Abstract
A chemically defined protocol requiring no animal-derived components allows for the easier derivation and enduring expansion of epicardial cells from human pluripotent stem cells.
Translational research has been revolutionized by induced pluripotent stem cell (iPSC) technology. After a myriad of refinements in the protocols1, today it is possible to generate iPSCs from human cells and to differentiate iPSCs into disease-relevant, organ-functioning cells, such as cardiomyocytes (CMs), endothelial cells (ECs) and vascular smooth muscle cells. However, iPSC-CMs and iPSC-ECs generated with current iPSC technology exhibit high levels of immaturity and heterogeneity, which can hamper disease modelling and drug screening. Furthermore, no method thus far has been capable of distinguishing atrial, ventricular and nodal subtypes of iPSC-CMs. Similarly, iPSC-ECs exhibit a heterogeneous mixture of arterial, venous and lymphatic characteristics. To overcome these obstacles, molecular and cellular juxtaposition of iPSC differentiation and mammalian development will be necessary to resolve the immaturity and heterogeneity of iPSC-derived cells and to generate cell types critical for cardiac development and repair — such as endocardial, pericardial and epicardial cells. So far, little is known of correlations between the iPSC differentiation process in vitro and the developmental biology of various tissue and cell types in vivo. In an effort that begins to address this gap in knowledge, Sean Palecek and colleagues report in Nature Biomedical Engineering a chemically defined, xeno-free method for the generation and expansion of epicardial cells derived from human PSCs (hPSCs)2.
The epicardium — a specialized epithelium that wraps and protects the heart3 — plays a critical role in heart development. In a developing heart, epicardial cells undergo the epithelial-to-mesenchymal transition (EMT) to form epicardial-derived cells (EPDCs), which invade the subepicardial matrix and migrate into the myocardium to give rise to cardiac fibroblasts, coronary vascular ECs, smooth muscle cells and cardiac muscle cells3–8. In fact, ablation of the epicardium or epicardial genes hampers myocardial growth and coronary-vessel formation during development3,4,6. Importantly, EPDCs are therapeutically beneficial during cardiac tissue repair in the adult mammalian heart; they modulate myocardial injury by secreting paracrine factors that enhance neovascularization and by differentiating directly into CMs3,9–12.
By taking cues from the mammalian cardiac developmental process (in which the pro-epicardium arises from multipotent progenitors expressing the ISL1+, NKX2.5+ and FLK-1+ genes), Palecek and co-authors sought to identify the minimal set of modulators of developmental signalling pathways that would be sufficient to generate epicardial cells from hPSC-derived cardiac progenitor cells (CPCs; Fig. 1). The authors treated ISL1+/NKX2.5+/FLK-1+ CPCs at day 6 of the cardiac differentiation protocol for 48 hours with modulators of the fibroblast growth factor, bone morphogenetic protein (BMP), Wnt, Hedgehog, retinoic acid and transforming growth factor-β (TGF-β) signalling pathways, and found that from days 7 to 9, the successful activation of canonical Wnt signalling by CHIR99021 (CHIR; a small-molecule inhibitor of the protein kinase GSK-3β) triggered the differentiation of putative WT1+ epicardial cells at high efficiency (>85%; WT1 is a protein required for the EMT to occur in the developing epicardium). Activation of canonical BMP signalling by BMP4 treatment was ineffective in producing epicardial cells. Inhibition of BMP signalling in CHIR-treated CPCs did not alter the generation of WT1+ epicardial cells, indicating that BMP is dispensable at this stage of epicardial development. Instead, canonical Wnt activation of CPCs (at day 7 of differentiation) was critical in guiding the fate of CPCs to the epicardial or cardiac lineages. The absence of CHIR at day 7 yielded robust beating sheets of CMs (which expressed the cardiac-specific form of troponin (cTnT), a protein that plays a role in cardiac muscle contraction), whereas treatment with CHIR produced WT1+ epicardial cells at the expense of CMs.
Figure 1.
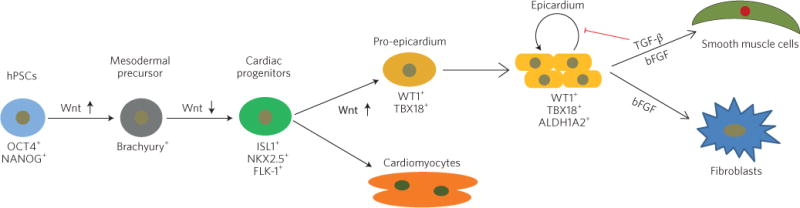
Generation of epicardial cells from human pluripotent stem cells (hPSCs). Canonical Wnt activation promotes mesodermal formation from OCT4+/NANOG+ pluripotent stem cells. Inhibition of canonical Wnt signalling then differentiates Brachyury+ mesoderm into ISL1+/NKX2.5+/FLK-1+ cardiac progenitor cells (CPCs). The activation of canonical Wnt signalling in CPCs is necessary and sufficient to generate pro-epicardial cells, whereas inhibition of canonical Wnt drives the differentiation of CPCs into cardiomyocytes. Differentiated epicardial cells express markers of the proteins WT1, TBX18 and ALDH1A2. These epicardial cells have the potential to differentiate into smooth muscle cell or fibroblast lineages in vitro and in vivo. The red symbol indicates inhibition. Brachyury is a transcription factor required for mesoderm formation. TGF-β, transforming growth factor beta; bFGF, basic fibroblast growth factor. Figure reproduced from ref.2, Nature Publishing Group.
Furthermore, the authors showed that the activation of β-catenin-mediated canonical Wnt signalling is necessary for the generation of WT1+ epicardial cells. Knockdown of β-catenin in CPCs significantly reduced the number of WT1+ cells and instead generated robust beating sheets of cTnT+ CMs. This is consistent with the mammalian cardiac developmental process, in which the inhibition of canonical Wnt and therefore activation of non-canonical Wnt signalling are necessary for the terminal differentiation of CPCs to CMs13. Palecek and colleagues also demonstrated that canonical Wnt signalling regulates epicardial and CM specification of CPCs, at least during the hPSC differentiation process in vitro. The generated epicardial cells expressed the pro-epicardial genes WT1, TBX18 and TCF21. The cells exhibited cobblestone-like morphology and epithelial-cell junction proteins, similar to what occurs in the cultured primary epicardium. By day 12 of differentiation, the epicardial cells were expressing the retinaldehyde enzyme ALDH1A2, suggesting that they can produce retinoic acid. Moreover, the cells exhibited the potential to differentiate into fibroblasts (which expressed the intermediate-filament protein vimentin and the cell-surface protein CD90) or smooth muscle cells (which produced the cytoplasmic structural protein α-smooth muscle actin (αSMA) and the calcium-binding protein calponin).
One limitation of epicardial-cell culture is the cells’ tendency to undergo spontaneous EMT. The hPSC-derived epicardial cells were no exception, losing WT1 expression and naturally undergoing EMT during long-term culture. Yet Palecek and co-authors found that a treatment with TGF-β inhibitors (A83-01, SB431542, RepSox or SB505124) maintained the epicardial phenotype and enhanced the proliferative potential of the cells, allowing for 25 population doublings and generating more than 10 million cells from a single hPSC-derived epicardial-cell clone. This is not surprising, given the well-described role of TGF-β in EMT induction in vivo and in vitro. The authors also showed that epicardial cells cultured with TGF-β inhibitors for 50 days retained normal karyotypes and maintained the potential to differentiate into fibroblasts or smooth muscle cells.
The epicardial cells generated from hPSCs were functional in vivo. When transferred to the surface of the heart via extracellular matrix patches in a myocardial-infarction mouse model, hPSC-derived epicardial cells invaded the myocardium and underwent EMT to differentiate into αSMA+calponin+ smooth muscle cells and vimentin+ fibroblasts. This suggests that hPSC-derived epicardial cells may be able to invade the myocardium and form EPDCs that give rise to various cardiac cell types, which highlights their potential for cell-based therapies. In addition, transcriptomic analysis showed that hPSC-derived epicardial cells resembled primary epicardial cells.
It is important to note that, unlike previous differentiation protocols for epicardial differentiation14,15, Palecek and co-authors’ protocol is a xeno-free and chemically defined protocol compatible with current good-manufacturing-practice standards, which is essential in assuring the efficiency and reproducibility of iPSC differentiation techniques. Indeed, independent iPSC lines have been shown to display varying responses to identical differentiation protocols, which necessitates the generation of chemically defined differentiation protocols to allow for optimization as necessary. In fact, using a WT1 fluorescence reporter cell line generated with the gene-editing CRISPR/Cas9 system, the authors determined both the concentration of CHIR and the initial seeding density of day 6 CPCs that yielded the greatest number of WT1+ epicardial cells. Other small-molecule activators of canonical Wnt signalling (CHIR98014 or BIO-acetoxime) were equally effective in generating the epicardial cells as CHIR. The Wnt3a recombinant protein was also sufficient in generating epicardial cells, though not as effectively as the small-molecule inhibitors.
The cardiogenic potential of EPDCs has long been a point of contention3,5,11. As Palecek and co-authors have illustrated the potential of transplantation of PSC-derived epicardial cells to the injured myocardium, it would be interesting to explore whether hPSC-derived EPDCs can differentiate into functional CMs at the expense of fibroblast and smooth-muscle lineages, and to identify the underlying mechanisms. Moreover, epicardial cells have been found to play an essential role in vasculogenesis and coronary-artery formation during development4,8, and in animal models a number of studies have described the beneficial paracrine role of epicardial cells in cardiac tissue repair3,9,10,12. A better understanding of the potential of hPSC-derived epicardial cells in endothelial differentiation or in coronary-vessel formation, and of the paracrine effects of hPSC-derived epicardial cells would thus be valuable.
All in all, Palecek and colleagues have addressed an important knowledge gap in the field of iPSC biology by elucidating the molecular pathways that regulate how CPCs transform into epicardial cells and CM lineages. The development of a xeno-free, chemically defined protocol for the generation of epicardial cells from hPSCs holds high potential in cell-based heart regenerative therapies and in disease modelling, and will pave the way for the use of iPSCs in cardiovascular precision medicine (Fig. 2).
Figure 2.
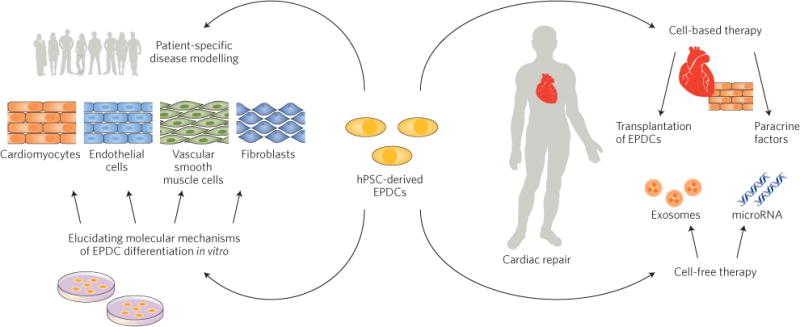
Applications of human pluripotent stem cell (hPSC)-derived epicardial-derived cells (EPDCs). Large-scale generation of EPDCs from hPSCs (including patient-specific cells and genome-edited-induced pluripotent stem cells) will boost cell-based (top right) and cell-free (bottom right) therapies, cardiovascular disease modelling (top left), and the characterization of the molecular mechanisms of human EPDC differentiation in vitro (bottom left).
References
- 1.Sayed N, Liu C, Wu JC. J Am Coll Cardiol. 2016;67:2161–2176. doi: 10.1016/j.jacc.2016.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao X, et al. Nat Biomed Eng. 2016;1:0003. doi: 10.1038/s41551-016-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Gise A, Pu WT. Circ Res. 2012;110:1628–1645. doi: 10.1161/CIRCRESAHA.111.259960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merki E, et al. Proc Natl Acad Sci USA. 2005;102:18455–18460. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai CL, et al. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Pomares JM, de la Pompa JL. Circ Res. 2011;109:1429–1442. doi: 10.1161/CIRCRESAHA.111.245589. [DOI] [PubMed] [Google Scholar]
- 7.Smith CL, Baek ST, Sung CY, Tallquist MD. Circ Res. 2011;108:e15–e26. doi: 10.1161/CIRCRESAHA.110.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh A, et al. Cell Rep. 2016;15:1384–1393. doi: 10.1016/j.celrep.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou B, et al. J Clin Invest. 2011;121:1894–1904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang GN, et al. Science. 2012;338:1599–1603. doi: 10.1126/science.1229765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smart N, et al. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei K, et al. Nature. 2015;525:479–485. doi: 10.1038/nature15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gessert S, Kuhl M. Circ Res. 2010;107:186–199. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- 14.Witty AD, et al. Nat Biotechnol. 2014;32:1026–1035. doi: 10.1038/nbt.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer D, et al. Development. 2015;142:1528–1541. doi: 10.1242/dev.119271. [DOI] [PMC free article] [PubMed] [Google Scholar]


