Abstract
Dysregulation of microRNAs has been confirmed to serve an important role in cancer development and progression. However, the role of microRNA (miR)-544 in colorectal cancer progression remains unknown. In the present study, it was observed that the expression level of miR-544 was increased in breast cancer cell lines and tissues using the quantitative polymerase chain reaction. Overexpression of miR-544 promoted cell proliferation and invasion in colorectal cancer, whereas inhibition of miR-544 suppressed colorectal cancer progression as determined using MTT, colony formation and Transwell assays. Furthermore, forkhead box O1 (FOXO1) was a direct target of miR-544. FOXO1 mediated miR-544-regulated colorectal cancer progression and cell cycle distribution. In conclusion, the results of the present study revealed that miR-544 serves an important role in promoting human colorectal cancer cell progression.
Keywords: colorectal cancer, progression, microRNA-544, forkhead box O1
Introduction
Colorectal cancer is one of the most common types of cancer and is the third leading cause of malignancy-associated mortality worldwide (1). Although screening methodologies have decreased the incidence of patients with advanced stage, treating late-stage colorectal cancer continues to challenge physicians. Understanding the molecular footprint of colorectal cancer is critically important for the development of novel treatments for this disease.
MicroRNAs (miRNAs/miRs) are small non-coding single-stranded RNAs that regulate gene expression by inhibiting mRNA translation by inducing mRNA degradation (2,3). The miRNAs target ~33% of all protein-coding genes, and each miRNA is able to potentially repress hundreds of target genes (4,5). miRNA is able to regulate multiple steps of cancer development and progression by modulating the expression of target genes. Recently, a large number of aberrantly expressed miRNAs have been identified in colorectal cancer and have been associated with colorectal cancer progression (6–9).
miRNA (miR)-544 has been identified to be involved in the regulation of numerous cellular processes, including polar over-dominance inheritance (10), stem cell self-renewal (11) and osteogenic differentiation (12). Although a number of previous studies have been reported that miR-544 serves an important role in various types of cancer, including breast cancer (13,14), lung cancer (15), cervical cancer (16), gastric cancer (17,18), osteosarcoma (19,20) and glioma (21), the role of miR-544 in colorectal cancer remains unknown. Recently, the role of miRNAs in the regulation of expression of cell cycle-associated genes has been investigated (22). For example, miR-196a promotes cervical cancer proliferation by regulating forkhead box O1 (FOXO1) and p27 (23). miR-142-3p has been suggested to act as a tumor suppressor that inhibits tumor proliferation by targeting cell division cycle 25C (24). However, there have been few detailed studies investigating the role of miR-544 in cell cycle-associated cancer cell proliferation (16,17).
The results of the present study identified miR-544 as a candidate oncogenic miRNA in colorectal cancer. The results of the present study revealed that miR-544 is upregulated in colorectal tissues. In vitro investigation demonstrated that miR-544 promotes colorectal cancer cell progression by binding to the 3′-untranslated region (UTR) of FOXO1 mRNA. Thus, miR-544 may serve an important role in the development and progression of colorectal cancer.
Materials and methods
Cell culture and specimens
HT29, SW480, LOVO and HCT116 colorectal cancer cell lines were obtained from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.). The cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
A total of 20 fresh colorectal cancer tissues and matched adjacent non-tumorous tissues were obtained from 9 males and 11 females with ages ranging between 40 and 65 years from January 2012 to December 2013. All tissues were stored at −80°C immediately following removal until use. None of the patients had received any treatment prior to surgery. The present study was approved by the Institutional Review Board of the Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) and written informed consent was obtained from all patients prior to enrollment in the present study.
Plasmid, short interfering RNA and transfection
To construct the FOXO1 expression vector, the entire coding sequence of the FOXO1 was amplified using the polymerase chain reaction (PCR) using Platinum SuperFi DNA (Thermo Fisher Scientific, Inc.) in the HT29 cell line according to the manufacturer's protocol using the following thermocycling conditions: 95°C for 3 min, then 95°C for 30 sec, 60°C for 30 sec and 72°C for 2 min for a total of 40 cycles. The primers were as following: Forward, 5′-GCGGATCCATGGCCGAGGCGCCTCAGGT-3′ and reverse, 5′-GCCTCGAGTCAGCCTGACACCCAGCTAT-3′. The PCR product was cloned into the pcDNA3 vector (Thermo Fisher Scientific, Inc.). The mutated miR-544-binding site sequence on the FOXO1 3′-UTR was cloned into the psiCHECK2 vector (Promega Corporation, Madison, WI, USA) [UTR-FOXO1-1/2wild-type (wt) and UTR-FOXO1-1/2mutant (mu)]. The construct was confirmed by sequencing. The miR-544 mimic, inhibitor and the negative control were purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). miRNAs or plasmids were transfected with Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol.
Western blot analysis
Cells were collected in lysis buffer (60 mM Tris/HCl, 2% SDS and 10% glycerol) with 0.1% protease inhibitor cocktail III (EMD Millipore, Billerica, CA, USA). The protein concentration was determined using the Bradford assay using a com mercial kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). A total of 50 µg protein was separated by SDS-PAGE (10% gel). Subsequently, the proteins were transferred onto polyvinylidene membranes (EMD Millipore). Following blocking with 5% skimmed milk in TBST at room temperature for 1 h, the membranes were probed with anti-FOXO1 (cat. no. sc-11350; dilution, 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-cyclin D1 (cat. no. sc-70899; dilution, 1:2,000; Santa Cruz Biotechnology, Inc.), anti-phosphorylated retinoblastoma protein (pRb; cat. no. 8516; dilution, 1:2,000; Cell Signaling Technology, Inc., Danvers, MA, USA) and anti-p27 antibodies (cat. no. 3686; dilution, 1:2,000; Cell Signaling Technology, Inc.) at 4°C overnight. The following day, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (cat. nos. 7076 and 7074; dilution, 1:2,500) at 4°C for 1 h, and the bands were visualized using enhanced chemiluminescence detection reagents (EMD Millipore). GAPDH (cat. no. sc-365062; dilution, 1:3,000; Santa Cruz Biotechnology, Inc.) served as a loading control.
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
miRNA from tissue samples and cultured cells was extracted using the mirVana miRNA Isolation kit (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Total RNA was extracted using TRIzol® (Thermo Scientific) reagent, according to the manufacturer's protocol. A 5 µg amount of total RNA was converted into first-strand cDNA using the TaqMan miRNA reverse transcription kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) or SuperScript II Reverse Transcriptase kit (Invitrogen; Thermo Fisher Scientific, Inc.). The expression level of miR-544 was quantified using the miRNA-specific TaqMan miRNA assay kit (Applied Biosystems; Thermo Fisher Scientific, Inc.), which is specific for hsa-miR-544. The RT-qPCR analysis of FOXO1 expression levels were performed using the Fast SYBR-Green Master Mix System (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol using the following thermocycling conditions: 95°C for 3 min, then 95°C for 15 sec and 60°C for 1 min for a total of 40 cycles. The following primers were used: GAPDH forward, 5′-GAAGGTGAAGGTCGGAGTC-3 and reverse, 5′-GAAGATGGTGATGGGATTTC-3; and FOXO1 forward, 5′-AAGAGCGTGCCCTACTTCAA-3′ and reverse, 5′-CTGTTGTTGTCCATGGATGC-3′. The targeted gene relative quantification was provided by the Cq values and the relative mRNA or miRNA expression levels of targeted genes were determined as 2−[(Cq of FOXO1 or miR-544)-(Cq of GAPDH or U6)] (25). The experiment was performed in triplicate.
Colony formation assay
A total of 1×103 cells were seeded in a 60-mm dish following transfection and cultured at 37°C in 5% CO2. Following a 2-week incubation, the cells were fixation with 10% formaldehyde for 30 min at room temperature and stained with 1% crystal violet for another 30 min at room temperature.
MTT assay
A total of 2×103 cells were seeded in 96-well plates per well after transfection. The cells were incubated in 10 µl MTT (0.5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at the indicated time points for 4 h at 37°C. The medium was then removed and 100 µl dimethyl sulfoxide was added. The absorbance at 570 nm was detected using a microplate auto-reader (Bio-Rad Laboratories, Inc.).
Invasion assay
The in vitro invasion assay was performed using an 8 µm pore size 24-well Transwell chamber coated with 150 µg Matrigel (BD Biosciences, San Jose, CA, USA). Cells (1×105) in serum-free DMEM were added to the inserts and 600 µl DMEM supplemented with 10% FBS was added to the lower wells to act as the nutritional attractant. Following incubation for 20 h at 37°C, the cells on the upper surface of the membrane were removed and the migratory cells that had attached to the lower surface were fixed with 100% methanol for 5 min and stained with 1% crystal violet for 30 min at room temperature. Cells in 3 selected microscopic fields (magnification, ×400) fields of view were counted and expressed as the average number of cells per field of view using a light microscope.
Bioinformatic prediction of miR-544 potential targets
The microRNA.org targets and expression (www.microrna.org) database was used to predict potential targets for miR-544, as previously described (26).
Flow cytometric analysis
Cells were collected by trypsinization and fixed in 70% ethanol in PBS at 4°C overnight. Cells were centrifuged at 500 × g for 1 min at room temperature, and then washed with cold PBS and re-centrifuged as before. The cells were incubated with 20 mg/ml propidium iodide (Sigma-Aldrich; Merck KGaA) supplemented with 2 mg/ml RNase A for 20 min at room temperature. The cells were then analyzed using a flow cytometer (FACSCalibur II; BD Biosciences). The data were processed using the ModFit LT 3.2 software (Verity Software House, Inc., Topsham, ME, USA).
Luciferase reporter assay
Cells (2×104) were seeded in triplicate in 24-well plates. A total of 200 ng UTR-FOXO1-1/2wt or UTR-FOXO1-1/2 mu was co-transfected with miR-544 mimic or control into the cells using Lipofectamine 3000 (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Luciferase and Renilla luciferase activities were evaluated 48 h after transfection using the Dual Luciferase Reporter Assay kit (Promega Corporation), according to the manufacturer's protocol. The results are expressed as a normalized ratio of Renilla to firefly luciferase.
Statistical analysis
The results are representative of three independent experiments and presented as the mean ± standard deviation. Student's t-test was used to perform comparisons between two groups of data. Multiple comparisons between data were performed using one-way analysis of variance, followed by Dunnett's test. P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA).
Results
miR-544 is upregulated in colorectal cancer cell lines
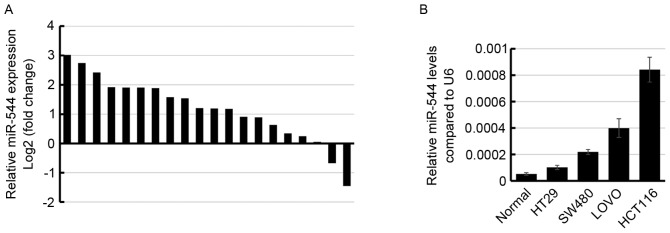
To examine the miR-544 expression level in colorectal cancer, the miR-544 expression level was determined in 20 pairs of colorectal cancer tissues and their adjacent normal tissues using RT-qPCR. The results demonstrated that miR-544 was higher in 60% (12/20) colorectal cancer tissue specimens compared with in normal tissues (Fig. 1A). Subsequently, the miR-544 expression level was determined in 4 colorectal cancer cell lines. Consistent with the results observed in the clinical tissue samples, miR-544 was significantly increased in the 4 colorectal cancer cell lines compared with the normal tissue samples (Fig. 1B), suggesting that miR-544 may be upregulated in human colorectal cancer.
Figure 1.
miR-544 expression is upregulated in colorectal cancer tissues and cell lines. (A) miR-544 expression levels in 20 paired colorectal cancer tissues and adjacent normal tissues determined using RT-qPCR. The relative miR-544 expression levels are presented as log2 (tumor/normal). (B) miR-544 expression levels in normal tissues and colorectal cancer cell lines (HT29, SW480, LOVO and HCT116) determined using RT-qPCR. miR, microRNA; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
miR-544 promotes colorectal cancer cell proliferation and invasion
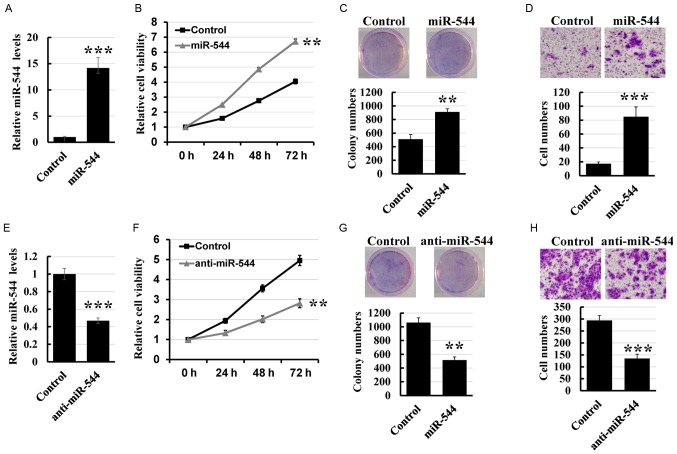
To determine the effect of miR-544 on colorectal cancer cell proliferation and invasion, miR-544 mimics were transfected into the HT29 cell line (Fig. 2A). MTT and colony formation assays revealed that overexpression of miR-544 markedly increased the growth rate compared with that of control cells (Fig. 2B and C). Furthermore, the Transwell assay demonstrated that overexpression of miR-544 increased the invasion rate of HT29 cells compared with the control cells (Fig. 2D). To further investigate the role of miR-544 on colorectal cancer cell proliferation and invasion, the miR-544 inhibitor was transfected into the HCT116 cell line (Fig. 2E). As presented in Fig. 2F-H, knockdown of miR-544 significantly decreased the proliferation and invasion of HCT116 cells compared with the control cells. Thus, these results indicated that miR-544 promoted colorectal cancer cell proliferation and invasion.
Figure 2.
miR-544 promotes colorectal cancer progression. (A) Expression levels of miR-544 in HT29 cells transfected with miR-544 mimic determined using RT-qPCR. (B) MTT analysis of miR-544-overexpressed HT29 and control cells. (C) Colony formation assay of miR-544-overexpressed HT29 and control cells. (D) Transwell assay of miR-544-overexpressed HT29 and control cells (magnification, ×400). (E) Expression levels of miR-544 in HCT116 cells transfected with miR-544 inhibitor determined using RT-qPCR. (F) MTT analysis of miR-544-depleted HCT116 and control cells. (G) Colony formation assay of miR-544-depleted HCT116 and control cells. (H) Transwell assay of miR-544-depleted HCT116 and control cells (magnification, ×400). **P<0.01, ***P<0.001 vs. control. miR, microRNA; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Effect of miR-544 on the cell cycle
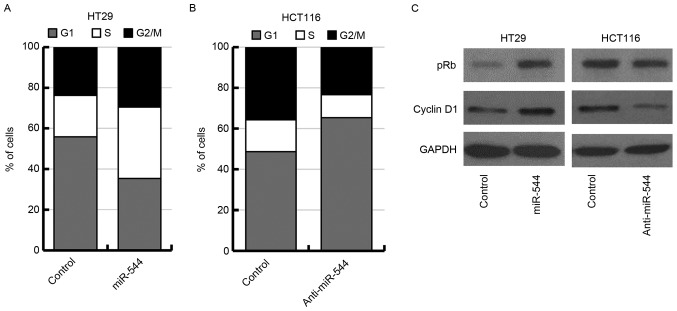
As miR-544 markedly affected cell proliferation in HT29 and HCT116 cells, the results of the present study suggested that miR-544 may function by regulating the cell cycle of colorectal cancer cells. Thus, the effect of miR-544 on cell cycle distribution was examined in miR-544-overexpressed HT29 cells, miR-544-depleted HCT116 cells and their corresponding control cells by flow cytometry. The results revealed that overexpression of miR-544 increased the percentage of cells in the S phase in HT29 cells (Fig. 3A). Knockdown of miR-544 increased the percentage of cells in G0/G1 phase in HCT116 cells (Fig. 3B). In the present study, it was investigated further whether the expression of pRb or cyclin D1 may be regulated by miR-544. The results indicated that miR-544-overexpressed HT29 cells exhibited a significant upregulation of pRb and cyclin D1 as determined using western blotting (Fig. 3C, left panel). Conversely, the expression levels of pRb and cyclin D1 were downregulated in miR-544-depleted HCT116 cells (Fig. 3C, right panel). Together, these results indicated that miR-544 may promote G1 to S phase transition in colorectal cancer cells.
Figure 3.
miR-544 induces G1 to S phase transition. Flow cytometric analysis of (A) miR-544-overexpressed HT29, (B) miR-544-depleted HCT116 and their control cells. (C) Western blot analysis of pRb and cyclin D1 expression levels in miR-544-overexpressed HT29 (left) and miR-544-depleted HCT116 (right), as well as their control cells. miR, microRNA; pRb, phosphorylated retinoblastoma protein.
FOXO1 is direct target of miR-544 in colorectal cancer cells
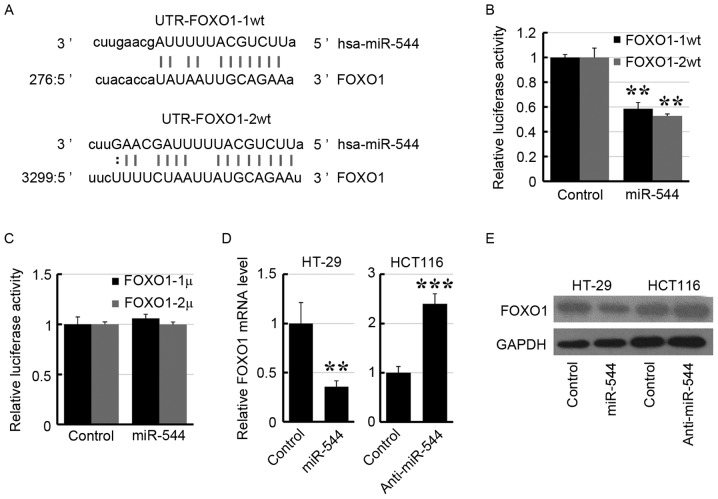
To identify the target gene of miR-544, a publically available database (http://www.microrna.org) was interrogated and it was revealed that FOXO1 was a potential target gene of miR-544 (Fig. 4A). Subsequently, the 3′-UTR of FOXO1 was subcloned into the psiCHECK2 vector. As presented in Fig. 4B, transfection of the miR-544 mimic suppressed the luciferase activity of UTR-FOXO1-1/2wt in 293FT cells. Furthermore, site mutations in the miR-544-binding site region of the FOXO1 3′-UTR abrogated the repressive effect of miR-544 (Fig. 4C). Furthermore, overexpression of miR-544 decreased the expression level of FOXO1 in HT29 cells, whereas knockdown of miR-544 increased the FOXO1 expression level in HCT116 cells by RT-qPCR (Fig. 4D) and western blotting (Fig. 4E), respectively. These results suggested that FOXO1 may be the target of miR-544.
Figure 4.
FOXO1 is a target of miR-544. (A) Predicted miR-544 target sequences of FOXO1 3′-UTR. (B) Luciferase assay on 293FT cells transfected with UTR-FOXO1-1/2wt and miR-544 mimic. (C) Luciferase assay of 293FT cells transfected with UTR-FOXO1-1/2mu and miR-544 mimic. (D) FOXO1 expression level in miR-544-overexpressed HT29 (left), miR-544-depleted HCT116 (right) cells and their control cells determined using the reverse transcription-quantitative polymerase chain reaction. (E) FOXO1 expression level in miR-544-overexpressed HT29 (left), miR-544-depleted HCT116 (right) cells and their control cells determined using western blotting. **P<0.01, ***P<0.001 vs. control. FOXO1, forkhead box O1; miR, microRNA; UTR, untranslated region; wt, wild-type; mu, mutant.
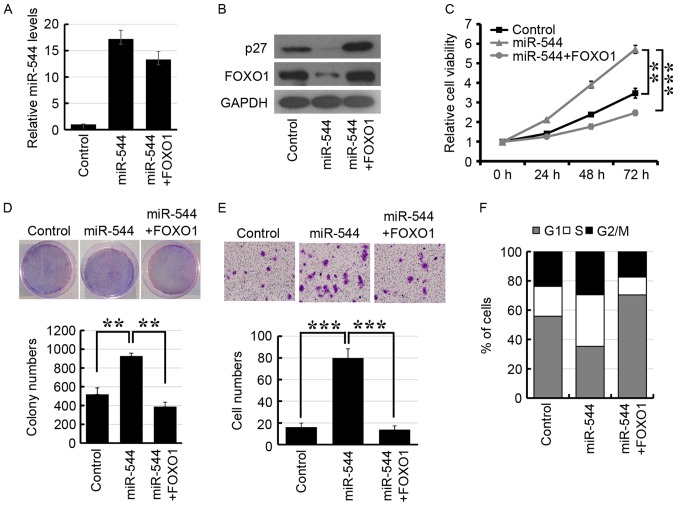
FOXO1 mediates miR-544-induced colorectal cancer progression
To further demonstrate whether miR-544 induced colorectal cancer progression via FOXO1 repression, FOXO1 was transfected into miR-544-overexpressed HT29 cells. As presented in Fig. 5A, RT-qPCR analysis revealed that the expression level of miR-544 was upregulated in miR-544 mimic-transfected HT29 cells. Furthermore, FOXO1 was significantly downregulated in miR-544-overexpressed HT29 cells and the expression of FOXO1 was re-established following transfection of FOXO1 (Fig. 5B). In addition, overexpression of miR-544 significantly decreased the expression of p27, which is the downstream target gene of FOXO1 (Fig. 5B). The MTT, colony formation and Transwell assays also indicated that co-transfection of miR-544 and FOXO1 may re-establish miR-544-promoted cell proliferation and invasion in HT29 (Fig. 5C-E). The analysis of cell cycle distribution revealed that overexpression of FOXO1 may re-establish the miR-544-induced increased proportion of cells in the S phase (Fig. 5F). Together, these results indicated that miR-544 may promote colorectal cancer progression by the inhibition of FOXO1.
Figure 5.
FOXO1 mediates miR-544-induced colorectal cancer cell proliferation and invasion. (A) Reverse transcription-quantitative polymerase chain reaction analysis of miR-544 expression levels in the indicated cells. (B) Western blot analysis of p27 and FOXO1 expression levels in the indicated HT29 cells. (C) MTT analysis of the indicated HT29 cells. (D) Colony formation assay of the indicated HT29 cells. (E) Transwell assay of the indicated HT29 cells (magnification, ×400). (F) Flow cytometric analysis of the indicated cells. **P<0.01, ***P<0.001. FOXO1, forkhead box O1; miR, microRNA.
Discussion
miRNAs are post-transcriptional regulators of oncogenes or tumor suppressors, and their deregulation is associated with cancer development and progression (27). The results of the present study indicated that miR-544 was upregulated in colorectal cancer cell lines and tissues. In addition, overexpression of miR-544 promoted G1 to S phase transition and cancer progression in colorectal cancer cell lines, whereas knockdown of miR-544 diminished these effects. Furthermore, FOXO1 is a direct target of miR-544. The results of the present study suggested that miR-544 may serve a critical role in colorectal cancer progression.
Aberrant expression of miR-544 has been observed in numerous cancer subtypes, suggesting that miR-544 may serve an important role in cancer development and progression (10,13–15,17–21). A previous study demonstrated that miR-544 promoted cell proliferation by downregulation of the tumor suppressor iroquois homeobox 1 in gastric cancer (17). As an oncogenic miRNA, miR-544 has also been revealed to be involved in the progression of nasopharyngeal carcinoma (28). Previous studies have demonstrated that miR-544 functions as a tumor suppressor and downregulates 14q32 miRNA (miR-382, miR-134 and miR-544), contributing to the biological behavior of osteosarcoma (19,20). These results indicated that dysregulation of miR-544 may occur in a tissue-specific manner in the various types of cancer; however, the role of miR-544 in colorectal cancer remains unknown. In the present study, the expression of miR-544 was upregulated in colorectal cancer tissues compared with the paired adjacent normal tissues. Furthermore, miR-544 promoted colorectal cancer cell proliferation and invasion. These results revealed that miR-544 may act as an oncogenic miRNA in colorectal cancer development and progression.
The forkhead box O (FOXO) family proteins, FOXO1, FOXO3a, FOXO4 and FOXO6, are key effectors of the phosphoinositide 3-kinase/protein kinase B signaling pathway and participate in numerous biological processes, including cell differentiation, oxidative stress responses, carcinogenesis and cell cycle regulation (29–32). Increased FOXO1 expression leads to G1/S cell cycle arrest, resulting in upregulated expression of the cyclin-dependent kinase inhibitors p21 and p27, and downregulation of cyclin D1 (33–35). FOXO1 has been reported to serve a crucial role in cancer development and progression. Repression of FOXO1 resulted in impaired cell death control and a dysregulated cell cycle, which may have a central role in tumorigenesis (23,36). Numerous miRNAs have been reported to regulate FOXO1 in colorectal cancer development and progression (37–39). To the best of our knowledge, the present study is the first to have identified FOXO1 as a target of miR-544, suggesting a crucial functional role of FOXO1 in colorectal carcinogenesis.
In conclusion, the results of the present study revealed that miR-544 promotes colorectal cancer development and progression by suppression of FOXO1 expression. Understanding the precise role that miR-544 serves in the induction of cancer cell proliferation will increase our understanding of the biology of colorectal cancer, and the inhibition of miR-544 may represent a novel therapeutic strategy in the treatment of colorectal cancer.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 4.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi M, Hisamori S, Oshima N, Sato F, Shimono Y, Sakai Y. miR-137 regulates the tumorigenicity of colon cancer stem cells through the inhibition of DCLK1. Mol Cancer Res. 2016;14:354–362. doi: 10.1158/1541-7786.MCR-15-0380. [DOI] [PubMed] [Google Scholar]
- 7.Pathak S, Meng WJ, Nandy SK, Ping J, Bisgin A, Helmfors L, Waldmann P, Sun XF. Radiation and SN38 treatments modulate the expression of microRNAs, cytokines and chemokines in colon cancer cells in a p53-directed manner. Oncotarget. 2015;6:44758–44780. doi: 10.18632/oncotarget.5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakai H, Sato A, Aihara Y, Ikarashi Y, Midorikawa Y, Kracht M, Nakagama H, Okamoto K. MKK7 mediates miR-493-dependent suppression of liver metastasis of colon cancer cells. Cancer Sci. 2014;105:425–430. doi: 10.1111/cas.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagaraju GP, Madanraj AS, Aliya S, Rajitha B, Alese OB, Kariali E, Alam A, El-Rayes BF. MicroRNAs as biomarkers and prospective therapeutic targets in colon and pancreatic cancers. Tumour Biol. 2016;37:97–104. doi: 10.1007/s13277-015-4346-6. [DOI] [PubMed] [Google Scholar]
- 10.Gao YQ, Chen X, Wang P, Lu L, Zhao W, Chen C, Chen CP, Tao T, Sun J, Zheng YY, et al. Regulation of DLK1 by the maternally expressed miR-379/miR-544 cluster may underlie callipyge polar overdominance inheritance; Proc Natl Acad Sci USA; 2015; pp. 13627–13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song W, Mu H, Wu J, Liao M, Zhu H, Zheng L, He X, Niu B, Zhai Y, Bai C, et al. miR-544 regulates dairy goat male germline stem cell self-renewal via targeting PLZF. J Cell Biochem. 2015;116:2155–2165. doi: 10.1002/jcb.25172. [DOI] [PubMed] [Google Scholar]
- 12.Song WQ, Gu WQ, Qian YB, Ma X, Mao YJ, Liu WJ. Identification of long non-coding RNA involved in osteogenic differentiation from mesenchymal stem cells using RNA-Seq data. Genet Mol Res. 2015;14:18268–18279. doi: 10.4238/2015.December.23.14. [DOI] [PubMed] [Google Scholar]
- 13.Haga CL, Velagapudi SP, Strivelli JR, Yang WY, Disney MD, Phinney DG. Small molecule inhibition of miR-544 biogenesis disrupts adaptive responses to hypoxia by modulating ATM-mTOR signaling. ACS Chem Biol. 2015;10:2267–2276. doi: 10.1021/acschembio.5b00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu P, Gu Y, Li L, Wang F, Qiu X. miR-544a promotes breast cancer cell migration and invasion reducing cadherin 1 expression. Oncol Res. 2016;23:165–170. doi: 10.3727/096504016X14519157902726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mo X, Zhang F, Liang H, Liu M, Li H, Xia H. miR-544a promotes the invasion of lung cancer cells by targeting cadherina 1 in vitro. Onco Targets Ther. 2014;7:895–900. doi: 10.2147/OTT.S61695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao L, Zhang Y, Deng X, Mo W, Yu Y, Lu H. Transcription factor KLF4 regulates microRNA-544 that targets YWHAZ in cervical cancer. Am J Cancer Res. 2015;5:1939–1953. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhi Q, Guo X, Guo L, Zhang R, Jiang J, Ji J, Zhang J, Zhang J, Chen X, Cai Q, et al. Oncogenic miR-544 is an important molecular target in gastric cancer. Anticancer Agents Med Chem. 2013;13:270–275. doi: 10.2174/1871520611313020013. [DOI] [PubMed] [Google Scholar]
- 18.Yanaka Y, Muramatsu T, Uetake H, Kozaki K, Inazawa J. miR-544a induces epithelial-mesenchymal transition through the activation of WNT signaling pathway in gastric cancer. Carcinogenesis. 2015;36:1363–1371. doi: 10.1093/carcin/bgv106. [DOI] [PubMed] [Google Scholar]
- 19.Sarver AL, Thayanithy V, Scott MC, Cleton-Jansen AM, Hogendoorn PC, Modiano JF, Subramanian S. MicroRNAs at the human 14q32 locus have prognostic significance in osteosarcoma. Orphanet J Rare Dis. 2013;8:7. doi: 10.1186/1750-1172-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thayanithy V, Sarver AL, Kartha RV, Li L, Angstadt AY, Breen M, Steer CJ, Modiano JF, Subramanian S. Perturbation of 14q32 miRNAs-cMYC gene network in osteosarcoma. Bone. 2012;50:171–181. doi: 10.1016/j.bone.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma R, Zhang G, Wang H, Lv H, Fang F, Kang X. Downregulation of miR-544 in tissue, but not in serum, is a novel biomarker of malignant transformation in glioma. Oncol Lett. 2012;4:1321–1324. doi: 10.3892/ol.2012.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattacharyya NP, Das E, Bucha S, Das S, Choudhury A. Regulation of cell cycle associated genes by microRNA and transcription factor. Microrna. 2016;5:180–200. doi: 10.2174/2211536605666161117112251. [DOI] [PubMed] [Google Scholar]
- 23.Hou T, Ou J, Zhao X, Huang X, Huang Y, Zhang Y. MicroRNA-196a promotes cervical cancer proliferation through the regulation of FOXO1 and p27Kip1. Br J Cancer. 2014;110:1260–1268. doi: 10.1038/bjc.2013.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao XC, Yu Y, Hou LK, Sun XH, Ge J, Zhang B, Wang X. miR-142-3p inhibits cancer cell proliferation by targeting CDC25C. Cell Prolif. 2016;49:58–68. doi: 10.1111/cpr.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008;36:D149–D153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markopoulos GS, Roupakia E, Tokamani M, Chavdoula E, Hatziapostolou M, Polytarchou C, Marcu KB, Papavassiliou AG, Sandaltzopoulos R, Kolettas E. A step-by-step microRNA guide to cancer development and metastasis. Cell Oncol (Dordr) 2017;40:303–339. doi: 10.1007/s13402-017-0341-9. [DOI] [PubMed] [Google Scholar]
- 28.Luo Z, Zhang L, Li Z, Li X, Li G, Yu H, Jiang C, Dai Y, Guo X, Xiang J, Li G. An in silico analysis of dynamic changes in microRNA expression profiles in stepwise development of nasopharyngeal carcinoma. BMC Med Genomics. 2012;5:3. doi: 10.1186/1755-8794-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao H, Mohamed EM, Xu GG, Waters M, Jing K, Ma Y, Zhang Y, Spiegel S, Idowu MO, Fang X. Carnitine palmitoyltransferase 1A functions to repress FoxO transcription factors to allow cell cycle progression in ovarian cancer. Oncotarget. 2016;7:3832–3846. doi: 10.18632/oncotarget.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bicknell KA. Forkhead (FOX) transcription factors and the cell cycle: Measurement of DNA binding by FoxO and FoxM transcription factors. Methods Mol Biol. 2005;296:247–262. doi: 10.1385/1-59259-857-9:247. [DOI] [PubMed] [Google Scholar]
- 31.Kuscu N, Celik-Ozenci C. FOXO1, FOXO3, and FOXO4 are differently expressed during mouse oocyte maturation and preimplantation embryo development. Gene Expr Patterns. 2015;18:16–20. doi: 10.1016/j.gep.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Sztukowska M, Ojo A, Scott DA, Wang H, Lamont RJ. FOXO responses to Porphyromonas gingivalis in epithelial cells. Cell Microbiol. 2015;17:1605–1617. doi: 10.1111/cmi.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, Burgering BM, Medema RH. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842–7852. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Gan B, Liu D, Paik JH. FoxO family members in cancer. Cancer Biol Ther. 2011;12:253–259. doi: 10.4161/cbt.12.4.15954. [DOI] [PubMed] [Google Scholar]
- 36.Myatt SS, Wang J, Monteiro LJ, Christian M, Ho KK, Fusi L, Dina RE, Brosens JJ, Ghaem-Maghami S, Lam EW. Definition of microRNAs that repress expression of the tumor suppressor gene FOXO1 in endometrial cancer. Cancer Res. 2010;70:367–377. doi: 10.1158/0008-5472.CAN-09-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao F, Wang W. MicroRNA-96 promotes the proliferation of colorectal cancer cells and targets tumor protein p53 inducible nuclear protein 1, forkhead box protein O1 (FOXO1) and FOXO3a. Mol Med Rep. 2015;11:1200–1206. doi: 10.3892/mmr.2014.2854. [DOI] [PubMed] [Google Scholar]
- 38.Wu L, Li H, Jia CY, Cheng W, Yu M, Peng M, Zhu Y, Zhao Q, Dong YW, Shao K, et al. MicroRNA-223 regulates FOXO1 expression and cell proliferation. FEBS Lett. 2012;586:1038–1043. doi: 10.1016/j.febslet.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 39.Roy UK, Henkhaus RS, Ignatenko NA, Mora J, Fultz KE, Gerner EW. Wild-type APC regulates caveolin-1 expression in human colon adenocarcinoma cell lines via FOXO1a and C-myc. Mol Carcinog. 2008;47:947–955. doi: 10.1002/mc.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]