Abstract
Syndecan-1 chondroitin sulfate glycopeptide was synthesized for the first time using the cassette approach. The sequence of glycosylation to form the octasaccharide serine cassette was critical. The glycopeptide was successfully assembled via a 2+ (3 + 3) glycosylation strategy followed by peptide chain elongation.
Graphical Abstract

Chondroitin sulfates (CSs) are polyanionic complex polysaccharides consisting of a disaccharide repeating unit of D-glucuronic acid (GlcA) β-1,3-linked to 2-acetamido-2-deoxy-D-galactosamine (GalNAc). CSs belong to the glycosaminoglycan family and exist as a part of chondroitin sulfate proteoglycans (CSPGs) in nature. In CSPG, CSs are covalently linked to the hydroxyl group of a serine residue in the core protein through a tetrasaccharide linker of GlcA-β-1,3-galactose (Gal)-β-1,3-Gal-β-1,4-xylose.1–3 A representative example of CSPG is syndecan-1, which interacts with a large variety of biological molecules such as growth factors, chemokines, amyloid β, and cell adhesion molecules.1–6 As a result, it plays important roles in mediating cell binding, signaling, proliferation, and cytoskeletal organization.

CS and CSPG on average contain one O-sulfate per disaccharide unit, which is installed by O-sulfotransferases in nature.7 The major types of CS have a 4- or 6-O-sulfo group on the GalNAc residue, which are referred to as CS-A and CS-C, respectively. Other sulfate variants are also known.1,3,8 Enzymatic sulfation reactions are often not complete, leading to high structural heterogeneities of naturally existing CSs. This significantly hinders the efforts to thoroughly understand the biological functions of CS/CSPG. Although CS oligosaccharides8–10 and the tetrasaccharide linkage region11 have been prepared, to date, the synthesis of CSPG glycopeptide bearing a homogeneous CS glycan chain has not been achieved. This paper addresses this critical need by providing a synthesis of the CSPG syndecan-112 glycopeptide 1, which bears a CS-A tetrasaccharide on a core protein sequence (corresponding to amino acid residues 45–53 of human syndecan-1) with two additional glycine residues at its N-terminal for future sortase-mediated ligation. In nature, syndecan-1 can bear CS chains on multiple possible serine locations. A successful completion of the synthesis of CSPG 1 lays important groundwork for the synthesis of other CSPGs.
To access CSPG glycopeptide 1, a cassette approach13 was chosen, in which the glycosyl amino acid would be prepared as a cassette for peptide elongation because although a short peptide can be glycosylated chemically,14 it is often challenging to directly glycosylate a large peptide acceptor. CS-A octasaccharide-linked serine 2, containing the full tetrasaccharide linkage region, galactosamine (GalN) β-linked GlcA, and O-sulfation, was designed as a cassette with the two sites for O-sulfation selectively protected as levulinoyl (Lev) esters.

With the large size of the glycan chain in 2, there are many potential reaction sequences for glyco-assembly. The first route investigated was through 3 + 2 + 3 glycosylation15 to access the octasaccharide with strategic disconnections at C/D and E/F linkages. Whereas most published CS syntheses take advantage of glycosyl trichloroacetimidates as building blocks,8–10 thioglycosides can be an attractive alternative due to their higher stabilities.16 The amine moiety of GalN was protected as a trichloroacetyl (TCA) amide, which could direct β stereo-selectivity8–10 and reduce the potential complications from the formation of side products/intermediates arising from the use of acetamide bearing building blocks.17,18 Thus, ABC trisaccharide 3, DE disaccharide 4, and FGH trisaccharide 519 were designed first to access octasaccharide 2.

Synthesis started with preparation of the DE disaccharide 4 by a glycosylation reaction between donor 6 and acceptor 7 (Scheme 1). Donor 6 was preactivated with p-TolSCl/AgOTf20 at −78 °C. Upon complete activation of donor (5 min), acceptor 7 was added together with a non-nucleophilic base tri-t-butylpyrimidine.21 Disaccharide 8 was obtained in an excellent yield of 92%. Protective group manipulation of 8 gave TBDPS-protected disaccharide 4. It was necessary to switch the PMB in 8 to TBDPS, as activation of 8 as a glycosyl donor in a subsequent glycosylation reaction gave the 1,6-anhydrosugar 10 as a major side product. The TBDPS group in 4 was chosen because it can be selectively removed and the resulting free primary hydroxyl groups can be oxidized after glyco-assembly to generate the corresponding GlcA.
Scheme 1.
Preparation of DE Disaccharide 4
To prepare the ABC trisaccharide (Scheme 2), glucoside donor 11 was preactivated for 5 min at −78 °C, and then disaccharide acceptor 4 was added over 2 min in DCM to generate the ABC trisaccharide 3 in 81% yield. Formation of the CD glycosyl linkage was tested next. However, glycosylation of donor 3 and disaccharide acceptor 4 gave very low yield (<10%) of the desired pentasaccharide. Extensive efforts in varying the protective groups on either 3 or 4 failed to improve the glycosylation yield. It should be noted that both AB and CD linkages are between glucosyl and GalN units. The much higher glycosylation yield in using donor 11 to glycosylate disaccharide 4, compared with the reaction between 3 and 4, suggests that it is difficult to use a trisaccharide donor to form the CD linkage, presumably due to the reduced reactivity of an oligosaccharide glycosyl donor (e.g., 3) vs the corresponding monosaccharide (e.g., 11).
Scheme 2.
Preparation of ABC Trisaccharide Donor 3
To overcome these setbacks, we designed a new 2 + (3 + 3) synthetic route, wherein the difficult CD linkage was formed earlier in the synthesis. Preactivation-based glycosylation of glucosyl donor 11 and disaccharide 12 gave the CDE trisaccharide 13 in 77% yield (Scheme 3a). Glycosylation between donor 13 and FGH trisaccharide acceptor 5,19 promoted by N-iodosuccinimide/TfOH, proceeded smoothly (Scheme 3b), forming the CDEFGH hexasaccharide 14 in an excellent yield (95%). The free hydroxyl group at C2 on hexasaccharide 14 was acetylated with Ac2O to produce 15, which was treated with HF/pyridine to form the triol hexasaccharide 16 in 92% yield. The regiostereoselectivity in the glycosylation of 13 and 5 was confirmed by comparing the chemical shift difference of H2″ in 14 vs 15. Selective oxidation of the two primary hydroxyl groups in triol 16 was tested first using TEMPO and [bis(acetoxy)-iodo]benzene.22 However, this reaction was slow, with multiple partial oxidation side products presumably due to the slow hydration of the aldehyde intermediates. Similar phenomena have been observed in synthesis of a Staphylococcus aureus associated trisaccharide.23 Switching the oxidation protocol to a two-step, one-pot oxidation (TEMPO, NaOCl followed by NaClO2)24 solved this problem and produced the desired product 17 in 87% yield after methyl ester formation.
Scheme 3.
Synthesis of (a) CDE Trisaccharide Donor 13 and (b) Hexasaccharide 17
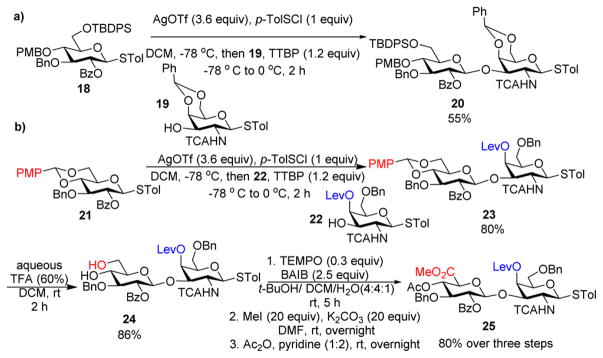
To access the AB module, donor 18 was preactivated by p-TolSCl/AgOTf and then glycosylated acceptor 19, producing the desired disaccharide 20 in 55% yield (Scheme 4a). When 20 was treated with Et3SiH/TFA25 to selectively open the benzylidene ring for future sulfation, the 4-O-PMB group was cleaved. To avoid this complication, we tried the glycosylation of donor 21 with acceptor 22, which smoothly formed the disaccharide 23 in 80% yield. Cleavage of the p-methoxybenzylidene ring in 23 using 60% aqueous TFA led to diol 24, which then underwent oxidation, methyl esterification, and acetylation, affording the AB disaccharide donor 25 in 80% yield over three steps (Scheme 4b).
Scheme 4.
Synthesis of AB Disaccharide Donor
With the AB and CDEFGH modules in hand, formation of the octasaccharide was explored. Glycosylation of the hexasaccharide acceptor 17 by disaccharide donor 25 produced octasaccharide 26 in 70% yield (Scheme 5). The successful assembly of 26 indicates that BC and EF linkages are suitable strategic linkage points for constructing the octasaccharide cassette.
Scheme 5.
Synthesis of the Octasaccharide Cassette
The next task was the conversion of the NHTCA in octasaccharide 26 to an acetamide and introduction of the O-sulfates. Radical reduction of NHTCA using tributyltin hydride (Bu3SnH)/AIBN26,27 is a popular method that has been successfully applied to CS synthesis.28,29 However, when 26 was treated with Bu3SnH/AIBN, the reaction gave several byproducts containing one or two residual chlorine atoms, which were difficult to separate. As an alternative, we tested the Zn–Cu couple reduction method.30,31 When octasaccharide 26 was treated with Zn–Cu couple in acetic acid, one or both of the benzylidene rings were cleaved, generating a complex mixture. To overcome this, octasaccharide 26 was first subjected to 80% aqueous acetic acid followed by acetylation of the tetraol, affording octasaccharide 27 (Scheme 5). Subsequent Zn–Cu couple mediated reduction of TCA led to the octasaccharide cassette 2 in 70% yield. The Lev groups in 2 were removed by hydrazine acetate, and the resulting diol 28 was sulfated to provide the octasaccharide 29 in 81% yield, which set the stage for peptide chain elongation.
One of the great challenges in CS glycopeptide assembly is the acid sensitivity of O-sulfates and the propensities of the glycosyl serine linkages and the glycan chains to undergo β-elimination under basic conditions. Thus, common acid-sensitive amino acid side chain protective groups such as Boc and trityl as well as strongly basic conditions for deprotection should be avoided. The Fmoc group in 29 was removed, and the resulting free amine 30 was coupled with tetrapeptide 31 using O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HATU) (Scheme 6). Subsequent catalytic hydrogenolysis was performed with Pearlman’s catalyst, which went smoothly to generate the carboxylic acid 32. Coupling of the carboxylic acid 32 and peptide 33 was promoted by HATU in the presence of diisopropylethyl amine (DIPEA) as the base. During this reaction, we observed a β-elimination side product32 due to cleavage of glycan from the glycopeptide. The extent of elimination could be reduced by lowering amounts of HATU and DIPEA from 3 equiv typically applied in peptide coupling to 1.5 equiv, leading to the glycopeptide 34 (60% yield).
Scheme 6.
Synthesis of Glycopeptide 1
Deprotection of 34 was first performed using LiOH at pH 9. Even when the reaction was carried out at 0 °C, partial cleavage of the sulfated glycan chain was observed. Switching to the reagent combination of H2O2/LiOH28,31,33 at pH 9 avoided the glycan cleavage problem and hydrolyzed the methyl esters as monitored by mass spectrometry. As removal of the benzoyl moieties was very slow under H2O2/LiOH, hydrazine was found to be a suitable agent to lead to the desired glycopeptide 1 in 71% yield (Scheme 6).

In conclusion, a strategy was successfully established for the first synthesis of a CS glycopeptide. Many obstacles were encountered in the synthesis of this highly complex structure. For glycan chain assembly, we found that it was difficult to form the CD linkage using trisaccharide donors. Rather this linkage was best assembled using a monosaccharide glycosyl donor, which gave the desired product in a high yield. A 2 + (3 + 3) route was developed to successfully prepare the octasaccharide cassette. Subsequently, suitable reaction conditions were identified for peptide chain elongation and deprotection to produce the CS-A bearing syndecan-1 glycopeptide 1. The successful establishment of a synthetic route to CS glycopeptide will now greatly facilitate understanding of their important biological functions.
Supplementary Material
Acknowledgments
We are grateful for the financial support from National Science Foundation (CHE 1507226) and the National Institute of General Medical Sciences, NIH (R01GM072667, U01GM116262).
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.7b02271.
Experimental procedures and analytical data and NMR spectra of new products (PDF)
References
- 1.Yamada S, Sugahara K. Curr Drug Discovery Technol. 2008;5:289. doi: 10.2174/157016308786733564. [DOI] [PubMed] [Google Scholar]
- 2.Volpi N. Chondroitin Sulfate: Structure, Role and Pharmacological Activity. Academic Press; San Diego, CA: 2006. [Google Scholar]
- 3.Kjellen L, Lindahl U. Annu Rev Biochem. 1991;60:443. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 4.Nandini CD, Mikami T, Ohta M, Itoh N, Akiyama-Nambu F, Sugahara K. J Biol Chem. 2004;279:50799. doi: 10.1074/jbc.M404746200. [DOI] [PubMed] [Google Scholar]
- 5.Kawashima H, Atarashi K, Hirose KM, Hirose J, Yamada S, Sugahara K, Miyasaka M. J Biol Chem. 2002;277:12921. doi: 10.1074/jbc.M200396200. [DOI] [PubMed] [Google Scholar]
- 6.Galtrey CM, Fawcett JW. Brain Res Rev. 2007;54:1. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Habuchi O. Biochim Biophys Acta, Gen Subj. 2000;1474:115. doi: 10.1016/s0304-4165(00)00016-7. [DOI] [PubMed] [Google Scholar]
- 8.Vibert A, Jacquinet JC, Lopin-Bon C. J Carbohydr Chem. 2011;30:393. [Google Scholar]
- 9.Mende M, Bednarek C, Wawryszyn M, Sauter P, Biskup MB, Schepers U, Bräse S. Chem Rev. 2016;116:8193. doi: 10.1021/acs.chemrev.6b00010. [DOI] [PubMed] [Google Scholar]
- 10.Yeung BKS, Chong PYC, Petillo PA. In: Glycochemistry. Principles, Synthesis, and Applications. Wang PG, Bertozzi CR, editors. Marcel Dekker; New York: 2001. p. 425. [Google Scholar]
- 11.Ramadan S, Yang W, Huang X. In: Chemical Biology of Glycoproteins. Tan Z, Wang L-X, editors. Royal Society of Chemistry; Cambridge: 2017. p. 209. [Google Scholar]
- 12.Kokenyesi R, Bernfield M. J Biol Chem. 1994;269:12304. [PubMed] [Google Scholar]
- 13.Chen XT, Sames D, Danishefsky SJ. J Am Chem Soc. 1998;120:7760. [Google Scholar]
- 14.Shimawaki K, Fujisawa Y, Sato F, Fujitani N, Kurogochi M, Hoshi H, Hinou H, Nishimura SI. Angew Chem, Int Ed. 2007;46:3074. doi: 10.1002/anie.200604909. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida K, Yang B, Yang W, Zhang Z, Zhang J, Huang X. Angew Chem, Int Ed. 2014;53:9051. doi: 10.1002/anie.201404625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garegg PJ. Adv Carbohydr Chem Biochem. 1997;52:179. doi: 10.1016/s0065-2318(08)60091-8. [DOI] [PubMed] [Google Scholar]
- 17.Tamura JI, Nakada Y, Taniguchi K, Yamane M. Carbohydr Res. 2008;343:39. doi: 10.1016/j.carres.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Liao L, Auzanneau FI. J Org Chem. 2005;70:6265. doi: 10.1021/jo050707+. [DOI] [PubMed] [Google Scholar]
- 19.Yang B, Yoshida K, Yin Z, Dai H, Kavunja H, El-Dakdouki MH, Sungsuwan S, Dulaney SB, Huang X. Angew Chem, Int Ed. 2012;51:10185. doi: 10.1002/anie.201205601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang X, Huang L, Wang H, Ye XS. Angew Chem, Int Ed. 2004;43:5221. doi: 10.1002/anie.200460176. [DOI] [PubMed] [Google Scholar]
- 21.Crich D, Smith M, Yao Q, Picione J. Synthesis. 2001;2001:323. [Google Scholar]
- 22.van den Bos LJ, Codee JD, van der Toorn JC, Boltje TJ, van Boom JH, Overkleeft HS, van der Marel GA. Org Lett. 2004;6:2165. doi: 10.1021/ol049380+. [DOI] [PubMed] [Google Scholar]
- 23.Hagen B, van Dijk JHM, Zhang Q, Overkleeft HS, van der Marel GA, Codée JDC. Org Lett. 2017;19:2514. doi: 10.1021/acs.orglett.7b00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang L, Teumelsan N, Huang X. Chem - Eur J. 2006;12:5246. doi: 10.1002/chem.200600290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeNinno MP, Etienne JB, Duplantier KC. Tetrahedron Lett. 1995;36:669. [Google Scholar]
- 26.Coutant C, Jacquinet JC. J Chem Soc, Perkin Trans. 1995;1:1573. [Google Scholar]
- 27.Blatter G, Beau JM, Jacquinet JC. Carbohydr Res. 1994;260:189. doi: 10.1016/0008-6215(94)84038-5. [DOI] [PubMed] [Google Scholar]
- 28.Lopin C, Jacquinet JC. Angew Chem, Int Ed. 2006;45:2574. doi: 10.1002/anie.200503551. [DOI] [PubMed] [Google Scholar]
- 29.Tully SE, Mabon R, Gama CI, Tsai SM, Liu X, Hsieh-Wilson LC. J Am Chem Soc. 2004;126:7736. doi: 10.1021/ja0484045. [DOI] [PubMed] [Google Scholar]
- 30.Vibert A, Lopin-Bon C, Jacquinet JC. Tetrahedron Lett. 2010;51:1867. [Google Scholar]
- 31.Ait-Mohand K, Mirault A, Jacquinet JC, Lopin-Bon C. Org Biomol Chem. 2016;14:7962. doi: 10.1039/c6ob01392a. [DOI] [PubMed] [Google Scholar]
- 32.Yang W, Yoshida K, Yang B, Huang X. Carbohydr Res. 2016;435:180. doi: 10.1016/j.carres.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucas H, Hasten JEM, van Dinther TG, Meuleman DG, van Aelst SF, van Boeckel CAA. Tetrahedron. 1990;46:8207. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.