Abstract
Activation of Wnt/β-catenin signaling plays a central role in the development and progression of colorectal cancer (CRC). The Wnt-transcription factor, TCF7L2, is overexpressed in primary rectal cancers that are resistant to chemoradiotherapy (CRT) and TCF7L2 mediates resistance to CRT. However, it is unclear whether the resistance is mediated by a TCF7L2 inherent mechanism or Wnt/β-catenin signaling in general. Here, inhibition of β-catenin by siRNAs or a small molecule inhibitor (XAV-939) resulted in sensitization of CRC cells to CRT. To investigate the potential role of Wnt/β-catenin signaling in controlling therapeutic responsiveness, non-tumorigenic RPE-1 cells were stimulated with Wnt-3a, a physiological ligand of Frizzled-receptors, which increased resistance to CRT. This effect could be recapitulated by overexpression of a degradation-resistant mutant of β-catenin (S33Y), also boosting resistance of RPE-1 cells to CRT, which was, conversely, abrogated by siRNA-mediated silencing of β-catenin. Consistent with these findings, higher expression levels of active β-catenin were observed as well as increased TCF/LEF reporter activity in SW1463 cells that evolved radiation resistance due to repeated radiation treatment. Global gene expression profiling identified several altered pathways, including PPAR signaling and other metabolic pathways, associated with cellular response to radiation. In summary, aberrant activation of Wnt/β-catenin signaling not only regulates the development and progression of colorectal cancer, but also mediates resistance of rectal cancers to chemoradiotherapy.
Implications
Targeting Wnt/β-catenin signaling or one of the downstream pathways represents a promising strategy to increase response to CRT.
Keywords: Colorectal cancer, chemoradiotherapy, treatment resistance, Wnt/β-catenin signaling
Introduction
Wnt/β-catenin signaling plays a central role during development and in maintaining homeostasis of multiple tissues throughout the body (1–3). Under physiological conditions, Wnt ligands such as Wnt-1 or Wnt-3a associate with trans-membrane Wnt receptors of the Frizzled family. This leads to an activation of dishevelled (DVL2), which in turn inhibits the so-called destruction complex. This multi-protein-complex consists of adenomatous polyposis coli (APC), glycogen synthase kinase 3β (GSK3β) as well as Axin, which, in the absence of Wnt signaling, promote ubiquitylation and proteasomal degradation of β-catenin. Inhibition of the destruction complex results in an accumulation of β-catenin, which subsequently enters the nucleus and binds to members of the T-cell factor/lymphoid enhancer factor (TCF/LEF) family of high-mobility-group transcription factors. Interaction with TCF/LEF factors promotes their ability to induce or repress the transcription of a plethora of target genes (1–3).
Aberrant Wnt/β-catenin signaling plays a critical role in the development and progression of various human malignancies. Importantly, mutations in and deregulation of components of this pathway are defining features of colorectal cancer (CRC) (4,5), which is the second leading cause of cancer related death in Europe and the U.S. (6). According to recent data from the Cancer Genome Atlas Network, more than 90% of CRC show alterations of the Wnt signaling pathway, including bi-allelic inactivation of APC or activating mutations of CTNNB1 (β-catenin) in approximately 80% (7).
In previous studies, we demonstrated that the Wnt transcription factor TCF7L2, formerly known as TCF4, was overexpressed in primary rectal cancers that were resistant to preoperative 5-fluorouracil (5-FU) based long-term chemoradiotherapy (50.4 Gy) (CRT) (8), and that shRNA-mediated silencing of TCF7L2 sensitized CRC cell lines to clinically relevant doses of 5-FU and radiation (9). These observations are of both clinical and scientific relevance: First, resistance to preoperative CRT, the standard treatment for locally advanced rectal cancer, is a major clinical problem in the management of this disease (4,10,11). Secondly, the role of aberrant Wnt/β-catenin signaling in treatment resistance in rectal cancer has not been fully elucidated. In the present study, we demonstrate that TCF7L2-associated radio-resistance is Wnt- and β-catenin-dependent, and we identified downstream pathways associated with response to radiation.
Materials and Methods
Cell lines and cell culture
Human CRC cell lines SW480, SW837, SW1463, and LS1034 were obtained in 2006 from the American Type Culture Collection (ATCC, Manassas, VA). In 2009, directly prior to freezing the cell stocks used in this study, cell line authenticity was reconfirmed using short tandem repeat profiling (12). Cells were cultured in their recommended media (Invitrogen, Carlsbad, Germany), supplemented with 10% fetal bovine serum (Pan, Aidenbach, Germany) and 2 mM L-glutamine (BioWhittaker, Verviers, Belgium), and discarded no later than after 15 passages. Periodically, mycoplasma contamination was determined using the MycoAlert® Mycoplasma Detection Kit (Lonza, Cologne, Germany). Wild-type L-cells and L-Wnt-3a cells (13), as well as hTERT-immortalized retinal pigment epithelial (RPE-1) cells (14), were kindly provided by Holger Bastians (Institute for Molecular Oncology, University Medical Center Goettingen). Cells were authenticated and stored and subsequently propagated as explained above. All cancer cell lines used in this study were characterized previously in regards to their radiation sensitivity and gene expression profiles (15).
siRNA transfection
Transfections with synthetic small interfering RNA (siRNA) duplexes were performed as previously described (16,17). Briefly, for cellular viability assays, cells were reverse transfected with siRNAs (Qiagen, Hilden, Germany; Dharmacon/Thermo Fisher Scientific, Schwerte, Germany) using RNAiMAX (Invitrogen) or HiPerFect (Qiagen). For colony formation assays and Western blot analyses, cells were transfected using nucleofector technology (Lonza). Additional information regarding transfection conditions and siRNA sequences can be found in Supplementary Tables S1, S2, and S3.
Small-molecule inhibitor XAV-939
The tankyrase inhibitor XAV-939 (Tocris Bioscience, Bristol, United Kingdom) was used to inhibit tankyrases 1 and 2 (18). Cells were treated with different inhibitor concentrations for various time spans, followed by a medium exchange to remove the inhibitor. DMSO treated cells served as a negative control. Details can be found in Supplementary Tables S1 and S2.
Western blot analysis
Western blot analysis was performed as previously described (9,17). Briefly, 20 μg of whole cell protein lysate was loaded and resolved on an 8% or 10% Bis-Tris polyacrylamide gel. Proteins were transferred by semi-dry blotting onto a polyvinylidene difluoride membrane (GE Healthcare, Little Chalfont, UK), probed with antibodies and detected using an ImageQuant LAS 4000 mini CCD camera system (GE Healthcare). The respective antibodies and experimental conditions are listed in Supplementary Table S4.
Cellular viability assay
Cellular viability following siRNA-transfection was assessed using the CellTiter-Blue® reagent (Promega, Madison, WI). Reduction of resazurin to resorufin was measured at various time points after the respective treatment using a plate reader (VICTOR™ X4, Perkin Elmer, Waltham, MA) per the manufacturer’s instructions. Cellular viability of siRNA-transfected cells was compared to cells transfected with a non-silencing control siRNA (siNEG). Detailed information can be found in Supplementary Table S1.
Dual luciferase reporter assay
Plasmid transfections were performed as follows: Cells were seeded into 12-well plates and allowed to adhere overnight. The next day, cells were forward transfected with the reporter plasmids SuperTopFlash or SuperFopFlash (Addgene, Cambridge, MA), and, to normalize for transfection efficiency, co-transfected with Renilla luciferase reporter plasmid (Promega) using X-tremeGENE HP DNA Transfection Reagent (Roche, Penzberg, Germany). Twenty-four hours after transfection, passive lysis buffer (Promega) was added, and both Firefly and Renilla luciferase activity was measured in a microplate reader (Mithras LB940, Berthold Technologies, Bad Wildbad, Germany). Relative transcription factor activity was calculated by dividing Renilla-normalized values of SuperTopFlash® reporter plasmid and SuperFopFlash control reporter plasmid. Details on experimental conditions are provided in Supplementary Table S1.
(Chemo-) Radiotherapy and determination of cell survival
To determine the respective surviving fractions after radiation treatment (RT) and CRT, a standard colony formation assay was performed. Defined numbers of cells growing in log-phase were seeded as single-cell suspensions into six-well plates (Supplementary Table S2), and subsequently radiated with a single dose of 1, 2, 4, 6 and 8 Gy of X-rays (Gulmay Medical, Camberley, UK). For CRT, cells were exposed to 3 μM of 5-FU (Sigma-Aldrich, Steinheim, Germany) for 16 hours prior to radiation. After defined time periods (Supplementary Table S2), cells were fixed with 70% ethanol and stained. Colonies with more than 50 cells were scored as survivors, and non-radiated cultures were used for data normalization. Resulting plating efficiencies for all colony formation assays can be found in Supplementary Table S5. Experiments were performed in triplicate (technical replicates) and independently repeated three times (biological replicates).
Exogenous stimulation of Wnt/β-catenin signaling using Wnt-3a
Wnt-3a-containing supernatant was produced from low-density seeded L-Wnt-3a cells, which were grown to confluence in DMEM/F12 medium. After approximately three days, medium was collected and stored at 4 °C (batch I). Subsequently, fresh DMEM/F12 medium was added and cells were grown for another three days, until medium was collected (batch II). Batches I and II were pooled and sterile filtered. The resulting medium was diluted 1:1 using fresh DMEM/F12 medium supplemented with 0.35% sodium bicarbonate (Biochrom, Berlin, Germany). As a negative control, wild-type L-cells were treated similarly to produce a supernatant without Wnt-3a. For colony formation assays, cells were seeded in 6-well plates in medium with or without Wnt-3a. 5-FU was added 16 hours prior to irradiation and removed by a media exchange, with or without Wnt-3a, 12 hours after irradiation (Supplementary Figure S4).
Overexpression of mutated β-catenin (S33Y)
RPE-1 cells were seeded into six-well plates and allowed to adhere overnight. Using 2.5 μl X-tremeGENE HP DNA Transfection Reagent (Roche), cells were transfected with 2.5 μg of pCl-neo-β-catenin (S33Y) (Addgene) or pCl-neo (empty vector control; Promega) plasmid DNA. Subsequently, cells were selected with 800 μg/ml G-418 (Biochrome) for two weeks.
Establishment of an isogenic radiation-resistant cell population (SW1463RES)
SW1463 cells were repeatedly radiated with 2 Gy of X-rays for 34 cycles, equaling to a cumulative dose of 68 Gy (Supplementary Figure S5A). Specifically, cells were seeded into six-well plates, radiated the next day, and cultured in fresh medium. At 70–80% confluence, they were exposed to the next cycle of radiation.
RNA isolation
For each condition (i.e., no irradiation (SW1463, SW1463RES) or 6 hours after exposure to 4 Gy of X-rays (SW1463-4 Gy and SW1463RES-4 Gy), total RNA was isolated from logarithmic growing cells using the RNeasy Mini kit (Qiagen, Hilden, Germany), per the manufacturer’s instructions, including the optional on-column DNase digestion (Supplementary Figure S5B). Quantity and purity of isolated RNA was assessed using a NanoDrop ND-1000 spectrophotometer (ThermoFischer, Waltham, MA); RNA integrity was assessed by Agilent 2100 Bioanalyzer electrophoresis (Agilent Technologies, Palo Alto, CA). Only samples with an RNA integrity number >9.5 were considered for additional experiments. Experiments were independently repeated three times (biological replicates).
Microarray-based global gene expression analyses
Gene expression profiling was performed as previously described (15,16,19). In brief, 200 ng of total RNA was reverse transcribed, amplified, and labeled with Cy3 using the Low RNA Input Linear Amplification Kit PLUS (Agilent Technologies). Subsequently, 1.65 μg of labeled cRNA was fragmentized and hybridized overnight to a 4 x 44 k gene expression microarray (Agilent Technologies). After a washing step, the arrays were scanned on an Agilent DNA microarray scanner G2505B (Agilent Technologies) at 5-micron resolution. The respective gene expression data has been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GSE97543).
Statistical analysis
For analyses of the irradiation data, a multiple linear regression model was used to describe the normalized surviving fraction as a dependent variable, given the independent variables of irradiation dose, treatment group (control group or treated group) and biological replicates. Two different models, including either only irradiation dose and replicates or, additionally, treatment effect and treatment and irradiation dose interaction term were compared using analysis of variance (ANOVA). All analyses were performed using the R statistical computing software R (version 3.1.0) using the R packages nlme and survival. For visualization, irradiation data are presented as mean and standard error of the mean (SEM) from at least three independent experiments using the software KaleidaGraph (version 4.1.0). P-values < 0.05 were considered significant, suggesting an influence of the treatment on the dose response.
Statistical analyses of cellular viability and luciferase reporter activity experiments were performed using an unpaired two-tailed Student’s t-test in Microsoft Excel and visualized in Grapher (version 8.2.460). Again, P-values < 0.05 were scored as significant.
To identify genes that are significantly altered in response to irradiation for each condition we performed a one sided, paired-sample student t-test to test whether the expression of each gene is increased or decreased as a response to irradiation. We then used false discovery rate (FDR) correction for multiple hypotheses with an FDR-level q < 0.05.
Overrepresented pathways were identified by using WikiPathway enrichment applying the hyper geometric enrichment test P-value < 0.05 (20).
Results
Inhibition of β-catenin increases treatment sensitivity of CRC cells
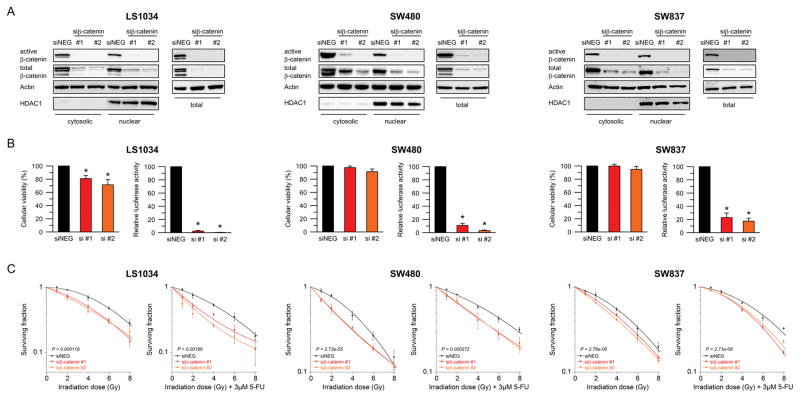
We previously reported a novel role for TCF7L2 in mediating responsiveness of CRC to CRT (8,9). To test whether the observed effect of radiosensitization is TCF7L2-specific or dependent on the Wnt/β-catenin pathway more generally, β-catenin expression was silenced in two colon cancer cell lines, SW480 and LS1034, and in the rectal cancer cell line SW837, using RNA interference. Successful silencing was confirmed for all cell lines at 24, 48, 72, and 96 hours post transfection by Western blot analysis (Supplementary Figure S1). Of note, we detected a pronounced reduction of nuclear, cytosolic and total expression levels of both active and total β-catenin (Figure 1A). siRNA-mediated silencing of β-catenin led to a mild reduction of cellular viability 48 hours after transfection, which was significant in LS1034 (Figure 1B, left panels), and was accompanied by a significantly decreased TCF/LEF reporter activity in all three cell lines (LS1034 si#1: P = 4.196e-08, si#2: P = 4.317e-11; SW480 si#1: P = 1.058e-05, si#2: P = 2.646e-08; SW837 si#1: P = 0.0004, si#2: P = 5.131 e-05) (Figure 1B, right panels). To assess the consequences of β-catenin inhibition on cellular sensitivity to CRT, we determined the respective surviving fractions following (chemo-) radiotherapy using a colony formation assay, as is standard in the field. Compared with the non-silencing control siRNA, silencing of β-catenin significantly increased the sensitivity of LS1034 (P = 0.000119), SW480 (P = 2.73e-05), and SW837 (P = 2.76e-06) cells to irradiation (Figure 1C, left panels). A similar effect was observed for a combination of 5-FU and irradiation (LS1034: P = 0.00186; SW480: P = 0.000272; SW837: P = 2.71e-08) (Figure 1C, right panels). Hereby, the addition of 5-FU only slightly increased the overall sensitivity to irradiation.
Figure 1.
siRNA-mediated silencing of β-catenin sensitizes CRC cells to (chemo-) radiotherapy. (A) Active β-catenin and total β-catenin protein levels decreased 48 hours after transfection with siRNAs targeting β-catenin compared to a non-specific negative-control (siNEG) in LS1034, SW480, and SW837. Proteins were isolated as cytosolic and nuclear fractions (left panel) and as whole protein lysates (right panel). (B) Cellular viability was measured 48 hours after transfection using a CellTiter-Blue® assay (left panel), and transcriptional activity of the TCF/LEF complex was determined using a dual luciferase reporter assay (right panel). While silencing of β-catenin resulted in a mild reduction of cellular viability, the TCF/LEF reporter activity decreased significantly (LS1034: P = 4.196e-08 (si#1), P = 4.317e-11 (si#2); SW480: P = 1.058e-05 (si#1), P = 2.646e-08 (si#2); SW837: P = 0.0004 (si#1), P = 5.131e-05 (si#2)). (C) Cell lines were irradiated 48 hours after transfection at various doses of X-rays (RT, left panel). For CRT, cells were pre-incubated, 24 hours after transfection, with 3 μM of 5-FU for 16 hours, and subsequently irradiated (right panel). Silencing of β-catenin significantly increased the sensitivity of LS1034 (RT: P = 0.000119, CRT: P = 0.00186; ANOVA model), SW480 (RT: P = 2.73e-05, CRT: P = 0.000272; ANOVA model), and SW837 (RT: P = 2.76e-06, CRT: P = 2.71e-08) to (chemo-) radiotherapy. Each experiment was repeated three times. Data are displayed as mean values, n = 3, error bars ± SEM.
Next, we aimed to test whether we can recapitulate this sensitization effect using a small-molecule inhibitor of Wnt/β-catenin signaling. Towards this goal, we used the potent and clinically promising tankyrase inhibitor XAV-939 (21). Tankyrase inhibitors have been previously identified to inhibit β-catenin-mediated transcription by blocking Tankyrase isoforms 1 and 2 thereby stabilizing Axin through preventing its degradation (18). SW837 and SW480 cells were incubated with varying doses of XAV-939 for multiple time periods (Supplementary Figure S2A, B), and both induction of Axin2 protein expression and reduction of unphosphorylated (active) β-catenin protein levels were validated by Western blot analysis. For each cell line two doses were established, one inducing Axin2 and consequently reducing (active) β-catenin by approx. 50%, the second (higher one) raising the Axin2 levels even more and reducing (active) β-catenin as much as possible without decreasing cellular viability (Supplementary Figure S3A, B). As previously described (18), treatment with XAV-939 resulted in a viability reduction of up to 20% (Supplementary Figure S3B). Importantly, treatment with XAV-939 led to a significant radio sensitization of SW480 (1 μM P = 0.016; 4 μM P = 0.0112) and SW837 (5 μM P = 0.0223; 10 μM P =0.000264). The sensitization effect to RT was stronger with higher doses of XAV-939, but less prominent as observed with siRNAs targeting β-catenin (Supplementary Figure S3C).
These results suggest that both TCF7L2, as previously demonstrated (8,9), and β-catenin influence responsiveness to CRT.
Wnt-3a-stimulation leads to treatment resistance in non-tumorigenic epithelial cells
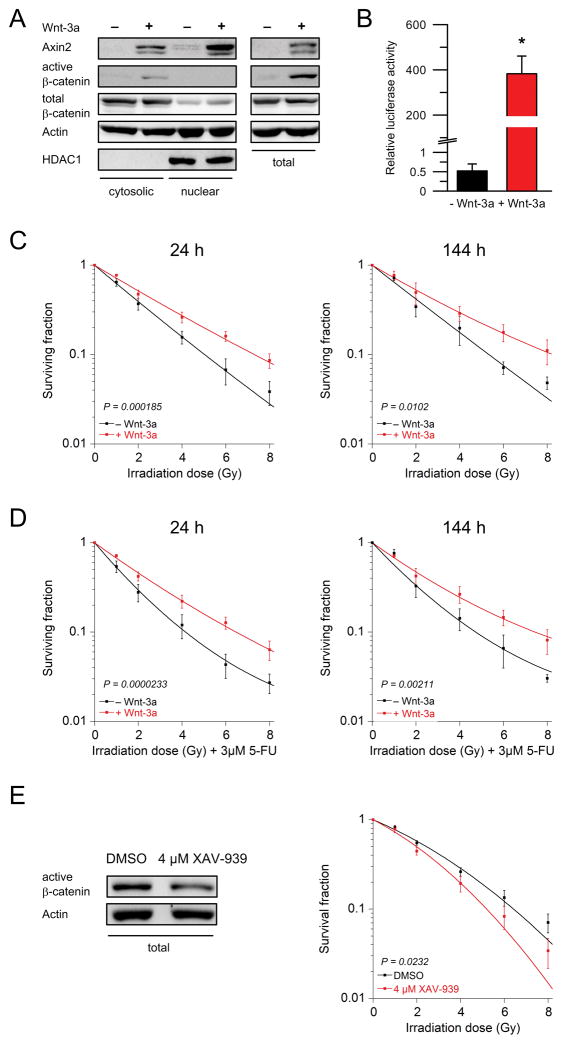
To further investigate whether responsiveness to CRT is not only β-catenin- and TCF7L2-dependent, but also Wnt/β-catenin-dependent, we used RPE-1 cells, which are highly sensitive to irradiation. RPE-1 cells represent a non-tumorigenic epithelial cell model system with a stable karyotype (13,22). Incubation with Wnt-3a, a physiological ligand of the Frizzled receptor family, induced protein expression of Axin2 and active (unphosphorylated) β-catenin (Figure 2A), and resulted in a ~800 fold increased TCF/LEF reporter activity (P = 0.0105, Figure 2B). Importantly, external stimulation of Wnt/β-catenin signaling with Wnt-3a significantly increased the resistance to both irradiation (24 h: P = 0.000185, 144 h: P = 0.0102; Figure 2C) and CRT (24 h: P = 0.0000233, 144 h: P = 0.00211; Figure 2D). Conversely, exposure to XAV-939 resulted in a significantly increased sensitivity of RPE-1 cells to irradiation (P = 0.0232; Figure 2E and Supplementary Figure S2C). This indicates that responsiveness to (chemo-) radiotherapy is Wnt/β-catenin dependent, and that the mechanism of Wnt/β-catenin-controlled responsiveness is not restricted to malignant cells but points to a universal phenomenon.
Figure 2.
Wnt-3a-stimulation induces treatment resistance in non-tumorigenic epithelial cells. (A) Incubation of RPE-1 cells with Wnt-3a for 24 hours (h) resulted in increased protein levels of Axin2 and active β-catenin. Proteins were isolated as cytosolic and nuclear fractions (left panel), and as whole protein lysates (right panel). (B) Similarly, stimulation with Wnt-3a resulted in a ~800 fold increased TCF/LEF reporter activity (P = 0.0105). (C, D) Treatment with Wnt-3a significantly increased resistance to both irradiation and CRT after 24 hours (RT: P = 0.000185, CRT: P = 0.0000233; ANOVA model) and 144 hours (RT: P = 0.0102, CRT: P = 0.00211; ANOVA model). (E) Exposure to 4 μM of XAV-939 for 24 hours significantly sensitized RPE-1 cells to irradiation (P = 0.0232). Each experiment was repeated three times. Data are displayed as mean values, n = 3, error bars ± SEM.
Overexpression of mutated β-catenin (S33Y) leads to treatment resistance in non-tumorigenic epithelial cells
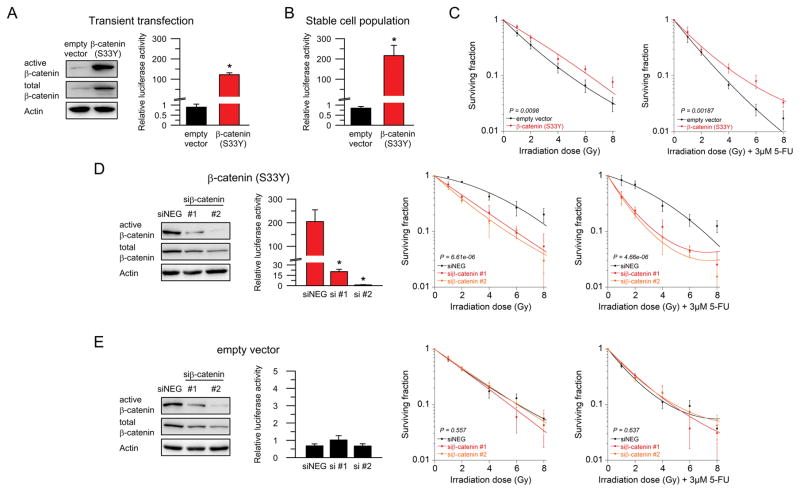
To obtain further evidence that (chemo-) radiotherapy responsiveness is Wnt/β-catenin dependent, and to rule out potential Wnt- or β-catenin-independent effects of Wnt-3a (3,23), we stimulated the pathway through overexpression of a constitutively active β-catenin (S33Y mutated) protein, which cannot be inactivated by the degradation complex. We first demonstrated that transient transfection of mutated β-catenin resulted in increased protein levels of active and total β-catenin, and in significantly elevated TCF/LEF reporter activity 24 hours after transfection of RPE-1 cells (P = 0.0002, Figure 3A). Consistent with these findings, stable overexpression of mutated β-catenin in RPE-1 cells resulted in a ~250 fold increased TCF/LEF reporter activity (P = 0.0017), as shown in Figure 3B. Importantly, stimulation of Wnt/β-catenin signaling significantly increased the resistance of RPE-1 cells to both irradiation (P = 0.0098; Figure 3C, left panel) and CRT (P = 0.00187; Figure 3C, right panel), further supporting our hypothesis that Wnt/β-catenin signaling, and not a β-catenin independent mechanism, controls responsiveness to CRT. To test whether this effect can be reversed, we transfected RPE-1 cells that stably express mutated β-catenin (S33Y) with siRNAs targeting β-catenin. As expected, this significantly reduced β-catenin protein levels and the TCF/LEF reporter activity (si#1: P = 0.010, si#2: P = 0.0063; Figure 3D, left panels), and abrogated the resistance effect after radiation (P = 6.61e-06; Figure 3D, middle panel) and CRT (P = 4.66e-06; Figure 3D, right panel). In contrast, silencing of β-catenin in RPE-1 cells that stably express the empty vector did neither affect TCF/LEF reporter activity nor treatment sensitivity (Figure 3E).
Figure 3.
Overexpression of mutated β-catenin (S33Y) induces treatment resistance in non-tumorigenic epithelial cells. (A) Transient transfection of mutated β-catenin resulted in increased protein levels of active and total β-catenin (left panel), and in elevated TCF/LEF reporter activity (P = 0.0002, right panel). (B, C) Stable overexpression of mutated β-catenin resulted in a ~250 fold increased TCF/LEF reporter activity (P = 0.0017), and significantly decreased the sensitivity of RPE-1 cells to both irradiation (P = 0.0098; ANOVA model) and CRT (P = 0.00187; ANOVA model). (D) Silencing of β-catenin in RPE-1 cells that stably express mutated β-catenin (S33Y) significantly decreased β-catenin protein expression and TCF/LEF reporter activity (si#1: P = 0.010, si#2: P = 0.0063; left panels), and abrogated the resistance to radiotherapy (P = 6.61e-06; middle panel) and CRT (P = 4.66e-06; right panel). (E) In control RPE-1 cells that stably express the empty vector, silencing of β-catenin did neither affect TCF/LEF reporter activity (left panel) nor treatment sensitivity (RT: P = 0.557; CRT P = 0.637). Each experiment was repeated three times. Data are displayed as mean values, n = 3, error bars ± SEM.
Isogenic radiation-resistant cells show elevated Wnt/β-catenin signaling activity
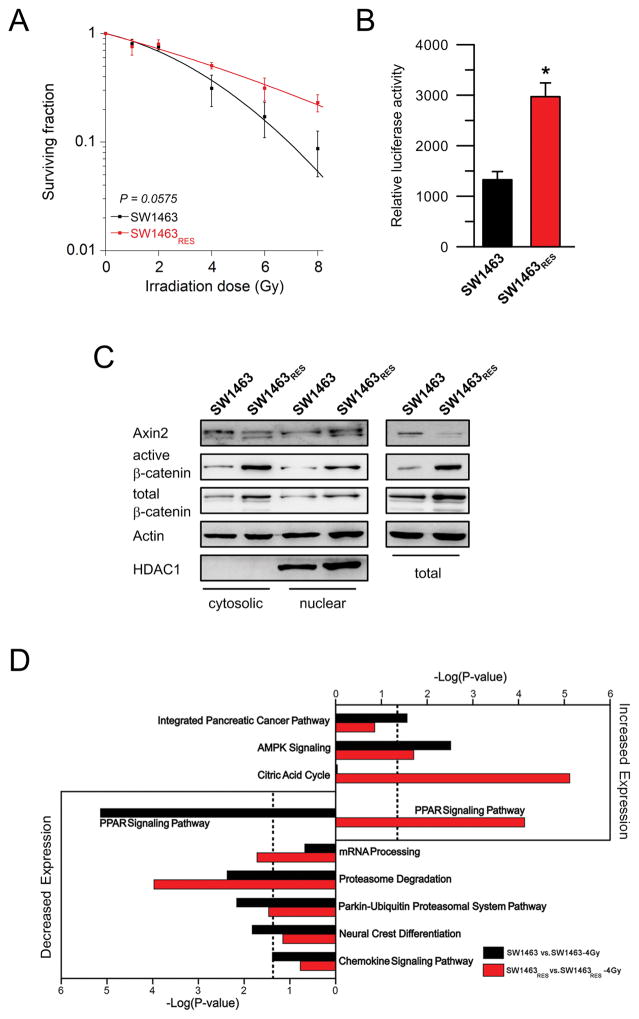
To obtain further evidence that Wnt/β-catenin signaling regulates responsiveness of CRC cells to CRT, we repetitively irradiated SW1463 cells in order to establish an isogenic radioresistant cell population (Supplementary Figure S5A), an approach well described in the literature (24). As shown in Figure 4A, repeated irradiation with 2 Gy (total dose of 68 Gy after 35 weeks) resulted in the development of a SW1463 cell population with increased resistance to irradiation (P = 0.0575), referred to as SW1463RES. Importantly, and consistent with our hypothesis, this increased resistance was accompanied by a significantly increased TCF/LEF transcriptional activity (P = 0.002, Figure 4B), and elevated nuclear, cytosolic and total protein levels of both active and total β-catenin, as measured by Western blot analysis (Figure 4C). Protein levels of Axin2, a negative feedback regulator of Wnt/β-catenin (25), were not increased. These findings suggest that increased signaling activity of the Wnt/β-catenin pathway confers a selective advantage to survive irradiation.
Figure 4.
Isogenic radiation-resistant CRC cells show elevated Wnt/β-catenin signaling activity. (A) SW1463 cells were repetitively irradiated with 2 Gy (total dose of 68 Gy), which resulted in a cell population (SW1463RES) with a decreased sensitivity to irradiation (P = 0.0575; ANOVA model). (B) This increased treatment resistance was accompanied by a significantly increased TCF/LEF transcriptional activity (P = 0.002). (C) Protein levels of both active and total β-catenin were elevated in radiation-resistant SW1463RES cells. Proteins were isolated as cytosolic and nuclear fractions (left panel) and whole protein lysates (right panel). Each experiment was repeated three times. Data are displayed as mean values, n = 3, error bars ± SEM. (D) SW1463RES cells activate distinct pathways in response to a single dose of 4 Gy of X-Rays compared to SW1463 cells. Pathway analysis is based on gene expression profiles of three biological replicates of each condition (SW1463, SW1463-4 Gy, SW1463RES, SW1463RES-4 Gy).
Radioresistant colorectal cancer cells show deregulated RNA expression profiles and activate different pathways in response to irradiation
To elucidate how Wnt/β-catenin signaling mediates resistance, we established gene expression profiles of SW1463 and radio-resistant SW1463 (SW1463RES), both prior to and 6 h after exposure to a single dose of 4 Gy (Supplementary Figure S5B). As expected, we found a significant (P = 0.05) overexpression of Wnt/β-catenin signature genes in SW1463RES compared to SW1463, including increased expression of LEF1, part of the TCF/LEF1 transcription factor complex, and WNT5B (Supplementary Table S6A)
To now assess transcriptional changes in response to radiation and to detect different activation of signaling pathways, we first identified genes differentially expressed between SW1463 cells and SW1463 cells six hours after exposure to 4 Gy (SW1463-4 Gy; Supplementary Table S6B). Next, we profiled our radiation resistant SW1463 cells, prior to (SW1463RES) and six hours after exposure to 4 Gy (SW1463RES-4 Gy) (Supplementary Table S6C). Both populations responded to radiation with a significant up and downregulation of numerous genes and pathways, which can be found in Figure 4D and Supplementary Table S6B and S6C. In contrast to SW1463 cells, which responded to radiation with 4 Gy with significantly decreased expression of genes belonging to the PPAR signaling, we observed increased expression of PPAR pathway genes in SW1463RES in response to radiation with 4 Gy (Figure 4D). Additionally, SW1463RES cells responded with overexpression of genes belonging to metabolic pathways, such as AMPK signaling or the citric acid cycle (Figure 4D).
Discussion
Preoperative CRT followed by radical surgical resection represents the standard of care for patients with locally advanced rectal cancer (4,11,26). However, the response of individual tumors to preoperative multimodal treatment is highly heterogeneous and ranges from complete clinical response to absence of any histopathological tumor regression (complete resistance). This poses a clinical dilemma, because patients with resistant tumors are exposed to the potential side effects of chemotherapy and irradiation with no clear benefit. It is therefore critical to uncover mechanisms and pathways of treatment resistance for the identification of strategies to increase the fraction of patients with rectal cancer who benefit from multimodal neoadjuvant treatment (10).
In an attempt to identify novel molecular targets and pathways that may be manipulated to sensitize tumors to CRT, we previously demonstrated that the Wnt transcription factor TCF7L2 is overexpressed in CRT-resistant rectal cancers (8). Subsequently, we showed that RNAi-mediated silencing of TCF7L2 sensitizes colon and rectal cancer cell lines to CRT (9). This radio-sensitization was the consequence of a transcriptional deregulation of Wnt/TCF7L2 target genes, and a compromised DNA double strand break repair. In addition, silencing of TCF7L2 resulted in an increased fraction of cells in the G2/M phase of the cell cycle, which is known for increased vulnerability to radiation-induced DNA damage (27). However, it remained unclear whether this effect was a TCF7L2-inherent function or Wnt/β-catenin signaling-dependent. In the present study, we confirm that the Wnt/β-catenin pathway mediates resistance of CRC cells to irradiation and 5-FU-based CRT.
We demonstrated that inhibition of β-catenin, either mediated through siRNAs or the small-molecule inhibitor XAV-939, sensitizes CRC cells to irradiation. This adds weight to the growing body of evidence suggesting that Wnt/β-catenin signaling mediates treatment responsiveness, in addition to its central role in tumor development and progression (1,2). Recently, Cojoc and colleagues established gene expression profiles of prostate cancer cell lines and derived radio-resistant clones and discovered that β-catenin regulates aldehyde dehydrogenase (ALDH1A3) (28). RNAi-mediated silencing of β-catenin and ALDH1A3 led to a pronounced radiosensitization of a priori resistant cells (28). Fitting, in our experiment, radio-sensitive SW1463 cells responded with a significant downregulation of ALDH1A3 to radiation. Dong and colleagues demonstrated a role for Wnt/β-catenin signaling in radiation-induced invasion of glioblastoma cells (29). In their model, radiation mediated nuclear accumulation of β-catenin and an up-regulation of Wnt/β-catenin downstream genes. Pathway inhibition abrogated the pro-invasion effects of radiotherapy (29). Similar to our approach, Ahn et al. repeatedly irradiated lung cancer cells to obtain resistant cell populations. Using gene expression profiling, they identified multiple genes that were differentially expressed between resistant cells and their parental cell lines. Wnt/β-catenin pathway genes were the most frequently altered (30). In our model, the increased resistance of SW1463RES was accompanied by elevated levels of active and total β-catenin, and increased TCF/LEF transcriptional activity. Interestingly, several of the genes described by Ahn and colleagues (24) were differentially deregulated in a similar fashion in our model, including many Wnt pathway genes. This points to an involvement of Wnt/β-catenin signaling in radiation resistance in multiple tumor entities. Of note, we previously characterized the CRT-sensitivity of 12 colorectal cancer cell lines, which we correlated with gene expression profiles. Importantly, and nicely fitting with the observations reported here, we detected an overrepresentation of Wnt-pathway and Wnt-target genes within this signature of chemoradiosensitivity (15).
From a clinical point of view, one goal is to improve sensitivity to CRT. Of equal importance is to decrease potential side effects of irradiation, specifically, to decrease normal tissue toxicity (31). In this respect, Hai and colleagues demonstrated that transient activation of Wnt/β-catenin signaling prevents radiation-induced damage to salivary glands (32). Zhao and colleagues observed that an upregulation of the Wnt/β-catenin pathway accelerates mucosal repair following radiotherapy-induced oral mucositis (33). Very recently, Chandra and colleagues demonstrated that an activated Wnt/β-catenin pathway blocks radiation-induced apoptosis in osteoblasts through enhanced DNA repair, suggesting that Wnt agonists may be clinically used to block radiation-induced osteoporosis (34). In a similar attempt, we stimulated phenotypically “normal” RPE-1 cells, which are highly sensitive to irradiation, with Wnt-3a, a physiological ligand of the Frizzled receptor family. Treatment with Wnt-3a resulted in a strong activation of Wnt/β-catenin signaling, with increased expression levels of both Axin2 and β-catenin, accompanied by a ~800-fold increase of TCF/LEF reporter activity. Importantly, however, Wnt-3a stimulation resulted in significantly increased resistance to irradiation, while inhibition of β-catenin by XAV-939 sensitized RPE-1 to irradiation. A similar effect was observed through overexpression of constitutively active (S33Y-mutated) β-catenin in RPE-1 cells, which also resulted in a strong activation of Wnt/β-catenin signaling and which increased radiation resistance. This effect could be rescued by siRNA-mediated silencing of β-catenin. These results further support the notion that Wnt/β-catenin signaling controls responsiveness to CRT, and that this effect is not due to a β-catenin-independent branch of Wnt signaling (23).
However, open questions remain: Firstly, the underlying molecular mechanisms through which the Wnt/β-catenin pathway functionally mediates responsiveness to CRT are still not fully understood. Preliminary evidence indicates that Wnt/β-catenin signaling may trigger epithelial to mesenchymal transition (EMT), which has been implicated in increased resistance to radiotherapy (28,35–38). In our gene expression data, we observed an overexpression of genes associated with EMT in resistant cell populations, underscoring the importance of the Wnt/β-catenin EMT axis (data not shown). Recently, Jun et al. suggested a role for non-homologous end joining (NHEJ) in Wnt/β-catenin mediated radiation resistance (39). By screening DNA repair genes and β-catenin targets they identified LIG4, whose expression was directly regulated by β-catenin and observed that deregulation of LIG4 mediates resistance of colon cancer cell lines to radiation. However, we did not observe changes in LIG4 expression when comparing SW1463 and Wnt-active radiation resistant SW1463RES. In addition, LIG4 expression was not associated with treatment response in a set of 161 primary rectal cancers treated with 5-FU-based chemoradiotherapy (Emons et al., unpublished data). This leads to the conclusion that, while the activation of LIG4 may represent one possible mechanism through which Wnt/β-catenin signaling controls responsiveness in colon cancer cells, there likely exist other resistance mechanisms in rectal cancer.
Of note, our data suggests a role for PPAR signaling in mediating radiation resistance. We observed increased expression of genes from the PPAR pathway in response to 4 Gy in SW1463RES, while SW1463 cells reacted in a completely opposite manner, i.e., with a decrease in expression of these genes. It has been previously shown that PPAR is a downstream pathway of Wnt/β-catenin/TCF7L2 signaling (40), and that PPAR signaling, besides its prominent role in providing metabolic advantages for cancer cells (41), prevents radiation induced cellular damage. For example, a PPAR-gamma agonist has been recently shown to protect normal tissue from radiation injury (42). Finally, the pathway is associated with a poor prognosis in a variety of human carcinomas (43,44). Therefore, we speculate that PPAR signaling might be a novel mechanism through which Wnt signaling mediates radio resistance.
Our study did not address the question whether Wnt/β-catenin pathway inhibition in vivo sensitizes to CRT. However, several studies reported an association between treatment resistance and the expression of members of the Wnt/β-catenin pathway (45,46). As described above, we previously used pre-therapeutic gene expression profiling of tumor biopsies to demonstrate that TCF7L2 was expressed at higher levels in resistant compared to responsive primary rectal cancers (8). Using immunohistochemical analyses, Kriegl and colleagues reported that TCF7L2 expression was a negative prognostic factor associated with a shorter overall survival of CRC patients (47). Similarly, Gomez-Millan and colleagues demonstrated that overexpression of β-catenin following CRT was associated with a decreased disease-free survival and a poor prognosis of rectal cancer patients (45).
In summary, our data demonstrate that Wnt/β-catenin signaling mediates responsiveness of rectal cancers to CRT. We suggest that targeting Wnt/β-catenin signaling or one of the downstream pathways represent a promising strategy to increase therapeutic responsiveness of rectal cancers to CRT, the standard treatment for patients with locally advanced stages of this disease (4,11,26).
Supplementary Material
Acknowledgments
Financial support: This work was supported by the Deutsche Forschungsgemeinschaft (Klinische Forschergruppe 179) and the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
The authors are grateful to Jessica Eggert and Stefanie Müller for excellent technical support, to Markus Brown and Reinhard Ebner for critical discussions on the manuscript, and to Michael Klintschar for short-tandem repeat analyses. Materials and data from this manuscript are part of the doctoral theses of Janneke Möller and Sebastian Reinecke.
Abbreviations
- CRC
colorectal cancer
- APC
adenomatous polyposis coli
- TCF
T-cell factor
- LEF
lymphoid enhancer factor
- RT
radiotherapy
- CRT
chemoradiotherapy
- 5-FU
5-fluorouracil
- RPE
retinal pigment epithelial
References
- 1.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 2.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Nusse R, Clevers H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169(6):985–99. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet. 2010;375(9719):1030–47. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 5.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361(25):2449–60. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–7. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghadimi BM, Grade M, Difilippantonio MJ, Varma S, Simon R, Montagna C, et al. Effectiveness of gene expression profiling for response prediction of rectal adenocarcinomas to preoperative chemoradiotherapy. J Clin Oncol. 2005;23(9):1826–38. doi: 10.1200/JCO.2005.00.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendziorra E, Ahlborn K, Spitzner M, Rave-Frank M, Emons G, Gaedcke J, et al. Silencing of the Wnt transcription factor TCF4 sensitizes colorectal cancer cells to (chemo-) radiotherapy. Carcinogenesis. 2011;32(12):1824–31. doi: 10.1093/carcin/bgr222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grade M, Wolff HA, Gaedcke J, Ghadimi BM. The molecular basis of chemoradiosensitivity in rectal cancer: implications for personalized therapies. Langenbecks Arch Surg. 2012;397(4):543–55. doi: 10.1007/s00423-012-0929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodel C, Hofheinz R, Liersch T. Rectal cancer: state of the art in 2012. Curr Opin Oncol. 2012;24(4):441–7. doi: 10.1097/CCO.0b013e328352ea02. [DOI] [PubMed] [Google Scholar]
- 12.Masters JR, Thomson JA, Daly-Burns B, Reid YA, Dirks WG, Packer P, et al. Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci U S A. 2001;98(14):8012–7. doi: 10.1073/pnas.121616198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423(6938):448–52. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 14.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279(5349):349–52. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 15.Spitzner M, Emons G, Kramer F, Gaedcke J, Rave-Frank M, Scharf JG, et al. A gene expression signature for chemoradiosensitivity of colorectal cancer cells. Int J Radiat Oncol Biol Phys. 2010;78(4):1184–92. doi: 10.1016/j.ijrobp.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grade M, Hummon AB, Camps J, Emons G, Spitzner M, Gaedcke J, et al. A genomic strategy for the functional validation of colorectal cancer genes identifies potential therapeutic targets. Int J Cancer. 2011;128(5):1069–79. doi: 10.1002/ijc.25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spitzner M, Roesler B, Bielfeld C, Emons G, Gaedcke J, Wolff HA, et al. STAT3 inhibition sensitizes colorectal cancer to chemoradiotherapy in vitro and in vivo. Int J Cancer. 2014;134(4):997–1007. doi: 10.1002/ijc.28429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–20. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 19.Camps J, Pitt JJ, Emons G, Hummon AB, Case CM, Grade M, et al. Genetic amplification of the NOTCH modulator LNX2 upregulates the WNT/beta-catenin pathway in colorectal cancer. Cancer Res. 2013;73(6):2003–13. doi: 10.1158/0008-5472.CAN-12-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pico AR, Kelder T, van Iersel MP, Hanspers K, Conklin BR, Evelo C. WikiPathways: pathway editing for the people. PLoS Biol. 2008;6(7):e184. doi: 10.1371/journal.pbio.0060184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afifi MM, Austin LA, Mackey MA, El-Sayed MA. XAV-939: from a small inhibitor to a potent drug bioconjugate when delivered by gold nanoparticles. Bioconjug Chem. 2014;25(2):207–15. doi: 10.1021/bc400271x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang XR, Jimenez G, Chang E, Frolkis M, Kusler B, Sage M, et al. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat Genet. 1999;21(1):111–4. doi: 10.1038/5056. [DOI] [PubMed] [Google Scholar]
- 23.Acebron SP, Niehrs C. beta-Catenin-Independent Roles of Wnt/LRP6 Signaling. Trends Cell Biol. 2016;26(12):956–67. doi: 10.1016/j.tcb.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 24.McDermott N, Meunier A, Lynch TH, Hollywood D, Marignol L. Isogenic radiation resistant cell lines: development and validation strategies. Int J Radiat Biol. 2014;90(2):115–26. doi: 10.3109/09553002.2014.873557. [DOI] [PubMed] [Google Scholar]
- 25.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol. 2015;33(16):1797–808. doi: 10.1200/JCO.2014.60.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seiwert TY, Salama JK, Vokes EE. The concurrent chemoradiation paradigm--general principles. Nat Clin Pract Oncol. 2007;4(2):86–100. doi: 10.1038/ncponc0714. [DOI] [PubMed] [Google Scholar]
- 28.Cojoc M, Peitzsch C, Kurth I, Trautmann F, Kunz-Schughart LA, Telegeev GD, et al. Aldehyde Dehydrogenase Is Regulated by beta-Catenin/TCF and Promotes Radioresistance in Prostate Cancer Progenitor Cells. Cancer Res. 2015;75(7):1482–94. doi: 10.1158/0008-5472.CAN-14-1924. [DOI] [PubMed] [Google Scholar]
- 29.Dong Z, Zhou L, Han N, Zhang M, Lyu X. Wnt/beta-catenin pathway involvement in ionizing radiation-induced invasion of U87 glioblastoma cells. Strahlenther Onkol. 2015;191(8):672–80. doi: 10.1007/s00066-015-0858-7. [DOI] [PubMed] [Google Scholar]
- 30.Ahn SJ, Choi C, Choi YD, Kim YC, Kim KS, Oh IJ, et al. Microarray analysis of gene expression in lung cancer cell lines treated by fractionated irradiation. Anticancer Res. 2014;34(9):4939–48. [PubMed] [Google Scholar]
- 31.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11(4):239–53. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 32.Hai B, Yang Z, Shangguan L, Zhao Y, Boyer A, Liu F. Concurrent transient activation of Wnt/beta-catenin pathway prevents radiation damage to salivary glands. Int J Radiat Oncol Biol Phys. 2012;83(1):e109–16. doi: 10.1016/j.ijrobp.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao J, Kim KA, De Vera J, Palencia S, Wagle M, Abo A. R-Spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical Wnt/beta-catenin pathway. Proc Natl Acad Sci U S A. 2009;106(7):2331–6. doi: 10.1073/pnas.0805159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandra A, Lin T, Zhu J, Tong W, Huo Y, Jia H, et al. PTH1-34 blocks radiation-induced osteoblast apoptosis by enhancing DNA repair through canonical Wnt pathway. J Biol Chem. 2015;290(1):157–67. doi: 10.1074/jbc.M114.608158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su H, Jin X, Zhang X, Zhao L, Lin B, Li L, et al. FH535 increases the radiosensitivity and reverses epithelial-to-mesenchymal transition of radioresistant esophageal cancer cell line KYSE-150R. J Transl Med. 2015;13:104. doi: 10.1186/s12967-015-0464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27(9):2059–68. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 37.Marie-Egyptienne DT, Lohse I, Hill RP. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: potential role of hypoxia. Cancer Lett. 2013;341(1):63–72. doi: 10.1016/j.canlet.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Gomez-Casal R, Bhattacharya C, Ganesh N, Bailey L, Basse P, Gibson M, et al. Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes. Mol Cancer. 2013;12(1):94. doi: 10.1186/1476-4598-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jun S, Jung YS, Suh HN, Wang W, Kim MJ, Oh YS, et al. LIG4 mediates Wnt signalling-induced radioresistance. Nat Commun. 2016;7:10994. doi: 10.1038/ncomms10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jansson EA, Are A, Greicius G, Kuo IC, Kelly D, Arulampalam V, et al. The Wnt/beta-catenin signaling pathway targets PPARgamma activity in colon cancer cells. Proc Natl Acad Sci U S A. 2005;102(5):1460–5. doi: 10.1073/pnas.0405928102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer. 2012;12(3):181–95. doi: 10.1038/nrc3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangoni M, Sottili M, Gerini C, Desideri I, Bastida C, Pallotta S, et al. A PPAR gamma agonist protects against oral mucositis induced by irradiation in a murine model. Oral Oncol. 2017;64:52–8. doi: 10.1016/j.oraloncology.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Nath A, Chan C. Genetic alterations in fatty acid transport and metabolism genes are associated with metastatic progression and poor prognosis of human cancers. Sci Rep. 2016;6:18669. doi: 10.1038/srep18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dey N, Young B, Abramovitz M, Bouzyk M, Barwick B, De P, et al. Differential activation of Wnt-beta-catenin pathway in triple negative breast cancer increases MMP7 in a PTEN dependent manner. PLoS One. 2013;8(10):e77425. doi: 10.1371/journal.pone.0077425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez-Millan J, Perez L, Aroca I, Del Mar Delgado M, De Luque V, Roman A, et al. Preoperative chemoradiotherapy in rectal cancer induces changes in the expression of nuclear beta-catenin: prognostic significance. BMC Cancer. 2014;14:192. doi: 10.1186/1471-2407-14-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi H, Nakamura K, Usami A, Tsuruta T, Hashimura M, Matsumoto T, et al. Possible role of nuclear beta-catenin in resistance to preoperative chemoradiotherapy in locally advanced rectal cancer. Histopathology. 2017 doi: 10.1111/his.13227. [DOI] [PubMed] [Google Scholar]
- 47.Kriegl L, Horst D, Reiche JA, Engel J, Kirchner T, Jung A. LEF-1 and TCF4 expression correlate inversely with survival in colorectal cancer. J Transl Med. 2010;8:123. doi: 10.1186/1479-5876-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.