Abstract
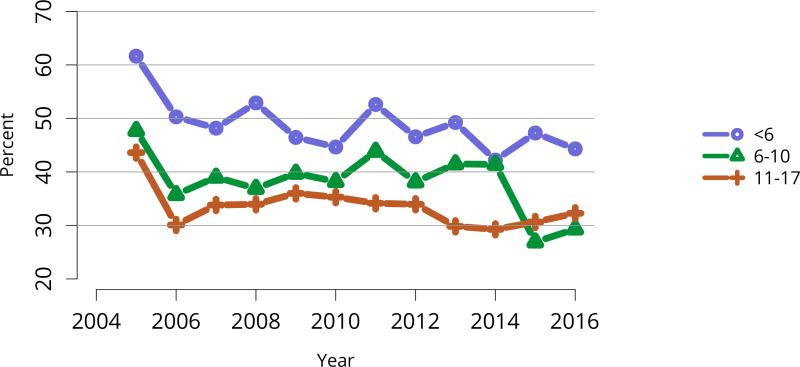
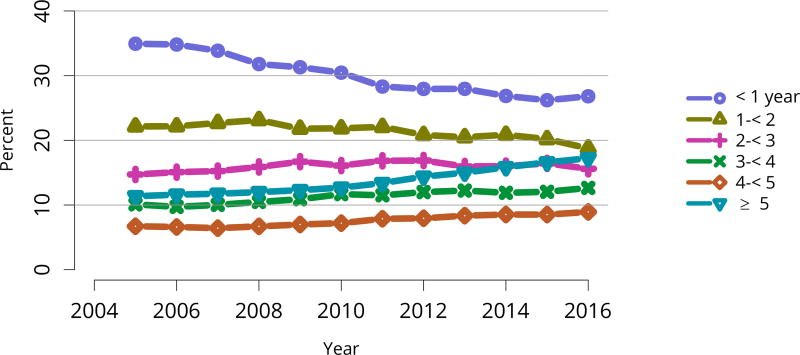
Data from 2016 show ongoing positive trends in short- and long-term allograft survival, and a decrease in the number of active listed candidates for the first time in more than a decade, with a concomitant increase in deceased donor kidney transplants. Transplant rates that had changed dramatically for some groups after implementation of the new kidney allocation system in 2014 are stabilizing, allowing for evaluation of new steady states and trends. Many challenges remain in adult kidney transplantation, including stagnant rates of living donor transplant, geographic disparities in access to transplant, racial disparities in living donor transplant, and overall a continuing demand for kidneys that far outpaces the supply. For pediatric recipients, a decline in the proportion of living donor transplants is of concern. In 2016, only 34.2% of pediatric transplants were from living donors, compared with 47.2% in 2005. The number of related donors decreased dramatically over the past decade, and the number of unrelated directed transplants performed in pediatric candidates remained low (50).
1 Introduction
The 2016 Annual Data Report kidney chapter provides a second year of data following implementation of the new kidney allocation system (KAS) in December 2014. Examination of 2015 data revealed “bolus effects,” or rapid changes in transplant rates before they leveled out at a new steady state. We can now begin to assess both intended and potential unintended consequences of the new policy. These data also show where the new KAS achieved its aims, for example in increasing deceased donor transplant rates among racial minorities, and where the kidney transplant community should continue its efforts beyond the KAS to achieve equity, such as increasing access to transplant for blood group B candidates and reducing the ongoing marked disparity for black patients in access to living donor transplant and allograft survival.
The 2016 data show other encouraging trends and concerns that warrant further investigation. For the first time in more than a decade, the number of candidates, both active and inactive, on the deceased donor waiting list declined, and the number of deceased donor transplants increased notably. Both short- and long-term unadjusted allograft survival continued to improve, although the short-term effect of KAS may not have stabilized, and long-term effects are unknown. However, the number of living donor transplants remained flat. Geographic variation in access to transplant remained high, and fewer candidates were willing to accept kidneys with a high kidney donor profile index (KDPI) score despite an aging waitlist population with more years on dialysis and higher prevalence of comorbid conditions. The potential long-term graft survival benefits of longevity matching with kidney donor risk index (KDRI) and expected posttransplant survival scores will be difficult to assess for several years. In summary, the 2016 data show both progress and ongoing challenges for the transplant community in providing this life-saving treatment to patients with end-stage kidney disease.
2 Adult Kidney Transplant
2.1 Waiting List
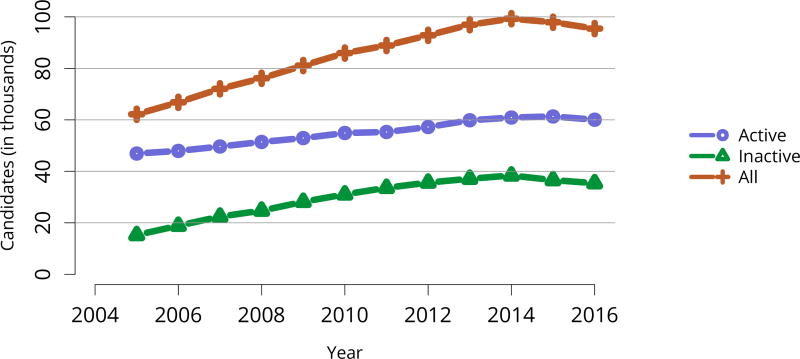
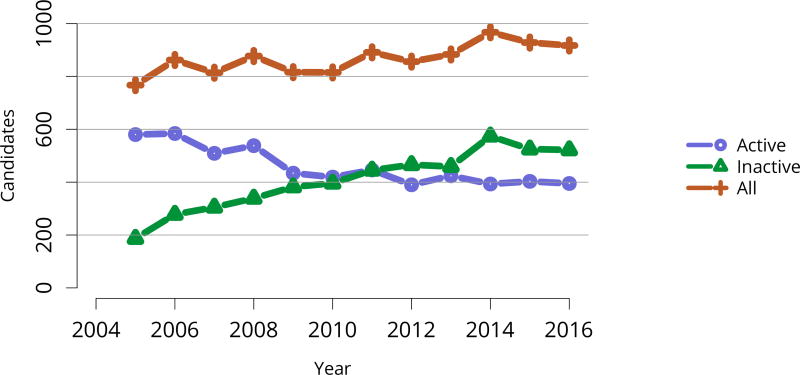
Perhaps the most striking trend apparent in the 2016 waitlist data is the decrease in listed candidates for the second year in a row, after a peak of nearly 100,000 in 2014 (Figure KI 2). Unlike in 2015, numbers of both active and inactive candidates decreased. In total, 30,869 adult candidates were added to and 33,291 removed from the list, and deceased donor transplants increased from 12,279 in 2015 to 13,501 in 2016 (Table KI 5, Table KI 6). The number of new inactive listings declined for the second year in a row, likely due to the new KAS, which eliminated the utility of newly listing as inactive for candidates already on dialysis undergoing pretransplant workup (Figure KI 1). Credit given for time on dialysis may also explain the ongoing increase in numbers of adult patients removed from the list due to being too sick to undergo transplant, 4411 in 2016 versus 3325 in 2014. Unfortunately, more than one-fourth of the 33,291 adult patients removed from the list were removed due to death or deteriorating medical condition, reflecting the ongoing organ shortage despite gains in numbers of deceased donor transplants (Table KI 6). Removals for other reasons also increased, and given that more than 13.4% of waitlist removals were for other reasons, a closer examination of how reporting categories are used may be warranted to ensure that clinically relevant trends are not missed.
Figure KI 2. Adults listed for kidney transplant on December 31 each year.
Candidates concurrently listed at multiple centers are counted once. Those with concurrent listings and active at any program are considered active. Includes kidney and kidney-pancreas listings.
Table KI 5. Kidney transplant waitlist activity among adults.
Candidates concurrently listed at more than one center are counted once, from the time of earliest listing to the time of latest removal. Candidates who are listed, undergo transplant, and are relisted are counted more than once. Candidates are not considered to be on the list on the day they are removed; counts on January 1 may differ from counts on December 31 of the prior year. Candidates listed for multi-organ transplants are included.
| Waiting list state | 2014 | 2015 | 2016 |
|---|---|---|---|
| Patients at start of year | 96,920 | 99,239 | 97,878 |
| Patients added during year | 31,267 | 30,221 | 30,869 |
| Patients removed during year | 28,893 | 31,538 | 33,291 |
| Patients at end of year | 99,294 | 97,922 | 95,456 |
Table KI 6. Removal reason among adult kidney transplant candidates.
Removal reason as reported to the OPTN. Candidates with death dates that precede removal dates are assumed to have died waiting.
| Removal reason | 2014 | 2015 | 2016 |
|---|---|---|---|
| Deceased donor transplant | 11,589 | 12,279 | 13,501 |
| Living donor transplant | 5084 | 5331 | 5335 |
| Transplant outside US | 46 | 50 | 77 |
| Patient died | 4953 | 4976 | 4830 |
| Patient refused transplant | 474 | 524 | 479 |
| Improved, transplant not needed | 194 | 211 | 195 |
| Too sick for transplant | 3325 | 4099 | 4411 |
| Other | 3228 | 4068 | 4463 |
Figure KI 1. New adult candidates added to the kidney transplant waiting list.
A new candidate is one who first joined the list during the given year, without having been listed in a previous year. Previously listed candidates who underwent transplant and subsequently relisted are considered new. Candidates concurrently listed at multiple centers are counted once. Active and inactive patients are included; active status is determined on day 7 after first listing. Includes kidney and kidney-pancreas listings.
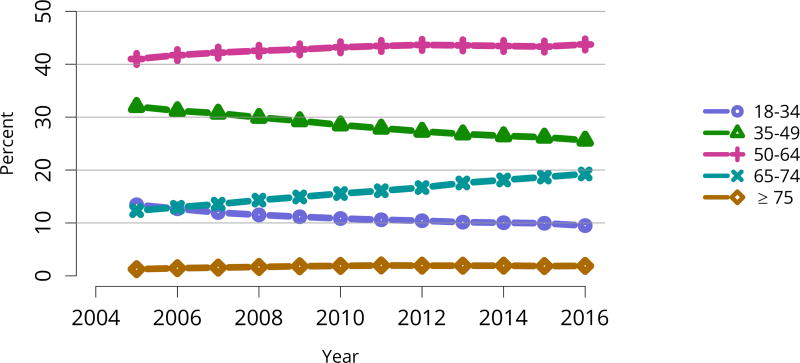
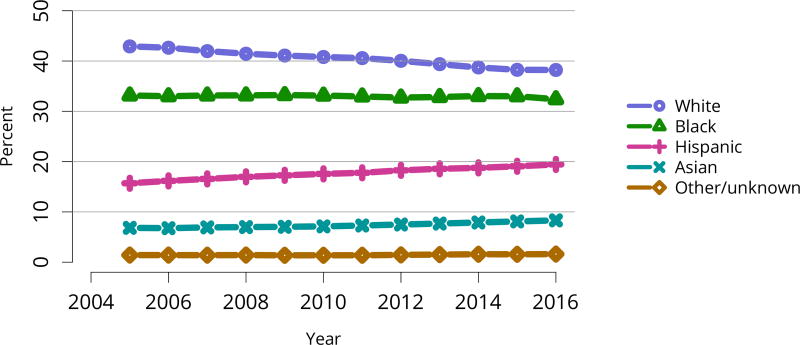
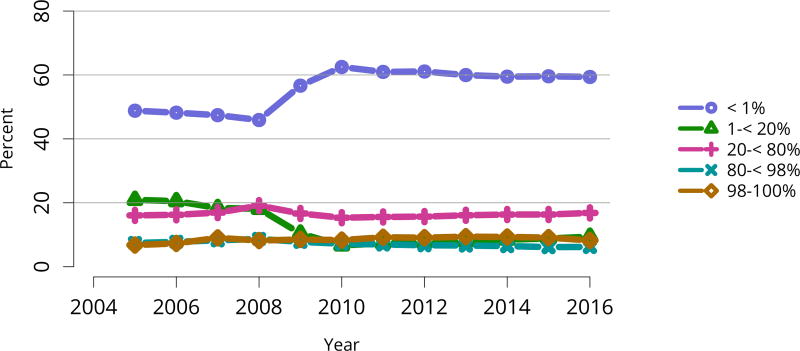
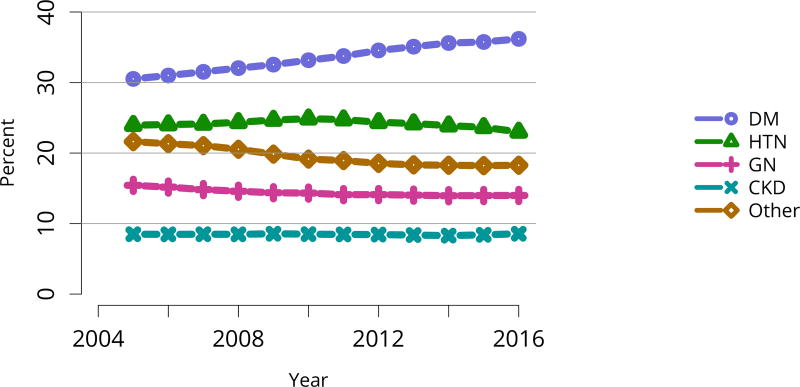
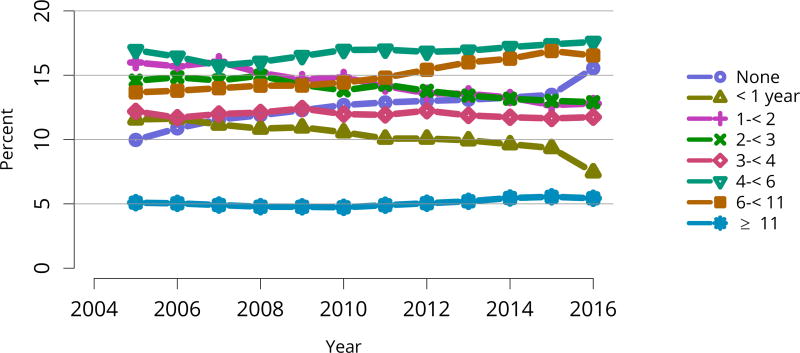
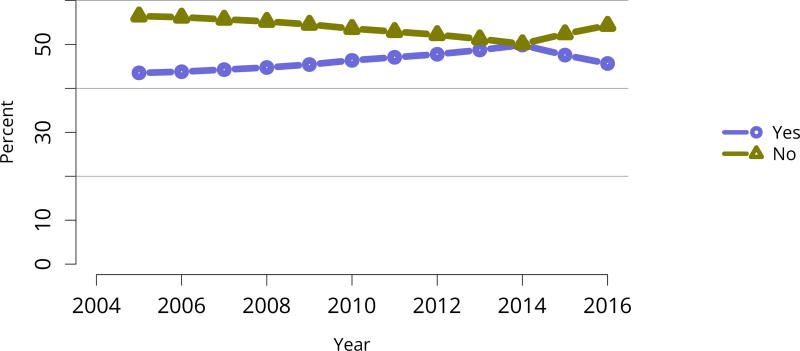
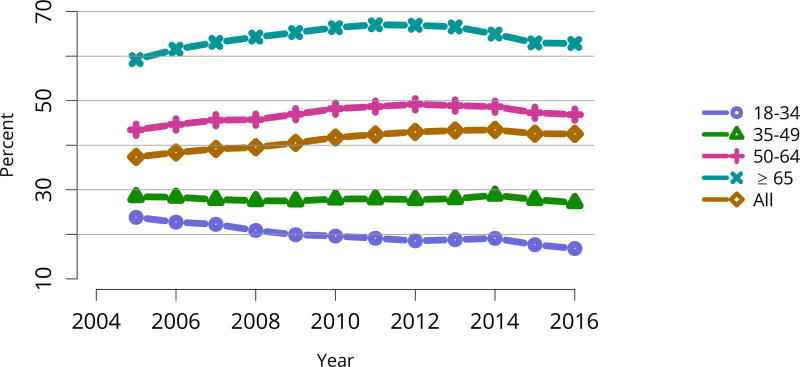
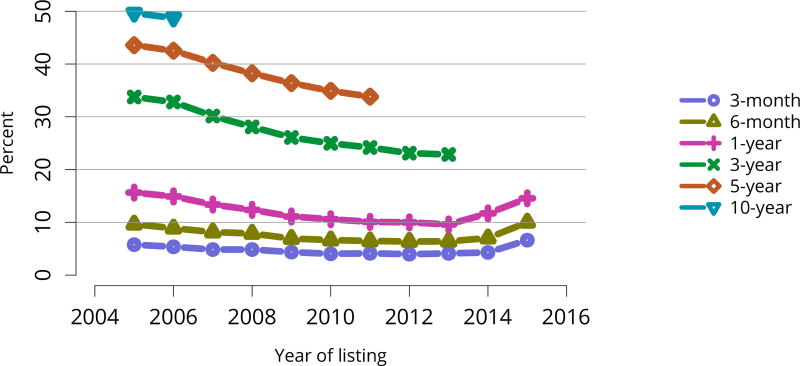
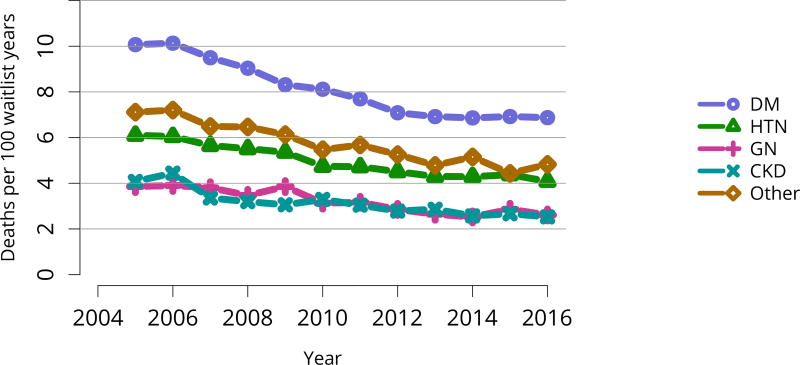
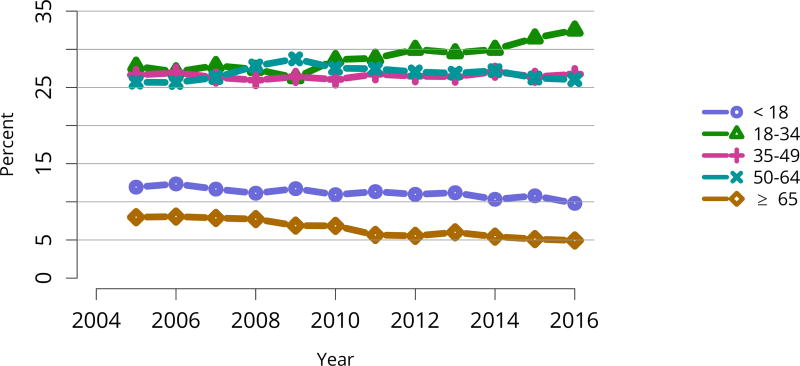
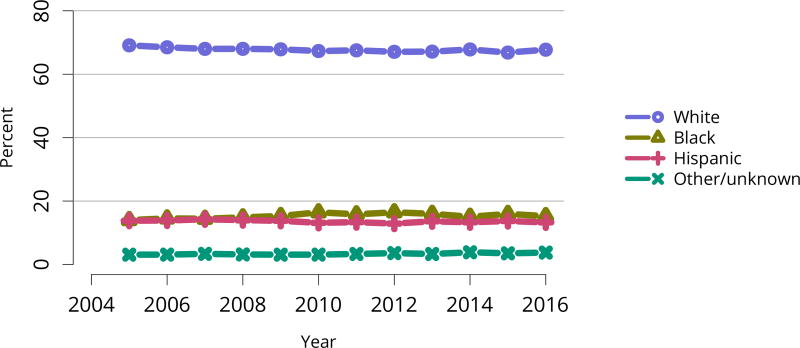
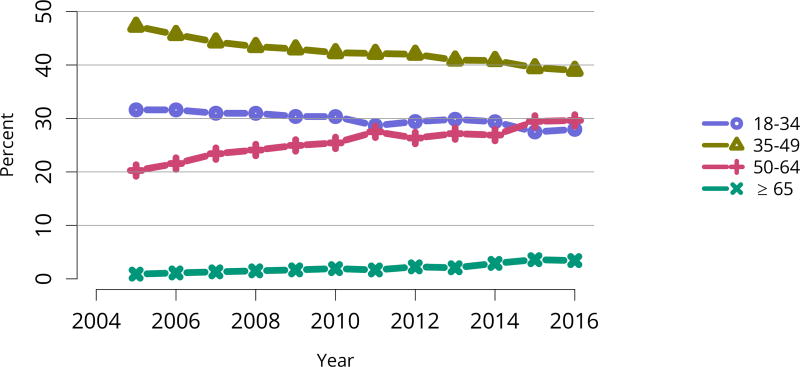
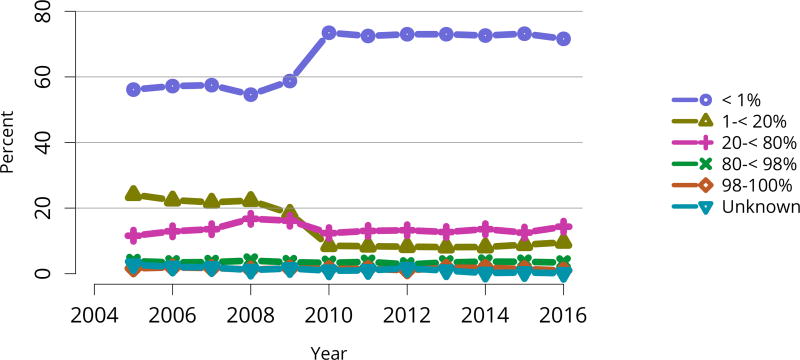
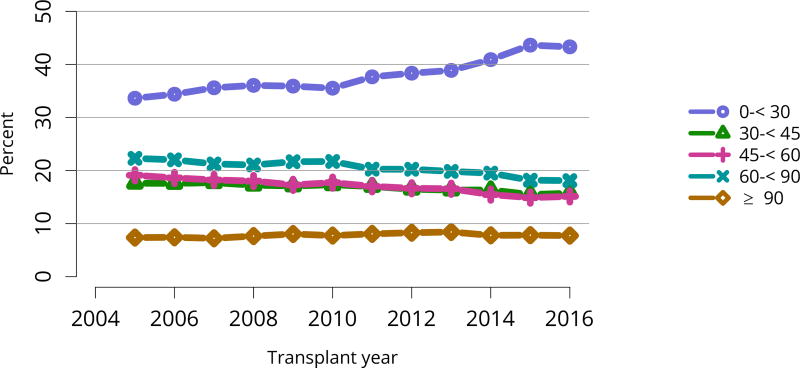
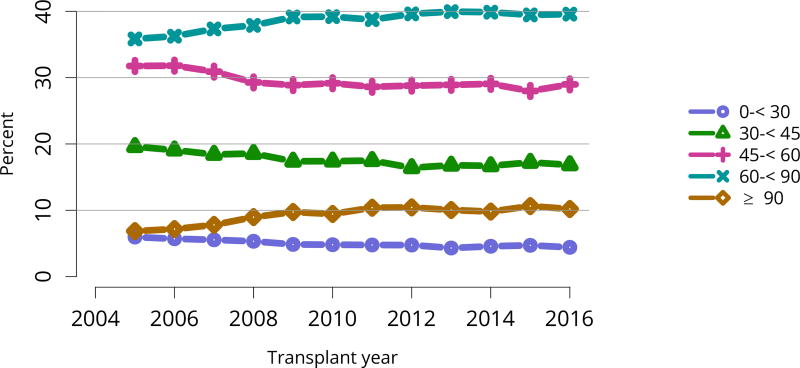
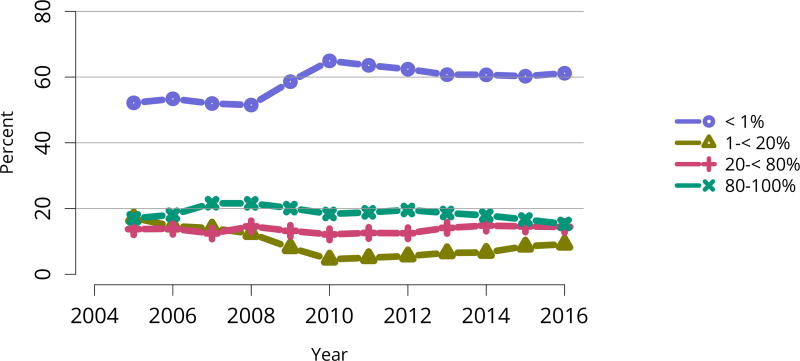
The kidney transplant waiting list continued to age, with ongoing increases in the proportions of candidates aged 50–74 years (Figure KI 3). While overall the racial composition of the list changed little, the trend toward increasing proportions of Hispanic candidates continued, from 15.7% in 2005 to 19.4% in 2016 (Figure KI 4). Proportions of waitlisted candidates with calculated panel-reactive antibodies (cPRA) 98%–100% declined from 9.4% in 2013 to 8.2% in 2016, likely reflecting increases in transplants for these candidates due to the new KAS (Figure KI 7). The proportion of candidates with diabetes as a cause of kidney disease increased to 36.2% pf waitlisted candidates (Figure KI 5). Time on the waiting list and on dialysis also continued to increase; more than 20% of listed candidates had been on dialysis for at least 6 years from their most recent listing (Figure KI 8). Considering that more listed candidates are older, have diabetes, and have longer dialysis duration, perhaps the most concerning recent waitlist trend is a decrease for the second year in a row in the proportion willing to accept a high-KDPI kidney, down from 49.9 % in 2014 to 45.7% in 2016 (Figure KI 9). Counter-intuitively, this decline was more dramatic among candidates aged 65 years or older (Figure KI 19).
Figure KI 3. Distribution of adults waiting for kidney transplant by age.
Candidates waiting for transplant at any time in the given year. Candidates listed concurrently at multiple centers are counted once. Age is determined at the later of listing date or January 1 of the given year. Active and inactive candidates are included.
Figure KI 4. Distribution of adults waiting for kidney transplant by race.
Candidates waiting for transplant at any time in the given year. Candidates listed concurrently at multiple centers are counted once. Active and inactive candidates are included.
Figure KI 7. Distribution of adults waiting for kidney transplant by C/PRA.
Candidates waiting for transplant at any time in the given year. Candidates listed concurrently at multiple centers are counted once. From December 5, 2007, through September 30, 2009, CPRA was used if greater than 0; otherwise, the maximum pretransplant PRA was used. Before December 5, 2007, the maximum pretransplant PRA was used unconditionally. CPRA is used after September 30, 2009. C/PRA is the highest value during the year. Active and inactive candidates are included.
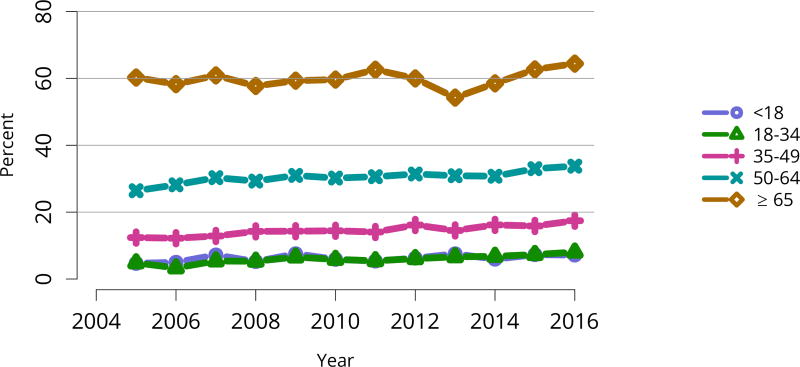
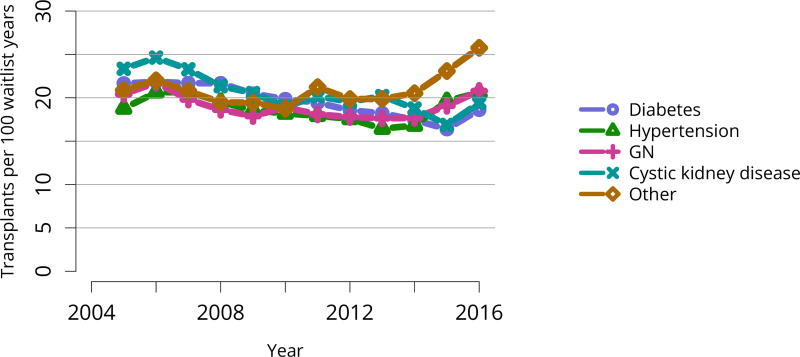
Figure KI 5. Distribution of adults waiting for kidney transplant by diagnosis.
Candidates waiting for transplant at any time in the given year. Candidates listed concurrently at multiple centers are counted once. Active and inactive candidates are included. CKD, cystic kidney disease; DM, diabetes. HTN, hypertension. GN, glomerulonephritis.
Figure KI 8. Distribution of adults waiting for kidney transplant by time on dialysis.
Candidates waiting for transplant at any time in the given year. Candidates listed concurrently at multiple centers are counted once. Time on dialysis begins at the more recent of first ESRD service date and most recent graft failure, and ends at the earlier of December 31 or removal from the waiting list. Active and inactive candidates are included.
Figure KI 9. Distribution of adults waiting for kidney transplant by willingness to accept ECD or KDPI > 85% kidney.
Candidates waiting for transplant at any time in the given year. Candidates listed concurrently at multiple centers are counted once. Active and inactive candidates are included. Willingness to accept ECD at time of listing or willingness to accept a local non-zero HLA mismatch KDPI >85% kidney for at least one day during the year, beginning in 2014. ECD, expanded criteria donor.
Figure KI 19. Adults willing to accept a kidney designated ECD or KDPI > 85% by age.
Adults waiting for kidney transplant on December 31 of the given year. Candidates concurrently listed at more than one center are counted once, from the time of earliest listing to the time of latest removal. Willingness to accept ECD at time of listing or willingness to accept a local non-zero HLA mismatch KDPI >85% kidney for at least one day during the year, beginning in 2014. ECD, expanded criteria donor.
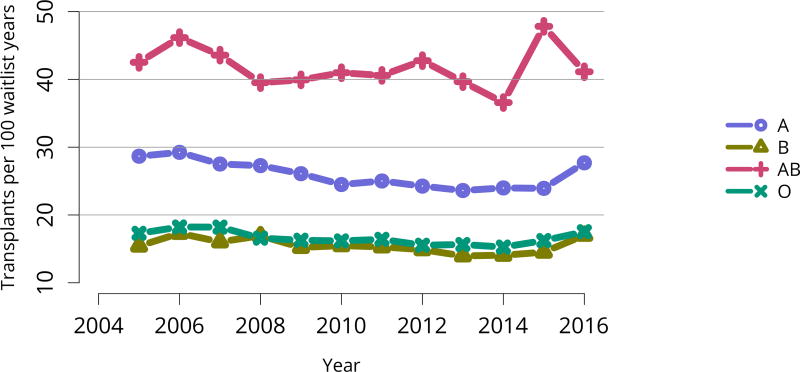
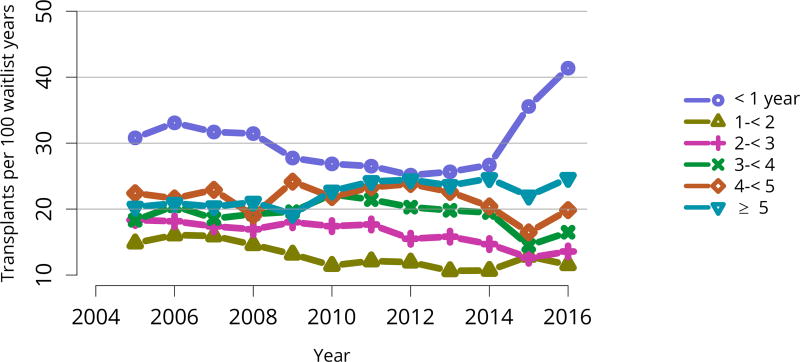
Deceased donor transplant rates, or transplants per 100 waitlist-years, changed dramatically for some groups after KAS implementation. After an initially large increase in 2015 for candidates aged 18–34 years, the rate increased again in 2016, but to a degree similar to increases for all other age groups (Figure KI 11). The rate for candidates with cPRA 98%–100% was essentially equal to the rate in 2015, when a dramatic increase followed KAS implementation (Figure KI 13). Transplant rates remained higher for candidates with blood type AB (Figure KI 14). Interestingly, the rate for candidates listed for less than 1 year soared after 2014, perhaps reflecting many more transplants in newly listed candidates who had been on dialysis for many years (Figure KI 15).
Figure KI 11. Deceased donor kidney transplant rates among active adult waitlist candidates by age.
Transplant rates are computed as the number of deceased donor transplants per 100 patient-years of active wait time in a given year. Individual listings are counted separately. Age is determined at the later of listing date or January 1 of the given year. Rates with less than 10 patient-years of exposure are not shown.
Figure KI 13. Deceased donor kidney transplant rates among active adult waitlist candidates by C/PRA.
Transplant rates are computed as the number of deceased donor transplants per 100 patient-years of active wait time in a given year. Individual listings are counted separately. From December 5, 2007, through September 30, 2009, CPRA was used if greater than 0; otherwise, the maximum pretransplant PRA was used. Before December 5, 2007, the maximum pretransplant PRA was used unconditionally. CPRA is used after September 30, 2009. Rates with less than 10 patient-years of exposure are not shown.
Figure KI 14. Deceased donor kidney transplant rates among active adult waitlist candidates by blood type.
Transplant rates are computed as the number of deceased donor transplants per 100 patient-years of active wait time in a given year. Individual listings are counted separately. Rates with less than 10 patient-years of exposure are not shown.
Figure KI 15. Deceased donor kidney transplant rates among active adult waitlist candidates by time on the waitlist.
Transplant rates are computed as the number of deceased donor transplants per 100 patient-years of active wait time in a given year. Individual listings are counted separately. Rates with less than 10 patient-years of exposure are not shown.
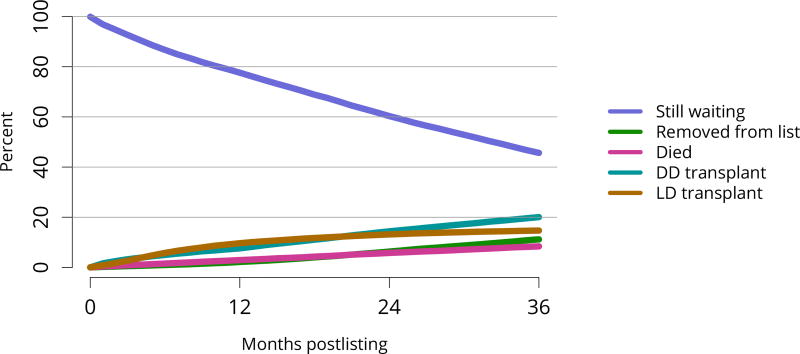
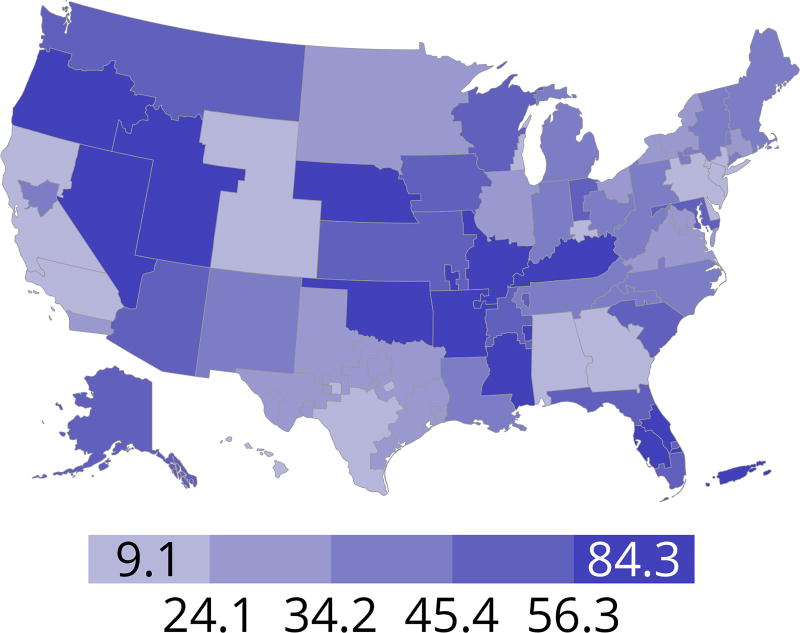
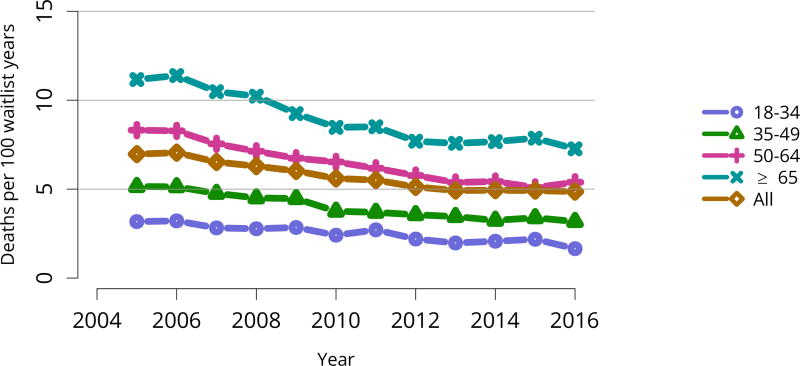
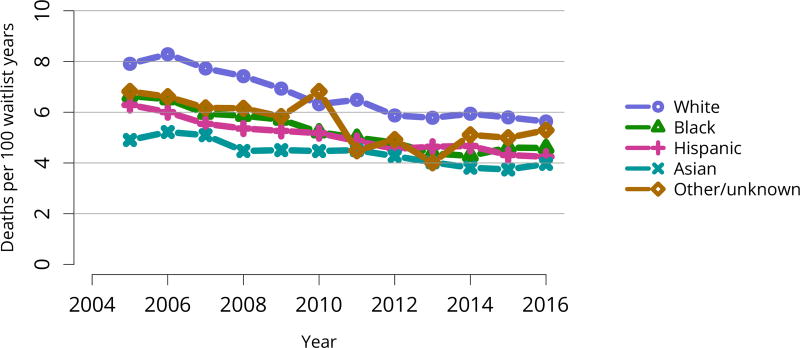
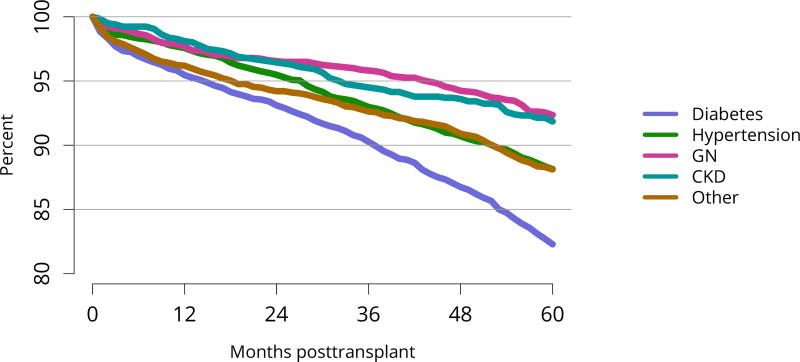
Cumulatively, for candidates listed in 2013, fewer than 50% were still waiting in 2016; 20% underwent deceased donor transplant, 15% underwent living donor transplant, 8% died, and 11% were removed from the list for other reasons (Figure KI 16). These competing risks reflect the difficulty of calculating a national median time to transplant, as half of newly listed candidates in 2005 had not undergone transplant by 2016 (Figure KI 17). Geographic variability in access to transplant remained high, making national averages for waitlist outcomes less relevant than from region to region. The percentage of patients who underwent deceased donor kidney transplant within 5 years varied from 9.1% to 84.3% across donation service areas (DSAs) (Figure KI 18); waitlist mortality rates also varied, ranging from 0 to 12.7 per 100 patient-years across DSAs (Figure KI 23). Overall and by age, race, and diagnosis, mortality rates for listed patients decreased over the past 10 years (Figure KI 20, Figure KI 21, Figure KI 22). However, given recent increases in removals from the waiting list for reasons other than death or transplant, it is notable that deaths within 6 months of removal have also declined since 2014, suggesting that, at the very least, transplant programs are not compensating for changed waitlist demographics post-KAS by more rapidly delisting candidates at higher mortality risk.
Figure KI 16. Three-year outcomes for adults waiting for kidney transplant, new listings in 2013.
Adults waiting for any kidney transplant and first listed in 2013. Candidates concurrently listed at more than one center are counted once, from the time of earliest listing to the time of latest removal. Removed from list includes all reasons except transplant and death. DD, deceased donor; LD, living donor.
Figure KI 17. Percentage of adults who underwent deceased donor kidney transplant within a given time period of listing.
Candidates concurrently listed at more than one center are counted once, from the time of earliest listing to the time of latest removal.
Figure KI 18. Percentage of adults who underwent deceased donor kidney transplant within 5 years of listing in 2011 by DSA.
Candidates listed concurrently in a single DSA are counted once in that DSA, from the time of earliest listing to the time of latest removal; candidates listed in multiple DSAs are counted separately per DSA.
Figure KI 23. Pretransplant mortality rates among adults waitlisted for kidney transplant in 2016, by DSA.
Mortality rates are computed as the number of deaths per 100 patient-years of waiting in the given year. Patients censored at waitlist removal. Individual listings are counted separately. Rates with less than 10 patient-years of exposure are not shown.
Figure KI 20. Pretransplant mortality rates among adults waitlisted for kidney transplant by age.
Mortality rates are computed as the number of deaths per 100 patient-years of waiting in the given year. Individual listings are counted separately. Rates with less than 10 patient-years of exposure are not shown. Age is determined at the later of listing date or January 1 of the given year.
Figure KI 21. Pretransplant mortality rates among adults waitlisted for kidney transplant by race.
Mortality rates are computed as the number of deaths per 100 patient-years of waiting in the given year. Individual listings are counted separately. Rates with less than 10 patient-years of exposure are not shown.
Figure KI 22. Pretransplant mortality rates among adults waitlisted for kidney transplant by diagnosis.
Mortality rates are computed as the number of deaths per 100 patient-years of waiting in the given year. Individual listings are counted separately. Rates with less than 10 patient-years of exposure are not shown. CKD, cystic kidney disease; DM, diabetes. HTN, hypertension. GN, glomerulonephritis.
2.2 Deceased Donation
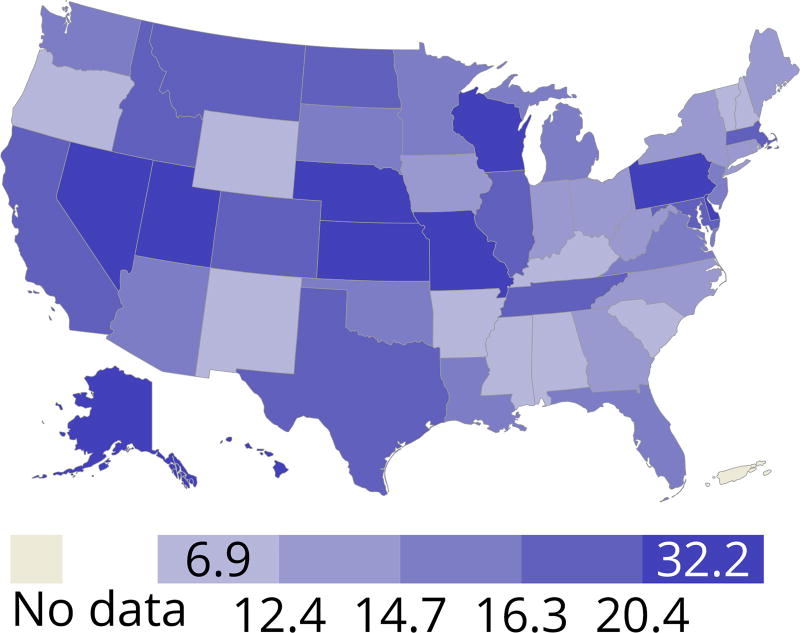
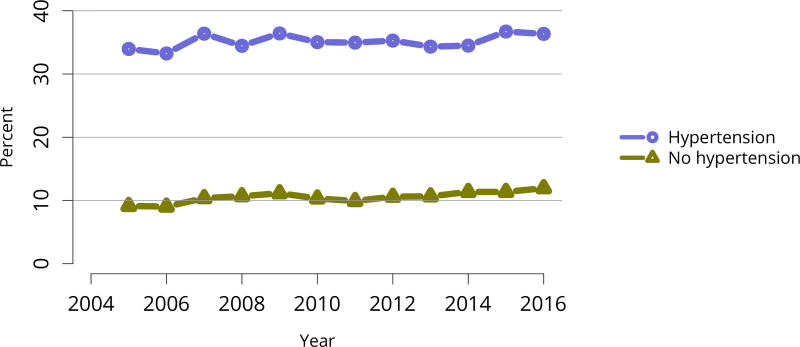
Overall, the demographics of deceased kidney donors remained stable, with a slight decline over 10 years in the proportions in the youngest and oldest age groups, and an increase in the proportion aged 18–34 years (Figure KI 25, Figure KI 26). Donation rates continued to vary greatly by state, from 6.9 to 32.2 per 1000 deaths (Figure KI 27). The previously noted trend of a slow 10-year increase in discard rates continued across age group, comorbidity, cause of death, donor type, and KDPI. Implementation of the new KAS raised concerns about increasing discard rates in the setting of increased geographic sharing of kidneys and longer cold ischemia time. While the current discard trend preceded the new KAS, the rate of increase appears to have worsened post-KAS in some groups (Figure KI 28, Figure KI 29, Figure KI 30, Figure KI 31, Figure KI 32, Figure KI 33). In particular, discards of kidneys recovered from donors aged 65 years or older, from donors with diabetes, and with KDPI above 85% increased more rapidly in the 2 years since KAS implementation.
Figure KI 25. Deceased kidney donors by age.
Deceased donors with at least one kidney recovered for transplant. Donors whose kidneys were recovered en-bloc are counted once, and donors whose kidneys were recovered separately are counted twice.
Figure KI 26. Deceased kidney donors by race.
Deceased donors with at least one kidney recovered for transplant. Donors whose kidneys were recovered en-bloc are counted once, and donors whose kidneys were recovered separately are counted twice.
Figure KI 27. Deceased donor kidney donation rates (per 1000 deaths) by state, 2013–2015.
Numerator: Deceased donors aged < 70 years, by state of death, whose kidneys were recovered for transplant from 2013 through 2015. Denominator: US deaths aged < 70 years, by state of death, from 2013 through 2015. State death data by age obtained through agreement with NAPHSIS (https://www.naphsis.org/research-requests). Donors whose kidneys were recovered en-bloc are counted once, and donors whose kidneys were recovered separately are counted twice.
Figure KI 28. Rates of kidneys recovered for transplant and not transplanted by age.
Percentages of kidneys not transplanted out of all kidneys recovered for transplant. Kidneys recovered en-bloc are counted once, and kidneys recovered separately are counted twice.
Figure KI 29. Rates of kidneys recovered for transplant and not transplanted by diabetes status.
Percentages of kidneys not transplanted out of all kidneys recovered for transplant. Kidneys recovered en-bloc are counted once, and kidneys recovered separately are counted twice.
Figure KI 30. Rates of kidneys recovered for transplant and not transplanted by hypertension status.
Percentages of kidneys not transplanted out of all kidneys recovered for transplant. Kidneys recovered en-bloc are counted once, and kidneys recovered separately are counted twice.
Figure KI 31. Rates of kidneys recovered for transplant and not transplanted by terminal creatinine.
Percentages of kidneys not transplanted out of all kidneys recovered for transplant. Kidneys recovered en-bloc are counted once, and kidneys recovered separately are counted twice.
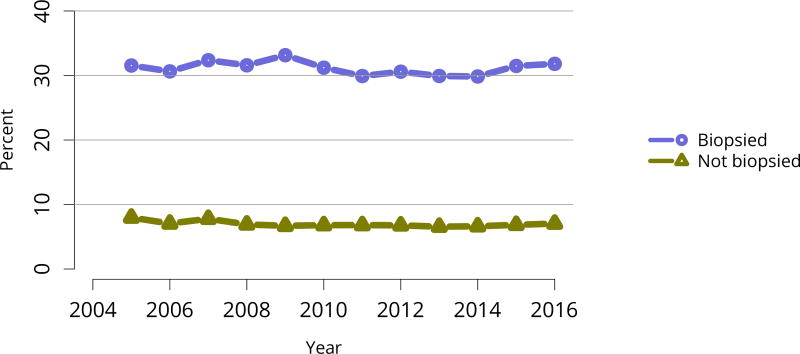
Figure KI 32. Rates of kidneys recovered for transplant and not transplanted by biopsy status.
Percentages of kidneys not transplanted out of all kidneys recovered for transplant. Kidneys recovered en-bloc are counted once, and kidneys recovered separately are counted twice.
Figure KI 33. Rates of kidneys recovered for transplant and not transplanted by cause of death.
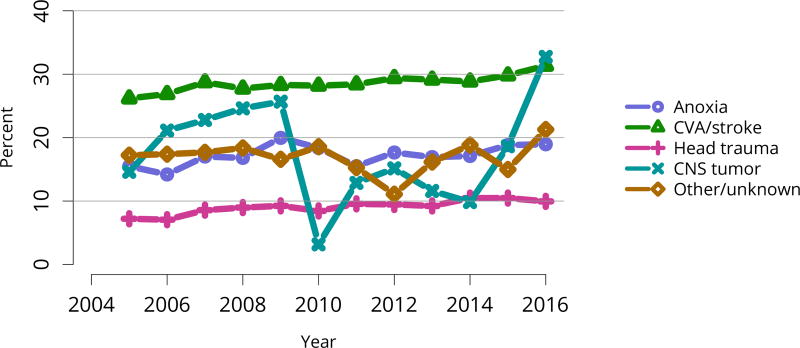
Percentages of kidneys not transplanted out of all kidneys recovered for transplant. Kidneys recovered en-bloc are counted once, and kidneys recovered separately are counted twice. CNS, central nervous system; CVA, cerebrovascular accident.
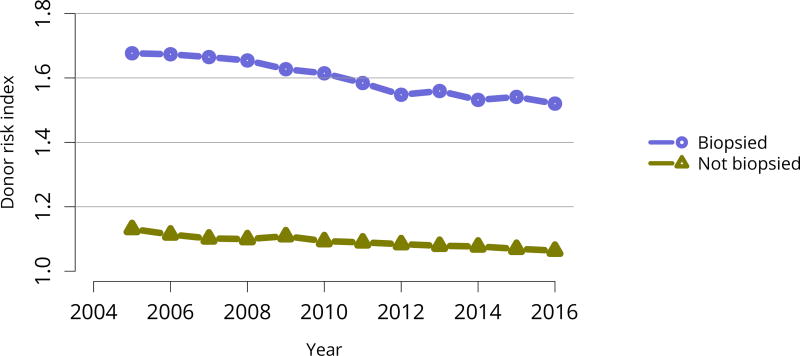
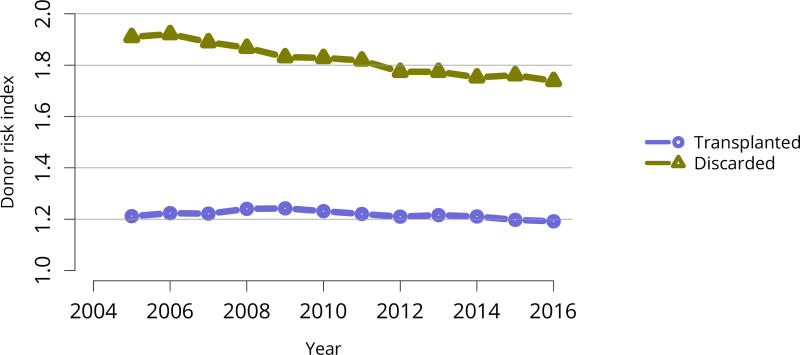
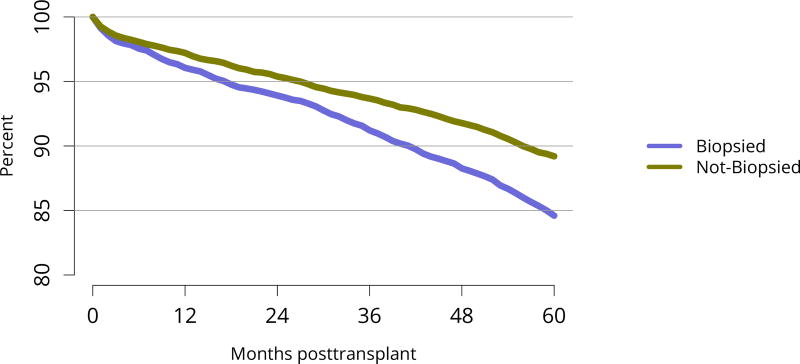
The discard rate for biopsied kidneys remained markedly higher than the rate for non-biopsied kidneys; nearly one-third of biopsied kidneys were discarded in 2016, despite declines in the KDRI of biopsied kidneys over the past 10 years, from 1.61 in 2005 to 1.45 in 2016 (Figure KI 32, Figure KI 38). This suggests that kidneys discarded based on biopsy could likely have benefitted listed candidates. Of similar concern is a trend toward decreasing KDRI of discarded kidneys (Figure KI 37). This may be an unintended consequence of the clinical use of KDPI rather than KDRI; KDPI assigns a percentile score of 0–100 based on the previous years’ recovered kidney donors (for the purpose of transplant) and can result in “drift.” Specifically, if recovery practice nationwide becomes more conservative in a single year, the definition of a KDPI > 85 kidney will be more conservative the next year (i.e., have a relatively lower KDRI than the prior year). The meaning of a KDPI > 85 kidney is redefined each year and always tends in the direction of the previous year, driving an ongoing process.
Figure KI 38. Average kidney donor risk index by biopsy status.
Kidneys recovered for transplant. Kidney donor risk index is computed using only donorspecific components, and is not converted to KDPI.
Figure KI 37. Average kidney donor risk index.
Kidneys recovered for transplant. Kidney donor risk index is computed using only donor-specific components.
2.3 Living Donation
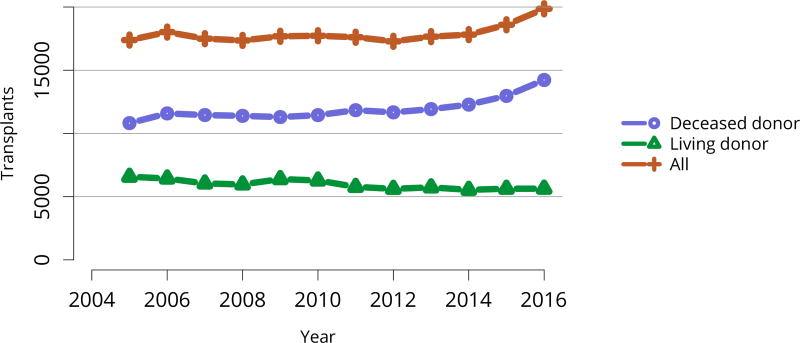
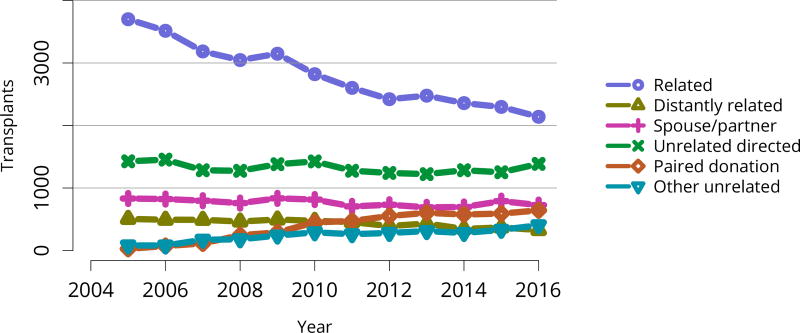
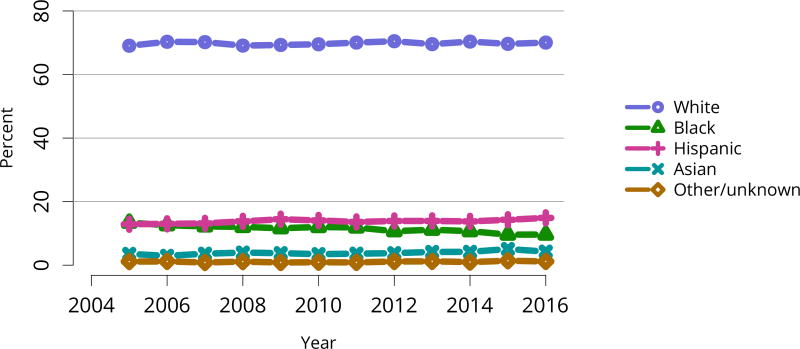
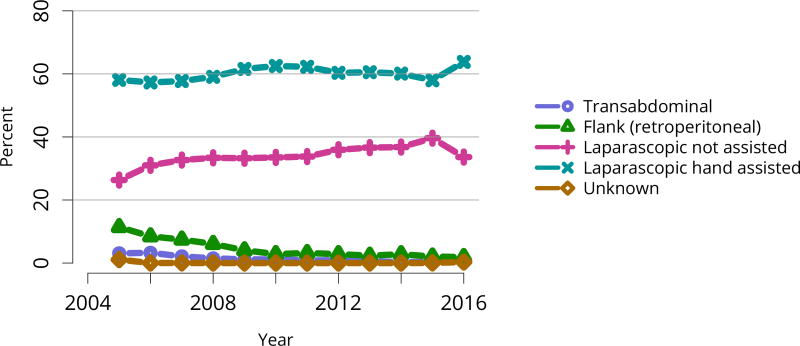
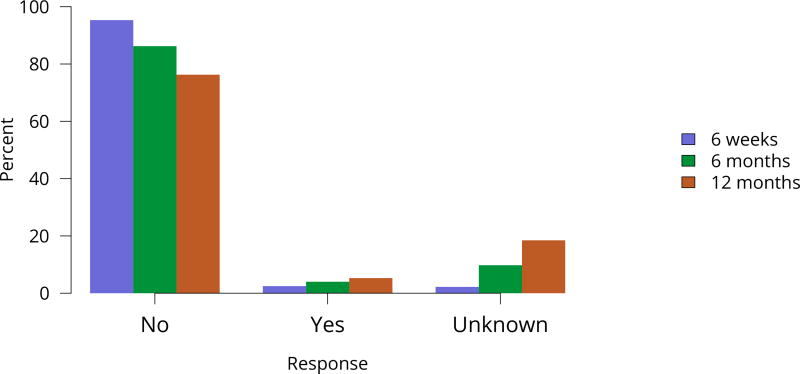
The total number of living donor transplants, in adults and children, has remained flat since 2011, and represents a declining proportion of all kidney transplants (Figure KI 48). Unrelated donations continued to make up a greater proportion of living donor kidney transplants; paired donations increased from 27 in 2005 to 642 in 2016 (Figure KI 40). White donors continued to donate most living donor kidneys (70%); proportions of black living donors declined from 13.4% in 2005 to 9.6% in 2016 (Figure KI 43). The extent of this decrease due to medical contraindications or psychosocial barriers needs further study. In addition, the proportion of donors aged 50 years or older increased (Figure KI 41), possibly due to concern that the long-term risks for younger donors may be greater than for older donors. More comprehensive follow-up of these living donors, along with appropriate controls as proposed by SRTR’s Living Donor Collective (see Kasiske et al, The Living Donor Collective: A scientific registry for living donors. Am J Transplant. In press. DOI: 10.1111/ajt.14365) will provide better insights into the short- and long-term risks of donation, especially given improvements in surgical techniques and the near elimination of retroperitoneal nephrectomy (Figure KI 44). Readmission after donor nephrectomy within the first year remains uncommon, at 5.3% with complications reported in 9%; However, readmission rates at 12 months are unknown for nearly one-fifth of donors (Figure KI 45), illustrating the need to better ascertain living donor outcomes.
Figure KI 48. Total kidney transplants.
All kidney transplant recipients, including adult and pediatric, retransplant, and multi-organ recipients.
Figure KI 40. Kidney transplants from living donors by donor relation.
As reported on the OPTN Living Donor Registration Form.
Figure KI 43. Living kidney donors by race.
As reported on the OPTN Living Donor Registration Form.
Figure KI 41. Living kidney donors by age.
As reported on the OPTN Living Donor Registration Form.
Figure KI 44. Intended living kidney donor procedure type.
As reported on the OPTN Living Donor Registration Form.
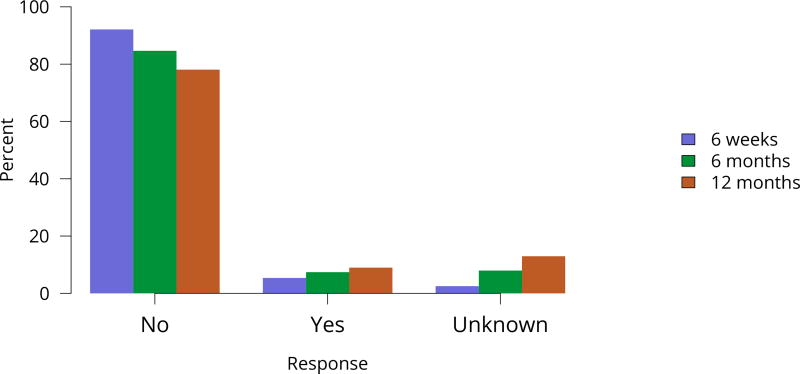
Figure KI 45. Rehospitalization in the first 6 weeks, 6 months, and 1 year among living kidney donors, 2011–2015.
Cumulative hospital readmission. The 6-week time point is recorded at the earliest of discharge or 6 weeks after donation.
2.4 Kidney Transplants
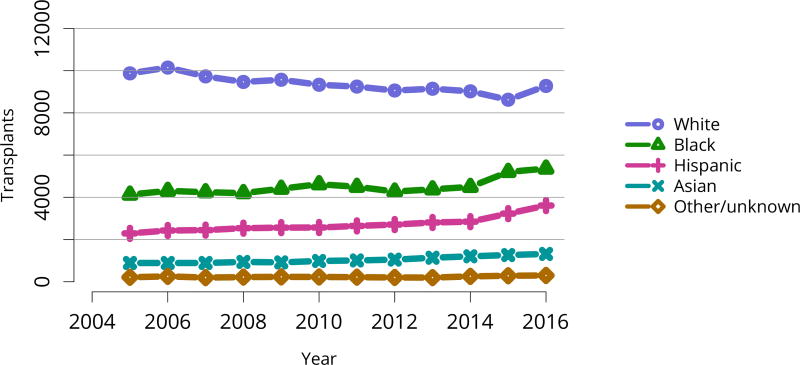
Encouragingly, after at least a decade of stasis in numbers of transplants despite an expanding waiting list, the total number of kidney transplants rose notably in 2015 and 2016. This increase is entirely attributable to an increase in deceased donor transplants, as living donor transplants did not increase (Figure KI 48). The increase in transplants occurred across most levels of age, sex, racial/ethnic, and diagnosis groups (Figure KI 49, Figure KI 50, Figure KI 51, Figure KI 52). Also encouraging are apparently accelerated gains in numbers of transplants in black and Hispanic patients since 2014 (Figure KI 51). These gains appear to be related to intentional KAS policies aimed at reducing racial disparities in access to deceased donor transplant, such as credit given for time on dialysis before listing. However, disparity in access to living donation persists; only 12.3% of living donor kidney transplants were performed in black recipients, compared with 65.1% in white recipients (Table KI 8). Meanwhile, white candidates made up only 36.4% of the waiting list, and black candidates 33.2% (Table KI 2).
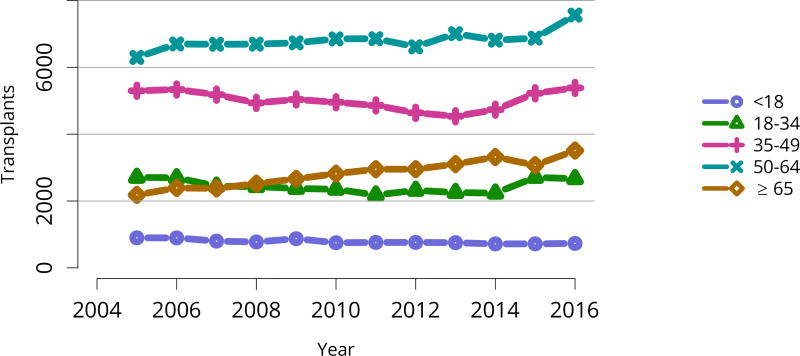
Figure KI 49. Total kidney transplants by age.
All kidney transplant recipients, including adult and pediatric, retransplant, and multi-organ recipients.
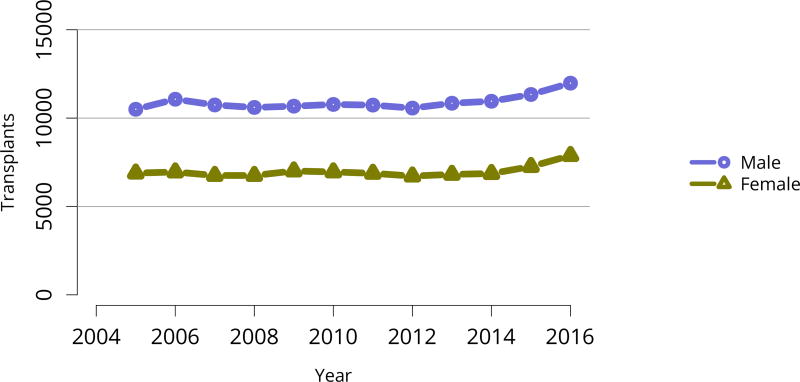
Figure KI 50. Total kidney transplants by sex.
All kidney transplant recipients, including adult and pediatric, retransplant, and multi-organ recipients.
Figure KI 51. Total kidney transplants by race.
All kidney transplant recipients, including adult and pediatric, retransplant, and multi-organ recipients.
Figure KI 52. Total kidney transplants by diagnosis.
All kidney transplant recipients, including adult and pediatric, retransplant, and multi-organ recipients. GN, glomerulonephritis; CKD, cystic kidney disease.
Table KI 8. Demographic characteristics of adult kidney transplant recipients, 2016.
Adult kidney transplant recipients, including retransplants.
| Characteristic | Deceased | Living | All | |||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Age | ||||||
| 18–34 years | 1721 | 12.5% | 948 | 17.6% | 2669 | 14.0% |
| 35–49 years | 3842 | 27.9% | 1542 | 28.7% | 5384 | 28.1% |
| 50–64 years | 5552 | 40.4% | 2013 | 37.4% | 7565 | 39.5% |
| ≥65 years | 2633 | 19.2% | 877 | 16.3% | 3510 | 18.4% |
| Sex | ||||||
| Female | 5546 | 40.3% | 2012 | 37.4% | 7558 | 39.5% |
| Male | 8202 | 59.7% | 3368 | 62.6% | 11,570 | 60.5% |
| Race/ethnicity | ||||||
| White | 5406 | 39.3% | 3505 | 65.1% | 8911 | 46.6% |
| Black | 4576 | 33.3% | 660 | 12.3% | 5236 | 27.4% |
| Hispanic | 2561 | 18.6% | 858 | 15.9% | 3419 | 17.9% |
| Asian | 985 | 7.2% | 297 | 5.5% | 1282 | 6.7% |
| Other/unknown | 220 | 1.6% | 60 | 1.1% | 280 | 1.5% |
| Insurance | ||||||
| Private | 3005 | 21.9% | 2989 | 55.6% | 5994 | 31.3% |
| Medicare | 9452 | 68.8% | 2032 | 37.8% | 11,484 | 60.0% |
| Medicaid | 897 | 6.5% | 223 | 4.1% | 1120 | 5.9% |
| Other government | 243 | 1.8% | 72 | 1.3% | 315 | 1.6% |
| Unknown | 151 | 1.1% | 64 | 1.2% | 215 | 1.1% |
| All recipients | 13,748 | 100.0% | 5380 | 100.0% | 19,128 | 100.0% |
Table KI 2. Demographic characteristics of adults on the kidney transplant waiting list on December 31, 2006, December 31, 2011 and December 31, 2016.
Candidates waiting for transplant on December 31, 2006, December 31, 2011, and December 31, 2016, regardless of first listing date; multiple listings are collapsed.
| Characteristic | 2006 | 2011 | 2016 | |||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Age | ||||||
| 18–34 years | 7749 | 11.6% | 8692 | 9.8% | 8018 | 8.4% |
| 35–49 years | 20,636 | 30.9% | 24,356 | 27.4% | 23,691 | 24.8% |
| 50–64 years | 28,230 | 42.2% | 38,731 | 43.6% | 42,254 | 44.3% |
| ≥ 65 years | 10,203 | 15.3% | 17,105 | 19.2% | 21,493 | 22.5% |
| Sex | ||||||
| Female | 27,997 | 41.9% | 36,343 | 40.9% | 37,384 | 39.2% |
| Male | 38,821 | 58.1% | 52,541 | 59.1% | 58,072 | 60.8% |
| Race/ethnicity | ||||||
| White | 26,299 | 39.4% | 33,991 | 38.2% | 34,745 | 36.4% |
| Black | 23,357 | 35.0% | 30,329 | 34.1% | 31,692 | 33.2% |
| Hispanic | 11,415 | 17.1% | 16,480 | 18.5% | 18,990 | 19.9% |
| Asian | 4795 | 7.2% | 6811 | 7.7% | 8505 | 8.9% |
| Other/unknown | 952 | 1.4% | 1273 | 1.4% | 1524 | 1.6% |
| All candidates | 66,818 | 100.0% | 88,884 | 100.0% | 95,456 | 100.0% |
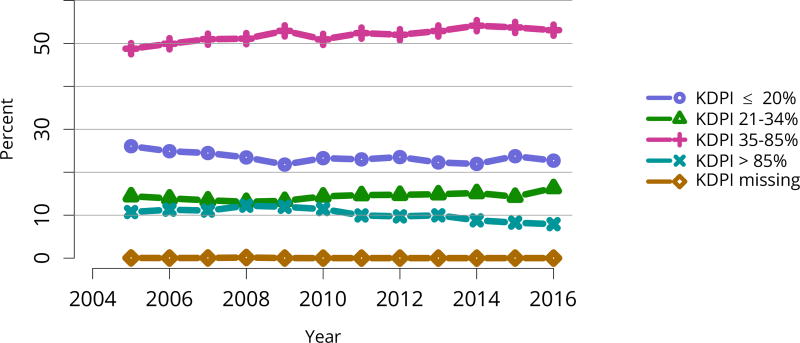
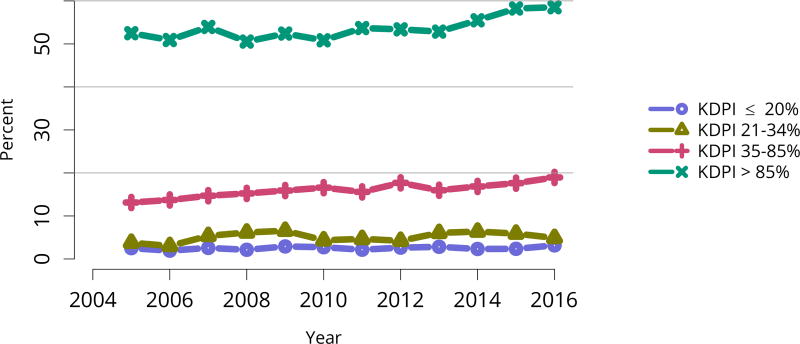
Nearly half of deceased donor recipients in 2016 had been on dialysis for at least 5 years; the proportion of deceased donor recipients who had waited more than 5 years was only 19.2%, likely reflecting the credit given for time on dialysis under the new KAS (Table KI 9, Table KI 10). Consistent with the higher rate of discards for kidneys with KDPI above 85%, the proportion of transplants using high-KDPI kidneys declined from 10.7% in 2005 to 7.9% in 2016 (Figure KI 53). This trend again suggests that kidneys that could benefit some candidates may be unnecessarily discarded.
Table KI 9. Clinical characteristics of adult kidney transplant recipients, 2016.
Adult kidney transplant recipients, including retransplants.
| Characteristic | Deceased | Living | All | |||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Diagnosis | ||||||
| Diabetes | 4240 | 30.8% | 1209 | 22.5% | 5449 | 28.5% |
| Hypertension | 3363 | 24.5% | 865 | 16.1% | 4228 | 22.1% |
| GN | 2188 | 15.9% | 1279 | 23.8% | 3467 | 18.1% |
| CKD | 1278 | 9.3% | 969 | 18.0% | 2247 | 11.7% |
| Other | 2679 | 19.5% | 1058 | 19.7% | 3737 | 19.5% |
| Blood type | ||||||
| A | 4942 | 35.9% | 2073 | 38.5% | 7015 | 36.7% |
| B | 1914 | 13.9% | 696 | 12.9% | 2610 | 13.6% |
| AB | 638 | 4.6% | 221 | 4.1% | 859 | 4.5% |
| O | 6254 | 45.5% | 2390 | 44.4% | 8644 | 45.2% |
| Dialysis time | ||||||
| None | 1555 | 11.3% | 1906 | 35.4% | 3461 | 18.1% |
| < 1 year | 858 | 6.2% | 1023 | 19.0% | 1881 | 9.8% |
| < 3 years | 2493 | 18.1% | 1342 | 24.9% | 3835 | 20.0% |
| < 5 years | 2657 | 19.3% | 385 | 7.2% | 3042 | 15.9% |
| ≥ 5 years | 6185 | 45.0% | 724 | 13.5% | 6909 | 36.1% |
| CPRA | ||||||
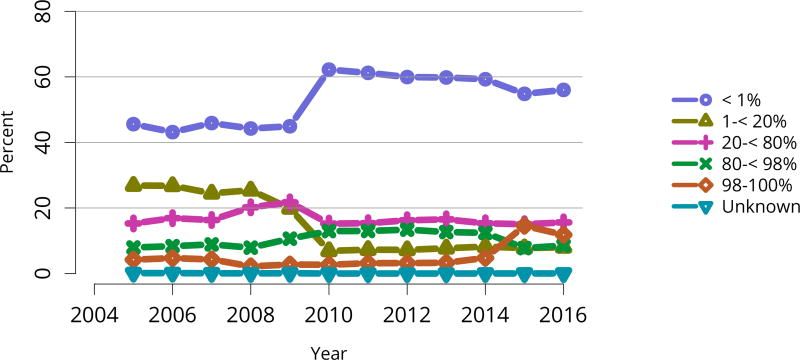
| < 1% | 8075 | 58.7% | 3852 | 71.6% | 11,927 | 62.4% |
| 1–< 20% | 1084 | 7.9% | 514 | 9.6% | 1598 | 8.4% |
| 20–< 80% | 2067 | 15.0% | 771 | 14.3% | 2838 | 14.8% |
| 80–< 98% | 1081 | 7.9% | 183 | 3.4% | 1264 | 6.6% |
| 98–100% | 1439 | 10.5% | 53 | 1.0% | 1492 | 7.8% |
| Unknown | 2 | 0.0% | 7 | 0.1% | 9 | 0.0% |
| All recipients | 13,748 | 100.0% | 5380 | 100.0% | 19,128 | 100.0% |
CKD, cystic kidney disease; GN, glomerulonephritis.
Table KI 10. Transplant characteristics of adult kidney transplant recipients, 2016.
Adult kidney transplant recipients, including retransplants.
| Characteristic | Deceased | Living | All | |||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Wait time | ||||||
| < 1 year | 124 | 0.9% | 100 | 1.9% | 224 | 1.2% |
| < 3 years | 5301 | 38.6% | 3361 | 62.5% | 8662 | 45.3% |
| < 5 years | 3838 | 27.9% | 1505 | 28.0% | 5343 | 27.9% |
| ≥ 5 years | 2634 | 19.2% | 311 | 5.8% | 2945 | 15.4% |
| Unknown | 1851 | 13.5% | 103 | 1.9% | 1954 | 10.2% |
| KDPI | ||||||
| ≤ 20% | 3120 | 22.7% | ||||
| 21–34% | 2244 | 16.3% | ||||
| 35–85% | 7296 | 53.1% | ||||
| > 85% | 1087 | 7.9% | ||||
| Unknown | 1 | 0.0% | ||||
| DCD status | ||||||
| DBD | 11,204 | 81.5% | ||||
| DCD | 2544 | 18.5% | ||||
| Transplant history | ||||||
| First | 11,921 | 86.7% | 4816 | 89.5% | 16,737 | 87.5% |
| Retransplant | 1827 | 13.3% | 564 | 10.5% | 2391 | 12.5% |
| All recipients | 13,748 | 100.0% | 5380 | 100.0% | 19,128 | 100.0% |
DBD, donation after brain death; DCD, donation after circulatory death; KDPI, kidney donor profile index. DCD status and KDPI scores apply to deceased donor transplants only.
Figure KI 53. Kidney transplants by kidney donor profile index (KDPI).
All adult recipients of deceased donor kidneys, including multi-organ transplants. The reference population for the KDRI to KDPI conversion is all deceased donor kidneys recovered for transplant in the US in 2016. Kidneys recovered en-bloc are counted once. KDPI, kidney donor profile index; KDRI, kidney donor risk index.
Nearly 70% of deceased donor recipients in 2016 were on Medicare, compared with only 37.8% of living donor recipients. Conversely, 21.9% of deceased donor recipients had private insurance, compared with 55.6% of living donor recipients. A small but similar proportion of deceased and living donor recipients were covered by Medicaid, 6.5% and 4.1%, respectively (Table KI 8).
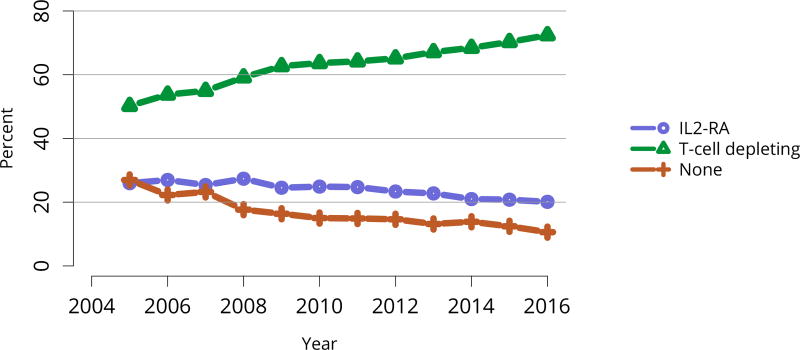
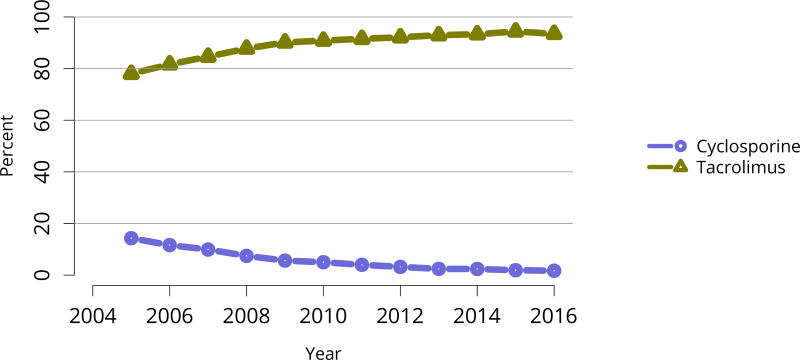
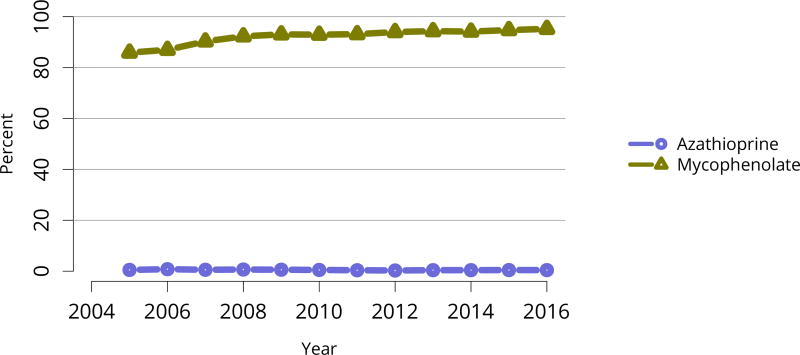
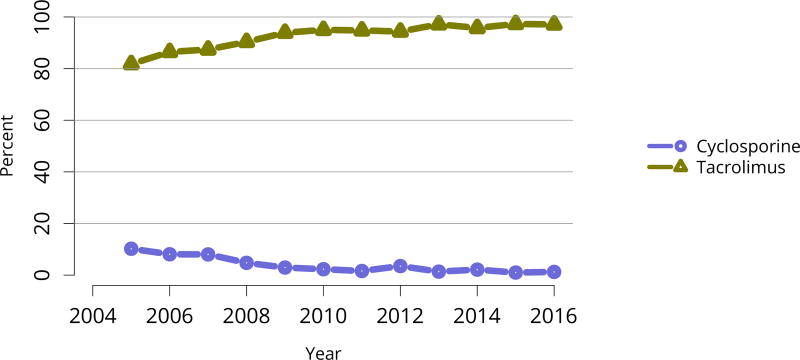
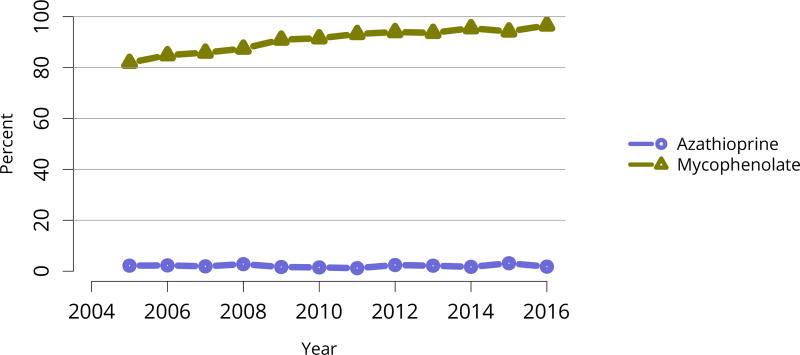
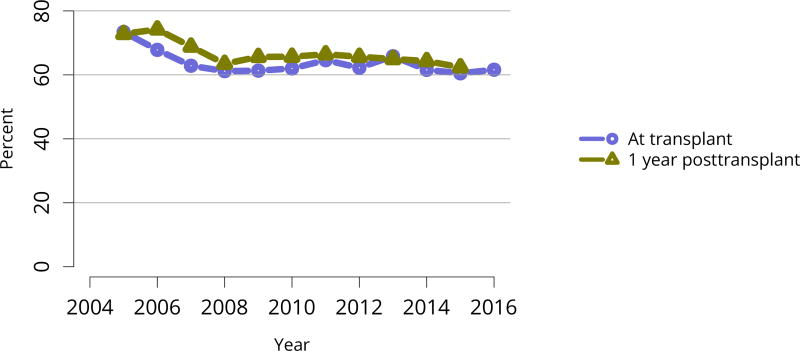
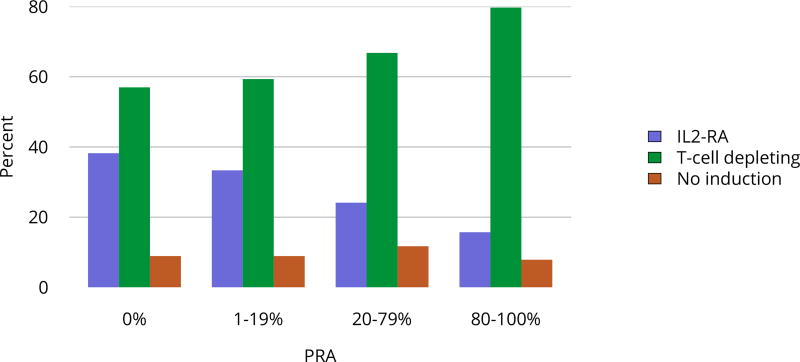
Nearly 75% of transplant recipients underwent immunosuppression induction with T-cell depleting agents in 2016, and IL2 receptor antagonists (IL-2-RA) or no induction became increasingly uncommon (Figure KI 54). Similarly, tacrolimus remained the calcineurin inhibitor of choice over cyclosporine, prescribed for only 1.7% of recipients (Figure KI 55). Ten years ago, mTOR inhibitors were more commonly used, but only 1.9% of recipients were prescribed them at transplant in 2016, increasing to 4.3% at 1 year posttransplant (Figure KI 57). Mycophenolate use continued to increase, to 95.2% in 2016 (Figure KI 56). Steroid use also continued to increase. After a nadir of 63.8% recipients using steroids at 1 year posttransplant in 2007, 71.8% were using steroids in 2016 (Figure KI 58).
Figure KI 54. Induction agent use in adult kidney transplant recipients.
Immunosuppression at transplant reported to the OPTN. IL2-RA, interleukin-2 receptor antagonist.
Figure KI 55. Calcineurin inhibitor use in adult kidney transplant recipients.
Immunosuppression at transplant reported to the OPTN.
Figure KI 57. mTOR inhibitor use in adult kidney transplant recipients.
Immunosuppression at transplant reported to the OPTN. One-year posttransplant data are limited to patients alive with graft function at 1 year posttransplant. mTOR, mammalian target of rapamycin.
Figure KI 56. Anti-metabolite use in adult kidney transplant recipients.
Immunosuppression at transplant reported to the OPTN. Mycophenolate includes mycophenolate mofetil and mycophenolate sodium.
Figure KI 58. Steroid use in adult kidney transplant recipients.
Immunosuppression at transplant reported to the OPTN. One-year posttransplant data are limited to patients alive with graft function at 1 year posttransplant.
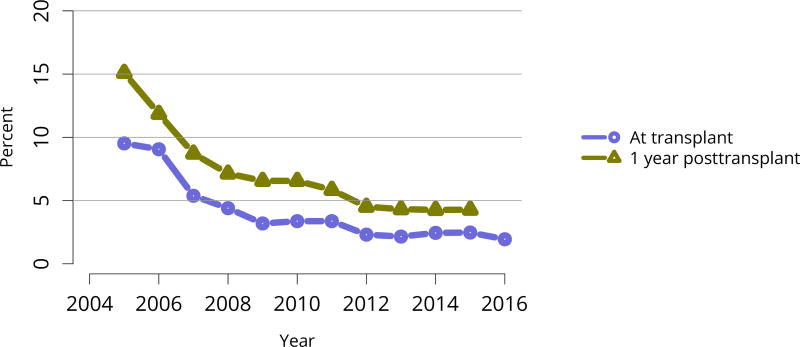
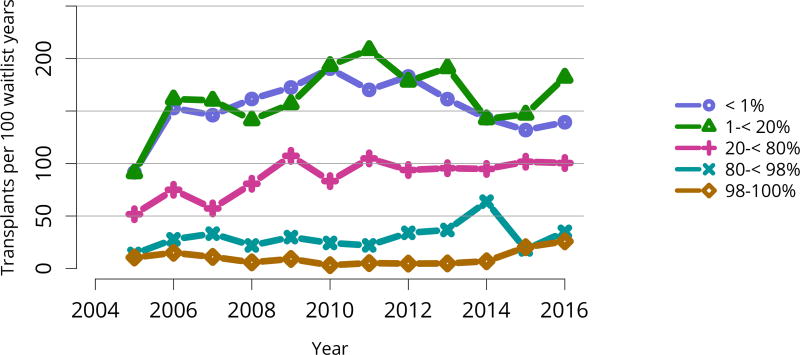
Due to the new KAS, the proportion of deceased donor transplants among candidates with cPRA 98%–100% increased dramatically in 2015, to 14.6%. In 2016, the proportion decreased to 11.8%, still well above the 2014 proportion of 4.8% (Figure KI 59). Monthly data from the OPTN 2-year KAS report shows minimal fluctuation during 2016, suggesting that a steady state may have been reached for this group (see https://www.transplantpro.org/wp-content/uploads/sites/3/KAS_First-two-years_041917.pdf).
Figure KI 59. C/PRA at time of kidney transplant in adult deceased donor recipients.
From December 5, 2007, through September 30, 2009, CPRA was used if greater than 0; otherwise, the maximum pretransplant PRA was used. Before December 5, 2007, the maximum pretransplant PRA was used unconditionally. CPRA is used after September 30, 2009, unless it is missing; if it is missing, the maximum pretransplant PRA is used. Kidney-alone transplants only.
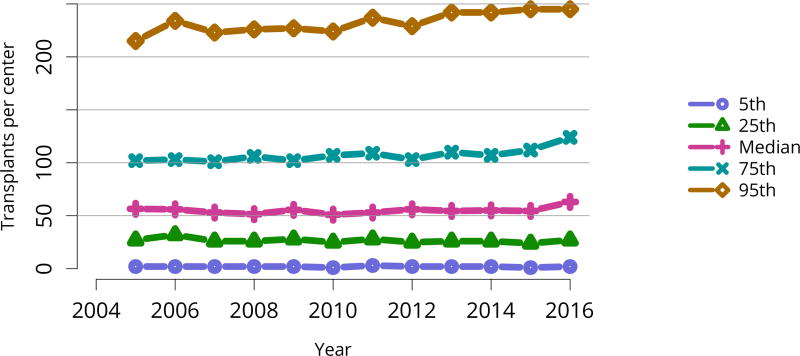
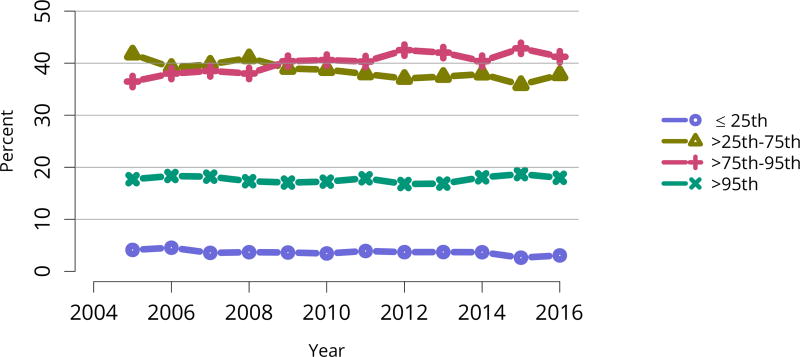
Transplants were performed at a variety of transplant programs; 5% of kidney transplants occurred at programs that performed at least 245 transplants, and 5% at programs that performed 2 or fewer. The 75th and 95th percentile program volumes increased over time, while numbers of transplants performed at programs of median or smaller size remained relatively stable (Figure KI 62). More than half of all transplants occurred at programs in the 75th or higher percentile, with 18% occurring at programs in the 95th percentile and 41% occurring at programs in the 75th to 95th percentile (Figure KI 63).
Figure KI 62. Annual adult kidney transplant center volumes, by percentile.
Annual volume data are limited to recipients aged 18 or older.
Figure KI 63. Distribution of adult kidney transplants by percentile of center volume.
Percentiles are based on annual volume data among recipients aged 18 or older.
2.5 Outcomes
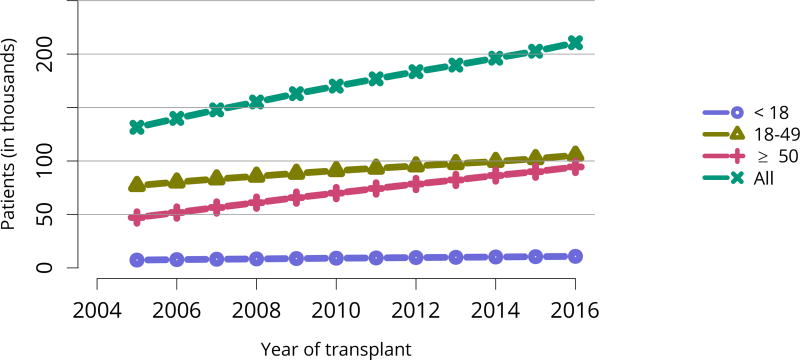
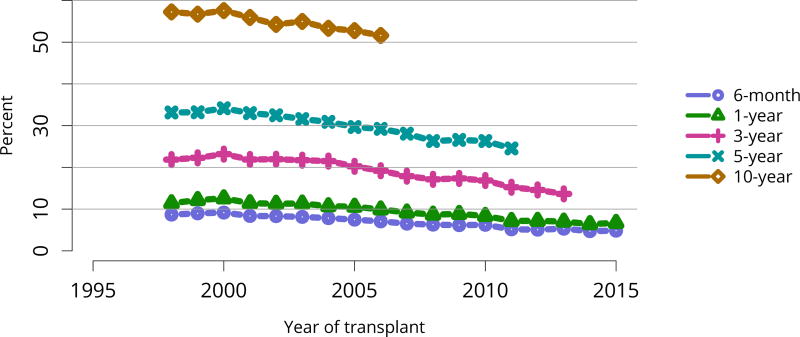
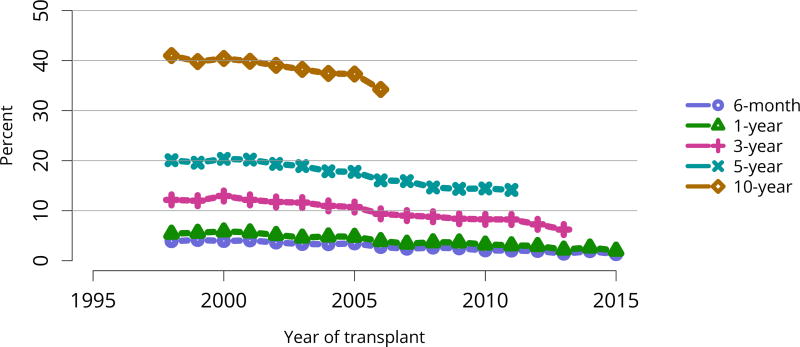
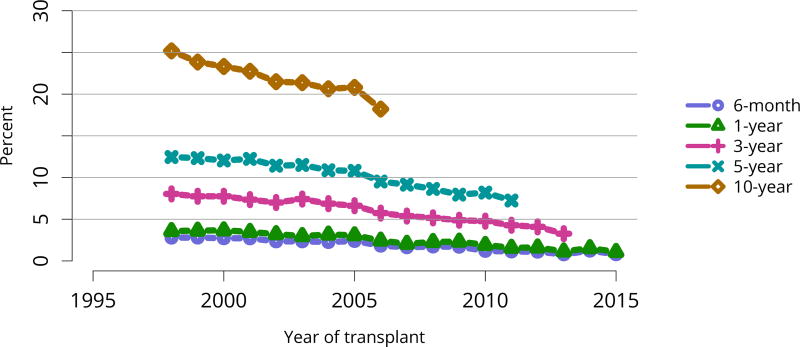
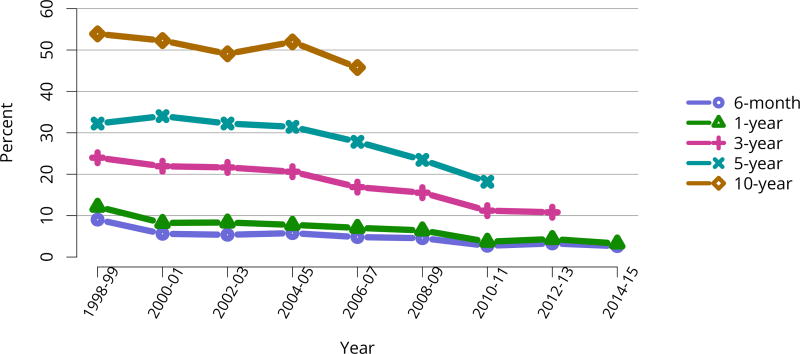
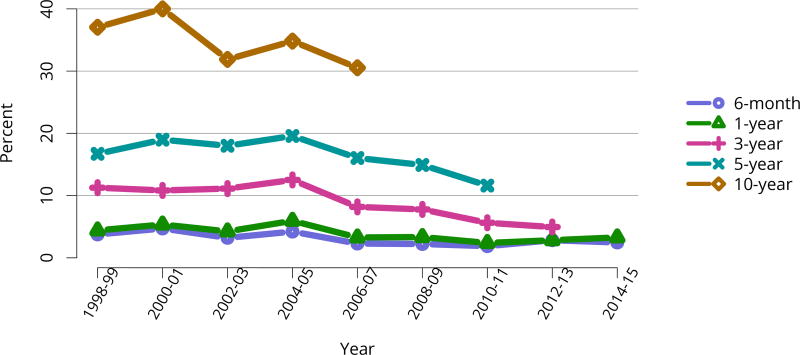
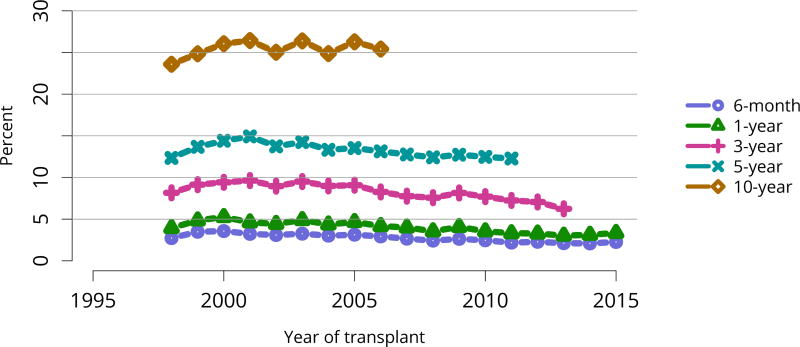
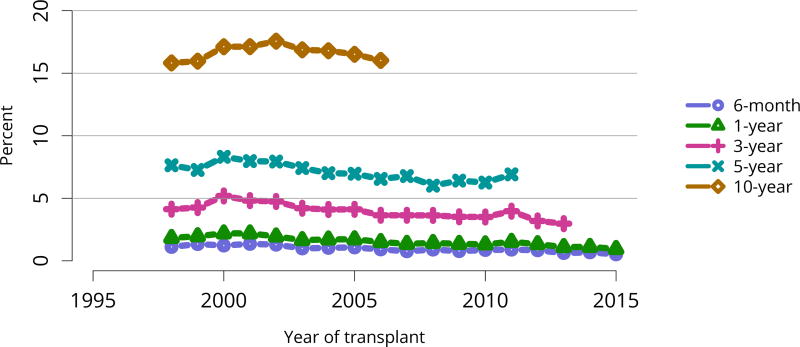
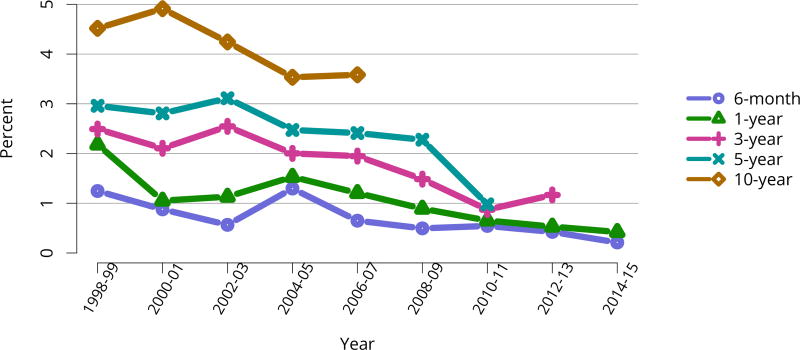
In mid-2016, 210,615 recipients were alive with a functioning graft, nearly twice as many as in 2005 (Figure KI 79). The longstanding improvement in unadjusted short- and long-term deceased donor graft survival continued in 2016; 6-month all-cause and death-censored graft failure for deceased donor recipients in 2015 was nearly half what it was 10 years ago. All-cause graft failure declined from 7.5% in 2005 to 4.8% in 2015, with a similar decline in 6-month death-censored graft failure from 4.3% to 2.6% over the same period. Long-term failure rates improved; 10-year all-cause graft failure for recipients in 2006 declined to 51.6% from 57.2% 8 years earlier, and 10-year death-censored graft failure declined from 33.7% to 26.2% (Figure KI 64, Figure KI 65). Similarly positive trends continued for living donor recipients, with 6-month and 10-year all-cause graft failure only 1.3% and 34.2% (Figure KI 67). Censoring for death, nearly 82% of living donor kidneys transplanted in 2006 were still functioning in 2016 (Figure KI 68).
Figure KI 79. Recipients alive with a functioning kidney graft on June 30 of the year, by age at transplant.
Recipients are assumed to be alive with function unless a death or graft failure is recorded. A recipient may experience a graft failure and be removed from the cohort, undergo retransplant, and reenter the cohort.
Figure KI 64. Graft failure among adult deceased donor kidney transplant recipients.
Estimates are unadjusted, computed using Kaplan-Meier competing risk methods. Recipients are followed to the earliest of kidney graft failure; kidney retransplant; return to dialysis; death; or 6 months, 1, 3, 5, or 10 years posttransplant. All-cause graft failure (GF) is defined as any of the prior outcomes prior to 6 months, 1, 3, 5, or 10 years, respectively.
Figure KI 65. Death-censored graft failure among adult deceased donor kidney transplant recipients.
Estimates are unadjusted, computed using Kaplan-Meier competing risk methods. Recipients are followed to the earliest of kidney graft failure; kidney retransplant; return to dialysis; death; or 6 months, 1, 3, 5, or 10 years posttransplant. Death-censored graft failure (DCGF) is defined as a return to dialysis, reported graft failure, or kidney retransplant.
Figure KI 67. Graft failure among adult living donor kidney transplant recipients.
Estimates are unadjusted, computed using Kaplan-Meier competing risk methods. Recipients are followed to the earliest of kidney graft failure; kidney retransplant; return to dialysis; death; or 6 months, 1, 3, 5, or 10 years posttransplant. All-cause graft failure (GF) is defined as any of the prior outcomes prior to 6 months, 1, 3, 5, or 10 years, respectively.
Figure KI 68. Death-censored graft failure among adult living donor kidney transplant recipients.
Estimates are unadjusted, computed using Kaplan-Meier competing risk methods. Recipients are followed to the earliest of kidney graft failure; kidney retransplant; return to dialysis; death; or 6 months, 1, 3, 5, or 10 years posttransplant. Death-censored graft failure (DCGF) is defined as a return to dialysis, reported graft failure, or kidney retransplant.
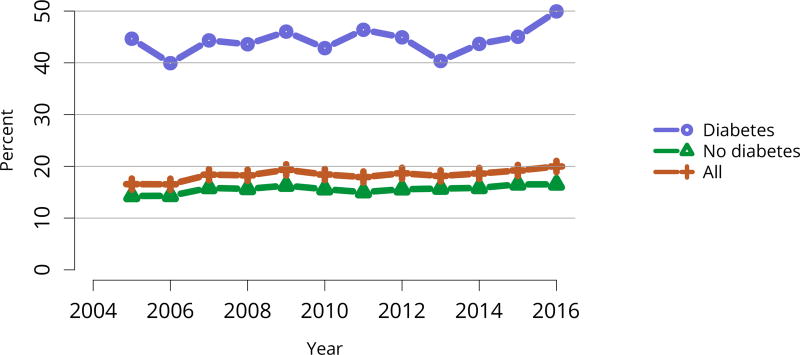
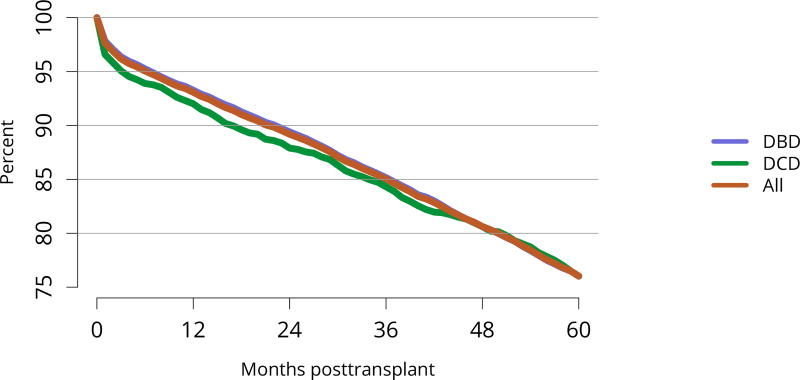
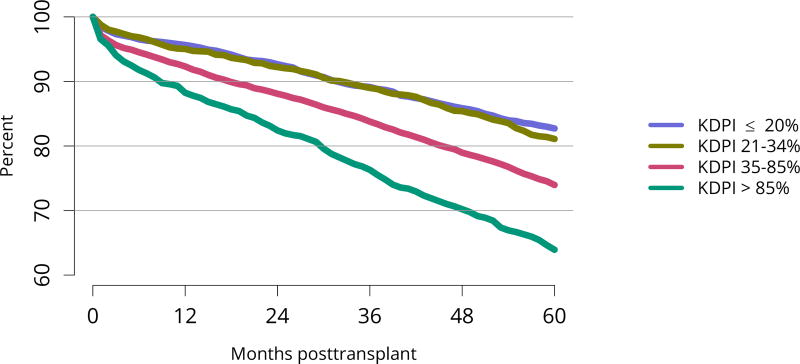
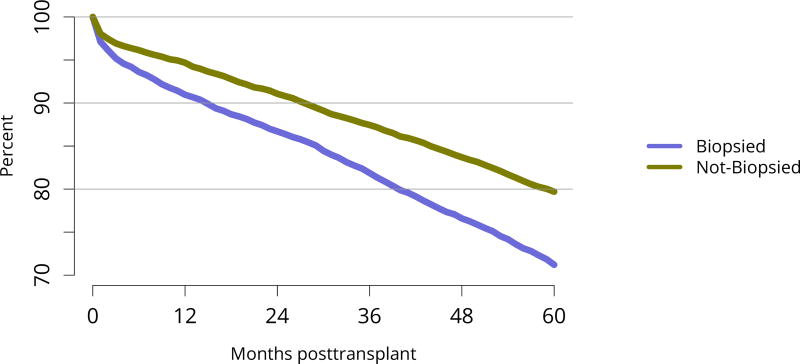
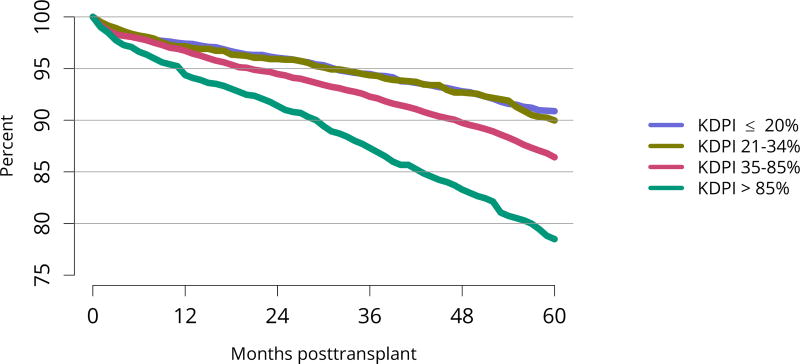
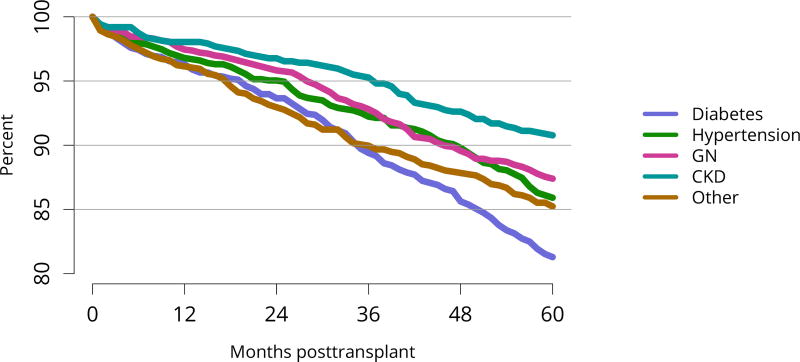
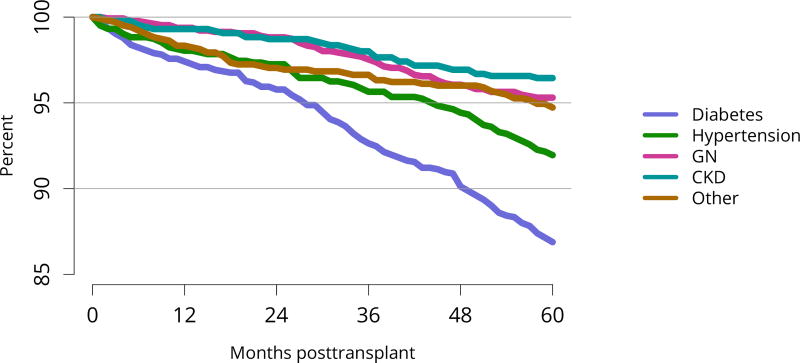
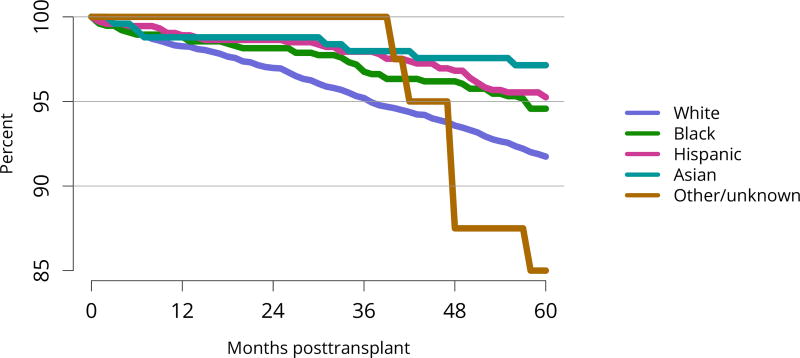
Five-year graft survival among recipients who underwent deceased donor transplant in 2011 was lower for those with diabetes and hypertension as cause of kidney failure than for those with cystic disease or glomerulonephritis (Figure KI 70). Graft survival did not differ for donation-after-circulatory-death versus donation-after-brain-death kidneys (Figure KI 72). While graft survival for KDPI 35%–85% and > 85% was notably lower than for KDPI = 20% and 21%–34% (63.9% for KDPI > 85%, 82.7% for KDPI = 20%), graft survival differed little between the two lowest KDPI groups (82.7% and 81.1% for KDPI = 20% and 21%–34%, respectively) (Figure KI 71). Observed 5-year graft survival was lower for biopsied than for non-biopsied kidneys (71.2% versus 79.7%), suggesting that biopsies are more often performed when kidneys are medically likely to be of lower quality (Figure KI 73). Given that the 5-year survival for biopsied vs. non-biopsied kidneys was nearly equivalent to calculated survival for KDPI 35–85% vs. KDPI 21–34%, this again raises concern that biopsy may not add to available clinical information with regard to predicting subsequent graft failure rates. While still better than deceased donor graft survival, 5-year living donor graft survival was lower for black recipients than for any other racial/ethnic group, at 82.0% compared with 92.3% for Asian, 89.9% for Hispanic, and 85.7% for white recipients (Figure KI 75).
Figure KI 70. Graft survival among adult deceased donor kidney transplant recipients, 2011, by diagnosis.
Graft survival estimated using unadjusted Kaplan-Meier methods. CKD, cystic kidney disease; GN, glomerulonephritis.
Figure KI 72. Graft survival among adult deceased donor kidney transplant recipients, 2011, by DCD status.
Graft survival estimated using unadjusted Kaplan-Meier methods. DCD, donation after circulatory death; DBD, donation after brain death.
Figure KI 71. Graft survival among adult deceased donor kidney transplant recipients, 2011, by KDPI.
Graft survival estimated using unadjusted Kaplan-Meier methods. The reference population for the KDRI to KDPI conversion is all deceased donor kidneys recovered for transplant in the US in 2016. KDPI, kidney donor profile index.
Figure KI 73. Graft survival among adult deceased donor kidney transplant recipients, 2011, by biopsy status.
Graft survival estimated using unadjusted Kaplan-Meier methods.
Figure KI 75. Graft survival among adult living donor kidney transplant recipients, 2011, by race.
Graft survival estimated using unadjusted KaplanMeier methods.
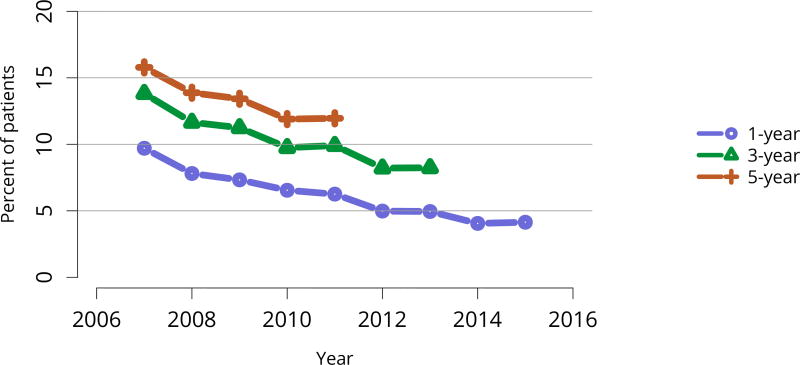
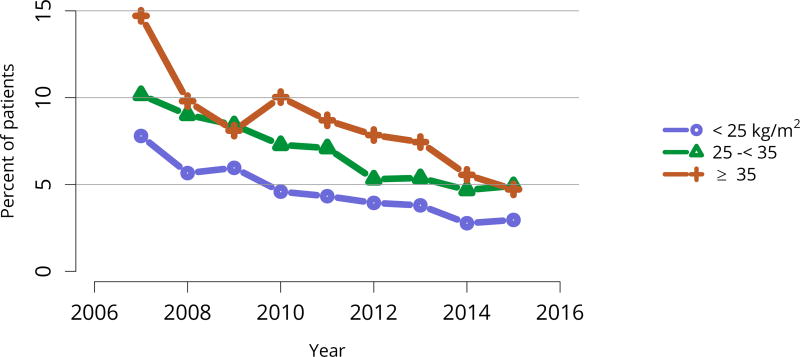
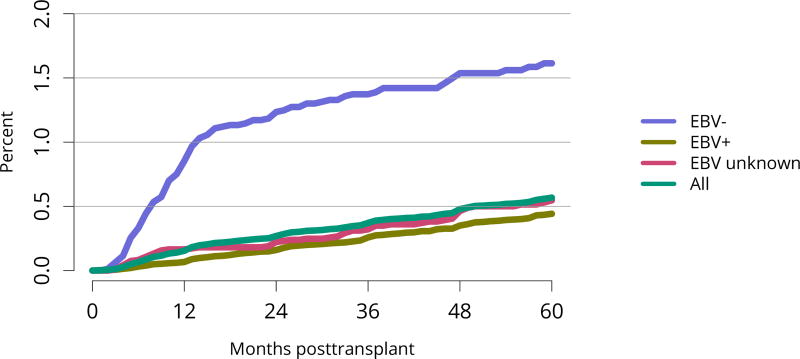
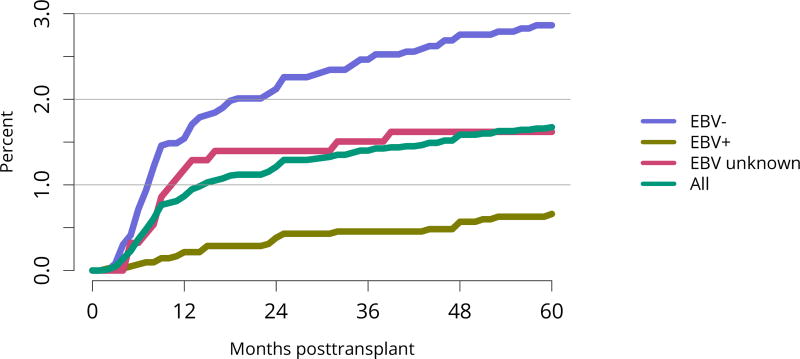
Posttransplant diabetes continued to decline, especially among recipients with the highest body mass index (BMI); 1-year incidence in recipients with BMI = 35 kg/m2 was essentially the same as for recipients with BMI 25–34 kg/m2 (Figure KI 81, Figure KI 82). This trend is particularly encouraging given the increased use of tacrolimus in lieu of cyclosporine for immunosuppression. Incidence of posttransplant lymphoproliferative disorder (PTLD) remained low overall at 0.6% at 5 years posttransplant. However, 5-year incidence was substantially higher for recipients who were Epstein-Barr virus (EBV) negative, albeit still low at 1.6% (Figure KI 83).
Figure KI 81. Posttransplant diabetes among adult kidney transplant recipients.
Percentage of adult deceased donor kidney recipients who were nondiabetic at transplant and developed diabetes posttransplant. Posttransplant diabetes is reported on the Transplant Recipient Follow-up Form. Death and graft failure are treated as competing events.
Figure KI 82. Posttransplant diabetes within 1 year among adult kidney transplant recipients by BMI at transplant.
Percentage of adult deceased donor kidney recipients who were nondiabetic at transplant and developed diabetes posttransplant. Posttransplant diabetes is reported on the Transplant Recipient Follow-up Form. Death and graft failure are treated as competing events.
Figure KI 83. Incidence of PTLD among adult kidney transplant recipients by recipient EBV status at transplant, 2010–2014.
Cumulative incidence is estimated using the Kaplan-Meier competing risk method. PTLD is identified as a reported complication or cause of death on the OPTN Transplant Recipient Follow-up Form or the Posttransplant Malignancy Form as polymorphic PTLD, monomorphic PTLD, or Hodgkin disease. Only the earliest date of PTLD diagnosis is considered. EBV, Epstein-Barr virus; PTLD, posttransplant lymphoproliferative disorder.
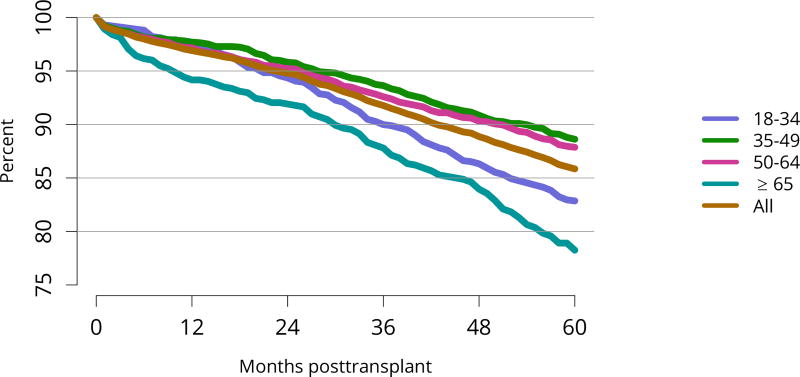
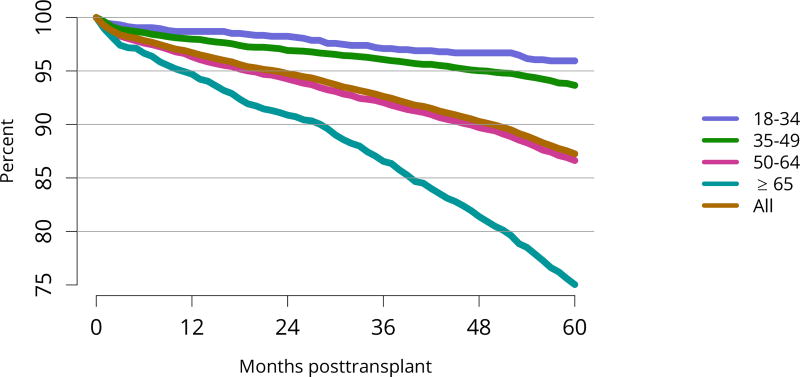
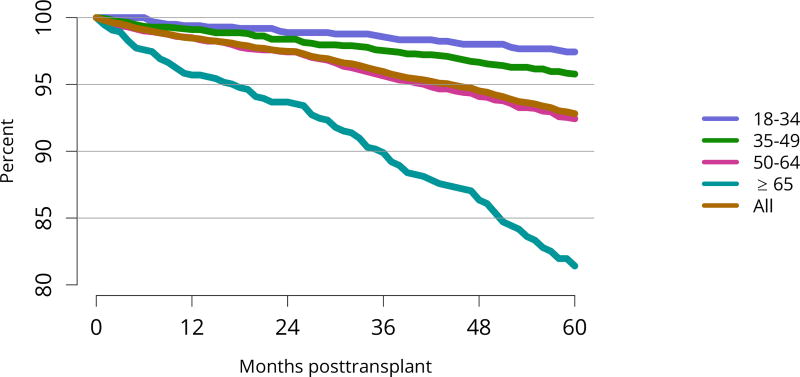
Patient survival closely mirrored graft survival. Five-year deceased donor recipient survival was lowest for patients with diabetes (Figure KI 85) and for those who received a high-KDPI or biopsied kidney (Figure KI 86, Figure KI 87). Patient and living donor graft survival were lowest for recipients aged 65 years or older. The next worse graft survival was for recipients aged 18–34 years (Figure KI 74), but not surprisingly patient survival was highest for these recipients after both living and deceased donor transplant (Figure KI 84, Figure KI 88).
Figure KI 85. Patient survival among adult deceased donor kidney transplant recipients, 2011, by diagnosis.
Patient survival estimated using unadjusted Kaplan-Meier methods. For recipients of more than one transplant during the period, only the first is considered. CKD, cystic kidney disease; GN, glomerulonephritis.
Figure KI 86. Patient survival among adult deceased donor kidney transplant recipients, 2011, by KDPI.
Patient survival estimated using unadjusted Kaplan-Meier methods. For recipients of more than one transplant during the period, only the first is considered. The reference population for the KDRI to KDPI conversion is all deceased donor kidneys recovered for transplant in the US in 2016. KDPI, kidney donor profile index.
Figure KI 87. Patient survival among adult deceased donor kidney transplant recipients, 2011, by biopsy status.
Patient survival estimated using unadjusted Kaplan-Meier methods. For recipients of more than one transplant during the period, only the first is considered.
Figure KI 74. Graft survival among adult living donor kidney transplant recipients, 2011, by age.
Graft survival estimated using unadjusted KaplanMeier methods.
Figure KI 84. Patient survival among adult deceased donor kidney transplant recipients, 2011, by age.
Patient survival estimated using unadjusted Kaplan-Meier methods. For recipients of more than one transplant during the period, only the first is considered.
Figure KI 88. Patient survival among adult living donor kidney transplant recipients, 2011, by age.
Patient survival estimated using unadjusted KaplanMeier methods. For recipients of more than one transplant during the period, only the first is considered.
3 Pediatric Kidney Transplant
3.1 Waiting List
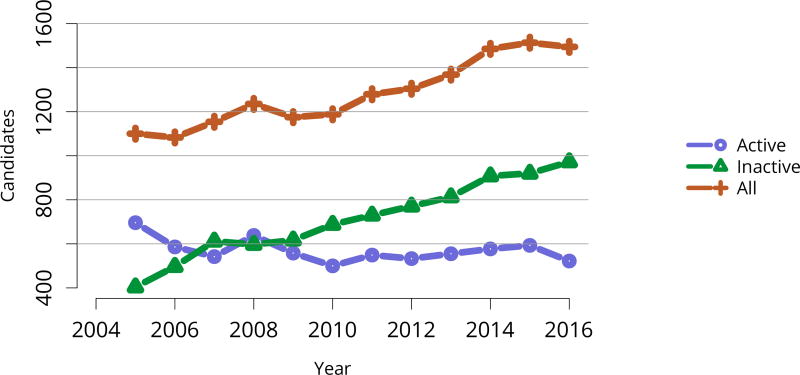
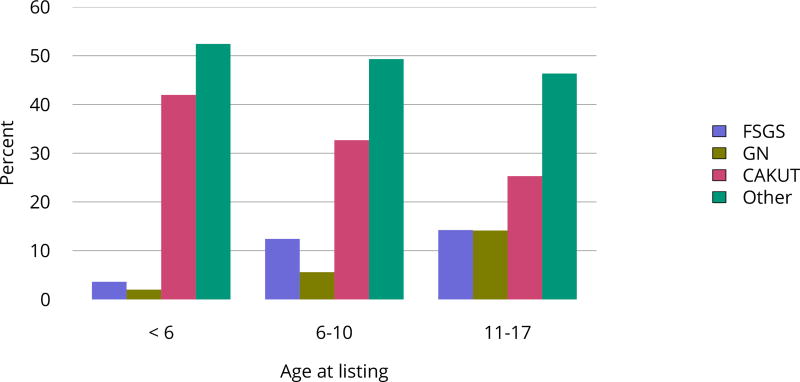
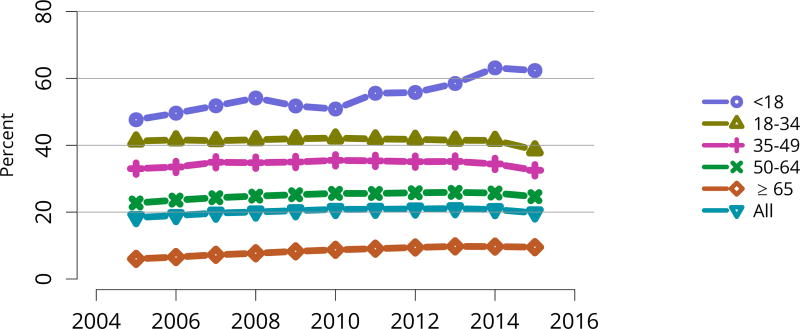
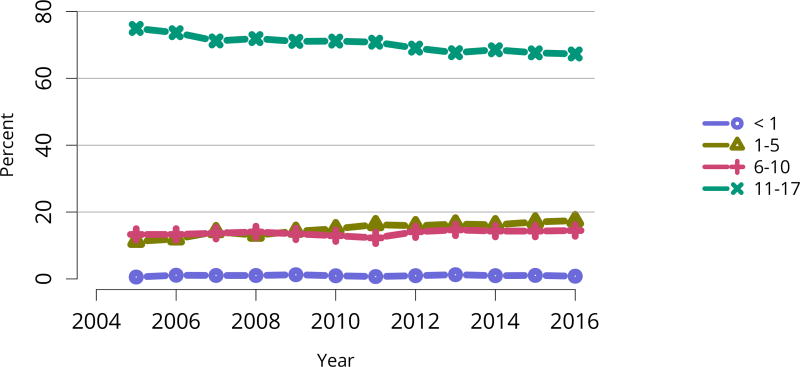
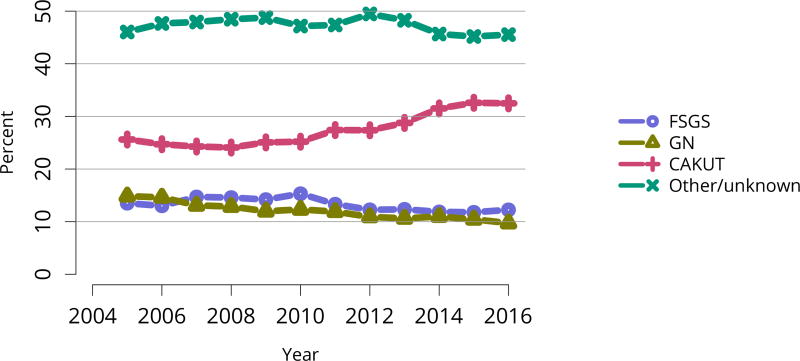
In 2016, 917 pediatric candidates were added to the kidney transplant waiting list, 522 (57%) as inactive (Figure KI 91). The number of prevalent pediatric candidates (listed at age < 18 years and on the list on December 31 of the given year) has been steadily increasing and reached 1,494 on December 31, 2016 (Figure KI 92). The most common reason for inactive status among newly listed candidates in 2016 was incomplete work-up (52.1%), followed by living donor candidate status (16.8%), and too well to need transplant (11.6%) (Table KI 13). Over the past decade, the age of pediatric candidates on the list at year-end shifted, with an increase in those aged 1–5 years (14.9% to 24.6%) and a decrease in those aged 11–17 years (66.3% to 54.3%) (Table KI 14). Proportions of candidates with congenital anomalies of the kidney and urinary tract (CAKUT) as primary cause of disease increased from 27.8% in 2006 to 37.3% in 2016, and proportions with glomerulonephritis decreased from 12.3% to 7.1%. Most candidates (65.7%) had a cPRA of less than 1% (Table KI 15). The proportion of pediatric candidates waiting for retransplant decreased from 26.4% on December 31, 2006, to 15.0% on December 31, 2016. Multi-organ listing was uncommon; only 2.4% of pediatric candidates were awaiting multi-organ transplant on December 31, 2016 (Table KI 16). The leading cause of end-stage kidney disease changed with age; CAKUT was most common in children aged younger than 6 years, while focal segmental glomerulosclerosis and glomerulonephritis were more common in older children (Figure KI 98).
Figure KI 91. New pediatric candidates added to the kidney transplant waiting list.
A new candidate is one who first joined the list during the given year, without having been listed in a previous year. Previously listed candidates who underwent transplant and subsequently relisted are considered new. Candidates concurrently listed at multiple centers are counted once. Active and inactive patients are included. Age determined at listing.
Figure KI 92. Pediatric candidates listed for kidney transplant on December 31 each year.
Candidates concurrently listed at multiple centers are counted once. Those with concurrent listings and active at any program are considered active. Active status is determined on day 7 after first listing; age determined at first listing.
Table KI 13. Reasons for inactive status among new pediatric kidney transplant listings, 2016.
Candidates first listed as inactive. Each listing is counted separately.
| Reasons for inactive status | N | Percent |
|---|---|---|
| Candidate work-up incomplete | 282 | 52.1% |
| Candidate for LD transplant only | 91 | 16.8% |
| Too well | 63 | 11.6% |
| Too sick | 37 | 6.8% |
| Candidate choice | 29 | 5.4% |
| Insurance issues | 15 | 2.8% |
| Medical non-compliance | 10 | 1.8% |
| Weight inappropriate | 9 | 1.7% |
| Transplant pending | 4 | 0.7% |
| Candidate could not be contacted | 1 | 0.2% |
LD, living donor.
Table KI 14. Demographic characteristics of pediatric candidates on the kidney transplant waiting list on December 31, 2006, December 31, 2011, and December 31, 2016.
Candidates aged younger than 18 years waiting for transplant on December 31 of given year, regardless of first listing date; multiple listings are collapsed. Age calculated at snapshot.
| Characteristic | 2006 | 2011 | 2016 | |||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Age | ||||||
| < 1 year | 4 | 0.5% | 0 | 0.0% | 1 | 0.1% |
| 1–5 years | 109 | 14.9% | 181 | 21.8% | 241 | 24.6% |
| 6–10 years | 133 | 18.2% | 151 | 18.2% | 206 | 21.0% |
| 11–17 years | 484 | 66.3% | 497 | 60.0% | 533 | 54.3% |
| Sex | ||||||
| Female | 290 | 39.7% | 329 | 39.7% | 384 | 39.1% |
| Male | 440 | 60.3% | 500 | 60.3% | 597 | 60.9% |
| Race/ethnicity | ||||||
| White | 287 | 39.3% | 344 | 41.5% | 410 | 41.8% |
| Black | 179 | 24.5% | 180 | 21.7% | 201 | 20.5% |
| Hispanic | 232 | 31.8% | 263 | 31.7% | 291 | 29.7% |
| Asian | 22 | 3.0% | 30 | 3.6% | 56 | 5.7% |
| Other/unknown | 10 | 1.4% | 12 | 1.4% | 23 | 2.3% |
| All candidates | 730 | 100.0% | 829 | 100.0% | 981 | 100.0% |
Table KI 15. Clinical characteristics of pediatric candidates on the kidney transplant waiting list on December 31, 2006, December 31, 2011, and December 31, 2016.
Candidates aged younger than 18 years waiting for transplant on December 31, 2006, December 31, 2011, and December 31, 2016, regardless of first listing date; multiple listings are collapsed.
| Characteristic | 2006 | 2011 | 2016 | |||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Diagnosis | ||||||
| FSGS | 79 | 10.8% | 80 | 9.7% | 97 | 9.9% |
| GN | 90 | 12.3% | 74 | 8.9% | 70 | 7.1% |
| CAKUT | 203 | 27.8% | 268 | 32.3% | 366 | 37.3% |
| Other | 358 | 49.0% | 407 | 49.1% | 448 | 45.7% |
| Blood type | ||||||
| A | 215 | 29.5% | 258 | 31.1% | 279 | 28.4% |
| B | 97 | 13.3% | 132 | 15.9% | 166 | 16.9% |
| AB | 19 | 2.6% | 29 | 3.5% | 23 | 2.3% |
| O | 399 | 54.7% | 410 | 49.5% | 513 | 52.3% |
| CPRA | ||||||
| < 1% | 406 | 55.6% | 550 | 66.3% | 645 | 65.7% |
| 1–< 20% | 93 | 12.7% | 32 | 3.9% | 92 | 9.4% |
| 20–< 80% | 91 | 12.5% | 93 | 11.2% | 143 | 14.6% |
| 80–< 98% | 53 | 7.3% | 64 | 7.7% | 39 | 4.0% |
| 98–100% | 53 | 7.3% | 79 | 9.5% | 60 | 6.1% |
| Unknown | 34 | 4.7% | 11 | 1.3% | 2 | 0.2% |
| All candidates | 730 | 100.0% | 829 | 100.0% | 981 | 100.0% |
FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; CAKUT, congenital anomalies of the kidney and urinary tract.
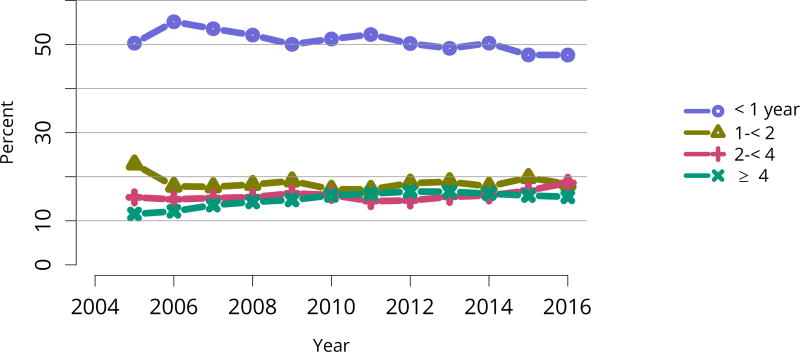
Table KI 16. Listing characteristics of pediatric candidates on the kidney transplant waiting list on December 31, 2006, December 31, 2011, and December 31, 2016.
Candidates aged younger than 18 years waiting for transplant on December 31, 2006, December 31, 2011, and December 31, 2016, regardless of first listing date; multiple listings are collapsed.
| Characteristic | 2006 | 2011 | 2016 | |||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Transplant history | ||||||
| First | 537 | 73.6% | 633 | 76.4% | 834 | 85.0% |
| Retransplant | 193 | 26.4% | 196 | 23.6% | 147 | 15.0% |
| Wait time | ||||||
| < 1 year | 432 | 59.2% | 482 | 58.1% | 510 | 52.0% |
| 1–< 2 years | 164 | 22.5% | 166 | 20.0% | 210 | 21.4% |
| 2–< 3 years | 68 | 9.3% | 68 | 8.2% | 118 | 12.0% |
| 3–< 4 years | 40 | 5.5% | 45 | 5.4% | 68 | 6.9% |
| 4–< 5 years | 15 | 2.1% | 28 | 3.4% | 34 | 3.5% |
| ≥ 5 years | 11 | 1.5% | 40 | 4.8% | 41 | 4.2% |
| Tx type | ||||||
| Kidney alone | 712 | 97.5% | 799 | 96.4% | 956 | 97.5% |
| Kidney-liver | 12 | 1.6% | 21 | 2.5% | 21 | 2.1% |
| Kidney-heart | 3 | 0.4% | 4 | 0.5% | 1 | 0.1% |
| Other | 3 | 0.4% | 4 | 0.5% | 2 | 0.2% |
| All candidates | 730 | 100.0% | 829 | 100.0% | 981 | 100.0% |
Figure KI 98. Primary cause of ESRD in pediatric candidates waiting for kidney transplant by age, 2011–2015.
Candidates who joined the list 2011–2015. Candidates concurrently listed at more than one center are counted once. Patients who were listed, underwent transplant, and were relisted during the time period are counted more than once. Age is computed at earliest listing date. FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; CAKUT, congenital anomalies of the kidney and urinary tract.
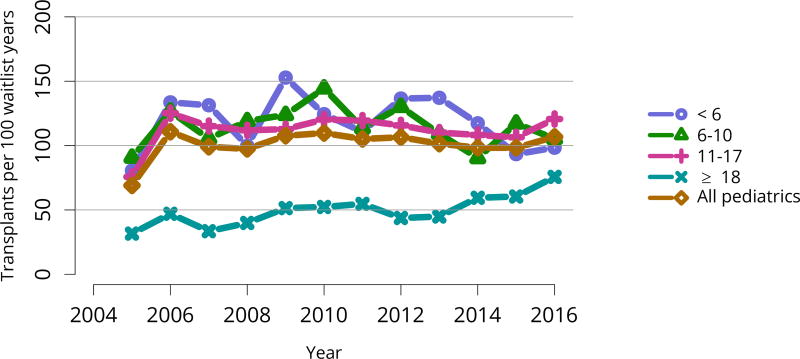
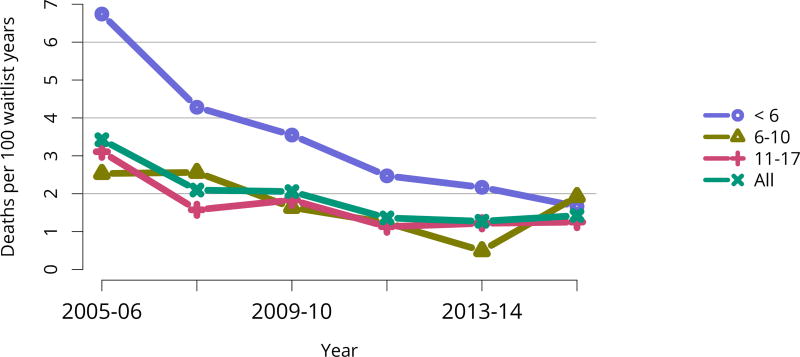
Of the 972 pediatric candidates removed from the waiting list in 2016, 598 (61.5%) received a deceased donor kidney, 273 (28.1%) received a living donor kidney, 27 (2.8%) died, 23 (2.4%) were considered too sick to undergo transplant, and 7 (0.7%) were removed from the list because their condition improved (Table KI 17, Table KI 18). Among patients newly listed in 2013, 57.4% underwent deceased donor transplant within 3 years, 22.5% underwent living donor transplant, 16.5% were still waiting, 2.3% were removed from the list for other reasons, and 1.2% died (Figure KI 99). The rate of deceased donor transplant in 2016 among pediatric waitlisted candidates was 106.8 per 100 active waitlist years, up from 98.3 in 2015 (Figure KI 100), compared with 20.7 for adult candidates (Figure KI 11). One aim of the KAS was to maintain the high level of access to transplant for pediatric candidates that was present pre-KAS. Transplant rates varied by age. In 2016, transplant rates were highest for candidates aged 11–17 years (120.7 per 100 active waitlist years), followed by candidates aged 6–10 years (105.3). Mirroring 2015, transplant rates among pediatric candidates were lowest for children aged younger than 6 years (98.4 per 100 active waitlist years). Rates also varied by cPRA (Figure KI 101), further demonstrating the effects of new priority for highly sensitized candidates under the KAS. For pediatric candidates with cPRA greater than 98%, the transplant rate increased from 6.9 per 100 active waitlist years in 2014 to 25.9 in 2016. Transplant rates for pediatric candidates with cPRA 80%–97% declined from 63.7 in 2014 to 18.2 in 2015, and increased to 34.9 in 2016. In contrast to mortality among candidates waiting for other organs, pretransplant mortality among pediatric candidates waiting for kidney transplant was low: 1.4 per 100 waitlist years in 2015–2016 (Figure KI 102).
Table KI 17. Kidney transplant waitlist activity among pediatric candidates.
Candidates concurrently listed at more than one center are counted once, from the time of earliest listing to the time of latest removal. Candidates who are listed, undergo transplant, and are relisted are counted more than once. Candidates are not considered to be on the list on the day they are removed; counts on January 1 may differ from counts on December 31 of the prior year. Candidates listed for multi-organ transplants are included.
| Waiting list state | 2014 | 2015 | 2016 |
|---|---|---|---|
| Patients at start of year | 1365 | 1482 | 1513 |
| Patients added during year | 1002 | 976 | 953 |
| Patients removed during year | 882 | 945 | 972 |
| Patients at end of year | 1485 | 1513 | 1494 |
Table KI 18. Removal reason among pediatric kidney transplant candidates.
Removal reason as reported to the OPTN. Candidates with death dates that precede removal dates are assumed to have died waiting.
| Removal reason | 2014 | 2015 | 2016 |
|---|---|---|---|
| Deceased donor transplant | 575 | 586 | 598 |
| Living donor transplant | 238 | 261 | 273 |
| Transplant outside US | 0 | 1 | 0 |
| Patient died | 22 | 20 | 27 |
| Patient refused transplant | 2 | 2 | 1 |
| Improved, transplant not needed | 2 | 8 | 7 |
| Too sick for transplant | 8 | 12 | 23 |
| Other | 35 | 55 | 43 |
Figure KI 99. Three-year outcomes for newly listed pediatric candidates waiting for kidney transplant, 2013.
Pediatric candidates who joined the waitlist in 2013. Candidates concurrently listed at more than one center are counted once, from the time of earliest listing to the time of latest removal. DD, deceased donor; LD, living donor.
Figure KI 100. Deceased donor kidney transplant rates among active pediatric waitlist candidates by age.
Transplant rates are computed as the number of deceased donor transplants per 100 patient-years of active waiting in a given year. Individual listings are counted separately. Age is determined at the later of listing date or January 1 of the given year. Rates with less than 10 patient-years of exposure are not shown. The age category 18 years or older includes candidates listed when pediatrics but still on the list in the given year.
Figure KI 101. Deceased donor kidney transplant rates among active pediatric waitlist candidates by C/PRA.
Transplant rates are computed as the number of deceased donor transplants per 100 patient-years of active waiting in a given year. Individual listings are counted separately. Rates with less than 10 patient-years of exposure are not shown.
Figure KI 102. Pretransplant mortality rates among pediatrics waitlisted for kidney transplant by age.
Mortality rates are computed as the number of deaths per 100 patient-years of waiting in the given year. Individual listings are counted separately. Age is determined at the later of listing date or January 1 of the given year. Rates with less than 10 patient-years of exposure are not shown.
3.2 Transplant
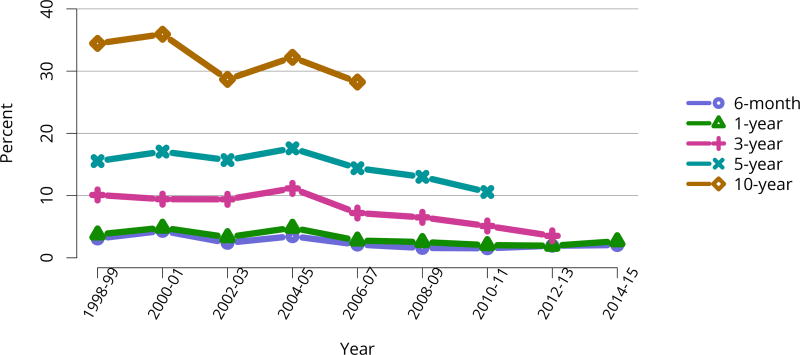
The number of total pediatric kidney transplants decreased from a peak of 899 in 2005 to 731 in 2016 (Figure KI 103). The decline in the proportion of living donor kidney transplants in pediatric recipients is of concern. In 2016, only 34.2% of pediatric transplants were from living donors, compared with 47.2% in 2005. Similar to adults, the number of related donors decreased dramatically over the past decade. The number of unrelated directed transplants performed in pediatric candidates remained low (50 in 2016) (Figure KI 104). Children aged younger than 6 years made up the largest group of living donor kidney recipients (44.3%) (Figure KI 105).
Figure KI 103. Pediatric kidney transplants by donor type.
All pediatric kidney transplant recipients, including retransplant, and multi-organ recipients.
Figure KI 104. Pediatric kidney transplants from living donors by relation.
Relationship of living donor to recipient is as indicated on the OPTN Living Donor Registration Form.
Figure KI 105. Percent of pediatric kidney transplants from living donors by recipient age.
All pediatric living kidney transplant recipients, including retransplant, and multi-organ recipients.
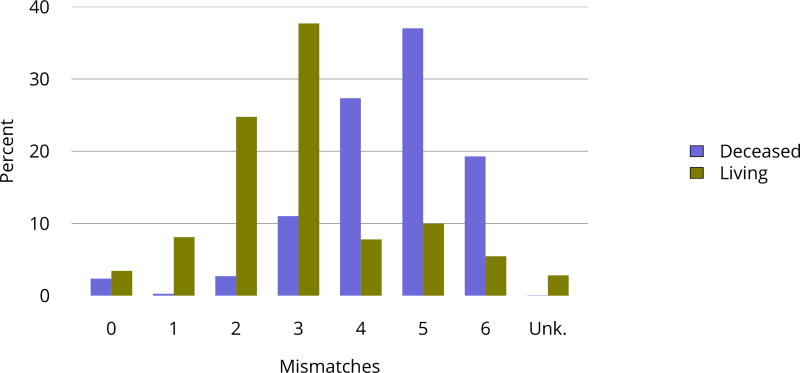
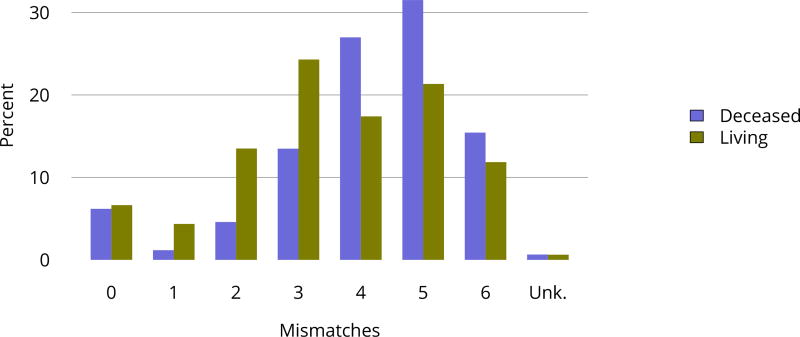
In 2016, 30 programs were performing only pediatric kidney transplants, compared with 130 performing only adult transplants and 58 performing transplants in both adults and children (Figure KI 106). In 2016, 14.2% of transplants in candidates aged 0–14 years were performed at programs with volumes of 5 or fewer pediatric transplants in that year (Figure KI 107). A higher proportion of living donor transplants were in recipients aged 1–5 years; this group accounted for 28.5% of pediatric living donor transplants and 18.5% of pediatric deceased donor transplants, compared with 17.8% and 19.4%, respectively, for recipients aged 6–10 years. While most pediatric transplants were in recipients aged 11–17 years (59.0%), deceased donor transplants were more common than living donor transplants (62.0% vs. 53.3%) (Table KI 19). The racial distribution differed for deceased and living donor transplant recipients. A higher proportion of living donor than deceased donor recipients were white (69.6% vs. 39.8%) and a higher proportion of deceased donor recipients than living donor recipients were black (23.7% vs. 9.2%) and Hispanic (27.9% vs. 16.1%). Private insurance was more common among living donor recipients and Medicare/Medicaid among deceased donor recipients. Most deceased donor recipients (66.3%) underwent transplant with a kidney from a donor with KDPI = 20%. The number of HLA mismatches was higher among deceased donor recipients than among living donor recipients; 83.6% of deceased donor recipients and 23.2% of living donor recipients had more than three HLA mismatches in 2012–2016 (Figure KI 114).
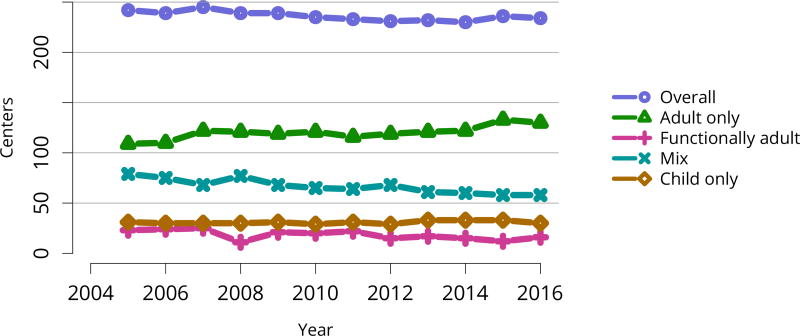
Figure KI 106. Number of centers performing pediatric and adult kidney transplants by center’s age mix.
Adult centers transplanted only recipients aged 18 years or older. Functionally adult centers transplant 80% adults or more, and the remainder were children aged 15–17 years. Mixed included adults and children of any age groups. Child only centers transplanted recipeints aged 0–17 years, and small number of adults up to age 21 years.
Figure KI 107. Pediatric kidney recipients at programs that perform 5 or fewer pediatric transplants annually.
Age groups are cumulative.
Table KI 19. Demographic characteristics of pediatric kidney transplant recipients, 2014–2016.
Kidney transplant recipients, including retransplants.
| Characteristic | Deceased | Living | All | |||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Age | ||||||
| < 1 year | 2 | 0.1% | 3 | 0.4% | 5 | 0.2% |
| 1–5 years | 264 | 18.5% | 210 | 28.5% | 474 | 21.9% |
| 6–10 years | 277 | 19.4% | 131 | 17.8% | 408 | 18.8% |
| 11–17 years | 886 | 62.0% | 393 | 53.3% | 1279 | 59.0% |
| Sex | ||||||
| Female | 602 | 42.1% | 298 | 40.4% | 900 | 41.6% |
| Male | 827 | 57.9% | 439 | 59.6% | 1266 | 58.4% |
| Race/ethnicity | ||||||
| White | 569 | 39.8% | 513 | 69.6% | 1082 | 50.0% |
| Black | 338 | 23.7% | 68 | 9.2% | 406 | 18.7% |
| Hispanic | 399 | 27.9% | 119 | 16.1% | 518 | 23.9% |
| Asian | 81 | 5.7% | 23 | 3.1% | 104 | 4.8% |
| Other/unknown | 42 | 2.9% | 14 | 1.9% | 56 | 2.6% |
| Insurance | ||||||
| Private | 402 | 28.1% | 419 | 56.9% | 821 | 37.9% |
| Medicare | 427 | 29.9% | 142 | 19.3% | 569 | 26.3% |
| Medicaid | 486 | 34.0% | 130 | 17.6% | 616 | 28.4% |
| Other government | 93 | 6.5% | 32 | 4.3% | 125 | 5.8% |
| Unknown | 21 | 1.5% | 14 | 1.9% | 35 | 1.6% |
| All recipients | 1429 | 100.0% | 737 | 100.0% | 2166 | 100.0% |
Figure KI 114. Total HLA A, B, and DR mismatches among pediatric kidney transplant recipients, 2012–2016.
Donor and recipient antigen matching is based on OPTN antigen values and split equivalences policy as of 2016.
The combination of a donor who was positive for cytomegalovirus and a pediatric recipient who was negative occurred in 22.7% of deceased donor transplants and in 19.5% of living donor transplants (Table KI 22, Table KI 23). The combination of a donor who was positive for EBV and a recipient who was negative occurred in 36.9% of deceased donor transplants and in 46.0% of living donor transplants.
Table KI 22. Pediatric deceased donor kidney donor-recipient serology matching, 2012–2016.
Donor serology is reported on the OPTN Donor Registration Form and recipient serology on the OPTN Transplant Recipient Registration Form. There may be multiple fields per serology. Any evidence for a positive serology is treated as positive for that serology. If all fields are unknown, incomplete, or pending, the person is categorized as unknown for that serology; otherwise, serology is assumed negative.
| Donor | Recipient | CMV | EBV |
|---|---|---|---|
| D− | R− | 16.0% | 4.7% |
| D− | R+ | 10.3% | 7.2% |
| D− | R unk | 14.4% | 0.3% |
| D+ | R− | 22.7% | 36.9% |
| D+ | R+ | 12.7% | 47.4% |
| D+ | R unk | 23.0% | 3.4% |
| D unk | R− | 0.3% | 0.0% |
| D unk | R+ | 0.3% | 0.1% |
| D unk | R unk | 0.3% | 0.0% |
CMV, cytomegalovirus; EBV, Epstein-Barr virus.
Table KI 23. Pediatric living donor kidney donor-recipient serology matching, 2012–2016.
Donor serology is reported on the OPTN Donor Registration Form and recipient serology on the OPTN Transplant Recipient Registration Form. There may be multiple fields per serology. Any evidence for a positive serology is treated as positive for that serology. If all fields are unknown, incomplete, or pending, the person is categorized as unknown for that serology; otherwise, serology is assumed negative.
| Donor | Recipient | CMV | EBV |
|---|---|---|---|
| D− | R− | 21.5% | 7.8% |
| D− | R+ | 4.8% | 2.5% |
| D− | R unk | 18.7% | 0.5% |
| D+ | R− | 19.5% | 46.0% |
| D+ | R+ | 13.5% | 34.9% |
| D+ | R unk | 18.9% | 3.7% |
| D unk | R− | 2.1% | 2.8% |
| D unk | R+ | 0.5% | 1.3% |
| D unk | R unk | 0.6% | 0.6% |
CMV, cytomegalovirus; EBV, Epstein-Barr virus.
3.3 Immunosuppressive Medication Use
Trends in immunosuppressive medications used in children and adolescents were similar to trends for adults. In 2016, use of T-cell depleting agents continued to increase, reaching 65.1%; IL-2-RA therapy use remained steady at 34.7%. The percentage of recipients receiving no induction therapy continued to decline, reaching a low of 5.2% in 2016 (Figure KI 108). In 2016, tacrolimus was used as part of the initial maintenance immunosuppressive medication regimen in 97.1% of pediatric transplant recipients and mycophenolate in 96.5% (Figure KI 109, Figure KI 110). Mammalian target of rapamycin inhibitors were used in 5.6% of 2015 pediatric recipients at 1 year posttransplant (Figure KI 111). Corticosteroids were used in 61.6% of 2016 pediatric recipients at the time of transplant and in 62.4% of 2015 recipients at 1 year posttransplant (Figure KI 112). T-cell depleting agents were more common with increasing cPRA and IL-2-RA use more common with decreasing cPRA (Figure KI 113).
Figure KI 108. Induction agent use in pediatric kidney transplant recipients.
Immunosuppression at transplant reported to the OPTN. IL2-RA, interleukin-2 receptor antagonist.
Figure KI 109. Calcineurin inhibitor use in pediatric kidney transplant recipients.
Immunosuppression at transplant reported to the OPTN.
Figure KI 110. Anti-metabolite use in pediatric kidney transplant recipients.
Immunosuppression at transplant reported to the OPTN. Mycophenolate includes mycophenolate mofetil and mycophenolate sodium.
Figure KI 111. mTOR inhibitor use in pediatric kidney transplant recipients.
Immunosuppression at transplant reported to the OPTN. One-year posttransplant data are limited to patients alive with graft function at 1 year posttransplant. mTOR, mammalian target of rapamycin.
Figure KI 112. Steroid use in pediatric kidney transplant recipients.
Immunosuppression at transplant reported to the OPTN. One-year posttransplant data are limited to patients alive with graft function at 1 year posttransplant.
Figure KI 113. Induction use by C/PRA among pediatric kidney transplant recipients, 2012–2016.
IL2-RA, interleukin-2 receptor antagonist.
3.4 Outcomes
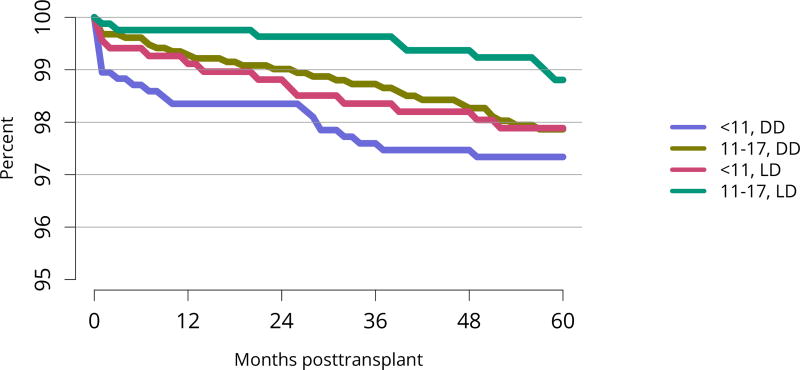
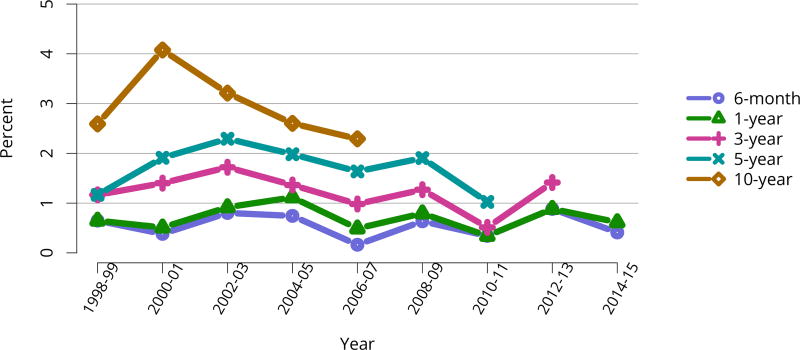
All-cause graft failure after deceased donor transplant in pediatric recipients was 2.6% at 6 months and 3.3% at 1 year for transplants in 2014–2015, 10.8% at 3 years for transplants in 2012–2013, 18.2% at 5 years for transplants in 2010–2011, and 45.8% at 10 years for transplants in 2006–2007 (Figure KI 117). Corresponding graft failure after living donor transplant was 2.5% at 6 months and 3.3% at 1 year for transplants in 2014–2015, 4.9% at 3 years for transplants in 2012–2013, 11.5% at 5 years for transplants in 2010–2011, and 30.5% at 10 years for transplants in 2006–2007 (Figure KI 120). For the cohort of recipients who underwent transplant in 2007–2011, graft survival was highest for living donor recipients aged younger than 11 years (91.1% at 5 years) and lowest for deceased donor recipients aged 11–17 years (74.5% at 5 years) (Figure KI 123). Over the past 6 years, the incidence of acute rejection in the first year remained relatively stable between 11.4% and 12%. In the youngest age group (< 6 years), incidence of reported acute rejection in the first posttransplant year increased over time from 9.3% in 2010–2011 to 11.9% in 2014–2015, the highest incidence by age (Figure KI 124). Short-term renal function, measured by estimated glomerular filtration rate (eGFR), improved substantially over the past decade. The proportion of recipients with eGFR 90 mL/min/1.73 m2 or higher at discharge increased from 20.6% in 2005 to 35.9% in 2016, and at 1 year posttransplant from 13.0% to 27.7% (Figure KI 115, Figure KI 116). Of recipients in the 2015 cohort, 74.7% had chronic kidney disease stage 1–2 at 1 year posttransplant, with eGFR 60 mL/min/1.73 m2 or higher. Incidence of PTLD among EBV-negative recipients was 2.9% at 5 years posttransplant, compared with 0.7% among EBV-positive recipients (Figure KI 125). Overall 5-year patient survival among pediatric kidney transplant recipients in 2007–2011 was 98.0% (Figure KI 126).
Figure KI 117. Graft failure among pediatric deceased donor kidney-alone transplant recipients.
Estimates are unadjusted, computed using KaplanMeier competing risk methods. Recipients are followed to the earliest of kidney graft failure; kidney retransplant; return to dialysis; death; or 6 months, 1, 3, 5, or 10 years posttransplant. All-cause graft failure (GF) is defined as any of the prior outcomes prior to 6 months, 1, 3, 5, or 10 years, respectively.
Figure KI 120. Graft failure among pediatric living donor kidney-alone transplant recipients.
Estimates are unadjusted, computed using KaplanMeier competing risk methods. Recipients are followed to the earliest of kidney graft failure; kidney retransplant; return to dialysis; death; or 6 months, 1, 3, 5, or 10 years posttransplant. All-cause graft failure (GF) is defined as any of the prior outcomes prior to 6 months, 1, 3, 5, or 10 years, respectively.
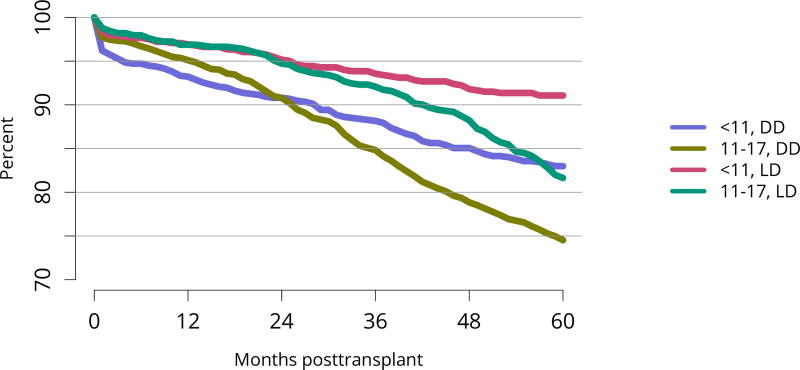
Figure KI 123. Graft survival among pediatric kidney transplant recipients by age and donor type, 2007–2011.
Graft survival estimated using unadjusted Kaplan-Meier methods. DD, deceased donor; LD, living donor.
Figure KI 124. Incidence of acute rejection by 1 year posttransplant among pediatric kidney transplant recipients by age.
Acute rejection is defined as a record of acute or hyperacute rejection, as reported on the OPTN Transplant Recipient Registration Form or Transplant Recipient Followup Form. Only the first rejection event is counted. Cumulative incidence is estimated using the Kaplan-Meier competing risk method.
Figure KI 115. Distribution of eGFR at discharge among pediatric kidneyalone transplant recipients.
GFR (mL/min/1.73 m2) estimated using the bedside Schwartz equation, and computed for patients alive with graft function at discharge. Equation: eGFR = 0.413*Height(cm)/Creatinine (mg/dL).
Figure KI 116. Distribution of eGFR at 12 months posttransplant among pediatric kidney-alone transplant recipients.
GFR (mL/min/1.73 m2) estimated using the bedside Schwartz equation, and computed for patients alive with graft function at 12 months posttransplant. Equation: eGFR = 0.413*Height(cm)/Creatinine (mg/dL).
Figure KI 125. Incidence of PTLD among pediatric kidney transplant recipients by recipient EBV status at transplant, 2004–2014.
Cumulative incidence is estimated using the Kaplan-Meier competing risk method. Posttransplant lymphoproliferative disorder (PTLD) is identified as a reported complication or cause of death on the OPTN Transplant Recipient Follow-up Form or on the Posttransplant Malignancy Form as polymorphic PTLD, monomorphic PTLD, or Hodgkin disease. Only the earliest date of PTLD diagnosis is considered. EBV, Epstein-Barr virus.
Figure KI 126. Patient survival among pediatric kidney transplant recipients, 2007–2011, by age and donor type.
Recipient survival estimated using unadjusted Kaplan-Meier methods. DD, deceased donor; LD, living donor.
Figure KI 6. Distribution of adults waiting for kidney transplant by waiting time.
Candidates waiting for transplant at any time in the given year. Candidates listed concurrently at multiple centers are counted once. Time on the waiting list is determined at the earlier of December 31 or removal from the waiting list. Active and inactive candidates are included.
Figure KI 10. Prevalent dialysis patients waitlisted for kidney transplant by age.
Estimated percentage of prevalent dialysis patients waitlisted for kidney or kidney-pancreas transplant. Percentage calculated as the sum of point prevalent waitlist candidates divided by the sum of point prevalent dialysis patients on December 31 of each year. Dialysis data from the Consolidated Renal Operations in a Web-enabled Network (CROWN) dataset. Age calculated on December 31 of given year.
Figure KI 12. Deceased donor kidney transplant rates among active adult waitlist candidates by diagnosis.
Transplant rates are computed as the number of deceased donor transplants per 100 patient-years of active wait time in a given year. Individual listings are counted separately. Rates with less than 10 patient-years of exposure are not shown. GN, glomerulonephritis.
Figure KI 24. Deaths within six months after removal among adult kidney waitlist candidates.
Denominator includes only candidates removed from the waiting list for reasons other than transplant or death while on the list.
Figure KI 34. Rates of kidneys recovered for transplant and not transplanted by DCD status.
Percentages of kidneys not transplanted out of all kidneys recovered for transplant. Kidneys recovered en-bloc are counted once, and kidneys recovered separately are counted twice. DBD, donation after brain death; DCD, donation after circulatory death.
Figure KI 35. Rates of kidneys recovered for transplant and not transplanted by KDPI.
Percentages of kidneys not transplanted out of all kidneys recovered for transplant, by KDPI classification. The reference population for the KDRI to KDPI conversion is all deceased donor kidneys recovered for transplant in the US in 2016. Kidneys recovered en-bloc are counted once. KDPI, kidney donor profile index; KDRI, kidney donor risk index.
Figure KI 36. Donor-specific components of the kidney donor risk index.
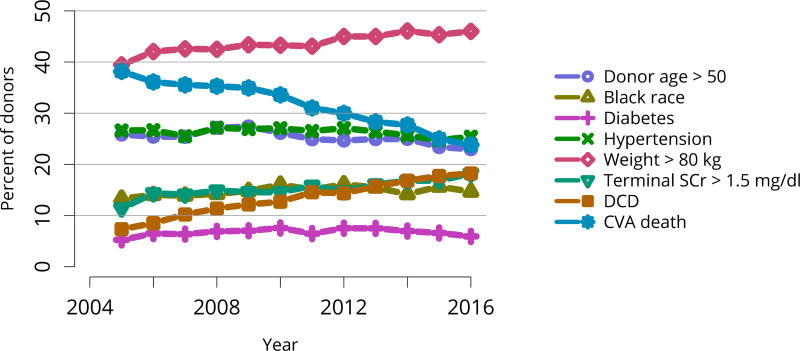
Donors with at least one transplanted kidney. The donor-specific components of the kidney donor risk index are shown, except for donor height and hepatitis C virus status. CVA, cerebrovascular accident; DCD, donation after circulatory death; SCr, serum creatinine.
Figure KI 39. Cause of death among deceased kidney donors.
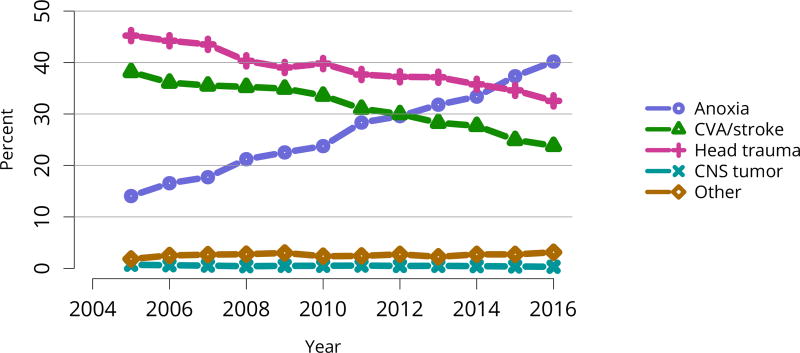
Deceased donors whose kidneys were transplanted. Each donor is counted once. CNS, central nervous system; CVA, cerebrovascular accident.
Figure KI 42. Living kidney donors by sex.
As reported on the OPTN Living Donor Registration Form.
Figure KI 46. Kidney complications among living kidney donors, 2011–2015.
Complications reported on the OPTN Living Donor Registration and Living Donor Follow-up Forms at each time point. Complications include readmission, re-operation, vascular complications, and other complications requiring intervention. Multiple complications may be reported at any time point.
Figure KI 47. BMI among living kidney donors.
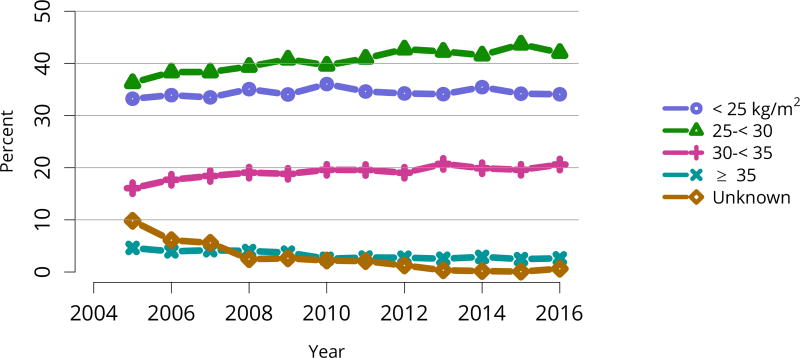
Donor height and weight reported on the OPTN Living Donor Registration Form.
Figure KI 60. C/PRA at time of kidney transplant in adult living donor recipients.
From December 5, 2007, through September 30, 2009, CPRA was used if greater than 0; otherwise, the maximum pretransplant PRA was used. Before December 5, 2007, the maximum pretransplant PRA was used unconditionally. CPRA is used after September 30, 2009, unless it is missing; if it is missing, the maximum pretransplant PRA is used. Kidney-alone transplants only.
Figure KI 61. Total HLA A, B, and DR mismatches among adult kidney transplant recipients, 2012–2016.
Donor and recipient antigen matching is based on OPTN antigen values and split equivalences policy as of 2016.
Figure KI 66. Death with function among adult deceased donor kidney transplant recipients.
Estimates are unadjusted, computed using KaplanMeier competing risk methods. Recipients are followed to the earliest of kidney graft failure; kidney retransplant; return to dialysis; death; or 6 months, 1, 3, 5, or 10 years posttransplant. Death with function (DWF) is defined as death without prior graft failure, return to dialysis, or retransplant.
Figure KI 69. Death with function among adult living donor kidney transplant recipients.
Estimates are unadjusted, computed using Kaplan-Meier competing risk methods. Recipients are followed to the earliest of kidney graft failure; kidney retransplant; return to dialysis; death; or 6 months, 1, 3, 5, or 10 years posttransplant. Death with function (DWF) is defined as death without prior graft failure, return to dialysis, or retransplant.
Figure KI 76. Graft survival among adult living donor kidney transplant recipients, 2011, by diagnosis.
Graft survival estimated using unadjusted Kaplan-Meier methods. CKD, cystic kidney disease; GN, glomerulonephritis.
Figure KI 77. Distribution of eGFR at discharge among adult kidney transplant recipients.
GFR (mL/min/1.73 m2) estimated using the Chronic Kidney Disease Epidemiology Collaboration equation, and computed for patients alive with graft function at discharge.
Figure KI 78. Distribution of eGFR at 6 months posttransplant among adult kidney transplant recipients.
GFR (mL/min/1.73 m2) estimated using the Chronic Kidney Disease Epidemioogy Collaboration equation, and computed for patients alive with graft function at 6 months posttransplant.
Figure KI 80. Incidence of acute rejection by 1 year posttransplant among adult kidney transplant recipients by donor type.
Acute rejection is defined as a record of acute or hyperacute rejection, as reported on the OPTN Transplant Recipient Registration or Transplant Recipient Follow-up Form. Only the first rejection event is counted. Cumulative incidence is estimated using the Kaplan-Meier competing risk method.
Figure KI 89. Patient survival among adult living donor kidney transplant recipients, 2011, by diagnosis.
Patient survival estimated using unadjusted Kaplan-Meier methods. For recipients of more than one transplant during the period, only the first is considered. CKD, cystic kidney disease; GN, glomerulonephritis.
Figure KI 90. Patient survival among adult living donor kidney transplant recipients, 2011, by race.
Patient survival estimated using unadjusted Kaplan-Meier methods. For recipients of more than one transplant during the period, only the first is considered.
Figure KI 93. Distribution of pediatric candidates waiting for kidney transplant by age.
Candidates waiting for transplant at any time in the given year. Candidates listed concurrently at multiple centers are counted once. Age is determined at the later of listing date or January 1 of the given year. Active and inactive candidates are included.
Figure KI 94. Distribution of pediatric candidates waiting for kidney transplant by race.
Candidates waiting for transplant any time in the given year. Candidates listed concurrently at multiple centers are counted once. Active and inactive candidates are included.
Figure KI 95. Distribution of pediatric candidates waiting for kidney transplant by diagnosis.
Candidates waiting for transplant any time in the given year. Candidates listed concurrently at multiple centers are counted once. Diagnosis categories follow North American Pediatric Renal Trials and Collaborative Studies recommendations. Active and inactive candidates are included. FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; CAKUT, congenital anomalies of the kidney and urinary tract.
Figure KI 96. Distribution of pediatric candidates waiting for kidney transplant by waiting time.
Candidates waiting for transplant any time in the given year. Candidates listed concurrently at multiple centers are counted once. Time on the waiting list is determined at the earlier of December 31 or removal from the waiting list. Active and inactive candidates are included.
Figure KI 97. Distribution of pediatric candidates waiting for kidney transplant by C/PRA.
Candidates waiting for transplant at any time in the given year. Candidates listed concurrently at multiple centers are counted once. From December 5, 2007, through September 30, 2009, CPRA was used if greater than 0; otherwise, the maximum pretransplant PRA was used. Before December 5, 2007, the maximum pretransplant PRA was used unconditionally. CPRA is used after September 30, 2009. C/PRA is the highest value during the year. Active and inactive candidates are included.
Figure KI 118. Death-censored graft failure among pediatric deceased donor kidney-alone transplant recipients.
Estimates are unadjusted, computed using Kaplan-Meier competing risk methods. Recipients are followed to the earliest of kidney graft failure; kidney retransplant; return to dialysis; death; or 6 months, 1, 3, 5, or 10 years posttransplant. Death-censored graft failure (DCGF) is defined as a return to dialysis, reported graft failure, or kidney retransplant.
Figure KI 119. Death with function among pediatric deceased donor kidney-alone transplant recipients.
Estimates are unadjusted, computed using Kaplan-Meier competing risk methods. Recipients are followed to the earliest of kidney graft failure; kidney retransplant; return to dialysis; death; or 6 months, 1, 3, 5, or 10 years posttransplant. Death with function (DWF) is defined as death without prior graft failure, return to dialysis, or retransplant.
Figure KI 121. Death-censored graft failure among pediatric living donor kidney-alone transplant recipients.
Estimates are unadjusted, computed using Kaplan-Meier competing risk methods. Recipients are followed to the earliest of kidney graft failure; kidney retransplant; return to dialysis; death; or 6 months, 1, 3, 5, or 10 years posttransplant. Death-censored graft failure (DCGF) is defined as a return to dialysis, reported graft failure, or kidney retransplant.
Figure KI 122. Death with function among pediatric living donor kidneyalone transplant recipients.
Estimates are unadjusted, computed using Kaplan-Meier competing risk methods. Recipients are followed to the earliest of kidney graft failure; kidney retransplant; return to dialysis; death; or 6 months, 1, 3, 5, or 10 years posttransplant. Death with function (DWF) is defined as death without prior graft failure, return to dialysis, or retransplant.
Table KI 1. Reasons for inactive status among new adult kidney transplant listings, 2016.
Candidates first listed as inactive. Each listing is counted separately.
| Reasons for inactive status | N | Percent |
|---|---|---|
| Candidate work-up incomplete | 5707 | 68.2% |
| Insurance issues | 732 | 8.7% |
| Too sick | 565 | 6.8% |
| Too well | 459 | 5.5% |
| Weight inappropriate | 274 | 3.3% |
| Candidate for LD transplant only | 205 | 2.5% |
| Candidate choice | 203 | 2.4% |
| Transplant pending | 134 | 1.6% |
| Unknown | 34 | 0.4% |
| Medical non-compliance | 30 | 0.4% |
| Inappropriate substance abuse | 17 | 0.2% |
| Candidate could not be contacted | 5 | 0.1% |
| Physician/surgeon unavailable | 1 | 0.0% |
LD, living donor.
Table KI 3. Clinical characteristics of adults on the kidney transplant waiting list on December 31, 2006, December 31, 2011 and December 31, 2016.
Candidates waiting for transplant on December 31, 2006, December 31, 2011, and December 31, 2016, regardless of first listing date; multiple listings are collapsed.
| Characteristic | 2006 | 2011 | 2016 | |||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Diagnosis | ||||||
| Diabetes | 20,636 | 30.9% | 30,133 | 33.9% | 34,811 | 36.5% |
| Hypertension | 16,648 | 24.9% | 22,362 | 25.2% | 22,301 | 23.4% |
| GN | 10,059 | 15.1% | 12,572 | 14.1% | 13,320 | 14.0% |
| CKD | 5489 | 8.2% | 7366 | 8.3% | 8106 | 8.5% |
| Other | 13,986 | 20.9% | 16,451 | 18.5% | 16,918 | 17.7% |
| Diabetes* | 26,343 | 39.4% | 38,620 | 43.4% | 43,883 | 46.0% |
| Blood type | ||||||
| A | 18,925 | 28.3% | 25,777 | 29.0% | 26,931 | 28.2% |
| B | 10,885 | 16.3% | 14,166 | 15.9% | 15,644 | 16.4% |
| AB | 1904 | 2.8% | 2581 | 2.9% | 2573 | 2.7% |
| O | 35,104 | 52.5% | 46,360 | 52.2% | 50,308 | 52.7% |
| CPRA | ||||||
| < 1% | 31,929 | 47.8% | 49,823 | 56.1% | 58,590 | 61.4% |
| 1–< 20% | 12,982 | 19.4% | 9148 | 10.3% | 8932 | 9.4% |
| 20–< 80% | 10,125 | 15.2% | 13,673 | 15.4% | 14,918 | 15.6% |
| 80–< 98% | 5250 | 7.9% | 5846 | 6.6% | 5533 | 5.8% |
| 98–100% | 5188 | 7.8% | 8421 | 9.5% | 7155 | 7.5% |
| Unknown | 1344 | 2.0% | 1973 | 2.2% | 328 | 0.3% |
| All candidates | 66,818 | 100.0% | 88,884 | 100.0% | 95,456 | 100.0% |
CKD, cystic kidney disease; GN, glomerulonephritis.
Diabetes status based on diagnosis and comorbid conditions.
Table KI 4. Listing characteristics of adults on the kidney transplant waiting list on December 31, 2006, December 31, 2011 and December 31, 2016.
Candidates waiting for transplant on December 31, 2006, December 31, 2011, and December 31, 2016, regardless of first listing date; and multiple listings are collapsed.
| Characteristic | 2006 | 2011 | 2016 | |||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Transplant history | ||||||
| First | 55,812 | 83.5% | 75,496 | 84.9% | 83,505 | 87.5% |
| Retransplant | 11,006 | 16.5% | 13,388 | 15.1% | 11,951 | 12.5% |
| Wait time | ||||||
| < 1 year | 23,062 | 34.5% | 25,472 | 28.7% | 25,134 | 26.3% |
| 1–< 2 years | 15,202 | 22.8% | 20,426 | 23.0% | 18,818 | 19.7% |
| 2–< 3 years | 10,144 | 15.2% | 14,900 | 16.8% | 15,415 | 16.1% |
| 3–< 4 years | 6393 | 9.6% | 9867 | 11.1% | 12,160 | 12.7% |
| 4–< 5 years | 4367 | 6.5% | 6729 | 7.6% | 8347 | 8.7% |
| ≥ 5 years | 7650 | 11.4% | 11,490 | 12.9% | 15,582 | 16.3% |
| Will accept KDPI*> 85% | 29,294 | 43.8% | 41,859 | 47.1% | 45,219 | 47.4% |
| Tx type | ||||||
| Kidney alone | 64,124 | 96.0% | 86,052 | 96.8% | 92,621 | 97.0% |
| Kidney-pancreas | 2297 | 3.4% | 2081 | 2.3% | 1720 | 1.8% |
| Kidney-liver | 325 | 0.5% | 635 | 0.7% | 909 | 1.0% |
| Kidney-heart | 58 | 0.1% | 107 | 0.1% | 191 | 0.2% |
| Other | 14 | 0.0% | 9 | 0.0% | 15 | 0.0% |
| All candidates | 66,818 | 100.0% | 88,884 | 100.0% | 95,456 | 100.0% |
KDPI, kidney donor profile index.
Prior to 2014, includes willingness to accept expanded criteria donor (ECD) kidney. KDPI $>$85% is local non-zero HLA mismatch only.
Table KI 7. Living kidney donor deaths, 2012–2016, by number of days after donation.
Living kidney donors. Numbers of deaths reported to OPTN or the Social Security Administration. Donation-related deaths are included in the Medical category.
| Cause | 0–30 days | 31–90 days | 91–365 days |
|---|---|---|---|
| Suicide | 1 | 0 | 1 |
| Accident/homicide | 0 | 0 | 7 |
| Overdose | 0 | 0 | 1 |
| Medical | 3 | 0 | 0 |
| Cancer | 0 | 0 | 0 |
| Unknown | 0 | 1 | 1 |
| TOTAL | 4 | 1 | 10 |
Table KI 11. Adult deceased donor kidney donor-recipient serology matching, 2012–2016.
Donor serology is reported on the OPTN Donor Registration Form and recipient serology on the OPTN Transplant Recipient Registration Form. There may be multiple fields per serology. Any evidence for a positive serology is treated as positive for that serology. If all fields are unknown, incomplete, or pending, the person is categorized as unknown for that serology; otherwise, serology is assumed negative.
| Donor | Recipient | CMV | EBV | HB core | HB surf. ant. | HCV | HIV |
|---|---|---|---|---|---|---|---|
| D− | R− | 7.6% | 0.7% | 80.6% | 96.7% | 91.3% | 92.0% |
| D− | R+ | 15.2% | 5.8% | 8.1% | 1.9% | 4.1% | 1.0% |
| D− | R unk | 16.4% | 0.8% | 7.7% | 1.3% | 1.8% | 5.8% |
| D+ | R− | 10.7% | 8.1% | 2.7% | 0.0% | 0.3% | 0.0% |
| D+ | R+ | 24.9% | 75.6% | 0.6% | 0.0% | 2.5% | 0.0% |
| D+ | R unk | 24.8% | 9.0% | 0.2% | 0.0% | 0.0% | 0.0% |
| D unk | R− | 0.1% | 0.0% | 0.0% | 0.1% | 0.0% | 1.2% |
| D unk | R+ | 0.1% | 0.1% | 0.0% | 0.0% | 0.0% | 0.0% |
| D unk | R unk | 0.2% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
CMV, cytomegalovirus; EBV, Epstein-Barr virus; HB, hepatitis B; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Table KI 12. Adult living donor kidney donor-recipient serology matching, 2012–2016.
Donor serology is reported on the OPTN Donor Registration Form and recipient serology on the OPTN Transplant Recipient Registration Form. There may be multiple fields per serology. Any evidence for a positive serology is treated as positive for that serology. If all fields are unknown, incomplete, or pending, the person is categorized as unknown for that serology; otherwise, serology is assumed negative.
| Donor | Recipient | CMV | EBV | HB core | HB surf. ant. | HCV | HIV |
|---|---|---|---|---|---|---|---|
| D− | R− | 15.1% | 2.0% | 80.1% | 93.2% | 94.0% | 55.9% |
| D− | R+ | 13.0% | 6.7% | 3.6% | 1.2% | 2.0% | 0.3% |
| D− | R unk | 17.7% | 0.5% | 8.6% | 1.3% | 1.6% | 1.8% |
| D+ | R− | 10.3% | 7.1% | 1.5% | 0.4% | 0.4% | 0.0% |
| D+ | R+ | 21.7% | 72.0% | 0.4% | 0.0% | 0.0% | 0.0% |
| D+ | R unk | 19.6% | 4.7% | 0.1% | 0.0% | 0.0% | 0.0% |
| D unk | R− | 0.6% | 0.7% | 3.5% | 3.6% | 1.9% | 38.9% |
| D unk | R+ | 1.0% | 2.7% | 0.2% | 0.1% | 0.0% | 0.2% |
| D unk | R unk | 1.1% | 3.6% | 2.1% | 0.1% | 0.2% | 2.9% |
CMV, cytomegalovirus; EBV, Epstein-Barr virus; HB, hepatitis B; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Table KI 20. Clinicial characteristics of pediatric kidney transplant recipients, 2014–2016.
Kidney transplant recipients, including retransplants. Diagnosis categories follow North American Pediatric Renal Trials and Collaborative Studies recommendations.
| Characteristic | Deceased | Living | All | |||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Diagnosis | ||||||
| FSGS | 177 | 12.4% | 73 | 9.9% | 250 | 11.5% |
| GN | 167 | 11.7% | 65 | 8.8% | 232 | 10.7% |
| CAKUT | 507 | 35.5% | 264 | 35.8% | 771 | 35.6% |
| Other | 578 | 40.4% | 335 | 45.5% | 913 | 42.2% |
| Blood type | ||||||
| A | 468 | 32.8% | 267 | 36.2% | 735 | 33.9% |
| B | 182 | 12.7% | 103 | 14.0% | 285 | 13.2% |
| AB | 60 | 4.2% | 36 | 4.9% | 96 | 4.4% |
| O | 719 | 50.3% | 331 | 44.9% | 1050 | 48.5% |
| Dialysis time | ||||||
| None | 347 | 24.3% | 303 | 41.1% | 650 | 30.0% |
| < 1 year | 283 | 19.8% | 203 | 27.5% | 486 | 22.4% |
| < 3 years | 492 | 34.4% | 156 | 21.2% | 648 | 29.9% |
| < 5 years | 147 | 10.3% | 16 | 2.2% | 163 | 7.5% |
| ≥ 5 years | 160 | 11.2% | 59 | 8.0% | 219 | 10.1% |
| CPRA | ||||||
| < 1% | 1054 | 73.8% | 554 | 75.2% | 1608 | 74.2% |
| 1–< 20% | 129 | 9.0% | 68 | 9.2% | 197 | 9.1% |
| 20–< 80% | 176 | 12.3% | 84 | 11.4% | 260 | 12.0% |
| 80–< 98% | 43 | 3.0% | 16 | 2.2% | 59 | 2.7% |
| 98–100% | 27 | 1.9% | 9 | 1.2% | 36 | 1.7% |
| Unknown | 0 | 0.0% | 6 | 0.8% | 6 | 0.3% |
| All recipients | 1429 | 100.0% | 737 | 100.0% | 2166 | 100.0% |
FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; CAKUT, congenital anomalies of the kidney and urinary tract.
Table KI 21. Transplant characteristics of pediatric kidney transplant recipients, 2014–2016.
Kidney transplant recipients, including retransplants.
| Characteristic | Deceased | Living | All | |||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Wait time | ||||||
| < 1 year | 8 | 0.6% | 79 | 10.7% | 87 | 4.0% |
| < 3 years | 991 | 69.3% | 549 | 74.5% | 1540 | 71.1% |
| < 5 years | 353 | 24.7% | 99 | 13.4% | 452 | 20.9% |
| ≥ 5 years | 57 | 4.0% | 8 | 1.1% | 65 | 3.0% |
| Unknown | 20 | 1.4% | 2 | 0.3% | 22 | 1.0% |
| KDPI | ||||||
| ≤ 20% | 947 | 66.3% | ||||
| 21–34% | 334 | 23.4% | ||||
| 35–85% | 145 | 10.1% | ||||
| > 85% | 3 | 0.2% | ||||
| DCD status | ||||||
| DBD | 1376 | 96.3% | ||||
| DCD | 53 | 3.7% | ||||
| DGF | ||||||
| None | 1310 | 91.7% | 710 | 96.3% | 2020 | 93.3% |
| Yes | 119 | 8.3% | 27 | 3.7% | 146 | 6.7% |
| Tx type | ||||||
| Kidney only | 1378 | 96.4% | 737 | 100.0% | 2115 | 97.6% |
| Kidney-liver | 43 | 3.0% | 0 | 0.0% | 43 | 2.0% |
| Other | 8 | 0.6% | 0 | 0.0% | 8 | 0.4% |
| Transplant history | ||||||
| First | 1302 | 91.1% | 681 | 92.4% | 1983 | 91.6% |
| Retransplant | 127 | 8.9% | 56 | 7.6% | 183 | 8.4% |
| All recipients | 1429 | 100.0% | 737 | 100.0% | 2166 | 100.0% |
DBD, donation after brain death; DCD, donation after circulatory death; DGF, delayed graft function; KDPI, kidney donor profile index. DCD status and KDPI scores apply to deceased donor transplants only.
Footnotes
The publication was produced for the U.S. Department of Health and Human Services, Health Resources and Services Administration, by the Minneapolis Medical Research Foundation (MMRF) and by the United Network for Organ Sharing (UNOS) under contracts HHSH250201500009C and 234-2005-37011C, respectively.
This publication lists non-federal resources in order to provide additional information to consumers. The views and content in these resources have not been formally approved by the U.S. Department of Health and Human Services (HHS) or the Health Resources and Services Administration (HRSA). Neither HHS nor HRSA endorses the products or services of the listed resources.
OPTN/SRTR 2016 Annual Data Report is not copyrighted. Readers are free to duplicate and use all or part of the information contained in this publication. Data are not copyrighted and may be used without permission if appropriate citation information is provided.
Pursuant to 42 U.S.C. §1320b-10, this publication may not be reproduced, reprinted, or redistributed for a fee without specific written authorization from HHS.
Suggested Citations Full citation: Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2016 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration; 2017. Abbreviated citation: OPTN/SRTR 2016 Annual Data Report. HHS/HRSA.