Abstract
Purpose of review
The influence of gut bacteria upon host physiology is increasingly recognized, but mechanistic links are lacking. Diseases of energetic imbalance such as obesity and diabetes represent major risk factors for cardiovascular diseases such as hypertension. Thus, here we review current mechanistic contributions of the gut microbiota to host energetics.
Recent findings
Gut bacteria generate a multitude of small molecules which can signal to host tissues within and beyond the gastrointestinal tract to influence host physiology, and gut bacteria can also influence host digestive efficiency by altering the bioavailability of polysaccharides, yet the quantitative energetic effects of these processes remain unclear. Recently our team has demonstrated that gut bacteria constitute a major anaerobic thermogenic biomass, which can quantitatively account for obesity.
Summary
Quantitative understanding of the mechanisms by which gut bacteria influence energy homeostasis may ultimately inform the relationship between gut bacteria and cardiovascular dysfunction.
Keywords: Microbiome, Microbiota, Bacteria, Metabolism, Energy, Energetics
Introduction
Energy balance and hypertension
Obesity and hypertension are strongly correlated in humans [1–3]. A gross majority (71%) of patients with diabetes also experience hypertension [4]. The American Heart Association has published that obesity and related metabolic disorders represent the single greatest challenge to improving cardiovascular health [5, 6]. Thus, it is becoming increasingly clear that cardiovascular health is inextricably linked to metabolic health. Understanding the obesity epidemic and developing novel approaches to prevent weight gain and maintain weight loss should therefore be of growing interest to cardiovascular researchers.
Obesity is the result of a small but chronic imbalance of energy input and output [7]. While extensive lifestyle interventions can improve the effectiveness of behavioral interventions such as diet and exercise, successful long-term weight loss using these approaches in isolation have been routinely demonstrated as insufficiently efficacious for most adults [8]. Surgical interventions are highly effective but due to risks of complications are only reserved for morbidly obese patients. Pharmacological interventions which modify digestive efficiency, such as the FDA-approved compound Orlistat (Alli, Xenical, Tetrahydrolipstatin) are useful to promote a few kilograms of weight loss, but insufficient to completely reverse obesity [9]. Notably, a recent multi-year follow-up study of human subjects participating in an extreme weight-loss program discovered that all of the patients regained essentially all of their pre-program participation weight specifically due to a suppression of baseline resting metabolic rate (RMR) (known as RMR adaptation) [10]. Pharmaceutical interventions which modify RMR are exceptionally powerful at promoting weight loss, yet currently-available compounds to promote RMR are woefully dangerous due to small therapeutic dosing windows [11]. Collectively these findings underscore the complex array of interacting behavioral and biological mechanisms which influence energy balance, and highlight the promise of targeting RMR – we simply need safer interventions (drugs, supplements, diets, or other approaches) which work through stimulating RMR.
A role for gut bacteria in energy balance
Strong evidence supports a role for gut bacteria (the gut microbiota) in energy balance [12]. Early studies investigating a role for altered gut bacteria in host metabolism demonstrated that the host environment contributes to the composition of the gut microbiota, and that the gut microbiota contribute to digestive efficiency of the host.
While there is substantial diversity in the composition of gut bacteria across individual humans, there exists a relatively consistent “core” genetic composition of the microbiome that is consistent between individuals, and disease states such as obesity are associated with phylum-level shifts [13]. Whether these shifts are the cause or effect of the disease state is unclear. For example, animals made obese due to loss of the leptin gene (ob/ob mice) exhibit shifts in the gut microbiome [14]. Similarly, diet changes can cause major shifts in the gut microbiome and body weight [15–18]. Antibiotic administration to promote accelerated growth has also been used for many decades in the agricultural industry, and chronic low-dose antibiotic administration in laboratory mice similarly potentiates weight gain [19, 20]. In contrast, transplant of fecal bacteria from obese vs lean humans [18] or obese vs lean donor mice [21] to naïve mice causes differential weight gain in the recipients. The composition of the gut microbiome may also change to meet the metabolic demands of the host organism, such as during reproduction and growth [22], or to predispose the host toward specific immune profiles and growth trajectories [23]. Thus it is most likely that the microbiota-host relationship is bi-directional. The concept that the gut bacteria and host are commensal and co-evolved is supported by the divergence in abundance and identity of gut bacteria at the species level between hosts (eg – mouse vs human), but the generalized maintenance of similar gut bacteria at the division/superkingdom level in these same hosts [14].
Collectively these studies have led to the working hypotheses that the gut microbiota contributes to whole-organism energetics, including effects upon host tissue energy flux and upon energy flux through the biomass of bacteria itself, and that the relative abundance of distinct species of bacteria modifies the contributions of the gut microbiota to host energetics.
Modulation of host energetics by gut bacteria: Digestive efficiency
Digestive efficiency, or the efficiency of the gastrointestinal tract to extract calories from ingested food, is a major contributor to energy balance. Inhibition of fatty acid absorption by the pancreatic lipase inhibitor Orlistat results in relatively rapid weight loss in humans with normal to high dietary fat content [9]. The ability of bacteria to metabolize polysaccharides, which are otherwise unavailable for absorption by the host, [24] and the location of bacteria within the gastrointestinal tract prompt the hypothesis that these microorganisms may contribute to digestive efficiency.
Multiple studies have supported the concept that gut bacteria contribute to the regulation of digestive efficiency, and that changes in the composition of the gut microbiota may affect host weight changes through this mechanism [24]. Compared to the digestive efficiency of germ-free mice colonized with gut bacteria from wildtype control mice, germ-free mice colonized with gut bacteria from ob/ob mice exhibited a significant reduction in digestive efficiency and increased fat gain [25]. Germ-free animals express higher levels of angiopoietin-like 4 (Angptl4), an endogenous inhibitor of lipoprotein lipase, which reduces triglyceride absorption and deposition in adipose tissues [26, 27]. In addition, angiopoietin-like 4 levels inversely correlate with body mass index in matched human twin pairs [28].
In addition to processing polysaccharides to make them bioavailable to the host, the gut bacteria process various substrates such as lipids, carbohydrates, proteins and amino acids to produce various substrates for the host organism [29–31]. Examples include short-chain fatty acids like acetate, butyrate, and proprionate, in addition to vitamins such as folate, biotin, riboflavin, cobalamin, vitamin K, and bile acids (reviewed in [12]).
Together these studies clearly and qualitatively document a role for the gut microbiota in the control of digestive efficiency, but critically these studies do not attempt to determine whether observed changes in digestive efficiency quantitatively account for the observed weight changes in the host organisms. In other words, what fraction of host weight gain/loss is due to changes in digestive efficiency caused by the modification of the gut microbiome? It remains unclear whether other energy flux mechanisms may quantitatively contribute to the effects of gut bacteria upon host energetics, and the failure of the field to comprehensively assess other mechanisms may result from the technical challenges of measuring the forms of energy metabolism performed by bacteria within the lumen of the gastrointestinal tract.
Energy utilization by gut bacteria: Anaerobic resting metabolism
Gut bacteria are constantly growing and dividing, and thus it would be naïve to ignore the flux of energy through these organisms when calculating total energy utilization of a host. The NIH Human Microbiome Project has estimated that bacteria contribute roughly 1 – 3% of the host’s total body mass [32, 33]. For a standard 90 kg man, that equates to between 0.9 – 2.7 kg of bacteria. Using the estimated metabolic rates of prokaryotes of approximately 8 W/kg (or 7 kcal/hr/kg) [34], the biomass of bacteria could therefore be expected to contribute between 7 – 22 W (or 6 – 19 kcal/hr, or 144 – 456 kcal/d), which represents between 7 – 22% of a typical 97 W (or 83 kcal/hr, or 2,000 kcal/d) adult human caloric turnover. As obesity in developed countries such as the United States has been estimated to be the result of a chronic energy imbalance gap on the order of 30 kJ/d (or 7 kcal/d) [7], this implies that the energy flux through the gut bacteria biomass itself is between 20 – 65 times greater than required to explain human obesity, even without considering any of the complex signaling interactions between the microbiota and the host or the effects of the bacteria upon digestive efficiency. Therefore, it is prudent to investigate how small changes to the composition of the gut microbiota could influence energy flux, and thus, body mass.
The gut microbiota exists in a largely anaerobic environment within the lumen of the gastrointestinal tract. Thus it is expected that any energy flux through this biomass will be anaerobic in form. Unfortunately, the field is ill-equipped to assess anaerobic metabolism in the in vivo setting, as the majority of researchers utilize only respirometry-based ‘indirect’ calorimetry methods. Respirometry-based methods involve the measurement of respiratory gasses (oxygen consumption and carbon dioxide production), and then the application of empirically-derived formulae to estimate metabolic rates. Because these methods estimate metabolic rate based upon oxygen consumption, they remain blind to anaerobic processes [35–37]. By extension, many investigators fall into the trap of intentionally or unintentionally ignoring the energy flux through the biomass of the gut bacteria, simply because it is an endpoint for which they are unequipped to measure.
In contrast to respirometry-based methods to assess metabolic rates, direct calorimetry methods involve the assessment of all forms of energy flux through a subject by measuring all forms of heat retention and dissipation by the subject [35]. By coupling a direct calorimetry chamber (to measure total metabolism) with a series of respiratory gas analyzers (to simultaneously assess aerobic metabolism), investigators can estimate the contribution of anaerobic metabolism to the total. While no commercially-available sources of such equipment currently exist, such systems have been independently developed in a handful of physiology laboratories around the globe.
In the 1970’s, Jéquier’s group utilized a combined calorimetry system to assess metabolic rate in human subjects. They demonstrated that in lean men [38] and women [39], aerobic resting metabolism accounts for roughly 90% of total resting metabolic rate. Interestingly, total resting metabolic rate is reduced in obese women compared to lean women, and the relative contribution of anaerobic metabolism to the total was completely abolished in obese women [39]. These findings establish a physiologically significant contribution of anaerobic metabolism to total energy expenditure (roughly 10%), and correlate the loss of anaerobic metabolism with obesity in humans.
Anaerobic metabolism has also been documented in non-human species. In 2005–2006, Walsberg and Hoffman demonstrated that in selected endothermic organisms (kangaroo rats, quail, and doves), anaerobic metabolism contributes between 15 – 40% of total resting metabolism [40]. The team then demonstrated that anaerobic metabolism did not contribute any substantial fraction of total energy expenditure in an ectotherm (ball python) [41]. The van Breukelen team has also recently demonstrated anaerobic metabolism in desert pupfish even in the absence of anoxia (paradoxical anaerobism) [42]. In 2013, our team demonstrated that anaerobic metabolism accounted for roughly 10% of total energy expenditure in laboratory mice (both C57BL/6J and FVB/NCrl strains) [43]. In addition, we have subsequently determined that various interventions modify the contribution of anaerobic metabolism to total metabolism in these inbred mouse strains. For example, genetic disruption of the angiotensin II type 2 receptor (Agtr2) on the FVB/NCrl background strain significantly reduced anaerobic metabolism [43]. Similarly, feeding mice a 45% high fat diet (HFD) for just one week resulted in the complete loss of anaerobic metabolism in C57BL/6J mice [44]. We have subsequently demonstrated that the Agtr2 and diet composition are both critically important in the control of digestive efficiency [45], which leads back to the hypothesis that gut bacteria may be responsible for the changes in anaerobic metabolism observed with these manipulations.
To further probe the hypothesis that gut bacteria contribute to total energy expenditure through anaerobic energy utilization, we have also examined the effect of direct manipulations of the gut microbiota upon resting metabolism. Risperidone is an antipsychotic drug used in humans for the treatment of hyperactivity disorders and schizophrenia, but its use frequently leads to the unintended side-effect of excessive weight gain. We have recently demonstrated that risperidone treatment in children results in robust changes in the composition of the gut microbiome [46]. Similarly, we have documented robust effects of risperidone upon the gut microbiome in mice which correlate with excess weight gain [21]. To assess a role for the transformed gut microbiome in the excess weight gain associated with risperidone, we performed a fecal transplant experiment. Mice were treated with vehicle or risperidone, and stool samples were collected. Bacteria isolated from these fecal samples were then transplanted into the gut of naïve recipient mice via gastric gavage. Critically, we determined that the amount of active risperidone remaining in the transplanted fecal bacteria sample was >100-fold less than the dose required to cause any body mass changes. Mice receiving fecal bacteria from vehicle-treated donor mice exhibited no changes in resting metabolism. In contrast, mice receiving bacteria from risperidone-treated donor mice exhibited a 16% reduction in total resting metabolic rate, due almost entirely to the loss of anaerobic metabolism [21]. (Notably a 16% reduction in resting metabolism in a human would be the functional equivalent of consuming an extra 300 kcal cheeseburger per day.) Excitingly, we also determined that transplant of bacteriophage isolated from the feces of risperidone- but not vehicle-treated mice was also sufficient to cause excess weight gain through a suppression of energy expenditure [21]. Collectively, these results illustrate the very large contribution of gut bacteria to total energy flux, highlight the predominant role of anaerobic metabolism in this effect, and prompt the consideration of the potential metabolically-therapeutic benefits of manipulating the gut microbiome via bacteria or phage transplant procedures.
The gut microbiome and hypertension
Dysfunctional energy homeostasis disorders including obesity and diabetes (which are linked to gut dysbiosis) represent major risk factors leading to, and resisting the reversal of, cardiovascular diseases such as hypertension [47]. A team led by Raizada and Pepine have provided experimental evidence supporting a role for the gut microbiota in hypertension and possibly resistant hypertension. Spontaneously hypertensive rats (SHR), rats infused with exogenous angiotensin II, and human hypertensive patients exhibit changes in their gut microbiome including a shift toward an increased firmicutes/bacteroidetes ratio [48]. Chronic administration of the antibiotic, minocycline [48], or the short chain fatty acid, butyrate [49], to rats infused with angiotensin reversed this ratio and attenuate the hypertension in this model. In a recent case-report, a potent antihypertensive effect was observed in a human with resistant hypertension [50]. Further, changes in intestinal structure and function correlate with hypertension in the SHR model [51], and thus it is proposed that a bi-directional relationship between host cardiovascular function and gut bacteria exists, which may involve a complex interaction among hypothalamic inflammation, the autonomic nervous system, and host immune functions [52, 53]. How these mechanisms cross-talk with energy homeostasis remains speculative but not unexpected, given the implication of such systems and pathways in the control of host RMR [54–57].
Conclusions
In summary, increasing evidence supports a multifaceted role for the gut microbiota in the control of energy homeostasis including direct actions to influence both digestive efficiency and anaerobic resting metabolism (Figure 1). It has also been hypothesized that the gut microbiota functions as a previously unappreciated endocrine organ, producing and releasing an enormous array of compounds which may act upon host tissues to alter metabolic function and appetite [58]. In addition, the host-microbiome relationship is bi-directional, as diets and host genetics have considerable effects upon the composition of the gut microbiota.
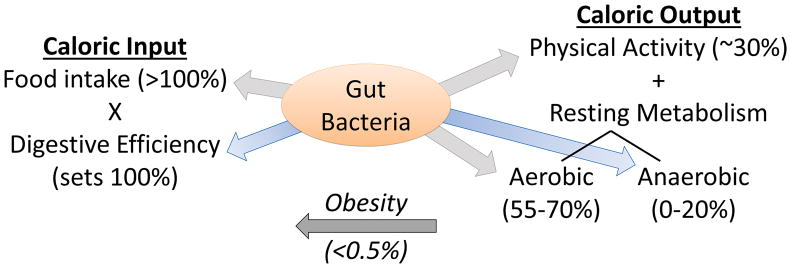
Figure 1. Evidence supports effects of the gut microbiota upon almost all forms of energy input or output.
Food intake maximally limits the calories input into the host animal, but digestive efficiency scales the fraction of calories actually absorbed from food, versus those lost to the stool, which subsequently defines (100%) the calories that must be balanced by output mechanisms. Physical activity typically accounts for roughly 30% of caloric output in mammals, whereas resting metabolic processes account for the balance of energy expenditure, including both aerobic (55–70% of output) and anaerobic (0–20% of output) processes. Because the relative contribution of each of these processes is in gross excess of the imbalance between input and output which accounts for human obesity (<0.5%), all of these processes are positioned to significantly contribute to the pathogenesis, maintenance, and reversal of obesity. Evidence reviewed herein highlight the major contributions of the gut microbiota to the control of digestive efficiency and anaerobic resting metabolism.
Therefore, mechanisms by which the gut microbiota influences energy homeostasis may directly or indirectly influence cardiovascular function and dysfunction. Understanding whether such cardiovascular effects are mediated through interactions between gut bacteria and the host immune system or enteric nervous system, through a complex regulation of energy substrate format and availability, or some other unknown mechanism remains to be determined. Nonetheless, the quantitative involvement of the gut microbiota in multiple aspects of animal energetics is undeniable, which identifies these microorganisms as novel and largely untapped therapeutic targets for a wide array of cardiometabolic diseases.
Acknowledgments
RAR and CMLB were supported by research fellowships from the University of Iowa Carver College of Medicine (SUMR, MSRP), and SNA was supported by a predoctoral fellowship from the University of Iowa Graduate College. This project was supported by grants from the NIH (HL084207, HL098276, HL134850 to JLG, and AI108255 to JRK), NSF (MCB-1244021 to JRK), American Diabetes Association (1-14-BS-079 to JLG), American Heart Association (15SFRN23730000 to JLG), the University of Iowa Center for Hypertension Research (to JLG), the University of Iowa/Fraternal Order of Eagles’ Diabetes Research Center (to JLG & JRK), and the Departments of Pharmacology and Microbiology at the University of Iowa.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2015 update: a report from the american heart association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/cir.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Bramlage P, Pittrow D, Wittchen HU, Kirch W, Boehler S, Lehnert H, et al. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. American journal of hypertension. 2004;17(10):904–10. doi: 10.1016/j.amjhyper.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention; US Department of Health and Human Services, editor. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: 2014. [Google Scholar]
- 5.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/circulationaha.109.192703. [DOI] [PubMed] [Google Scholar]
- 6.Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd-Jones DM. Cardiovascular health behavior and health factor changes (1988–2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation. 2012;125(21):2595–602. doi: 10.1161/CIRCULATIONAHA.111.070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826–37. doi: 10.1016/s0140-6736(11)60812-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan Y, Head V, Jacob D, Bachmann MO, Diu S, Ford J. Lifestyle interventions for weight loss in adults with severe obesity: a systematic review. Clinical obesity. 2016 doi: 10.1111/cob.12161. [DOI] [PubMed] [Google Scholar]
- 9.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes care. 2004;27(1):155–61. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 10*.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring, Md) 2016;24(8):1612–9. doi: 10.1002/oby.21538. The human body resists weight loss predominantly through the adaptation/suppression of RMR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grundlingh J, Dargan PI, El-Zanfaly M, Wood DM. 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. Journal of medical toxicology : official journal of the American College of Medical Toxicology. 2011;7(3):205–12. doi: 10.1007/s13181-011-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheithauer TP, Dallinga-Thie GM, de Vos WM, Nieuwdorp M, van Raalte DH. Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Molecular metabolism. 2016;5(9):759–70. doi: 10.1016/j.molmet.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(31):11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmody RN, Gerber GK, Luevano JM, Jr, Gatti DM, Somes L, Svenson KL, et al. Diet dominates host genotype in shaping the murine gut microbiota. Cell host & microbe. 2015;17(1):72–84. doi: 10.1016/j.chom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell host & microbe. 2008;3(4):213–23. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Science translational medicine. 2009;1(6):6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–6. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahana D, Trent CM, Kurtz ZD, Bokulich NA, Battaglia T, Chung J, et al. Antibiotic perturbation of the murine gut microbiome enhances the adiposity, insulin resistance, and liver disease associated with high-fat diet. Genome medicine. 2016;8(1):48. doi: 10.1186/s13073-016-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Bahr SM, Weidemann BJ, Castro AN, Walsh JW, deLeon O, Burnett CM, et al. Risperidone-induced weight gain is mediated through shifts in the gut microbiome and suppression of energy expenditure. EBioMedicine. 2015;2(11):1725–34. doi: 10.1016/j.ebiom.2015.10.018. Excess weight gain with risperidone is caused by changes in the gut microbiome, and subsequent loss of anaerobic energy expenditure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amato KR, Leigh SR, Kent A, Mackie RI, Yeoman CJ, Stumpf RM, et al. The role of gut microbes in satisfying the nutritional demands of adult and juvenile wild, black howler monkeys (Alouatta pigra) American journal of physical anthropology. 2014;155(4):652–64. doi: 10.1002/ajpa.22621. [DOI] [PubMed] [Google Scholar]
- 23.Mach N, Berri M, Estelle J, Levenez F, Lemonnier G, Denis C, et al. Early-life establishment of the swine gut microbiome and impact on host phenotypes. Environmental microbiology reports. 2015;7(3):554–69. doi: 10.1111/1758-2229.12285. [DOI] [PubMed] [Google Scholar]
- 24.Cox LM, Blaser MJ. Pathways in microbe-induced obesity. Cell metabolism. 2013;17(6):883–94. doi: 10.1016/j.cmet.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 26.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(44):15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattijssen F, Alex S, Swarts HJ, Groen AK, van Schothorst EM, Kersten S. Angptl4 serves as an endogenous inhibitor of intestinal lipid digestion. Molecular metabolism. 2014;3(2):135–44. doi: 10.1016/j.molmet.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robciuc MR, Naukkarinen J, Ortega-Alonso A, Tyynismaa H, Raivio T, Rissanen A, et al. Serum angiopoietin-like 4 protein levels and expression in adipose tissue are inversely correlated with obesity in monozygotic twins. Journal of lipid research. 2011;52(8):1575–82. doi: 10.1194/jlr.P015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cani PD, Knauf C. How gut microbes talk to organs: The role of endocrine and nervous routes. Molecular metabolism. 2016;5(9):743–52. doi: 10.1016/j.molmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broussard JL, Devkota S. The changing microbial landscape of Western society: Diet, dwellings and discordance. Molecular metabolism. 2016;5(9):737–42. doi: 10.1016/j.molmet.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bashiardes S, Shapiro H, Rozin S, Shibolet O, Elinav E. Non-alcoholic fatty liver and the gut microbiota. Molecular metabolism. 2016;5(9):782–94. doi: 10.1016/j.molmet.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDougall R. [Accessed May 2016];NIH Human Microbiome Project defines normal bacterial makeup of the body. 2012 https://www.nih.gov/news-events/news-releases/nih-human-microbiome-project-defines-normal-bacterial-makeup-body.
- 33.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–10. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makarieva AM, Gorshkov VG, Li BL. Energetics of the smallest: Do bacteria breathe at the same rate as whales? Proceedings Biological sciences/The Royal Society. 2005;272(1577):2219–24. doi: 10.1098/rspb.2005.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiyala KJ, Ramsay DS. Direct animal calorimetry, the underused gold standard for quantifying the fire of life. Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2011;158(3):252–64. doi: 10.1016/j.cbpa.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLean JA, Tobin G. Animal and human calorimetry. New York: Cambridge University Press; 1988. [Google Scholar]
- 37.Lighton JRB. Measuring metabolic rates. New York: Oxford University Press; 2008. [Google Scholar]
- 38.Pittet P, Gygax PH, Jequier E. Thermic effect of glucose and amino acids in man studied by direct and indirect calorimetry. The British journal of nutrition. 1974;31(3):343–9. doi: 10.1079/bjn19740042. [DOI] [PubMed] [Google Scholar]
- 39.Pittet P, Chappuis P, Acheson K, De Techtermann F, Jequier E. Thermic effect of glucose in obese subjects studied by direct and indirect calorimetry. The British journal of nutrition. 1976;35(2):281–92. doi: 10.1079/bjn19760033. [DOI] [PubMed] [Google Scholar]
- 40.Walsberg GE, Hoffman TC. Direct calorimetry reveals large errors in respirometric estimates of energy expenditure. The Journal of experimental biology. 2005;208(Pt 6):1035–43. doi: 10.1242/jeb.01477. [DOI] [PubMed] [Google Scholar]
- 41.Walsberg GE, Hoffman TC. Using direct calorimetry to test the accuracy of indirect calorimetry in an ectotherm. Physiological and biochemical zoology : PBZ. 2006;79(4):830–5. doi: 10.1086/505514. [DOI] [PubMed] [Google Scholar]
- 42.Heuton M, Ayala L, Burg C, Dayton K, McKenna K, Morante A, et al. Paradoxical anaerobism in desert pupfish. The Journal of experimental biology. 2015;218(Pt 23):3739–45. doi: 10.1242/jeb.130633. [DOI] [PubMed] [Google Scholar]
- 43.Burnett CM, Grobe JL. Direct calorimetry identifies deficiencies in respirometry for the determination of resting metabolic rate in C57Bl/6 and FVB mice. American journal of physiology Endocrinology and metabolism. 2013;305(7):E916–24. doi: 10.1152/ajpendo.00387.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnett CM, Grobe JL. Dietary effects on resting metabolic rate in C57BL/6 mice are differentially detected by indirect (O2/CO2 respirometry) and direct calorimetry. Molecular metabolism. 2014;3(4):460–4. doi: 10.1016/j.molmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weidemann BJ, Voong S, Morales-Santiago FI, Kahn MZ, Ni J, Littlejohn NK, et al. Dietary Sodium Suppresses Digestive Efficiency via the Renin-Angiotensin System. Sci Rep. 2015;5:11123. doi: 10.1038/srep11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bahr SM, Tyler BC, Wooldridge N, Butcher BD, Burns TL, Teesch LM, et al. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Translational psychiatry. 2015;5:e652. doi: 10.1038/tp.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pevsner-Fischer M, Blacher E, Tatirovsky E, Ben-Dov IZ, Elinav E. The gut microbiome and hypertension. Current opinion in nephrology and hypertension. 2016 doi: 10.1097/mnh.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 48*.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–40. doi: 10.1161/hypertensionaha.115.05315. Hypertensive humans and rats exhibit similar changes in gut microbiome, and antibiotic treatment prevents hypertension in rat model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S, Wang G, Lobaton G, Li E, Yang T, Raizada M. OS 05-10 THE MICROBIAL METABOLITE, BUTYRATE ATTENUATES ANGIOTENSIN II-INDUCED HYPERTENSION AND DYSBIOSIS. Journal of hypertension. 2016;34(Suppl 1):e60–1. doi: 10.1097/01.hjh.0000500010.38755.52. [DOI] [Google Scholar]
- 50*.Qi Y, Aranda JM, Rodriguez V, Raizada MK, Pepine CJ. Impact of antibiotics on arterial blood pressure in a patient with resistant hypertension - A case report. International journal of cardiology. 2015;201:157–8. doi: 10.1016/j.ijcard.2015.07.078. Antibiotic administration can reverse resistant hypertension in a human. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart DC, Rubiano A, Santisteban MM, Shenoy V, Qi Y, Pepine CJ, et al. Hypertension-linked mechanical changes of rat gut. Acta biomaterialia. 2016;45:296–302. doi: 10.1016/j.actbio.2016.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santisteban MM, Kim S, Pepine CJ, Raizada MK. Brain-Gut-Bone Marrow Axis: Implications for Hypertension and Related Therapeutics. Circulation research. 2016;118(8):1327–36. doi: 10.1161/circresaha.116.307709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, et al. Hypertension-Linked Pathophysiological Alterations in the Gut. Circulation research. 2016 doi: 10.1161/circresaha.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seoane-Collazo P, Ferno J, Gonzalez F, Dieguez C, Leis R, Nogueiras R, et al. Hypothalamic-autonomic control of energy homeostasis. Endocrine. 2015;50(2):276–91. doi: 10.1007/s12020-015-0658-y. [DOI] [PubMed] [Google Scholar]
- 55.Claflin KE, Grobe JL. Control of energy balance by the brain renin-angiotensin system. Current hypertension reports. 2015;17(5):38. doi: 10.1007/s11906-015-0549-x. [DOI] [PubMed] [Google Scholar]
- 56.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, et al. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension. 2011;57(3):600–7. doi: 10.1161/hypertensionaha.110.165829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, et al. The brain Renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell metabolism. 2010;12(5):431–42. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donia MS, Fischbach MA. HUMAN MICROBIOTA. Small molecules from the human microbiota. Science. 2015;349(6246):1254766. doi: 10.1126/science.1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]