Abstract
Objective
Despite high-grade intravesical prostatic protrusion (IPP) being closely related to bladder outlet obstruction (BOO), up to 21% of patients with low IPP remain obstructed. This study evaluates the characteristics and urodynamic findings of men with small prostates and low IPP.
Methods
One hundred and fourteen men aged >50 years old with lower urinary tract symptoms (LUTS) were assessed with symptoms, uroflowmetry, serum prostate-specific antigen (PSA), transabdominal ultrasound measurement of prostate volume (PV), IPP and post-void residual urine (PVRU). All patients underwent pressure flow studies. Patients with PV < 30 mL and IPP ≤ 10 mm were examined for parameters correlating with BOO or impaired detrusor contractility.
Results
Thirty-six patients had PV < 30 mL and IPP <10 mm. Nine patients (25.0%) had urodynamic BOO, all with normal bladder contractility. Fourteen patients (38.9%) had poor detrusor contractility and all had no BOO. PV, PVRU and IPP were significantly associated with BOO, with IPP showing greatest positive correlation. Both Qmax and IPP were significantly associated with detrusor contractility. At 5-year follow-up, most patients responded to medical therapy. Only three out of nine patients (33.3%) with BOO eventually underwent surgery, and all had a high bladder neck seen on the resectoscope. Only one patient (7.1%) with poor detrusor contractility eventually required surgery after repeat pressure flow study revealed BOO.
Conclusion
In men with small prostates and low IPP, the presence of BOO is associated with higher PV, PVRU and IPP, and most respond well to medical management. BOO can possibly be explained by elevation of the bladder neck by a small subcervical adenoma.
Keywords: Male, Prostate hyperplasia, Pathology, Prostate, Ultrasonography, Urodynamics, Intravesical prostatic protrusion
1. Introduction
Benign prostatic enlargement (BPE) is a common cause of bladder outlet obstruction (BOO) and poor urinary flow in elderly men presenting with lower urinary tract symptoms (LUTS) [1].
The relationship between prostate size and BOO has been shown to be controversial [2], [3], and indeed, the concept of benign prostate hyperplasia (BPH) has gradually been replaced by the idea of a prostate adenoma arising from nodular hyperplasia that distorts the prostatic urethra rather than compresses it [4], [5]. As a result, an adenoma at the submucosal region of the bladder can cause significant obstruction despite being small, while an adenoma in the prostatic stroma would have to grow to a large size before causing significant symptoms and obstruction. Unlike prostate volume (PV), the intravesical prostatic protrusion (IPP) has been shown to correlate well with poor urinary flow and the presence of BOO [4], [5], [6].
However, a proportion of patients with small prostates and low IPP complain of significant urinary disturbances and poor flow, and up to 21% of patients with small prostates and low IPP still present with urodynamic BOO [5]. In this subgroup of patients, it is understandable that poor urinary flow might be due to BOO or poor detrusor contractility, but reports distinguishing both aspects as causes for poor urinary flow have been scarce.
This study therefore aims to evaluate the characteristics and urodynamic findings of men with small prostates and low IPP, but presenting with LUTS and poor urinary flow. This may further elucidate a cause of BOO in this specific subgroup of patients, and aid in directing further management.
2. Methods
Between November 2001 and November 2002, a total of 114 patients above 50 years of age presenting with LUTS suggestive of BPH were assessed for baseline symptoms using the International Prostate Symptoms Score (IPSS) including the quality of life (QoL) score, digital rectal examination, uroflowmetry findings, and serum prostate-specific antigen (PSA) measurements. Transabdominal ultrasound was done by a single operator (K.T. Foo) to evaluate PV, IPP, and post-void residual urine (PVRU). PV and IPP were measured when the bladder was sufficiently distended with between 100 and 200 mL of urine. The IPP was measured as previously described [5].
Patients specifically included in this study were selected based on a small prostate size of <30 mL and a low IPP of ≤10 mm, yet presenting with poor urinary flow of <15 mL/s. Only patients who were able to void spontaneously with a voided volume of ≥100 mL were eligible. Exclusion criteria included men with neurological diseases, previous surgery to the prostate/bladder neck or urethral instrumentation, prostate/bladder cancer, pelvic radiotherapy, or use of medications that interfere with voiding. All patients with PSA that were raised underwent transrectal-ultrasound guided biopsy to exclude malignancy. All patients with incomplete datasets were also excluded from this study.
All patients underwent urodynamic investigations according to recommendations by the International Continence Society (ICS). BOO was defined according to the Bladder Outlet Obstruction Index (BOOI), calculated as (Pdet Qmax) − 2 (Qmax). Patients were characterized as obstructed when BOOI was >40, and not obstructed when BOOI was ≤20 [7]. All values in between were considered equivocal, and considered as unobstructed in this study.
In addition, detrusor contractility was also calculated from urodynamic findings, based on the Bladder Contractility Index (BCI) described by Abrams [7]. This was derived from the formula (Pdet Qmax) + 5 (Qmax), such that a strong contractility was given by a BCI of >150, normal contractility a BCI of 100–150, and weak contractility a BCI of <100.
Statistical analysis was carried out with SPSS version 23 (IBM, Chicago, CA, USA). Numerical data were presented as mean values with SD and range. The Wilcoxon rank sum test was used for comparisons between variables, where a p-value of <0.05 was taken as significant. Pearson's correlation coefficients were used to assess the relationship between BOOI and the following variables: PV, PSA, IPP and PVRU.
This study received IRB approval, and informed consent was obtained from all patients prior to involvement in the study and before urodynamic studies were performed.
3. Results
A total of 36 patients with complete datasets had a small prostate of <30 mL, low-grade IPP of ≤10 mm, and poor urinary flow of <15 mL/s.
Table 1 shows the general characteristics of the patients included in this study. Results of urodynamic studies with causes for poor urinary flow are summarised in Table 2. Urodynamic abnormalities were found in 32 patients (88.9%), where nine patients (25.0%) were found to have BOO, while 27 patients had no obstruction. All patients with BOO had normal bladder contractility. Poor detrusor contractility was observed in 14 out of 36 patients (38.9%), and all of them did not have BOO.
Table 1.
General characteristics of patients with small prostates and low IPP (n = 36).
| Index | Mean | SD | Range |
|---|---|---|---|
| Age (year) | 66.0 | 8.3 | 54.0–85.0 |
| IPSS | 12.0 | 6.5 | 4.0–32.0 |
| QoL score | 3.0 | 1.1 | 2.0–6.0 |
| PV (mL) | 22.3 | 5.1 | 9.0–29.0 |
| IPP (mm) | 5.4 | 2.6 | 1.0–9.5 |
| Qmax (mL/s) | 12.0 | 2.4 | 6.0–14.9 |
| PVRU (mL) | 52.2 | 26.6 | 10.0–118.0 |
| PSA (ng/mL) | 1.3 | 1.5 | 0.3–7.8 |
IPSS, international prostate symptom score; IPP, intravesical prostatic protrusion; Qmax, maximum urinary flow rate; PVRU, post-void residual urine; PSA, prostate-specific antigen; PV, prostate volume; QoL score, quality of life score.
Table 2.
Distribution of patients according to causes for voiding disturbances (n (%)).
| No obstruction (BOOI < 20) | Equivocal obstruction (BOOI 21–40) | BOO (BOOI > 40) | Total | |
|---|---|---|---|---|
| Normal/good contractility | 4 | 9 | 9 | 22 (61.1) |
| Poor contractility | 9 | 5 | 0 | 14 (38.9) |
| Total | 13 (36.1) | 14 (38.9) | 9 (25.0) | 36 |
BOO, bladder outlet obstruction; BOOI, bladder outlet obstruction Index.
3.1. Characteristics of patients with and without BOO
Clinical parameters of patients with and without BOO were compared to determine associations with BOO in this group of patients; findings are summarised in Table 3. The mean age of patients with and without BOO were 69.0 ± 10.0 and 64.0 ± 7.0 years, respectively (p = 0.122). PV (p = 0.001), IPP (p < 0.001) and residual urine (p = 0.011) all showed a significant difference between patients who had BOO (BOOI > 40) and patients who did not (BOOI ≤ 40). There was no significant difference noted for IPSS, QoL, PSA and Qmax.
Table 3.
Comparison of variables in patients with or without urodynamic BOO.
| Patients without BOOa (BOOI ≤ 40) (n = 27) |
Patients with BOOa (BOOI > 40) (n = 9) |
p-Value | |
|---|---|---|---|
| Age | 64.0 ± 7.0 | 69.0 ± 10.0 | 0.122 |
| IPSS | 11.5 ± 6.5 | 13.0 ± 6.7 | 0.932 |
| QoL score | 3.0 ± 1.2 | 3.0 ± 1.0 | 0.511 |
| PV (mL) | 20.6 ± 5.0 | 27.1 ± 1.8 | 0.001b |
| IPP (mm) | 4.0 ± 2.1 | 8.5 ± 0.8 | <0.001b |
| Qmax (mL/s) | 12.2 ± 2.3 | 11.7 ± 2.7 | 0.801 |
| PVRU (mL) | 46.9 ± 22.8 | 60.0 ± 29.2 | 0.011b |
| PSA (ng/mL) | 1.0 ± 1.5 | 1.9 ± 1.2 | 0.065 |
IPSS, international prostate symptom score; IPP, intravesical prostatic protrusion; Qmax, maximum urinary flow rate; PVRU, post-void residual urine; PSA, prostate-specific antigen; PV, prostate volume; QoL score, quality of life score.
All values were shown as mean ± SD.
p < 0.05, Wilcoxon rank sum test.
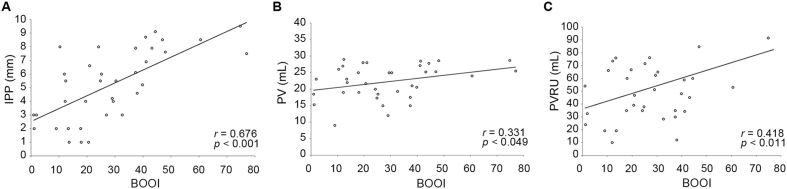
BOOI was positively correlated to IPP, PV and PVRU. The correlation coefficients ranged from 0.331 to 0.676 (Fig. 1), with IPP having the most significant correlation (r = 0.676).
Figure 1.
Scatter plot of relationship between bladder outlet obstruction index (BOOI) and intravesical prostatic protrusion (IPP) (A), prostate volume (PV) (B), and post-void residual urine (PVRU) (C), respectively.
3.2. Characteristics of patients with and without poor detrusor function
As 14 patients were found to have poor detrusor contractility, the characteristics of these patients were evaluated as well to determine which parameters may predict impairments in detrusor contractility. This is summarised in Table 4. Patients with poor detrusor contractility were found to have significantly smaller Qmax (p = 0.006) and IPP (p = 0.031).
Table 4.
Comparison of variables in patients with or without poor detrusor function.
| Patients with good detrusor contractilitya (n = 22) |
Patients with poor detrusor contractilitya (n = 14) |
p-Value | |
|---|---|---|---|
| Age | 66.0 ± 9.5 | 65.5 ± 6.3 | 0.879 |
| IPSS | 12.0 ± 5.8 | 12.0 ± 7.7 | 0.633 |
| QoL score | 3.0 ± 1.0 | 3.0 ± 1.2 | 0.416 |
| PV (mL) | 23.1 ± 5.1 | 22.5 ± 5.4 | 0.898 |
| IPP (mm) | 6.5 ± 2.8 | 4.0 ± 2.0 | 0.031b |
| Qmax (mL/s) | 12.7 ± 2.2 | 10.7 ± 2.2 | 0.006b |
| PVRU (mL) | 49.7 ± 27.7 | 59.5 ± 25.8 | 0.938 |
| PSA (ng/mL) | 1.4 ± 1.7 | 1.0 ± 0.8 | 0.175 |
IPSS, international prostate symptom score; IPP, intravesical prostatic protrusion; Qmax, maximum urinary flow rate; PVRU, post-void residual urine; PSA, prostate-specific antigen; PV, prostate volume; QoL score, quality of life score.
All values were shown as mean ± SD.
p < 0.05, Wilcoxon rank sum test.
3.3. Further evaluation of patients with BOO
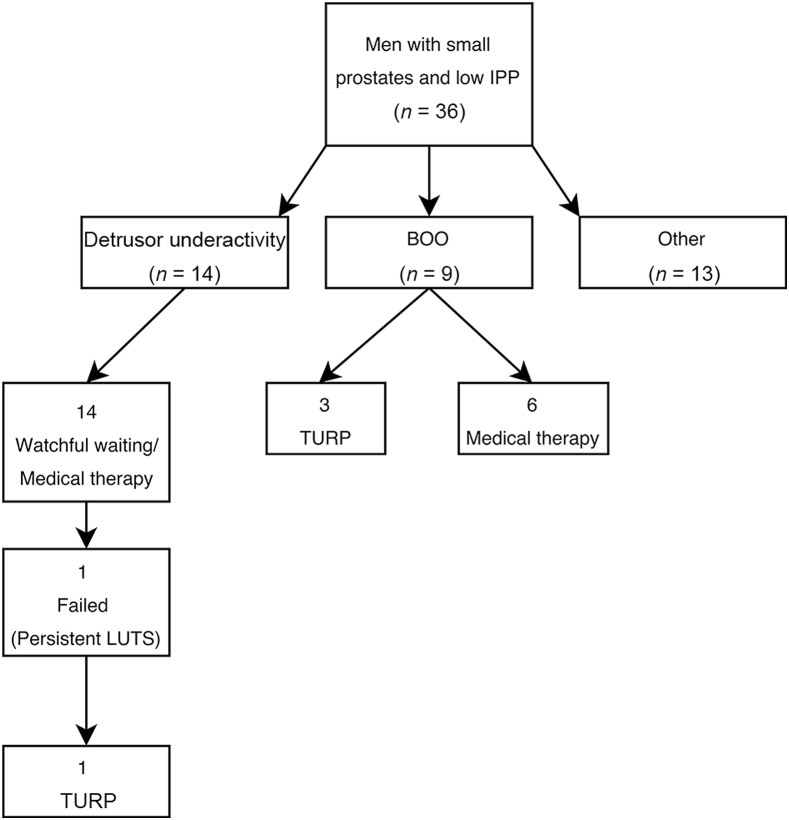
The patients with BOO were followed up for a period of 5 years, and this is summarised in Fig. 2. There were nine patients in total; all were started on α-blockers, and three patients (33.3%) eventually underwent surgery. In all patients, surgery was offered due to urodynamic diagnosis of BOO, and these patients were keen as symptoms severely affected QoL. With a mean follow-up time of 42.7 months, only one patient experienced acute retention of urine (ARU) 6 years later right before his death from a cerebrovascular accident. The six patients (66.7%) who were managed medically were maintained on α-blockers for the duration of 5 years with no complications, and were either subsequently discharged or continued follow-up without requiring surgery. Among the 14 patients with poor detrusor contractility, only one (7.7%) underwent surgery after 4.2 years due to progression of symptoms. Surgery was offered as a repeat urodynamic study done revealed a BOOI of 48, suggesting BOO.
Figure 2.
Outcome and management of patients with small prostates and low intravesical prostatic protrusion (IPP). BOO, bladder outlet obstruction; LUTS, lower urinary tract symptoms; PV, prostate volume; TURP, transurethral resection of the prostate.
For the three patients with BOO who underwent surgery, the mean time to surgery was 2.0 ± 1.7 months. Data collected at the end of 5-year follow-up as compared to that taken at the time of presentation is summarised in Table 5. There was no significant difference noted in terms of symptoms or uroflow findings, although a marginal benefit could be noted for IPSS, QoL, Qmax and PVRU after 1 year of follow-up.
Table 5.
Comparison of variables during presentation and follow-up in three patients with BOO who underwent TURP.
| At presentationa | 1-Year follow-upa | p-Value | 5-Year follow-upa | p-Value | |
|---|---|---|---|---|---|
| Age (year) | 79.0 ± 23.0 | 80.0 ± 23.0 | – | 85.0 ± 23.0 | – |
| IPSS | 7.0 ± 11.0 | 4.0 ± 6.0 | 0.208 | 7.0 ± 3.0 | 0.414 |
| QoL score | 3.0 ± 1.0 | 2.0 ± 2.0 | 0.184 | 3.0 ± 2.0 | 0.180 |
| PV (mL) | 27.8 ± 4.6 | 14.4 ± 5.9 | 0.088 | 16.0 ± 11.0 | 0.109 |
| IPP (mm) | 8.5 ± 2.0 | 3.0 ± 2.0 | 0.002b | 3.0 ± 1.0 | 0.109 |
| Qmax (mL/s) | 10.8 ± 2.7 | 12.5 ± 5.5 | 0.295 | 8.9 ± 9.1 | 0.593 |
| PVRU (mL) | 60.0 ± 46.0 | 34.0 ± 38.0 | 0.219 | 30.0 ± 40.0 | 0.109 |
IPSS, international prostate symptom score; IPP, intravesical prostatic protrusion; Qmax, maximum urinary flow rate; PVRU, post-void residual urine; PSA, prostate-specific antigen; PV, prostate volume; QoL score, quality of life score.
All values were shown as mean ± SD.
p < 0.05, Wilcoxon rank sum test.
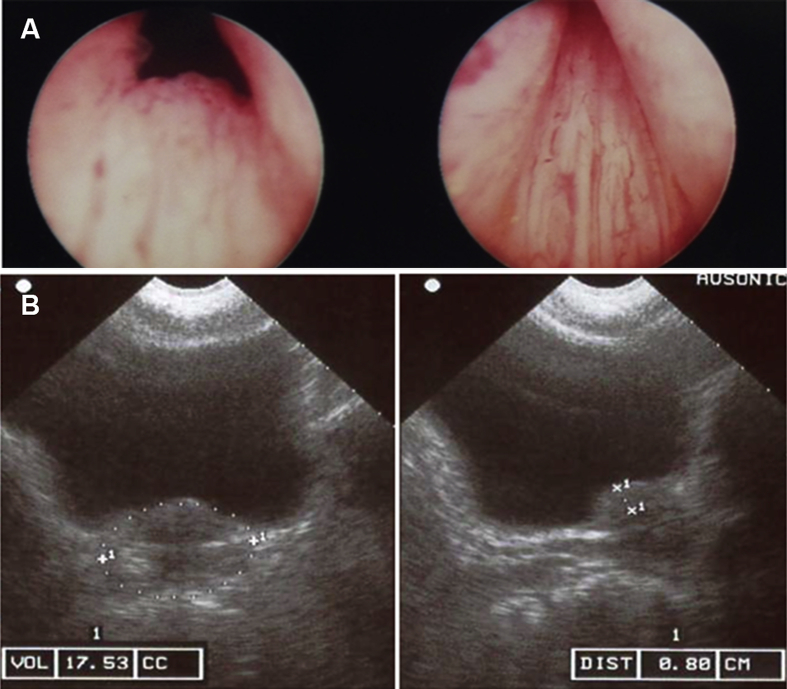
Photographs taken during resection were also examined and one example is shown in Fig. 3A. It was noted that all of the patients who underwent TURP demonstrated a “high bladder neck”, seen as an elevation of the bladder neck. All patients had enlarged lateral lobes as well. The transabdominal ultrasound image of the same patient was also shown in Fig. 3B, showing a prostate adenoma with a small PV and IPP at the edge of the bladder neck.
Figure 3.
Image taken during TURP demonstrating a high bladder neck (A) and transabdominal ultrasound showing a prostate adenoma with small PV and IPP (B). IPP, intravesical prostatic protrusion; PV, prostate volume; TURP, transurethral resection of the prostate.
4. Discussion
The relationship between prostate size and BOO has previously been investigated, but there are few studies evaluating BOO in patients with small prostates presenting with significant symptoms and poor flow, and none to our knowledge looking into patients with a low IPP. Determining the cause for symptoms in patients with small prostates is important as it ultimately affects management if symptoms were due to urodynamically-proven BOO, patients have been shown to respond well to surgery [10], [11]. On the other hand, patients with impaired detrusor function have been known to experience worse outcomes after prostatectomy [10], [11], [12]. Since IPP is increasingly being used as a surrogate to predict BOO especially within Asia, it becomes prudent to evaluate the population of patients that, despite a low IPP, are symptomatic and experience BOO on UDS.
In our series, prostate size and IPP were measured with trans-abdominal ultrasound, which is simple and non-invasive, and allows for evaluation of the bladder and PVRU at the same time. In order to eliminate inter-observer variability, all measurements were carried out by a single experienced operator (K.T. Foo). It is suggested that IPP may be affected by bladder volumes, with small volumes causing inaccurate estimation of IPP length, and larger volumes causing under-estimation of the IPP as it recedes behind the symphysis pubis, but it is our general practice to measure the IPP when the bladder is sufficiently distended between 100 and 200 mL, although this exact value is not reflected in the results above. A prostate size of <30 mL was selected for this study, as it provides a sufficient sample size for analysis and guidelines generally dictate use of 5 α-reductase inhibitors or surgery in prostates above 30 mL [13].
Detrusor contractility was evaluated with the BCI, a measure introduced by Abrams and derived from the 7 qualitative grades of Schafer's normogram [7]. Although the BCI is simple and its use has not been extensively validated, it has previously been applied by other studies to good effect [9], [14]. There is currently no gold standard for determining detrusor contractility, and the BCI was used in this case for its ease of administration in statistical analysis, although we are aware that it is highly dependent on Qmax [15], and artefacts during measurement of Qmax or straining may cause the BCI values to be overestimated. Another possible alternative is the use of the Watts Factor [16] which has a less pronounced reliance on Qmax, but its calculation is complex and requires additional parameters not commonly recorded in urodynamic studies.
In this study, we sought to characterise patients with small prostates/low IPP by determining the clinical parameters associated with BOO, in order to aid in clinical prediction of patients who may be obstructed. BOO has been shown to not correlate well with IPSS and QoL [10], [21] in multiple instances; this is re-confirmed again in this study, where symptoms were not associated with either BOO or poor detrusor contractility. IPSS and QoL scores reflect subjective experiences in men with LUTS or LUTS/BPH, but obstruction is objective; this suggests that although symptom relief is important in LUTS/BPH, patients should not be over-managed as this does not correlate with obstruction and its potential complications. Among the other parameters associated with BOO, PV, IPP and PVRU; IPP still had the greatest correlation to BOO, further reinforcing its use as a useful clinical adjunct for predicting the presence of obstruction even in the subgroup of patients with a low IPP.
Qmax did not have a significant association with BOO but was instead associated with the presence of poor detrusor contractility. This potentially suggests that in patients with small prostates/low IPP, an isolated decrease in Qmax without significant abnormalities in PV, IPP, PSA and PVRU may indicate that a patient's symptoms are due to poor detrusor function which is usually treated medically, as compared to outlet obstruction which may necessitate surgery. Our results however differ from previous studies [8], [9], where Qmax was seen to be associated with BOO instead. Nevertheless, Hirayama et al. [8] have found that even in men with a Qmax of less than 5 mL, 20% had impaired detrusor contractility, so the presence of a low Qmax should always be considered in association with other clinical parameters as discussed above.
In 5 years, it was found that only three patients (33.3%) with BOO underwent surgery, which was offered due to a urodynamic diagnosis of BOO. At the end of 5 years, the clinical variables recorded however showed no significant difference. The need for surgery among the population with poor detrusor contractility was also small with only one patient undergoing surgery for progression of symptoms, and decision for TURP was based on a repeat urodynamic study revealing a BOOI of 48. Currently, there has been no study following the progress of patients with small prostates/low IPP, and these results suggest that in this specific subgroup of patients, medical management is still the preferred choice.
A secondary objective of this study was to examine the cause for BOO in men with small prostates/low IPP. In our series, 25.0% of patients were noted to have urodynamic BOO, despite having a small PV and IPP. The causes of BOO in a male patient are variable, but conflicting etiologies were eliminated when the data were examined, including conditions originating from the prostate (infections and neoplasia) and the urethra (strictures, structural abnormalities). One possible mechanism was originally proposed by Turner–Warwick [17], who suggested that the mechanism behind BOO in small prostates originated from the bladder neck itself – termed “bladder neck dyssynergia”, where obstruction occurs due to ineffective bladder neck opening from an abnormal arrangement of detrusor musculature. However, this was noted to be present throughout a patient's lifetime, and patients were generally younger with a mean age ranging from 30 to 50 years [18], and was thus considered unlikely. It was also previously reported by Kaplan et al. [19] that PV at the transitional zone, determined by the Transitional Zone Index (TZI), was correlated to symptom score and flow rate. However, the series done by Lepor et al. [20] showed no significant difference between TZI and IPSS, and only a weak association with flow rate, while Hirayama et al. [8] showed that TZI was not associated with urodynamic BOO as well. Measurements of TZI were therefore not included in this study, as it requires the use of invasive trans-rectal ultrasonography as well. Nevertheless, it was fascinating to note that the patients in our study with BOO undergoing surgery had a “high bladder neck” when viewed from the verumontanum of the bladder through the resectoscope. When the bladder neck was incised, we found prostatic adenoma tissue below the raised bladder mucosa, and this was correlated to ultrasound findings of a prostatic adenoma with small PV and IPP in the same location. This “subcervical adenoma” distorting the bladder neck upwards could therefore be a possible cause for BOO despite a small PV and low IPP, and could be a possible area to explore in future studies.
Although this study was prospective, it is not without drawbacks – the sample size, especially in a sub-population of patients with small prostates and low IPP lengths, is small. This may affect the statistical significance of the findings observed, and the number of patients who underwent surgery was also small, limited our ability to truly characterise them. Nevertheless, we feel that this study is interesting, and larger scale studies can certainly be carried out using data obtained from this study as a means to design a more precise methodology.
5. Conclusion
In men with small prostates and low IPP, the presence of BOO on urodynamic study is significantly associated with a higher PV, PVRU and IPP, while poor detrusor contractility is significantly associated with a smaller Qmax and IPP. In general, most patients responded well to medical management, but for patients undergoing surgery, BOO can possibly be explained by elevation of the bladder neck by a small subcervical adenoma.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
We would like to thank the National Medical Council, the Goh Foundation and the Lee Foundation for their support in our BPH research, and Dr Hong Hong Huang and Ms Mei Ying Ng for their statistical and editorial support.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Eckhardt M., van Venrooji G., Boon T. Symptoms, prostate volume, and urodynamic findings in elderly male volunteers without and with LUTS and in patients with LUTS suggestive of benign prostatic hyperplasia. Urology. 2001;58:966–971. doi: 10.1016/s0090-4295(01)01413-3. [DOI] [PubMed] [Google Scholar]
- 2.Madersbacher S., Klingler H.C., Djavan B., Stulnig T., Schatzl G., Schmidbauer C.P. Is obstruction predictable by clinical evaluation in patients with lower urinary tract symptoms? Br J Urol. 1997;80:72–77. doi: 10.1046/j.1464-410x.1997.00220.x. [DOI] [PubMed] [Google Scholar]
- 3.Chancellor M.B., Rivas D.A. American Urological Association Symptom Index for women with voiding symptoms: lack of index specificity for benign prostate hyperplasia. J Urol. 1993;150:1706–1709. doi: 10.1016/s0022-5347(17)35872-x. [DOI] [PubMed] [Google Scholar]
- 4.Foo K.T., Lim K.B., Ho H., Fook S. Co-relationship between the size of the prostate, the intravesical prostatic protrusion and benign prostatic obstruction. Eur Urol Suppl. 2005;4:71. [Google Scholar]
- 5.Chia S.J., Heng C.T., Chan S.P., Foo K.T. Correlation of intravesical prostatic protrusion with bladder outlet obstruction. BJU Int. 2003;91:371–374. doi: 10.1046/j.1464-410x.2003.04088.x. [DOI] [PubMed] [Google Scholar]
- 6.Kuo T.L.C., Teo J.S.M., Foo K.T. The role of intravesical prostatic protrusion (IPP) in the evaluation and treatment of bladder outlet obstruction (BOO) Neurourol Urodyn. 2016;35:535–537. doi: 10.1002/nau.22741. [DOI] [PubMed] [Google Scholar]
- 7.Abrams P. Bladder outlet obstruction index, bladder contractility index and bladder voiding efficiency: three simple indices to define bladder voiding function. BJU Int. 1999;84:14–15. doi: 10.1046/j.1464-410x.1999.00121.x. [DOI] [PubMed] [Google Scholar]
- 8.Hirayama A., Samma S., Fujimoto K., Yamaguchi A., Akiyama T., Fukui Y. Comparison of parameters to determine the cause of urinary disturbance in men with prostate volume less than 20 milliliters. Int J Urol. 2002;9:554–559. doi: 10.1046/j.1442-2042.2002.00524.x. [DOI] [PubMed] [Google Scholar]
- 9.Gomes C.M., Nunes R.V., Araújo R.M., Sacomani C.R., Trigo-Rocha F.E., Bruschini H. Urodynamic evaluation of patients with lower urinary symptoms and small prostate volume. Urol Int. 2008;81:129–134. doi: 10.1159/000144049. [DOI] [PubMed] [Google Scholar]
- 10.Abrams P.H., Farrar D.J., Turner W.R., Whiteside C.G., Feneley R.C. The results of prostatectomy: a symptomatic and urodynamic analysis of 152 patients. J Urol. 1979;121:640–642. doi: 10.1016/s0022-5347(17)56918-9. [DOI] [PubMed] [Google Scholar]
- 11.Seki N., Takei M., Yamaguchi A., Naito S. Analysis of prognostic factors regarding the outcome after a transurethral resection for symptomatic benign prostatic enlargement. Neurourol Urodyn. 2006;25:428–432. doi: 10.1002/nau.20262. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues P., Lucon A.M., Freire G.C., Arap S. Urodynamic pressure flow studies can predict the clinical outcome after transurethral prostatic resection. J Urol. 2001;165:499–502. doi: 10.1097/00005392-200102000-00033. [DOI] [PubMed] [Google Scholar]
- 13.Gravas S., Bach T., Bachmann A., Drake M., Gacci M., Gratzke C. 2015. Guidelines on the management of non-neurogenic male lower urinary tract symptoms (LUTS), incl. benign prostatic obstruction (BPO); European Association of Urology.https://uroweb.org/wp-content/uploads/EAU-Guidelines-Non-Neurogenic-Male-LUTS-Guidelines-2015-v2.pdf Available at: [Accessed 28 June 2016] [DOI] [PubMed] [Google Scholar]
- 14.Groen J., Bosch J.L. Bladder contraction strength parameters poorly predict the necessity of long-term catheterization after a pubovaginal rectus fascial sling procedure. J Urol. 2004;172:1006–1009. doi: 10.1097/01.ju.0000135339.90689.e8. [DOI] [PubMed] [Google Scholar]
- 15.Oelke M., Rademakers K.L.J., van Koeveringe G.A., on behalf of the FORCE Research Group, on behalf of the FORCE Research Group, Maastricht & Hannover Unravelling detrusor underactivity: development of a bladder outlet resistance—bladder contractility nomogram for adult male patients with lower urinary tract symptoms. Neurourol Urodyn. 2016;35:980–986. doi: 10.1002/nau.22841. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths D., van Mastrigt R., Bosch R. Quantification of urethral resistance and bladder function during voiding, with special reference to the effects of prostate size reduction on urethral obstruction due to benign prostatic hyperplasia. Neurourol Urodyn. 1989;8:17–27. [Google Scholar]
- 17.Turner-Warwick R. Clinical problems associated with urodynamic abnormalities. In: Lutzeyer W., Melchior H., editors. Urodynamics. Springer Verlag; Berlin: 1970. pp. 237–263. [Google Scholar]
- 18.Nitti V.W. Primary bladder neck obstruction in men and women. Rev Urol. 2005;7(Suppl. 8):S12–S17. [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan S.A., Te A.E., Pressler L.B., Olsson C.A. Transition zone index as a method of assessing benign prostatic hyperplasia: correlation with symptoms, urine flow and detrusor pressure. J Urol. 1995;154:1764–1769. [PubMed] [Google Scholar]
- 20.Lepor H., Nieder A., Feser J., O'Connell C., Dixon C. Total prostate and transition zone volumes, and transition zone index are poorly correlated with objective measures of clinical benign prostatic hyperplasia. J Urol. 1997;158:85–88. doi: 10.1097/00005392-199707000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Chancellor M.B., Rivas D.A., Keeley F.X., Lotfi M.A., Gomella L.G. Similarity of the American Urological Association Symptom Index among men with benign prostate hyperplasia (BPH), urethral obstruction not due to BPH and detrusor hyperreflexia without outlet obstruction. Br J Urol. 1994;74:200–203. doi: 10.1111/j.1464-410x.1994.tb16586.x. [DOI] [PubMed] [Google Scholar]