Abstract
Purpose
Our objective was to determine the association of protein intake by source (dairy, non-dairy animal, plant) with bone strength and bone microarchitecture among older men.
Methods
We used data from 1016 men (mean 84.3 years) who attended the Year 14 exam of the Osteoporotic Fractures in Men (MrOS) study, completed a food frequency questionnaire (500–5000 kcal/d), were not taking androgen or androgen agonists and had high resolution peripheral quantitative computed tomography (HR-pQCT) scans of the distal radius and distal or diaphyseal tibia. Protein was expressed as % of total energy intake (TEI); mean±SD for TEI = 1548±607 kcal/d and for total protein = 16.2±2.9%TEI. We used linear regression with standardized HR-pQCT parameters as dependent variables and adjusted for age, limb length, center, education, race/ethnicity, marital status, smoking, alcohol intake, physical activity level, corticosteroids use, supplement use (calcium and vitamin D), and osteoporosis medications.
Results
Higher dairy protein intake was associated with higher estimated failure load at the distal radius and distal tibia [radius effect size=0.17 (95% CI: 0.07, 0.27), tibia effect size=0.13 (95% CI: 0.03, 0.23)], while higher non-dairy animal protein was associated with higher failure load at only the distal radius. Plant protein intake was not associated with failure load at any site.
Conclusion
The association between protein intake and bone strength varied by source of protein. These results support a link between dairy protein intake and skeletal health but an intervention study is needed to evaluate causality.
Keywords: protein intake, bone microarchitecture, bone strength, older men
INTRODUCTION
Experimental studies have shown that protein source has differential effects on markers of bone mineral metabolism [1]. We have previously shown that there is heterogeneity of the association between protein intake in older men and fracture by protein source[2]. We also found that the relationship between protein and fracture differed by skeletal site of the fracture (i.e. there was a strong association for hip fracture, a null association for clinical spine fracture). The exact effects of these different bone marker profiles on trabecular and cortical bone is unknown, as is whether the effect varies by weight-bearing vs. non-weight bearing status. Differential effects on bone compartment might also explain the observed heterogeneity by fracture site if protein acts differentially on cortical vs. trabecular bone. Further, our previous results showed that the relationship between protein and fracture was predominately but only partially mediated by total hip bone mineral density (BMD) [2]. A potential explanation is that the causal pathway also includes compromised bone strength due to specific changes in bone microarchitecture. Such compromised bone strength could be attributable to changes in trabecular architecture, thinning of cortices, or cortical porosity, but the exact mechanism are unknown.
Our specific aim was to characterize the association of total protein intake and protein intake by source (dairy, non-dairy animal, plant) with trabecular and cortical bone microarchitecture at weight bearing (tibia) and non-weight bearing (radius) skeletal sites among older men. Our hypothesis was that low protein intake is associated with poorer measures of bone microarchitecture at both sites, and that the associations will differ by protein source.
METHODS
Study Population
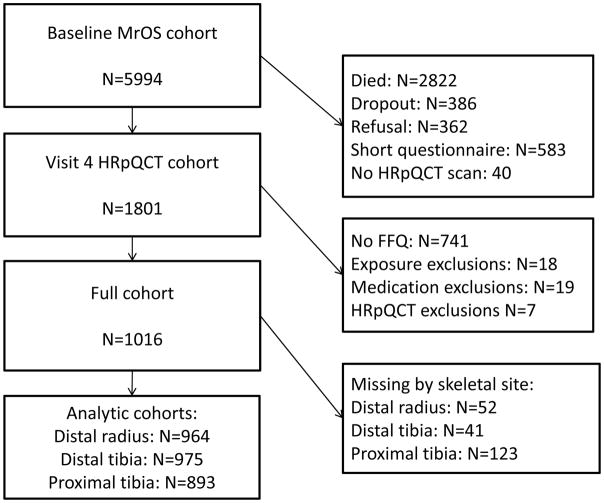
From 2000 to 2002, the Osteoporotic Fractures in Men (MrOS) study enrolled 5994 ambulatory community-dwelling men, aged 65 years and older, living in one of six U.S. metropolitan areas[3,4]. Those with a history of bilateral hip replacement or the inability to walk without the assistance of another person were not eligible to participate. The institutional review board at each participating institution approved the study protocol and written informed consent was obtained from all participants. All active MrOS participants were invited to participate in Visit 4 (clinic visits occurred between May 2014 and May 2016). Of the 2424 participants who completed a visit, 1801 are included in the high-resolution peripheral quantitative computed tomography (HR-pQCT) dataset (Figure 1). Men were eligible for the present study if they had HR-pQCT data and they 1) completed a food frequency questionnaire (FFQ) with estimated total energy intake of 500–5000 kcal/d and 2) were not taking androgens or androgen agonists.
Figure 1.
Participant flow
Protein Intake
Participants completed a brief version of the Block FFQ at Visit 4. The FFQ includes 69 individual food item questions, including an additional 10 questions about food preparation and low-fat foods which were used to refine nutrient calculations. There were nine categories of frequency responses for foods and beverages and four categories of portion size responses. A graphic representation of standard portion sizes was included with the questionnaire. Total energy intake, total protein intake, and protein intake by source were derived from the responses to the questionnaire by Block Dietary Data Systems (Berkeley CA, USA), with dietary reference data from the United States Department of Agriculture Nutrient Database for Standard Reference (Version 16) and the Nutrient Database for Dietary Studies (Version 1). We considered the following subcategories of intake: protein from dairy products, non-dairy animal protein (e.g. meat, fish, poultry, eggs), and protein from plant sources (e.g. legumes, grains, nuts). We excluded those with extremely low or high estimated total energy intake (<500kcal/d or >5000 kcal/d).
Measurement of HR-pQCT
HR-pQCT scans were done using Scanco XtremeCT II machines (Scanco Medical AG, Brüttisellen, Switzerland), which have nominal voxel size of 61μm. Centrally trained operators acquired scans of the distal radius (9 mm from the articular surface), distal tibia (22 mm from the articular surface), and diaphyseal tibia (30% offset). The radius from the non-dominant arm and the tibia from the ipsilateral leg were scanned except in the case of prior fracture, metal shrapnel or implant, or recent non-weight bearing loads > 6 weeks. Machines were calibrated prior to being used in the present study, and a single cross-calibration density phantom was circulated among the study sites. The between site calibration coefficients were all <0.6%, and therefore pooled data was used without transformations [5]. The standard local density phantom was scanned on a daily basis to monitor for values that fall outside of the nominal range (8 mg HA/cm3). Centralized quality assurance and standard analysis of all image data, including micro finite element analysis (μFEA), was performed.
A central observer read all images for motion artifacts and used an established semi-quantitative 5-point grading system (1=superior, 5=poor) to score image quality and images with 4 or 5 were deemed to be of insufficient quality and were excluded from the analytic data set (97% of scans image grade ≤3) [6]. A fully automated analysis pipeline was developed to segment the radius and tibia for quantification of bone density and structure [7]. Segmentation failures were detected automatically by measuring slice-wise variation in total cross-sectional area; cases with an absolute slice-wise difference of 2mm2 at the diaphysis, and 4mm2 at the distal sites, were visually reviewed and manually corrected, as needed. Observed failure rates were < 2% and < 6%, for diaphyseal and distal scans, respectively.
Volumetric BMD and cross sectional area of the total, cortical, and trabecular compartments were measured. Cortical porosity and thickness, and trabecular thickness, separation and number were calculated directly [8,9], Linear elastic micro-finite element analysis of a 1% uniaxial compression was performed using a homogenous elastic modulus of 10 GPa and a Poisson’s ratio of 0.3 (Scanco FE Software v1.12, Scanco Medical). The failure load was estimated by calculation of the reaction force at which 7.5% of the elements exceed a local effective strain of 0.7% [10].
All participants with outliers (difference from mean of greater than 3 standard deviations) were reviewed and those with abnormal anatomic findings at a given skeletal site (e.g. severe inflammatory arthritis, osteolytic lesions, injuries with ossification, unreported fracture) were excluded (distal radius n=72, distal tibia n=54, diaphyseal tibia n=62) from the analysis for that skeletal site.
Other variables
Based on a literature review, we chose the following variables for inclusion in the fully adjusted models: age, race/ethnicity (non-Hispanic white vs. other), limb length, study center, education, marital status, smoking, alcohol consumption, physical activity, bisphosphonate use, oral corticosteroid use, and supplement use (calcium and vitamin D). Information on demographics, lifestyle, and medical and family history was obtained by questionnaire and interview by trained clinical staff. Race/ethnicity (non-Hispanic white vs. other) was self-identified. Participants were classified into ever smoker (100+ cigarettes) vs. never smokers. Self-reported alcohol intake was divided into five categories: <12 drinks/year, ≥ 1 drink/month and < 1 drink/week, 1–2 drinks/week, 3–5 drinks/week, ≥6 drinks/week. Physical activity was measured by computing the Physical Activity Scale for the Elderly (PASE)[11]. Participants were asked to bring all current (any use within the past 30 days) prescription medications with them to the clinic. All non-prescription and prescription medications were recorded in an electronic medication inventory database and matched to its ingredients(s) based on the Iowa Drug Information Service drug vocabulary (College Pharmacy, University of Iowa, Iowa City, IA)[12]. Information on calcium and vitamin D supplement use was determined from the medication inventory.
Statistical Analysis
We first used chi-square tests for comparisons of categorical variables across quartiles of protein intake and ANOVA for comparisons of continuous variables across quartiles of protein intake. We then used multiple linear regression models and the nutrient density method[13] to determine the association between protein intake (expressed as % of total energy intake[TEI]) and HR-pQCT parameters, with both exposure and outcome modeled as continuous variables. Based on previous literature, including findings in this cohort, we posited that there would be heterogeneity by source. We tested this heterogeneity (dairy vs. non-dairy animal, dairy vs. plant, non-dairy animal vs. plant) by comparing estimates for the primary HR-pQCT outcome (failure load). If heterogeneity was present for failure load, then protein intake by source (dairy, non-dairy animal, plant) would be included in all models for the remaining outcomes. Effect size was defined to be the beta coefficient from a regression model where both exposure and outcome were parameterized to have mean=0 and SD=1. For comparison purposes, models considering protein by source were parameterized to have the same units as for total protein, i.e. 1 SD=2.9% TEI. Macronutrient intake is strongly correlated with TEI, a variable that is in turn associated with body size and fracture risk, thus all models were adjusted for TEI. We considered base models (adjusted for age, TEI, and center) and full models (further adjusted for additional covariates including, race/ethnicity (non-Hispanic white vs. other), limb length, education, marital status, smoking, alcohol consumption, physical activity, bisphosphonate use, oral corticosteroid use, use of calcium and vitamin D supplements). We assessed all continuous variables for possible non-linearity using higher order terms and fractional polynomials. Analysis was performed using Stata Version 14.0 (College Station, TX).
RESULTS
Among the 1016 men at the Visit 4 (Year 14) exam who comprised the analytical cohort, the mean (SD) age was 84.3 (4.0) years (with age range 78–98) years and the mean (SD) TEI was 1548 (607) kcal/d. The mean (SD) absolute total protein intake was 62.2 (25.3) g/d, while the mean (SD) %TEI intake was 16.2 (2.9), and the mean (SD) relative intake by body weight was 0.79 (0.34) (g/kg)/day. There were 590 (58.1%) men with protein intake below the recommended daily allowance of 0.8 (g/kg)/day and 402 men with intakes below the estimated average requirement of 0.66 (g/kg)/day[14]. The mean (SD) of protein intake by source as %TEI were as follows: dairy protein 3.5 (2.1), non-dairy animal protein 6.3 (2.9), and plant protein 6.4 (1.8). Characteristics of the cohort by quartile of total protein intake are shown in Table 1. Those with lower protein intake were less likely to have a college education or to have alcohol intake ≥ 1 drink/day. Those in the highest and lowest quartiles of protein intake were less likely to be married. Age, race/ethnicity, limb length, smoking, physical activity, medication use, and supplement use did not vary by protein intake quartiles. The percentage of men below the estimated average requirement for protein intake increased in a graded fashion with decreasing quartile of energy intake as expressed as %TEI.
Table 1.
Characteristics of Study Sample by Quartile of Total Protein Intake
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-value* | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age, years | 84.7 | 4.2 | 84.3 | 4.0 | 84.1 | 3.9 | 84.2 | 4.0 | 0.403 |
| Radius length (mm) | 286 | 16 | 288 | 15 | 287 | 14 | 285 | 14 | 0.471 |
| Tibia length (mm) | 403 | 26 | 405 | 24 | 407 | 26 | 404 | 24 | 0.231 |
| PASE score | 113 | 63 | 118 | 64 | 118 | 65 | 118 | 71 | 0.743 |
| Non-Hispanic White (n, %) | 223 | 87.8 | 237 | 93.3 | 232 | 91.3 | 221 | 87.0 | 0.061 |
| Married (n, %) | 171 | 67.3 | 188 | 74.0 | 198 | 78.0 | 164 | 64.6 | 0.003 |
| College Education (n, %) | 122 | 48.0 | 150 | 59.1 | 159 | 62.6 | 178 | 70.1 | <0.001 |
| Ever Smoker (n, %) | 142 | 55.9 | 145 | 57.1 | 139 | 54.7 | 139 | 54.7 | 0.941 |
| Alcohol ≥ 1 drink/day (n, %) | 100 | 39.4 | 123 | 48.4 | 134 | 52.7 | 136 | 53.5 | 0.005 |
| Calcium/Vitamin D (n, %) | 59 | 23.2 | 65 | 25.6 | 76 | 29.9 | 75 | 29.5 | 0.261 |
| Osteoporosis medications (n, %) | 9 | 3.5 | 9 | 3.5 | 8 | 3.1 | 6 | 2.4 | 0.856 |
| Corticosteroids (n, %) | 9 | 3.5 | 9 | 3.5 | 4 | 1.6 | 5 | 2.0 | 0.368 |
| Total Energy Intake (kcal) | 1658 | 678 | 1581 | 575 | 1509 | 574 | 1445 | 577 | <0.001 |
| Total protein (g) | 53.2 | 22.4 | 60.0 | 21.8 | 63.9 | 24.2 | 71.8 | 28.3 | <0.001 |
| Protein intake < EAR (n, %) | 134 | 52.8 | 106 | 41.7 | 94 | 37.0 | 68 | 26.8 | <0.001 |
| Total protein (%TEI) | 12.8% | 1.1% | 15.2% | 0.5% | 16.9% | 0.5% | 20.0% | 1.9% | <0.001 |
| Dairy protein (%TEI) | 2.7% | 1.4% | 3.4% | 1.7% | 3.8% | 1.7% | 4.3% | 2.8% | <0.001 |
| Non-dairy animal (%TEI) | 4.1% | 1.5% | 5.5% | 1.8% | 6.5% | 2.1% | 9.2% | 3.1% | <0.001 |
| Plant protein (%TEI) | 6.0% | 1.6% | 6.3% | 1.5% | 6.7% | 1.9% | 6.5% | 2.0% | <0.001 |
Abbreviations: PASE, Physical Activity Scale for the Elderly; BMD, bone mineral density; TEI, total energy intake; EAR, estimated average requirement (EAR for protein=0.66(g/kg)/day).
Protein intake Q1=9.5%–14.2% TEI, Q2=14.3%–16.0% TEI, Q3=16.1%–18.0% TEI, Q4=18.1%–28.4% TEI
ANOVA for continuous variables and chi-square test for categorical variables
Distal Radius
The association between protein intake and failure load of the distal radius varied by source (p=0.012 for dairy vs. plant and p=0.070 for dairy vs. non-dairy animal). In fully adjusted models using continuous variables, there was an association between higher dairy protein intake and higher failure load with effect size=0.17 (95% CI: 0.07, 0.27); while there was no significant association between plant protein intake and failure load in either the base or fully adjusted models (Table 2). Higher dairy protein intake was also associated with a lower total area (with higher cortical area and lower trabecular area), higher total BMD, higher compartmental BMD (cortical and trabecular BMD), thicker cortices and trabeculae, and increased trabecular number. There was no significant association between dairy protein intake and cortical porosity. The association between non-dairy animal protein intake and failure load was more modest than that for dairy protein with effect size=0.07 (95% CI: 0.00, 0.13) in the fully adjusted model. Higher non-dairy animal protein intake was associated with a higher total BMD, higher cortical BMD, higher cortical area, and thicker cortices.
Table 2.
The Association Between Protein Intake (by Source) and Bone Strength Parameters from High Resolution Peripheral Quantitative Computed Tomography (HR-pQCT) of the Distal Radius.
| Distal Radius | Base Model | Multivariate model | ||||
|---|---|---|---|---|---|---|
| Dairy protein | Non-dairy animal protein | Plant protein | Dairy protein | Non-dairy animal protein | Plant protein | |
| Failure load | 0.15 (0.05, 0.25) | 0.06 (−0.01, 0.13) | 0.00 (−0.11, 0.11) | 0.17 (0.07, 0.27) | 0.07 (0.00, 0.13) | 0.03 (−0.09, 0.14) |
| BMD | 0.18 (0.08, 0.28) | 0.07 (0.01, 0.14) | 0.04 (−0.08, 0.16) | 0.20 (0.11, 0.30) | 0.07 (0.01, 0.13) | 0.02 (−0.09, 0.14) |
| Total area | −0.10 (−0.20, 0.00) | −0.05 (−0.12, 0.02) | −0.12 (−0.24, −0.01) | −0.10 (−0.19, −0.01) | −0.03 (−0.09, 0.03) | −0.06 (−0.16, 0.05) |
| Trabecular BMD | 0.13 (0.03, 0.23) | 0.03 (−0.04, 0.10) | 0.03 (−0.09, 0.14) | 0.14 (0.04, 0.24) | 0.03 (−0.04, 0.10) | 0.04 (−0.07, 0.16) |
| Trabecular area | −0.13 (−0.23, −0.03) | −0.07 (−0.14, 0.00) | −0.11 (−0.22, 0.01) | −0.15 (−0.24, −0.05) | −0.06 (−0.12, 0.00) | −0.07 (−0.18, 0.04) |
| Trabecular number | 0.12 (0.02, 0.22) | 0.00 (−0.07, 0.07) | 0.01 (−0.12, 0.11) | 0.12 (0.02, 0.22) | 0.01 (−0.06, 0.08) | 0.02 (−0.10, 0.13) |
| Trabecular thickness | 0.12 (0.02, 0.22) | 0.04 (−0.03, 0.11) | 0.07 (−0.04, 0.19) | 0.13 (0.03, 0.24) | 0.04 (−0.03, 0.11) | 0.07 (−0.04, 0.19) |
| Cortical BMD | 0.18 (0.08, 0.27) | 0.07 (0.01, 0.14) | 0.08 (−0.03, 0.19) | 0.20 (0.11, 0.30) | 0.07 (0.00, 0.14) | 0.07 (−0.05, 0.18) |
| Cortical area | 0.14 (0.05, 0.24) | 0.08 (0.02, 0.15) | −0.07 (−0.18, 0.04) | 0.17 (0.07, 0.27) | 0.09 (0.02, 0.15) | −0.05 (−0.16, 0.07) |
| Cortical thickness | 0.15 (0.05, 0.25) | 0.09 (0.02, 0.16) | −0.02 (−0.13, 0.10) | 0.17 (0.07, 0.27) | 0.09 (0.02, 0.15) | −0.05 (−0.16, 0.07) |
| Cortical porosity | −0.02 (−0.12, 0.08) | 0.00 (−0.07, 0.07) | −0.02 (−0.13, 0.10) | −0.03 (−0.14, 0.07) | −0.02 (−0.09, 0.05) | −0.04 (−0.15, 0.08) |
Effect size (beta coefficient with unit=SD). Protein measured as percentage of total energy intake (TEI), SD=2.9% TEI.
Base model adjusted for age, clinical center, total calories
Multivariate model adjusted for age, clinical center, total calories, limb length, education, race/ethnicity, marital status, smoking, alcohol intake, physical activity level, corticosteroid use, supplement use (calcium and vitamin D), and osteoporosis medications.
Distal Tibia
The association between protein intake and failure load of the distal tibia varied by source (p=0.005 for dairy vs. plant and p=0.008 for dairy vs. non-dairy animal). In fully adjusted models using continuous variables, there was an association between higher dairy protein intake and higher failure load with effect size=0.13 (95% CI: 0.03, 0.23); while there was no significant association between either non-dairy animal protein or plant protein intake and failure load or any of the other HR-pQCT measures in either the base or fully adjusted models (Table 3). Higher dairy protein intake was also associated with a lower total area (with higher cortical area and lower trabecular area), higher total BMD, higher compartmental BMD (cortical and trabecular BMD), and thicker cortices. There was no significant association between dairy protein intake and the other HR-pQCT measures.
Table 3.
The Association Between Protein Intake (by Source) and Bone Strength Parameters from High Resolution Peripheral Quantitative Computed Tomography (HR-pQCT) of the Distal Tibia.
| Distal Tibia | Base Model | Multivariate model | ||||
|---|---|---|---|---|---|---|
| Dairy protein | Non-dairy animal protein | Plant protein | Dairy protein | Non-dairy animal protein | Plant protein | |
| Failure load | 0.13 (0.03, 0.23) | −0.01 (−0.07, 0.06) | −0.04 (−0.15, 0.08) | 0.13 (0.03, 0.23) | −0.01 (−0.07, 0.06) | −0.01 (−0.12, 0.11) |
| BMD | 0.17 (0.07, 0.27) | 0.01 (−0.06, 0.08) | 0.00 (−0.11, 0.12) | 0.19 (0.10 0.29) | 0.01 (−0.05, 0.07) | 0.01 (−0.10, 0.11) |
| Total area | −0.08 (−0.18, 0.02) | −0.05 (−0.12, 0.02) | −0.09 (−0.21, 0.02) | −0.12 (−0.21, −0.04) | −0.05 (−0.10, 0.01) | −0.04 (−0.13, 0.06) |
| Trabecular BMD | 0.15 (0.04, 0.25) | −0.03 (−0.09, 0.05) | 0.04 (−0.08, 0.15) | 0.13 (0.03, 0.23) | −0.01 (−0.08, 0.06) | 0.08 (−0.03, 0.19) |
| Trabecular area | −0.11 (−0.21, −0.00) | −0.05 (−0.12, 0.02) | −0.07 (−0.18, 0.05) | −0.15 (−0.23, −0.06) | −0.05 (−0.10, 0.01) | −0.02 (−0.11, 0.08) |
| Trabecular number | 0.09 (−0.01, 0.19) | −0.05 (−0.12, 0.02) | −0.03 (−0.14, 0.09) | 0.07 (−0.03, 0.17) | −0.04 (−0.10, 0.03) | 0.04 (−0.08, 0.15) |
| Trabecular thickness | 0.06 (−0.04, 0.16) | 0.02 (−0.05, 0.09) | 0.06 (−0.06, 0.17) | 0.07 (−0.04, 0.17) | 0.02 (−0.05, 0.09) | 0.07 (−0.05, 0.18) |
| Cortical BMD | 0.10 (0.01, 0.20) | 0.04 (−0.01, 0.11) | −0.01 (−0.12, 0.10) | 0.15 (0.05, 0.24) | 0.04 (−0.02, 0.11) | −0.03 (−0.14, 0.08) |
| Cortical area | 0.13 (0.03, 0.23) | 0.01 (−0.05, 0.09=8) | −0.08 (−0.20, 0.03) | 0.16 (0.06, 0.25) | 0.01 (−0.06, 0.07) | −0.09 (−0.20, 0.02) |
| Cortical thickness | 0.13 (0.03, 0.23) | 0.02 (−0.05, 0.09) | −0.04 (−0.16, 0.07) | 0.16 (0.06, 0.26) | 0.01 (−0.06, 0.07) | −0.06 (−0.17, 0.04) |
| Cortical porosity | 0.01 (−0.09, 0.11) | −0.03 (−0.09, 0.04) | 0.07 (−0.04, 0.19) | 0.00 (−0.11, 0.10) | −0.03 (−0.10, 0.04) | 0.06 (−0.06, 0.18) |
Effect size (beta coefficient with unit=SD). Protein measured as percentage of total energy intake (TEI), SD=2.9% TEI.
Base model adjusted for age, clinical center, total calories
Multivariate model adjusted for age, clinical center, total calories, limb length, education, race/ethnicity, marital status, smoking, alcohol intake, physical activity level, corticosteroid use, supplement use (calcium and vitamin D), and osteoporosis medications.
Diaphyseal Tibia
The association between protein intake and failure load of the diaphyseal tibia varied by source (p=0.011 for dairy vs. plant and p=0.056 for dairy vs. non-dairy animal). There was no significant association between any source-specific protein intake (dairy, non-dairy animal, plant) and failure load in the fully adjusted models (Table 4). Higher dairy protein intake was associated with higher total BMD and thicker cortices. Higher plant protein intake was associated with higher cortical BMD, but this association was attenuated and not significant for overall BMD.
Table 4.
The Association Between Protein Intake (by Source) and Bone Strength Parameters from High Resolution Peripheral Quantitative Computed Tomography (HR-pQCT) of the Diaphyseal Tibia.
| Diaphyseal Tibia | Base Model | Multivariate model | ||||
|---|---|---|---|---|---|---|
| Dairy protein | Non-dairy animal protein | Plant protein | Dairy protein | Non-dairy animal protein | Plant protein | |
| Failure load | 0.10 (0.00, 0.20) | 0.00 (−0.07, 0.07) | −0.06 (−0.17, 0.06) | 0.09 (−0.01, 0.18) | 0.00 (−0.07, 0.06) | −0.03 (−0.15, 0.08) |
| BMD | 0.18 (0.07, 0.28) | 0.01 (−0.06, 0.08) | 0.10 (−0.01, 0.22) | 0.18 (0.08, 0.29) | 0.00 (−0.07, 0.07) | 0.11 (−0.01, 0.22) |
| Total area | −0.05 (−0.15, 0.06) | −0.01 (−0.08, 0.06) | −0.11 (−0.23, 0.01) | −0.07 (−0.17, 0.03) | −0.01 (−0.08, 0.06) | −0.09 (−0.20, 0.03) |
| Cortical BMD | 0.06 (−0.04, 0.16) | −0.03 (−0.10, 0.04) | 0.17 (0.06, 0.29) | 0.06 (−0.04, 0.17) | −0.04 (−0.11, 0.03) | 0.15 (0.04, 0.27) |
| Cortical area | 0.10 (−0.00, 0.20) | 0.01 (−0.06, 0.08) | −0.06 (−0.18, 0.05) | 0.08 (−0.01, 0.18) | 0.01 (−0.06, 0.07) | −0.03 (−0.15, 0.08) |
| Cortical thickness | 0.16 (0.06, 0.26) | 0.00 (−0.07, 0.07) | 0.02 (−0.12, 0.11) | 0.16 (0.05, 0.26) | 0.00 (−0.07, 0.07) | 0.01 (−0.10, 0.13) |
| Cortical porosity | −0.01 (−0.11, 0.10) | 0.00 (−0.07, 0.07) | −0.04 (−0.16, 0.08) | −0.01 (−0.12, 0.09) | 0.01 (−0.06, 0.08) | −0.02 (−0.14, 0.10) |
Effect size (beta coefficient with unit=SD). Protein measured as percentage of total energy intake (TEI), SD=2.9% TEI.
Base model adjusted for age, clinical center, total calories.
Multivariate model adjusted for age, clinical center, total calories, limb length, education, race/ethnicity, marital status, smoking, alcohol intake, physical activity level, corticosteroid use, supplement use (calcium and vitamin D), and osteoporosis medications.
DISCUSSION
In this cross-sectional study of older men, we found that the association between protein intake and bone strength parameters varied by protein source. The strongest and most consistent associations were observed for dairy protein intake and bone strength parameters. The associations between non-dairy animal protein intake and bone strength parameters were smaller in magnitude and much less consistent, while the associations between plant protein intake and bone strength parameters were for the most part null. The associations were similar by weight-bearing status (radius vs. tibia) and bone compartment (cortical vs. trabecular). The results are consistent with previous results on heterogeneity of the association between protein and fracture[2,15]. Many nutrients have non-linear effects (no further effect after adequate intake or negative effect at high intake). In the present study we have found a linear association for all protein specific sources. Lower intakes vs. higher intakes of dairy protein (and in some cases non-dairy animal protein) as a percentage of total intake are associated with lower bone strength and associated microstructural parameters at all levels of intake. Thus, effects are not obviously related to protein deficiency or excess per se.
Based on a cross-sectional study of 749 younger healthy post-menopausal women, Durosier-Izart et al[16] reported that both dairy protein intake and non-dairy animal protein intake were associated with failure load, areal BMD, and modifications in cortical and trabecular microstructure. Our study is consistent with these previous results for women, which also showed that protein source plays a key role in the relationship between protein intake and measures of bone strength and geometry. In particular, relatively strong and consistent associations were also observed between dairy protein and bone strength parameters among women. Likewise, among women, null associations were observed between plant protein and bone strength parameters, while the associations observed between non-dairy animal protein and bone strength parameters were more modest in magnitude and not consistent across all measures. Analysis by sex has shown the trajectories of bone microarchitecture over the lifetime may differ in men and in women[17,18]. Thus consistency of these results in both men and women is likely due to global effects of food nutrient profiles on bone which is not modified by sex-specific risk factors and hormones.
Longitudinal analysis has shown that cortical porosity increases over time in older men and women[19]. In this study we found no association of any source of protein intake with cortical porosity. There may be age-related changes in bone that are not impacted by protein or other nutrient intakes. Alternatively, cross-sectional studies of bone microarchitecture might not be adequate to detect effects that are evident when images occur in time sequences. Thus a longitudinal increase in cortical porosity might lead to a shift in the boundary between cortical and trabecular bone and thus read from a cross-sectional sample as relatively lower cortical area and relatively higher trabecular area. In contrast, a nutrient profile that was protective might show a relatively higher cortical area and lower trabecular area, as was observed for dairy and non-dairy animal protein in the present study.
The heterogeneity noted above is likely due several source-dependent nutrient differences, including underlying amino acid profiles and concurrent micro-nutrients including calcium, vitamin D, and phosphorus. Plant protein includes protein from all non-animal sources (grains, nuts, vegetables, and legumes). These plants sources do not typically provide all essential amino acids and those that do are less frequently consumed[20]. Thus it is possible that the predominant plant protein profile does not provide sufficient amounts of the necessary amino acids for maintenance of muscle and bone in older men. In order to further elucidate the potential causal factors we need also to consider experimental studies. A study in male rats by Gaffney-Stromberg et al found systematic differences in markers of bone mineral metabolism specific to eight different experimental scenarios (normal vs. high protein, milk protein vs. soy protein, ad libitum vs. energy restriction)[1]. PTH was higher and bone turnover was lower in rats assigned to a milk protein group vs. those assigned to a soy protein group. Bone turnover was lower and trabecular volumetric BMD was higher in rats assigned to a high protein vs. those assigned a normal protein group. In humans, protein impacts calcium absorption as demonstrated in an experimental study showing that increasing protein intake from 10% to 20% of TEI increased fractional calcium absorption among those with low calcium intake[21]. Conversely, another randomized trial in elderly men and women noted that the addition of calcium and vitamin D appears to modify the association between protein and bone loss[22]. Thus, it is likely that dairy foods as a source of dairy protein are related to bone health outcomes due to the interaction of calcium, vitamin D, and protein.
There have been few clinical trials assessing the effect of dairy protein per se on longitudinal change in BMD among older men. Kerstetter et al [23] considered whether a protein supplement vs. an isocaloric maltodextrin supplement was associated with longitudinal changes in muscle and bone outcomes. There were no between group differences for BMD, but there were between group differences in muscle outcomes. The main limitations of the study were drop out (50/208) and non-compliance (37/158). Zhu et al[24] considered whether a high protein drink vs. a lower protein isocaloric drink was associated with bone health outcomes in older post-menopausal women. There was a decrease in areal hip BMD (by dual-energy x-ray absorptiometry [DXA]) and volumetric BMD (by QCT) over 2 years with no between-group differences, while femoral neck BMD was unchanged in either group over time. Other clinical studies have considered higher vs. lower protein diets in the context of weight loss [25–27]. The specifics of these study populations and the low compliance with dietary interventions make it difficult to interpret or generalize the findings of these studies.
The strengths of the present study are the inclusion of major potential confounders and a large sample of community-dwelling older men with a sufficient sample size to detect even small differences in bone strength. We assessed protein intake by source, thus accounting for differences in source-specific micro-nutrient and amino acid profiles of the three major sources (dairy, non-dairy animal, and plant). The HR-pQCT assessments had excellent quality control. Study limitations include the cross-sectional design and the use of a brief FFQ to assess diet. A further limitation was the measurement of diet at a single time point, since the causal relationship between diet and bone is likely cumulative and single measurement may not adequately capture long-term dietary intake as the causal exposure. FFQs have limitations in the assessment of absolute and relative intake with likely bias towards the null. The study design was an observational cohort study, and therefore limitations include the possibility of selection bias and residual confounding. The generalizability of the present study is limited to healthy community-dwelling older men. The cohort was also mostly non-Hispanic white, and therefore we were unable to assess potential racial/ethnic differences, and this will also limit generalizability. Finally, we were unable in this analysis to assess whether the differential effects for dairy vs. non-dairy sources of protein is attributable to the calcium (and vitamin D) or due to different amino acid profiles.
In summary, dairy protein but not plant protein was associated with bone strength and bone strength parameters at the distal radius and tibia in older men, consistent with previously observed HR-pQCT results in women and fracture results in men and women. These results on bone strength parameters are likely to be relevant to bone strength in general as there were no clear weight-bearing or compartmental specific effects. Differences in the association between protein and fracture by skeletal site might then be due to proximity of bone failure loads to loads applied to bone by day-to-day activities and typical fall trauma. Further research is necessary to establish causal pathways and magnitude of effect.
Acknowledgments
Source of Funding:
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128, R01 AR060700.
This manuscript is the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Disclosures: L. Langsetmo, J. Shikany, A. Burghardt, P. Cawthon, E. Orwoll, J. Cauley, B. Taylor, J. Schousboe, D. Bauer, T. Vo, K. Ensrud declare that they have no conflict of interest.
Authors’ roles:
Study concept and design: LL, JS, KEE
Data collection: JS, JAC, EO, KEE
Data analysis and interpretation: LL
Drafting manuscript: LL
Critical review and final approval of manuscript content: all authors
Statistical Analysis: Lisa Langsetmo performed the statistical analyses and her work is independent of any commercial funder. She had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Reference List
- 1.Gaffney-Stomberg E, Cao JJ, Lin GG, Wulff CR, Murphy NE, Young AJ, McClung JP, Pasiakos SM. Dietary protein level and source differentially affect bone metabolism, strength, and intestinal calcium transporter expression during ad libitum and food-restricted conditions in male rats. J Nutr. 2014;144:821–29. doi: 10.3945/jn.113.188532. [DOI] [PubMed] [Google Scholar]
- 2.Langsetmo L, Shikany JM, Cawthon PM, Cauley JA, Taylor BC, Vo TN, Bauer DC, Orwoll ES, Schousboe JT, Ensrud KE. The Association Between Protein Intake by Source and Osteoporotic Fracture in Older Men: A Prospective Cohort Study. J Bone Miner Res. 2017;32:592–600. doi: 10.1002/jbmr.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Burghardt AJ, Pialat JB, Kazakia GJ, Boutroy S, Engelke K, Patsch JM, Valentinitsch A, Liu D, Szabo E, Bogado CE, Zanchetta MB, McKay HA, Shane E, Boyd SK, Bouxsein ML, Chapurlat R, Khosla S, Majumdar S. Multicenter precision of cortical and trabecular bone quality measures assessed by high-resolution peripheral quantitative computed tomography. J Bone Miner Res. 2013;28:524–36. doi: 10.1002/jbmr.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S. Visual grading of motion induced image degradation in high resolution peripheral computed tomography: impact of image quality on measures of bone density and micro-architecture. Bone. 2012;50:111–18. doi: 10.1016/j.bone.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47:519–28. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hildebrand T, Laib A, Muller R, Dequeker J, Ruegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res. 1999;14:1167–74. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- 9.Manske SL, Zhu Y, Sandino C, Boyd SK. Human trabecular bone microarchitecture can be assessed independently of density with second generation HR-pQCT. Bone. 2015;79:213–21. doi: 10.1016/j.bone.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Mueller TL, Christen D, Sandercott S, Boyd SK, van RB, Eckstein F, Lochmuller EM, Muller R, van Lenthe GH. Computational finite element bone mechanics accurately predicts mechanical competence in the human radius of an elderly population. Bone. 2011;48:1232–38. doi: 10.1016/j.bone.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 12.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 13.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 14.Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102:1621–30. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 15.Langsetmo L, Barr SI, Berger C, Kreiger N, Rahme E, Adachi JD, Papaioannou A, Kaiser SM, Prior JC, Hanley DA, Kovacs CS, Josse RG, Goltzman D. Associations of Protein Intake and Protein Source with Bone Mineral Density and Fracture Risk: A Population-Based Cohort Study. J Nutr Health Aging. 2015;19:861–68. doi: 10.1007/s12603-015-0544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durosier-Izart C, Biver E, Merminod F, van RB, Chevalley T, Herrmann FR, Ferrari SL, Rizzoli R. Peripheral skeleton bone strength is positively correlated with total and dairy protein intakes in healthy postmenopausal women. Am J Clin Nutr. 2017;105:513–25. doi: 10.3945/ajcn.116.134676. [DOI] [PubMed] [Google Scholar]
- 17.Burt LA, Liang Z, Sajobi TT, Hanley DA, Boyd SK. Sex- and Site-Specific Normative Data Curves for HR-pQCT. J Bone Miner Res. 2016;31:2041–47. doi: 10.1002/jbmr.2873. [DOI] [PubMed] [Google Scholar]
- 18.Shanbhogue VV, Brixen K, Hansen S. Age- and Sex-Related Changes in Bone Microarchitecture and Estimated Strength: A Three-Year Prospective Study Using HRpQCT. J Bone Miner Res. 2016;31:1541–49. doi: 10.1002/jbmr.2817. [DOI] [PubMed] [Google Scholar]
- 19.Burt LA, Hanley DA, Boyd SK. Cross-sectional Versus Longitudinal Change in a Prospective HR-pQCT Study. J Bone Miner Res. 2017 doi: 10.1002/jbmr.3129. [DOI] [PubMed] [Google Scholar]
- 20.Pasiakos SM, Agarwal S, Lieberman HR, Fulgoni VL., III Sources and Amounts of Animal, Dairy, and Plant Protein Intake of US Adults in 2007–2010. Nutrients. 2015;7:7058–69. doi: 10.3390/nu7085322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunt JR, Johnson LK, Fariba Roughead ZK. Dietary protein and calcium interact to influence calcium retention: a controlled feeding study. Am J Clin Nutr. 2009;89:1357–65. doi: 10.3945/ajcn.2008.27238. [DOI] [PubMed] [Google Scholar]
- 22.Dawson-Hughes B, Harris SS. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr. 2002;75:773–79. doi: 10.1093/ajcn/75.4.773. [DOI] [PubMed] [Google Scholar]
- 23.Kerstetter JE, Bihuniak JD, Brindisi J, Sullivan RR, Mangano KM, Larocque S, Kotler BM, Simpson CA, Cusano AM, Gaffney-Stomberg E, Kleppinger A, Reynolds J, Dziura J, Kenny AM, Insogna KL. The Effect of a Whey Protein Supplement on Bone Mass in Older Caucasian Adults. J Clin Endocrinol Metab. 2015;100:2214–22. doi: 10.1210/jc.2014-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu K, Meng X, Kerr DA, Devine A, Solah V, Binns CW, Prince RL. The effects of a two-year randomized, controlled trial of whey protein supplementation on bone structure, IGF-1, and urinary calcium excretion in older postmenopausal women. J Bone Miner Res. 2011;26:2298–306. doi: 10.1002/jbmr.429. [DOI] [PubMed] [Google Scholar]
- 25.Tirosh A, de Souza RJ, Sacks F, Bray GA, Smith SR, LeBoff MS. Sex Differences in the Effects of Weight Loss Diets on Bone Mineral Density and Body Composition: POUNDS LOST Trial. J Clin Endocrinol Metab. 2015;100:2463–71. doi: 10.1210/jc.2015-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jesudason D, Nordin BC, Keogh J, Clifton P. Comparison of 2 weight-loss diets of different protein content on bone health: a randomized trial. Am J Clin Nutr. 2013;98:1343–52. doi: 10.3945/ajcn.113.058586. [DOI] [PubMed] [Google Scholar]
- 27.Sukumar D, Ambia-Sobhan H, Zurfluh R, Schlussel Y, Stahl TJ, Gordon CL, Shapses SA. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: a randomized, controlled trial. J Bone Miner Res. 2011;26:1339–48. doi: 10.1002/jbmr.318. [DOI] [PMC free article] [PubMed] [Google Scholar]