Abstract
Purpose of review
Notch signaling is an important component of retinal progenitor cell maintenance and MG specification during development, and its manipulation may be critical for allowing MG to re-enter the cell cycle and regenerate neurons in adults. In mammals, MG respond to retinal injury by undergoing a gliotic response rather than a regenerative one. Understanding the complexities of Notch signaling may allow for strategies that enhance regeneration over gliosis.
Recent findings
Notch signaling is regulated at multiple levels, and is interdependent with various other signaling pathways in both the receptor and ligand expressing cells. The precise spatial and temporal patterning of Notch components is necessary for proper retinal development. Regenerative species undergo a dynamic regulation of Notch signaling in MG upon injury, whereas non-regenerative species fail to productively regulate Notch.
Summary
Notch signaling is malleable, such that the altered composition of growth and transcription factors in the developing and mature retinas result in different Notch mediated responses. Successful regeneration will require the manipulation of the retinal environment to foster a dynamic rather than static regulation of Notch signaling in concert with other reprogramming and differentiation factors.
Keywords: Notch, Muller glia, gliosis, retina, regeneration, stem cells
Introduction
Notch signaling drives the proliferation of progenitor cell populations and cell fate specification [1, 2]. As a result, proper Notch signaling is critically important during development [3] and for the maintenance of stem cell populations throughout the life of an organism [4]. The ability to effectively manipulate Notch holds promise for the creation of future therapeutic interventions aimed at promoting tissue regeneration, by unlocking the latent stem cell potential of progenitor populations. In order to properly design these therapeutics, it is essential to understand how Notch signaling is regulated and its downstream effects within a given tissue. This task is complicated by the context dependent nature of Notch signaling, in which it is more permissive than instructive [5]. The combination of Notch with other signaling pathways and the overall tissue environment ultimately determines cell fate decisions [3]. Furthermore, Notch signaling has both cell autonomous and non-autonomous effects [6, 7] and uses a receptor-ligand signaling system that links the fate of one cell to its neighbors [3]. In vertebrates, there are four Notch receptor paralogues (Notch 1-4), and two families of ligands (Delta and Jagged) [8]. The spatial and temporal specific expression of Notch receptors and ligands determines some of the variability associated with Notch, both between tissues, and amongst different cell populations in the same tissue.
The activation of Notch signaling is a multi-step process initiated by the binding of one of the Notch ligands to its receptor [9]. This interaction triggers the cleavage of the Notch receptor extracellular domain by a furin-like protease [10] and a metalloprotease [11-13], resulting in transendocytosis of this domain by the ligand expressing cell in a process involving the ubiquitin E3-ligase, mindbomb [14]. This event then triggers the cleavage of the Notch receptor intracellular domain (NICD) by the gamma secretase complex [15], which allows it to translocate to the nucleus and regulate gene transcription [16-18]. In the nucleus the NICD forms a complex with Rbpj [19] and mastermind/MAML [20-22] to drive expression of genes that include the Hes and Hey families of transcriptional repressors [23].
This process provides for multiple points of regulation, such that Notch signaling is influenced by a variety of feedback loops in order to ensure the proper dosage of Notch in a given tissue at a given time [8]. Advances have been made in recent years with regard to our understanding of Notch signaling in the retina during development and in the context of regeneration. Muller glia (MG) have the capacity to reprogram and regenerate the retina in some species [24]. Notch signaling appears to play a vital role in both the specification and reprogramming of MG, therefore a detailed understanding of mechanisms underlying Notch signaling in these cells may hold the key to developing effective therapeutics for patients with retinal degenerative diseases.
Development
The basis for understanding the role of Notch signaling in the development of the vertebrate retina stems from studies manipulating Notch signaling through inhibition or forced overexpression. The expression of an active version of Xenopus Notch in retinal progenitor cells (RPCs) first revealed a role for Notch in maintaining this progenitor pool by preventing differentiation [25]. Furthermore, this early work in Xenopus indicated that Notch signaling is involved at several stages throughout retinal development, with the two major roles of RPC maintenance and MG specification. This iterative role for Notch is supported by similar findings in mice and fish. Typically, Notch1 is expressed by proliferating progenitors and undifferentiated progenitors during development, and then retained in MG in the mature retina [25-27].
The overexpression of the NICD from Notch1 in the RPCs of mice through a conditional Cre recombinase system promotes the appropriate early progenitor state of RPCs in the embryonic retina, but the maintenance of Notch signaling in RPCs in the postnatal retina leads to an overproduction of glia with inappropriate expression of both progenitor and glial genes [28]. In contrast, the inhibition of Notch signaling through a gamma secretase inhibitor, or a mindbomb mutant in zebrafish, results in normal neuronal differentiation, but leads to disorganization of the retinal architecture due to the loss of MG, which serve as a scaffold for migrating neurons [29]. This suggests that while neuronal differentiation requires the loss of Notch, the dynamic regulation of Notch is necessary for proper retinal development, likely through the interaction of Notch with other prominent developmental signaling pathways. Indeed, the oscillation of the Notch effector Hes1 is necessary to ensure the correct balance of differentiation and neural progenitor maintenance by regulating the cycling of Notch ligands and pro-neural differentiation factors [30]. The sustained expression of Hes1is actually inhibitory to progenitor proliferation by slowing the cell cycle, and is instead associated with dormancy [31]. The mechanisms of the feedback loops underlying Hes1 cycling are unclear, but several other proteins associated with developmental signaling pathways also have oscillatory expression and may be important drivers of this process [32]. Consequently, the effect of disrupting the Notch signaling cascade is dependent on the total milieu of signaling factors present in the developing retina at any given time. The dynamic feature of Notch signaling also has important implications for the utility and interpretation of studies using constitutive loss or activation of Notch, since they interfere with these complex interactions and regulatory loops. In spite of this limitation, recent work has helped uncover several of the other factors that regulate or influence Notch activity, which is starting to provide a framework for separating its pro-proliferative and pro-glial features.
Receptor cleavage regulation
Since the activation of Notch signaling is a multi-step process, there are many prospective points of regulation. RanBP9 is an adaptor protein which helps to bridge membrane bound receptors with intracellular components that regulate the signaling of the receptors, and has been implicated in the regulation of brain development [33]. In the zebrafish retina, RanBP9 has been identified as a binding partner of mindbomb, facilitating the interaction with the intracellular domain of the Notch ligand, delta, necessary for delta’s internalization [34]. Similar to the mindbomb mutant, loss of RanBP9 results in defective proliferation and premature neuronal differentiation in the retina. Snx5 co-localizes with mindbomb in endosomal compartments and is important for its trafficking and thus formation of mindbomb/delta complexes [35]. The microRNA miR-216a is expressed in a complimentary pattern to snx5 in the retina, and has been shown to regulate snx5 [36]. Consequently, knockdown of miR-216a results in increased Notch signaling. Notably, JAK2 has also been shown to be a target of miR-216a in other systems [37, 38], and this dual regulation of the Notch and JAK/Stat pathways may allow for a more potent inhibition of proliferation, as there is considerable cross-talk between these pathways. The Notch effectors Hes1 and Hes5 bind to Stat3 to facilitate its complex formation with JAK2 and subsequent activation [39]. Additionally, Stat3 has been found to co-oscillate with Hes1 and has been proposed to regulate Hes1 expression either directly or indirectly [32].
The mindbomb mediated internalization of ligand is accompanied by the transendocytosis of the extracellular domain of the Notch receptor, but first requires the cleavage of this ectodomain from the receptor expressing cell [11]. The ADAM family of metalloendopeptidases mediates the extracellular cleavage of Notch receptors in a tissue specific manner [40]. The activity of ADAMs then facilitates the subsequent cleavage of the NICD by the gamma-secretase complex [41]. While in the brain the cleavage activities of both ADAM17 and ADAM10 are important for pro-proliferative signaling, only ADAM10 is required in the retina for proper Notch signaling [42]. The retinas of mice with a retina specific knockout of ADAM10 exhibit a decrease in the number of proliferating progenitors and increased differentiation of photoreceptors and retinal ganglion cells (RGCs), which can be rescued by the expression of Notch1 NICD. The regulation of ADAM10 involves interaction with the Wnt pathway. Sfrps are secreted glycoproteins which block the activation of Wnt mediated signaling cascades by preventing the interaction between Wnts and their receptors [43]. Sfrp1 and Sfrp2 can also bind ADAM10 and inhibit its interaction with Notch receptors [44]. Loss of these Wnt antagonists leads to a transient upregulation of Notch signaling and a disruption of retinal neurogenesis. The over-activation of Notch results in a pulse of proliferation followed by a compensatory downregulation of ligand, and eventually, differentiation, creating a bias toward early born neurons. Sfrps also facilitate interactions between Wnts with BMPs in the process of retinal dorsal-ventral patterning [45]. Sfrps, then, may be critical players in regulating the coordinated activities of multiple signaling pathways during retinogenesis, thereby influencing the context for associated Notch activity.
RPC maintenance
In post-embryonic Xenopus, activation of the Wnt pathway increases proliferation of retinal progenitors, whereas activation of the sonic hedgehog (Shh) pathway results in an overproduction of MG [46]. Shh signaling antagonizes Wnt signaling by enhancing expression of Sfrp1, which would also be expected to affect Notch signaling. The cross-talk between these various developmental signaling pathways creates a series of interdependent feedback loops which ultimately control the extent of proliferation within the retina. In the developing retina, Shh can also promote proliferation in a context dependent manner [47] and works in cooperation with Notch. In the postnatal mouse retina, Notch can induce the Shh effector Gli2 [48], which in turn can stabilize Hes1 [49]. This Notch-Gli2 axis is necessary to sustain a proliferative response to Shh in RPCs and MG [48], and reinforces the notion that Notch is permissive, rather than instructive. Consequently, signaling pathways that facilitate proliferation and growth during development can be repurposed to restrict proliferation in a mature organism, and this switch is mediated by changes in the way pathways are coupled to one another.
MG specification
The transition of Notch from pro-proliferative to pro-glial also involves the dynamic changes in signaling cascades and the associated induction of transcription factors over the course of retinal development. A genome wide analysis of the postnatal mouse retina has revealed that Notch expression peaks several days after MG are born, suggesting that sustained Notch activity is necessary for proper glial maturation following cell cycle exit [50]. The LIM homeodomain transcription factor, Lhx2, may mediate this Notch dependent role in MG maturation through its direct regulation of a variety of Notch associated genes [51]. While constitutive activation of Notch permissively maintains cells in a RPC like state, Lhx2 regulation of Notch controls MG differentiation. The retinas of Lhx2 knockout mice lack Notch signaling and MG, but this phenotype can be rescued by the expression of the NICD [52]. Notably, overexpression of the potently gliogenic Hes5 is insufficient for rescue, indicating that multiple Notch target genes are involved in the specification of MG. Furthermore, Lhx2 must act in coordination with multiple intrinsic factors during this process. LIM domain binding (Ldb) proteins are cofactors which associate with LIM domain containing proteins and stabilize protein complexes [53]. In the mouse retina, Ldb1 and Ldb2 interact with Lhx2. While the loss of both proteins depletes the pool of RPCs leading to premature cell cycle exit and differentiation, Ldb2 is sufficient to sustain Lhx2 expression in MG [54].
Although many experiments manipulating Notch signaling focus on the NICD of Notch1, there is an increasing amount of evidence that the combined activity of multiple Notch components is necessary for proper retinogenesis. miR-7a is expressed in an opposing gradient to the transcription factor Pax6 and is involved in the specification of distinct types of mature neurons in the brain [55]. In the zebrafish retina it has been shown to play a role in the de-differentiation of MG, and knockdown of miR-7a phenocopies Pax6 mutants [56]. In the developing mouse retina, miR-7a negatively regulates MG differentiation by targeting Notch3, without affecting RPC proliferation [57]. Consequently, it is not simply the temporal pattern of signaling events, but also the spatial patterning of the signaling components that drives retinal development.
Spatial and temporal patterning of Notch
In the embryonic rat retina the Notch ligands delta and jagged are expressed in complimentary regions such that different combinations of ligand and receptor direct the development of distinct subsets of cells within the retina [26]. Jagged is expressed in the lens and ciliary margin, while delta is most strongly expressed in the central retina, in a coincident pattern with Notch1. The receptors have both distinct and overlapping patterns with Notch2 expressed strongly in the retinal pigment epithelium (RPE), and Notch3 expressed at a low level throughout the retina from E12.5 to E14.5. In the embryonic mouse retina, the interaction of RPE expressed ligand and Notch1 on RPCs has recently been shown to influence the cell fates of RPC derived daughter cells [58]. The daughter cell in closest proximity to the RPE receives extra ligand, thereby increasing Notch activity and maintaining progenitor status. Meanwhile, the other daughter experiences a lower level of receptor-ligand engagement and proceeds toward differentiation. The disruption of this Notch promoting niche through the loss of mindbomb in RPE cells leads to retinal disorganization and an imbalance of cellular subtypes biased toward more neurons, particularly photoreceptors.
The differential expression of Notch components in combination with other signaling and transcription factors can control the wave of neurogenesis in the developing retina. In the embryonic mouse retina, the Notch ligands delta-like 1 (Dll1) and Dll4 are expressed sequentially in a manner that prevents premature differentiation and contributes to cell type diversity [59]. This sequential expression may explain why Dll1 is critical for the differentiation of RGCs, but not other retinal cell types [60]. Dll1 exerts both cell autonomous and non-autonomous effects, both of which are necessary for retinal expression of Hes1. However, only the cell autonomous effects are needed for RGC differentiation. The transcription factors Atoh7 and Neurog2 are also critically important for the differentiation of RGCs [61, 62]. Although they are both activated by Pax6 and have coincident expression and onset in the mammalian retina, they are differentially regulated by Notch [63]. Atoh7 is required for the specification of early cell fates in RPCs, and is inhibited by Notch in a canonical lateral inhibition mediated manner involving Notch1 and Hes1. The differentiation of these initial neurons also depends on a feedback loop between Atoh7 and Notch, as it has been shown that in chick Atoh7 activates Hes5.3, which allows for the build-up of the necessary differentiation factors by retarding cell cycle progression [64]. The regulation of Neurog2 is more complex, as it participates in an oscillatory expression loop with Hes1 [30], but its patterning in the distal retina requires both Notch1 and Notch3, and is mediated by Rbpj in a Hes independent manner [63]. In this way, the spatial restriction of Notch components regulates Neurog2 expression and the propagation of neurogenesis across the retina. Additionally, a mathematical model of this process, suggests that the neurogenic wave propagation is produced through a combination of Notch mediated lateral inhibition and growth factor diffusion [65].
Regeneration
Regeneration is often characterized as a process involving the reactivation of developmental programs, but this description is an oversimplification of the reality. While many of the same signaling pathways and molecular players are involved, progenitor cells within developing and mature nervous systems exist in vastly different environments. As a result, activation of some of the same pathways that facilitate neural development could actually inhibit productive regeneration. The differential interactions amongst signaling pathways in the mature versus the developing retina may also contribute to the species variation in regenerative capacity, with pro-regenerative species harboring an environment that more fully replicates the spectrum of developmental dynamics (see Figure 1). Due to its context dependency, Notch signaling is likely to be a key mediator of these differences. This could involve differential regulation, responses of downstream effectors, and/or coupling to other signaling pathways. Unfortunately, most studies use broad methods of Notch repression or activation, making it difficult to uncover potentially crucial differences in the timing and molecular details of Notch’s contribution to the regenerative response. Side-by-side studies comparing pro- and non-regenerative species are key to determining which aspects are most relevant.
Figure 1. Notch signaling dynamics in retinal development and regeneration.
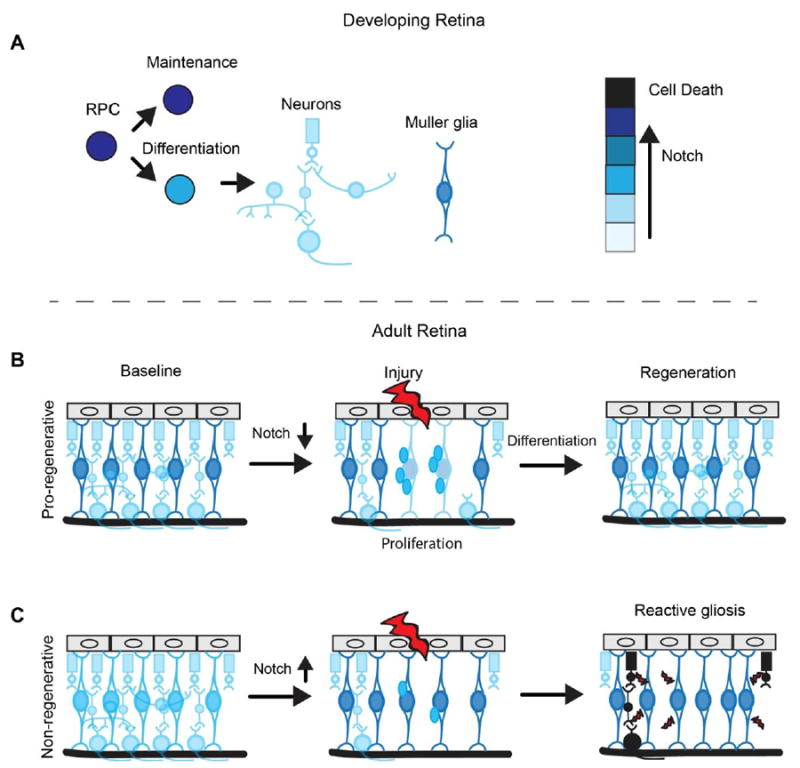
(A) During development the level of Notch in retinal progenitors (RPCs) determines whether they will be maintained or differentiate. A decrease in Notch is necessary for proper differentiation.
(B) In the adult retina of pro-regenerative species, like zebrafish, high levels of Notch signaling maintain the quiescence of Muller glia (MG). Following injury MG downregulate Notch signaling and up-regulate reprogramming factors, allowing them to re-enter the cell cycle and proliferate. In a low Notch environment the MG-derived progenitors are capable of differentiating into neurons and regenerating the retina.
(C) In the adult retina of a non-regenerative species (mammals), Notch is low in quiescent MG. If Notch increases endogenously (chick) or through exogenous intervention (mouse), MG can re-enter the cell cycle and proliferate at low levels. However, sustained Notch results in reactive gliosis and the release of neurotoxins by MG, furthering retinal damage.
Pro-regenerative species
Currently, our best understanding about Notch signaling in the regenerating retina comes from zebrafish. In the zebrafish retina, MG respond to injury by re-entering the cell cycle, and these MG-derived progenitors then go on to differentiate and regenerate the retina [24]. Notch signaling is dynamically regulated during this process, characterized by an initial decrease followed by an up-regulation of specific Notch components [66-68]. Unlike in mammals, Hes1 expression is maintained in MG in the mature retina [29], and in the absence of damage, Notch maintains MG in a state of quiescence [69] . In the context of injury, Notch signaling is primarily involved in regulating the expansion of the MG-derived progenitor pool, as the zone of proliferation is expanded by global repression and limited by constitutive activation of Notch [66]. Additionally, an analysis of retinal regeneration associated miRNA expression has identified miRNAs that contribute to MG-derived progenitor proliferation, and several of these, including miR-7a, miR-146a, and miR-31 have been shown to regulate Notch in other systems [56]. Although repression of Notch can stimulate a small increase in the expression of de-differentiation markers including Ascl1a, this is not sufficient to produce a strong proliferative response in the retina; additional damage related signals are necessary [66, 69, 68]. The identities of many of these damage induced molecules have been uncovered in recent years [66, 68, 70-72] , but more work is needed in understanding the details of how they interact with the Notch signaling pathway.
The release of cytokines following injury, including IL-6, leads to stimulation of gp130-coupled cytokine receptors, and activation of the Jak/Stat pathway [70]. The repression of the Notch pathway also induces Stat3, suggesting that in the mature fish retina Notch may inhibit Jak/Stat ligands [69]. Although active phosphorylated Stat3 is associated with proliferation in both developmental and regenerative contexts, the interaction with the Notch pathway may be altered, as Notch activity is typically associated with Stat3 activation during development [39]. Notably, the cocktail of growth factors present in a tissue can influence the location(s) of Stat3 phosphorylation, and this differential phosphorylation is associated with either the proliferative or glial properties of Notch [73].
Notch’s participation in a feedback loop with the growth factor HB-EGF has been shown to be critical for its ability to confine the zone of proliferation to the area immediately adjacent to the injury site [66]. Retinal damage stimulates the production of HB-EGF, which activates various Notch components via Ascl1a. Notch signaling, in turn, represses both HB-EGF and Ascl1a, thereby limiting proliferative capacity to MG-derived progenitors experiencing the greatest concentration of damage induced signals. It has recently been demonstrated that the initial decrease in Notch signaling following injury is dependent on Fgf8a, which itself undergoes an age-dependent switch from the facilitation to the inhibition of proliferation by progenitor cells [68]. Since Fgf8 is not expressed in the mature mammalian retina, this interaction between Notch and Fgf8 may be related to the unique feature of zebrafish retinal regeneration in which Notch is inhibitory, rather than stimulatory.
Non-regenerative species
The success of regeneration in zebrafish lies in the ability of MG-derived progenitors to both proliferate and differentiate into the appropriate retinal cell subtypes. In some species, such as the chick, MG are capable of de-differentiating and proliferating, but fail to properly differentiate into neurons [74]. This difference appears to be mediated, at least in part, by Notch signaling. The details of the interactions and mechanism by which Notch signaling participates in the differentiation process during regeneration in the zebrafish retina still need to be worked out, and could offer critical insight into how to elicit successful regeneration in other species. It is important to note that most studies use Notch components such as receptor or Hes gene expression as read-outs, but this approach does not convey the full breadth of Notch related changes, and could potentially be misleading. An accurate depiction requires the use of a Notch signaling reporter, which at this point has only been used in zebrafish [68]. Therefore, a comprehensive understanding of Notch signaling in the MG of non-regenerative species is still lacking, and is likely to be more complex than is currently appreciated. In the chick retina, MG normally express low levels of Notch1, which is upregulated following retinal damage, reaching a peak of expression during the period of greatest progenitor proliferation [75]. While Notch signaling can facilitate the de-differentiation process initially, its persistence prevents neuronal differentiation later on. Similar to zebrafish, signaling through gp130 and activation of the Jak/Stat pathway is necessary for the proliferation of MG-derived progenitors, however, in chick, gp130 promotes Notch and inhibits the neurogenic potential of the progenitors [76]. The reasons for the discrepancy need to be determined, but may involve differences between the coupling of the Notch and Jak/Stat pathways as well as the sites of Stat3 phosphorylation.
Although extremely limited, MG reprogramming in the rat retina also involves the interaction of Notch with growth factors and developmental signaling pathways. MG from retinal explants cultured in the presence of the Notch ligand Jagged1, and the Wnt signaling protein Wnt3a, are capable of de-differentiating and proliferating [77]. These activated MG can also differentiate into photoreceptors upon removal of the ligands. The ability of Notch to induce cell cycle re-entry may be mediated, at least in part, by its regulation of the cyclin dependent kinase inhibitor, p27Kip1. The activation of Notch signaling leads to the transcriptional repression of p27Kip1 and induction of Skp2, which further inhibits p27Kip1 by directing its post-translational degradation [78]. In vivo, the ability to precisely regulate the temporal variations in Notch signaling necessary to achieve both MG activation and neuronal differentiation is far more limited and represents a real barrier in the effort to develop effective therapeutic strategies for retinal repair. Following NaIO3 induced retinal injury, some rat MG down-regulate expression of p27Kip1 and re-enter the cell-cycle [79]. There is also a transient peak of NGF a few days after injury, and a more sustained decrease in protein levels of Notch1 and Hes1 which slowly re-accumulate over the course of a month. Similar to development, this biphasic feature of Notch facilitates the transition from proliferating progenitors to differentiated neurons, as prolonged Notch signaling leads to gliosis.
Reactive gliosis can promote inflammation, exacerbate damage, and prevent neurogenesis [80]. Consequently, there is strong interest in understanding the mechanism underlying gliosis as well as developing methods to modulate Notch activity in the context of retinal repair. The transcription factor Lhx2, which is essential for MG specification during development, also appears to play a key role in preventing gliosis in the mouse retina. The loss of Lhx2 in mature MG blocks the expression of injury induced MG-derived neuroprotective factors and results in a program of reactive gliosis [81]. Since Lhx2 is associated with Notch activation in the developing retina and relies on the coordinated activities of several intrinsic factors, the interaction between Lhx2 and Notch signaling may be altered in the context of the mature retina. The transplantation of olfactory ensheathing cells into the subretinal space of rats with retinal degeneration can improve neuronal survival and prevent gliosis [82]. This appears to be due, in part, to the down-regulation of Notch signaling by MG through Notch3, and this decrease in the expression of a subset of Notch components may be mediated by matrix metalloproteinases. More work is needed to understand exactly how Notch signaling contributes to non-regenerative gliosis.
Conclusions
Notch interacts with a variety of signaling pathways during development to pattern the retina. The complex process of activation following receptor-ligand binding involving many accessory proteins and multiple cleavage events provides for an extensive network of regulation that serve as check-points to ensure proper development. The coordinated activities of Notch signaling and growth factors facilitate the specification of retinal cells both during development and regeneration. The altered environmental milieu of growth factors and signaling molecules in a damaged mature retina as compared to a developing retina can impact the regulation and activity of Notch signaling in these two contexts. In the mammalian retina, reactivation of Notch typically leads to nonproductive gliosis, but more dynamic and transient methods of Notch manipulation that better recapitulate changes exhibited in the retinas of more regenerative species could be ameliorative. Overall, a better understanding of cell type specific Notch targets may help uncover differences in the intrinsic reprogramming capacity of MG from different species and improve the development of therapeutics in the future.
Acknowledgments
The authors were supported by the NIH (NEI grant RO1 EY 018132, Kirschstein-NRSA 4T32HD007505-20), a Research to Prevent Blindness Innovative Ophthalmic Research Award, and gifts from the Marjorie and Maxwell Jospey Foundation and Shirlye and Peter Helman Fund.
Footnotes
Conflict of Interest
The authors declare they have no conflicts of interest.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References
Papers of particular interest, published recently, have been highlighted as:
*Of importance
**Of major importance
- 1.Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268(5208):225–32. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 2.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131(5):965–73. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 3.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 4.Koch U, Lehal R, Radtke F. Stem cells living with a Notch. Development. 2013;140(4):689–704. doi: 10.1242/dev.080614. [DOI] [PubMed] [Google Scholar]
- 5.Cagan RL, Ready DF. Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 1989;3(8):1099–112. doi: 10.1101/gad.3.8.1099. [DOI] [PubMed] [Google Scholar]
- 6.Baker R, Schubiger G. Autonomous and nonautonomous Notch functions for embryonic muscle and epidermis development in Drosophila. Development. 1996;122(2):617–26. doi: 10.1242/dev.122.2.617. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds-Kenneally J, Mlodzik M. Notch signaling controls proliferation through cell-autonomous and non-autonomous mechanisms in the Drosophila eye. Dev Biol. 2005;285(1):38–48. doi: 10.1016/j.ydbio.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 8.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7(2):93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 9.Fehon RG, Kooh PJ, Rebay I, Regan CL, Xu T, Muskavitch MA, et al. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990;61(3):523–34. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- 10.Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, et al. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci U S A. 1998;95(14):8108–12. doi: 10.1073/pnas.95.14.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, et al. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5(2):197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- 12.Rooke J, Pan D, Xu T, Rubin GM. KUZ, a conserved metalloprotease-disintegrin protein with two roles in Drosophila neurogenesis. Science. 1996;273(5279):1227–31. doi: 10.1126/science.273.5279.1227. [DOI] [PubMed] [Google Scholar]
- 13.Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, et al. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5(2):207–16. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 14.Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4(1):67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 15.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398(6727):518–22. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 16.Stifani S, Blaumueller CM, Redhead NJ, Hill RE. Artavanis-Tsakonas S. Human homologs of a Drosophila Enhancer of split gene product define a novel family of nuclear proteins. Nat Genet. 1992;2(2):119–27. doi: 10.1038/ng1092-119. [DOI] [PubMed] [Google Scholar]
- 17.Struhl G, Adachi A. Nuclear access and action of notch in vivo. Cell. 1998;93(4):649–60. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- 18.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393(6683):382–6. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 19.Fortini ME, Artavanis-Tsakonas S. The suppressor of hairless protein participates in notch receptor signaling. Cell. 1994;79(2):273–82. doi: 10.1016/0092-8674(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 20.Smoller D, Friedel C, Schmid A, Bettler D, Lam L, Yedvobnick B. The Drosophila neurogenic locus mastermind encodes a nuclear protein unusually rich in amino acid homopolymers. Genes Dev. 1990;4(10):1688–700. doi: 10.1101/gad.4.10.1688. [DOI] [PubMed] [Google Scholar]
- 21.Petcherski AG, Kimble J. Mastermind is a putative activator for Notch. Curr Biol. 2000;10(13):R471–3. doi: 10.1016/s0960-9822(00)00577-7. [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD. MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet. 2000;26(4):484–9. doi: 10.1038/82644. [DOI] [PubMed] [Google Scholar]
- 23.Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134(7):1243–51. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- 24.Goldman D. Muller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15(7):431–42. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorsky RI, Rapaport DH, Harris WA. Xotch inhibits cell differentiation in the Xenopus retina. Neuron. 1995;14(3):487–96. doi: 10.1016/0896-6273(95)90305-4. [DOI] [PubMed] [Google Scholar]
- 26.Lindsell CE, Boulter J, diSibio G, Gossler A. Weinmaster G. Expression patterns of Jagged, Delta1, Notch1, Notch2, and Notch3 genes identify ligand-receptor pairs that may function in neural development. Mol Cell Neurosci. 1996;8(1):14–27. doi: 10.1006/mcne.1996.0040. [DOI] [PubMed] [Google Scholar]
- 27.Furukawa T, Mukherjee S, Bao ZZ, Morrow EM, Cepko CL. rax, Hes1, and notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron. 2000;26(2):383–94. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- 28.Jadhav AP, Cho SH, Cepko CL. Notch activity permits retinal cells to progress through multiple progenitor states and acquire a stem cell property. Proc Natl Acad Sci U S A. 2006;103(50):18998–9003. doi: 10.1073/pnas.0608155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernardos RL, Lentz SI, Wolfe MS, Raymond PA. Notch-Delta signaling is required for spatial patterning and Muller glia differentiation in the zebrafish retina. Dev Biol. 2005;278(2):381–95. doi: 10.1016/j.ydbio.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58(1):52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Baek JH, Hatakeyama J, Sakamoto S, Ohtsuka T, Kageyama R. Persistent and high levels of Hes1 expression regulate boundary formation in the developing central nervous system. Development. 2006;133(13):2467–76. doi: 10.1242/dev.02403. [DOI] [PubMed] [Google Scholar]
- 32.Yoshiura S, Ohtsuka T, Takenaka Y, Nagahara H, Yoshikawa K, Kageyama R. Ultradian oscillations of Stat, Smad, and Hes1 expression in response to serum. Proc Natl Acad Sci U S A. 2007;104(27):11292–7. doi: 10.1073/pnas.0701837104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palavicini JP, Lloyd BN, Hayes CD, Bianchi E, Kang DE, Dawson-Scully K, et al. RanBP9 Plays a Critical Role in Neonatal Brain Development in Mice. PLoS One. 2013;8(6):e66908. doi: 10.1371/journal.pone.0066908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoo KW, Thiruvarangan M, Jeong YM, Lee MS, Maddirevula S, Rhee M, et al. Mind Bomb-Binding Partner RanBP9 Plays a Contributory Role in Retinal Development. Mol Cells. 2017 doi: 10.14348/molcells.2017.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoo KW, Kim EH, Jung SH, Rhee M, Koo BK, Yoon KJ, et al. Snx5, as a Mind bomb-binding protein, is expressed in hematopoietic and endothelial precursor cells in zebrafish. FEBS Lett. 2006;580(18):4409–16. doi: 10.1016/j.febslet.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Olena AF, Rao MB, Thatcher EJ, Wu SY, Patton JG. miR-216a regulates snx5, a novel notch signaling pathway component, during zebrafish retinal development. Dev Biol. 2015;400(1):72–81. doi: 10.1016/j.ydbio.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Chen X, Tang M. MicroRNA-216a inhibits pancreatic cancer by directly targeting Janus kinase 2. Oncol Rep. 2014;32(6):2824–30. doi: 10.3892/or.2014.3478. [DOI] [PubMed] [Google Scholar]
- 38.Hou BH, Jian ZX, Cui P, Li SJ, Tian RQ, Ou JR. miR-216a may inhibit pancreatic tumor growth by targeting JAK2. FEBS Lett. 2015;589(17):2224–32. doi: 10.1016/j.febslet.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 39.Kamakura S, Oishi K, Yoshimatsu T, Nakafuku M, Masuyama N, Gotoh Y. Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signalling. Nat Cell Biol. 2004;6(6):547–54. doi: 10.1038/ncb1138. [DOI] [PubMed] [Google Scholar]
- 40.Groot AJ, Vooijs MA. The role of Adams in Notch signaling. Adv Exp Med Biol. 2012;727:15–36. doi: 10.1007/978-1-4614-0899-4_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Six E, Ndiaye D, Laabi Y, Brou C, Gupta-Rossi N, Israel A, et al. The Notch ligand Delta1 is sequentially cleaved by an ADAM protease and gamma-secretase. Proc Natl Acad Sci U S A. 2003;100(13):7638–43. doi: 10.1073/pnas.1230693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toonen JA, Ronchetti A, Sidjanin DJ. A Disintegrin and Metalloproteinase10 (ADAM10) Regulates NOTCH Signaling during Early Retinal Development. PLoS One. 2016;11(5):e0156184. doi: 10.1371/journal.pone.0156184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esteve P, Sandonis A, Ibanez C, Shimono A, Guerrero I, Bovolenta P. Secreted frizzled-related proteins are required for Wnt/beta-catenin signalling activation in the vertebrate optic cup. Development. 2011;138(19):4179–84. doi: 10.1242/dev.065839. [DOI] [PubMed] [Google Scholar]
- 44.Esteve P, Sandonis A, Cardozo M, Malapeira J, Ibanez C, Crespo I, et al. SFRPs act as negative modulators of ADAM10 to regulate retinal neurogenesis. Nat Neurosci. 2011;14(5):562–9. doi: 10.1038/nn.2794. [DOI] [PubMed] [Google Scholar]
- 45.Holly VL, Widen SA, Famulski JK, Waskiewicz AJ. Sfrp1a and Sfrp5 function as positive regulators of Wnt and BMP signaling during early retinal development. Dev Biol. 2014;388(2):192–204. doi: 10.1016/j.ydbio.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 46.Borday C, Cabochette P, Parain K, Mazurier N, Janssens S, Tran HT, et al. Antagonistic cross-regulation between Wnt and Hedgehog signalling pathways controls post-embryonic retinal proliferation. Development. 2012;139(19):3499–509. doi: 10.1242/dev.079582. [DOI] [PubMed] [Google Scholar]
- 47.Locker M, Agathocleous M, Amato MA, Parain K, Harris WA, Perron M. Hedgehog signaling and the retina: insights into the mechanisms controlling the proliferative properties of neural precursors. Genes Dev. 2006;20(21):3036–48. doi: 10.1101/gad.391106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ringuette R, Atkins M, Lagali PS, Bassett EA, Campbell C, Mazerolle C, et al. A Notch-Gli2 axis sustains Hedgehog responsiveness of neural progenitors and Muller glia. Dev Biol. 2016;411(1):85–100. doi: 10.1016/j.ydbio.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Wall DS, Mears AJ, McNeill B, Mazerolle C, Thurig S, Wang Y, et al. Progenitor cell proliferation in the retina is dependent on Notch-independent Sonic hedgehog/Hes1 activity. J Cell Biol. 2009;184(1):101–12. doi: 10.1083/jcb.200805155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson BR, Ueki Y, Reardon S, Karl MO, Georgi S, Hartman BH, et al. Genome-wide analysis of Muller glial differentiation reveals a requirement for Notch signaling in postmitotic cells to maintain the glial fate. PLoS One. 2011;6(8):e22817. doi: 10.1371/journal.pone.0022817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51*.de Melo J, Zibetti C, Clark BS, Hwang W, Miranda-Angulo AL, Qian J, et al. Lhx2 Is an Essential Factor for Retinal Gliogenesis and Notch Signaling. J Neurosci. 2016;36(8):2391–405. doi: 10.1523/JNEUROSCI.3145-15.2016. This study identifies the transcription factor Lhx2 as critical to the pro-glial aspect of Notch signaling in the developing retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.de Melo J, Clark BS, Blackshaw S. Multiple intrinsic factors act in concert with Lhx2 to direct retinal gliogenesis. Sci Rep. 2016;6:32757. doi: 10.1038/srep32757. This study demonstrates that the coordinated activities of multiple signaling pathways and associated transcription factors are necessary for proper MG specification, and highlights the concept that individual Notch effectors are only effective in the context of functional Notch signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matthews JM, Visvader JE. LIM-domain-binding protein 1: a multifunctional cofactor that interacts with diverse proteins. EMBO Rep. 2003;4(12):1132–7. doi: 10.1038/sj.embor.7400030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gueta K, David A, Cohen T, Menuchin-Lasowski Y, Nobel H, Narkis G, et al. The stage-dependent roles of Ldb1 and functional redundancy with Ldb2 in mammalian retinogenesis. Development. 2016;143(22):4182–92. doi: 10.1242/dev.129734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Chevigny A, Core N, Follert P, Gaudin M, Barbry P, Beclin C, et al. miR-7a regulation of Pax6 controls spatial origin of forebrain dopaminergic neurons. Nat Neurosci. 2012;15(8):1120–6. doi: 10.1038/nn.3142. [DOI] [PubMed] [Google Scholar]
- 56.Rajaram K, Harding RL, Bailey T, Patton JG, Hyde DR. Dynamic miRNA expression patterns during retinal regeneration in zebrafish: reduced dicer or miRNA expression suppresses proliferation of Muller glia-derived neuronal progenitor cells. Dev Dyn. 2014;243(12):1591–605. doi: 10.1002/dvdy.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baba Y, Aihara Y, Watanabe S. MicroRNA-7a regulates Muller glia differentiation by attenuating Notch3 expression. Exp Eye Res. 2015;138:59–65. doi: 10.1016/j.exer.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 58.Ha T, Moon KH, Dai L, Hatakeyama J, Yoon K, Park HS, et al. The Retinal Pigment Epithelium Is a Notch Signaling Niche in the Mouse Retina. Cell Rep. 2017;19(2):351–63. doi: 10.1016/j.celrep.2017.03.040. [DOI] [PubMed] [Google Scholar]
- 59.Rocha SF, Lopes SS, Gossler A, Henrique D. Dll1 and Dll4 function sequentially in the retina and pV2 domain of the spinal cord to regulate neurogenesis and create cell diversity. Dev Biol. 2009;328(1):54–65. doi: 10.1016/j.ydbio.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 60.Riesenberg AN, Brown NL. Cell autonomous and nonautonomous requirements for Delltalike1 during early mouse retinal neurogenesis. Dev Dyn. 2016;245(6):631–40. doi: 10.1002/dvdy.24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128(13):2497–508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hufnagel RB, Le TT, Riesenberg AL, Brown NL. Neurog2 controls the leading edge of neurogenesis in the mammalian retina. Dev Biol. 2010;340(2):490–503. doi: 10.1016/j.ydbio.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63*.Maurer KA, Riesenberg AN, Brown NL. Notch signaling differentially regulates Atoh7 and Neurog2 in the distal mouse retina. Development. 2014;141(16):3243–54. doi: 10.1242/dev.106245. This study proposes a mechanism by which Notch regulates transcription factor patterning and the wave of neurogenesis in the developing retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiodini F, Matter-Sadzinski L, Rodrigues T, Skowronska-Krawczyk D, Brodier L, Schaad O, et al. A positive feedback loop between ATOH7 and a Notch effector regulates cell-cycle progression and neurogenesis in the retina. Cell Rep. 2013;3(3):796–807. doi: 10.1016/j.celrep.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 65.Sato M, Yasugi T, Minami Y, Miura T, Nagayama M. Notch-mediated lateral inhibition regulates proneural wave propagation when combined with EGF-mediated reaction diffusion. Proc Natl Acad Sci U S A. 2016;113(35):E5153–62. doi: 10.1073/pnas.1602739113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Muller glia dedifferentiation and retina regeneration. Dev Cell. 2012;22(2):334–47. doi: 10.1016/j.devcel.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68**.Wan J, Goldman D. Opposing Actions of Fgf8a on Notch Signaling Distinguish Two Muller Glial Cell Populations that Contribute to Retina Growth and Regeneration. Cell Rep. 2017;19(4):849–62. doi: 10.1016/j.celrep.2017.04.009. This is the first study using a reporter line to visualize the dynamics of Notch signaling in the context of zebrafish retina regeneration and demonstrates a key role for Fgf8 in this process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69*.Conner C, Ackerman KM, Lahne M, Hobgood JS, Hyde DR. Repressing notch signaling and expressing TNFalpha are sufficient to mimic retinal regeneration by inducing Muller glial proliferation to generate committed progenitor cells. J Neurosci. 2014;34(43):14403–19. doi: 10.1523/JNEUROSCI.0498-14.2014. This study highlights a role for Notch in zebrafish in the maintenance of MG quiescence in the adult retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao XF, Wan J, Powell C, Ramachandran R, Myers MG, Jr, Goldman D. Leptin and IL-6 family cytokines synergize to stimulate Muller glia reprogramming and retina regeneration. Cell Rep. 2014;9(1):272–84. doi: 10.1016/j.celrep.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramachandran R, Zhao XF, Goldman D. Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration. Proc Natl Acad Sci U S A. 2011;108(38):15858–63. doi: 10.1073/pnas.1107220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nelson CM, Ackerman KM, O’Hayer P, Bailey TJ, Gorsuch RA, Hyde DR. Tumor necrosis factor-alpha is produced by dying retinal neurons and is required for Muller glia proliferation during zebrafish retinal regeneration. J Neurosci. 2013;33(15):6524–39. doi: 10.1523/JNEUROSCI.3838-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagao M, Sugimori M, Nakafuku M. Cross talk between notch and growth factor/cytokine signaling pathways in neural stem cells. Mol Cell Biol. 2007;27(11):3982–94. doi: 10.1128/MCB.00170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4(3):247–52. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- 75.Hayes S, Nelson BR, Buckingham B, Reh TA. Notch signaling regulates regeneration in the avian retina. Dev Biol. 2007;312(1):300–11. doi: 10.1016/j.ydbio.2007.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Todd L, Squires N, Suarez L, Fischer AJ. Jak/Stat signaling regulates the proliferation and neurogenic potential of Muller glia-derived progenitor cells in the avian retina. Sci Rep. 2016;6:35703. doi: 10.1038/srep35703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Del Debbio CB, Balasubramanian S, Parameswaran S, Chaudhuri A, Qiu F, Ahmad I. Notch and Wnt signaling mediated rod photoreceptor regeneration by Muller cells in adult mammalian retina. PLoS One. 2010;5(8):e12425. doi: 10.1371/journal.pone.0012425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78*.Del Debbio CB, Mir Q, Parameswaran S, Mathews S, Xia X, Zheng L, et al. Notch Signaling Activates Stem Cell Properties of Muller Glia through Transcriptional Regulation and Skp2-mediated Degradation of p27Kip1. PLoS One. 2016;11(3):e0152025. doi: 10.1371/journal.pone.0152025. This study provides evidence for a molecular mechanism by which Notch influences MG quiescence in a non-regenerative mammal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jian Q, Tao Z, Li Y, Yin ZQ. Acute retinal injury and the relationship between nerve growth factor, Notch1 transcription and short-lived dedifferentiation transient changes of mammalian Muller cells. Vision Res. 2015;110(Pt A):107–17. doi: 10.1016/j.visres.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 80.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 81.de Melo J, Miki K, Rattner A, Smallwood P, Zibetti C, Hirokawa K, et al. Injury-independent induction of reactive gliosis in retina by loss of function of the LIM homeodomain transcription factor Lhx2. Proc Natl Acad Sci U S A. 2012;109(12):4657–62. doi: 10.1073/pnas.1107488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie J, Huo S, Li Y, Dai J, Xu H, Yin ZQ. Olfactory ensheathing cells inhibit gliosis in retinal degeneration by down-regulation of the Muller cell Notch signaling pathway. Cell Transplant. 2017 doi: 10.3727/096368917X694994. [DOI] [PMC free article] [PubMed] [Google Scholar]


