Abstract
In certain patients with advanced colorectal cancer, loss of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) activity is observed. PTEN is a major gatekeeper gene of the AKT serine/threonine kinase (AKT) signaling pathway responsible for the proliferative activity of cells. The assessment of AKT activity may be a prognostic factor or a predictor of response to the targeted therapies against particular signaling proteins. To precisely identify the cause and the place of the pathway deregulation, it is necessary to identify phosphorylation states and concentrations of several proteins located at different levels of the regulatory cascade. In the present study, we propose the simultaneous use of specific antibodies conjugated with different quantum dots to highlight the nature of AKT/PKB cascade deregulation in patients with colorectal cancer and the loss of PTEN expression in tumor tissue. Fifty patients with colorectal cancer of no specific location were enrolled in the study. The expression of the PTEN protein, and concentrations of phosphorylated/activated forms of 3-Phosphoinositide-dependent kinase 1 (PDK1) and AKT were assessed using quantum dot-conjugated antibodies. In patients with a diminished or complete loss of the PTEN expression in the tumor tissue increased levels of activated/phosphorylated forms of PDK1 (Phospho-PDK1-Ser241) and AKT (Phospho-AKT-Thr308) proteins were found, which are responsible for the permanent activation of the phosphoinositide 3-kinase/AKT/PTEN signaling pathway in certain cases of colorectal cancer.
Keywords: AKT, PKB, Quantum dots, colorectal cancer, PTEN
Introduction
Much evidence demonstrates dysregulation in the PI3K/AKT signaling pathway in colorectal cancer (CRC) (1). It seems that in some cases activation of AKT downstream is an initial step in the development of CRC. On the other hand, PI3K/AKT activation is found in more advanced stages of CRC carcinogenesis, which in many cases is caused by PTEN protein loss or downregulation.
Phosphatase and tensin homolog deleted on chromosome ten (PTEN) is an important suppressor gene. Protein product of the PTEN gene is a dual-specific phosphatase which plays a crucial role in the signal transduction from the membrane receptors to the intracellular downstream cascades. Its role in the development of many cancers was doubtlessly confirmed with a mechanism of action via the catalysis of phosphate detachment from (3–5)-tri-phospho-inositol (PIP3) in the PI3K/AKT signaling pathway (2,3).
In our previous studies we demonstrated that PTEN activity may be diminished as early as in the phase of an intestinal glandular polyp and is frequently observed in CRC (4,5). These observations justify the following questions: How much the loss of PTEN activates AKT signaling and how it is reflected in the levels and states of its signaling protein members.
This problem is very important in the context of cytotoxic therapy in CRC which is administered concurrently with directed therapy aimed to compensate molecular disturbances in the activity of the PI3K/AKT/PTEN pathway. Identification of the characteristic pattern of signaling protein expression and phosphorylation states could be a predictive marker for target therapies based on specific signaling kinase inhibitors. The simultaneous quantitative or at least semi-quantitative assessment of many proteins with preservation of information about their spatial location is within the interest of the system biology. Unfortunately, it suffers from a lack of appropriate methodology to achieve these goals. Some hope with severe restrictions is placed on immunofluorescence techniques. However, it is possible to visualize only 3–4 proteins with these methods. Significantly greater possibilities are connected with the use of antibodies conjugated with semiconducting quantum dots (QDs) which are within the interest of nanotechnology.
Quantum dots are semiconducting nanocrystals sized 2–10 nm which became a promising research, diagnostic and possibly therapeutic tool due to their unique possibilities of light absorption and emission in the process known as the quantum confinement effect. By changing the composition and/or the size of QDs emission spectrum may be freely modified starting from ultraviolet (UV) to infrared (IR). Simultaneously QDs are characterized by a very broad absorption spectrum and a narrow emission spectrum, which allows synchronic excitation of several QDs by the light of the same wavelength. Due to all these properties, QDs could be used in the precise detection of a plethora of biomolecules such as nucleic acids, carbohydrates, enzymes and antibodies (6–8).
The aim of the present study was to visualize dysregulation of signaling protein members of PI3K/AKT/PTEN pathway in CRC in response to the loss of activity of its major suppressor that is PTEN using QD-conjugated antibodies. In the future perspective the presented method could be utilized in visualization of subtle changes of concentrations and activation states of crucial regulatory proteins in other signaling cascades which are important in oncogenesis.
Materials and methods
Study group
A total of fifty patients diagnosed with adenocarcinoma of the large intestine after major surgery were enrolled in the study. Clinico-pathological characteristics of the study group are presented in Table I. Immediately after the excision intestinal biopsy samples with tumors were fixed in neutral buffered formalin with the addition of commercially-available pan-phosphatase inhibitor PhosSTOP (Phosphatase Inhibitor Coctail tablets; Roche, Mannheim, Germany). Preparations were dissected longitudinally to ensure quick penetration of the fixative to the tumor tissues. During the grossing process the representative tumor samples were taken by the pathologist then dehydrated and embedded in paraffin.
Table I.
Clinicopathological characteristics of the study group.
| Feature | Value |
|---|---|
| Age, years | 67 (range, 42–85) |
| Sex | |
| Male | 27 |
| Female | 23 |
| Location | |
| Colon | 32 |
| Rectum | 18 |
| Histologic type | |
| Adenocarcinoma | 55 |
| Mucinosum | 5 |
| Dysplasia grade | |
| G1 | 13 |
| G2 | 29 |
| G3 | 8 |
| TNM staging | |
| I | 14 |
| II | 17 |
| III | 9 |
| IV | 10 |
TNM, tumor node metastasis.
Preparing QD-conjugated antibodies
During QD-conjugation of antibodies commercially-available reagent kits were used (QDots Antibody Labeling kit, Molecular Probes; Invitrogen, Carlsbad, CA, USA). Quantum dots with maxima of emission located at 525, 565 and 585 nm were utilized in the present study. The procedure of antibody labeling was carried out according to the manufacturer's instructions and consisted of the following five steps: i) Antibody concentration and buffer exchange in order to eliminate other proteins as well as azide ions used as an antibody preservative, ii) modification of the carbohydrate domain of the antibody using galactosidase enzyme, iii) azide domain connection to the modified antibody, iv) purification of the activated antibody amd v) conjugation of the activated antibody with QD molecule using dibenzo-cyclo-octane (DIBO) residue as an interconnector.
Final concentration of the conjugated antibodies was estimated to be about 1 mg/ml according to the manufacturer's specifications assuming 2 µmol/l molarity of the obtained conjugate (9).
Immunofluorescence
Formalin-fixed paraffin-embedded tumor samples were cut with a microtome into 5 µm-thick histological sections and fixed on microscope subject glasses. The slides were rehydrated in xylene, graded alcohols and finally in phosphate-buffered saline (PBS). Next the process of antigen retrieval was carried out in citric buffer for 30 min in a microwave oven. Finally, the remains of citric buffers were washed out using PBS and the slides were exposed to QD-labeled antibodies. In order to demonstrate the concentration and spatial location of subject proteins (PTEN, phosphorylated PDK1 and partially-phosphorylated AKT) specific QD-labeled monoclonal antibodies were used. The brief characteristics of the used antibodies and their concentrations are presented in the Table II.
Table II.
Summary and the characteristics of primary antibodies used in the study.
| Target | Antibody type/clone | Host | Antibody dilution | Manufacturer |
|---|---|---|---|---|
| PTEN (pan) | Monoclonal 138G6 | Rabbit IgG | 1:500 | Cell Signaling Technology, Inc. |
| Phospho-Akt Thr308 | Monoclonal D52E6 | Rabbit IgG | 1:1,500 | Cell Signaling Technology, Inc., no. 13038 |
| PhosphoPDK1 Ser241 | Monoclonal C49H2 | Rabbit IgG | 1:1,000 | Cell Signaling Technology, Inc., no. 3438 |
AKT, AKT serine/threonine kinase; PDK-1, 3-Phosphoinositide-dependent kinase 1; PTEN, phosphatase and tensin homolog deleted on chromosome 10.
Incubation of the tumor sections with primary antibodies was performed for 12 h in a humidified chamber in the fridge at the temperature of 6°C. After the incubation antibody solutions were washed out with PBS and the preparations were mounted with fluorescence-preserving mounting medium dedicated to QDs (Qmount Qdot Mounting Medium; Invitrogen).
Fluorescence acquisition and fluorescence signal assessment
Visualization of the fluorescence signal of the QD-labeled antibodies was performed with epi-fluorescence microscope (AxioImager M.2; Carl Zeiss, Jena, Germany) equipped with a set of excitation/emission filters with spectral characteristics compatible with used QDs band-pass filters with the transmission bandwidth of 20 nm (Chroma, Augsburg, Germany), Plan-apochromat objectives with magnification of 63× NA=0.95 and 100× NA=1.3 oil immersion as well as AxioCam MRm 1.4 Mega pixel monochrome camera (Carl Zeiss).
Fluorescence signal measurement was made in a region-based manner. At least 20 manually selected points corresponding to submembrane or cytoplasmic regions of individual neoplastic cells were sampled and averaged for the particular region and case. As a measure of antibody concentrations the mean pixel values ranging from 0 (no fluorescence) to 255 (maximal fluorescence signal strength) was reported and included in the further analysis.
Statistical analysis
Statistical analysis of the results was performed using Statistica software v8.0, (Statsoft Inc., Palo Alto, CA, USA). The comparisons of the mean values of the analyzed parameters were performed using Student's t-test. Interrelationships between fluorescence signals were investigated by calculating of Pearson's correlation coefficient (R). P<0.05 was considered to indicate a statistically significant difference.
Ethical approval
The present study was conducted in accordance with the guidelines of the Declaration of Helsinki and its subsequent amendments, and informed consent was obtained from all patients. Project of the study was approved by institutional review board of the Medical University of Silesia, Katowice (approval no. KNW/0022KBI/178/09).
Results
In the cases with a complete loss or a diminished expression of the PTEN protein (defined as pixel values of PTEN immunofluorescence less than median) fluorescence signals corresponding to phospho-PDK1 (Ser241) and phospho-AKT (Thr308) were higher than in the group with normal PTEN expression (above median; t-Student test P=0.002 and P=0.01 respectively). Maxima of fluorescence signals corresponding to phospho-PDK1 and phospho-AKT were localized in a submembrane cellular compartment and were colocalized with each other (Fig. 1). We also found a positive correlation between fluorescence signals corresponding to phospho-PDK1 and phospho-AKT (R=0.65 P=0.047) as well as a negative correlation between PTEN signal and the above-mentioned signaling proteins (R=−0.47, P=0.05; and R=−0.51, P=0.055, respectively) in the group of all the examined samples.
Figure 1.
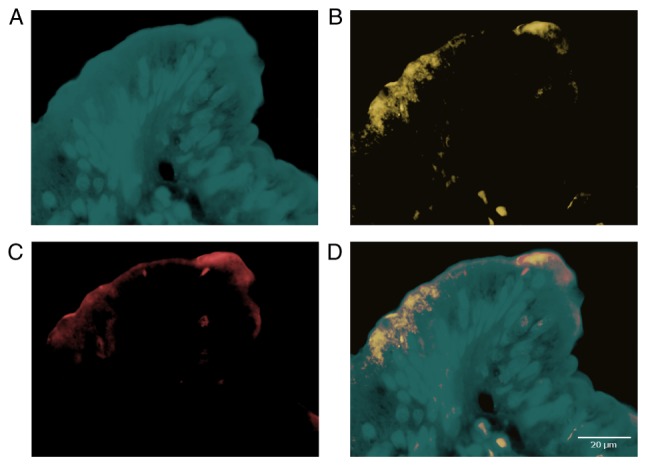
Representative immunofluorescence images of the colorectal cancer cells showing expression of activated PDK1, AKT and total PTEN expression in separated (A, B, C respectively) and combined (D) fluorescence channels. Note primarily submembrane location of the markers (less then 10 µm from the cell membrane).
The summary of the obtained results is presented in Table III.
Table III.
Fluorescent signal strength of the analyzed signaling proteins in relation to the expression of the PTEN protein.
| PTEN expression | ||
|---|---|---|
| Marker | Negative (n=26) | Positive (n=24) |
| PTEN (pan) | Mean=156.29 (49–250)a | |
| MarkerMedian=167 | ||
| SD=57.1 | ||
| Mean=108.08 (49–167) | Mean=206.5 (173–250) | |
| Median=105 | Median=211.5 | |
| SD=33.58 | SD=21.68 | |
| phospho-AKT (Thr308) | Mean=75.92 (28–153) | Mean=49.04 (18–117) |
| SD=27.98 | SD=29.16 | |
| phospho-PDK1 (Ser241) | Mean=74.16 (43–107) | Mean=61.00 (34–101) |
| SD=16.47 | SD=18.25 | |
range; SD, standard deviation; P-value, level of statistical significance; AKT, AKT serine/threonine kinase; PDK-1, 3-Phosphoinositide-dependent kinase 1; PTEN, phosphatase and tensin homolog deleted on chromosome 10; SD, standard deviation.
Discussion
A dynamic development of imaging techniques utilizing QDs in medicine and biological sciences has been observed since the end of the 20th century, especially from the moment of the first synthesis of water-soluble QDs with biologically-active coating molecules. Since then QDs with adequately modified surface have been used in labeling of cell surface and intracellular structures in live cells or fixed specimens. Unique features of QDs connected with wideband excitation spectra and a narrow emission of excited light allowed to use their functionalized conjugates in detection and visualization of virtually all biomolecules, cells and tissues both in vivo and in vitro. In medicine QDs are seen as modern markers which could be used to visualize and track spreading of neoplastic cells or viruses in the human body (7,10,11). Quantum dots also possess a therapeutic potential in oncology (12,13). It seems that QDs are more precise and stable markers than widely used organic chromogens (6).
By increasing the diameter of the core of the QD the wavelength of emitted light can be increased. Based on this phenomenon, a broad range of QDs with different spectral characteristics can be easily synthesized in one technological process. Using a single monochromatic excitation source all of these QDs can be excited producing fluorescence light from UV to IR in narrow, non-overlapping bands eliminating the problem of cross-talk between channels (6).
In life sciences the following are used in fluorescence microscopy: Cadmium selenide (core) and cadmium sulfide (shell) QDs (CdSe/ZnS) with emission between 450–650 nm. The second type of QDs with emission of longer wavelengths between 500 and 750 nm are cadmium telluride QDs (CdTe) (6,8). Broad absorption spectra of QDs allow at the same time to effectively excite them using single light source emitting in the range of UV, violet or blue light equipped with a short-pass excitation filter. Simultaneously, a narrow emission spectrum with almost arbitrary shaping of its width and placement within both visible and IR light allows creating many non-overlapping acquisition channels with the aid of standard single-band interference emission filters with a bandwidth of 20 or 40 nm. In the present experiment only three simultaneous channels were used, however it confirms the concept in the field of bioimaging of protein concentration and activation state with preserving information about their spatial location in single cells.
Many cancers possess elevated levels of PtdIns(3,4,5)P(3), the second messenger that induces activation of the protein kinase PKB/AKT and thereby stimulates cell proliferation, growth, and survival. The importance of this pathway in tumorigenesis has been highlighted by the finding that PTEN, the lipid phosphatase that breaks down PtdIns(3,4,5)P(3) to PtdIns(4,5)P(2), is frequently mutated in human cancer. Cells lacking PTEN possess elevated levels of PtdIns(3,4,5)P(3) correlated with PKB activation (14,15).
3-Phosphoinositide-dependent kinase 1 (PDK1) is the first node of the PI3K/AKT/PTEN signal cascade and is required for activation of AKT. PIP(3) recruits PDK1 and AKT to the cell membrane through interactions with their pleckstrin homology domains, allowing PDK1 to activate AKT by phosphorylating it at residue threonine-308 (16).
It was demonstrated in breast cancer patients that increased PDK1 copy number is associated with upstream pathway lesions (ERBB2 amplification, PTEN loss, or PIK3CA mutation), as well as patient survival. The examination of an independent set of breast cancers and tumor cell lines derived from multiple forms of human cancers also found increased PDK1 protein levels associated with such upstream pathway lesions. In human mammary cells, PDK1 enhanced the ability of upstream lesions to signal to AKT, stimulate cell growth and migration, and rendered cells more resistant to PDK1 and PI3K inhibition (17).
The role of PTEN in the CRC pathology has been postulated by many authors. The loss of PTEN expression is postulated to be an early event in CRC carcinogenesis which is described as early as in precancerous lesions-large intestine adenomatous polyps (adenomas) (4) and its frequency increases in more advanced stages (5). An association between PTEN status and histological grade, tumor size and clinical outcome was also demonstrated by other authors (18–20).
In our present experiment we tried to assess the functional state of the top-most elements of PI3K/AKT/PTEN pathway in CRC. They include gate-keeping, pacemaker gene PTEN and its two effector proteins PDK1 and AKT.
In the analyzed samples of large intestine adenocarcinoma we found that a lower concentration of PTEN in the cytoplasm neighboring the cell membrane correlates with the accumulation of activated by phosphorylation forms of downstream signaling proteins: Phospho-PDK1-Ser241 and phospho-AKT-Thr308. This observation is indirect evidence of tri-phosphatydyl-inositol (PIP3) concentration due to the loss of phosphatase activity of PTEN in cancer cells described by other authors as separate observations. To our best knowledge, this is the first report demonstrating the above-described changes concomitantly in the same cellular compartments of cancer cells as a response to the loss of PTEN activity.
In patients with a diminished or complete loss of the PTEN expression in the tumor tissue we found increased levels of activated/phosphorylated forms of PDK1 (Phospho-PDK1-Ser241) and AKT (Phospho-AKT-Thr308) proteins which are responsible for a permanent activation of the PI3K/AKT/PTEN pathway in some cases of CRC.
Application of QDs in fluorescence bioimaging is a more flexible and precise method in comparison to the traditionally utilized organic chromophores. Due to their unique properties QDs form a new class of optical markers successfully applied in life sciences imaging with specific antibodies allowing simultaneous visualization of location, concentration and activity states of specified signaling proteins in cellular compartments.
References
- 1.Davies EJ, Marsh Durban V, Meniel V, Williams GT, Clarke AR. PTEN loss and KRAS activation leads to the formation of serrated adenomas and metastatic carcinoma in the mouse intestine. J Pathol. 2014;233:27–38. doi: 10.1002/path.4312. [DOI] [PubMed] [Google Scholar]
- 2.Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–198. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- 3.Leslie NR, Downes CP. PTEN function: How normal cells control it and tumour cells lose it. Biochem J. 2004;382:1–11. doi: 10.1042/BJ20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waniczek D, Śnietura M, Młynarczyk-Liszka J, Pigłowski W, Kopeć A, Lange D, Rudzki M, Arendt J. PTEN expression profiles in colorectal adenocarcinoma and its precancerous lesions. Pol J Pathol. 2013;64:15–20. doi: 10.5114/pjp.2013.34598. [DOI] [PubMed] [Google Scholar]
- 5.Waniczek D, Snietura M, Kopec A, Scieglinska D, Piglowski W, Lorenc Z, Muc-Wierzgon M, Nowakowska-Zajdel E. A novel quantitative method of pten expression assessment in tumor tissue. J Biol Regul Homeost Agents. 2016;30:79–90. [PubMed] [Google Scholar]
- 6.Smith AM, Duan H, Mohs AM, Nie S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev. 2008;60:1226–1240. doi: 10.1016/j.addr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang M, Peng CW, Pang DW, Li Y. Quantum dots for cancer research: Current status, remaining issues, and future perspectives. Cancer Biol Med. 2012;9:151–163. doi: 10.7497/j.issn.2095-3941.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iga AM, Robertson JH, Winslet MC, Seifalian AM. Clinical potential of quantum dots. J Biomed Biotechnol. 2007;10:76087. doi: 10.1155/2007/76087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molecular Probes Inc., corp-author; Molecular Probes, Inc., corp-editor Qdot antibody conjugation kits. 2008. Separating the conjugated antibody from unconjugated antibody; pp. 7–8. [Google Scholar]
- 10.Wang Y, Chen L. Quantum dots, lighting up the research and development of nanomedicine. Nanomedicine. 2011;7:385–402. doi: 10.1016/j.nano.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Liu SL, Wang ZG, Zhang ZL, Pang DW. Tracking single viruses infecting their host cells using quantum dots. Chem Soc Rev. 2016;45:1211–1224. doi: 10.1039/C5CS00657K. [DOI] [PubMed] [Google Scholar]
- 12.Tholouli E, Sweeney E, Barrow E, Clay V, Hoyland JA, Byers RJ. Quantum dots light up pathology. J Pathol. 2008;216:275–285. doi: 10.1002/path.2421. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Qiu X, Wang Z, Pan J, Chen J, Han J. Application of quantum dots as vectors in targeted survivin gene siRNA delivery. Onco Targets Ther. 2013;3:303–309. doi: 10.2147/OTT.S38453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayascas JR, Leslie NR, Parsons R, Fleming S, Alessi DR. Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN(+/-) mice. Curr Biol. 2005;15:1839–1846. doi: 10.1016/j.cub.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 15.Haas-Kogan D, Shalev N, Wong M, Mills G, Yount G, Stokoe D. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998;8:1195–1198. doi: 10.1016/S0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 16.Bayascas JR. Dissecting the role of the 3-phosphoinositide-dependent protein kinase-1 (PDK1) signalling pathways. Cell Cycle. 2008;7:2978–2982. doi: 10.4161/cc.7.19.6810. [DOI] [PubMed] [Google Scholar]
- 17.Maurer M, Su T, Saal LH, Koujak S, Hopkins BD, Barkley CR, Wu J, Nandula S, Dutta B, Xie Y, et al. 3-Phosphoinositide-dependent kinase 1 potentiates upstream lesions on the phosphatidylinositol 3-kinase pathway in breast carcinoma. Cancer Res. 2009;69:6299–6306. doi: 10.1158/0008-5472.CAN-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yazdani Y, Farazmandfar T, Azadeh H, Zekavatian Z. The prognostic effect of PTEN expression status in colorectal cancer development and evaluation of factors affecting it: miR-21 and promoter methylation. J Biomed Sci. 2016;23:9. doi: 10.1186/s12929-016-0228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colakoglu T, Yildirim S, Kayaselcuk F, Nursal TZ, Ezer A, Noyan T, Karakayali H, Haberal M. Clinicopathological significance of PTEN loss and the phosphoinositide 3-kinase/Akt pathway in sporadic colorectal neoplasms: Is PTEN loss predictor of local recurrence? Am J Surg. 2008;195:719–725. doi: 10.1016/j.amjsurg.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 20.Hsu CP, Kao TY, Chang WL, Nieh S, Wang HL, Chung YC. Clinical significance of tumor suppressor PTEN in colorectal carcinoma. Eur J Surg Oncol. 2011;37:140–147. doi: 10.1016/j.ejso.2010.12.003. [DOI] [PubMed] [Google Scholar]


