Abstract
Purpose of the review
Radiation became a pillar of oncologic treatment in the last century and provided a powerful and effective locoregional treatment of solid malignancies. After achieving some of the first cures in lymphomas and skin cancers, it assumed a key role in curative treatment of epithelioid malignancies. Despite success across a variety of histologic types, glioblastoma (GBM), the most common primary brain tumor afflicting adults, remains ultimately resistant to current radiation strategies. While GBMs demonstrate an initial response, recurrence is essentially universal and fatal, and typically reoccur in the areas that received the most intense radiation.
Recent Findings
Glioma stem cells (GSCs), a subpopulation of tumor cells with expression profiles similar to neural stem cells and marked self-renewal capacities, have been shown to drive tumor recurrence and preclude curative radiotherapy. Recent research has shown that these cells have enhanced DNA repair capacity, elevated resistance to cytotoxic ion fluxes and escape multi-modality therapies.
Summary
We will analyze the current understanding of GSCs and radiation by highlighting key discoveries probing their ability to withstand radiotherapy. We then speculate on novel mechanisms by which GSC can be made sensitive to or specifically targeted by radiation therapy.
Keywords: Cancer Stem Cells, Radiation Oncology, Glioblastoma Multiforme, Translational Oncology
Introduction
Following Roentgen’s discovery of x-rays in 1898, radiation was identified as an effective treatment for cancer. After early treatment of skin malignancies, it led to curative treatment for Hodgkin’s lymphoma and ushered in a new era of lymphoma treatment(1). With the advent of the linear accelerator, powerful, penetrating beams could be delivered focally, increasing dose and efficacy while reducing toxicity. With image-guidance and computer control radiotherapy has become critical for curative treatments of multiple epithelioid cancers including head-and-neck, gastrointestinal, and gynecologic cancers. While successful for the treatment of the aforementioned neoplasms, radiation has proven ineffective for curing glioblastoma (GBM), the most common and lethal brain tumor in adults. GBM demonstrates a remarkable ability to resist radiotherapy and generate aggressive recurrent tumors following treatment. This resistance persists despite the use of modern image-guided dose-escalated conformal radiotherapy, radiosurgery, and brachytherapy.
The inability of radiation to prevent recurrence is not from insufficient doses. Pivotal trials by the Brain Tumor Study Group (BTSG) and the Medical Research Council (MRC) demonstrated that doses up to 60Gy, led to some improvement in survival. However, further attempts at dose intensification failed(2, 3). Subsequent studies of 70Gy, 72Gy, or higher doses failed to demonstrate improvement in outcomes(4). Indeed, the University of Michigan trial examining focal dose escalation to 90Gy – exceeding currently considered “safe” dose limits of normal brain– found that 91% of recurrences occurred in the high dose region(5). I-125 high dose brachytherapy (GliaSite) to the surgical bed was also without significant improvement(6). No matter the dose, the majority of these tumors recurred. This paradigm differs from nearly every other solid malignancy, in which dose escalation has enhanced patient survival and limited recurrence(7, 8). Clearly, some powerful mechanisms must endow GBM tumors with this ability to survive extremely high dose radiotherapy.
A growing body of evidence indicates that a specific subpopulations of glioma cells, termed glioma stem cells (GSCs), underlie this recurrence in the face of aggressive multimodal therapies. A landmark study conducted by Rich and colleagues demonstrated that GSCs isolated from human tumor samples survive irradiation better than non-GSC matched populations(9, 10). This resistance in the face of radiation has been confirmed by many studies(11, 12). As GSCs have enhanced tumor-forming abilities in engraftment studies carried out in immunocompromised mice(13), the following theory has been proposed: GSCs, having survived the onslaught of radiation treatment, repopulate the tumor and generate more aggressive treatment-resistant recurrences. Such a notion is supported by recent clinical observation where both recurrent tumors and tumors examined immediately after chemo- and radiotherapy have been shown to contain a higher proportion of GSCs(14). These finding can be easily explained by the Darwinian selection, where therapy resistant GSC population can survive anti-cancer therapies and thus repopulate the tumor and generate GSC-enriched recurrent disease. However, recent findings complicate this story, as our group and others have demonstrated that stemness in cancer is a dynamic process, readily influenced by microenvironmental factors like hypoxia, intratumoral pH, and even therapeutic stress(15–20). Thus, available experimental evidence suggests that not only are some cells intrinsically resistant to radiation, but differentiated nonresistant GBM cells can also attain a radiation-resistant-GSC state in response to therapeutic stress. Given this ability of GSC to resist radiation therapy, we must engage two questions: how do GSCs resist current radiation treatments and how can novel therapeutics disrupt these defenses? This review will probe these two questions, analyze our current understanding of GSC biology and radiation, and speculate on forthcoming innovations in radiotherapy for GBM.
I. Mechanisms of radiation-induced cytotoxicity
Before discussing how GSCs resist current radiation strategies, we must first characterize the mechanisms by which radiotherapy kills cancer cells. Three key factors play a role in this process—i) direct DNA damage, ii) indirect damage of DNA induced by the formation of reactive oxygen species (ROS), and iii) secondary dysregulation of intracellular ion buffering(21). In order for these effects to lead to apoptosis, a specific series of molecular events and cellular processes are required. An examination of both the initial cellular insult caused by radiotherapy and subsequent cellular responses will foreshadow many of the key factors underlying the ability of GSCs to resist radiation.
It has been established for decades that radiation damages DNA in two ways—direct damage of the DNA strand and indirect damage secondary to the formation of reactive oxygen species (ROS)(22). With direct damage, the energy of the radioactive particle strikes the electron cloud of target molecules, displacing electrons and breaking covalent bonds necessary for the molecule’s structure and function(23). In the case of DNA, where the particular structure of each nucleotide is crucial for the successful replication and transcription of genes, such damage can be catastrophic. Indirect DNA damage relies on the ability of radiation to generate ROS—molecular species characterized by the presence of unbound, free electrons. Like direct damage, these ROS can dismantle covalent bonds in the DNA backbone. While this damage can come in many forms, including double-stranded breaks (DSB) and single stranded breaks (SSB), it is thought that DSBs are responsible for the majority of cell death (and overall clinical efficacy) of radiation treatment(24). Given the extreme problems caused by broken chromosomes, healthy cells will go to great lengths to avoid passing on this DSB to daughter cells.
The cellular response to DNA damage, whether from direct radiation damage or indirect ROS-dependent damage, can be broken into two critical cellular events—sensing and repairing. A full analysis of all of the known mechanisms for DNA repair is beyond the scope of this review (an expert review of these mechanisms can be found here(25)). As such, we will focus on the DNA damage checkpoint, which is well-established for its role in oncogenesis. Briefly, damage to genomic DNA is detected by a variety of sensory proteins; sites of damage are tagged by phosphorylation of Histone2AX, generating γH2AX (expertly reviewed by Bonner(26)). This phosphorylation occurs incredibly rapidly (within 30 minutes in vivo(27)) and relies on the activity of several kinases including ataxia telangiectasia mutated (ATM), ataxia telangiectasia and Rad3-related (ATR) and DNA-dependent protein kinase (DNA-PK). These kinases will, in turn, activate several downstream pathways with the purpose of halting the cell cycle to provide time for repair to occur and physically fixing damaged DNA. ATM activation of checkpoint kinase 1 and 2 (CHK1 and CHK2) leads to increased proteasomal activity, which subsequently reduces the levels of cyclin-dependent kinases responsible for cell cycle progression(28). This cell cycle arrest buys the cell critical time to repair DNA damage. This repair process can occur via either non-homologous end joining (NHEJ) or homologous recombination (HR), with the latter maintaining genetic code most effectively (expertly reviewed by Chapman(29)). These processes rely on the accumulation of a wide range of proteins. One of the key regulatory genes in this process is microcephalin 1 (MCPH1), which has been shown to orchestrate the recruitment of DNA repair proteins(30). When possible, homologous recombination will occur under the control of the well-established tumor suppressor BRCA1(31) and its complex partner RAD51, deregulation of which has been implicated in breast cancer progression(32). In addition, it has been shown that ATM, ATR, and DNA-PK can activate p53, the so-called guardian of the genome, allowing it to activate protein complexes that drive DSB repair(33). Clearly, the management of DNA repair is an immensely complex process involving many signaling pathways. This complexity creates a vast array of potential mechanisms by which GSCs may avoid and repair radiation-induced damage. Deciphering this molecular code remains a critical challenge to the field of neuro-oncology.
II. Glioma stem cell radioresistance— something old and something new
Given the aforementioned ability of GSCs to resist radiation therapy, it is perhaps not surprising that GSCs have enhanced canonical DNA damage responses, both in the ability to sense and repair damaged DNA. However, these cells do not simply rely on conventional mechanisms. GSCs, as we shall see, appear to utilize novel and at times surprising mechanisms to activate and drive these repair processes. Like a clever DJ taking old standards and making them new, GSCs have put their own spin on classical DNA repair.
In a seminal work in the field, Bao and colleagues demonstrated that GSCs, as defined by expression of the surface marker CD133(34), are more capable of surviving radiation both in vitro and in vivo; this resistance allows for the expansion of the GSC population after treatment via Darwinian selection. While both GSCs and differentiated GBM cells showed comparable amounts of DNA damage in response to radiation, comet-tail assays revealed that CD133+ cells more rapidly repair this damage. Mechanistically, radiation led to an increased phosphorylation of many of the well-studied DNA damage response proteins, including ATM, Chk1, and Chk2 in CD133+ cells relative to matched CD133- cells. Phosphorylation of these proteins is crucial for initiating DNA damage checkpoint; this increased activation shows that GSCs more effectively activate DNA damage responses to avoid radiation-induced apoptosis. Further, GSCs had higher basal activation of protein responsible for marking damaged DNA for repair such as Rad17, suggesting that these cells are on a high alert for detecting DNA damage prior or during exposure to radiation(9). This paper set the stage for an explosion of research into the ability of GSCs to activate DNA damage repair mechanisms.
Indeed, since Bao’s publication, a chorus of other studies has confirmed that GSCs exhibit a heightened ability to activate the DNA damage response and has posited many mechanisms underlying this phenotype. Some have confirmed increased activation of canonical DNA damage response proteins in GSCs, and have shown that compounds capable of inhibiting these proteins can increase survival in preclinical murine models of GBM. For example, it has been demonstrated that small molecules inhibiting the phosphorylation of ATM sensitize GSCs to radiation(35). In addition to ATM, other DNA damage proteins are upregulated in GSCs. Recently, it was shown that a DNA damage protein critical for DSB repair, RAD51, is a key player in GSC radioresistance. King and colleagues demonstrated that RAD51 is selectively expressed in GSCs and that RAD51 levels increase in GSC following radiation. Further, this expression is positively correlated with SOX2 expression. Inhibition of RAD51 via small molecules increased the susceptibility of GBM cells to radiation both in vitro and in orthotopic flank xenografts (the drugs tested reportedly do not cross blood-brain-barrier, a major issue precluding the development of anti-glioma drug(36)).
Clearly, GSCs have enhanced DNA damage response activity following radiotherapy. It is not yet known what specific mechanism activate the DNA repair pathway and endow GSCs with these abilities. Several interesting mechanisms by which GSCs increased the activation of this pathway have been suggested. One recent report linked enhanced DNA damage response in GSCs to the presence of prostaglandins. Cook et al. demonstrated that treatment with exogenous PGE2, a key prostaglandin, was sufficient to increase phosphorylation of ATM in GSCs via activation of its receptor EP4. In animal models, pretreatment with PGE2 reduced the effectiveness of high dose radiation; this effect was reversed by treatment with an EP4 antagonist(37). Beyond prostaglandin signaling, it has repeatedly been shown that proteins previously identified as GSC markers, but with no previously known role in radiation or DNA damage response, enhance the activity of DNA repair proteins in GSCs. For example, it was recently shown that MET, a marker of GSCs, plays a critical role in the ability of GSCs to resist radiation. GSCs expressing MET withstand radiation by activating ATM. Critically, xenograft mice treated with MET inhibitors and radiation did markedly better than control or monotherapy treated mice(10). In addition, another study on cancer stem cells from adenoid cystic carcinoma, which are also identified by expression of CD133, showed that expression of Notch and Sox, two GSC regulators, are key players in radioresistance. Panaccione and colleagues demonstrated that Notch and Sox are critical for cancer stem cell self-renewal; targeting their downstream effectors increased the effectiveness of radiation(38). Another recent report highlighted the role of newly identified Tumor Associated Long Non-coding RNA Expressed on Chromosome 2 (TALNEC2) in the ability of GSCs to respond to radiation. Brodie et al. identified TALNEC2 levels are higher in GSCs than that of normal neural stem cells, indicating a potentially oncogenic specific mechanism. Via small interfering RNA, this study showed that TALNEC2 participates in the regulation of GSC expression profiles, with siRNA-treated cells demonstrating reduced expression of several well-known GSC markers and increased the radiosensitive of GSCs in vitro(39). These results are promising; in vivo xenograft models showed that siRNA-treated cells increased median survival. However, these authors did not irradiate their tumor-bearing mice. Overall, these studies demonstrate that the GSC state is strongly and causally linked with the ability to cells to resist radiotherapy. This connection, however, is controversial, as it has recently been shown that ABCG2, a drug efflux pump implicated in drug resistance, participates in regulating the glioma stem cell niche, but does not have a role in radioresistance. While ABCG2+ cells had higher expression of canonical GSC markers and overexpression of ABCG2 increased sphere formation, this overexpression had no effect on the ability of GSCs to resist radiotherapy(40). These results suggest that some, but not all, GSC markers have the ability to influence resistance to radiotherapy. More work is needed to delineate which GSC markers are true drivers of the stem cell phenotype, including radio-resistance, and which are secondary players.
Beyond activation of the established DNA repair pathway, other protective mechanisms have been proposed. A recent report linked radioresistance to epidermal growth factor receptor (EGFR), a well-known player in glioblastoma. Elevated levels of EGFR are known to influence the progression of several different brain tumors(41, 42). Mutations increasing the activity of EGFR have been shown to inflate proliferation rates and reduce apoptotic signaling in GBM(43). A recent report from Want and associates demonstrated a novel role for EGFR in radioresistance. Their work in lung cancer stem cells, which are also characterized by the CD133 expression, showed that EGFR signaling increases chromatin condensation and leads to the formation of mitosis-like condensed chromatin (MLCC), which protects cells from radiation induced DSBs, specifically in the CD133 expressing population(44). Treatment with erlotinib, an EGFR inhibitor, blocked MLCC formation and increased the sensitivity of cells to radiation. While this study was performed in lung cancer stem cells, it raises the intriguing possibility that EGFR participates in radioresistance in GSCs via modulating epigenetic structure.
The evidence demonstrating that GSCs resist radiation is overwhelming. As these studies show, many novel mechanisms are under investigation and have begun to identify possible strategies for sensitizing GSCs to radiotherapy. In addition, other studies have highlighted other potential mechanisms including enhanced activity of L1CAM(12), elevated autophagy(45), and increases in Notch signaling(46). Given the wide range of proteins and pathways involved in DNA damage repair, it is unsurprising that so many unique and novel participants have been shown to participate in GSC radiation resistance. We remain hopeful that continued research will bring these exciting studies from preclinical models to effective clinical use.
III. Radiation, hypoxia, and cellular plasticity: A complex equilibrium contributing to radioresistance
Another key factor in the ability of GSCs to withstand ionizing radiation is the level of oxygenation within the tumor microenvironment. As previously discussed, the generation of ROS enables indirect DNA damage by radiation; this process is oxygen-dependent. Critically, the tumor microenvironment in GBM is highly variable and contains areas of very low oxygen, termed the hypoxic niche(47–49). These alterations in oxygen concentration content have a pronounced effect on radiosensitivity—without oxygen, no ROS can be generated. Two clinical investigations have shown low oxygen content and large hypoxic regions are significant predictors of time to recurrence and overall survival in human patients undergoing radiotherapy (50, 51), corroborating the idea that oxygen is required for effective radiotherapy. These results have led investigators to explore increased oxygenation as an adjuvant treatment for radiation treatment. Clarke and colleagues reported that pretreatment with pure oxygen increased the effectiveness of radiation in nude mice bearing U87 xenograft tumors in a HIF dependent manner(52). They showed that pretreatment with oxygen reduced levels of hypoxia-inducible factor 1 alpha (HIF1α) and made GSCs more sensitive to radiation. This observation of HIFs role in radioresistance highlights the connection between GSCs and hypoxia.
GSCs are well established to preferentially reside in hypoxic niches within GBM tumors and hypoxia has been shown to influence the phenotype of GBM cells, shifting them towards a GSC fate. This alteration is an example of cellular plasticity, a mechanism by which GBM cells can transition from differentiated, treatment-sensitive states to treatment-resistant, undifferentiated GSC states. Hypoxia-induced factors (HIF) have been shown to participate in the regulation of the GSC niche(17, 50, 53–55). This ability of HIF to influence cellular plasticity has been confirmed in breast cancer stem cells(56). It has also been shown that hypoxia exposure alone is sufficient to activate HIF-dependent increases in the CD133+ GSC population(54). These studies raise an interesting chicken-and-egg question about hypoxia and radioresistant GSCs. Does the existence of hypoxia produce GSCs which, by nature of their cell state, are resistant to radiation or do already resistant GSCs simply preferentially occupy the hypoxic niche? We suspect that dynamic cellular plasticity may provide some explanation.
As discussed above, HIFs are well established players in controlling the GSC niche. We have previously shown that therapeutic stress induced by chemotherapy can activate cellular plasticity via HIF signaling under normoxic conditions(17). Several studies have recently confirmed that radiation also alters cellular plasticity. Dahan et al. illustrated that radiation treatment can cause dedifferentiation of GBM cells to a stem cell state, as demonstrated by the ability of previously irradiated GBM cells to generate secondary tumor spheres. These authors demonstrate that this plasticity endows induced-GSCs with radioresistance(57). Radiation-induced conversion was recently confirmed in head and neck cancers(58). HIF expression, whether from the presence of low-oxygen microenvironment or induced by therapeutic stress, can activate the expression of GSC markers and lead to radioresistance. Rather than the chicken or the egg, it appears that either one or both can lead to the formation of a population of cells capable of withstanding the onslaught of irradiation.
In addition to HIF-mediated plasticity, radiation has been shown to play a role in another key conversion process—the epithelial-to-mesenchymal transition (EMTs), a plastic event associated with heightened aggressiveness and resistance to therapy (expertly reviewed by Lee et al.(59)). It was recently shown that radiation-generating ROS increases the frequency of EMTs in glioma. Kesanakurti and colleagues showed that ionizing radiation increases EMT events via the translocation of PAK4, a key regulator of nuclear signaling and transcription, to the nucleus, where it binds with novel partner PPARγ, another transcription regulator. This complex in turn binds to the promoter of Nox1 to induce EMT conversion(60). Another study recently suggested that mesenchymal transition and subsequent radioresistance depends on activation of MLK4(61). Radiation’s ability to induce EMT events was recently confirmed in head and neck cancer(62).
Finally, epigenetic alterations in response to radiation have arisen as another form of cellular plasticity limiting the effectiveness of radiation in GBM. Plasticity depends upon the ability of cells to alter their transcriptomes, which in turn relies on epigenetic modifications—a set of posttranslational modifications to histones that determine the ability of specific genomic regions to be transcribed to RNA(63). This process has been heavily implicated in gliomagenesis and therapeutic resistance(64). Specifically, it has been shown that the polycomb repressor complex (PRC), which includes EZH2, is critical for radioresistance in GSCs. Kim and associates reveled that GSCs overexpress maternal embryonic leucine-zipper kinase (MELK) and further increase expression after treatment with radiation, which in turn activates EZH2. MELK overexpression was sufficient to enhance radioresistance; however, pretreatment with EZH2 inhibitor abolished this radioresistance(65). Thus, it appears that radiation treatment activates a variety of pathways, including HIF signaling, capable of influencing cellular plasticity. This activation of plasticity expands the GSC population and promotes radioresistance.
IV. Calcium management—an emerging influence in radioresistance
The ability of GSCs to repair DNA damage and the capacity of GBM cells to undergo plastic events to acquire GSC state appear critical for promoting radioresistance. However, they are not the only exciting cellular processes currently being studied. A recent report proposed that intracellular calcium management enables GBM to withstand radiation. Elevation of intracellular calcium levels can promote apoptosis, and it has been shown that radiation and other anti-cancer therapies depend on this increase for cytotoxicity(66). A landmark study recently identified that GBM cells interconnect to form a functional network, capable of generating and spreading intercellular calcium waves (ICWs). Osswald and colleagues demonstrated that these ICWs endow GBM cells with the ability to resist radiotherapy by dispersing calcium among many cells, thereby preventing intracellular calcium from reaching cytotoxic levels. Those GBM cells maintaining functional connections with one another were more resistant to radiation than their non-connected counterparts. Reduced expression of the connexin responsible for calcium exchange extended animal survival(67). This study highlights the importance of calcium management in radioresistance. Interestingly, a large genomic analysis study demonstrated that GSCs have elevated expression of several key genes related to calcium transport, calcium reuptake, and calcium-responding proteins(68); these results suggest that GSCs may have the ability to resist radiotherapy by more efficient calcium management. Given the importance of calcium levels in radiation induced apoptosis, this theory seems like a promising avenue for further investigation.
V. Remaining challenges and future directions
The ability of GSCs to resist radiotherapy, despite the effective use of radiation for curative treatments in many other cancers, remains a key problem in the treatment of brain cancer. A wealth of published reports have indicated that GSCs exhibit remarkable capabilities to activate DNA repair pathways following radiotherapy. This activation appears to rely on many unique pathways, including prostaglandin signaling, GSC signaling, and hypoxia. Many of the papers cited and discussed end with figures toting impressive increases in median survival in mouse models. Despite these vast mechanistic insights as well as exciting preclinical results, the majority of these breakthroughs have failed to translate in the clinical setting. As shown in this review, an immense amount of creative and innovative work has gone into the question of GSC radioresistance since that landmark 2006 paper. However, we must reconcile with the fact that these preclinical results have overwhelming failed to improve outcomes in human trials. We suspect that this failure results from three factors—limitations of current models, complications from the blood-brain-barrier (BBB), and our lack of holistic understanding of adaptation mechanisms of cancer cells towards therapies.
One of the major issues contributing to inefficacy of clinical translation is the currently available animal model of human cancer. Much of the work discussed in this review relies on in vitro experiments with human glioma cells and murine models. However, it has been shown that glioma cell lines, even gold standard patient-derived lines (PDX), are susceptible to alterations in gene expression, produce variable results after passaging, and fail to recapitulate molecular characteristics of GBM faithfully(69). Mouse models are also flawed; human cells must be implanted into immunocompromised mice, thereby removing critical immune-related factors from the equation. Moreover, experimental methods like shRNA are often utilized to probe the role of specific genes in GSC radioresistance. Even though shRNAs technology is a great tool for studying complex biological process, clinical utilization of such shRNA-based anti-cancer therapeutics are far from reality. While new techniques like CRIPSR can remove entire genes, they still suffer from the fact that these alterations are likely to disrupt the finely-tuned processes governing cells. These tools remain far more shovel than scalpel. Incomplete models, both in culture and in animals, are likely to hamper the effective and rapid transfer of bench results to the bedside.
Second, the presence of the BBB is likely to prevent drugs capable of sensitizing other cancers to radiation from being applied to GBM. The BBB is a highly selective gate keeper, controlling the composition of the brain microenvironment. Thus, many drugs successful in other cancers cannot access brain tumors. This fact clearly limits our ability to increase the radiosensitivity of GSCs, as demonstrated in the RAD51 paper (36). This study had to demonstrate an effect in flank tumors, as their drug is BBB impermeable. There are several strategies to overcome this issue. First, chemical modifications of drugs can be employed to selectively maintain active sites while tailoring compounds to clear the BBB. This method has been successful applied to drugs targeting prion diseases(70). Second, repackaging of BBB-impermeable drugs with nanoparticle platforms has been shown to successfully penetrate into tumors(71). This same strategy can and should be applied to radiation-enhancing drugs. Finally, focused ultrasound has been shown to enable focal delivery of systematically injected particles. This strategy may be applicable to radio-sensitizers during radiation treatment. By combining traditional radiation with focused ultra-sound, specific sections of the BBB abutting the tumor can be opened to allow inhibitors to penetrate the tumor.
Finally, and, in our opinion, most importantly, we currently lack of a holistic understanding of adaptation mechanisms of GSCs, or cancer in general, to therapy. Cancer, like us, is defined by its ability to adapt to various microenvironments. While we are busy identifying individual targets and pathways, plastic GBMs and GSCs are constantly developing multiple fail-safes and back up plans in response to various microenvironmental changes such those that occur during therapy. While we are busy looking for a single chink in GSCs current suit of armor, GSCs are already developing a second and third suit. We believe that the only logical pathway forward is to seek holistic understanding of the adaptation process of cancer, identifying the connections between these seemingly disparate mechanisms. Rather than looking for a single target and a molecular silver bullet, we must first develop a unified understanding of cancer cells’ ability to adapt to various therapies. In order to address this problem, we must expand our research and harness the power of so called big-data analysis such as RNA-seq, CHIP-seq, and single cell genomic analyses. These approaches have begun being applied to various cancers including GBM and have produced new, comprehensive network-based understandings of how GSCs adapt to anti-cancer therapies. For example, a group at MIT recently demonstrated that GSCs respond to target therapies with a wide range of chromatin modifications. By examining binding on the whole genome and subsequent RNA expression patters, they identified a multitude of transcriptome changes that participate in the GSC response to therapy (72). Critically, their big-data approach allowed them to identify certain proteins as key convergence points for these adaptation processes. Using large scale analyses to identify critical signaling convergence points is the key to understanding the larger forces at play in therapy resistance.
In light of GSCs’ ability to rapidly adapt to therapeutic stressors, we must aim for therapies that limit such adaption. First and foremost, pharmacological targeting of DNA repair proteins remains a potent option when applied in combination. As several of the aforementioned studies show, blocking DNA repair proteins can increase the effectiveness of radiation. By combining several inhibitors, it may be possible to prevent adaptation and escape. Second, cellular plasticity in glioma cells should be further studied and targeted. As discussed, HIF signaling plays a key role in plasticity-enhanced radioresistance, and enables GBM cells to rapidly respond to their environment. Targeting HIFs remains a promising strategy, given the large body of evidence indicating these transcription factors in GBM onset and therapeutic resistance (HIF targeting expertly reviewed by Wilson and Hay(55)); indeed, the preclinical evidence presented above demonstrating the effects of oxygenation in glioma xenografts demonstrates that HIF remains an attractive target. However, the status of HIFs as master transcription factors complicates their use as adjuvant therapies, because any drug targeting malignant HIF expression is more than likely to dysregulate healthy, basal HIF activity.
These side effects suggest that more work should instead focus on controlling the epigenetic changes that underlie cellular plasticity in response to radiotherapy. Targeting epigenetics to enhance radiosensitivity has already produced promising preclinical results in prostate cancer. Recent research by Peitzch and colleagues found that prostate cancer cells respond to radiation with specific histone modifications, enabling a shift in the transcriptome of these cells and subsequent stem cell state and radioresistance. Blocking these histone modifications via DZNep prevented this induction and sensitized cells to radiation(73). It could be argued that targeting such a broad and widely utilized process like histone methylation is subject to the same off-target problem found in targeting HIFs. While some off-target effects may occur, we believe they will be substantially less problematic. Drugs targeting the epigenome are becoming more precise, with drug development moving away from broad-acting agents like DZNep and HDAC inhibitors to targeted, cancer-specific agents. This new strategy will enable oncologists to selectively drug those epigenetic changes that are overactive in specific tumors. The same cannot be argued for HIF-targeting, in which tumors co-opt a canonical pathway broadly utilized by healthy somatic cells. We strongly believe that with further research, targeting epigenetic modifications regulating GSC plasticity has potential as an adjuvant therapy in radioresistant GBM.
Finally, network formation and calcium exchange in GBM also prevent a novel, potentially exploitable mechanism. As stated above, GBMs utilize gap junctions to dissipate calcium following radiation, and GSCs express elevated levels of various proteins related to controlling calcium levels. Similarly to HIF and epigenetics, targeting calcium signaling is not a simple solution, as calcium management is critical to a variety of physiological processes including normal astrocyte ICWs(74), regulation of cardiac rhythms(75), and renal ion buffering(76). However, we suspect that, as in the case of epigenetics, increased research will uncover tumor-specific alterations in calcium management and allow for targeted therapy. Each of these avenues can and should be exploited to generate novel therapies for the radiation oncologist’s toolbox.
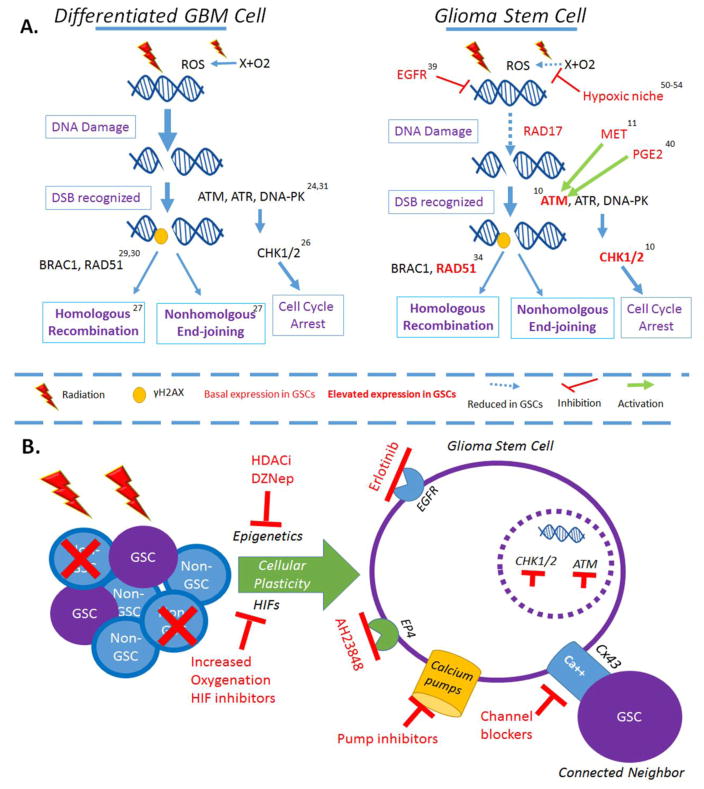
Overall, GSCs resist radiation in both expected and surprising ways (Figure 1). From simply increasing the levels and activity of well-studied DNA repair proteins, to coiling their chromosomes tightly to hide the precious DNA strand, GSCs have a wide and remarkable range of ways to evade current radiotherapy. Further, these strategies are not static, set pieces, but rather constantly evolving and changing. This complexity and constant flux demand that we strive to understand GSCs holistically and generate novel and creative combination strategies for preventing adaptation. Figure 1 highlights some potential avenues for developing these strategies. In order to defeat GSCs, we ourselves must be ready to adapt and adjust. We must be ready to beat them at their own game.
Figure 1.
Glioma stem cells resist radiation via increased activity of canonical DNA repair mechanisms under the control of novel activators. A. Comparison of differentiated GBM cells and glioma stem cells (GSCs). Differentiated, non-glioma stem cells utilize traditional repair mechanisms to respond to radiotherapy. Radiation induces double strand breaks, which are sensed by proteins including ATM, ATR, and DNA-PK; these proteins mark DSBs for repair by phosphorylation of Histone2. These marked sites are then recognized by DNA repair proteins and fixed by either homologous recombination or nonhomologous endjoining. Further, ATM- and ATR-activated CHK1/2 increase proteasome activity and force arrest of the cell cycle. In the event that HR or NHEJ fail to repair the DSBs, the cell will undergo apoptosis. Glioma stem cells have been shown to increase activation of many proteins involved in DNA damage response, including increased activity of ATM, CHK1/2, and RAD51. Further, they demonstrate selective activity of RAD17, which predisposes them to sensing and repairing DSBs. Recent research has shown several novel mechanisms by which GSCs increases their radioresistance. Both MET and PGE2 have been shown to increase the phosphorylation of ATM. Further, EGFR activity has been shown to induce the formation of heterochromatin. This chromatin condensing is thought to protect DNA from the damaging effects of radiation. B. On the population level, radiation effectively kills differentiated, non-GSC cells. However, the therapeutic stress induced by radiation is sufficient to cause some of these cells to undergo conversion to the GSC state. Blocking this process, either by inhibiting epigenetic adaption or the transcription effects of hypoxia inducible factors (HIFs), may offer novel strategies for enhancing the efficiency of existing radiotherapy modalities. In terms of targeting GSCs, several small molecules have been demonstrated to target specific mechanisms of GSC radioresistance. Finally, GSCs exchange calcium via gap junctions and have high levels of calcium pumps, suggesting that they are efficient calcium managers. Targeting these mechanisms has great potential for sensitizing GSCs to radiation. Superscripts correspond to references.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke grant 1R01NS096376-01A1 and the American Cancer Society grant RSG-16-034-01-DDC (to A.U.A.) grant.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Seamus P. Caragher, Sean Sachdev, and Atique Ahmed declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
*Of importance
**Of major importance
- 1.Hoppe RT. Evolution of the techniques of radiation therapy in the management of lymphoma. Int J Clin Oncol. 2013;18(3):359–63. doi: 10.1007/s10147-013-0556-3. [DOI] [PubMed] [Google Scholar]
- 2.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5(10):1725–31. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 3.Bleehen NM, Stenning SP. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The Medical Research Council Brain Tumour Working Party. Br J Cancer. 1991;64(4):769–74. doi: 10.1038/bjc.1991.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson DF, Diener-West M, Horton J, Chang CH, Schoenfeld D, Nelson JS. Combined modality approach to treatment of malignant gliomas--re-evaluation of RTOG 7401/ECOG 1374 with long-term follow-up: a joint study of the Radiation Therapy Oncology Group and the Eastern Cooperative Oncology Group. NCI Monogr. 1988;(6):279–84. [PubMed] [Google Scholar]
- 5.Chan JL, Lee SW, Fraass BA, Normolle DP, Greenberg HS, Junck LR, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20(6):1635–42. doi: 10.1200/JCO.2002.20.6.1635. [DOI] [PubMed] [Google Scholar]
- 6.Gabayan AJ, Green SB, Sanan A, Jenrette J, Schultz C, Papagikos M, et al. GliaSite brachytherapy for treatment of recurrent malignant gliomas: a retrospective multi-institutional analysis. Neurosurgery. 2006;58(4):701–9. doi: 10.1227/01.NEU.0000194836.07848.69. discussion -9. [DOI] [PubMed] [Google Scholar]
- 7.Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, Lopez C, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63(2):324–33. doi: 10.1016/j.ijrobp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Zelefsky MJ, Leibel SA, Gaudin PB, Kutcher GJ, Fleshner NE, Venkatramen ES, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41(3):491–500. doi: 10.1016/s0360-3016(98)00091-1. [DOI] [PubMed] [Google Scholar]
- 9.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 10.De Bacco F, D’Ambrosio A, Casanova E, Orzan F, Neggia R, Albano R, et al. MET inhibition overcomes radiation resistance of glioblastoma stem-like cells. EMBO Mol Med. 2016;8(5):550–68. doi: 10.15252/emmm.201505890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardee ME, Marciscano AE, Medina-Ramirez CM, Zagzag D, Narayana A, Lonning SM, et al. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-beta. Cancer Res. 2012;72(16):4119–29. doi: 10.1158/0008-5472.CAN-12-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng L, Wu Q, Huang Z, Guryanova OA, Huang Q, Shou W, et al. L1CAM regulates DNA damage checkpoint response of glioblastoma stem cells through NBS1. EMBO J. 2011;30(5):800–13. doi: 10.1038/emboj.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 14.Tamura K, Aoyagi M, Ando N, Ogishima T, Wakimoto H, Yamamoto M, et al. Expansion of CD133-positive glioma cells in recurrent de novo glioblastomas after radiotherapy and chemotherapy. J Neurosurg. 2013;119(5):1145–55. doi: 10.3171/2013.7.JNS122417. [DOI] [PubMed] [Google Scholar]
- 15.Hjelmeland AB, Wu Q, Heddleston JM, Choudhary GS, MacSwords J, Lathia JD, et al. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2011;18(5):829–40. doi: 10.1038/cdd.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auffinger B, Tobias AL, Han Y, Lee G, Guo D, Dey M, et al. Conversion of differentiated cancer cells into cancer stem-like cells in a glioblastoma model after primary chemotherapy. Cell Death Differ. 2014;21(7):1119–31. doi: 10.1038/cdd.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee G, Auffinger B, Guo D, Hasan T, Deheeger M, Tobias AL, et al. Dedifferentiation of Glioma Cells to Glioma Stem-like Cells By Therapeutic Stress-induced HIF Signaling in the Recurrent GBM Model. Mol Cancer Ther. 2016;15(12):3064–76. doi: 10.1158/1535-7163.MCT-15-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olmez I, Shen W, McDonald H, Ozpolat B. Dedifferentiation of patient-derived glioblastoma multiforme cell lines results in a cancer stem cell-like state with mitogen-independent growth. J Cell Mol Med. 2015;19(6):1262–72. doi: 10.1111/jcmm.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safa AR, Saadatzadeh MR, Cohen-Gadol AA, Pollok KE, Bijangi-Vishehsaraei K. Glioblastoma stem cells (GSCs) epigenetic plasticity and interconversion between differentiated non-GSCs and GSCs. Genes Dis. 2015;2(2):152–63. doi: 10.1016/j.gendis.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim RK, Suh Y, Cui YH, Hwang E, Lim EJ, Yoo KC, et al. Fractionated radiation-induced nitric oxide promotes expansion of glioma stem-like cells. Cancer Sci. 2013;104(9):1172–7. doi: 10.1111/cas.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritzell JA, Narayanan L, Baker SM, Bronner CE, Andrew SE, Prolla TA, et al. Role of DNA mismatch repair in the cytotoxicity of ionizing radiation. Cancer Res. 1997;57(22):5143–7. [PubMed] [Google Scholar]
- 22.Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol. 1985;32:115–54. doi: 10.1016/s0079-6603(08)60347-5. [DOI] [PubMed] [Google Scholar]
- 23.Kempner ES. Direct Effects of Ionizing Radiation on Macromolecules. J Polym Sci B Polym Phys. 2011;49(12):827–31. doi: 10.1002/polb.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40(2):179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–8. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, et al. GammaH2AX and cancer. Nat Rev Cancer. 2008;8(12):957–67. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146(5):905–16. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14(12):1448–59. [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell. 2012;47(4):497–510. doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 30.Peng G, Lin SY. BRIT1/MCPH1 is a multifunctional DNA damage responsive protein mediating DNA repair-associated chromatin remodeling. Cell Cycle. 2009;8(19):3071–2. doi: 10.4161/cc.8.19.9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Willers H, Feng Z, Ghosh JC, Kim S, Weaver DT, et al. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol Cell Biol. 2004;24(2):708–18. doi: 10.1128/MCB.24.2.708-718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin RW, Orelli BJ, Yamazoe M, Minn AJ, Takeda S, Bishop DK. RAD51 up-regulation bypasses BRCA1 function and is a common feature of BRCA1-deficient breast tumors. Cancer Res. 2007;67(20):9658–65. doi: 10.1158/0008-5472.CAN-07-0290. [DOI] [PubMed] [Google Scholar]
- 33.Serrano MA, Li Z, Dangeti M, Musich PR, Patrick S, Roginskaya M, et al. DNA-PK, ATM and ATR collaboratively regulate p53-RPA interaction to facilitate homologous recombination DNA repair. Oncogene. 2013;32(19):2452–62. doi: 10.1038/onc.2012.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, et al. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14(1):123–9. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 35.Carruthers R, Ahmed SU, Strathdee K, Gomez-Roman N, Amoah-Buahin E, Watts C, et al. Abrogation of radioresistance in glioblastoma stem-like cells by inhibition of ATM kinase. Mol Oncol. 2015;9(1):192–203. doi: 10.1016/j.molonc.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.King HO, Brend T, Payne HL, Wright A, Ward TA, Patel K, et al. RAD51 Is a Selective DNA Repair Target to Radiosensitize Glioma Stem Cells. Stem Cell Reports. 2017;8(1):125–39. doi: 10.1016/j.stemcr.2016.12.005. This study highlights the ability of glioma stem cells to co-opt and ehnahce canonical DNA repair mechanisms to resist radiation-induced apoptosis. It demonstrates the potential for targeting these proteins in sensitizing GBM tumors to radiotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook PJ, Thomas R, Kingsley PJ, Shimizu F, Montrose DC, Marnett LJ, et al. Cox-2-derived PGE2 induces Id1-dependent radiation resistance and self-renewal in experimental glioblastoma. Neuro Oncol. 2016;18(10):1379–89. doi: 10.1093/neuonc/now049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panaccione A, Chang MT, Carbone BE, Guo Y, Moskaluk CA, Virk RK, et al. NOTCH1 and SOX10 are Essential for Proliferation and Radiation Resistance of Cancer Stem-Like Cells in Adenoid Cystic Carcinoma. Clin Cancer Res. 2016;22(8):2083–95. doi: 10.1158/1078-0432.CCR-15-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brodie S, Lee HK, Jiang W, Cazacu S, Xiang C, Poisson LM, et al. The novel long non-coding RNA TALNEC2, regulates tumor cell growth and the stemness and radiation response of glioma stem cells. Oncotarget. 2017;8(19):31785–801. doi: 10.18632/oncotarget.15991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wee B, Pietras A, Ozawa T, Bazzoli E, Podlaha O, Antczak C, et al. ABCG2 regulates self-renewal and stem cell marker expression but not tumorigenicity or radiation resistance of glioma cells. Sci Rep. 2016;6:25956. doi: 10.1038/srep25956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci U S A. 1998;95(10):5724–9. doi: 10.1073/pnas.95.10.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Han J, Marcar L, Black J, Liu Q, Li X, et al. Radiation Resistance in KRAS-Mutated Lung Cancer Is Enabled by Stem-like Properties Mediated by an Osteopontin-EGFR Pathway. Cancer Res. 2017;77(8):2018–28. doi: 10.1158/0008-5472.CAN-16-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lomonaco SL, Finniss S, Xiang C, Decarvalho A, Umansky F, Kalkanis SN, et al. The induction of autophagy by gamma-radiation contributes to the radioresistance of glioma stem cells. Int J Cancer. 2009;125(3):717–22. doi: 10.1002/ijc.24402. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Wakeman TP, Lathia JD, Hjelmeland AB, Wang XF, White RR, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28(1):17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans SM, Judy KD, Dunphy I, Jenkins WT, Nelson PT, Collins R, et al. Comparative measurements of hypoxia in human brain tumors using needle electrodes and EF5 binding. Cancer Res. 2004;64(5):1886–92. doi: 10.1158/0008-5472.can-03-2424. [DOI] [PubMed] [Google Scholar]
- 48.Cruickshank GS, Rampling RP, Cowans W. Direct measurement of the PO2 distribution in human malignant brain tumours. Adv Exp Med Biol. 1994;345:465–70. doi: 10.1007/978-1-4615-2468-7_62. [DOI] [PubMed] [Google Scholar]
- 49.Rampling R, Cruickshank G, Lewis AD, Fitzsimmons SA, Workman P. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int J Radiat Oncol Biol Phys. 1994;29(3):427–31. doi: 10.1016/0360-3016(94)90432-4. [DOI] [PubMed] [Google Scholar]
- 50.Evans SM, Judy KD, Dunphy I, Jenkins WT, Hwang WT, Nelson PT, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res. 2004;10(24):8177–84. doi: 10.1158/1078-0432.CCR-04-1081. [DOI] [PubMed] [Google Scholar]
- 51.Spence AM, Muzi M, Swanson KR, O’Sullivan F, Rockhill JK, Rajendran JG, et al. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin Cancer Res. 2008;14(9):2623–30. doi: 10.1158/1078-0432.CCR-07-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clarke RH, Moosa S, Anzivino M, Wang Y, Floyd DH, Purow BW, et al. Sustained radiosensitization of hypoxic glioma cells after oxygen pretreatment in an animal model of glioblastoma and in vitro models of tumor hypoxia. PLoS One. 2014;9(10):e111199. doi: 10.1371/journal.pone.0111199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8(20):3274–84. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28(45):3949–59. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 55.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 56.Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m(6)A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A. 2016;113(14):E2047–56. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Dahan P, Martinez Gala J, Delmas C, Monferran S, Malric L, Zentkowski D, et al. Ionizing radiations sustain glioblastoma cell dedifferentiation to a stem-like phenotype through survivin: possible involvement in radioresistance. Cell Death Dis. 2014;5:e1543. doi: 10.1038/cddis.2014.509. This paper demonstrates the remarkable ability to glioma cells to adapt to therapeutic stress and attain the glioma stem cell state following radiation. It further highlights the complexes inherent in treating a tumor in which the cells are highly plastic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vlashi E, Chen AM, Boyrie S, Yu G, Nguyen A, Brower PA, et al. Radiation-Induced Dedifferentiation of Head and Neck Cancer Cells Into Cancer Stem Cells Depends on Human Papillomavirus Status. Int J Radiat Oncol Biol Phys. 2016;94(5):1198–206. doi: 10.1016/j.ijrobp.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, et al. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer. 2017;16(1):10. doi: 10.1186/s12943-016-0577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kesanakurti D, Maddirela D, Banasavadi-Siddegowda YK, Lai TH, Qamri Z, Jacob NK, et al. A novel interaction of PAK4 with PPARgamma to regulate Nox1 and radiation-induced epithelial-to-mesenchymal transition in glioma. Oncogene. 2017 doi: 10.1038/onc.2016.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Kim SH, Ezhilarasan R, Phillips E, Gallego-Perez D, Sparks A, Taylor D, et al. Serine/Threonine Kinase MLK4 Determines Mesenchymal Identity in Glioma Stem Cells in an NF-kappaB-dependent Manner. Cancer Cell. 2016;29(2):201–13. doi: 10.1016/j.ccell.2016.01.005. This work demosntrates the remarkable plasticity of GBM cells, including the ability to attain mutliple distinct cell states and phenotypes. Critcally, these data accentuate the complex nature of targeing glioma stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Su Z, Li G, Liu C, Ren S, Tian Y, Liu Y, et al. Ionizing radiation promotes advanced malignant traits in nasopharyngeal carcinoma via activation of epithelial-mesenchymal transition and the cancer stem cell phenotype. Oncol Rep. 2016;36(1):72–8. doi: 10.3892/or.2016.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones B. Epigenetics: Histones pass the message on. Nat Rev Genet. 2015;16(1):3. doi: 10.1038/nrg3876. [DOI] [PubMed] [Google Scholar]
- 64.Nagarajan RP, Costello JF. Epigenetic mechanisms in glioblastoma multiforme. Semin Cancer Biol. 2009;19(3):188–97. doi: 10.1016/j.semcancer.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Kim SH, Joshi K, Ezhilarasan R, Myers TR, Siu J, Gu C, et al. EZH2 protects glioma stem cells from radiation-induced cell death in a MELK/FOXM1-dependent manner. Stem Cell Reports. 2015;4(2):226–38. doi: 10.1016/j.stemcr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tombal B, Denmeade SR, Gillis JM, Isaacs JT. A supramicromolar elevation of intracellular free calcium ([Ca(2+)](i)) is consistently required to induce the execution phase of apoptosis. Cell Death Differ. 2002;9(5):561–73. doi: 10.1038/sj.cdd.4400999. [DOI] [PubMed] [Google Scholar]
- 67•.Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528(7580):93–8. doi: 10.1038/nature16071. This paper was the first to demonstrate the ability of astrocytomas to form functional cellular networks and evinced the role these networks play in resisting current radiotherapy. It also provides several novel targets for enhancing radiosensitivity via targeting cell-to-cell interactions. [DOI] [PubMed] [Google Scholar]
- 68.Robil N, Petel F, Kilhoffer MC, Haiech J. Glioblastoma and calcium signaling--analysis of calcium toolbox expression. Int J Dev Biol. 2015;59(7–9):407–15. doi: 10.1387/ijdb.150200jh. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Romero N, Gonzalez-Tejedo C, Carrion-Navarro J, Esteban-Rubio S, Rackov G, Rodriguez-Fanjul V, et al. Cancer stem cells from human glioblastoma resemble but do not mimic original tumors after in vitro passaging in serum-free media. Oncotarget. 2016;7(40):65888–901. doi: 10.18632/oncotarget.11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gallardo-Godoy A, Gever J, Fife KL, Silber BM, Prusiner SB, Renslo AR. 2-Aminothiazoles as therapeutic leads for prion diseases. J Med Chem. 2011;54(4):1010–21. doi: 10.1021/jm101250y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng Y, Dai Q, Morshed RA, Fan X, Wegscheid ML, Wainwright DA, et al. Blood-brain barrier permeable gold nanoparticles: an efficient delivery platform for enhanced malignant glioma therapy and imaging. Small. 2014;10(24):5137–50. doi: 10.1002/smll.201400654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liau BB, Sievers C, Donohue LK, Gillespie SM, Flavahan WA, Miller TE, et al. Adaptive Chromatin Remodeling Drives Glioblastoma Stem Cell Plasticity and Drug Tolerance. Cell Stem Cell. 2017;20(2):233–46. e7. doi: 10.1016/j.stem.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peitzsch C, Cojoc M, Hein L, Kurth I, Mabert K, Trautmann F, et al. An Epigenetic Reprogramming Strategy to Resensitize Radioresistant Prostate Cancer Cells. Cancer Res. 2016;76(9):2637–51. doi: 10.1158/0008-5472.CAN-15-2116. [DOI] [PubMed] [Google Scholar]
- 74.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247(4941):470–3. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 75.Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, et al. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci U S A. 2005;102(23):8089–96. doi: 10.1073/pnas.0502506102. discussion 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nordin BE, Peacock M. Role of kidney in regulation of plasma-calcium. Lancet. 1969;2(7633):1280–3. doi: 10.1016/s0140-6736(69)90813-7. [DOI] [PubMed] [Google Scholar]