Abstract
Objective
Anorexia nervosa is associated with social-emotional functioning deficits and low levels of the social neurohormone oxytocin, even after weight gain. The relationship between low oxytocin levels and social-emotional functioning impairment has not been studied.
Method
We performed a cross-sectional study of 79 women (19 who were less than 85% of ideal body weight [IBW] with anorexia nervosa [AN], 26 who were 90–120% IBW with a history of AN [AN-WR], and 34 who were 90–120% IBW with no eating disorder history [H]). We administered the Eating Disorder Examination–Questionnaire (EDE-Q), Liebowitz Social Anxiety Scale–Self Report (LSAS-SR), Dimensional Assessment of Personality Pathology–Basic Questionnaire (DAPP-BQ; suspiciousness and insecure attachment subscales), and the Toronto Alexithymia Scale (TAS-20). We also analyzed fasting serum oxytocin levels.
Results
Most measures of social-emotional functioning showed impairment in women with AN and AN-WR compared to H. Oxytocin levels were low in AN-WR compared to H. Across groups, low oxytocin levels were associated with difficulty identifying feelings (r=−0.45, p=0.008) and overall alexithymia (r=−0.34, p=0.0489).
Discussion
We speculate that low oxytocin levels may contribute to alexithymia in women with anorexia nervosa.
Women with anorexia nervosa (AN) are at risk for premature mortality (Crow et al., 2009; Keshaviah et al., 2014) and demonstrate social functioning impairments, including social anxiety (Bulik, Beidel, Duchmann, Weltzin, & Kaye, 1991), suspiciousness and insecure attachment (Holliday, Uher, Landau, Collier, & Treasure, 2006), difficulty recognizing others’ emotions (Jansch, Harmer, & Cooper, 2009; Russell, Schmidt, Doherty, Young, & Tchanturia, 2009), and alexithymia (difficulty understanding one’s own emotions; Bourke, Taylor, Parker, & Bagby, 1992; Lule et al., 2014). Social-emotional functioning deficits predict poorer long-term outcomes in AN (Speranza, Loas, Wallier, & Corcos, 2007) and do not resolve with weight gain (Beadle, Paradiso, Salerno, & McCormick, 2013; Beales & Dolton, 2000; Coulon, Jeammet, & Godart, 2009; Holliday et al., 2006; Parling, Mortazavi, & Ghaderi, 2010; Speranza et al., 2007; Tchanturia et al., 2012).
Oxytocin is an anorexigenic neurohormone that reduces food intake in animals and humans (Blevins & Baskin, 2015) and regulates social-emotional functioning. In animals, oxytocin has anxiolytic (Ring et al., 2006; Windle, Shanks, Lightman, & Ingram, 1997) and pro-social effects (Lukas et al., 2011; Pedersen & Prange, 1979). When administered to humans, oxytocin increases gaze toward the eye region (Guastella, Mitchell, & Dadds, 2008), trust (Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005), and, particularly in individuals with higher alexithymia scores, the ability to recognize emotional expressions (Aoki et al., 2014; Fischer-Shofty, Levkovitz, & Shamay-Tsoory, 2013; Guastella et al., 2010; Lischke et al., 2012; Luminet, Grynberg, Ruzette, & Mikolajczak, 2011; Marsh, Yu, Pine, & Blair, 2010). Further, oxytocin administration increases the sharing of emotional experiences with others (Lane et al., 2013). We have reported low basal serum oxytocin levels (compared to healthy women, H) in women with AN (Lawson et al., 2011) and those with a history of AN who are now weight-restored and in partial or full recovery (AN-WR; Afinogenova et al., 2016 [same study sample as described here]; Lawson et al., 2012). These data imply that basal oxytocin levels may be low in women with AN regardless of weight status and raise the question of whether low oxytocin levels may contribute to symptoms.
Whether low oxytocin levels in women with AN are related to difficulties in social-emotional functioning has not been studied. We therefore investigated the relationship between oxytocin levels and social-emotional functioning in women with AN, AN-WR, and H, hypothesizing that lower oxytocin levels would be associated with increased severity of social-emotional functioning impairment.
Methods
Participants
We studied 79 ambulatory women (19 AN, 26 AN-WR, and 34 H), 18–45 years old. Clinical characteristics and oxytocin levels have been reported (Afinogenova et al., 2016). Social-emotional functioning measures and their relationship to oxytocin levels have not been published. Participants with AN met DSM-5 criteria (American Psychological Association, 2013) and were <85% ideal body weight (IBW). Participants with AN-WR were 90–120% IBW and met DSM-5 criteria for past AN. H were 90–120% IBW, had regular menstrual cycles, and reported no eating disorder history (assessed by the Structural Clinical Interview for DSM-IV [SCID]; First, Spitzer, M., & Williams, 2002) and no significant medical problems. Exclusion criteria for all participants included diabetes, pregnancy, breastfeeding, 3,4-methylenedioxymethamphetamine (MDMA) use, and oral contraceptive use.
Methods
This study was approved by Partners HealthCare, Inc. and Harvard Medical School Institutional Review Boards. Written informed consent was obtained prior to procedures.
Participants presented to the Massachusetts General Hospital Clinical Research Center, where trained personnel confirmed a diagnosis of AN using the SCID. Amenorrhea was not required, making the diagnosis consistent with DSM-5 criteria (not yet published at the time of data collection). We administered a medical history questionnaire and self-report psychological measures, including the Eating Disorder Examination–Questionnaire (EDE-Q; Fairburn & Beglin, 1994), the Liebowitz Social Anxiety Scale–Self Report (LSAS-SR; Rytwinski et al., 2009), the Dimensional Assessment of Personality Pathology–Basic Questionnaire (DAPP-BQ; suspiciousness and insecure attachment subscales; Kushner, Quilty, Tackett, & Bagby, 2011), and the Toronto Alexithymia Scale (TAS-20; Bagby, Parker, & Taylor, 1994).
Bionutritionists measured height, weight, and frame size (determined by comparing elbow breadth with race-specific norms derived from US National Health and Nutritional Examination Survey-I; Frisancho & Flegel, 1983) and calculated %IBW (using the “Metropolitan height and weight tables,” 1983) and body mass index (BMI). A fasting blood draw was performed.
Psychological Measures
On all of these well-validated self-report measures, higher scores indicate increased symptom severity. The EDE-Q, completed by all participants, measures eating disorder psychopathology (Fairburn & Beglin, 1994). A global score plus subscales (dietary restraint, eating concern, shape concern, and weight concern) are calculated, ranging from 0 to 6. The LSAS-SR, completed by 71 participants (19 AN, 24 AN-WR, 28 H), uses 4-point Likert-type scales to assess fear and avoidance of 11 social situations and 13 situations of public performance over the past week (Rytwinski et al., 2009). Scores are calculated by summing the fear or avoidance ratings for each type of situation. For the DAPP-BQ (Kushner et al., 2011), completed by 71 participants (19 AN, 24 AN-WR, 28 H), participants rate 14 suspiciousness and 16 insecure attachment items on a 5-point Likert-type scale from 1 (“Very unlike me”) to 5 (“Very like me”). Scale scores are sum scores. For the TAS-20, 34 participants (9 AN, 14 AN-WR, 11 H) rated 20 items on a 5-point Likert-type scale ranging from 1 (“Strongly disagree”) to 5 (“Strongly agree”). Sum scores are calculated for three subscales (difficulty identifying feelings [7 items], difficulty describing feelings [5 items], and externally oriented thinking [8 items]) and a global score (≤51: non-alexithymia, 52–60: possible alexithymia, ≥61: alexithymia; Bagby, Parker, et al., 1994; Bagby, Taylor, & Parker, 1994). Internal consistency was good for the TAS-20 (α=0.857) and excellent for DAPP-BQ, LSAS-SR, and EDE-Q (α=0.944, 0.968, and 0.942, respectively).
Biochemical Analysis
Serum was stored at −80°C. Oxytocin was measured in unextracted serum by ELISA (Assay Designs, Inc., Ann Arbor, MI, USA; Afinogenova et al., 2016). We have demonstrated a robust correlation between extracted and unextracted serum oxytocin levels (Lawson et al., 2013). The detection limit was 12.5 pg/mL. In-house QCs had a mean of 152 and 338 pg/mL and a between-assay CV of 15 and 18%, respectively.
Data Analysis
We used JMP 12 (SAS Institute, Inc., Cary, NC, USA) for statistical analyses. We compared clinical characteristics, psychiatric measures, and oxytocin levels using Fisher’s Least Significant Difference test. We utilized Hedges’ g to determine effect sizes for pairwise comparisons and Pearson’s correlations to examine the relationship between oxytocin levels and psychological measures. To control for potential confounders (BMI and amenorrhea), we used multivariate least square analyses. To analyze contributors to TAS-20 scores, we used hierarchical regression. Data are reported as mean±SEM. Statistical significance is defined as a two-tailed p-value <0.05.
Results
Clinical Characteristics
Table 1 shows clinical characteristics as reported in Afinogenova et al. (2016). Self-reported mean time since recovery of AN-WR was 40.8±6.6 months (range: 5–104 months). Amenorrhea was reported by 14 participants with AN (of 19 who provided this information), 3 of 26 with AN-WR, and 0 of 34 H.
Table 1.
Clinical Characteristics and Assessments of Psychopathology
| P Values, Hedges’ g | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| H (n=34) | AN-WR (n=26) | AN (n=19) | AN-WR vs. H | AN vs. H | AN-WR vs. AN | Overall ANOVA | |
| Age (Years) | 23.9 ± 0.8 | 22.9 ± 0.5 | 25.1 ± 1.7 | - | - | - | 0.38 |
| Weight (kg) | 61.5 ± 0.9 | 60.7 ± 1.4 | 47.2 ± 1.1 | 0.59, 0.13 | <0.0001, 2.72 | <0.0001, 2.09 | <0.0001 |
| % Ideal body weight | 101.2 ± 1.1 | 100.2 ± 1.6 | 80.7 ± 1.1 | 0.58, 0.14 | <0.0001, 3.36 | <0.0001, 2.76 | <0.0001 |
| Body mass index (kg/m2) | 22.6 ± 0.3 | 22.5 ± 0.4 | 17.7 ± 0.2 | 0.72, 0.07 | <0.0001, 3.36 | <0.0001, 2.93 | <0.0001 |
| Lowest adult body mass index (kg/m2) | 20.8 ± 0.3 | 17.1 ± 0.5 | 14.9 ± 0.5 | <0.0001, 1.78 | <0.0001, 3.09 | 0.001, 1.01 | <0.0001 |
| Oxytocin | 1501 ± 114 | 984 ± 93 | 1230 ± 86 | 0.0005, 0.86 | 0.09, 0.46 | 0.14, 0.56 | 0.002 |
| Eating Disorder Examination - Questionnaire | |||||||
| Dietary Restraint | 0.5 ± 0.1 | 2.1 ± 0.3 | 2.7 ± 0.3 | <0.0001, 1.32 | <0.0001, 2.04 | 0.16, 0.34 | <0.0001 |
| Eating Concern | 0.14 ± 0.03 | 1.8 ± 0.3 | 2.8 ± 0.3 | <0.0001, 1.59 | <0.0001, 3.42 | 0.002, 0.72 | <0.0001 |
| Shape Concern | 0.9 ± 0.2 | 3.3 ± 0.3 | 3.8 ± 0.3 | <0.0001, 1.64 | <0.0001, 2.13 | 0.31, 0.28 | <0.0001 |
| Weight Concern | 0.6 ± 0.2 | 2.9 ± 0.4 | 2.7 ± 0.4 | <0.0001, 1.57 | <0.0001, 1.64 | 0.73, 0.08 | <0.0001 |
| Global Score | 0.5 ± 0.1 | 2.5 ± 0.3 | 3.0 ± 0.3 | <0.0001, 1.70 | <0.0001, 2.50 | 0.20, 0.32 | <0.0001 |
| Dimensional Assessment of Personality Pathologya | |||||||
| Suspiciousness | 17.8 ± 1.0 | 24.0 ± 2.3 | 29.8 ± 2.6 | 0.02, 0.70 | <0.0001, 1.44 | 0.047, 0.51 | 0.0002 |
| Insecure Attachment | 22.6 ± 1.4 | 24.8 ± 1.4 | 30.8 ± 2.7 | 0.35, 0.31 | 0.002, 0.86 | 0.03, 0.63 | 0.007 |
| Leibowitz Social Anxiety Scalea | |||||||
| Social Fear | 5.4 ± 1.0 | 9.7 ± 1.1 | 15.8 ± 1.6 | 0.01, 0.80 | <0.0001, 1.70 | 0.001, 0.96 | <0.0001 |
| Public Fear | 6.8 ± 1.0 | 11.2 ± 1.0 | 17.4 ± 2.0 | 0.01, 0.84 | <0.0001, 1.52 | 0.002, 0.89 | <0.0001 |
| Social Avoidance | 5.0 ± 0.7 | 8.5 ± 1.2 | 14.5 ± 1.8 | 0.03, 0.71 | <0.0001, 1.63 | 0.001, 0.86 | <0.0001 |
| Public Avoidance | 5.0 ± 0.9 | 8.0 ± 1.1 | 14.3 ± 2.0 | 0.10, 0.58 | <0.0001, 1.37 | 0.002, 0.88 | <0.0001 |
| Toronto Alexithymia Scaleb | |||||||
| Difficulty Describing Feelings | 9.3 ± 0.8 | 14.6 ± 1.0 | 14.4 ± 1.0 | 0.0004, 1.50 | 0.002, 1.72 | 0.89, 0.05 | 0.0006 |
| Difficulty Identifying Feelings | 9.4 ± 1.2 | 18.5 ± 2.1 | 20.8 ± 1.4 | 0.0005, 1.38 | 0.0001, 2.77 | 0.37, 0.33 | 0.0002 |
| Externally Orienting Thinking | 15.5 ± 1.2 | 15.0 ± 1.1 | 18.7 ± 2.1 | - | - | - | 0.17 |
| Total Score | 34.2 ± 2.7 | 48.1 ± 3.2 | 53.9 ± 3.1 | 0.002, 1.26 | 0.0002, 2.09 | 0.20, 0.50 | 0.0005 |
Values expressed as mean ± SEM. Boldface indicates significance.
Based on 28 H, 24 AN-WR, and 19 AN.
Based on 11 H, 14 AN-WR, and 9 AN.
Abbreviation: ANOVA, analysis of variance.
Fasting oxytocin levels were lower in participants with AN-WR (984±93 pg/mL, range: 115–1,962 pg/mL) than H (1,501±114 pg/mL, range: 882–4,110 pg/mL). This difference remained significant after controlling for BMI and amenorrhea (p=0.003). Oxytocin levels in women with AN were numerically between AN-WR and H (mean: 1,230±86 pg/mL, range: 690–1,938 pg/mL) but did not significantly differ from either group.
Social-Emotional Functioning (Table 1)
LSAS-SR
On the social fear, public fear, and social avoidance subscales, scores were lowest in H, intermediate in women with AN-WR, and highest in women with AN (p≤0.03). On the public avoidance subscale, scores were higher in women with AN than women with AN-WR and H (p≤0.002), who did not differ on this measure (p=0.10).
DAPP-BQ
Suspiciousness was lowest in H, intermediate in participants with AN-WR, and highest in participants with AN (p≤0.047). Insecure attachment scores were higher in participants with AN than participants with AN-WR and H (p≤0.03), and did not differ between participants with AN-WR and H (p=0.35).
TAS-20
Difficulty describing feelings, difficulty identifying feelings, and total alexithymia scores were higher in participants with AN and AN-WR than H (p≤0.002) and did not differ between participants with AN and AN-WR (p≥0.20). Groups did not differ on the externally oriented thinking subscale (p=0.17).
Relationship Between Oxytocin and Social-Emotional Functioning
Across groups, oxytocin was negatively associated with difficulty identifying feelings (r=−0.45, p=0.008) and TAS-20 total score (r=−0.34, p=0.049; Figure 1). These relationships remained significant after controlling for BMI and estrogen status (presence or absence of amenorrhea; p=0.005 and 0.02, respectively). There were no significant associations between oxytocin and other social-emotional measures (p≥0.12).
Figure 1. Relationship Between Oxytocin and (A) Total TAS-20 Score and (B) Difficulty Identifying Feelings.
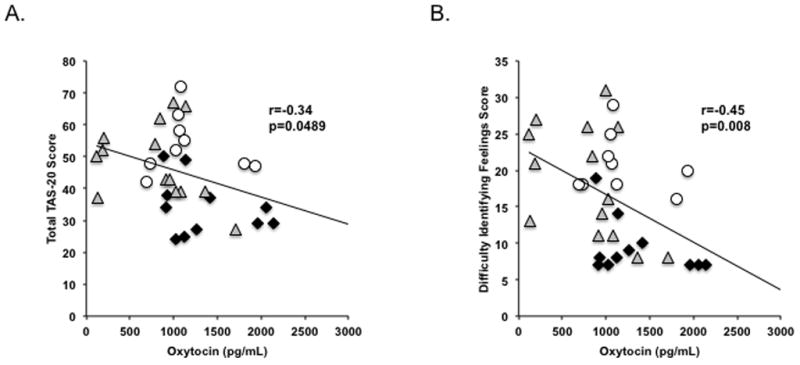
Oxytocin levels were negatively associated with (A) total alexithymia and (B) the difficulty identifying feelings subscale of the TAS-20. Black diamond, H; white circle, AN; gray triangle, AN-WR. These relationships were significant after controlling for BMI and amenorrhea (p≤0.02). Abbreviation: TAS-20, Toronto Alexithymia Scale–20.
In a hierarchical multiple regression model with Oxytocin (step 1), BMI (step 2), and Estrogen Status (step 3), Oxytocin and BMI accounted for 13.3% (p=0.02) and 13.1% (p=0.01) of variance in TAS-20 total score, respectively. Using the same model, Oxytocin and BMI accounted for 20.3% (p=0.005) and 11.3% (p=0.07) of variance in difficulty identifying feelings, respectively.
Discussion
To the best of our knowledge, our study is the first to show that low levels of the neurohormone oxytocin are associated with greater severity of alexithymia. This novel finding is important, because it raises the question of whether oxytocin might be a potential mediator of alexithymia, which predicts poorer outcomes in women with AN.
Oxytocin, a key hormone involved in social interaction, has been implicated in disorders involving social deficits, e.g., autism. Females with AN have elevated autistic traits (Baron-Cohen et al., 2013; Tchanturia et al., 2013), suggesting overlap between these disorders, which have both been associated with single-nucleotide polymorphism (SNP) variations in the oxytocin receptor gene (Acevedo, Valencia, Lutter, & McAdams, 2015; Jacob et al., 2007; Wu et al., 2005). Here, we report significantly lower fasting serum oxytocin levels in women with AN-WR than healthy controls, while oxytocin levels in AN were numerically lower than those of healthy controls at the trend level. Across all groups, we found that lower oxytocin levels were associated with increased alexithymia, in particular in identifying one’s own feelings, independent of BMI and estrogen status. Whether oxytocin plays a role in the social-emotional functioning deficits observed in individuals with a life-time diagnosis of AN merits investigation.
Alexithymia is a well-described social-emotional functioning deficit in adult women and adolescents with AN (Corcos et al., 2000; Speranza et al., 2005; Speranza et al., 2007) and a poor prognostic factor. In a three-year prospective study of 63 women with AN and 39 with bulimia nervosa, the difficulty identifying feelings subscale of the TAS-20 was negatively associated with clinical improvement at follow-up (Speranza et al., 2007). Furthermore, it has been hypothesized that deficits in emotional processing serve as a barrier to effective cognitive therapy (Jansch et al., 2009). The recognition of oxytocin as a potential mediator of these processes is important, as identifying the underlying neurobiology is a critical step in developing targeted treatments and improving outcomes. A prior study showed an interaction between oxytocin receptor genotype and childhood attachment in predicting adult alexithymia as well as brain structure and functioning in regions involved in salience processing and mentalizing (Schneider-Hassloff et al., 2016). Others have shown that administration of oxytocin improves key processes that are impaired in alexithymia: emotion recognition (Aoki et al., 2014; Fischer-Shofty et al., 2013; Guastella et al., 2010; Lischke et al., 2012; Luminet et al., 2011; Marsh et al., 2010) and emotion expression (Lane et al., 2013), further emphasizing the link between oxytocin and social-emotional functioning. Based on this, oxytocin has recently been proposed as a potential novel therapy in patients with alexithymia. Our data linking low serum oxytocin levels to higher alexithymia scores in women with AN are consistent with these findings and support the hypothesis that low oxytocin might contribute to these symptoms. A study investigating the impact of single-dose intranasal oxytocin (40 IU) did not find evidence for an improvement of social-emotional functioning in AN (Leppanen, Cardi, et al., 2017), and a recent meta-analysis showed improvements of social-emotional functioning following intranasal oxytocin administration in healthy individuals but not in clinical populations (Leppanen, Ng, Tchanturia, & Treasure, 2017). However, it is important to note that these studies used supraphysiologic levels of oxytocin, while our study investigated the link between physiologic levels of oxytocin and social-emotional functioning. It is conceivable that the revealed link between oxytocin levels and total alexithymia and difficulty identifying feelings only exists within the physiologic range of oxytocin, which emphasizes the need for studies of endogenous oxytocin levels in this population.
Limitations of this study include its small sample size, which may have introduced bias and/or reduced our ability to detect associations between oxytocin levels and social-emotional functioning. Even so, we demonstrated correlations between oxytocin levels and measures of alexithymia. This is a cross-sectional study, and therefore causality cannot be determined. Longitudinal studies in larger sample sizes of women with AN will be important to investigate the role of oxytocin in impaired social-emotional functioning. In addition, future studies are required to identify factors explaining individual variance in oxytocin levels within individuals with current or past AN.
In summary, we demonstrate that low oxytocin levels are associated with increased alexithymia, independent of BMI and estrogen status. Further investigation is needed to determine whether low oxytocin levels contribute to symptoms of alexithymia in AN.
Acknowledgments
EAL has a financial interest in OXT Therapeutics, a company developing an intranasal oxytocin and long-acting analogs of oxytocin to treat obesity and metabolic disease. Other authors report no conflicts of interest. The study was supported by NIH Grants K23 MH092560, P30 DK40561, and UL1 RR025758. The funding agencies had no direct role in the performance of the reported study and write-up of this manuscript. We gratefully acknowledge Anne Klibanski, MD for her constructive review of the manuscript. We also thank the MGH Clinical Research Center staff and study participants.
References
- Acevedo SF, Valencia C, Lutter M, McAdams CJ. Severity of eating disorder symptoms related to oxytocin receptor polymorphisms in anorexia nervosa. Psychiatry Research. 2015;228(3):641–648. doi: 10.1016/j.psychres.2015.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afinogenova Y, Schmelkin C, Plessow F, Thomas JJ, Pulumo R, Micali N, … Lawson EA. Low fasting oxytocin levels are associated with psychopathology in anorexia nervosa in partial recovery. Journal of Clinical Psychiatry. 2016;77(11):e1483–e1490. doi: 10.4088/JCP.15m10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC, USA: American Psychiatric Association; 2013. [Google Scholar]
- Aoki Y, Yahata N, Watanabe T, Takano Y, Kawakubo Y, Kuwabara H, … Yamasue H. Oxytocin improves behavioural and neural deficits in inferring others’ social emotions in autism. Brain. 2014;137(Pt 11):3073–3086. doi: 10.1093/brain/awu231. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Parker JDA, Taylor GJ. The twenty-item Toronto Alexithymia Scale: I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research. 1994;38(1):23–32. doi: 10.1016/0022-3999%2894%2990005-18126686. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Taylor GJ, Parker JDA. The twenty-item Toronto Alexithymia Scale: II. Convergent, discriminant, and concurrent validity. Journal of Psychosomatic Research. 1994;38(1):33–40. doi: 10.1016/0022-3999%2894%2990006-X8126688. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Jaffa T, Davies S, Auyeung B, Allison C, Wheelwright S. Do girls with anorexia nervosa have elevated autistic traits? Molecular Autism. 2013;4(1):24. doi: 10.1186/2040-2392-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle JN, Paradiso S, Salerno A, McCormick LM. Alexithymia, emotional empathy, and self-regulation in anorexia nervosa. Annals of Clinical Psychiatry. 2013;25(2):107–120. [PMC free article] [PubMed] [Google Scholar]
- Beales DL, Dolton R. Eating disordered patients: Personality, alexithymia, and implications for primary care. British Journal of General Practice. 2000;50(450):21–26. [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Baskin DG. Translational and therapeutic potential of oxytocin as an anti-obesity strategy: Insights from rodents, nonhuman primates and humans. Physiology & Behavior. 2015;152(Pt B):438–449. doi: 10.1016/j.physbeh.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke MP, Taylor GJ, Parker JD, Bagby RM. Alexithymia in women with anorexia nervosa. A preliminary investigation. British Journal of Psychiatry. 1992;161:240–243. doi: 10.1192/bjp.161.2.240. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Beidel DC, Duchmann E, Weltzin TE, Kaye WH. An analysis of social anxiety in anorexic, bulimic, social phobic, and control women. Journal of Psychopathology and Behavioral Assessment. 1991;13(3):199–211. doi: 10.1007/bf00960784. [DOI] [Google Scholar]
- Corcos M, Guilbaud O, Speranza M, Paterniti S, Loas G, Stephan P, Jeammet P. Alexithymia and depression in eating disorders. Psychiatry Research. 2000;93(3):263–266. doi: 10.1016/s0165-1781(00)00109-8. [DOI] [PubMed] [Google Scholar]
- Coulon N, Jeammet P, Godart N. Social phobia in anorexia nervosa: Evolution during the care. L’Encephale: Revue de psychiatrie clinique biologique et therapeutique. 2009;35(6):531–537. doi: 10.1016/j.encep.2008.09.00520004283. [DOI] [PubMed] [Google Scholar]
- Crow SJ, Peterson CB, Swanson SA, Raymond NC, Specker S, Eckert ED, Mitchell JE. Increased mortality in bulimia nervosa and other eating disorders. American Journal of Psychiatry. 2009;166(12):1342–1346. doi: 10.1176/appi.ajp.2009.09020247. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? International Journal of Eating Disorders. 1994;16(4):363–370. [PubMed] [Google Scholar]
- First MB, Spitzer RLMG, Williams JBW. N. Y. P. Institute, editor. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York, NY, USA: Biometrics Research; 2002. Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. [Google Scholar]
- Fischer-Shofty M, Levkovitz Y, Shamay-Tsoory SG. Oxytocin facilitates accurate perception of competition in men and kinship in women. Social Cognitive and Affective Neuroscience. 2013;8(3):313–317. doi: 10.1093/scan/nsr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisancho AR, Flegel PN. Elbow breadth as a measure of frame size for US males and females. American Journal of Clinical Nutrition. 1983;37(2):311–314. doi: 10.1093/ajcn/37.2.311. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biological Psychiatry. 2010;67(7):692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry. 2008;63(1):3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Holliday J, Uher R, Landau S, Collier D, Treasure J. Personality pathology among individuals with a lifetime history of anorexia nervosa. Journal of Personality Disorders. 2006;20(4):417–430. doi: 10.1521/pedi.2006.20.4.417. [DOI] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neuroscience Letters. 2007;417(1):6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansch C, Harmer C, Cooper MJ. Emotional processing in women with anorexia nervosa and in healthy volunteers. Eating Behaviors. 2009;10(3):184–191. doi: 10.1016/j.eatbeh.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Keshaviah A, Edkins K, Hastings ER, Krishna M, Franko DL, Herzog DB, … Eddy KT. Re-examining premature mortality in anorexia nervosa: A meta-analysis redux. Comprehensive Psychiatry. 2014;55(8):1773–1784. doi: 10.1016/j.comppsych.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kushner SC, Quilty LC, Tackett JL, Bagby RM. The hierarchical structure of the Dimensional Assessment of Personality Pathology (DAPP-BQ) Journal of Personality Disorders. 2011;25(4):504–516. doi: 10.1521/pedi.2011.25.4.504. [DOI] [PubMed] [Google Scholar]
- Lane A, Luminet O, Rime B, Gross JJ, de Timary P, Mikolajczak M. Oxytocin increases willingness to socially share one’s emotions. International Journal of Psychology. 2013;48(4):676–681. doi: 10.1080/00207594.2012.677540. [DOI] [PubMed] [Google Scholar]
- Lawson EA, Ackerman KE, Estella NM, Guereca G, Pierce L, Sluss PM, … Misra M. Nocturnal oxytocin secretion is lower in amenorrheic athletes than nonathletes and associated with bone microarchitecture and finite element analysis parameters. European Journal of Endocrinology. 2013;168(3):457–464. doi: 10.1530/EJE-12-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Donoho DA, Blum JI, Meenaghan EM, Misra M, Herzog DB, … Klibanski A. Decreased nocturnal oxytocin levels in anorexia nervosa are associated with low bone mineral density and fat mass. Journal of Clinical Psychiatry. 2011;72(11):1546–1551. doi: 10.4088/JCP.10m06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Holsen LM, Santin M, Meenaghan E, Eddy KT, Becker AE, … Klibanski A. Oxytocin secretion is associated with severity of disordered eating psychopathology and insular cortex hypoactivation in anorexia nervosa. Journal of Clinical Endocrinology and Metabolism. 2012;97(10):E1898–1908. doi: 10.1210/jc.2012-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen J, Cardi V, Ng KW, Paloyelis Y, Stein D, Tchanturia K, Treasure J. Effects of intranasal oxytocin on the interpretation and expression of emotions in anorexia nervosa. Journal of Neuroendocrinology. 2017;29(3) doi: 10.1111/jne.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen J, Ng KW, Tchanturia K, Treasure J. Meta-analysis of the effects of intranasal oxytocin on interpretation and expression of emotions. Neuroscience and Biobehavioral Reviews. 2017;78:125–144. doi: 10.1016/j.neubiorev.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Lischke A, Berger C, Prehn K, Heinrichs M, Herpertz SC, Domes G. Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology. 2012;37(4):475–481. doi: 10.1016/j.psyneuen.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36(11):2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lule D, Schulze UM, Bauer K, Scholl F, Muller S, Fladung AK, Uttner I. Anorexia nervosa and its relation to depression, anxiety, alexithymia and emotional processing deficits. Eating and Weight Disorders. 2014;19(2):209–216. doi: 10.1007/s40519-014-0101-z. [DOI] [PubMed] [Google Scholar]
- Luminet O, Grynberg D, Ruzette N, Mikolajczak M. Personality-dependent effects of oxytocin: Greater social benefits for high alexithymia scorers. Biological Psychology. 2011;87(3):401–406. doi: 10.1016/j.biopsycho.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Blair RJ. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology. 2010;209(3):225–232. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- Metropolitan height and weight tables. Statistical Bulletin (Metropolitan Life Foundation) 1983;64(1):2–9. [PubMed] [Google Scholar]
- Parling T, Mortazavi M, Ghaderi A. Alexithymia and emotional awareness in anorexia nervosa: Time for a shift in the measurement of the concept? Eating Behaviors. 2010;11(4):205–210. doi: 10.1016/j.eatbeh.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(12):6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, … Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: Behavioral and autonomic evidence, therapeutic implications. Psychopharmacology. 2006;185(2):218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Russell TA, Schmidt U, Doherty L, Young V, Tchanturia K. Aspects of social cognition in anorexia nervosa: Affective and cognitive theory of mind. Psychiatry Research. 2009;168(3):181–185. doi: 10.1016/j.psychres.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Rytwinski NK, Fresco DM, Heimberg RG, Coles ME, Liebowitz MR, Cissell S, … Hofmann SG. Screening for social anxiety disorder with the self-report version of the Liebowitz Social Anxiety Scale. Depression and Anxiety. 2009;26(1):34–38. doi: 10.1002/da.20503. [DOI] [PubMed] [Google Scholar]
- Schneider-Hassloff H, Straube B, Jansen A, Nuscheler B, Wemken G, Witt SH, … Kircher T. Oxytocin receptor polymorphism and childhood social experiences shape adult personality, brain structure and neural correlates of mentalizing. NeuroImage. 2016;134:671–684. doi: 10.1016/j.neuroimage.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Speranza M, Corcos M, Loas G, Stephan P, Guilbaud O, Perez-Diaz F, … Jeammet P. Depressive personality dimensions and alexithymia in eating disorders. Psychiatry Research. 2005;135(2):153–163. doi: 10.1016/j.psychres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Speranza M, Loas G, Wallier J, Corcos M. Predictive value of alexithymia in patients with eating disorders: A 3-year prospective study. Journal of Psychosomatic Research. 2007;63(4):365–371. doi: 10.1016/j.jpsychores.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Tchanturia K, Davies H, Harrison A, Fox JR, Treasure J, Schmidt U. Altered social hedonic processing in eating disorders. International Journal of Eating Disorders. 2012;45(8):962–969. doi: 10.1002/eat.22032. [DOI] [PubMed] [Google Scholar]
- Tchanturia K, Smith E, Weineck F, Fidanboylu E, Kern N, Treasure J, Baron Cohen S. Exploring autistic traits in anorexia: A clinical study. Molecular Autism. 2013;4(1):44. doi: 10.1186/2040-2392-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138(7):2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, … Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biological Psychiatry. 2005;58(1):74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]


