-1alpha expression in experimental stroke. Neuropharmacology. 2010;59:70–76. doi: 10.1016/j.neuropharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Wang T, Gu J, Wu PF, Wang F, Xiong Z, Yang YJ, Wu WN, Dong LD, Chen JG. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: Involvement of JNK, SIRT1, and NF-kappaB pathways and inhibition of intracellular ROS/RNS generation. Free Radic Biol Med. 2009;47:229–240. doi: 10.1016/j.freeradbiomed.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Deng A, Zhou T, Ding F. Pretreatment with 2-(4-methoxyphenyl)ethyl-2-acetamido-2-deoxy-β-D-pyranoside attenuates cerebral ischemia/reperfusion-induced injury in vitro and in vivo. PLoS One. 2014;1:e100126. doi: 10.1371/journal.pone.0100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han T. Effects of salidroside pretreatment on expression of tumor necrosis factor-alpha and permeability of blood brain barrier in rat model of focal cerebralischemia-reperfusion injury. Asian Pac J Trop Med. 2013;6:156–158. doi: 10.1016/S1995-7645(13)60014-0. [DOI] [PubMed] [Google Scholar]
- 27.Koronowski KB, Perez-Pinzon MA. Sirt1 in cerebral ischemia. Brain Circ. 2015;1:69–78. doi: 10.4103/2394-8108.162532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakka VP, Gusain A, Mehta SL, Raghubir R. Molecular mechanisms of apoptosis in cerebral ischemia: Multiple neuroprotective opportunities. Mol Neurobiol. 2008;37:7–38. doi: 10.1007/s12035-007-8013-9. [DOI] [PubMed] [Google Scholar]
- 29.Zhao JJ, Song JQ, Pan SY, Wang K. Treatment with isorhamnetin protects the brain against ischemic injury in mice. Neurochem Res. 2016;41:1939–1948. doi: 10.1007/s11064-016-1904-2. [DOI] [PubMed] [Google Scholar]
- 30.Ahn SM, Kim HN, Kim YR, Choi YW, Kim CM, Shin HK, Choi BT. Emodin from Polygonum multiflorum ameliorates oxidative toxicity in HT22 cells and deficits in photothrombotic ischemia. J Ethnopharmacol. 2016;188:13–20. doi: 10.1016/j.jep.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 31.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo Y, Zhao Y, Zheng C, Meng Y, Yang Y. Synthesis, biological activity of salidroside and its analogues. Chem Pharm Bull (Tokyo) 2010;58:1627–1629. doi: 10.1248/cpb.58.1627. [DOI] [PubMed] [Google Scholar]
- 33.Lai W, Zheng Z, Zhang X, Wei Y, Chu K, Brown J, Hong G, Chen L. Salidroside-mediated neuroprotection is associated with induction of early growth response genes (Egrs) across a wide therapeutic window. Neurotox Res. 2015;28:108–121. doi: 10.1007/s12640-015-9529-9. [DOI] [PubMed] [Google Scholar]
- 34.Shi N, Zhu C, Li L. Rehabilitation training and resveratrol improve the recovery of neurological and motor function in rats after cerebral ischemic injury through the Sirt1 signaling pathway. Biomed Res Int. 2016;2016:1732163. doi: 10.1155/2016/1732163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu H, Wang B. SIRT1 exerts neuroprotective effects by attenuating cerebral ischemia/reperfusion-induced injury via targeting p53/microRNA-22. Int J Mol Med. 2017;39:208–216. doi: 10.3892/ijmm.2016.2806. [DOI] [PubMed] [Google Scholar]
- 36.Mellini P, Valente S, Mai A. Sirtuin modulators: An updated patent review (2012–2014) Expert Opin Ther Pat. 2015;25:5–15. doi: 10.1517/13543776.2014.982532. [DOI] [PubMed] [Google Scholar]
- 37.Xue F, Huang JW, Ding PY, Zang HG, Kou ZJ, Li T, Fan J, Peng ZW, Yan WJ. Nrf2/antioxidant defense pathway is involved in the neuroprotective effects of Sirt1 against focal cerebral ischemia in rats after hyperbaric oxygen preconditioning. Behav Brain Res. 2016;309:1–8. doi: 10.1016/j.bbr.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 38.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Kreutzenberg SV, Ceolotto G, Papparella I, Bortoluzzi A, Semplicini A, Dalla Man C, Cobelli C, Fadini GP, Avogaro A. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: Potential biochemical mechanisms. Diabetes. 2010;59:1006–1015. doi: 10.2337/db09-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lv H, Wang L, Shen J, Hao S, Ming A, Wang X, Su F, Zhang Z. Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Res Bull. 2015;115:30–36. doi: 10.1016/j.brainresbull.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Lam EW, Francis RE, Petkovic M. FOXO transcription factors: Key regulators of cell fate. Biochem Soc Trans. 2006;34:722–726. doi: 10.1042/BST0340722. [DOI] [PubMed] [Google Scholar]
- 42.Gomes AR, Yong JS, Kiew KC, Aydin E, Khongkow M, Laohasinnarong S, Lam EW. Sirtuin1 (SIRT1) in the acetylation of downstream target proteins. Methods Mol Biol. 2016;1436:169–188. doi: 10.1007/978-1-4939-3667-0_12. [DOI] [PubMed] [Google Scholar]
- 43.Zakhary SM, Ayubcha D, Dileo JN, Jose R, Leheste JR, Horowitz JM, Torres G. Distribution analysis of deacetylase SIRT1 in rodent and human nervous systems. Anat Rec (Hoboken) 2010;293:1024–1032. doi: 10.1002/ar.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukunaga K, Shioda N. Pathophysiological relevance of forkhead transcription factors in brain ischemia. Adv Exp Med Biol. 2009;665:130–142. doi: 10.1007/978-1-4419-1599-3_10. [DOI] [PubMed] [Google Scholar]
- 45.Wang XX, Wang XL, Tong MM, Gan L, Chen H, Wu SS, Chen JX, Li RL, Wu Y, Zhang HY, et al. SIRT6 protects cardiomyocytes against ischemia/reperfusion injury by augmenting FoxO3α-dependent antioxidant defense mechanisms. Basic Res Cardiol. 2016;111:13. doi: 10.1007/s00395-016-0531-z. [DOI] [PubMed] [Google Scholar]
- 46.Zheng F, Tang Q, Wu J, Zhao S, Liang Z, Li L, Wu W, Hann S. p38α MAPK-mediated induction and interaction of FOXOa and p contribute to the inhibited-growth and induced-apoptosis of human lung adenocarcinoma cells by berberine. J Exp Clin Cancer Res. 2014;33:36. doi: 10.1186/1756-9966-33-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JH, Moon JH, Nazim UM, Lee YJ, Seol JW, Eo SK, Lee JH, Park SY. Melatonin protects skin keratinocyte from hydrogen peroxide-mediated cell death via the SIRT1 pathway. Oncotarget. 2016;7:12075–12088. doi: 10.18632/oncotarget.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu L, Li Q, Yu B, Yang Y, Jin Z, Duan W, Zhao G, Zhai M, Liu L, Yi D, et al. Berberine attenuates myocardial ischemia/reperfusion injury by reducing oxidative stress and inflammation response: Role of silent information regulator 1. Oxid Med Cell Longev. 2016;2016:1689602. doi: 10.1155/2016/1689602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng JH, Viacava Follis A, Kriwacki RW, Moldoveanu T. Discoveries and controversies in BCL-2 protein-mediated apoptosis. FEBS J. 2016;283:2690–2700. doi: 10.1111/febs.13527. [DOI] [PubMed] [Google Scholar]
- 51.Gross A. BCL-2 family proteins as regulators of mitochondria metabolism. Biochim Biophys Acta. 2016;1857:1243–1246. doi: 10.1016/j.bbabio.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 52.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]