Abstract
(−)-Epigallocatechin-3-gallate (EGCG) is a polyphenol monomer compound extracted and separated from green tea, and is a key catechin in green tea. Recent research has identified that EGCG is equipped with important biological activities, including antitumor, antioxidant, anti-inflammation, blood fat reduction and radiation protection abilities. In the current study, it was investigated whether EGCG exerts a neuroprotective effect on AIT and examined the possible underlying mechanism. The present study sought to establish an experimental autoimmune thyroiditis (AIT) rat model and to investigate the neuroprotective effect of EGCG in this model. EGCG was demonstrated to inhibit urinary iodine values and thyroid pathological features in AIT model rats. Treatment with EGCG significantly reduced interleukin-1β, interferon-γ (INF-γ) and tumor necrosis factor-α (TNF-α) levels in the AIT rats through suppression of nuclear factor-κB (NF-κB) pathway. In addition, pretreatment with EGCG significantly increased B-cell lymphoma-2 protein expression, and suppressed caspase-3 activity and TNF-α-related apoptosis-inducing ligand (TRAIL) protein expression levels in the AIT model rats. In conclusion, these results suggested that the neuroprotective effect of EGCG protects against AIT through its anti-inflammatory ability, anti-apoptosis and TRAIL signaling pathway in model rats, and it may be used as a therapeutic agent against AIT caused by inflammation.
Keywords: (−)-epigallocatechin-3-gallate, autoimmune thyroiditis, inflammation, apoptosis, TRAIL
Introduction
Autoimmune thyroiditis (AIT) is also known as Hashimoto's thyroiditis or chronic lymphocytic thyroiditis, and is a relatively common specific immunity disease of the organs (1). AIT is the second most common thyroid disorder and accounts for 21% of all thyroid disorders (2). Recently, due to environmental and multiple other factors, the morbidity of the disease has been increasing year by year (3). Traditional Chinese medicine (TCM) considers that the etiology of the disease is closely associated with internal injury and eating disorders (4). Due to the disease not having clear clinical symptoms at an early stage, the majority of patients only present increase of thyroid autoantibodies (4). AIT is often diagnosed as a subclinical stage of chronic lymphocytic thyroiditis. Currently, there are no specific conventional medicine strategies for effective treatment of AIT. TCM applies the therapeutic principle of treatment based on syndrome differentiation and specimen consideration, so as to improve immunocompetence and develop an effective therapy (5). However, no drugs are currently available in clinical practice for the effective treatment of the disease (5). At present, medical treatment for AIT mainly adopts the replacement of thyroid hormones, immunization therapy and surgical treatment; however, toxicity, side effects and recurrence restrain the development of an effective strategy (6). Therefore, alternative therapies have certain superiority with regards to safety and curative stability in the treatment of AIT (7).
Apoptosis is a spontaneous and programmed cell death process that occurs under the induction of specific physiological or pathological factors in cells (8). Morphological characteristics of apoptosis are mainly reflected in the following five aspects: Vesicles on cytomembrane, concentration of chromatin, release of cytochrome c, potential reduction of mitochondrial membrane, and DNA bond fission and loss of contact with neighboring cells (8). The role of apoptosis is mainly reflected in the normal differentiation of cell (9). In addition, the immune response of autoimmune T and B lymphocytes also depends on apoptosis (10).
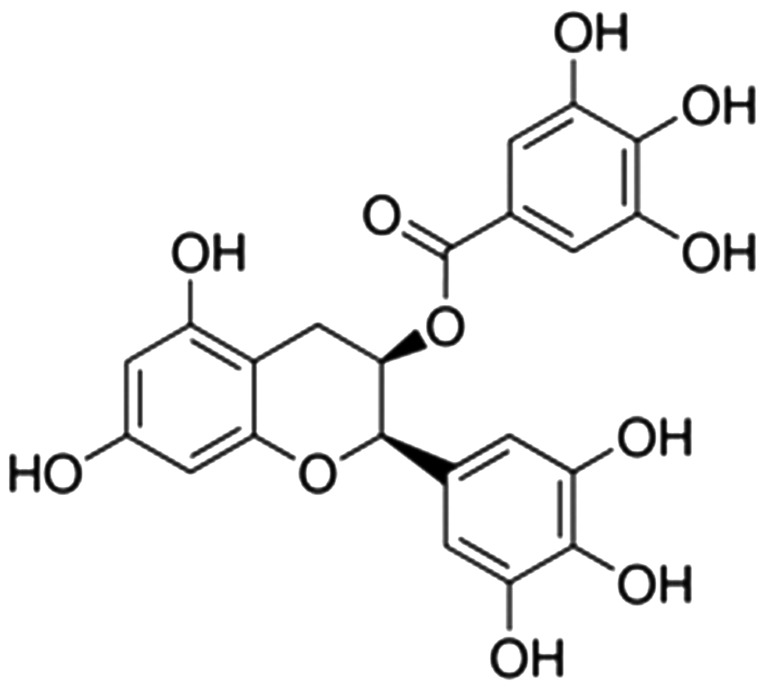
Polyphenols are components of green tea, accounting for 22–30% of the tea dry weight, and are mainly composed of catechins, flavonoid, phenolic acid and anthocyanidins (11). As the key components of green tea, catechins account for 60–80% of the total tea polyphenol content. (−)-Epigallocatechin-3-gallate (EGCG) is a catechin present in green tea that can relieve the inflammatory process in skin diseases associated with blood vessels, and its structural formula is shown in Fig. 1. In dermatitis, vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) serve an important role (12), EGCG and soy isoflavone inhibit NHK proliferation, but do not induce apoptosis. EGCG can also inhibit the IL-8 release in NHK that is induced by preventing TNF-α expression, while isoflavone only has a weak effect (11). Green tea contains various natural active materials, and as the catechin compound with the highest content in green tea, EGCG has multiple important physiological and pharmacologic activities (13). In the current study, it was investigated whether EGCG exerts a neuroprotective effect on AIT and examined the possible underlying mechanism.
Figure 1.
Structural formula of (−)-epigallocatechin-3-gallate.
Materials and methods
Animals and chemicals
A total of 30 female Lewis rats (4-weeks-old; weight, 80±2 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Animals were kept under a temperature of 24±2°C and humidity of 55±5% with a 12 h light/dark cycle, and were allowed free access to food and water. The rats were randomly divided into three groups (n=10 rats per group): Sham control, AIT model and EGCG groups.
Next, rats in the AIT model group and EGCG group were immunized by subcutaneous injection of 0.1 ml pig thyroglobulin (8 mg/ml) in incomplete Freund's adjuvant (both from Sigma-Aldrich; Merck, Darmstadt, Germany) once every 2 weeks for 6 weeks. Rats in the EGCG group were injected intraperitoneally with 0.5 mg/kg EGCG three times with a 1-h interval at 3 h. Rats in the Sham control or AIT group were injected intraperitoneally with 100 µl of PBS. The animal experiments were conducted according to the ethical guidelines approved by the Medical Ethics Review Committee of the First Center Hospital of Tianjin (Tianjin, China).
Iodine intake level
Prior to euthanasia, urine was collected from the rats. Urine iodine levels were determined by arsenic cerium catalytic spectrophotometry method (Victor 3 Multilabel Counter system; PerkinElmer, Inc., Waltham, MA, USA) as mentioned previously (14).
Body and thyroid weight measurement
The weights of the body and thyroid of the rats were measured following euthanasia. Next, the relative thyroid weight was calculated as the thyroid wet weight (mg)/rat body weight (g).
Thyroid pathological features measurement
The thyroid was fixed using 4% paraformaldehyde for 24 h at room temperature, embedded into paraffin, and sectioned into 0.5-µm thick sections. The thyroid pathological features were classified into four grades, as follows: 0, indicating normal physiology; 1, slight disruption of thyroid follicles (two or more thyroid follicles); 2, moderate destruction of thyroid follicles (10–40% of thyroid sections); 3, severe disruption of thyroid physiology (>40% of thyroid sections) (14).
Antibody-capture ELISA for detection of IL-1β, interferon-γ (IFN-γ) and TNF-α levels
Thyroid tissue samples (20 mg) were collected from rats after euthanasia and homogenized in radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich; Merck). IL-1β (EK-R30172), IFN-γ (EK-R30149) and TNF-α (EK-R31092) levels were determined with sandwich ELISA kits (Shanghai BlueGene Biotech Co., Ltd., Shanghai, China). The absorbance of samples was determined using an ELISA reader (Model ELX800, BioTek Instruments, Inc., Winooski, VT, USA) at 450 nM.
Western blot analysis
Thyroid tissue samples were collected from rats following euthanasia and homogenized in RIPA buffer (Sigma-Aldrich). Total protein was acquired following centrifugation at 12,000 × g for 10 min at 4°C, and protein concentration was measured using a bicinchoninic acid assay method (Beyotime Institute of Biotechnology, Haimen, China). Equal amounts of protein (50 µg) were subjected to 10–15% SDS-polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane (EMD Millipore, Bedford, MA, USA). The membranes were blocked with 5% skimmed milk powder in Tris-buffered saline with Tween-20 for 1 h at 37°C and incubated with primary antibodies against nuclear factor-κB (NF-κB; cat. no. sc-71675; dilution, 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), B-cell lymphoma-2 (Bcl-2; cat. no. sc-7382; dilution, 1:500; Santa Cruz Biotechnology, Inc.), TNF-α-related apoptosis-inducing ligand (TRAIL; cat. no. sc-8440; dilution, 1:500, Santa Cruz Biotechnology, Inc.) and GAPDH (cat. no. B661204; dilution, 1:500; Sangon Biotech Co., Ltd., Shanghai, China) at 4°C overnight. The blots were then washed with Tris-buffered saline/Tween-20 and incubated with a horseradish peroxidase-conjugated secondary antibody (cat. no. sc-2314; dilution, 1:2,000; Sangon Biotech Co., Ltd.). Protein was detected with BeyoECL Star Plus reagent (Beyotime Institute of Biotechnology) and analyzed using Bio-Rad Laboratories Q 3.0 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Results are expressed as the mean ± standard error of the mean using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Statistical analyses were performed using one-way analysis of variance followed by a Student-Newman-Keuls test for multiple comparisons. Differences with P<0.05 were considered as statistically significant.
Results
Effect of EGCG treatment on urinary iodine values in AIT rats
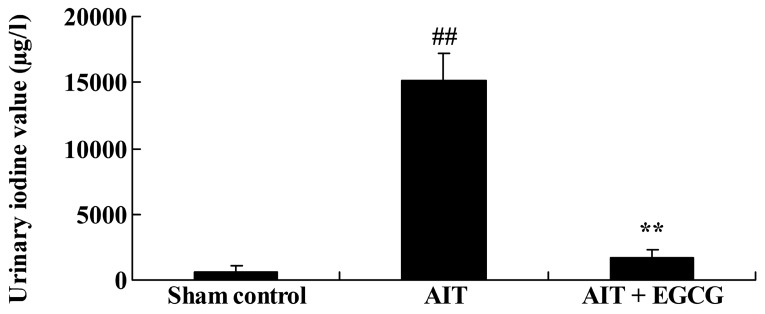
The neuroprotective effect of EGCG in AIT rats was initially evaluated by investigating the urinary iodine values. Compared with the sham control group, urinary iodine values of the AIT rat model were significantly increased (Fig. 2). However, pretreatment with EGCG significantly inhibited the AIT-induced urinary iodine values, compared with those in the untreated AIT model group (Fig. 2).
Figure 2.
Effect of EGCG treatment on urinary iodine values in AIT rats. The AIT + EGCG group was pretreated with 0.5 mg/kg EGCG prior to AIT induction. ##P<0.01 vs. sham control group; **P<0.01 vs. AIT + EGCG group. EGCG, (−)-epigallocatechin-3-gallate; AIT, autoimmune thyroiditis.
Body and thyroid weights in AIT rats
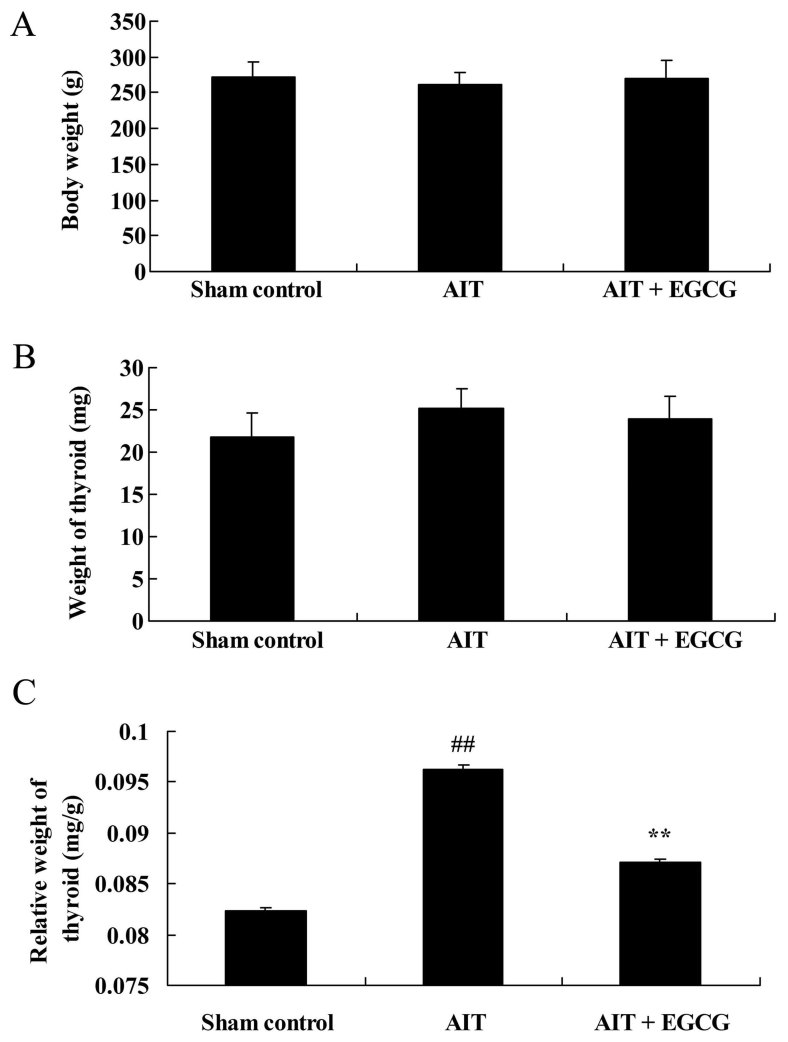
The study also examined the effect of EGCG on the body and thyroid weights in AIT rats. There was no significant difference in the body and thyroid weights among the three experimental groups (Fig. 3A and B). However, AIT significantly increased the relative thyroid weight in model rats as compared with the sham control group. Pretreatment with EGCG markedly reduced the relative thyroid weight of AIT rats, as compared with the untreated AIT model group (Fig. 3C).
Figure 3.
Effect of EGCG treatment on the (A) body weight, (B) thyroid weight and (C) relative thyroid weight in AIT model rats. The AIT + EGCG group was pretreated with 0.5 mg/kg EGCG prior to AIT induction. EGCG was found to exert a neuroprotective effect. ##P<0.01 vs. sham control group; **P<0.01 vs. AIT + EGCG group. EGCG, (−)-epigallocatechin-3-gallate; AIT, autoimmune thyroiditis.
Thyroid pathological features in AIT rats
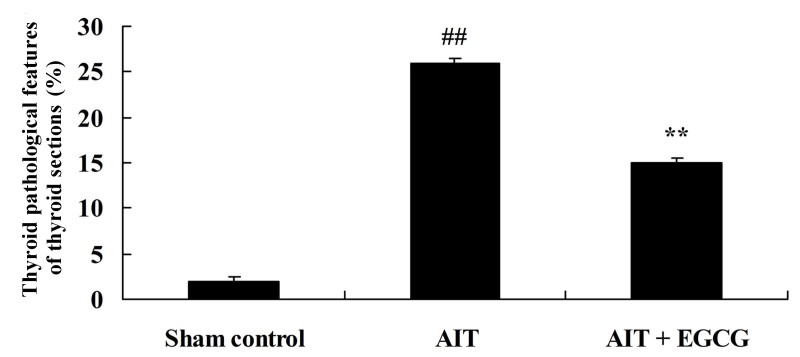
The present study further examined whether EGCG treatment affected the thyroid pathological features in AIT rats. As shown in Fig. 4, thyroid pathological features of AIT model rat were significantly more severe in comparison with those of the control sham group. However, the AIT-induced thyroid pathological features were significantly suppressed by treatment with EGCG, as compared with the AIT model group (Fig. 4).
Figure 4.
Effect of EGCG treatment on thyroid pathological features in AIT rats. The AIT + EGCG group was pretreated with 0.5 mg/kg EGCG prior to AIT induction. ##P<0.01 vs. sham control group; **P<0.01 vs. AIT + EGCG group. EGCG, (−)-epigallocatechin-3-gallate; AIT, autoimmune thyroiditis.
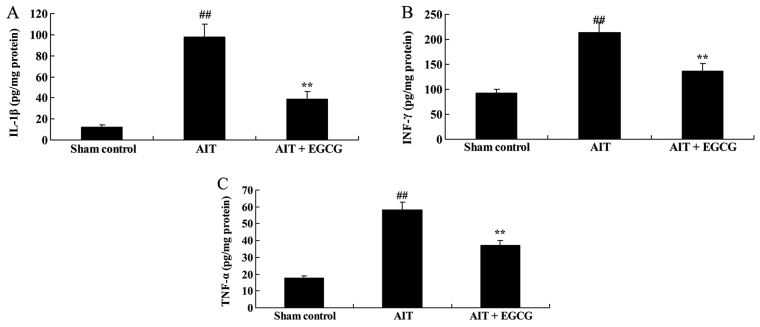
IL-1β, IFN-γ and TNF-α protein levels in AIT rats
The thyroid tissue protein levels of IL-1β, IFN-γ and TNF-α were measured using ELISA kits. The results indicated that IL-1β (Fig. 5A), IFN-γ (Fig. 5B) and TNF-α (Fig. 5C) levels in the thyroid tissue of AIT rats were significantly enhanced, compared with the sham control group. However, treatment with EGCG significantly inhibited the AIT-induced IL-1β, IFN-γ and TNF-α levels in AIT rats, as compared with the untreated AIT model group (Fig. 5).
Figure 5.
Effect of EGCG treatment on (A) IL-1β, (B) IFN-γ and (C) TNF-α in model rats, as determined by ELISA. ##P<0.01 vs. sham control group; **P<0.01 vs. AIT + EGCG group. EGCG, (−)-epigallocatechin-3-gallate; AIT, autoimmune thyroiditis; IL-1β, interleukin-1β; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α.
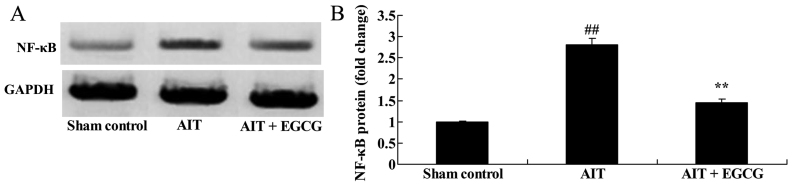
NF-κB protein expression in AIT rats
The mechanism underlying the anti-inflammatory effect of EGCG in AIT rats, NF-κB protein expression in thyroid tissue was measured using western blot analysis. Compared with the sham control group, NF-κB/p65 protein expression of AIT rats was significantly promoted, whereas pretreatment with EGCG significantly suppressed the AIT-induced NF-κB/p65 protein expression in rats (Fig. 6).
Figure 6.
Effect of EGCG treatment on NF-κB protein expression in AIT model rats. (A) Western blots and (B) quantified results of NF-κB protein expression are demonstrated. ##P<0.01 vs. sham control group; **P<0.01 vs. AIT + EGCG group. EGCG, (−)-epigallocatechin-3-gallate; AIT, autoimmune thyroiditis; NF-κB, nuclear factor-κB.
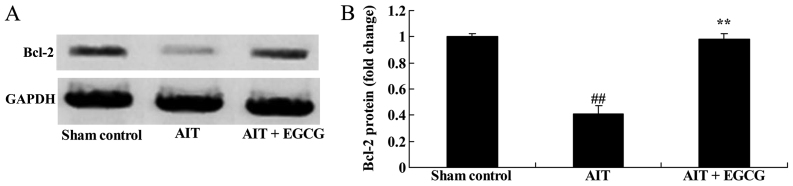
Bcl-2 protein expression in AIT rats
The role of Bcl-2 in the neuroprotective effect of EGCG on AIT rats was examined using western blot analysis. As illustrated in Fig. 7, Bcl-2 protein expression of AIT rats was significantly decreased, compared with the sham control group. However, the AIT-induced Bcl-2 protein expression was significantly increased by treatment with EGCG, as compared with untreated AIT model group (Fig. 7).
Figure 7.
Effect of EGCG treatment on Bcl-2 protein expression in AIT model rats. (A) Western blots and (B) quantified results of Bcl-2 protein expression are shown. ##P<0.01 vs. sham control group; **P<0.01 vs. AIT + EGCG group. EGCG, (−)-epigallocatechin-3-gallate; AIT, autoimmune thyroiditis; Bcl-2, B-cell lymphoma-2.
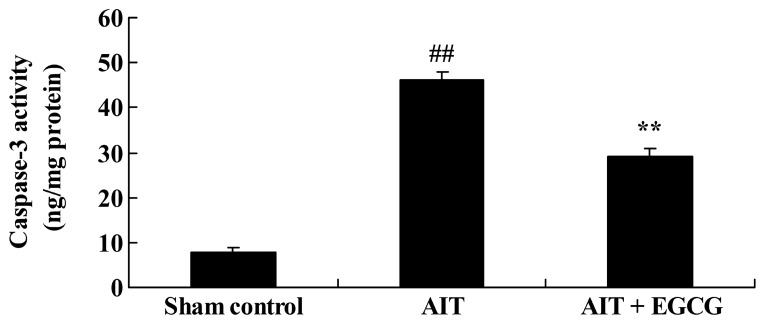
Caspase-3 activity in AIT rats
To investigate the effect of EGCG on apoptosis in AIT rats, caspase-3 activity was measured using ELISA. As expected, the caspase-3 activity of the AIT rat model group was evidently higher in comparison to that of the sham control group (Fig. 8). However, treatment with EGCG significantly reduced the AIT-induced caspase-3 activity in the rats (Fig. 8).
Figure 8.
Effect of EGCG treatment on caspase-3 activity in AIT model rats, as determined by ELISA. ##P<0.01 vs. sham control group; **P<0.01 vs. AIT + EGCG group. EGCG, (−)-epigallocatechin-3-gallate; AIT, autoimmune thyroiditis.
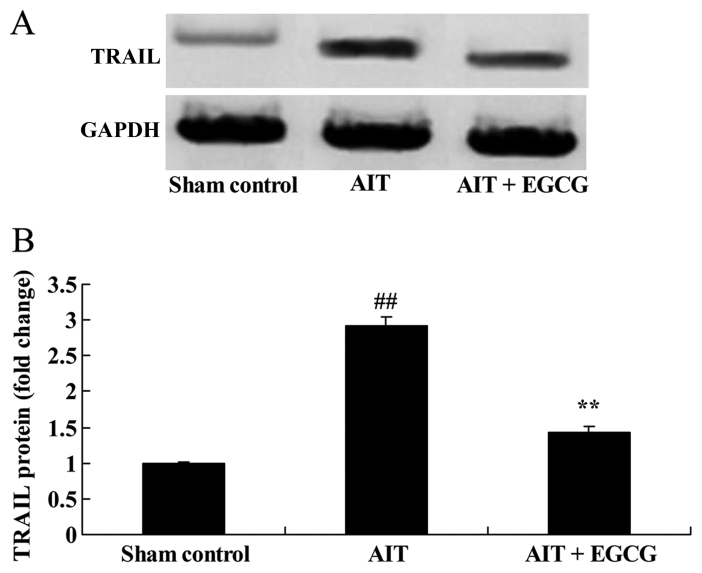
TRAIL protein expression in AIT rats
To further investigate the mechanism underlying the neuroprotective effect of EGCG in AIT rats, TRAIL expression was detected by western blot analysis. As demonstrated in Fig. 9, AIT significantly induced the TRAIL protein expression in AIT rats, as compared with the sham control group. By contrast, treatment with EGCG significantly suppressed the AIT-induced TRAIL protein expression in AIT rats, as compared with the AIT model group (Fig. 9).
Figure 9.
Effect of EGCG treatment on TRAIL protein expression in model rats. (A) Western blots and (B) quantified results of TRAIL protein expression are presented. ##P<0.01 vs. sham control group; **P<0.01 vs. AIT + EGCG group. EGCG, (−)-epigallocatechin-3-gallate; AIT, autoimmune thyroiditis; TRAIL, tumor necrosis factor-α-related apoptosis-inducing ligand.
Discussion
NF-κB is a nucleoprotein factor with pleiotropy transcription regulators, which participates in the expression and regulation of multiple genes, particularly genes associated with the immune and inflammatory responses, while it also serves an important role on the body inflammatory reaction process (15). In the present study, treatment with EGCG significantly inhibited the AIT-induced urinary iodine values, the relative thyroid weight and the thyroid pathological features in the AIT rats, while it suppressed IL-1β, IFN-γ and TNF-α levels, as well as NF-κB protein expression, which showed that EGCG had a neuroprotective effect against AIT (16). These results are in accordance with the findings of Liu et al, which suggested that EGCG prevents PCB-126-induced endothelial cell inflammation through the inhibition of NF-κB in human endothelial cells (12).
The Bcl-2 gene family is a group of genes that have a close association with apoptosis, and induce or inhibit the role of apoptosis by coding proteins (17). Bcl-2 and Bcl-2-associated X protein (Bax) are genes regulating apoptosis, positively or negatively. Bax gene serves a role in promoting apoptosis, whereas the Bcl-2 gene inhibits apoptosis (18). Therefore, the ratio of relative Bcl-2 and Bax levels (namely Bax/Bcl-2) is an important indicator of the apoptosis degree. When the Bax/Bcl-2 ratio value is higher (indicating increased Bax expression), then apoptosis is promoted (18). By contrast, a lower Bax/Bcl-2 ratio indicates reduced apoptosis (19). In the present study, EGCG treatment significantly increased Bcl-2 protein expression in AIT rats, and may reduce the AIT-induced apoptosis. Similarly, Adikesavan et al reported that EGCG stabilizes the mitochondrial enzymes and inhibits the apoptosis through upregulation of Bcl-2 in cigarette smoke-induced myocardial dysfunction (20).
The caspase family serves a main executer role in the process of apoptosis, with the main function of inactivating inhibitors of apoptosis (such as apoptin and Bcl-2), ultimately resulting in degradation of cell components and formation of apoptotic bodies by hydrolyzing the cell protein structure (21). Caspase-3 is an important effector molecule in apoptosis, which can activate DNA enzyme, degrade chromosome and ultimately cause apoptosis (22). Therefore, the present study investigated caspase-3 activity, and the results demonstrated that EGCG significantly reduced the AIT-induced caspase-3 activity in rats.
TRAIL selectively induces the apoptosis of various tumor cells; however, it does not have an impact on cell growth, development and differentiation under normal conditions (23). The TRAIL gene group mRNA is widely detected in various tissues, while regions of its cytomembrane present a homologous consistency with members of the TNF family (24). However, TRAIL differs from other members of the TNF family in that it is much shorter and only has 14 amino acid residues as the support (24). In addition, the broad role of TRAIL in inducing apoptosis can only be compared with Fas-L (25).
Apoptosis is caused by TRAIL by Fas and TNF receptor (TNFR) in AIT (26). At present, TRAIL is considered to result in apoptosis through activation of immune cells, leading to generation of TNF-α and IL-1 (24). TNF-α and IL-1 have a synergistic effect that improves thyroid cell sensitivity of TRAIL, resulting in the death of thyroid cells (27). TRAIL expression activates immune cells through thyroid autoantigen generation, in order to generate IFN-γ and TNF-α. The synergic induction of IFN-γ and TNF-α results in the expression of TRAIL by thyroid cells, increasing the sensitivity of TRAIL on immune cells, and ultimately resulting in apoptosis (27). Furthermore, TNF-α and IL-1 may result in the destruction of thyroid cells. IFN-γ, TNF-α and IL-1 inhibit TRAIL expression and thus induce the death of thyroid cells (28). The results of the present study indicated that EGCG treatment significantly suppressed the AIT-induced TRAIL protein expression in AIT rats. These results showed that EGCG reduced TRAIL expression, and may therefore reduce apoptosis in AIT. Similarly, Aktas et al (29) reported that EGCG mediates T cellular NF-κB inhibition and suppression of TRAIL in autoimmune encephalomyelitis.
In conclusion, the results of the present study indicated that EGCG exerted a potent neuroprotective effect in an AIT rat model, since it inhibited the AIT-induced urinary iodine values, the relative thyroid weight and the thyroid pathological features of the rats. Furthermore, it was demonstrated the EGCG suppressed IL-1β, IFN-γ, TNF-α and NF-κB protein expression levels, while it upregulated Bcl-2 expression, and reduced caspase-3 activity and TRAIL protein expression in AIT rats. Therefore, EGCG may be a potential novel drug for neuroinflammatory diseases.
Acknowledgements
The present study was supported by Scientific Research Task of Chinese Medicine and Chinese and Western Medicine Combined (grant no. 2015048).
References
- 1.Nordio M, Basciani S. Efficacy of a food supplement in patients with hashimoto thyroiditis. J Biol Regul Homeost Agents. 2015;29:93–102. [PubMed] [Google Scholar]
- 2.Krysiak R, Okopien B. The effect of levothyroxine and selenomethionine on lymphocyte and monocyte cytokine release in women with Hashimoto's thyroiditis. J Clin Endocrinol Metab. 2011;96:2206–2215. doi: 10.1210/jc.2010-2986. [DOI] [PubMed] [Google Scholar]
- 3.Hofling DB, Chavantes MC, Juliano AG, Cerri GG, Romão R, Yoshimura EM, Chammas MC. Low-level laser therapy in chronic autoimmune thyroiditis: A pilot study. Lasers Surg Med. 2010;42:589–596. doi: 10.1002/lsm.20941. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama A, Zhu BM, Takahara A, Satoh Y, Hashimoto K. Cardiac effects of salvia miltiorrhiza/dalbergia odorifera mixture, an intravenously applicable Chinese medicine widely used for patients with ischemic heart disease in China. Circ J. 2002;66:182–184. doi: 10.1253/circj.66.182. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki K, Kosaka K, Mori H, Okitsu R, Furukawa K, Manabe Y, Yoshita M, Kanamori A, Ito N, Wada K, et al. Open label trial to evaluate the efficacy and safety of Yokukansan, a traditional Asian medicine, in dementia with Lewy bodies. J Am Geriatr Soc. 2011;59:936–938. doi: 10.1111/j.1532-5415.2011.03373.x. [DOI] [PubMed] [Google Scholar]
- 6.Kristensen B, Hegedüs L, Madsen HO, Smith TJ, Nielsen CH. Altered balance between self-reactive T helper (Th)17 cells and Th10 cells and between full-length forkhead box protein 3 (FoxP3) and FoxP3 splice variants in Hashimoto's thyroiditis. Clin Exp Immunol. 2015;180:58–69. doi: 10.1111/cei.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scarpa V, Kousta E, Tertipi A, Vakaki M, Fotinou A, Petrou V, Hadjiathanasiou C, Papathanasiou A. Treatment with thyroxine reduces thyroid volume in euthyroid children and adolescents with chronic autoimmune thyroiditis. Horm Res Paediatr. 2010;73:61–67. doi: 10.1159/000271917. [DOI] [PubMed] [Google Scholar]
- 8.Vecchiatti SM, Lin CJ, Capelozzi VL, Longatto-Filho A, Bisi H. Prevalence of thyroiditis and immunohistochemistry study searching for a morphologic consensus in morphology of autoimmune thyroiditis in a 4613 autopsies series. Appl Immunohistochem Mol Morphol. 2015;23:402–408. doi: 10.1097/PAI.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 9.Wang SH, Cao Z, Wolf JM, Van Antwerp M, Baker JR., Jr Death ligand tumor necrosis factor-related apoptosis-inducing ligand inhibits experimental autoimmune thyroiditis. Endocrinology. 2005;146:4721–4726. doi: 10.1210/en.2005-0627. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Zheng T, Mao Y, Xu C, Wu F, Bu L, Mou X, Zhou Y, Yuan G, Wang S, et al. γδ T cells enhance B cells for antibody production in Hashimoto's thyroiditis, and retinoic acid induces apoptosis of the γδ T cell. Endocrine. 2016;51:113–122. doi: 10.1007/s12020-015-0631-9. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Paik JS, Yun M, Lee SB, Yang SW. The effect of (−)-epigallocatechin-3-gallate on IL-1β induced IL-8 expression in orbital fibroblast from patients with thyroid-associated ophthalmopathy. PLoS One. 2016;11:e0148645. doi: 10.1371/journal.pone.0148645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu D, Perkins JT, Hennig B. EGCG prevents PCB-126- induced endothelial cell inflammation via epigenetic modifications of NF-κB target genes in human endothelial cells. J Nutr Biochem. 2016;28:164–170. doi: 10.1016/j.jnutbio.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q, Qian Y, Chen F, Chen X, Chen Z, Zheng M. EGCG attenuates pro-inflammatory cytokines and chemokines production in LPS-stimulated L02 hepatocyte. Acta Biochim Biophys Sin (Shanghai) 2014;46:31–39. doi: 10.1093/abbs/gmt128. [DOI] [PubMed] [Google Scholar]
- 14.Cui SL, Yu J, Shoujun L. Iodine intake increases IP-10 expression in the serum and thyroids of rats with experimental autoimmune thyroiditis. Int J Endocrinol. 2014;2014:581069. doi: 10.1155/2014/581069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eguchi K. Apoptosis in autoimmune diseases. Intern Med. 2001;40:275–284. doi: 10.2169/internalmedicine.40.275. [DOI] [PubMed] [Google Scholar]
- 16.Arslan S, Korkmaz Ö, Özbilüm N, Berkan Ö. Association between NF-κBI and NF-κBIA polymorphisms and coronary artery disease. Biomed Rep. 2015;3:736–740. doi: 10.3892/br.2015.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaczmarek E, Lacka K, Jarmolowska-Jurczyszyn D, Sidor A, Majewski P. Changes of B and T lymphocytes and selected apopotosis markers in Hashimoto's thyroiditis. J Clin Pathol. 2011;64:626–630. doi: 10.1136/jcp.2010.086553. [DOI] [PubMed] [Google Scholar]
- 18.Myśliwiec J, Okota M, Nikołajuk A, Górska M. Soluble Fas, Fas ligand and Bcl-2 in autoimmune thyroid diseases: Relation to humoral immune response markers. Adv Med Sci. 2006;51:119–122. [PubMed] [Google Scholar]
- 19.Mysliwiec J, Okłota M, Nikołajuk A, Górska M. Age related changes of soluble Fas, Fas ligand and Bcl-2 in autoimmune thyroid diseases. Endokrynol Pol. 2007;58:492–495. (In Polish) [PubMed] [Google Scholar]
- 20.Adikesavan G, Vinayagam MM, Abdulrahman LA, Chinnasamy T. (−)-Epigallocatechin-gallate (EGCG) stabilize the mitochondrial enzymes and inhibits the apoptosis in cigarette smoke-induced myocardial dysfunction in rats. Mol Biol Rep. 2013;40:6533–6545. doi: 10.1007/s11033-013-2673-5. [DOI] [PubMed] [Google Scholar]
- 21.Bossowski A, Czarnocka B, Bardadin K, Stasiak-Barmuta A, Urban M, Dadan J, Ratomski K, Bossowska A. Identification of apoptotic proteins in thyroid gland from patients with Graves' disease and Hashimoto's thyroiditis. Autoimmunity. 2008;41:163–173. doi: 10.1080/08916930701727749. [DOI] [PubMed] [Google Scholar]
- 22.Minatoguchi S, Kariya T, Uno Y, Arai M, Nishida Y, Hashimoto K, Wang N, Aoyama T, Takemura G, Fujiwara T, Fujiwara H. Caspase-dependent and serine protease-dependent DNA fragmentation of myocytes in the ischemia-reperfused rabbit heart: These inhibitors do not reduce infarct size. Jpn Circ J. 2001;65:907–911. doi: 10.1253/jcj.65.907. [DOI] [PubMed] [Google Scholar]
- 23.Zhitao J, Long L, Jia L, Yunchao B, Anhua W. Temozolomide sensitizes stem-like cells of glioma spheres to TRAIL-induced apoptosis via upregulation of casitas B-lineage lymphoma (c-Cbl) protein. Tumour Biol. 2015;36:9621–9630. doi: 10.1007/s13277-015-3720-8. [DOI] [PubMed] [Google Scholar]
- 24.Zauli G, Tisato V, Melloni E, Volpato S, Cervellati C, Bonaccorsi G, Radillo O, Marci R, Secchiero P. Inverse correlation between circulating levels of TNF-related apoptosis-inducing ligand and 17β-estradiol. J Clin Endocrinol Metab. 2014;99:E659–E664. doi: 10.1210/jc.2013-4193. [DOI] [PubMed] [Google Scholar]
- 25.Ge Y, Yan D, Deng H, Chen W, An G. Novel molecular regulators of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in NSCLC cells. Clin Lab. 2015;61:1855–1863. doi: 10.7754/Clin.Lab.2015.150328. [DOI] [PubMed] [Google Scholar]
- 26.El-Karaksy SM, Kholoussi NM, Shahin RM, El-Ghar MM, Gheith Rel-S. TRAIL mRNA expression in peripheral blood mononuclear cells of Egyptian SLE patients. Gene. 2013;527:211–214. doi: 10.1016/j.gene.2013.05.084. [DOI] [PubMed] [Google Scholar]
- 27.Zhang N, Wang X, Huo Q, Li X, Wang H, Schneider P, Hu G, Yang Q. The oncogene metadherin modulates the apoptotic pathway based on the tumor necrosis factor superfamily member TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) in breast cancer. J Biol Chem. 2013;288:9396–9407. doi: 10.1074/jbc.M112.395913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramamurthy V, Yamniuk AP, Lawrence EJ, Yong W, Schneeweis LA, Cheng L, Murdock M, Corbett MJ, Doyle ML, Sheriff S. The structure of the death receptor 4-TNF-related apoptosis-inducing ligand (DR4-TRAIL) complex. Acta Crystallogr F Struct Biol Commun. 2015;71:1273–1281. doi: 10.1107/S2053230X15016416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aktas O, Prozorovski T, Smorodchenko A, Savaskan NE, Lauster R, Kloetzel PM, Infante-Duarte C, Brocke S, Zipp F. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J Immunol. 2004;173:5794–5800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]