Abstract
The purpose of this study was to evaluate the accuracy of diffusion-weighted imaging (DWI) for predicting locoregional failure of chemoradiotherapy in patients with head and neck squamous cell carcinoma (HNSCC). A comprehensive search was conducted through the EMBASE, PubMed and Cochrane Library databases for relevant publications. Stata software was used to calculate the pooled sensitivity, specificity, likelihood ratios and diagnostic odds ratios, and to construct a summary receiver operating characteristics (sROC) curve for DWI. A total of 9 studies comprising 421 patients were included. The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio and diagnostic odds ratio were 0.82 [95% confidence interval (CI): 0.72–0.88], 0.70 (95% CI: 0.62–0.77), 2.7 (95% CI: 2.1–3.6), 0.26 (95% CI: 0.17–0.41), and 10.48 (95% CI: 5.35–20.53), respectively. The area under the sROC curve was 0.84 (95% CI: 0.81–0.87). Therefore, DWI appears to be a promising imaging modality for predicting local failure of chemoradiotherapy in patients with HNSCC.
Keywords: head and neck squamous cell carcinoma, chemoradiotherapy, diffusion-weighted imaging, locoregional failure
Introduction
Head and neck squamous cell carcinoma (HNSCC) accounts for ~3% of all malignancies (1). Currently, chemoradiotherapy (CRT) is considered as a good approach to the treatment of locally advanced HNSCC. However, ~25–30% of patients have local residual disease or develop relapse at the primary or lymph node sites after treatment (2), which represents a challenge in the management of HNSCC. Therefore, there is a need for biomarkers that can provide accurate and timely prediction of therapeutic outcome prior to treatment or in the early stages of treatment, with discontinuation of ineffective approaches and timely application of alternative therapeutic strategies.
Recently, diffusion-weighted imaging (DWI) was proposed as an imaging biomarker for predicting treatment outcome in multiple malignancies, including rectal cancer (3), breast cancer (4) and glioblastoma (5). DWI quickly measures the Brownian motion of extracellular water molecules in biological tissues, which may be quantified with the apparent diffusion coefficient (ADC) (6). Previous studies demonstrated that higher pretreatment ADCs were correlated with poor response to treatment (7,8). In addition, due to its ability to depict the range of ADCs, ADC histograms may be used to evaluate the heterogeneity of the whole tumor. This feature enables evaluation of the degree of necrosis and viability, which may be crucial when planning a radiation dose boost (9).
Over the past few years, a number of studies have investigated the role of DWI in predicting response to CRT in patients with HNSCC. Given the varied characteristics of the patients and studies, individual studies are unable to provide a reliable estimate of DWI performance for the prediction of treatment response. Hence, the present meta-analysis was performed to determine the diagnostic performance of DWI for the prediction of locoregional failure of CRT in patients with HNSCC, which may be helpful in optimizing the management of this disease.
Data collection methods
Search strategy
A comprehensive search was conducted through the EMBASE, PubMed and Cochrane Library databases to identify relevant publications on the accuracy of DWI in the prediction of response to CRT in patients with HNSCC. The EMTREE terms (for EMBASE), medical subject heading terms (for Medline), and text words (for other databases) included (‘head and neck cancer’ OR ‘head and neck neoplasms’ OR ‘lip cancer’ OR ‘lip neoplasms’ OR ‘oropharynx cancer’ OR ‘oropharyngeal neoplasms’ OR ‘hypopharyngeal cancer’ OR ‘hypopharyngeal neoplasms’ OR ‘nasopharynx cancer’ OR ‘nasopharyngeal neoplasms’ OR ‘laryngeal cancer’ OR ‘laryngeal neoplasms’ OR ‘salivary gland cancer’ OR ‘salivary gland neoplasms’) AND (‘diffusion weighted magnetic resonance imaging’ OR ‘diffusion weighted MRI’ OR ‘diffusion MRI’ OR ‘DWI’) AND (‘concurrent chemoradiotherapy’ OR ‘synchronous chemoradiotherapy’ OR ‘concomitant chemoradiotherapy’ OR ‘chemoradiotherapy’ OR ‘radiotherapy’). The search was updated on March 21, 2017. To extend the search, the reference lists of the articles that were identified after the selection process were screened for additional suitable articles.
Study selection
Studies were considered eligible for inclusion if the accuracy of DWI in the prediction of response to CRT was investigated in patients with HNSCC. The eligible studies were also required to have a defined reference standard for local failure, which included residual disease and local recurrence. Accordingly, local failure was required to be histopathologically confirmed, or at least clinically suspected, in terms of the presence of any mass or a persisting and/or increasing existing residual mass on serial imaging during follow-up. In addition, the studies had to include at least 25 patients and yield sufficient information, including true and false positive and negative values, in order to construct a 2×2 contingency table to calculate sensitivity and specificity in the prediction of local failure. Moreover, only articles published in English were included. Review articles, comments, letters, case reports and animal experiments were excluded. When overlapping data from the same authors were presented among different articles, the article with the most recent details or the largest number of patients was selected.
Data extraction and quality assessment
Two reviewers (Q.M.Z. and Y.D.) independently extracted the relevant data and assessed the quality of the retrieved studies. To resolve discrepancies between the two reviewers, a consensus meeting was held; if agreement could not be reached, a third reviewer (F.F.Z.) was consulted. The extracted data were as follows: Patient characteristics (sample size, mean or median age and range), study characteristics [first author, publication year, country of origin, study design (prospective, retrospective or unknown), patient enrollment (consecutive or not), whether blinding was used in the study, number of patients, tumor location, TNM stage, number of reviewers, radiotherapy regimen and chemotherapy regimen], and technical details of magnetic resonance imaging (MRI) protocols [magnetic field strength, repetition time/echo time (TR/TE), MRI vendor, slice thickness, field of view (FOV), matrix and b value]. The Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) was used to assess the methodological quality of the included studies. QUADAS-2 has four key domains: Patient selection, index test, reference standard, and flow and timing.
Statistical analysis
A meta-analysis was performed using Stata software, version 13.0 (Stata Corp., College Station, TX, USA). Data were combined to obtain pooled sensitivity, specificity, diagnostic odds ratios (DORs) and likelihood ratios (LRs). A summary receiver operating characteristics (sROC) curve was constructed and the area under the curve (AUC) obtained. An inconsistency index (I2) test was performed to assess the heterogeneity between studies. An I2 value >50% indicated heterogeneity, in which case a random-effects model was used; otherwise, a fixed-effects model was applied. As an important source of heterogeneity, the threshold effect was assessed by calculating the Spearman's correlation coefficient. If there was no threshold effect, a meta-regression analysis was then performed to investigate other potential sources of heterogeneity. Publication bias was investigated by constructing a Deeks' funnel plot. P-values of <0.05 were considered to indicate statistically significant differences.
Results
Literature search and study selection
Our database search and extensive reference list cross-check yielded 365 studies. After excluding duplicates and reviewing the titles and abstracts, 65 studies remained, and their full texts were retrieved. After the full texts were reviewed, 56 studies were excluded for the following reasons: Patient number <25 (n=19); the same data were presented in another article by the same group (n=5); the studies were reviews, letters, or proceedings of a symposium (n=19); the articles were not written in English (n=9); and sufficient data were not extractable to construct a 2×2 contingency table to calculate sensitivity and specificity in the prediction of local failure (n=4). Ultimately, a total of 9 eligible studies involving 421 patients were included in the meta-analysis (7,8,10–16). A search of the reference lists of these eligible articles did not yield other potentially relevant articles.
Study description
The detailed characteristics of the 9 included studies are summarized in Tables I and II. The median number of patients per study was 37 (range, 26–134). Of the 9 studies, 6 enrolled patients prospectively and 3 retrospectively. A total of 4 studies enrolled patients consecutively, whereas the remaining 5 studies enrolled patients in an unknown manner. In 4 studies, treatment response was assessed by reviewers who had been blinded to the results of the DWI analysis; in the other 5 studies, this detail was not provided. In 8 studies, DWI examinations were conducted with 1.5 T devices, whereas in 1 study, both 3.0 T and 1.5 T devices were used.
Table I.
Patient and study characteristics.
| Authors | Year | Country | Study design | Patient enrollment | Blinding status | No. of patients | Mean/median age, years (range) | Tumor location | TNM stage(s) | Reviewers (experience) | Radiotherapy regimen | Chemotherapy regimen | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xiao-ping et al | 2016 | China | Prospective | Consecutive | Blind | 50 | 48.9±11.1 | Nasopharynx | T2-4N0-3M0-1 | Two radiologists (5 and 20 years) | 7,000–7,600 cGy/30-33 f | TN, TNF, NF | (10) |
| Scalco et al | 2016 | Italy | Retrospective | NA | NA | 30 | NA | HNSCCa | T1-4N1-3 | One radiologist (15 years) | 7,000 cGy/33 f | Cisplatin | (11) |
| Hou et al | 2016 | China | Prospective | Consecutive | Blind | 43 | 49.0 (26–68) | Nasopharynx | T2-4N0-3M0 | Two radiologists (>10 years) | 7,000–7,600 cGy/30-33 f | TN | (12) |
| Hong et al | 2013 | China | Prospective | NA | NA | 134 | 47.0 (18–79) | Nasopharynx | T1-4 | NA | 6,600–7,875f cGy/30-33 | TP, cisplatin | (14) |
| King et al | 2013 | China | Prospective | Consecutive | NA | 37 | 57.0 (45–71) | HNSCCb | III–IV | One radiologist (>15 years) | NA | NA | (13) |
| Nakajo et al | 2012 | Japan | Retrospective | NA | NA | 26 | 65.0 (45–89) | HNSCCc | T1-4N0-3 | NA | 6,000 cGy/30 f | NA | (15) |
| Hatakenaka et al | 2011 | Japan | Retrospective | NA | Blind | 38 | 64.0 (37–85) | HNSCCd | T1-4N0-3 | Two radiologists (unknown) | 6,000 cGy | S-1, cisplatin | (8) |
| Vandecaveye et al | 2010 | Belgium | Prospective | Consecutive | Blind | 30 | 53.0 (38–66) | HNSCCe | T1-4N1-3 | One radiologist (6 years) | 7,200 cGy | NA | (16) |
| Kim et al | 2009 | USA | Prospective | NA | NA | 33 | 61.0±10.8 | HNSCCf | T0-4N1-2bM0 | NA | 7,040 cGy/32 f | Cisplatin | (7) |
Oropharynx, nasopharynx, hypopharynx, larynx, and unknown primary.
Oropharynx, hypopharynx, larynx, nasal cavity, oral cavity, and maxillary sinus.
Oropharynx, nasopharynx, hypopharynx, larynx, oral cavity and maxillary sinus.
Oropharynx, hypopharynx, larynx and oral cavity.
Oropharynx, tonsil, base of tongue, supraglottic, glottic and piriform sinus.
Larynx, vallecula, tonsil, base of tongue and unknown primary. NA, not available; HNSCC, head and neck squamous cell carcinoma; TN, Taxol + nedaplatin; TNF, Taxol + nedaplatin + 5-fluorouracil; NF, nedaplatin + 5-fluorouracil; TP, Taxol + cisplatin.
Table II.
Technical details of MRI protocols.
| Authors | Magnet strength (T) | TR/TE (msec) | MRI vendor | Slice thickness (mm) | FOV (mm) | Matrix | b value (sec/mm2) | (Refs.) |
|---|---|---|---|---|---|---|---|---|
| Xiao-ping et al | 1.5 | 4,225/106 | GE | 5 | Unknown | 128×130 | 0, 200, 400, 600, 800, 1,000 | (10) |
| Scalco et al | 1.5 | 4,500/77 | GE | 4 | 260–280 | 128×128 | 0, 500, 800 | (11) |
| Hou et al | 1.5 | 4,225/106 | GE | 5 | 220 | 128×130 | 0, 50, 80, 100, 150, 200, 400, 600, 800, 1,000 | (12) |
| Hong et al | 1.5 | Unknown | GE | Unknown | 240×240 | 128×128 | 0, 800 | (14) |
| King et al | 1.5 | 2,000/75 | Philips | 4 | 230 | 112×112 | 0, 100, 200, 300, 400, 500 | (13) |
| Nakajo et al | 1.5 | 6,000/68 | Siemens | 6 | 370 | 112×168 | 0, 800 | (15) |
| Hatakenaka et al | 1.5 | 3,000/73 | Philips | 3–5 | 200–230 | 112×79 | 0, 300, 1,000 | (8) |
| Vandecaveye et al | 1.5 | 7,100/84 | Siemens | 4 | 200×250 | 104×128 | 0, 50, 100, 500, 750, 1,000 | (16) |
| Kim et al | 1.5 or 3.0a | 4,000/89 | Siemens | 5 | 260 | NA | 0, 500, 1,000 | (7) |
1.5 T (n= 24), 3.0 T (n=9); NA, not available; MRI, magnetic resonance imaging; TE, echo time; TR, repetition time.
Assessment of study quality
The QUADAS-2 scores of each study are listed in Table III. The quality of the 9 studies varied. A total of 4 studies had a low risk of bias regarding patient selection, whereas 5 studies had an unclear risk of bias due to insufficient information. All 9 studies reported a low risk of bias regarding the index test. The risk of bias regarding the reference standard was low in 4 studies and unclear in 5 studies, as the latter studies provided insufficient information on blinding. The risk of bias regarding flow and timing was low in 4 studies and high in 5 studies, as different reference standards were applied during follow-up in the latter studies. As regards risk in applicability, all 9 studies had a low risk of bias.
Table III.
Quality assessment of included studies: Summarized risk of bias and applicability concerns.
| Risk of bias | Applicability | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | (Refs.) | ||
| Xiao-ping et al | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | (10) | ||
| Scalco et al | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | (11) | ||
| Hou et al | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | (12) | ||
| Hong et al | Unclear risk | Low risk | Unclear risk | High risk | Low risk | Low risk | Low risk | (14) | ||
| King et al | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | (13) | ||
| Nakajo et al | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | (15) | ||
| Hatakenaka et al | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | (8) | ||
| Vandecaveye et al | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | (16) | ||
| Kim et al | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | (7) | ||
Meta-analysis
The sensitivity and specificity of DWI in the prediction of locoregional failure of CRT in HNSCC patients ranged from 0.69 to 0.95 and from 0.55 to 0.89, respectively, with pooled estimates of 0.82 [95% confidence interval (CI): 0.72–0.88] and 0.70 (95% CI: 0.62–0.77), respectively. The forest plots of sensitivities and specificities are shown in Fig. 1. Using the sROC curve, the AUC was 0.84 (95% CI: 0.81–0.87; Fig. 2). The positive LR, negative LR and the DOR of DWI were 2.7 (95% CI: 2.1–3.6), 0.26 (95% CI: 0.17–0.41) and 10.48 (95% CI: 5.35–20.53), respectively. Among all 9 studies, no significant heterogeneity was found in terms of pooled sensitivity (P=0.42, I2=1.4%), specificity (P=0.05, I2=48.06%), or DOR (P=0.26, I2=20.6%). The Deeks' funnel plot asymmetry test revealed no strong evidence for publication bias (P=0.10; Fig. 3).
Figure 1.
Forest plots of sensitivity and specificity with corresponding 95% confidence intervals for diffusion-weighted imaging to predict locoregional failure of chemoradiotherapy in patients with head and neck squamous cell carcinoma. CI, confidence interval; df, degree of freedom.
Figure 2.
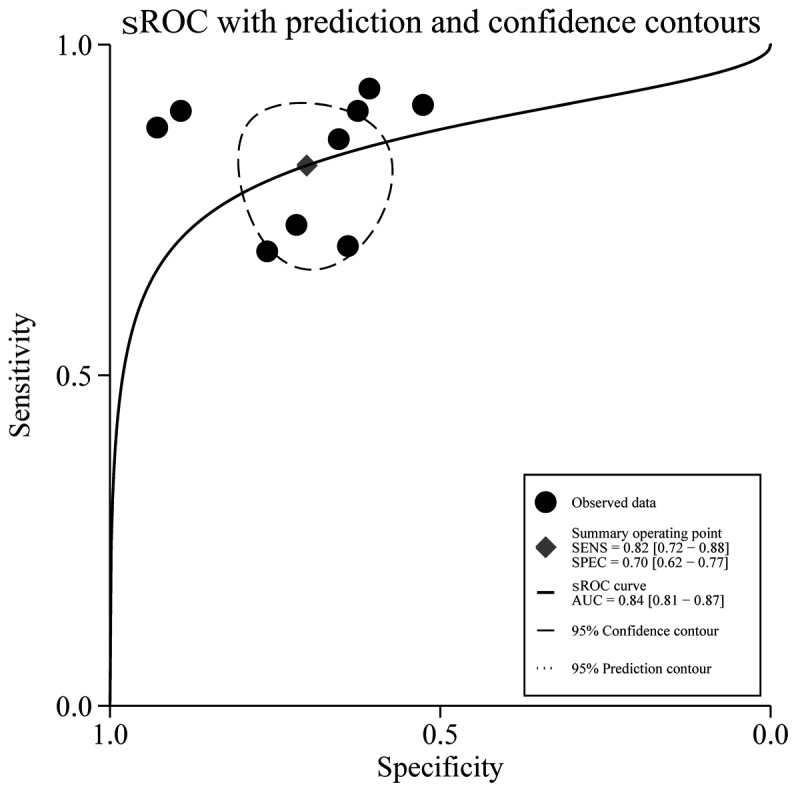
Summary receiver operating characteristic (sROC) curve for all 9 studies. The area under the ROC curve (AUC) of the apparent diffusion coefficient (ADC) was 0.84 (95% confidence interval: 0.81–0.87), indicating good accuracy in predicting locoregional failure of chemoradiotherapy in patients with head and neck squamous cell carcinoma. SENS, sensitivity; SPEC, specificity.
Figure 3.
Results of Deeks' funnel plot from the asymmetry test for publication bias. The non-significant slope indicates that no significant bias was observed. ESS, effective sample size.
Discussion
The present meta-analysis investigated the feasibility of ADC as an imaging biomarker for the assessment of locoregional failure of CRT in HNSCC patients. Accurate prediction or monitoring of therapeutic efficacy before or during the early stages of treatment is crucial for individual patients to modify the ongoing treatment regimen in a timely manner. In addition, patients with radioresistant tumors may be offered an escalated radiation dose or alternative treatment options, such as early surgical intervention.
To the best of our knowledge, this is the first meta-analysis to determine the ability of DWI to predict local failure of CRT in HNSCC patients. A pooled sensitivity of 0.82 (95% CI: 0.72–0.88) and a specificity of 0.70 (95% CI: 0.62–0.77) were calculated from a total of 421 patients in 9 studies who fulfilled all the inclusion and exclusion criteria. Furthermore, according to a widely accepted interpretation (17), a good predictive accuracy (AUC=0.84) was observed between local failure and locally controlled lesions.
A number of studies have investigated the role of DWI in the prediction of response to therapeutic interventions. A preclinical study by Hamstra et al (18) reported that a significant increase in ADC was noted in an animal model of HNSCC 5 days after initiation of CRT, which may be used as a marker to predict treatment response. Kim et al (7) also reported that patients with complete response to CRT had significantly lower pretreatment ADC values than did patients with partial response. In addition, the changes in ADC between baseline and early intratreatment measurement may also demonstrate higher test accuracy in separating favorable from unfavorable responders. Contrary to Kim et al, King et al (19) reported that ADC values were more effective after treatment, compared with before or during treatment, in predicting response to CRT in HNSCC patients. All these studies indicated that ADC may be used as an important imaging biomarker for personalized treatment in HNSCC.
From a clinical perspective, optimized clinical benefit may be achieved by adopting the most appropriate treatment strategies or timely modification of a treatment plan on the basis of an individual patient's response assessment. Therefore, ADC values have been proposed to be of higher value as biomarkers before treatment or in early intratreatment than they are in a post-treatment setting. In our meta-analysis, only studies using either pretreatment ADC (n=6) (7,8,10–12,15) or percentage change in ADC values between baseline and early intratreatment measurement (n=3) (13,14,16) were included to predict locoregional failure of CRT in HNSCC patients, with the optimum cutoff (threshold) values for ADC ranging from 0.86 to 1.11 for pretreatment and from 14 to 52.7% for percentage change in ADC. According to the histopathological analysis (20), high numbers of tumor-stromal cells, which contribute to reducing the restriction of water molecule motion in tumors, are significantly associated with high pretreatment ADC values. Stromal cells play a crucial role in supporting the growth of tumors by promoting tumor cell invasion, protecting apoptosis, and then creating a barrier to systemic therapy. This may partly explain the biological mechanisms underlying the association between high pretreatment ADC values and poor treatment response to CRT in HNSCC patients.
Currently, in addition to DWI, multiple imaging modalities, including dynamic contrast-enhanced MRI (21), phosphorous MR spectroscopy (22), and fluorine-18-fluorodeoxyglucose positron emission tomography with computed tomography (23) have been used to predict and monitor treatment efficacy in HNSCC patients. The notable advantages of DWI over these other imaging modalities are its simplicity and the fact that the use of contrast agents is not required. Injection of contrast agents may increase the cost and their use may not be indicated in patients who have severe renal function impairment. Therefore, it is more advantageous to use DWI to predict and monitor the treatment response.
Several limitations should be acknowledged in this meta-analysis. First, the number of included studies and samples was moderate. This limitation may lead to an overestimation of predictive accuracy (24). Second, the reference standards regarding locoregional failure of CRT included histopathological findings or clinical and imaging results during the follow-up period, which may affect the predictive value of DWI. Third, although the heterogeneity among the included studies was not significant, it was present. Human papillomavirus (HPV) status was not detailed in all the included studies. HPV status is the primary etiological determinant of chemoradiotherapy response, demonstrating a 25% improvement in 3-year overall survival in large-scale cooperative group datasets (25). While not exclusively HPV-associated, domestically, the vast majority of oropharyngeal cancers are HPV-related (26). However, at present, HPV status does not define a differential in terms of standard of care treatment, which remains CRT for the majority of head and neck cancers. Consequently, pooled analysis is appropriate until results from cooperative group studies (such as NRG HN002) (27) are mature enough to define whether HPV status-based de-intensification is efficacious. Finally, factors associated with the scanning protocol and analysis method differed among the studies. To further establish DWI as a routine clinical assessment tool, DWI scanning methods and analysis protocols should be validated or standardized. Recently, the Radiological Society of North America Quantitative Imaging Biomarker Alliance have developed a standardized ADC measurement phantom (28). The use of this device to standardize ADC measurements across facilities/devices/sequences is imperative for future DWI implementation.
In conclusion, our meta-analysis suggests that DWI is a promising imaging modality for predicting local failure of CRT in HNSCC patients. However, considering the limitations of the present study, the results must be interpreted with caution. Larger-scale, prospective, randomized-controlled studies are required to confirm the clinical value of DWI in predicting treatment outcome in HNSCC.
Acknowledgements
The authors would like to thank Mrs. Tamara K. Locke at the Department of Scientific Publications of MD Anderson Cancer Center for providing editorial support in the preparation of this manuscript.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.King AD, Thoeny HC. Functional MRI for the prediction of treatment response in head and neck squamous cell carcinoma: Potential and limitations. Cancer Imaging. 2016;16:23. doi: 10.1186/s40644-016-0080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu LM, Zhu J, Hu J, Yin Y, Gu HY, Hua J, Chen J, Xu JR. Is there a benefit in using magnetic resonance imaging in the prediction of preoperative neoadjuvant therapy response in locally advanced rectal cancer? Int J Colorectal Dis. 2013;28:1225–1238. doi: 10.1007/s00384-013-1676-y. [DOI] [PubMed] [Google Scholar]
- 4.Weis JA, Miga MI, Arlinghaus LR, Li X, Abramson V, Chakravarthy AB, Pendyala P, Yankeelov TE. Predicting the response of breast cancer to neoadjuvant therapy using a mechanically coupled reaction-diffusion model. Cancer Res. 2015;75:4697–4707. doi: 10.1158/0008-5472.CAN-14-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoo RE, Choi SH, Kim TM, Lee SH, Park CK, Park SH, Kim IH, Yun TJ, Kim JH, Sohn CH. Independent poor prognostic factors for true progression after radiation therapy and concomitant temozolomide in patients with glioblastoma: Subependymal enhancement and low ADC value. AJNR Am J Neuroradiol. 2015;36:1846–1852. doi: 10.3174/ajnr.A4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia. 2009;11:102–125. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, Loevner L, Quon H, Sherman E, Weinstein G, Kilger A, Poptani H. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res. 2009;15:986–994. doi: 10.1158/1078-0432.CCR-08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatakenaka M, Nakamura K, Yabuuchi H, Shioyama Y, Matsuo Y, Ohnishi K, Sunami S, Kamitani T, Setoguchi T, Yoshiura T, et al. Pretreatment apparent diffusion coefficient of the primary lesion correlates with local failure in head-and-neck cancer treated with chemoradiotherapy or radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81:339–345. doi: 10.1016/j.ijrobp.2010.05.051. [DOI] [PubMed] [Google Scholar]
- 9.Matoba M, Tuji H, Shimode Y, Toyoda I, Kuginuki Y, Miwa K, Tonami H. Fractional change in apparent diffusion coefficient as an imaging biomarker for predicting treatment response in head and neck cancer treated with chemoradiotherapy. AJNR Am J Neuroradiol. 2014;35:379–385. doi: 10.3174/ajnr.A3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao-ping Y, Jing H, Fei-ping L, Yin H, Qiang L, Lanlan W, Wei W. Intravoxel incoherent motion MRI for predicting early response to induction chemotherapy and chemoradiotherapy in patients with nasopharyngeal carcinoma. J Magn Reson Imaging. 2016;43:1179–1190. doi: 10.1002/jmri.25075. [DOI] [PubMed] [Google Scholar]
- 11.Scalco E, Marzi S, Sanguineti G, Vidiri A, Rizzo G. Characterization of cervical lymph-nodes using a multi-parametric and multi-modal approach for an early prediction of tumor response to chemo-radiotherapy. Phys Med. 2016;32:1672–1680. doi: 10.1016/j.ejmp.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Hou J, Yu X, Hu Y, Li F, Xiang W, Wang L, Wang H, Lu Q, Zhang Z, Zeng W. Value of intravoxel incoherent motion and dynamic contrast-enhanced MRI for predicting the early and short-term responses to chemoradiotherapy in nasopharyngeal carcinoma. Medicine (Baltimore) 2016;95:e4320. doi: 10.1097/MD.0000000000004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King AD, Chow KK, Yu KH, Mo FK, Yeung DK, Yuan J, Bhatia KS, Vlantis AC, Ahuja AT. Head and neck squamous cell carcinoma: Diagnostic performance of diffusion-weighted MR imaging for the prediction of treatment response. Radiology. 2013;266:531–538. doi: 10.1148/radiol.12120167. [DOI] [PubMed] [Google Scholar]
- 14.Hong J, Yao Y, Zhang Y, Tang T, Zhang H, Bao D, Chen Y, Pan J. Value of magnetic resonance diffusion-weighted imaging for the prediction of radiosensitivity in nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 2013;149:707–713. doi: 10.1177/0194599813496537. [DOI] [PubMed] [Google Scholar]
- 15.Nakajo M, Nakajo M, Kajiya Y, Tani A, Kamiyama T, Yonekura R, Fukukura Y, Matsuzaki T, Nishimoto K, Nomoto M, Koriyama C. FDG PET/CT and diffusion-weighted imaging of head and neck squamous cell carcinoma: Comparison of prognostic significance between primary tumor standardized uptake value and apparent diffusion coefficient. Clin Nucl Med. 2012;37:475–480. doi: 10.1097/RLU.0b013e318248524a. [DOI] [PubMed] [Google Scholar]
- 16.Vandecaveye V, Dirix P, De Keyzer F, de Beeck KO, Vander Poorten V, Roebben I, Nuyts S, Hermans R. Predictive value of diffusion-weighted magnetic resonance imaging during chemoradiotherapy for head and neck squamous cell carcinoma. Eur Radiol. 2010;20:1703–1714. doi: 10.1007/s00330-010-1734-6. [DOI] [PubMed] [Google Scholar]
- 17.Jones CM, Athanasiou T. Summary receiver operating characteristic curve analysis techniques in the evaluation of diagnostic tests. Ann Thorac Surg. 2005;79:16–20. doi: 10.1016/j.athoracsur.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 18.Hamstra DA, Lee KC, Moffat BA, Chenevert TL, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: An imaging treatment response biomarker to chemoradiotherapy in a mouse model of squamous cell cancer of the head and neck. Transl Oncol. 2008;1:187–194. doi: 10.1593/tlo.08166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King AD, Mo FK, Yu KH, Yeung DK, Zhou H, Bhatia KS, Tse GM, Vlantis AC, Wong JK, Ahuja AT. Squamous cell carcinoma of the head and neck: Diffusion-weighted MR imaging for prediction and monitoring of treatment response. Eur Radiol. 2010;20:2213–2220. doi: 10.1007/s00330-010-1769-8. [DOI] [PubMed] [Google Scholar]
- 20.Driessen JP, Caldas-Magalhaes J, Janssen LM, Pameijer FA, Kooij N, Terhaard CH, Grolman W, Philippens ME. Diffusion-weighted MR imaging in laryngeal and hypopharyngeal carcinoma: Association between apparent diffusion coefficient and histologic findings. Radiology. 2014;272:456–463. doi: 10.1148/radiol.14131173. [DOI] [PubMed] [Google Scholar]
- 21.Chawla S, Kim S, Dougherty L, Wang S, Loevner LA, Quon H, Poptani H. Pretreatment diffusion-weighted and dynamic contrast-enhanced MRI for prediction of local treatment response in squamous cell carcinomas of the head and neck. AJR Am J Roentgenol. 2013;200:35–43. doi: 10.2214/AJR.12.9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King AD, Yeung DK, Yu KH, Mo FK, Bhatia KS, Tse GM, Vlantis AC, Wong JK, Hu CW, Ahuja AT. Pretreatment and early intratreatment prediction of clinicopathologic response of head and neck cancer to chemoradiotherapy using 1H-MRS. J Magn Reson Imaging. 2010;32:199–203. doi: 10.1002/jmri.22224. [DOI] [PubMed] [Google Scholar]
- 23.Wichmann G, Krüger A, Boehm A, Kolb M, Hofer M, Fischer M, Müller S, Purz S, Stumpp P, Sabri O, et al. Induction chemotherapy followed by radiotherapy for larynx preservation in advanced laryngeal and hypopharyngeal cancer: Outcome prediction after one cycle induction chemotherapy by a score based on clinical evaluation, computed tomography-based volumetry and (18)F-FDG-PET/CT. Eur J Cancer. 2017;72:144–155. doi: 10.1016/j.ejca.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Brazzelli M, Sandercock PA, Chappell FM, Celani MG, Righetti E, Arestis N, Wardlaw JM, Deeks JJ. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane Database Syst Rev. 2009;4:CD007424. doi: 10.1002/14651858.CD007424.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adelstein DJ, Rodriguez CP. Human papillomavirus: Changing paradigms in oropharyngeal cancer. Curr Oncol Rep. 2010;12:115–120. doi: 10.1007/s11912-010-0084-5. [DOI] [PubMed] [Google Scholar]
- 27.Yom SS. NRG-HN002: A randomized phase ii trial for patients with p16 positive, non-smoking associated, locoregionally advanced oropharyngeal cancer. NCT02254278. 2014. https://www.crcwm.org/Attachments/NRG%20HN002%20FastFacts.pdf. [Jun 20;2016 ]. https://www.crcwm.org/Attachments/NRG%20HN002%20FastFacts.pdf
- 28.Michael A, Boss TLC, Mark A, Rose Edward F, Jackson, et al. QIBA PDF MRI technical committee: Activities in diffusion MRI. 2014. https://qibawiki.rsna.org/images/b/bc/QIBA_PDF_DWI_Poster_2014_v1_0.pdf. [May 24;2014 ]. https://qibawiki.rsna.org/images/b/bc/QIBA_PDF_DWI_Poster_2014_v1_0.pdf




