Abstract
Serotonin (5-HT) functioning is associated with alcohol problems. However, the mechanisms underlying this association remain unclear. The current study tested whether five separate dimensions of impulsivity (UPPS-P) mediated the relation between a polygenic score indexing 5-HT functioning and alcohol problems and whether any of these paths were moderated by age. Results showed that a 5-HT polygenic score predicted alcohol problems indirectly through negative urgency, but not any other facet of impulsivity. The 5-HT polygenic score also directly predicted alcohol problems. No age moderation was found. Findings suggest that negative urgency might be one important mechanism underlying the relation between genetically-influenced 5-HT functioning and alcohol problems. However, genetically-influenced 5-HT functioning likely influences alcohol problems through additional mechanisms. More broadly, results suggest that the previously observed transdiagnostic nature of 5-HT functioning on diverse types of psychopathology might be, in part, explained by its effect on negative urgency.
Keywords: Serotonin, Polygenic Risk Score, Alcohol Problems, Negative Urgency, Impulsivity
Serotonin (5-HT) is a monoamine neurotransmitter that has been implicated in the etiology of alcohol consumption and dependence (LeMarquand et al., 1994). Although associations between 5-HT functioning and alcohol phenotypes have been demonstrated, the mechanisms underlying these associations remain unclear (e.g., Canli & Lesch, 2007; Carver, Johnson & Joormann, 2008; Heinz, Mann, Weinberger, & Goldman, 2001; Lesch, 2005; Wang et al., 2017). The present study attempted to elucidate the mechanism(s) underlying the relation between 5-HT functioning and alcohol problems, thereby informing the etiology and treatment of alcohol problems.
Association between 5-HT Functioning and Alcohol Phenotypes
The “serotonin hypothesis of alcoholism” states that a genetically transmitted biochemical abnormality, when expressed, might result in a deficit of 5-HT in the brain that subsequently increases the risk for problematic alcohol consumption (LeMarquand et al., 1994). Results from multiple studies corroborate this hypothesis. In cross-sectional studies, 5-HT functioning as measured by levels of the serotonin metabolite, 5-hydroxyindoleacetic acid (5-HIAA), in the cerebrospinal fluid (CSF) and drug challenge were related to human alcohol consumption and dependence (Balldin et al., 1994; Ballenger et al., 1979; Banki, 1981; Farren et al., 1995; Lee & Meltzer, 1991), such that lower levels of 5-HT functioning were related to greater risk for alcohol use and/or dependence.
Previous research also suggested that 5-HT functioning might be a premorbid, heritable risk factor for alcohol use. In animal research, non-alcohol exposed rodents bred with an alcohol preference showed lower 5-HT levels than rodents bred without an alcohol preference (Gongwer et al., 1989). Children of alcoholics who had never consumed alcohol also showed higher platelet 5-HT uptake when compared to controls, which indicates that there is less 5-HT available for neurotransmission (Ernouf et al., 1993). Genetic variants thought influence 5-HT functioning (e.g., 5-HTTLPR) are also related to alcohol phenotypes (Feinn, Nellisery, & Kranzler, 2005). In addition, Wang et al. (2017) found that a polygenic score indexing concentrations of 5-HIAA in the CSF was associated with adolescents’ alcohol use (both directly and indirectly through adolescents’ aggression/antisociality, and sometimes for specific demographic subgroups), such that scores indicating lower 5-HIAA (i.e., 5-HT functioning) were associated with greater alcohol use. These findings were replicated in two independent longitudinal samples of adolescents, one of which overlapped with the current subsample.
Mechanisms in the Relation Between 5-HT Functioning and Alcohol Problems
Although researchers have established an association between 5-HT functioning and alcohol use and dependence, the mechanisms underlying this relation have not yet been clearly explicated. Indeed, because 5-HT functioning is a premorbid and heritable risk factor, it is likely to have some impact on individuals’ functioning prior to the onset of alcohol use and disorder (which have a typical onset in the adolescent years or later; SAMHSA, 2014). Moreover, alcohol use disorders are extremely heterogeneous in their behavioral presentations. It is unlikely that a single biological vulnerability factor has only a simple direct effect on developing the disorder. Instead, 5-HT functioning might influence some earlier-appearing behavior(s) or trait(s) that predispose individuals to develop alcohol problems.
Previous researchers have hypothesized that 5-HT functioning might exert its influence on problematic alcohol use through various mechanisms. These include, among others, negative emotionality (Heinz et al., 2001), behavioral disinhibition (Depue & Spoont, 1986; Soubriè, 1986), top-down emotion- and self-regulation (Carver et al., 2008; Hariri & Holmes, 2006; Lesch, 2005), and impulsive reactivity to emotions (Carver, Johnson, Joormann, Kim, & Nam, 2011).
However, the exact mechanisms through which 5-HT functioning exerts its influence has been difficult to concretize. One reason for this difficulty is that the patterns of relations between 5-HT functioning and other types of psychopathology are oftentimes unexpected and do not logically lead to a single mechanism of action. Indeed, Cools, Roberts, and Robbins (2008) called this “the paradox of 5-HT.” For example, individuals with low levels of 5-HT are more likely to show aggression, problematic alcohol use, and mania (Coccaro, 1989; LeMarquand et al., 1994; Thakore, O’Keane, & Dinan, 1996). These findings are consistent with animal and human studies suggesting that 5-HT functioning mediates behavioral disinhibition (Soubriè, 1986). However, this hypothesis is at odds with findings that individuals with low levels of 5-HT functioning are also prone to depression (e.g., Munafò, Hayward, & Harmer, 2006; Neumeister et al., 2002), because individuals with depression often show less behavioral disinhibition (i.e., they are behaviorally inhibited; Wang, Chassin, Eisenberg & Spinrad, 2015).
In order to reconcile this discrepancy, Carver et al. (2008) posited that lower levels of 5-HT functioning might be associated with poorer self-regulation, which would allow one’s preexisting, bottom-up, tendency towards approach or avoidance to be expressed. Individuals with poor self-regulation (i.e., lower 5-HT functioning) as well as a tendency towards approach-related behaviors might be predisposed to externalizing-related behaviors. On the other hand, individuals with poor self-regulation (i.e., lower 5-HT functioning) as well as a tendency towards avoidance or withdrawal might be predisposed to depressive or internalizing-related behaviors. One previous study tested this theory in two longitudinal samples of adolescents by examining whether top-down self-regulation, depressive symptoms, and/or aggressive/antisocial behaviors mediated the relation between a 5-HT polygenic score and adolescents’ alcohol use. Wang et al (2017) found that the 5-HT polygenic score did not predict self-regulation. However, the polygenic score did predict depressive symptoms, aggression/antisociality, and alcohol use, especially within certain demographic high-risk groups. Thus, although the polygenic score predicted aspects of psychopathology as expected (suggesting that the genetic measure was valid), it did not predict top-down self-regulation as hypothesized by Carver et al. (2008).
Impulsive Reactivity to Negative Emotion as a Potential Mediator
Drawing from their original review, Carver et al. (2011) posited that 5-HT functioning (and specifically the 5-HTTLPR polymorphism) is related to impulsive reactivity to emotions, which describes the tendency to act impulsively in the face of strong positive or negative emotions. Although impulsivity in response to emotions typically implies rash actions, such as binge drinking or risky behaviors, Carver et al. (2011) argued that this impulsivity could also include inaction, as long as it was a reflexive and automatic response. For example, an individual might experience a negative emotion when thinking about entering a social situation, and subsequently decline from the social engagement without thinking through (i.e., acting impulsively or reflexively) the consequences (e.g., loneliness, isolation). A related construct is called urgency, which is one of five factors from a widely used five-factor model of impulsivity (i.g., UPPS-P; Lynam, Smith, Cyders, Fischer, & Whiteside, 2007; Whiteside & Lynam, 2001). Urgency describes “the disposition to act in rash, impulsive ways when highly emotional” (Smith & Cyders, 2016). Urgency consists of two sub-dimensions called positive and negative urgency, which each refer to the disposition to act impulsively when experiencing high positive or negative emotions, respectively. Although urgency was originally conceptualized as the tendency to respond to strong emotions with rash actions leading to externalizing-related dysfunction (e.g., problem drinking, risky sex), a recent study found that urgency likely also taps the tendency to respond to strong emotions with ill-advised inaction such as avoidance, passivity, and withdrawal (Smith et al., 2013). In other words, urgency is likely a marker of the impulsive reactivity to emotion construct described by Carver et al. (2011; Smith et al., 2013).
The theory that 5-HT functioning directly impacts impulsive reactivity to emotions would help explain why 5-HT functioning is related to divergent types of problem behaviors, thus helping to clarify “the paradox of 5-HT.” 1 This is because “impulsive” or reflexive actions in response to strong emotions could manifest as both externalizing-related (e.g., risky behavior) and internalizing-related (e.g., withdrawal) behaviors. Providing some support for the theory of impulsive responses to emotions, Carver et al. (2011) found that the 5-HTTLPR polymorphism interacted with childhood adversity to predict a latent factor reflecting the triggering of impulsive action or inaction by both positive and negative emotions (positive and negative urgency were included as indicators of this factor).
The possible link between 5-HT functioning and impulsive reactions to strong emotions (i.e., urgency) could help explain why 5-HT functioning ultimately influences problematic alcohol use. Several studies demonstrated that individuals with greater urgency exhibited greater levels of problematic alcohol use (see Smith & Cyders, 2016 for a review). Individuals with high levels of negative and positive urgency might impulsively use substances to escape from negative affect or to enhance existing positive mood, respectively (see Cooper, Frone, Russell, & Mudar, 1995).2
Thus, one purpose of the current study was to test whether negative and/or positive urgency (as markers of impulsive reactivity to emotions) mediated the relation between 5-HT functioning and alcohol problems in adolescence through young adulthood (12–21 years old). Although previous studies combined positive and negative urgency to index impulsive reactivity to emotions, in this study we test them separately because we wanted to test whether 5-HT functioning showed specificity to positive or negative urgency. If 5-HT functioning actually predicts urgency more broadly, it should predict both positive and negative urgency.
Multidimensionality of Impulsivity
The current study also tested the potential mediating role of other facets of impulsivity to assess whether 5-HT functioning showed specificity with regards to the prediction of impulsive behavior. Indeed, research suggests that impulsivity is a multidimensional construct consisting of correlated, but distinct, facets (Whiteside & Lynam, 2001). Moreover, some work suggests that 5-HT functioning is related to behavioral disinhibition not enacted in the context of emotion (Soubriè, 1986). Two studies found that tryptophan-depleted individuals with a family history of alcoholism made more commission errors on a go/no-go task and had longer stop reaction times on the Stop Task than tryptophan-depleted individuals without a family history of alcoholism (Crean, Richards, & de Wit, 2002; LeMarquand, Benkelfat, Pihl, Palmour, & Young, 1999). Netter, Hennig, and Roed (1996) also found that individuals with lower levels of 5-HT as assessed by drug-induced prolactin responses scored higher on questionnaire measures of disinhibition and novelty seeking. However, it is possible that these findings are spurious and simply reflect the association between 5-HT functioning and impulsive reactivity to emotion, given that dimensions of impulsivity are correlated. Thus, it is important to test the specificity of 5-HT functioning on different facets of impulsivity.
To do so, this study used the additional subscales of the UPPS-P, which is the scale from which positive and negative urgency are derived (Lynam et al., 2007). The UPPS-P measure contains the following five subscales: negative urgency, lack of premeditation, lack of perseverance, sensation seeking, and positive urgency. Each subscale has been refined so as to be distinct from each other scale, which is particularly useful for the current goal of assessing whether 5-HT functioning shows specificity in predicting a certain dimension(s) of impulsivity. In addition, this five-factor structure and measure has been validated in a number of samples varying in age and clinical severity (see Cyders et al, 2007; Jacob et al., 2010; Van der Linden et al., 2006). Besides positive and negative urgency, the UPPS-P subscales refer to difficulties in reflecting on consequences before engaging in certain behaviors (i.e., lack of premeditation), difficulties in remaining focused on tasks that might be difficult or boring (i.e., lack of perseverance), and the propensity to enjoy and engage in exciting activities or experiences, some of which might be dangerous (i.e., sensation seeking; Whiteside & Lynam, 2001).
In addition to the possibility that these other facets of impulsivity might be predicted by measures of 5-HT functioning, research also suggests that they are associated with various alcohol phenotypes. In a meta-analysis examining the role of UPPS-P traits in alcohol phenotypes, Coskunpinar, Dir, and Cyders (2013) found that positive urgency, negative urgency, and lack of premeditation were the most important facets in predicting alcohol problems and/or dependence. All five dimensions of impulsivity were associated with drinking frequency, and lack of perseverance was the strongest predictor of drinking quantity (Coskunpinar et al., 2013). Interestingly, these data corroborate this study’s hypotheses that negative and positive urgency are important mechanisms in the relation between 5-HT functioning and more severe and clinical alcohol phenotypes (i.e., alcohol problems). Regardless, it seemed important to test each impulsivity dimension as a potential mediator given that they all bear some relation to drinking phenotypes and could also be related to 5-HT functioning.
Age as a Potential Moderator
Genetic effects grow stronger with age (Bergen, Gardner, & Kendler, 2007), likely as a result of increasing autonomy that allows for greater genetically-influenced niche-picking, or the ability to select into one’s own environment. These self-selected environments might facilitate the expression of pre-existing genetic tendencies. For example, an adolescent with a high genetic prospensity for sensation seeking might select into a risky peer group, which might facilitate substance use. Thus, the effect of 5-HT functioning on impulsivity and alcohol problems might grow stronger with age. We tested whether 5-HT functioning was moderated by age in predicting impulsivity dimensions in 11–20 year olds and alcohol problems in 12–21 year olds. The wide age range of participants is advantageous for the study of age moderation because it might help elucidate differences among several developmental stages.
Early and mid-adolescents are also less likely to have experienced problems from their alcohol use when compared to older adolescents/young adults (Brown et al., 2008). Thus, the effect of impulsivity on alcohol problems might strengthen with age. Consistent with this notion, in a meta-analysis, Stautz and Cooper (2013) found that negative urgency predicted alcohol problems more strongly in later than in early adolescence. Thus, the current study also tested whether dimensions of impulsivity were moderated by age in predicting alcohol problems.
The Current Study
In summary, the current study tested whether five dimensions of impulsivity mediated the relation between 5-HT functioning and alcohol problems. To index 5-HT functioning, this study used results from an independent genome-wide association study on 5-HIAA in the cerebrospinal fluid (CSF, Luykx et al., 2014) to create a 5-HT polygenic score in a similar fashion to a previous study, where the score was shown to have predictive validity and demonstrated reproducibility across two samples (Wang et al., 2017). We also tested whether any of these mediated paths were moderated by age. We hypothesized that the 5-HT polygenic score would predict positive and negative urgency, but would not predict the other facets of impulsivity. Moreover, we hypothesized that the effect of the 5-HT polygenic score on positive and negative urgency would be stronger for older than for younger participants. We also hypothesized that only positive urgency, negative urgency, and lack of premeditation would predict alcohol problems. In addition, we expected that the effects of negative urgency, positive urgency and lack of premeditation would be moderated by age in predicting alcohol problems, with stronger effects at older ages. Finally, we hypothesized that the relation between the 5-HT polygenic score and alcohol problems would be mediated by both positive and negative urgency, and that these mediated effects would be stronger for older than for younger participants.
Method
Participants
Participants were drawn from a larger longitudinal study of familial alcoholism that spanned three generations (Chassin et al., 1992). The study followed adolescents (called Generation 2s or G2s) and their parents (Called Generation 1s or G1s) for three annual assessments (waves 1–3) as well as for three additional follow-up assessments separated by five year intervals (waves 4–6). During waves 4–6, siblings of G2s (also called G2s) were interviewed. G2s were, on average, 26.0 (SD = 2.2) and 32.3 years of age (SD = 2.4) at wave 5 and wave 6, respectively, and many had become parents. During waves 5 and 6, G2s’ children (called G3s), G3s’ “other” biological parents, and teachers participated in the study. Only G3s participated in three follow-up assessments (waves 7–9) occurring approximately 1.5 years, three years, and four years after wave 6. The main variables used in the current study were G3 data from the last three follow-up assessments, which are hereafter referred to as T1 (wave 7), T2 (wave 8; 1.5 years after wave 7), and T3 (wave 9; one year after wave 8). Some covariates were from wave 6, such as parental (G2) and demographic characteristics (e.g., parent substance use disorder, gender). Because G2s siblings were included, G3s were nested within families.
G3 participants were included if they had genetic data, self-reported their ethnicity as non-Hispanic Caucasian (to ensure that population stratification did not confound results), were 11–20 years old at T2, and were 12–21 at T3 (n = 238). When compared to those excluded, included participants were younger at T1 and T3, more likely to be of non-Hispanic Caucasian ancestry (consistent with inclusion criteria), had parents with higher levels of education, and self-reported higher levels of lack of premeditation (see Table 1). Included and excluded participants did not differ on any other study variables.
Table 1.
Descriptive Statistics, t-tests, χ2 tests, and Cronbach’s Alpha
| Included | Excluded | t-test | Cronbach’s Alpha |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| Continuous/Count Variables | N | M(SD) | N | M(SD) | ||
| T1 Age | 232 | 13.58(1.71) | 348 | 14.67(2.90) | 5.73* | -- |
| T2 Age | 224 | 15.13(2.49) | 325 | 15.62 (3.81) | 1.69 | -- |
| T3 Age | 208 | 16.54(2.12) | 295 | 17.71(3.18) | 4.94* | -- |
| Ancestry | 228 | 0.54(0.34) | 311 | −0.42(0.99) | −14.04** | -- |
| Parental Education | 234 | 7.52(2.26) | 540 | 6.69(2.29) | −4.67** | -- |
| 5-HT Polygenic Score | 238 | 7.77(7.79) | 332 | 7.86(8.14) | −0.13 | -- |
| Lack of Premeditation | 233 | 1.92(0.48) | 345 | 1.81(0.48) | −2.51* | 0.80 |
| Negative Urgency | 233 | 2.28(0.68) | 345 | 2.25(0.65) | −0.63 | 0.87 |
| Sensation Seeking | 233 | 2.91(0.62) | 345 | 2.84(0.65) | −1.45 | 0.80 |
| Lack of Perseverance | 233 | 1.81(0.40) | 345 | 1.75(0.41) | −1.57 | 0.72 |
| Positive Urgency | 232 | 2.14(0.74) | 345 | 2.12(0.66) | −0.50 | 0.93 |
| Alcohol Problems (Baseline, T1) | 232 | 0.14(0.66) | 348 | 0.11(0.61) | −0.61 | -- |
| Alcohol Problems (Outcome, T3) | 208 | 0.21(0.80) | 295 | 0.20(0.80) | −0.23 | -- |
|
| ||||||
| Dichotomous/Count Variables | % | % | χ2 | |||
|
| ||||||
| Gender | 238 | 51.7% males | 371 | 53.9% males | 0.29 | -- |
| Parents’ SUD | 237 | 52.3% parent with SUD | 524 | 53.6% parent with SUD | 0.11 | -- |
| Medication Use | 235 | 15.7% use prescription medications | 525 | 11.0% use prescription medications | 3.28 | -- |
Note.
p < 0.001,
p < 0.05.
SUD: Substance use disorder. Parental Education: 1 = 8th grade or less, 2 = some high school, 3 = GED (high school equivalency diploma), 4 = high school graduate, 5 = some vocational or technical school, 6 = completed vocational or technical school, 7 = some college, 8 = AA degree (2 year college), 9 = BA or BS (4 year college degree), 10 = some graduate or professional school, 11 = completed graduated or professional school.
Recruitment and Procedures
Court records, HMO wellness questionnaires, and community telephone surveys were used to recruit G1 and G2 children of alcoholic (COA) families. Reverse directories were used to recruit non-COA families who lived in the same neighborhoods as COA families. Non-COA families were matched to COA families on children’s age, ethnicity, family composition, and socioeconomic status. See Chassin, Barrera, Bech, and Kossak-Fuller (1999) for more details.
At T1, G3 participants were interviewed via the telephone. At T2, which occurred approximately 1.5 years after T1, G3 participants completed an online survey. At T3, which occurred approximately one year after T2, G3 participants were again interviewed via the telephone. Genetic samples were obtained either during the wave 6 home visit or via mail. Informed consent was obtained from G3 participants who were no longer minors and informed assent was obtained from G3 participants who were minors. Procedures were approved by the university’s institutional review board.
Genotyping
Genomic data were collected using Oragene collection kits (cheek brushing or saliva samples). DNA extraction, standardization and plating were completed in the Washington University School of Medicine Department of Psychiatry and genotyping was completed at the Washington University Genome Sequencing Center. The Illumina Golden Gate technology was used to design 1536 SNPs for genotyping. This drew on a previous collaboration (Hodgkinson et al., 2008) but added substitutions to reflect advances in the literature. Ambiguous genotype calls were ruled out by examining cluster plots. We excluded participants with incorrect gender assignments (i.e., sex as indicated by genetic data did not match self-reported sex, which might indicate that samples were labeled with incorrect identifying information), cryptic relatedness (i.e., kinship among participants that was unknown to the investigator), sample swaps, and/or Mendelian inconsistencies (N = 5). SNPs with low call rates (<95%), deviations from Hardy-Weinberg equilibrium (HWE; p<10−6) and minor allele frequencies < 2% were deleted.
Measures
Descriptive statistics are shown in Table 1.
Demographics
Participants self-reported their gender (0 = female, 1 = male), age, and ethnicity.
Parent substance use disorder (SUD)
At wave 6, participants’ biological parents reported their lifetime alcohol and drug abuse or dependence by DSM-IV criteria using the Computerized Diagnostic Interview Schedule (Robins et al., 2000). Non-interviewed parents were assessed using spousal reports on the Family History Research Diagnostic Criteria (Endicott, Andreasen, & Spitzer, 1975). If at least one biological parent met criteria for a lifetime alcohol or drug use disorder, participants were coded ‘1’ for parent SUD. All others were coded ‘0.’
Parental education
Parents’ highest level of education at wave 6 was used as an indicator of socioeconomic status. This was used as a continuous covariate.
Prescription medication use
At wave 6, parents reported on the participants’ use of prescription medications, excluding antibiotics or allergy medication [1 = Yes, 0 = No]. Prescription medication use was included as a covariate because many psychotropic medications influence neurotransmitter levels in the brain, including 5-HT.
Ancestry
Ancestry, measured using genetic markers, was included as a covariate. Although we only included self-identified non-Hispanic Caucasian participants, this covariate was still used to control for population admixture. To create the ancestry variable, we used 37 SNPs that have been shown to distinguish between non-Hispanic Caucasian and Mexican/Mexican-American ancestry (Tian, Gregersen, & Seldin, 2008), which are the two most highly represented ethnic groups in the current sample as well as in the geographic region of data collection. The SNPS were subjected to a principal components analysis, which is a commonly used dimension reduction technique in genetic studies to infer continuous axes of genetic variation (Tian et al., 2008). The first component explained 18.99% of the variance (eigenvalue = 7.03), whereas the second and third components explained 3.36% (eigenvalue = 1.24) and 3.11% (eigenvalue = 1.02), respectively. Thus, 32 SNPs that loaded onto the first principal component at 0.30 or greater were used as indicators of a one-factor model. The model fit the data well: χ2(464) = 824.99, p < 0.001, RMSEA = 0.03, CFI = 0.94, SRMR = 0.03. Factor scores were used as a covariate. These scores were highly correlated with self-reported ethnicity (r = −0.83, p < 0.001), confirming the validity of the ancestry measure and the use of these SNPs to control for ancestry. Higher scores on the ancestry variable reflect higher levels of Caucasian (as opposed to Mexican/Mexican-American) ancestry.
5-HT polygenic score
The 5-HT polygenic score was formed using results from a previous genome-wide association study (GWAS) of 5-HIAA concentrations in the CSF among 414 European participants between the ages of 18–60 years (Luykx et al., 2014).3 Thus, the polygenic score indexed the genetic propensity for differing levels of 5-HIAA in the CSF.
A commonly used approach to polygenic scoring is to create several scores using various p-value thresholds (e.g., 0.50, 0.10, 0.05, 0.001, etc), and to select the score with the strictest p-value that also maximizes the variance explained in the phenotype of interest (Evans et al., 2013). Unfortunately, we could not take this approach because the current study did not measure 5-HT functioning (i.e., phenotype of interest). Thus, SNPs were included in the score if they passed an a priori threshold of p < 0.05 in the discovery GWAS (Arpana Agrawal, personal communication). This threshold was chosen because it might better capture polygenicity than would a strict genome-wide significance threshold. It was also chosen because it is relatively conservative when compared to thresholds used in other studies (e.g., p < 0.50; Hamshere et al., 2013). Indeed, such liberal thresholds have recently been shown to increase the likelihood of spurious associations and Type I error (Evans et al., 2013). Note that these polygenic scoring methods are identical to those used in a previous study (Wang et al., 2017).4 See Wang et al., (2017) for more extensive details and further justifications for this approach.
We excluded palindromic SNPs due to strand ambiguity and used a pairwise r2 threshold of 0.25 within a 200-SNP sliding window to ensure that the SNPs were independent (Purcell et al., 2009). After applying these criteria and the p < 0.05 threshold, 26 SNPs remained. We computed 5-HT polygenic scores by weighting each SNP by its GWAS beta coefficient and summing these figures. We coded the score such that higher levels of the score indicated lower levels of 5-HIAA in the CSF (i.e., lower 5-HT functioning). See Supplementary Table 1 for a list of included SNPs.
Five facets of impulsivity
At T2, participants self-reported [1 = Agree Strongly, 4 = Disagree Strongly] on their impulsivity using the UPPS-P (Lynam et al., 2007). The five subscales measured included negative urgency (e.g., “When I feel bad, I often do things I later regret in order to make myself feel better now”), positive urgency (e.g., “When I am very happy, I can’t stop myself from going overboard”), lack of premeditation (e,g., “I tend to blurt out things without thinking”), lack of perseverance (e,g., “I like to see things through to the end”), and sensation seeking (e,g., “I like new, thrilling things to happen”). No items referenced substance use. Items within each subscale were averaged. See Table 1 for descriptive and reliability statistics.
Alcohol problems
At T1 and T3, participants self-reported on 18 alcohol consequences and 21 alcohol dependence symptoms [0 = No, 1 = Yes]. At T1, participants reported on whether they experienced these symptoms in their lifetime and at T3, participants reported on whether they experienced these symptoms in the past year (to ensure that we were assessing alcohol problems that occurred after the T2 measurement of impulsivity). These measures were summed to form a measure indexing alcohol problems. Some items included, “Have you ever gotten complaints from your family because of your alcohol use,” “Did you ever get in trouble at school or work because of your alcohol use,” and “Have you ever found that you needed larger amounts of alcohol to get an effect?” The T1 measure of alcohol problems was used as a covariate so that the T2 impulsivity measures predicted change in alcohol problems. Note that T1 alcohol problems were assessed approximately 1.5 years prior to T2 and was the most proximal alcohol assessment to T2. Unfortunately, alcohol problems were not assessed at T2.
Data Analytic Plan
Structural equation modeling was conducted using Mplus version 7.2 (Muthén & Muthén, 1998–2012). Models were analyzed using maximum likelihood estimation with robust standard errors as well as full information maximum likelihood to estimate missing data. We used the TYPE=COMPLEX command in Mplus to account for the fact that G3 participants were nested within G1 families. We centered all predictors and covariates to reduce non-essential multicollinearity.
Data plan for main effect model
The first structural equation model only analyzed main effects. All covariates except T1 alcohol problems (gender, age, parental education, parental SUD, prescription medication use, and ancestry) and the 5-HT polygenic score were specified as predictors of each facet of impulsivity. Next, each facet of impulsivity, all covariates (including T1 alcohol problems), and the 5-HT polygenic score were specified as predictors of T3 alcohol problems. The impulsivity facets, which are mediating variables, were all specified to be correlated with one another.
Data plan for interaction effects model
The second structural equation model added all predictor-by-age interaction terms to the main effects model. Thus, a 5-HT polygenic score-by-age interaction (cross-product) was added in predicting all five facets of impulsivity and alcohol problems. All five facets of impulsivity-by-age (five separate cross-products) interactions were added in predicting T3 alcohol problems. All other aspects of the model were identical to the main effect model. Interaction effects were examined using simple slope analyses, which were probed at 1 SD below the mean, at the mean, and 1 SD above the mean of each continuous predictor (Aiken & West, 1991).
Several steps were taken to ensure that any significant interactions were not spurious. First, for any significant predictor-by-age interactions, we added all relevant predictor-by-covariate and all age-by-covariate interactions in line with recommendations by Keller (2014). This is the proper way to control for the influence of confounding variables in interaction effects. Second, for any significant 5-HT polygenic score-by-age interactions, we also re-tested these interactions following monotone transformation of the interacting variables to rule out spuriousness related to scaling (Young-Wolff, Enoch, & Prescott, 2011).
Data plan for modeling alcohol problems
We also conducted preliminary analyses to determine the best way to model T1 and T3 alcohol problems, given that these are both count variables with high percentages of zeroes (T1: 91.4% had no alcohol problems; T3: 89.9% had no alcohol problems). We tested a model where only covariates (including T1 alcohol problems) predicted T3 alcohol problems. We alternatively tested this model using a zero-inflated poisson, zero-inflated negative binomial, poisson, negative binomial, or negative binomial hurdle specifications. The negative binomial model produced the lowest Akaike and Bayesian Information Criteria and the highest −2 log likelihood (i.e., it was the best fitting model) and was chosen.
Data plan for testing mediation and moderated mediation
Mediation was tested using the model indirect command in Mplus (delta method). If any paths in the mediational chain were moderated by age, we tested moderated mediation by calculating simple slopes for each path by age and examining mediation effects at differing levels of age.
Results
Zero-Order Correlations
Zero-order correlations are shown in Table 2. Higher scores on the 5-HT polygenic score (indexing lower 5-HT functioning) were significantly correlated with greater levels of negative urgency and greater levels of T1 and T3 alcohol problems. Greater levels of negative urgency and positive urgency were significantly correlated with greater levels of T3 alcohol problems. Note that age was significantly correlated with several of the covariates, highlighting the need to add predictor-by-covariate and age-by-covariate interaction terms to control for confounding influences. The five facets of impulsivity were significantly correlated with one another for the most part, except lack of perseverance did not significantly correlate with sensation seeking or positive urgency. T1 and T3 alcohol problems were also significantly correlated. Participants whose parents had an SUD had greater levels of lack of premeditation, negative urgency, positive urgency, and T1 alcohol problems. Males only differed from females on sensation seeking in terms of the main predictors.
Table 2.
Correlations Among Study Variables
| 5-HT Polygenic Score |
Age | Lack of Premeditation |
Negative Urgency |
Sensation Seeking |
Lack of Perseverance |
Positive Urgency |
T3 Alcohol Problems (Outcome) |
T1 Alcohol Problems (Control) |
Gender | Parent SUD |
Ancestry | Parent Education |
Prescription Medication |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT Polygenic Score | 1 | |||||||||||||
| Age | 0.11 | 1 | ||||||||||||
| Lack of Premeditation | −0.06 | −0.16* | 1 | |||||||||||
| Negative Urgency | 0.19** | 0.13 | 0.38** | 1 | ||||||||||
| Sensation Seeking | 0.01 | 0.09 | 0.26** | 0.17** | 1 | |||||||||
| Lack of Perseverance | −0.07 | −0.23** | 0.48** | 0.18** | −0.01 | 1 | ||||||||
| Positive Urgency | 0.09 | 0.01 | 0.28** | 0.62** | 0.26** | 0.11 | 1 | |||||||
| T3 Alcohol Problems (Outcome) | 0.28** | 0.28** | 0.03 | 0.23** | 0.11 | −0.07 | 0.18* | 1 | ||||||
| T1 Alcohol Problems (Control) | 0.14* | 0.23** | 0.13* | 0.22** | 0.10 | 0.04 | 0.13* | 0.18** | 1 | |||||
| Gender | −0.08 | −0.16* | 0.10 | −0.004 | 0.23** | 0.08 | 0.01 | −0.003 | −0.10 | 1 | ||||
| Parent SUD | 0.01 | 0.13 | 0.13* | 0.18** | 0.08 | −0.02 | 0.18** | −0.03 | 0.15* | −0.10 | 1 | |||
| Ancestry | −0.10 | −0.19** | −0.06 | −0.13 | −0.08 | −0.001 | −0.17* | −0.15* | −0.09 | 0.00 | −0.08 | 1 | ||
| Parent Education | −0.02 | 0.03 | −0.12 | −0.11 | −0.03 | −0.09 | −0.11 | 0.02 | −0.21** | 0.07 | −0.22** | 0.10 | 1 | |
| Prescription Medication | −0.03 | 0.15* | 0.04 | 0.12 | 0.05 | −0.08 | 0.11 | 0.11 | 0.05 | 0.12 | 0.09 | −0.12 | 0.02 | 1 |
Note.
p < 0.01,
p < 0.05.
n = 238. All impulsivity facets were measured at T2. SUD: Substance use disorder.
Structural Equation Modeling
Main effect model results
See Table 3 and Figure 1 for results and see Supplementary Table 2 for effect sizes. Regarding the prediction of the five facets of impulsivity by the 5-HT polygenic score, only negative urgency was significantly predicted by the 5-HT polygenic score. The association was such that greater levels of the 5-HT polygenic score (indexing lower levels of 5-HT functioning) were associated with greater levels of negative urgency. The size of this effect is considered small (f2 = 0.03; Cohen 1977, 1988; See Supplementary Table 2). In addition, we tested whether the prediction of impulsivity facets by the 5-HT polygenic score were significantly different from one another using the Wald χ2 test. The effect of the 5-HT polygenic score on negative urgency was significantly different from its effect on lack of perseverance, sensation seeking, and lack of premeditation (χ2(1) range = 4.43–9.82, p’s < 0.05), but not significantly different from its effect on positive urgency (χ2(1) = 2.32, p = 0.13).
Table 3.
Structural Equation Modeling Results
| Mediators | Outcome | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Lack of Premeditation | Negative Urgency | Sensation Seeking | Lack of Perseverance | Positive Urgency | T3 Alcohol Problems | |||||||
|
| ||||||||||||
| Predictors | b(SE) | 95% C.I. | b(SE) | 95% C.I. | b(SE) | 95% C.I. | b(SE) | 9 5% C.I. | b(SE) | 9 5% C.I. | b(SE) | 9 5% C.I. |
| Gender | 0.08(0.06) | −0.04, 0.20 | 0.04(0.08) | −0.12, 0.19 | 0.33(0.08)** | 0.17, 0.49 | 0.04(0.05) | −0.06, 0.15 | 0.03(0.09) | −0.14, 0.20 | 0.05(0.49) | −0.91, 1.01 |
| Age | −0.03(0.01)* | −0.06, −0.01 | 0.02(0.02) | −0.02, 0.05 | 0.03(0.02 )† | −0.004, 0.06 | −0.04(0.01)* | −0.06, −0.01 | −0.02(0.02) | −0.05, 0.02 | 0.92(0.24)** | 0.44, 1.39 |
| Parents’ SUD | −0.13(0.06)* | 0.01, 0.25 | 0.19(0.09)* | 0.02, 0.36 | 0.11(0.09) | −0.08, 0.29 | 0.00(0.06) | −0.11, 0.11 | 0.23(0.11)* | 0.02, 0.43 | −0.15(0.56) | −1.24, 0.95 |
| Ancestry | −0.10(0.11) | −0.31, 0.11 | −0.13(0.16) | −0.45, 0.19 | −0.09(0.13) | −0.34, 0.16 | −0.06(0.09) | −0.23, 0.12 | −0.31(0.14)* | −0.58, −0.04 | 0.12(0.59) | −1.03, 0.17 |
| Parents’ Education | −0.02(0.02) | −0.05, 0.01 | −0.02(0.02) | −0.06, 0.01 | −0.01(0.02) | −0.04, 0.02 | −0.02(0.01) | −0.04, 0.01 | −0.02(0.02) | −0.06, 0.02 | 0.10(0.13) | −0.15, 0.35 |
| Prescription Medication Use | 0.05(0.09) | −0.13, 0.22 | 0.18(0.14) | −0.10, 0.45 | −0.03(0.12) | −0.25, 0.20 | −0.06(0.06) | −0.19, 0.06 | 0.18(0.13) | −0.07, 0.43 | 0.37(0.58) | −0.77, 1.51 |
| 5-HT Polygenic Score | −0.003(0.004) | −0.01,0.01 | 0.02(0.01)* | 0.004, 0.03 | 0.00(0.01) | −0.01, 0.01 | −0.002(0.004) | −0.01, 0.01 | 0.01(0.01) | −0.004, 0.02 | 0.14(0.92)** | 0.07, 0.21 |
| T1 Alcohol Problems | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0.08(0.19) | −0.44, 0.29 |
| Lack of Premeditation | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0.79(0.66) | −0.50, 2.08 |
| Negative Urgency | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 1.39(0.47)* | 0.46, 2.31 |
| Sensation Seeking | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0.76(0.40)† | −0.03, 1.55 |
| Lack of Perseverance | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | −0.39(0.78) | −1.93, 1.14 |
| Positive Urgency | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | −0.66(0.40)† | −1.44, 0.12 |
Note.
p < 0.001,
p < 0.05,
p < 0.10.
n = 238. Unstandardized coefficients are shown. SUD: Substance use disorder.
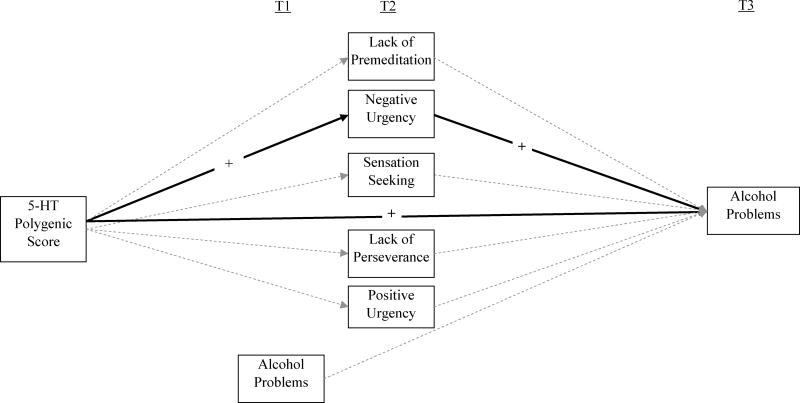
Figure 1.
Structural Equation Model. Black lines indicate significant paths. Grayed, dotted lines indicate non-significant or marginally significant paths. For significant paths, the plus signs refer to the direction of the association.
Significant predictors of T3 alcohol problems included the 5-HT polygenic score and negative urgency. The percent change in the incident rate of alcohol problems was a 15% increase for every unit increase in the 5-HT polygenic score (which equates to approximately 1/3-1/2 of a risk allele; incidence rate ratio = 1.15). The percent change in the incident rate of alcohol problems was a 299% increase for every unit increase in the negative urgency score (which equates to approximately 1 point on a likert scale averaged across all negative urgency items or 1.5 SDs; incidence rate ratio = 3.99). In addition, sensation seeking marginally significantly predicted T3 alcohol problems. These associations were such that greater levels of the 5-HT polygenic score, negative urgency, and sensation seeking were associated with greater levels of alcohol problems. Positive urgency also marginally significantly predicted T3 alcohol problems. Unexpectedly, this association was such that greater levels of positive urgency predicted fewer T3 alcohol problems.
Interaction effect model results
After entering interaction cross-products with age, we found that the polygenic score-by-age and lack of premeditation-by-age interactions were significant in predicting alcohol problems. However, after adding in the relevant predictor-by-covariate and age-by-covariate interactions to control for confounding influences, all interactions either became non-significant or, upon further probing, none of the simple slopes were significant. Thus, it appears that these interactions were not robust to the confounding influences of the other covariates. All interaction terms were removed for this reason.
Mediation results
T2 negative urgency significantly mediated the relation between the 5-HT polygenic score and T3 alcohol problems (indirect effect=0.55, p = 0.03). Positive urgency, sensation seeking, lack of premeditation, and lack of perseverance did not significantly mediate the relation between the 5-HT polygenic score and alcohol problems (indirect effect range = −0.13–0.02, ps = ns). To strengthen conclusions regarding unique mechanisms underlying 5-HT polygenic score and alcohol problems, we calculated whether the mediated effect involving negative urgency differed significantly from the other mediated effects using the Wald χ2 test. The mediated effect involving negative urgency significantly differed from the mediated effect involving positive urgency (χ2(1) = 4.02, p = 0.045), lack of premeditation (χ2(1) = 6.12, p = 0.01), sensation seeking (χ2(1) = 4.11, p = 0.04), and lack of perseverance (χ2(1) = 4.40, p = 0.04). Calculations of the proportion of the mediated effect (using standardized coefficients) showed that approximately 8.9% of the total effect of the 5-HT polygenic score on alcohol problems was through negative urgency.
Alternative analyses
This study’s method, design, and analytic plan were not pre-registered. Therefore, we report other analyses that were conducted, results, and the rationale for such analyses in the Supplementary Materials.
Discussion
This study sought to test mechanisms in the relation between 5-HT functioning (as indexed by a polygenic score) and alcohol problems. Partially consistent with original hypotheses, our results suggested that negative urgency might be an important mediating variable that underlies the relation between a 5-HT polygenic score and alcohol problems in adolescence through emerging adulthood. In contrast, the other dimensions of impulsivity, including positive urgency, lack of perseverance, lack of premeditation, and sensation seeking, did not mediate the relation between 5-HT polygenic scores and future alcohol problems.
5-HT Polygenic Score and Facets of Impulsivity
Our finding that higher 5-HT polygenic scores (indexing lower 5-HT functioning) predicted greater negative urgency is partially in line with one previous study that showed that the 5-HTTLPR polymorphism predicted a latent factor representing impulsive reactivity to strong emotions (which contained negative and positive urgency as indicators) at high levels of childhood adversity (Carver et al., 2011). Our results are also consistent with a previous investigation that studied a rat model of negative urgency. Yates et al. (2015) found that serotonin transporter (SERT) uptake in the orbitofrontal cortex was positively correlated with rats’ greater negative urgency behaviors. In other words, increased SERT activity, which likely leads to decreased extracellular 5-HT, was associated with greater levels of negative urgency.
We found that the 5-HT polygenic score predicted negative urgency, but not positive urgency. This finding was unexpected; theories and previous research have suggested that 5-HT functioning plays a role in individuals’ overall urgency (combination of negative and positive urgency; Carver et al., 2011; Cyders & Smith, 2008). However, the effect of the 5-HT polygenic score on negative urgency was not significantly different from its effect on positive urgency. Thus, it is possible that the 5-HT polygenic score would have had an effect on positive urgency with a greater sample size, a polygenic score with a greater number of variants, or a different measure of positive urgency.
Alternatively, despite a lack of statistically significant differentiation between these effects, it is possible that the effect of 5-HT functioning is specific to negative, not positive, urgency. Although negative and positive urgency are often moderately-to-highly correlated (in this study, r = 0.62), research also shows that they are distinct constructs. Thus, genetic and neural markers might predict these two facets of impulsivity differently in some cases. Consistent with this notion, social drinkers’ levels of negative urgency, but not positive urgency, were associated with their neural responses to alcohol cues in the ventromedial prefrontal cortex (Cyders et al., 2014) and to negative images in the right lateral orbitofrontal cortex and left amygdala (Cyders et al., 2015). If 5-HT functioning is a specific risk factor for negative urgency, this would suggest that perhaps 5-HT functioning confers transdiagnostic risk specifically due to dysregulation of behavior under negative affect. This model would still be consistent with the previous literature on the role of 5-HT functioning in various types of psychopathology, because many of the pathologies linked with 5-HT functioning have shown specific links with negative urgency (Coskunpinar et al., 2013; Smith & Cyders, 2016; Smith et al., 2013). Nonetheless, our results suggest that further research is needed to understand whether 5-HT functioning is a general predictor of overall urgency or a specific predictor of negative urgency.
Aligning with study hypotheses, we also found that the 5-HT polygenic score was not related to any of the other facets of impulsivity, including lack of premeditation, sensation seeking, and lack of perseverance, and these effects were significantly different from the effect of the 5-HT polygenic score on negative urgency. This provides some confidence that genetically-influenced 5-HT functioning might be a specific risk factor for negative urgency (or urgency more broadly). Previous studies have found associations between indices of 5-HT functioning and other dimensions of impulsivity that are not dependent upon strong negative or positive emotions (e.g., go/no-go task, stop task, novelty seeking, disinhibition; Crean et al., 2002; LeMarquand et al., 1999; Netter et al., 1996). However, perhaps these associations were due to the fact that impulsivity dimensions tend to be correlated. In other words, the tasks and measures used in previous studies might have picked up on negative urgency to a greater extent than did the other UPPS-P impulsivity facets used in the current study. Indeed, the UPPS-P measures were intentionally created so that each impulsivity facet reflected a unidimensional construct, whereas previous questionnaires and tasks measuring impulsivity might have contained more overlap with negative urgency. In sum, our study shows greater specificity of the 5-HT polygenic score on impulsivity in the context of strong emotions (particularly negative emotions) when compared to facets of impulsivity that do not involve emotion.
Impulsivity Facets and Alcohol Problems
We also found that individuals with greater levels of negative urgency tended to have greater alcohol problems, even after controlling for previous alcohol problems. This finding has been widely replicated across studies (e.g., Coskunpinar et al., 2013; Smith & Cyders, 2016). Individuals with high levels of negative urgency are likely prone to rashly engaging in problematic drinking to alleviate or escape from negative emotions. We also expected that positive urgency and lack of premeditation, but not sensation seeking or lack of perseverance, would predict alcohol problems based on a previous meta-analysis (Coskunpinar et al., 2013). Contrary to these hypotheses, we found that none of these impulsivity facets significantly predicted alcohol problems. However it is noteworthy that, in our study, negative urgency, positive urgency, and lack of premeditation were predicted by parent SUD, whereas lack of perseverance and sensation seeking were not. This pattern of associations is consistent with the aforementioned meta-analysis. Perhaps negative urgency, positive urgency, and lack of premeditation are markers of heightened risk for more severe alcohol outcomes in our sample, such as alcohol problems. It is possible that positive urgency and lack of premeditation did not predict alcohol problems in our sample because the participants were still quite young and had not fully progressed to the stage of experiencing problems and consequences from their use (Mage=15.13).
Note that, in the final model, lower levels of positive urgency were related to greater levels of alcohol problems (but this effect was marginally significant; 0.05 < p < 0.10). This finding is inconsistent with other studies and with our zero-order correlations, which both showed that greater levels of positive urgency were related to greater alcohol use (see Table 2 and Coskunpinar et al., 2013). Because of the moderate-to-high zero-order correlation between negative and positive urgency, this raises concerns that including both variables in the prediction model introduced problems with multicollinearity, and thus possibly influenced the validity of each predictor coefficient. However, the variance inflation factors of negative urgency (1.92) and positive urgency (1.74) were well below the recommended cut-off of < 7 (Neter, Wasserman, & Kutner, 1989), suggesting that multicollinearity was not a concern. To better understand whether including negative urgency caused the sign reversal of positive urgency on alcohol problems, we also tested a model that removed negative urgency as a predictor of alcohol problems. Analyses showed that positive urgency no longer had a negative effect on alcohol problems, but the effect was not statistically significant (b = 0.05, ns). Thus, the inclusion of negative urgency appears to cause the (non-significant) sign reversal of positive urgency on alcohol problems, but does not account for the lack of a significant relation between greater positive urgency and greater alcohol problems.
Indirect Effect of the 5-HT Polygenic Score on Alcohol Problems
Consistent with hypotheses, we also found that higher 5-HT polygenic scores (indexing lower 5-HT functioning) significantly and prospectively predicted greater alcohol problems through greater negative urgency. Note that this mediated effect significantly differed from the other mediated effects (i.e., through other impulsivity facets), strengthening conclusions that negative urgency is a unique mediator of the association between the 5-HT polygenic score and alcohol problems. This finding enhances our understanding of the mechanisms underlying biological markers and alcohol problems, and therefore, helps clarify the heterogeneity of alcohol use disorders and personalized treatment (Cuthbert & Insel, 2013; Litten et al., 2015). Specifically, this result elucidates one possible unique pathway to alcohol problems, in which individuals with lower levels of genetically-influenced 5-HT functioning are more likely to have high levels of negative urgency, which subsequently serves to increase the risk that they will experience alcohol problems. Individuals following this risk pathway might benefit from alcohol use treatments that specifically focus on skills to regulate negative urgency and strategies to respond to or modify the contexts that elicit greater negative urgency.
Researchers have posited that 5-HT functioning might also indirectly predict other types of psychopathology, such as depression and aggression/antisociality, through negative urgency (e.g., Carver et al., 2011). Unfortunately, we were unable to test whether negative urgency mediated the relation between the 5-HT polygenic score and depression and aggression/antisociality. This is because all other forms of symptomatology (other than alcohol use) were measured prior to the assessment of the UPPS-P impulsivity scales in our study. Interestingly, a post-hoc exploratory analysis showed that negative urgency was significantly correlated with adolescents’ aggression/antisociality and depression measured at an earlier time point. In addition, the 5-HT polygenic score predicted adolescents’ aggression/antisociality and depression in a previous investigation of the participants in this larger longitudinal study and another longitudinal sample (for certain demographic subgroups; Wang et al., 2017). Thus, our data support the possibility that 5-HT functioning (as indexed by this 5-HT polygenic score) and negative urgency might be transdiagnostic risk factors that could help explain the co-occurrence among different psychological problems.
Direct Effect of the 5-HT Polygenic Score on Alcohol Problems
After controlling for covariates and the five facets of impulsivity, the 5-HT polygenic score still had a direct effect on alcohol problems. This suggests that genetically-influenced variation in 5-HT functioning might also predict alcohol problems through additional mechanisms. Although many potential mechanisms might exist, one plausible candidate is low levels of response to the acute effects of alcohol. Indeed, Heinz et al (2001) found that 5-HT functioning might be implicated in individuals’ low levels of response to alcohol, and this vulnerability is a known risk factor for problematic alcohol use (Schuckit, 1994). Future research should explore whether the relation between 5-HT functioning and alcohol phenotypes operates through several distinct mechanisms and should attempt to identify these mechanisms.
Moderation by Age
We found that the 5-HT polygenic score and lack of premeditation interacted with age to predict alcohol problems. However, these interactions were not robust after controlling for confounding influences (see Keller, 2014). We tested age interactions using an age-heterogeneous sample because previous studies suggested that they might exist (Bergen et al., 2007; Stautz & Cooper, 2013). Results suggest that the effect of the 5-HT polygenic score on impulsivity facets and alcohol problems, and the effect of impulsivity facets on alcohol problems, do not differ based on age.
Limitations and Strengths
Our study had several limitations. Our polygenic score only contained 26 SNPs. Future studies that have genome-wide data should create polygenic scores that include more genetic variants. Moreover, future studies should include other types of genetic variants in their analyses, such as insertions/deletions, variable number tandem repeats, and rare variants. Given the small effect sizes of most genetic factors, future research using larger samples should be conducted to replicate this work. It is also possible that, with the current sample size, some true effects were not detected.
In addition, the wide age range of the sample within time points was a limitation. Although we tested age interactions, we note that our sample size might have impacted our ability to detect age interactions and mediated effects as well. Post-hoc Monte Carlo simulation studies that accounted for nesting of participants showed that our sample was well-powered (> 0.80) to detect age interactions of small-medium effect sizes in predicting impulsivity (standardized coefficients ranged from 0.20–0.22) and interactions of medium effect sizes in predicting alcohol problems (standardized coefficients ranged from 0.35–0.39). Based on power calculations conducted in Fritz and Mackinnon (2007), our sample was well-powered (> 0.80) to detect mediated effects wherein the effect sizes of the a or b paths were between small-and-medium, medium, or large (standardized coefficients greater than 0.26). Thus, findings should be considered within the context of these power limitations.
All measures were self-reported by participants because, unfortunately, no other informant reports were available. Incorporating information from other reporters and different methodologies in the future would strengthen the conclusions from this study. Only non-Hispanic Caucasian participants were included, which is both a limitation and a strength. Doing so limits the generalizability of findings to other ethnic groups. On the other hand, the original GWAS on which our polygenic score was based only included European participants, and thus, at least some of the identified SNPs might be specific to CSF 5-HIAA levels in European individuals.
Our study also had several strengths. This study created a polygenic score using an independent GWAS, and this polygenic score predicted several behavioral phenotypes in ways that are consistent with previous theories and research. This is notable given the difficulties with replication in the genetics literature (Ioannidis et al., 2001). Note that a very similar polygenic score was also shown to predict alcohol use (indirectly, and for certain subgroups) in another independent, longitudinal sample of adolescents (Wang et al., 2017).4 Another strength was the inclusion of multiple, empirically validated, facets of impulsivity as mediators. We also used a longitudinal and prospective design by controlling for previous alcohol problems. We note that the baseline measure of alcohol problems was assessed approximately 1.5 years prior to the T2 impulsivity measures and that it would have been preferable to use a measurement of alcohol problems at T2 if it had been possible. Thus, the predictive influence of negative urgency on T3 alcohol use might appear greater in the current study than if we had controlled for T2 alcohol use. Finally, this sample was well-suited to test the question of mechanisms to alcohol problems given that participants were at high-risk for alcohol use disorders due to familial history.
Conclusions
This study identified negative urgency as a possible mediating mechanism in the relation between a 5-HT polygenic score (indexing 5-HT functioning) and alcohol problems. We did not find that other facets of impulsivity (positive urgency, lack of perseverance, lack of premeditation, and sensation seeking) acted as mediators. Thus, genetically-influenced variation in 5-HT functioning does not appear to simply influence impulsivity more broadly, but shows some specificity to negative urgency. However, results showed that more research should be conducted on the role of 5-HT functioning in positive urgency. Moreover, the 5-HT polygenic score still had a direct effect on alcohol problems, suggesting that genetically-influenced variation in 5-HT functioning might influence other intermediate phenotypes that subsequently serve to increase risk for problematic alcohol use. Thus, the role of 5-HT functioning on alcohol phenotypes still remains to be more clearly elucidated. When considering the broader literature, our results suggest that genetically-influenced variation in 5-HT functioning might influence a host of psychological problems through its influence on impulsivity in the context of strong negative emotions.
Supplementary Material
Acknowledgments
This work was supported by Grants AA016213 and AA022097 to Laurie Chassin and AA023128 to Frances Wang from the National Institute on Alcohol Abuse and Alcoholism. Genotyping was supported by the Midwest Alcohol Research Center (P50 AA011998).
Footnotes
Author Contributions
F.L.W. developed the study concept, performed data analyses, and drafted the paper under the supervision of L.C. F.L.W. and L.C. both contributed to the interpretation and refinement of data analyses and L.C. provided critical revisions. Both authors approved the final version of the paper for submission.
Declaration of Conflicting Interests
The authors declared no conflicts of interest with respect to the authorship or the publication of this article.
It is important to note that this theory is similar in some ways to Carver et al. (2008)’s original theory that 5-HT functioning is related to poor self-regulation, which then unmasks preexisting tendencies towards approach or avoidance. Indeed, both of these theories emphasize the dysregulation of bottom-up control tendencies (i.e., approach and avoidance). However, the theory set forth by Carver et al. (2011) is distinct from the original theory because it posits that 5-HT functioning is related to impulsivity (a bottom-up form of control; Nigg, in press) and includes strong emotion as a precipitating factor, whereas the original theory posits that 5-HT functioning is related to the top-down regulatory systems that serve to control impulses and emotions.
Note that the present review of the literature suggests that it would be very interesting to test certain internalizing and externalizing problems as outcomes, in addition to alcohol problems. Unfortunately we were unable to do so because substance use outcomes were the only symptoms assessed after the measurement of urgency in the current study.
5-HIAA levels in the CSF have been shown to be associated with levels of 5-HT in the brains of post-mortem humans and non-human primates (Huggins et al., 2012; Stanley et al., 1985). This suggests that the polygenic score likely reflects individual genetic differences in 5-HT functioning in the brain as well. Research on non-human primates also shows that individual differences in CSF 5-HIAA are heritable and highly stable from infancy to early adulthood (Higley, Suomi, & Linoilla, 1992; Higley et al., 1993, 1996). This suggests that a GWAS of 18–60 year olds likely also reflects adolescents’ and emerging adults’ individual genetic differences in CSF 5-HIAA. Thus, the older age of the GWAS sample when compared to the current study sample is likely not problematic for creating polygenic scores.
Polygenic scores were slightly different between Wang et al. (2017) and the current study despite studying the G3 AFDP participants in both studies. This is because in Wang et al. (2017), genetic variants were only included in the score if they were also genotyped in the second replication sample, resulting in a polygenic score that had four fewer SNPs than in the current study.
References
- Aiken L, West S. Multiple Regression: Testing and Interpreting Interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Balldin J, Berggren U, Engel J, Eriksson M, Hård E, Söderpalm B. Effect of citalopram on alcohol intake in heavy drinkers. Alcoholism: Clinical and Experimental Research. 1994;18:1133–1136. doi: 10.1111/j.1530-0277.1994.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Goodwin FK, Major LF, Brown GL. Alcohol and central serotonin metabolism in man. Archives of General Psychiatry. 1979;36:224–227. doi: 10.1001/archpsyc.1979.01780020114013. [DOI] [PubMed] [Google Scholar]
- Banki CM. Factors influencing monoamine metabolites and tryptophan in patients with alcohol dependence. Journal of Neural Transmission. 1981;50:89–101. doi: 10.1007/BF01249132. [DOI] [PubMed] [Google Scholar]
- Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: a meta-analysis. Twin Research and Human Genetics. 2007;10.03:423–433. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Winters KC. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121:S290–S310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychological Bulletin. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J, Kim Y, Nam JY. Serotonin transporter polymorphism interacts with childhood adversity to predict aspects of impulsivity. Psychological Science. 2011;22:589–595. doi: 10.1177/0956797611404085. [DOI] [PubMed] [Google Scholar]
- Chassin L, Barrera M, Jr, Bech K, Kossak-Fuller J. Recruiting a community sample of adolescent children of alcoholics: a comparison of three subject sources. Journal of Studies on Alcohol. 1992;53(4):316–319. doi: 10.15288/jsa.1992.53.316. [DOI] [PubMed] [Google Scholar]
- Coccaro EF. Central serotonin and impulsive aggression. The British Journal of Psychiatry. 1989;155:52–62. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences (rev. ed.) New York Academic Press; 1977. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale NJ: Erlbaum; 1988. [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends in Cognitive Sciences. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. Journal of Personality and Social Psychology. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Coskunpinar A, Dir AL, Cyders MA. Multidimensionality in impulsivity and alcohol Use: a meta-analysis using the UPPS model of impulsivity. Alcoholism: Clinical and Experimental Research. 2013;37:1441–1450. doi: 10.1111/acer.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean J, Richards JB, de Wit H. Effect of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behavioural Brain Research. 2002;136:349–357. doi: 10.1016/s0166-4328(02)00132-8. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Dzemidzic M, Eiler WJ, Coskunpinar A, Karyadi K, Kareken DA. Negative urgency and ventromedial prefrontal cortex responses to alcohol cues: fMRI evidence of emotion-based impulsivity. Alcoholism: Clinical and Experimental Research. 2014;38:409–417. doi: 10.1111/acer.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Dzemidzic M, Eiler WJ, Coskunpinar A, Karyadi KA, Kareken DA. Negative urgency mediates the relationship between amygdala and orbitofrontal cortex activation to negative emotional stimuli and general risk-taking. Cerebral Cortex. 2015;25:4094–4102. doi: 10.1093/cercor/bhu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT, Spillane NS, Fischer S, Annus AM, Peterson C. Integration of impulsivity and positive mood to predict risky behavior: Development and validation of a measure of positive urgency. Psychological Assessment. 2007;19:107–118. doi: 10.1037/1040-3590.19.1.107. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Smith GT. Emotion-based dispositions to rash action: Positive and negative urgency. Psychological Bulletin. 2008;134:807–828. doi: 10.1037/a0013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue RA, Spoont MR. A Behavioral Dimension of Constraint. Annals of the New York Academy of Sciences. 1986;487:47–62. doi: 10.1111/j.1749-6632.1986.tb27885.x. [DOI] [PubMed] [Google Scholar]
- Endicott J, Anderson N, Spitzer RL. Family History Diagnostic Criteria. New York Biometrics Research, New York Psychiatric Institute; 1975. [Google Scholar]
- Ernouf D, Compagnon P, Lothion P, Narcisse G, Benard JY, Daoust M. Platelets 3H 5-HT uptake in descendants from alcoholic patients: a potential risk factor for alcohol dependence? Life Sciences. 1993;52:989–995. doi: 10.1016/0024-3205(93)90190-e. [DOI] [PubMed] [Google Scholar]
- Evans DM, Brion MJA, Paternoster L, Kemp JP, McMahon G, Munafò M TAG Consortium. Mining the human phenome using allelic scores that index biological intermediates. Plos Genetics. 2013;9:e1003919. doi: 10.1371/journal.pgen.1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farren CK, Ziedonis D, Clare AW, Hammeedi FA, Dinan TG. D-Fenfluramine-Induced Prolactin Responses in Postwithdrawal Alcoholics and Controls. Alcoholism: Clinical and Experimental Research. 1995;19:1578–1582. doi: 10.1111/j.1530-0277.1995.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;133:79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- Fritz MS, MacKinnon DP. Required sample size to detect the mediated effect. Psychological Science. 2007;18:233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongwer MA, Murphy JM, McBride WJ, Lumeng L, Li TK. Regional brain contents of serotonin, dopamine and their metabolites in the selectively bred high-and low-alcohol drinking lines of rats. Alcohol. 1989;6:317–320. doi: 10.1016/0741-8329(89)90089-x. [DOI] [PubMed] [Google Scholar]
- Hamshere ML, Langley K, Martin J, Agha SS, Stergiakouli E, Anney RJ, Thapar A. High loading of polygenic risk for ADHD in children with comorbid aggression. American Journal of Psychiatry. 2013;170:909–916. doi: 10.1176/appi.ajp.2013.12081129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Heinz A, Mann K, Weinberger DR, Goldman D. Serotonergic dysfunction, negative mood states, and response to alcohol. Alcoholism: Clinical and Experimental Research. 2001;25:487–495. [PubMed] [Google Scholar]
- Higley JD, King ST, Hasert MF, Champoux M, Suomi SJ, Linnoila M. Stability of interindividual differences in serotonin function and its relationship to severe aggression and competent social behavior in rhesus macaque females. Neuropsychopharmacology. 1996;14:67–76. doi: 10.1016/S0893-133X(96)80060-1. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biological Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Higley JD, Thompson WW, Champoux M, Goldman D, Hasert MF, Kraemer GW, Linnoila M. Paternal and maternal genetic and environmental contributions to cerebrospinal fluid monoaminemetabolites in rhesus monkeys (macaca mulatta) Archives of General Psychiatry. 1993;50:615–623. doi: 10.1001/archpsyc.1993.01820200025003. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Goldman D. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol and Alcoholism. 2008;43:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins KN, Mathews TA, Locke JL, Szeliga KT, Friedman DP, Bennett AJ, Jones SR. Effects of early life stress on drinking and serotonin system activity in rhesus macaques: 5-hydroxyindoleacetic acid in cerebrospinal fluid predicts brain tissue levels. Alcohol. 2012;46:371–376. doi: 10.1016/j.alcohol.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nature Genetics. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- Keller MC. Gene× environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biological Psychiatry. 2014;75:18–24. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob GA, Butz L, Bader K, Lieb K, Tuscher O, Stahl C. Impulsivity in borderline personality disorder: Impairment in self-report measures, but not behavioral inhibition. Psychopathology. 2010;43:180–188. doi: 10.1159/000304174. [DOI] [PubMed] [Google Scholar]
- Lee MA, Meltzer HY. Neuroendocrine responses to serotonergic agents in alcoholics. Biological Psychiatry. 1991;30:1017–1030. doi: 10.1016/0006-3223(91)90122-3. [DOI] [PubMed] [Google Scholar]
- LeMarquand DG, Benkelfat C, Pihl RO, Palmour RM, Young SN. Behavioral disinhibition induced by tryptophan depletion in nonalcoholic young men with multigenerational family histories of paternal alcoholism. American Journal of Psychiatry. 1999;156:1771–1779. doi: 10.1176/ajp.156.11.1771. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biological Psychiatry. 1994;36:326–337. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- Lesch KP. Alcohol dependence and gene x environment interaction in emotion regulation: Is serotonin the link? European Journal of Pharmacology. 2005;526:113–124. doi: 10.1016/j.ejphar.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcoholism: Clinical and Experimental Research. 2015;39:579–584. doi: 10.1111/acer.12669. [DOI] [PubMed] [Google Scholar]
- Luykx JJ, Bakker SC, Lentjes E, Neeleman M, Strengman E, Mentink L, Ophoff RA. Genome-wide association study of monoamine metabolite levels in human cerebrospinal fluid. Molecular Psychiatry. 2014;19:228–234. doi: 10.1038/mp.2012.183. [DOI] [PubMed] [Google Scholar]
- Lynam D, Smith GT, Cyders MA, Fischer S, Whiteside SA. The UPPS-P: A multidimensional measure of risk for impulsive behavior. 2007 Unpublished technical report. [Google Scholar]
- Munafò MR, Hayward G, Harmer C. Selective processing of social threat cues following acute tryptophan depletion. Journal of Psychopharmacology. 2006;20:33–39. doi: 10.1177/0269881105056667. [DOI] [PubMed] [Google Scholar]
- Muthén BO, Muthén LK. Version 7.2, Computer Software and Manual. Los Angeles, CA: Muthén and Muthén; 1998–2012. [Google Scholar]
- Neter J, Wasserman W, Kutner M. Applied Linear Regression Models. Boston, MA: Irwin; 1989. [Google Scholar]
- Netter P, Hennig J, Roed IS. Serotonin and dopamine as mediators of sensation seeking behavior. Neuropsychobiology. 1996;34:155–165. doi: 10.1159/000119318. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, et al. Association between serotonin transporter gene promoter polymorphism (5-HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Archives of General Psychiatry. 2002;59:613–620. doi: 10.1001/archpsyc.59.7.613. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On the relations among Self-Regulation, Self-control, Executive Functioning, Effortful Control, Cognitive Control, Impulsivity, Risk-Taking, and Inhibition for Developmental Psychopathology. Journal of Child Psychology and Psychiatry. doi: 10.1111/jcpp.12675. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Fraser G. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) Washington University School of Medicine. St. Louis, MO; 2000. [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. American Journal of Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Smith GT, Cyders MA. Integrating affect and impulsivity: The role of positive and negative urgency in substance use risk. Drug and Alcohol Dependence. 2016;163:S3–S12. doi: 10.1016/j.drugalcdep.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Guller L, Zapolski TC. A Comparison of Two Models of Urgency Urgency Predicts Both Rash Action and Depression in Youth. Clinical Psychological Science. 2013;1:266–275. doi: 10.1177/2167702612470647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubriè P. Reconciling the role of central serotonin neurons in human and animal behavior. Behavioral and Brain Sciences. 1986;9:319–335. [Google Scholar]
- Stanley M, Traskman-Bendz L, Dorovini-Zis K. Correlations between aminergic metabolites simultaneously obtained from human CSF and brain. Life Sciences. 1985;37:1279–1286. doi: 10.1016/0024-3205(85)90242-5. [DOI] [PubMed] [Google Scholar]
- Stautz K, Cooper A. Impulsivity-related personality traits and adolescent alcohol use: a meta-analytic review. Clinical Psychology Review. 2013;33:574–592. doi: 10.1016/j.cpr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: population substructure and genome-wide association studies. Human Molecular Genetics. 2008;17:R143–R150. doi: 10.1093/hmg/ddn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakore JH, O’keane V, Dinan TG. d-Fenfluramine-induced prolactin responses in mania: evidence for serotonergic subsensitivity. American Journal of Psychiatry. 1996;153:1460–1463. doi: 10.1176/ajp.153.11.1460. [DOI] [PubMed] [Google Scholar]
- Van der Linden M, D’Acremont M, Zermatten AN, Jermann F, Lari F, Willems S, Bechara A. A French adaptation of the UPPS Impulsive Behavior Scale: Confirmatory factor analysis in a sample of undergraduate students. European Journal of Psychological Assessment. 2006;22:38–42. doi: 10.1027/1015-5759.22.1.38. [DOI] [Google Scholar]
- Wang FL, Chassin L, Bates JE, Dick D, Lansford JE, Pettit GS, Dodge KA. Serotonin Functioning and Adolescents’ Alcohol Use: A Genetically Informed Study Examining Mechanisms of Risk. Development and Psychopathology. Advance online publication. 2017 doi: 10.1017/S095457941700058X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FL, Chassin L, Eisenberg N, Spinrad TL. Effortful Control Predicts Adolescent Antisocial-Aggressive Behaviors and Depressive Symptoms: Co-Occurrence and Moderation by Impulsivity. Child Development. 2015;86:1812–1829. doi: 10.1111/cdev.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and individual differences. 2001;30:669–689. [Google Scholar]
- Yates JR, Darna M, Gipson CD, Dwoskin LP, Bardo MT. Dissociable roles of dopamine and serotonin transporter function in a rat model of negative urgency. Behavioural Brain Research. 2015;291:201–208. doi: 10.1016/j.bbr.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Enoch MA, Prescott CA. The influence of gene- environment interactions on alcohol consumption and alcohol use disorders: a comprehensive review. Clinical Psychology Review. 2011;31:800–816. doi: 10.1016/j.cpr.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.