Abstract
Objective
Several inflammatory markers have been studied as potential biomarkers in renal cell carcinoma (RCC), however few reports have analyzed their prognostic value in aggregate and in non-clear cell histologies. We hypothesize that a combination of specific inflammatory markers into an RCC Inflammatory Score (RISK) could serve as a rigorous prognostic indicator of overall survival (OS) in patients with clear cell and non-clear cell RCC.
Methods
Combination of preoperative C-reactive protein (CRP), albumin, erythrocyte sedimentation rate (ESR), corrected calcium, and aspartate transaminase to alanine transaminase (AST/ALT) ratio was used to develop RISK. RISK was developed using grid-search methodology, receiver-operating-characteristic (ROC) analysis, and sensitivity-specificity trade-off analysis. Prognostic value of RISK was analyzed using the Kaplan–Meier method and Cox proportional regression models. Predictive accuracy was compared with RISK to Size, Size, Grade, and Necrosis (SSIGN) score, University of California-LOS Angeles (UCLA) Integrated Staging System (UISS), and Leibovich Prognosis Score (LPS).
Results
Among 391 RCC patients treated with nephrectomy, area under the curve (AUC) for RISK was 0.783, which was comparable to SSIGN (AUC 0.776, p = 0.82) and UISS (AUC 0.809, p = 0.317). Among patients with localized disease, AUC for RISK and LPS was 0.742 and 0.706, respectively (p = 0.456). On multivariate analysis, we observed a step-wise statistically significant inverse relationship between increasing RISK group and OS (all p < 0.001).
Conclusion
RISK is an independent and significant predictor of OS for patients treated with nephrectomy for clear cell and non-clear cell RCC, with accuracy comparable to other histopathological prognostic tools.
Keywords: Renal cell carcinoma, Inflammation, Prognosis, Biomarker
1. Introduction
Of the 61,500 new cases of renal cell carcinoma (RCC) each year in the US, 70% will have localized disease on initial presentation with potential for curative nephrectomy [1]. Assessment of clinical outcome after surgery, while much improved with modern tools, remains imperfect, with 30% of patients developing metastases and eventually succumbing to their disease [2]. In fact, the development of metastatic disease reduces 5-year survival from >80% to approximately 10% [2], [3]. With recent advancements in medical therapies, prognosis can be improved in select populations, with median overall survival (OS) surpassing 2 years [4]. Therefore, recent research has focused on methods to better identify high-risk patients for consideration of adjuvant therapy, increased surveillance, and enrollment into clinical trials.
Historically, risk stratification of patients with RCC was predominantly based on histopathological features as reflected by the TNM staging system and Fuhrman nuclear grade (FNG). However, recent studies attempting to validate the TNM staging system in patients with RCC showed mixed outcomes between stages and insufficient ability to differentiate high-risk disease [5]. In order to improve prognostic ability, various centers have developed integrative prognostic tools such as University of California Integrated Staging System (UCLA-UISS), Mayo Clinic Stage Size Grade Necrosis (SSIGN) score, and Leibovich Prognosis Score (LPS) [6], [7], [8], [9]. Though these tools are clinically useful and offer improved assessment of prognosis, they rely on surgical specimens obtained via invasive procedures, and also depend on potentially subjective pathological evaluations [10].
In the setting of a continued need for accurate prognostication for RCC patients, development of an affordable, convenient, and reliable preoperative prognostic tool is timely and indicated. With recent advancements in the understanding of cancer pathogenesis, it is well established that the host inflammatory response plays an integral role in disease progression. Over a decade of research has yielded several systemic inflammatory markers as prognostic biomarkers, most notably a combination of C-reactive protein (CRP) and albumin into the Glasgow Prognostic Score (GPS), which has been shown to be a robust predictor of OS in RCC and other solid organ cancers [11], [12]. Several other inflammatory markers, including erythrocyte sedimentation rate (ESR), calcium, aspartate transaminase to alanine transaminase (AST/ALT, DeRitis) ratio and various hematological markers have also been shown to have prognostic value [13], [14], [15], [16]. However, few reports have evaluated their prognostic value in aggregate, for non-clear cell histologies, and in comparison to currently utilized histopathological tools.
In this study, we hypothesize that a combination of preoperative CRP, albumin, ESR, corrected calcium, and AST/ALT ratio into an RCC Inflammatory Score (RISK) could serve as a rigorous prognostic indicator of OS in RCC. Notably, we aim to evaluate RISK in both clear cell and non-clear cell histologies, and compare the prognostic ability of RISK to commonly utilized histopathological prognostic tools.
2. Materials and methods
2.1. Patients
Patients that underwent nephrectomy for localized and metastatic RCC between 2007 and 2014 were retrospectively queried from our institutional database. The decision to perform radical, partial, or cytoreductive nephrectomy was based on tumor size and presence of metastases. All patients that developed metastases underwent targeted therapy according to standard of care. Patients with benign tumors or mucinous tubular and spindle cell carcinoma were excluded from the study cohort. We also excluded patients with unavailable preoperative laboratory results according to the criteria below or if age was less than 18 years. We identified sub-cohorts diagnosed with clear cell versus non-clear cell RCC (Fig. 1). Our Institutional Review Board approved this protocol and written consent was obtained from all participants before involvement in the study.
Figure 1.
Inclusion and exclusion criteria for the study. ALT, alanine transaminase; AST, aspartate transaminase; RCC, renal cell carcinoma.
2.2. Clinical and laboratory assessment
Patients were staged pathologically according to the 2009 TNM staging system for renal tumor classification [17]. All histological 2002 American Joint Committee on Cancer (AJCC) classifications were converted to 2009 classifications. Tumors were also graded via FNG criteria [18]. Preoperatively, we assessed clinical T stage, Eastern Cooperative Oncology Group (ECOG) performance status, and laboratory measurements (including CRP, albumin, ESR, AST, ALT, and serum-corrected calcium). Postoperatively, SSIGN score, UCLA Integrated Staging System (UISS), and LPS were calculated based on previously published methodologies [6], [7], [8]. Tumor size was recorded as the longest tumor diameter recorded in the pathology report.
Patients were followed postoperatively with physical examination, laboratory studies, and imaging. Follow-up for this study was terminated in June 2015. We defined OS as the time from surgery to either (a) death from any cause, or (b) last date of follow-up (i.e., censoring).
2.3. Development of RISK
Receiver operating characteristic (ROC) analysis identified albumin, CRP, ESR, AST/ALT ratio, and serum-corrected calcium as significant predictors of OS in RCC, later confirmed with grid-search methodology. Thus, the RISK biomarker panel consisted of those serum biomarkers measured preoperatively in accordance with their respective serum half-life. Kit information regarding specific commercial assays used at our institution and optimal collection time prior to surgery can be found in Table 1.
Table 1.
RISK biomarker assay methods, collection, and kit information.
| Laboratory value | Collection time before surgerya | Assay method and kit information |
|---|---|---|
| Albumin (g/dL) | Within 21 days | Beckman Coulter Synchron System |
| C-reactive protein (mg/L) | Within 14 days | Beckman Coulter Synchron System |
| Erythrocyte sedimentation rate (mm/h) | Within 30 days | ALCOR Scientific iSED Automated Sedimentation Rate Analyzer |
| AST (U/L) | Within 7 days | Beckman Coulter Synchron System |
| ALT (U/L) | Within 7 days | Beckman Coulter Synchron System |
| Calcium (mg/dL) | Within 30 days | Beckman Coulter Synchron System |
ALT, alanine transaminase; AST, aspartate transaminase; RCC, renal cell carcinoma; RISK: RCC Inflammatory Score.
Biomarker values used in analysis were collected defined time before nephrectomy.
Sensitivity-specificity trade-off curves, adjusted for interaction effect of gender, were used to find the optimal thresholds for effective discrimination of outcome variables and to dichotomize parameters for each variable. The primary differentiation threshold was fixed where sensitivity and specificity were equal. In order to identify more severe cases, a secondary threshold was fixed within 80%–90% specificity based on visual inspection of trade-off curves (Figs. S1–S5). We assigned each patient a total RISK score from 0 to 10 based on the sum of individual biomarker scores of 0, 1, or 2 (Table 2).
Table 2.
RISK calculation based on biomarker thresholds.
| Parameter | Biomarker Score |
||
|---|---|---|---|
| 0 | 1 | 2 | |
| Albumin (g/dL) | >3.5 | 2.5–3.5 | <2.5 |
| CRP (mg/L) | <10 | 10–25 | >25 |
| ESR (mm/h) | |||
| Male | <22 | 22–45 | >45 |
| Female | <29 | 29–55 | >55 |
| AST/ALT ratio | |||
| Male | <1.10 | 1.1–1.54 | >1.54 |
| Female | <1.23 | 1.23–1.54 | >1.54 |
| Corrected calcium (mg/dL) | <9.7 | 9.7–10.1 | >10.1 |
ALT, alanine transaminase; AST, aspartate transaminase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; RCC, renal cell carcinoma; RISK, RCC Inflammatory Score.
2.4. Statistical analysis
We estimated OS using the Kaplan–Meier method. Generalized chi-square test or Fisher's exact test was used to compare categorical variables, while Wilcoxon rank-sum test was used for continuous variables.
Cox proportional hazard regression models were fit to determine clinical, pathological, and laboratory features associated with OS. For our multivariable models, we planned to include pathological T stage, N stage, M stage, FNG, and age in the final model a priori based on widespread acceptance as prognostic factors. We then added any covariates with p < 0.05 on univariate testing of these models. For RISK score, we created a 4-level ordinal variable for risk stratification and interpretation: baseline risk (RISK 0), low risk (RISK 1–3), intermediate risk (RISK 4–6), and high risk (RISK 7–10).
We fit our final multivariable model using backward stepwise selection procedure with the selection threshold set at p = 0.05. This final model was validated using the bootstrap method in 500 equal sized samples selected with replacement. Harrell's Concordance index (C-index) was used to assess predictive accuracy of the final model. All statistical tests were two-sided with α-error set at 0.05. All analyses were performed using SAS version 9.4 (Cary, NC, USA) and R software version 3.1.2 (R Foundation, Vienna, Austria). Importantly, and uncommonly done for many biomarker studies in RCC, the REMARK criteria for tumor marker prognostic studies were rigorously followed [19].
3. Results
3.1. Cohort description
The overall study cohort was comprised of 391 patients (n = 280 clear cell RCC, n = 111 non-clear cell RCC, Table 3). The majority of patients were male and white with a mean age of 58.7 years. Of the study cohort, 207/370 (56.0%) presented with T1 disease, 38/370 (10.3%) with T2 disease, 114/370 (30.8%) with T3 disease, and 11/370 (3.0%) with T4 disease. Additionally, 10/391 (2.7%) patients had FNG 1, 149/391 (39.5%) had FNG 2, 162/391 (43.0%) had FNG 3, and 55/391 (14.6%) had FNG 4. After surgery, 89/391 (22.8%) developed metastases and 64/391 (16.4%) were deceased by the end of the study, likely a reflection of the advanced stage presentation at our tertiary care institution.
Table 3.
Description of clinical and pathological parameters of the overall study cohort (n = 391) and clear cell RCC sub-cohort (n = 280).
| Feature | Overall cohort∗ (n = 391) | Clear cell sub-cohort∗ (n = 280) |
|---|---|---|
| Primary tumor (1997 T stage)a | ||
| T1 | 207 (55.95) | 146 (54.48) |
| T2 | 38 (10.27) | 23 (8.58) |
| T3 | 114 (30.81) | 94 (35.07) |
| T4 | 11 (2.97) | 5 (1.87) |
| Regional lymph nodes (N stage)a | ||
| N0 | 328 (84.10) | 234 (83.47) |
| N1 | 57 (14.62) | 45 (16.17) |
| N2 | 5 (1.28) | 1 (0.36) |
| Distant metastases (M stage)a | ||
| M0 | 314 (80.51) | 216 (77.14) |
| M1 | 76 (19.49) | 64 (22.86) |
| Tumor size (cm) | ||
| Mean ± SD | 6.1 ± 4.1 | 6.1 ± 4 |
| Median (min–max) | 5.1 (0.4–23.0) | 5.3 (0.5–23.0) |
| Nuclear gradea | ||
| 1 | 10 (2.66) | 8 (2.88) |
| 2 | 149 (39.62) | 115 (41.37) |
| 3 | 162 (43.09) | 111 (39.93) |
| 4 | 55 (14.63) | 44 (15.82) |
| Necrosisa | ||
| No | 174 (57.24) | 133 (59.37) |
| Yes | 130 (42.76) | 91 (40.63) |
Participant's number may not add to 391 or 280 due to missing values.
Data presented as n (%).
3.2. Univariate and multivariate analysis
In the overall study cohort, we found that age, body mass index (BMI), ECOG performance status, pathological T stage, N stage, M stage, FNG, tumor size, RISK group, and each contributing component of RISK were significantly associated with OS on univariate analysis (all p < 0.05). These variables were included in the multivariable model. After adjusting for confounding variables, our analysis identified age, ECOG performance status, tumor size, and RISK group as significant and independent factors influencing OS (all p < 0.05, Table 4).
Table 4.
Univariate and multivariate association of overall survival in the overall cohort (N = 391).
| Covariate | Univariable |
Multivariable |
||
|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | |
| Age | 1.04 (1.02–1.06) | <0.001 | 1.04 (1.01–1.07) | 0.010 |
| BMI | 0.94 (0.90–0.98) | 0.003 | 0.97 (0.93–1.02) | 0.209 |
| Sex (ref: female) | 1.08 (0.64–1.82) | 0.767 | ||
| Race (ref: non-African-American) | 1.21 (0.64–2.26) | 0.556 | ||
| Pre-op ECOG performance status (ref: 0) | 3.35 (2.05–5.49) | <0.001 | 2.25 (1.31–3.86) | 0.003 |
| 2009 T stage | ||||
| pT2 | 2.20 (0.85–5.68) | 0.102 | 0.49 (0.15–1.58) | 0.229 |
| pT3 | 6.39 (3.50–11.68) | <0.001 | 0.88 (0.35–2.21) | 0.783 |
| pT4 | 10.96 (3.58–33.59) | <0.001 | 1.54 (0.36–6.60) | 0.564 |
| 2009 N stage (ref: N0) | 5.51 (3.33–9.12) | <0.001 | 1.82 (0.89–3.72) | 0.103 |
| 2009 M stage (ref: M0) | 6.01 (3.65–9.89) | <0.001 | 1.31 (0.61–2.80) | 0.483 |
| FNG (ref: G1–G2) | 3.64 (1.90–6.97) | <0.001 | 0.97 (0.39–2.41) | 0.951 |
| Tumor dimensions (ref: <5 cm) | 4.31 (2.29–8.12) | <0.001 | 2.63 (1.09–6.34) | 0.031 |
| RISK (ref: baseline) | ||||
| Low risk | 1.35 (0.37–4.92) | 0.645 | 0.72 (0.18–2.85) | 0.643 |
| Intermediate risk | 8.64 (2.57–29.02) | <0.001 | 3.29 (0.83–13.06) | 0.090 |
| High risk | 18.41 (5.60–60.58) | <0.001 | 4.80 (1.23–18.74) | 0.024 |
| C-reactive protein (ref: <10 mg/L) | ||||
| 10–25 mg/L | 3.27 (1.45–7.34) | 0.004 | ||
| >25 mg/L | 9.01 (5.05–16.07) | <0.001 | ||
| Albumin (ref: > 3.5 mg/dL) | ||||
| 2.5–3.5 mg/dL | 4.44 (2.61–7.53) | <0.001 | ||
| <2.5 mg/dL | 6.15 (2.64–14.32) | <0.001 | ||
| ESR (ref: 0–22 (male)/0–29 (female) mm/h) | ||||
| 22–45 (male)/29–55 (female) mm/h | 2.94 (1.29–6.70) | 0.01 | ||
| ≥45 (male)/≥55 (female) mm/h | 10.08 (5.02–20.24) | <0.001 | ||
| AST/ALT ratio (ref: 1.1 (male)/1.23 (female)) | ||||
| 1.1–1.54 (male)/1.23–1.54 (female) | 1.08 (0.59–1.97) | 0.803 | ||
| >1.54 | 2.65 (1.46–4.84) | 0.001 | ||
| Calcium (ref: <9.7 mg/dL) | ||||
| 9.7–10.11 mg/dL | 2.12 (1.02–4.39) | 0.044 | ||
| >10.11 mg/dL | 6.94 (3.58–13.42) | <0.001 | ||
AST/ALT, aspartate transaminase to alanine transaminase; BMI, body mass index; CI, confidence intervals; ECOG, Eastern Cooperative Oncology Group; ESR, erythrocyte sedimentation rate; FNG, Fuhrman nuclear grade; HR, hazard ratio; RCC, renal cell carcinoma; RISK, RCC Inflammatory Score.
In the sub-cohort of patients diagnosed with clear cell RCC, we found that age, BMI, African-American race, ECOG performance status, T stage, N stage, M stage, FNG, tumor size, RISK group, and each contributing component of RISK were significantly associated with OS on univariate analysis (all p < 0.05). These variables were included in the multivariable model. After adjusting for confounding variables, our analysis identified ECOG performance status, tumor size, and RISK group as significant and independent factors influencing OS (all p < 0.05, Table 5). Similar analysis was performed in the non-clear cell cohort, however due to limited sample size, a reliable model was difficult to develop and we were unable to produce adjusted hazard ratios.
Table 5.
Univariate and multivariate association of overall survival in the clear cell RCC sub-cohort (n = 280).
| Covariate | Univariate |
Multivariable |
||
|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95% CI) | p-Value | |
| Age | 1.03 (1.00–1.05) | 0.035 | 1.02 (0.98–1.05) | 0.346 |
| BMI | 0.94 (0.9–0.98) | 0.004 | 0.97 (0.92–1.01) | 0.132 |
| Sex (ref: female) | 1.15 (0.65–2.02) | 0.631 | ||
| Race (ref: non-African-American) | 2.85 (1.46–5.56) | 0.001 | ||
| Pre-op ECOG performance status (ref: 0) | 2.84 (1.66–4.86) | <0.001 | 2.15 (1.19–3.90) | 0.012 |
| 2009 T stage | ||||
| pT2 | 1.82 (0.59–5.59) | 0.295 | 0.32 (0.08–1.22) | 0.094 |
| pT3 | 5.85 (3.07–11.12) | <0.001 | 0.74 (0.28–1.99) | 0.554 |
| pT4 | 5.56 (0.72–43.12) | 0.101 | 0.68 (0.07–6.23) | 0.735 |
| 2009 N stage (ref: N0) | 5.18 (2.99–8.97) | <0.001 | 1.89 (0.87–4.11) | |
| 2009 M stage (ref: M0) | 5.24 (3.05–9.02) | <0.001 | 1.13 (0.50–2.55) | |
| FNG (ref: G1–G2) | 3.47 (1.79–6.73) | <0.001 | 0.83 (0.30–2.25) | 0.710 |
| Tumor dimensions (ref: <5 cm) | 4.88 (2.37–10.03) | <0.001 | 2.95 (1.15–7.55) | 0.024 |
| RISK (ref: baseline) | ||||
| Low risk | 1.31 (0.34–5.07) | 0.696 | 0.74 (0.17–3.26) | 0.886 |
| Intermediate risk | 9.55 (2.83–32.25) | <0.001 | 5.36 (1.23–23.46) | 0.026 |
| High risk | 16.44 (4.92–54.93) | <0.001 | 5.52 (1.29–23.55) | 0.021 |
| C-reactive protein (ref: <10 mg/L) | ||||
| 10–25 mg/L | 3.96 (1.71–9.18) | 0.001 | ||
| >25 mg/L | 7.62 (4.03–14.41) | <0.001 | ||
| Albumin (ref: > 3.5 mg/dL) | ||||
| 2.5–3.5 mg/dL | 4.33 (2.44–7.68) | <0.001 | ||
| <2.5 mg/dL | 4.62 (1.73–12.33) | 0.002 | ||
| ESR (ref: 0–22 (male)/0–29 (female) mm/h) | ||||
| 22–45 (male)/29–55 (female) mm/h | 2.84 (1.15–6.98) | 0.023 | ||
| ≥45 (male)/≥55 (female) mm/h | 8.72 (4.16–18.25) | <0.001 | ||
| AST/ALT ratio (ref: 1.1 (male)/1.23 (female)) | ||||
| 1.1–1.54 (male)/1.23–1.54 (female) | 1.41 (0.76–2.62) | 0.276 | ||
| >1.54 | 2.67 (1.32–5.39) | 0.006 | ||
| Calcium (ref: <9.7 mg/dL) | ||||
| 9.7–10.11 mg/dL | 2.43 (1.09–5.42) | 0.029 | ||
| >10.11 mg/dL | 6.90 (3.35–14.21) | <0.001 | ||
AST/ALT, aspartate transaminase to alanine transaminase; BMI, body mass index; CI, confidence intervals; ECOG, Eastern Cooperative Oncology Group; ESR, erythrocyte sedimentation rate; FNG, Fuhrman nuclear grade; HR, hazard ratio; RCC, renal cell carcinoma; RISK, RCC Inflammatory Score.
3.3. Kaplan–Meier survival analysis
OS was compared between RISK groups using the Kaplan–Meier method. In the overall study cohort, as well as the clear cell and non-clear cell RCC sub-cohorts, time to death was significantly reduced with increasing RISK group (p < 0.001, Figs. 2 and S6).
Figure 2.
Kaplan–Meier curves predicting overall survival in the overall cohort (n = 391), categorized by RISK group. RCC, renal cell carcinoma.
In the overall study cohort, median time-to-death for censored and non-censored subjects was 25.2 and 16.1 months, respectively. Median survival among the high-risk group was 7.2 months (95%CI: 4.9–11.4), compared with 14.5 months (95%CI: 10.1–21.5) among the intermediate-risk group (p = 0.008).
In the clear cell RCC sub-cohort, median time-to-death for censored and non-censored subjects was 25.1 and 16.4 months, respectively. Median survival among the high-risk group was 7.3 months (95%CI: 5.0–12.9), compared with 16.6 months (95%CI: 7.9–24.0) among the intermediate-risk group (p = 0.041).
In the non-clear cell RCC sub-cohort, median time-to-death for censored and non-censored subjects was 25.4 and 14.5 months, respectively. Median survival among the high-risk group was 4.9 months (95%CI: 2.4–27.6), compared with 10.6 months (95%CI: 6.0–33.5) among the intermediate-risk group (p = 0.010).
For all cohorts, median survival was not reached among the low-risk and baseline groups.
3.4. Comparison with established prognostic variables and nomograms
In the overall study cohort and clear cell RCC sub-cohort, RISK was significantly associated with ECOG performance status, histologic tumor necrosis, TNM stage, and FNG (all p < 0.001).
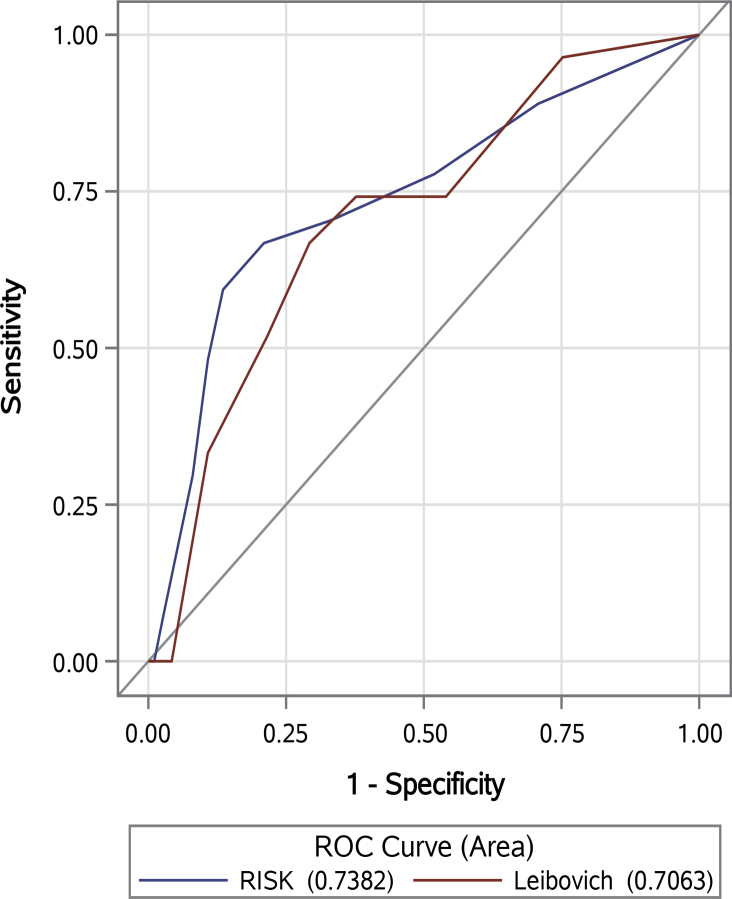
For the overall study cohort, AUC for RISK, SSIGN, and UISS was 0.783, 0.776, and 0.813, respectively. Among patients with localized RCC (i.e., T1-4N0M0), AUC for RISK and LPS was 0.742 and 0.706, respectively. There were no statistically significant differences between RISK, SSIGN, UISS, and LPS (p = 0.820, p = 0.317, p = 0.456, respectively, Figs. S7 and S8). The statistically significant (p = 0.019) addition of ESR, corrected calcium, and AST/ALT ratio to combination of CRP and albumin improved AUC from 0.737 to 0.783.
For the clear cell RCC sub-cohort, AUC for RISK, SSIGN, and UISS was 0.774, 0.776, and 0.809, respectively. Among patients with localized RCC, AUC for RISK and LPS was 0.738 and 0.706, respectively. Again, there were no statistically significant differences between RISK, SSIGN, UISS, and LPS (p = 0.975, p = 0.299, p = 0.561, respectively, Figs. S9 and S10).
For the non-clear cell RCC sub-cohort, AUC for RISK, SSIGN, and UISS was 0.809, 0.764, and 0.789, respectively. Among patients with localized RCC, AUC for RISK and LPS was 0.785 and 0.744, respectively. Again, there were no statistically significant differences between RISK, SSIGN, UISS, and LPS (p = 0.550, p = 0.776, and p = 0.597, respectively, Figs. S11 and S12).
4. Discussion
In this study, we developed a novel preoperative prognostic score termed RISK that aggregates measures of CRP, albumin, ESR, corrected calcium, and AST/ALT ratio in order to predict OS for all RCC patients treated with nephrectomy. We report four important findings. First, in evaluating prognostic performance, we found that RISK is an independent and significant predictor of OS in patients undergoing nephrectomy for RCC. Second, we found that this score is a valid predictor of OS among patients in the overall cohort, which included all histologies, as well as the clear cell RCC sub-cohort. Third, RISK was able to predict OS with similar certainty as established prognostic tools and nomograms such as SSIGN, UISS, and LPS. Finally, on Kaplan–Meier analysis, each increase in RISK group was associated with a significant reduction in OS, illustrating the score's ability to differentiate high-risk disease. Notably, this differentiation in prognosis is evident soon after surgery and is strongest between intermediate-risk and high-risk groups, demonstrating its utility even in the short-term postoperative setting. These findings emphasize the integral role the host inflammatory response plays in cancer pathogenesis and suggest that RISK provides clinically useful prognostic information comparable to other prognostic tools.
Our findings further support the current literature exploring the role of the host inflammatory response in cancer pathogenesis. Tumor infiltration by immune cells and production of inflammatory mediators promoting angiogenesis and immunosuppression have been strongly linked to tumor progression [20]. Notably, over 60 studies have focused on evaluating and validating the use of GPS [12], [21], [22]. Additionally, ESR has been shown in multiple studies to have prognostic value in RCC and other solid organ tumors with elevated levels predicting the presence of aggressive disease and decreased survival [23], [24]. Though several other inflammatory markers have been evaluated in singularity, few studies have analyzed their prognostic value in aggregate [16]. To our knowledge, this study is the first of its kind to analyze a comprehensive inflammatory score consisting of five well-studied inflammatory markers in RCC.
Though additional tumor and host factors have been added to TNM stage and FNG conferring improved prognostic ability with SSIGN and UISS, our data demonstrate that a prognostic score based solely on inflammatory markers can be just as accurate in predicting OS. In patients with metastatic RCC, Motzer et al. [3] and Eggener et al. [25] developed clinically useful prognostic models incorporating various inflammatory markers and performance status. Additionally, Chang et al. [26] developed the Systemic Inflammation Score (SIS) consisting of preoperative albumin and lymphocyte-to-monocyte ratio, and showed that elevated SIS was associated with high TNM stage, high SSIGN risk level, and poor outcomes. SIS was then combined with TNM stage, FNG, and lymphovascular invasion into a prognostic nomogram with predictive accuracy greater than SSIGN. These models as well as RISK serve as proof of principle that inflammatory markers can provide robust prognostic information in RCC.
Solely based on laboratory values commonly obtained during the preoperative workup, RISK provides considerable practical benefits over currently utilized prognostic tools. It is a simple and quantitative score that utilizes affordable, standardized, and widely available protein assays that are among the most frequently requested laboratory tests in the oncologic outpatient setting. These assays are fully automated, avoiding human error and subjectivity that may adversely influence histopathological prognostic tools as has been shown with FNG [27]. Most notably, no surgical specimen is required for scoring, and RISK can be calculated and interpreted prior to nephrectomy potentially allowing for improved patient counseling, medical decision-making, patient selection for surgery, and enrollment into clinical trials.
Following REMARK criteria, we have demonstrated that RISK is a powerful prognostic tool in patients undergoing nephrectomy for RCC [19]. A strength of our study is that RISK is based on standardized laboratory tests that are widely available and cost-effective. Further, the biomarker thresholds determined in our analysis align with previous studies. We also evaluated RISK in patients with clear cell and non-clear cell RCC, with localized and metastatic disease, illustrating the scope of its prognostic ability. Weaknesses of our study include its retrospective and single-centered nature, and lack of external validation. Importantly, our study used OS as the primary outcome as the use of cancer-specific survival would prevent inclusion of patients that underwent adjuvant therapy, including targeted therapy or radiation. Further investigation into the impact of RISK on cancer-specific survival is needed. The use of five inflammatory markers also limited the study cohort and may also restrict clinical applicability due to the select use of several of the biomarkers. This level of patient selection may also explain our finding that neither N stage, M stage, nor tumor grade are independent factors affecting OS in this study. Additionally, we were unable to adequately analyze RISK in the non-clear cell RCC cohort due to limited sample size.
In the future, RISK will need to be externally validated at other institutions and in distinct populations. Further research into the integration of RISK with additional inflammatory markers, host factors, and histopathological tools is warranted and could lead to even greater predictive accuracy. Given the preoperative utility of RISK, evaluation of its use in patients considering cytoreductive nephrectomy in the presence of metastasis or active surveillance in the setting of low risk disease is also warranted.
5. Conclusion
RISK is an independent and significant predictor of OS in RCC with predictive accuracy comparable to SSIGN, UISS, and LPS. Notably, RISK is composed of standardized laboratory markers that are easily and cost-effectively obtained preoperatively, allowing crucial prognostic information to be integrated into medical decision-making prior to surgery.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgement
Support of the John Robinson Family is greatly acknowledged.
Footnotes
Peer review under responsibility of Second Military Medical University.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ajur.2017.04.002.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Figure S1.

Sensitivity-specificity trade-off analysis for albumin.
Figure S2.
Sensitivity-specificity trade-off analysis for CRP.
Figure S3.
Sensitivity-specificity trade-off analysis for ESR.
Figure S4.
Sensitivity-specificity trade-off analysis for AST/ALT ratio.
Figure S5.
Sensitivity-specificity trade-off analysis for corrected calcium.
Figure S6.
Kaplan–Meier curves predicting overall survival in the clear cell RCC sub-cohort (n = 280), categorized by RISK group.
Figure S7.
Nomogram comparison (overall cohort).
Figure S8.
Nomogram comparison (clear cell RCC sub-cohort).
Figure S9.
Nomogram comparison (non-clear cell RCC sub-cohort).
Figure S10.

Leibovich comparison (overall cohort).
Figure S11.
Leibovich comparison (clear cell RCC sub-cohort).
Figure S12.
Leibovich comparison (non-clear cell RCC sub-cohort).
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey S., Lamb G.W., Aitchison M., McMillan D.C. Prospective study of the relationship between the systemic inflammatory response, prognostic scoring systems and relapse-free and cancer-specific survival in patients undergoing potentially curative resection for renal cancer. BJU Int. 2008;101:959–963. doi: 10.1111/j.1464-410X.2007.07363.x. [DOI] [PubMed] [Google Scholar]
- 3.Motzer R.J., Mazumdar M., Bacik J., Berg W., Amsterdam A., Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 4.Motzer R.J., Hutson T.E., Tomczak P., Michaelson M.D., Bukowski R.M., Oudard S. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novara G., Ficarra V., Antonelli A., Artibani W., Bertini R., Carini M. Validation of the 2009 TNM version in a large multi-institutional cohort of patients treated for renal cell carcinoma: are further improvements needed? Eur Urol. 2010;58:588–595. doi: 10.1016/j.eururo.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Zisman A., Pantuck A.J., Dorey F., Said J.W., Shvarts O., Quintana D. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19:1649–1657. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 7.Frank I., Blute M.L., Cheville J.C., Lohse C.M., Weaver A.L., Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 8.Leibovich B.C., Cheville J.C., Lohse C.M., Zincke H., Frank I., Kwon E.D. A scoring algorithm to predict survival for patients with metastatic clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. J Urol. 2005;174:1759–1763. doi: 10.1097/01.ju.0000177487.64651.3a. [DOI] [PubMed] [Google Scholar]
- 9.Kattan M.W., Reuter V., Motzer R.J., Katz J., Russo P. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166:63–67. [PubMed] [Google Scholar]
- 10.Vasdev N., Altal Y., Mafeld S., Wong K., Chadwick D., Gowda B.D. Can the Leibovich score for clear cell renal cell carcinoma (ccRCC) be accurately reported by a general pathologist? BJU Int. 2014;113:581–585. doi: 10.1111/bju.12337. [DOI] [PubMed] [Google Scholar]
- 11.Lamb G.W., Aitchison M., Ramsey S., Housley S.L., McMillan D.C. Clinical utility of the Glasgow Prognostic Score in patients undergoing curative nephrectomy for renal clear cell cancer: basis of new prognostic scoring systems. Br J Cancer. 2012;106:279–283. doi: 10.1038/bjc.2011.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMillan D.C. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Fox P., Hudson M., Brown C., Lord S., Gebski V., De Souza P. Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br J Cancer. 2013;109:147–153. doi: 10.1038/bjc.2013.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bezan A., Mrsic E., Krieger D., Stojakovic T., Pummer K., Zigeuner R. The preoperative AST/ALT (De Ritis) ratio represents a poor prognostic factor in a cohort of patients with nonmetastatic renal cell carcinoma. J Urol. 2015;194:30–35. doi: 10.1016/j.juro.2015.01.083. [DOI] [PubMed] [Google Scholar]
- 15.Guthrie G.J., Roxburgh C.S., Farhan-Alanie O.M., Horgan P.G., McMillan D.C. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. Br J Cancer. 2013;109:24–28. doi: 10.1038/bjc.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun M., Shariat S.F., Cheng C., Ficarra V., Murai M., Oudard S. Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol. 2011;60:644–661. doi: 10.1016/j.eururo.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 17.Edge S.B., Compton C.C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 18.Fuhrman S.A., Lasky L.C., Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Altman D.G., McShane L.M., Sauerbrei W., Taube S.E. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. BMC Med. 2012;10:51. doi: 10.1186/1741-7015-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proctor M.J., Horgan P.G., Talwar D., Fletcher C.D., Morrison D.S., McMillan D.C. Optimization of the systemic inflammation-based Glasgow prognostic score: a Glasgow inflammation outcome study. Cancer. 2013;119:2325–2332. doi: 10.1002/cncr.28018. [DOI] [PubMed] [Google Scholar]
- 22.Tai C.G., Johnson T.V., Abbasi A., Herrell L., Harris W.B., Kucuk O. External validation of the modified Glasgow prognostic score for renal cancer. Indian J Urol. 2014;30:33–37. doi: 10.4103/0970-1591.124203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cross B.W., Johnson T.V., Derosa A.B., Ogan K., Pattaras J.G., Nieh P.T. Preoperative erythrocyte sedimentation rate independently predicts overall survival in localized renal cell carcinoma following radical nephrectomy. Int J Surg Oncol. 2012;2012:524981. doi: 10.1155/2012/524981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sengupta S., Lohse C.M., Cheville J.C., Leibovich B.C., Thompson R.H., Webster W.S. The preoperative erythrocyte sedimentation rate is an independent prognostic factor in renal cell carcinoma. Cancer. 2006;106:304–312. doi: 10.1002/cncr.21617. [DOI] [PubMed] [Google Scholar]
- 25.Eggener S.E., Yossepowitch O., Pettus J.A., Snyder M.E., Motzer R.J., Russo P. Renal cell carcinoma recurrence after nephrectomy for localized disease: predicting survival from time of recurrence. J Clin Oncol. 2006;24:3101–3106. doi: 10.1200/JCO.2005.04.8280. [DOI] [PubMed] [Google Scholar]
- 26.Chang Y., An H., Xu L., Zhu Y., Yang Y., Lin Z. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br J Cancer. 2015;113:626–633. doi: 10.1038/bjc.2015.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Aynati M., Chen V., Salama S., Shuhaibar H., Treleaven D., Vincic L. Interobserver and intraobserver variability using the Fuhrman grading system for renal cell carcinoma. Arch Pathol Lab Med. 2003;127:593–596. doi: 10.5858/2003-127-0593-IAIVUT. [DOI] [PubMed] [Google Scholar]