Abstract
Objective
To determine the role of neutrophil-to-lymphocyte ratio (NLR) in prognosticating survival outcomes in patients with advanced/metastatic urothelial bladder cancer.
Methods
We retrospectively reviewed 84 patients undergoing radical cystectomy (RC) for UCB from January 2002 to June 2012. NLR was computed (median: 5 days) prior to surgery. No patients received neoadjuvant chemotherapy. NLR was analyzed as a continuous variable and a cut-off point of 2.7 was obtained, with a statistical receiver operating characteristics of 0.74. Kaplan–Meier curves, multivariate Cox proportional hazard and logistics regression models were used to predict NLR association with survival outcomes.
Results
The median follow-up period was 30.1 months (range: 3.2–161.7) owing to high recurrence rate and subsequent mortalities, compared to the median 64.7 months in patients alive at the end of study period. NLR ≥2.7 was associated with worse survival outcomes (5-year disease-specific survival: 22% vs 58%, p = 0.017, 95%CI: 1.193–6.009; 5-year overall survival: 23% vs 60%, p = 0.008, 95%CI: 1.322–6.147). Furthermore, on multivariate analyses, higher NLR was independently associated with higher recurrence rate (p = 0.007, HR =6.999, 95%CI: 1.712–28.606), higher T staging (p = 0.021, HR = 3.479, 95%CI: 1.212–9.990) and lymph node involvement (p = 0.009, HR = 4.534, 95%CI: 1.465–14.034).
Conclusion
This study suggests that NLR can be an inexpensive novel factor for patients risk stratification pre-operatively. This improves patient counseling and identifies patients who may benefit from multimodal treatment.
Keywords: Bladder cancer, Neutrophil-to-lymphocyte ratio, Radical cystectomy, Bladder carcinoma
1. Introduction
Radical cystectomy (RC) with pelvic lymph node dissection (PLND) remains the standard treatment for muscle-invasive urothelial cancer of the bladder (UCB) [1], [2], and a strong recommendation for patients with non-muscle-invasive disease with high risk of progression [3]. However, despite such aggressive curative surgeries, survival remained dismal over the decades [4]. Approximately 50% of patients will eventually develop recurrence or metastasis [5], and 5-year survival ranges from 26% to 64% [1], [6].
Adding to the challenge in managing UCB is the lack of pre-operative risk stratification. While most predictive models are based on pathological data, this information is insufficient to provide detailed prognosis for pre-operative decision making [7], [8]. Upstaging of RC on final histology was reported as high as 50% [9]. Given the high recurrence rate and surgical morbidities, newer prognostic factors should be considered to provide a better risk stratification for more informed patient counseling and patient selection for surgery.
On the other hand, there has been increasing evidence supporting the role of inflammation in cancer development and progression [10], and the neutrophil-to-lymphocyte ratio (NLR) has been identified as a marker of tumor activity [11]. A higher NLR has been associated with poorer disease specific and overall survival in gastric, hepatic, non-small cell and cervical cancer [12], [13], [14]. This evidence can be relevant in prognosticating UCB, given that inflammation appears to be an important role in the pathogenesis and progression of UCB [10].
Through this paper, we aim to determine the prognostic value of NLR in patients undergoing radical cystectomy for UCB, in particularly looking at the disease specific survival, overall survival and recurrence rate. This will allow us to better identify more suitable candidates for RC or other neoadjuvant or adjuvant therapies.
2. Materials and methods
Following Institutional Review Board approval (CIRB reference number: 2009/1027/D), we retrospectively reviewed our institutional database and identified 108 patients that underwent RC for UCB, between January 2002 to June 2012. Eight patients were excluded from the studies due to incomplete datasets. Given the small number and heterogeneity of neoadjuvant chemotherapeutic drugs and regimes, we excluded 16 patients who received neoadjuvant treatments prior to the definitive surgeries. All patients had histological proven UCB via transurethral resection of bladder tumor (TURBT). We excluded patients who underwent surgeries for UCB after June 2012 due to the short subsequent follow-up time, which would affect the interpretation of the survival outcomes and recurrence rates.
All patients had undergone staging scans with computed tomographies of the thorax, abdomen and pelvis to confirm the extent of disease. Magnetic resonance imagings were used as adjunct as deemed necessary by the surgeons.
Hematological and biochemical blood results were collected at a median of 5 days (range 1–16) prior to operation. All patients were cleared of any infection or active inflammation at the time of examination. We defined leukocytosis as total white blood cell counts ≥12 × 109/L, anemia as hemoglobin <12.5 g/dL for women and <13.5 g/dL for men. NLR was analyzed as a continuous variable and a cut-off point of 2.7 was obtained, with a statistical receiver operating characteristics (ROC) of 0.74 (area under curve).
All patients underwent RC and bilateral standard pelvic lymphadenectomies and achieve clear margins on final histology. Post-operatively, all patients were reviewed outpatient within 4 weeks after discharge. Given the retrospective nature of this study, follow-ups were not consistently standardized. However, in our institution's follow-up protocol, we recommended quarterly for the first 2 years post-operatively, semiannually for the subsequent 3 years and yearly follow-ups onwards. Evaluations included history and physical examination, urine cytology, repeated computed tomography scans of the thorax, abdomen and pelvis and cystoscopies to monitor for disease recurrence. Primary outcomes were disease-specific and overall survivals, and secondary outcome was recurrence rate. The time to recurrence, disease-specific and overall survivals were measured from the time of RC.
Statistical analyses were performed using IBM SPSS statistics version 20.0, United States, 2012. The relationship between clinicopathological features and pre-operative NLR was calculated with chi-square and Wilcoxon tests as appropriate. Survival curves were measured using Kaplan–Meier, with comparisons between patients with NLR <2.7 and ≥2.7 performed using log-rank test. Univariate and multivariate Cox proportional hazard regression models were used to find associations between NLR and survival outcomes, after adjusting for clinicopathologic factors. Univariate and multivariate logistic regression models were used to predict recurrence rate. All tests were two-sided with p < 0.05 considered to be statistically significant.
3. Results
The baseline patients' demographics and tumor characteristics were shown in Table 1. This study included 63 males and 21 females, with a median age of 67 years old (range 37–82). Seventy-six patients (90.5%) had muscle invasive bladder cancer, and the remaining eight patients had persistent BCG-refractory high-grade non-muscle invasive disease. Thirty-five patients (41.7%) had lymph node invasion and forty-three patients (51.2%) had extravesical involvement, defined as T3/4 disease, on final histology.
Table 1.
Demographics.
| Variable | Value |
|---|---|
| Age (year)a | 67 (37–82) |
| Genderb | |
| Male | 63 (75) |
| Female | 21 (25) |
| Pathological tumor stagingb | |
| T1 | 8 (9.5) |
| T2 | 33 (39.3) |
| T3 | 26 (31.0) |
| T4 | 17 (20.2) |
| No. of tumorsb | |
| 1 | 61 (72.6) |
| 2 or more | 23 (27.4) |
| Tumor gradeb | |
| Low–intermediate | 8 (9.5) |
| High | 76 (90.5) |
| Size (cm)b | |
| ≥3 | 59 (70.2) |
| <3 | 25 (29.8) |
| Hydronephrosisb | |
| Yes | 38 (45.2) |
| No | 46 (54.8) |
| Pathological LN involvementb | |
| Yes | 35 (41.7) |
| No | 49 (58.3) |
| Bloods takena | 5 (1–16) |
| Hemoglobin (g/dL) | |
| Male | 11.8 (8.1–15.4) |
| Female | 12.0 (8.3–14.0) |
| Platelet (×109/L)a | 282 (86–636) |
| Neutrophils (×109/L)a | 63.95 (44.3–87.3) |
| Lymphocytes (×109/L)a | 23.3 (6.7–42.5) |
| Neutrophil-lymphocyte ratioa | 2.69 (1.00–12.39) |
| NLR breakdownb | |
| ≥2.7 | 45 (53.6) |
| <2.7 | 39 (46.4) |
LN, lymph node; NLR, neutrophil-to-lymphocyte ratio.
Presented as median (range).
Presented as n (%).
The clinicopathologic features of patients with NLR <2.7 and ≥2.7 were shown in Table 2. Patients with NLR ≥2.7 were associated with larger tumor size, hydronephrosis, pathological extravesical (T3/4) tumors and lymph node involvement.
Table 2.
Clinicopathologic characteristics and pathological outcomes (n).
| NLR <2.7 | NLR ≥2.7 | p-Value | |
|---|---|---|---|
| Gender | 0.899 | ||
| Male | 29 | 34 | |
| Female | 10 | 11 | |
| T staging | <0.001 | ||
| T1-2 | 27 | 14 | |
| T3-4 | 12 | 31 | |
| Size | 0.036 | ||
| <3 cm | 16 | 9 | |
| ≥3 cm | 23 | 36 | |
| No. of tumors | 0.875 | ||
| 1 | 28 | 33 | |
| 2 or more | 11 | 12 | |
| Tumor grade | 0.594 | ||
| Low–intermediate | 3 | 5 | |
| High | 36 | 40 | |
| Hydronephrosis | 0.041 | ||
| Yes | 13 | 25 | |
| No | 26 | 20 | |
| LN involvement | <0.001 | ||
| Yes | 8 | 27 | |
| No | 31 | 18 | |
| Haemoglobin | 0.243 | ||
| Abnormal | 23 | 32 | |
| Normal | 16 | 13 | |
LN, lymph node; NLR, neutrophil-to-lymphocyte ratio.
On multivariate analyses, in particularly, controlling other variables that could affect the pathological outcomes, NLR ≥2.7 was shown to be significantly associated with extravesical disease and lymph node involvement. Patients with NLR ≥2.7 were 2.5 times more likely to have extravesical disease (p = 0.021, HR = 3.479, 95%CI: 1.212–9.990) and 3.5 times more likely to have associated lymph node involvement (p = 0.009, HR = 4.535, 95%CI: 1.465–14.034) (Table 3).
Table 3.
NLR and pathologial outcomes.
| Pathological T3/4 disease |
Pathological LN involvement |
|||||
|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||
| p-Value | p-Value | HR (95%CI) | p-Value | p-Value | HR (95%CI) | |
| Gender | 0.257 | 0.147 | 0.160 | 0.086 | ||
| Size ≥3 cm | 0.070 | 0.349 | 0.242 | 0.972 | ||
| No. of tumors ≥2 | 0.175 | 0.359 | 0.685 | 0.826 | ||
| High grade tumor | 0.944 | 0.848 | 0.079 | 0.157 | ||
| Hydronephrosis | 0.264 | 0.669 | 0.001 | 0.029 | 3.338 (1.134–9.828) | |
| Anemia | 0.332 | 0.211 | 0.332 | 0.608 | ||
| NLR ≥2.7 | <0.001 | 0.021 | 3.479 (1.212–9.990) | <0.001 | 0.009 | 4.534 (1.465–14.034) |
| Clinical T3/4 disease | 0.007 | 0.084 | 2.786 (0.872–8.901) | |||
| Pre-op LN involvement | 0.007 | 0.086 | 2.823 (0.864–9.219) | |||
CI, confidence interval; HR, hazard ratio; LN, lymph node; NLR, neutrophil-to-lymphocyte ratio; Pre-op, pre-operative.
3.1. Primary outcomes
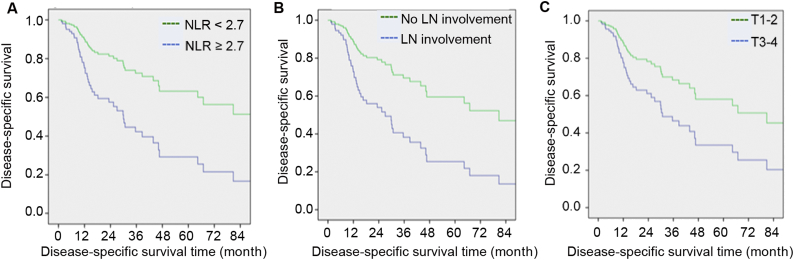
In univariate Cox analyses, tumor size (≥3 cm), presence of hydronephrosis, anemia, higher pathological T staging, lymph node involvement and higher NLR (≥2.7) were associated with poorer disease-specific survival (Table 4). However, on multivariate analyses, only lymph node involvement (p = 0.007, HR = 2.641, 95%CI: 1.303–5.356) and NLR ≥2.7 (p = 0.017, HR = 2.677, 95%CI: 1.193–6.009) were associated with a shorter disease-specific survival (Fig. 1). In fact, the 5-year disease-specific survival for patients with NLR <2.7 was 58%, significantly greater than the dismal 22% with NLR ≥2.7. The median disease-specific survival (DSS) for patients with NLR ≥2.7 was 21.8 months (range 3.4–142.0) compared to 67.4 months (range 3.8–161.8) in patients with NLR <2.7. Patients with higher T staging were associated with a shorter disease-specific survival (DSS), albeit not statistically significant (p = 0.058, HR = 2.013, 95%CI: 0.976–4.152).
Table 4.
Disease-specific survival and overall survival.
| Disease-specific survival |
Overall survival |
|||||
|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||
| p-Value | p-Value | HR (95%CI) | p-Value | p-Value | HR (95%CI) | |
| Size ≥3 cm | 0.015 | 0.618 | 0.024 | 0.845 | ||
| No. of tumors ≥2 | 0.531 | 0.842 | 0.645 | 0.997 | ||
| High grade tumor | 0.70 | 0.098 | 0.118 | 0.180 | ||
| Hydronephrosis | 0.002 | 0.961 | 0.003 | 0.872 | ||
| Anemia | 0.008 | 0.097 | 0.001 | 0.010 | 2.570 (1.250–5.286) | |
| NLR ≥2.7 | <0.001 | 0.017 | 2.677 (1.193–6.009) | <0.001 | 0.008 | 2.850 (1.322–6.147) |
| T3/4 disease (extravesical) | <0.001 | 0.058 | 2.013 (0.976–4.152) | <0.001 | 0.159 | |
| LN involvement | <0.001 | 0.007 | 2.641 (1.303–5.356) | <0.001 | 0.005 | 2.648 (1.348–5.201) |
CI, confidence interval; LN, lymph node; NLR, neutrophil-to-lymphocyte ratio.
Figure 1.
Disease-specific survival. NLR, neutrophil-to-lymphocyte; LN, lymph node.
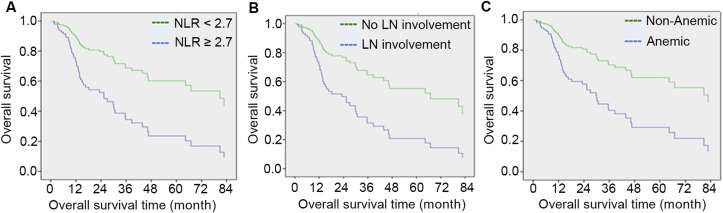
On the other hand, on multivariate analyses, lymph node involvement (p = 0.005, HR = 2.648, 95%CI: 1.348–5.201), NLR ≥2.7 (p = 0.008, HR = 2.850, 95%CI: 1.322–6.147) and anemia (p = 0.010, HR = 2.570, 95%CI: 1.250–5.286) were associated with a shorter overall survival (Table 4 and Fig. 2). Similarly, patients with NLR ≥2.7 had significantly worse 5-year overall survival (23% vs 60%). The median overall survival for patients with NLR ≥2.7 was 22.3 months (range 3.4–142.0) compared to 64.8 months (range 3.8–161.8) in patients with NLR <2.7.
Figure 2.
Overall survival. NLR, neutrophil-to-lymphocyte; LN, lymph node.
3.2. Secondary outcome
The median follow-up time was 30.1 months (range 3.2–161.7). This shortened follow-up period was largely due to the high recurrence and subsequent mortality rates in the study. More importantly, the median follow-up time for those alive was 64.7 months (range 22.6–161.7). Forty-seven patients (56.0%) developed recurrence, at a median time of 7.0 months (range 2.2–61.8).
In univariate Cox models, greater tumor size (≥3 cm), presence of hydronephrosis, anemia, higher pathological T staging, lymph node involvement and higher NLR (≥2.7) were associated with higher risk of recurrence (Table 5). Upon adjusting for confounders in multivariate analyses, higher pathological T staging (p = 0.007, HR = 7.223, 95% CI: 1.701–30.662), presence of lymph node involvement (p = 0.004, HR = 12.519, 95%CI: 2.218–70.650) and NLR ≥2.7 (p = 0.007, HR = 6.999, 95%CI: 1.712–28.606) were found to remain significant with recurrence rates. All 47 patients subsequently passed away within the study period. In addition, there were five more patients who died from non-bladder cancer related causes.
Table 5.
Recurrence.
| Yes (n) | No (n) | Univariate |
Multivariate |
||
|---|---|---|---|---|---|
| p-Value | p-Value | HR (95%CI) | |||
| Gender | 0.965 | ||||
| Male | 36 | 27 | |||
| Female | 11 | 10 | |||
| Size | 0.016 | 0.262 | |||
| <3 cm | 9 | 16 | |||
| ≥3 cm | 38 | 21 | |||
| No. of tumors | 0.949 | 0.334 | |||
| 1 | 34 | 27 | |||
| 2 or more | 13 | 10 | |||
| Tumor grade | 0.064 | 0.101 | |||
| Low–intermediate | 2 | 6 | |||
| High | 45 | 31 | |||
| Hydronephrosis | 0.001 | 0.539 | |||
| Yes | 23 | 10 | |||
| No | 19 | 27 | |||
| Haemoglobin | 0.016 | 0.076 | |||
| Abnormal | 36 | 19 | |||
| Normal | 11 | 18 | |||
| NLR | <0.001 | 0.007 | 6.999 (1.712–28.606) | ||
| <2.7 | 11 | 28 | |||
| ≥2.7 | 36 | 9 | |||
| T staging | 0.003 | 0.007 | 7.223 (1.701–30.662) | ||
| T1-2 | 28 | 10 | |||
| T3-4 | 19 | 27 | |||
| LN involvement | <0.001 | 0.004 | 12.519 (2.218–70.650) | ||
| Yes | 31 | 4 | |||
| No | 16 | 33 | |||
CI, confidence interval; HR, hazard ratio; LN, lymph node; NLR, neutrophil-to-lymphocyte ratio.
4. Discussion
Cumulative evidence have demonstrated the role of inflammation in tumor progression [15]. A heightened neutrophilic response is associated with increased cytokines, interleukins (IL-1, IL-6) and pro-angiogenic vascular endothelial growth factor (VEGF) which promote tumor migration and proliferation [16]. Müller et al. [17] also commented that neutrophils release reactive oxygen species, nitric oxide and arginase, all of which compromise T cell function. A relative leukocytopenia could weaken the body's immune response to malignancy, and increase the potential for tumor progression [18]. Henceforth, the combinatory effects of these two cellular mechanisms, or the NLR, can be a strong predictor of tumor biology and outcomes of cancer patients.
A higher NLR has been associated with poorer disease specific and overall survival in gastric, hepatic, non-small cell and cervical cancer [12], [13], [14]. Anti-inflammatory therapy, on the other hand, has proven to be cancer protective [19]. The role of nonsteroidal anti-inflammatory drugs (NSAIDS) in reducing incidence of colon cancer and breast cancer is widely recognized [20], [21], but its association with bladder cancer is less clear-cut.
The discussion between NLR and bladder cancer has been recent with limited data. Gondo et al. [22] published the first series, correlating NLR >2.5 with worse cancer-specific survival. However, the study was limited by the short follow-up period (median 25 months). Demirtas et al. [23] reported no correlation between NLR >2.5 and overall survival; Krane et al. [24] noted that NLR >2.5 was associated with extravesical disease and worse overall survival, although the study was limited by the small sample size (68 patients) and 15% had neoadjuvant chemotherapy which could have affected the actual NLR value. Hermanns et al. [25] and Viers et al. [26] produced more recent evidence that NLR ≥3.0 and ≥2.7 respectively could impact recurrence rate, disease-specific survival and overall survival.
Nonetheless, our analyses expanded on existing data on the validity of NLR in predicting outcomes following RC for UCB. We had demonstrated a significant correlation of NLR ≥2.7 with worse disease-specific and overall survivals, our primary endpoints, with an estimated 5-year survival rates of 22% vs. 58% and 23% vs. 60% respectively on multivariate analyses.
Furthermore, NLR ≥2.7 was also associated with higher recurrence rate, higher pathological T staging and lymph node involvement. Although our study design had a small population, we had a rigorous methodology and long follow-up period. None of the patients had concomitant inflammatory conditions, infections or neoadjuvant therapies that would confound the NLR value.
In our study, a cut-off NLR value of 2.7 was chosen, with a ROC of 0.74. At this ROC value, the sensitivity of NLR in predicting for disease-specific survival was 73%, specificity of 75%, positive predictive value of 78% and negative predictive value of 69%. The heterogeneity of NLR thresholds might suggest variations in host response at different disease stages and population groups [25]. However, Templeton et al. [27] also commented that the decisions behind certain cut-off values were not well elaborated. Krane et al. [24] chose an NLR cut-off of 2.5 for consistency with Gondo's study design [22], while Demirtas et al [23] did not elaborate on his NLR value. The optimal sensitivity and specificity of NLR in relation to cancer outcomes were also not explicitly published in many studies.
Similar to literature [28], [29], our analyses demonstrated that lymph node involvement remains the single most consistent predictor of disease recurrence, disease-specific survival and overall survival, despite clear margins on final histology. Patients with lymph node positivity were more than 10 times likely to have recurrence, with significant poorer 5-year disease-specific survival (19% vs. 52%) and overall survival (20% vs. 56%).
While Thrasher et al. [30] identified preoperative anemia with reduced survival, we noted that anemia was correlated with worse overall survivial but not disease-specific survival. Five patients died from other causes during the study period (3 malignancies, 2 end stage renal diseases). This might suggest anemia could be associated with other diseases rather than UCB.
Although some authors [22] had reported the association of hydronephrosis in predicting survival, there was no correlation observed in our study. We believed the strong association with other factors (lymph node involvement and NLR) could have confounded its value.
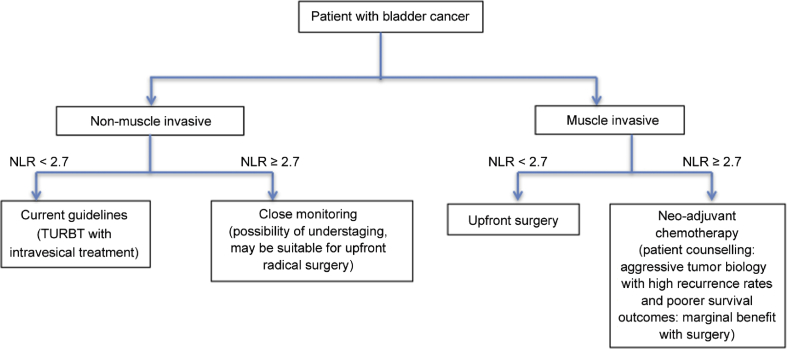
Ultimately, given the high risk of understaging, high recurrence rate and surgical morbidity [3], [4], [9], we hope that NLR can offer a simple alternative method of prognosticating survival outcomes and better risk stratify patients with UCB. Patients with high disease volume (high T staging, lymph node involvement) and raised NLR should consider alternative approaches such as neoadjuvant and/or adjuvant chemotherapy rather than upfront surgical resection. These patients need to be thoroughly counseled regarding the high recurrence rates and poor survival outcomes, in addition to the significant surgical morbidities. Conversely, for patients who have clinical non-muscle invasive bladder cancer (NMIBC) and high NLR values, clinicians should consider the increased likelihood of understaging. This may potentially identify a selected group of patients who are suitable for more aggressive upfront radical surgeries, although further validation is still required. Fig. 3 provides a schematic illustration of using NLR to identify patients with different tumour biology and the possible alternative treatments.
Figure 3.
NLR and possible treatment options. NLR, neutrophil-to-lymphocyte ratio; TURBT, transurethral resection of bladder tumor.
We recognize that our study is limited by the small sample size, and its retrospective and non-randomized nature, which requires further external validation. We have also excluded patients who underwent neoadjuvant chemotherapy, which limits the applicability of NLR. Henceforth, we hope that there can be continual research efforts to understand the role of NLR in other aspects of treatment for bladder cancer. Firstly, we can study the role of NLR in predicting survival outcomes of patients who underwent neoadjuvant chemotherapy. We postulate that a change in NLR (from high to low) maybe able to select a particular group of patients with more responsive tumor biology. They may benefit from subsequent radical surgical resections. Secondly, for patients who had undergone upfront surgical resections, a persistently high NLR value postoperatively may suggest residual disease. These patients may require closer monitoring for recurrence and adjuvant chemotherapy should be considered.
5. Conclusion
This present study had shown that elevated preoperative NLR was associated with worse disease-specific survival and overall survival. Also, NLR was an independent predictor of higher T staging, lymph node involvement and recurrence rate. These results suggested that NLR could be a novel preoperative factor to stratify and identify patients who might benefit from multimodal therapies in the treatment of UCB.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Stein J.P., Lieskovsky G., Cote R., Groshen S., Feng A.C., Boyd S. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 2.Dinney C.P. Therapy of invasive bladder cancer. Urology. 2006;67:56–61. doi: 10.1016/j.urology.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 3.Clark P.E., Agarwal N., Biagioli M.C., Eisenberger M.A., Greenberg R.E., Herr H.W. National comprehensive cancer N (2013) bladder cancer. J Natl Compr Cancer Netw. 2013;11:446–475. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 4.Zehnder P., Studer U.E., Skinner E.C., Thalmann G.N., Miranda G., Roth B. Unaltered oncological outcomes of radical cystectomy with extended lymphadenectomy over three decades. BJU Int. 2013;112:E51–E58. doi: 10.1111/bju.12215. [DOI] [PubMed] [Google Scholar]
- 5.Malkowicz S.B., van Poppel H., Mickisch G., Pansadoro V., Thüroff J., Soloway M.S. Muscle-invasive urothelial carcinoma of the bladder. Urology. 2007;69:3–16. doi: 10.1016/j.urology.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Gakis G., Efstathiou J., Lerner S.P., Cookson M.S., Keegan K.A., Guru K.A. International consultation on urologic disease-European association of urology consultation on bladder C ICUD-EAU international consultation on bladder cancer 2012: radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63:45–57. doi: 10.1016/j.eururo.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Shariat S.F., Palapattu G.S., Karakiewicz P.I., Rogers C.G., Vazina A., Bastian P.J. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol. 2007;51:137–149. doi: 10.1016/j.eururo.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Ficarra V., Dalpiaz O., Alrabi N., Novara G., Galfano A., Artibani W. Correlation between clinical and pathological staging in a series of radical cystectomies for bladder carcinoma. BJU Int. 2005;95:786–790. doi: 10.1111/j.1464-410X.2005.05401.x. [DOI] [PubMed] [Google Scholar]
- 9.Svatek R.S., Shariat S.F., Novara G., Skinner E.C., Fradet Y., Bastian P.J. Discrepancy between clinical and pathological stage: external validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int. 2011;107:898–904. doi: 10.1111/j.1464-410X.2010.09628.x. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A., Allavena P., Sica A., Pagani G.A. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 11.Zahorec R. Ratio of neutrophil to lymphocyte counts—rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 12.Jung M.R., Park Y.K., Jeong O., Seon J.W., Ryu S.Y., Kim D.Y. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;104:504–510. doi: 10.1002/jso.21986. [DOI] [PubMed] [Google Scholar]
- 13.Gomez D., Farid S., Malik H.Z., Young A.L., Toogood G.J., Lodge J.P. Preoperative neutrophil to- lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–1762. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- 14.Tomita M., Shimizu T., Ayabe T., Yonei A., Onitsuka T. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res. 2011;31:2995–2998. [PubMed] [Google Scholar]
- 15.Aggarwal B.B., Vijayalekshmi R.V., Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 16.Cho H., Hur H.W., Kim S.W., Kim S.H., Kim J.H., Kim Y.T. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller I., Munder M., Kropf P., Hänsch G.M. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol. 2009;30:522–530. doi: 10.1016/j.it.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Kim R., Emi M., Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thun M.J., Henley S.J., Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 20.Ahnen D.J. Colon cancer prevention by NSAIDs: what is the mechanism of action. Eur K Surg Suppl. 1998;(582):111–114. doi: 10.1080/11024159850191544. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs E.J., Thun M.J., Connell C.J., Rodriguez C., Henley S.J., Feigelson H.S. Aspirin and other nonsteroidal anti-inflammatory drugs and breast cancer in a large US cohort. Cancer Epidemiol Biomark Prev. 2005;14:261–264. [PubMed] [Google Scholar]
- 22.Gondo T., Nakashima J., Ohno Y., Choichiro O., Horiguchi Y., Namiki K. Prognostic value of neutrophil to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. 2012;79:1085–1091. doi: 10.1016/j.urology.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 23.Demirtas A., Sabur V., Akinsal E.C., Demirci D., Ekmekcioglu O., Gulmez I. Can neutrophil-lymphocyte ratio and lymph node density be used as prognostic factors in patients undergoing radical cystectomy? Sci World J. 2013;31:703579. doi: 10.1155/2013/703579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krane L.S., Richards K.A., Kader A.K., Davis R., Balaji K.C., Hemal A.K. Preoperative neutrophil lymphocyte ratio predicts overall survival and extravesical disease in patients undergoing radical cystectomy. J Endourol. 2013;27:1046–1050. doi: 10.1089/end.2012.0606. [DOI] [PubMed] [Google Scholar]
- 25.Hermanns T., Bhindi B., Wei Y., Yu J., Noon A.P., Richard P.O. Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br J Cancer. 2014;111:444–451. doi: 10.1038/bjc.2014.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viers B.R., Boorjian S.A., Frank I., Tarrell R.F., Thapa P., Karnes R.J. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol. 2014;66(6):1157–1164. doi: 10.1016/j.eururo.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 27.Templeton A.J., McNamara M.G., Šeruga B., Vera-Badillo F.E., Aneja P., Ocaña A. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 28.Hautmann R.E., Gschwend J.E., de Petriconi R.C., Kron M., Volkmer B.G. Cystectomy for transitional cell carcinoma of the bladder: results of a surgery only series in the neobladder era. J Urol. 2006;176:486–492. doi: 10.1016/j.juro.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 29.Madersbacher S., Hochreiter W., Burkhard F., Thalmann G.N., Danuser H., Markwalder R. Radical cystectomy for bladder cancer today—a homogeneous series without neoadjuvant therapy. J Clin Oncol. 2003;21:690–696. doi: 10.1200/JCO.2003.05.101. [DOI] [PubMed] [Google Scholar]
- 30.Thrasher J.B., Frazier H.A., Robertson J.E., Dodge R.K., Paulson D.F. Clinical variables which serve as predictors of cancer-specific survival among patients treated with radical cystectomy for transitional cell carcinoma of the bladder and prostate. Cancer. 1994;73:1708–1715. doi: 10.1002/1097-0142(19940315)73:6<1708::aid-cncr2820730626>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]