Abstract
Early adversity is associated with biological and behavioral dysregulation in early childhood. We examined whether early adversity (i.e., poverty and involvement with child protective services [CPS]) had an indirect effect on externalizing behavior through HPA axis dysregulation, specifically blunted diurnal cortisol patterns. Participants included 94 children between the ages of 3.94 and 6.52 years old, who had a history of CPS involvement (n = 53) or no history of CPS involvement (n = 41). Cortisol samples were collected at wake-up and bedtime across 3 days, and parent-reported externalizing behavior was assessed using the Child Behavior Checklist. Results showed that history of CPS involvement and poverty were associated with blunted cortisol patterns, which in turn led to elevated externalizing behavior. The indirect effect of CPS involvement on externalizing behavior through blunted cortisol was significant, whereas the indirect effect of poverty on externalizing behavior was non-significant. Findings add to our understanding of neurobiological mechanisms linking early adversity to psychopathology.
Keywords: maltreatment, stress, cortisol, externalizing behavior
INTRODUCTION
Externalizing behavior problems have been associated with exposure to various types of early adversity, including maltreatment (Cicchetti & Toth, 2005; Manly, Kim, Rogosch, & Cicchetti, 2001) and poverty (Ackerman, Brown, & Izard, 2004; Plybon & Kliewer, 2001; Slopen, Fitzmaurice, Williams, & Gilman, 2010). It is critical to identify biological mechanisms that explain the link between these early exposures and later behavioral problems, as such mechanisms may serve as targets for early intervention efforts. As a key arm of the body’s stress response system, the hypothalamic–pituitary–adrenal (HPA) axis has received significant attention among researchers who examine the effects of early life stress. Given evidence that (1) exposure to maltreatment (Bernard, Butzin-Dozier, Rittenhouse, & Dozier, 2010; Bruce, Fisher, Pears, & Levine, 2009) and poverty (Blair, Berry, Mills-Koonce, & Granger, 2013; Zalewski, Lengua, Kiff, & Fisher, 2012) are associated with dysregulation of the HPA axis, and (2) HPA axis dysregulation (i.e., blunted diurnal cortisol) is associated with externalizing behavior (Alink et al., 2008; Martin, Kim, Bruce, & Fisher, 2014; Zalewski, Lengua, Kiff et al., 2012), the present study examined whether dysregulation of diurnal cortisol patterns explained the association between early adversity and externalizing behavior.
Early Adversity and Externalizing Behavior
Risky family contexts (i.e., those characterized by poverty, low social support, parental psychopathology, and/or harsh parenting) confer risk for externalizing behavior problems, such as bullying, impulsivity and hyperactivity, and oppositional behavior (Repetti, Taylor, & Seeman, 2002; Teisl & Cicchetti, 2008). Exposure to poverty, specifically, is associated with elevated externalizing behavior (e.g., Ackerman et al., 2004; Slopen et al., 2010). The association between poverty and externalizing behavior may be explained by a number of mechanisms, such as problematic parenting (Tremblay et al., 2004; Trentacosta et al., 2008). Exposure to neglect and/or abuse, which, by definition, reflect disruptions in parenting, is linked to elevated externalizing across development, from early childhood (Godinet, Li, & Berg, 2013; Kotch et al., 2008; Woodruff & Lee, 2011) to adolescence (Mills et al., 2013). Similar to the sample examined in the present study, Campbell, Thomas, Cook, & Keenan (2012) examined the prevalence of externalizing behavior among children who remained in their homes following their first investigation by child protective services, and found that children who remained in their homes demonstrated elevated externalizing behavior relative to national norms. These findings are consistent with a broader body of literature demonstrating that CPS referral, regardless of substantiation status, is a significant risk indicator—predictive of future reports of maltreatment (Drake, Jonson-Reid, Way, & Chung, 2003; Kohl, Jonson-Reid, & Drake, 2009) and problematic outcomes (Hussey et al., 2005).
Early Adversity and HPA Axis Dysregulation
Stressful experiences in early childhood interfere with children’s development of regulatory functions, especially in the absence of a responsive caregiver (Bruce, Gunnar, Pears, & Fisher, 2013; Gunnar & Donzella, 2002). Caregivers play a critical role in infancy as a co-regulator of emotions, behaviors, and physiology (Hofer, 1994, 2006; Winberg, 2005). Thus, when children do not receive adequate input in the form of sensitive caregiving, which is often the case among caregivers living in poverty (Zalewski, Lengua, Kiff et al., 2012) as well as caregivers involved with CPS (Baer & Martinez, 2006; Moss et al., 2011), biological and behavioral regulation is compromised.
The HPA axis is one of the physiological systems involved in mounting a response to threat. In addition to being produced following an acute stressor, cortisol follows a diurnal rhythm: increasing before awakening, peaking approximately 30 min after awakening, declining across the morning, and reaching near zero levels by bedtime (Bailey & Heitkemper, 1991; Schmidt-Reinwald et al., 1999; Watamura, Donzella, Kertes, & Gunnar, 2004). Early adversity disrupts the typical regulation of diurnal cortisol production, with children exposed to early life stressors tending to show blunted patterns of cortisol production across the day (Bernard et al., 2010; Bruce et al., 2009; Carlson et al., 1995; Gunnar & Vazquez, 2001). For example, Zalewski, Lengua, Kiff et al. (2012) found that income was positively associated with morning levels of cortisol in a sample of 3-year-old children; further, there was an indirect effect of cumulative family risk on blunted diurnal cortisol slope through maternal negativity. Similarly, Bernard et al. (2010) found that young children who remained with birth parents following involvement with child protective services had lower wake-up cortisol and higher bedtime cortisol than children living in foster care and children living with low-risk biological parents. These patterns of low/blunted cortisol (indicating hypo-activation of the HPA axis) may follow repeated or chronic hyper-activation earlier in development, reflecting a process of down-regulation (Fries, Hesse, Hellhammer, & Hellhammer, 2005; Miller, Chen, & Zhou, 2007).
HPA Axis Functioning and Externalizing Behavior
A recent meta-analysis examined the association between basal levels of cortisol and externalizing behavior in childhood (Alink et al., 2008). Although the combined effect size of this association was small, there was evidence of moderation by age. Specifically, for school age children (i.e., age 5 and above), lower basal cortisol was associated with higher externalizing behavior. In contrast, among children younger than 5 years of age, higher basal cortisol was associated with higher externalizing behavior (Alink et al., 2008). One challenge of interpreting the results of this meta-analysis is that basal cortisol was assessed differently across studies (i.e., number of samples, timing of sample, location [home vs. lab]). Additionally, the meta-analysis examined studies that reported average basal levels, rather than diurnal patterns, of cortisol production.
The Present Study
Although a number of studies have examined individual pathways from early risk to cortisol patterns or from cortisol patterns to externalizing behavior, few studies have tested these pathways simultaneously and modeled indirect effects. One exception is a recent study by Martin et al. (2014), who examined associations between parenting quality, cortisol rhythms, and externalizing behavior during middle childhood. Findings suggested that poor parental monitoring predicted blunted diurnal cortisol rhythms, which, in turn, predicted heightened levels of externalizing behavior. This study provided support for the mediating role of diurnal cortisol rhythms in linking the quality of early care to later behavior problems.
In the present study, we aimed to examine whether diurnal cortisol dysregulation explained the association between early adversity and externalizing behavior in early childhood. Thus, we examined whether involvement with CPS and/or poverty (i.e., a continuous measure of income-to-needs ratio) predicted elevated externalizing behavior via an indirect effect through cortisol dysregulation (i.e., more blunted patterns from wake-up to bedtime).
METHODS
Participants
The full sample recruited for the study included 101 participants: 56 children involved with CPS and 45 children recruited from the community with no history of CPS involvement according to parent-report. Primary analyses included 94 of these children (CPS-referred: 53; low-risk comparison: 41) due to the exclusion of seven children whose parents did not provide saliva samples (six not completed/returned; one returned but with insufficient amounts of saliva for assay). Within the CPS-referred group, there were three sibling pairs (each with CPS involvement) and within the low-risk group there was one sibling pair; due to issues related to the nonindependence of these data, follow-up analyses were conducted randomly excluding one sibling from each pair. At the time of the present study, children ranged in age from 3.94 to 6.52 years old (M = 4.93, SD = .58).
The CPS-referred children were recruited from an ongoing longitudinal sample assessing the efficacy of an attachment-based intervention for infants who continue to live with their birth mothers following involvement with child protective services. Parents of CPS-referred children were referred to the study after enrollment in a city program intended to divert children from foster care. Records were not available for coding of maltreatment history, but when making referrals, caseworkers primarily noted concerns about neglect, as well as other issues such as homelessness, parent substance use, and domestic violence. Low-risk community comparison children (n = 45) were recruited to be of comparable race and similar ages to the CPS-referred sample. Descriptive statistics of demographic data separated by CPS-referred and low-risk comparison groups are presented in Table 1.
Table 1.
Demographic Characteristics and Externalizing Behavior Scores of CPS-Referred and Low-Risk Comparison Groups
| Variable | CPS-Referred (n = 53)
|
Low-Risk Comparison (n = 41)
|
||
|---|---|---|---|---|
| n | % | n | % | |
| Child ethnicity | ||||
| African-American | 40 | 75.5 | 28 | 68.3 |
| Asian | 0 | 0 | 1 | 2.4 |
| Bi-Racial | 4 | 7.5 | 1 | 2.4 |
| Caucasian | 6 | 11.3 | 8 | 19.5 |
| Hispanic | 3 | 5.7 | 2 | 4.9 |
| Other | 0 | 0 | 1 | 2.4 |
| Child gender | ||||
| Male | 34 | 64.2 | 17 | 41.5 |
| Female | 19 | 35.8 | 24 | 58.5 |
|
| ||||
| Mean (SD) | Min–Max | Mean (SD) | Min–Max | |
|
| ||||
| Household income | $15,645 (12,694) | $2000–60,000 | $53,489 (50,544) | $2400–200,000 |
| Income-to-needs ratio | .56 (.45) | .10–2.15 | 2.28 (2.33) | .12–10.11 |
| Mother age (in years) | 31.7 (7.73) | 20.0–49.0 | 31.6 (6.20) | 22.7–45.3 |
| Child age (in years) | 4.84 (.57) | 3.94–6.20 | 5.03 (.58) | 4.13–6.52 |
| Externalizing behavior (raw score) | 16.09 (9.57) | 2–38 | 10.44 (8.14) | 0–38 |
As part of the larger longitudinal study, parents of children in the CPS-referred group had been randomly assigned to receive either an attachment-based intervention (n = 27; Attachment and Biobehavioral Catch-up; Dozier and the Infant Caregiver Project Lab, 2006) or a control intervention (n = 26). Given that examining intervention effectiveness was not the target question of the present report, the two intervention groups were collapsed into one group for primary analyses, with intervention group included as a covariate.
Procedure
At the time of the present study, CPS-referred children were already enrolled in the longitudinal study. They were invited to participate in the current study via phone calls and home visits during which procedures were described. The low-risk community comparison participants expressed interest by phone or email after being informed by varying recruitment sources, such as postings on a University website and fliers distributed at a University child care center and other community day care centers. The research staff initially contacted each family by phone, confirmed via parent report that children met criteria (i.e., in age range, lack of CPS involvement), provided information regarding the research protocol, and scheduled a home visit if the parent expressed interest. Home visits were identical for CPS-referred and low-risk comparison families. First, the researcher obtained consent from the parent and assent from the child. After obtaining consent, the researcher read questionnaires to the parent that assessed demographic/risk information and child behavior problems. The researcher also explained the procedure and provided the materials for collecting diurnal cortisol samples. At the home visit, additional assessments were conducted that were components of other studies (e.g., collection of other biological samples, completion of additional questionnaires). Parents received $75 for completion of the home visit, and $25 upon return of the cortisol samples.
Measures
Diurnal Cortisol
The researcher explained the procedure for collecting saliva samples in detail at the end of the home visit. Although most of the parents in the CPS-referred group had participated in cortisol assessments previously as part of the longitudinal study, instructions were still explained in detail. Parents were instructed to collect saliva samples from children at wake-up and bedtime across 3 consecutive days. Specifically, the researcher instructed parents to collect a wake-up sample each day immediately when the child woke up, before even getting out of bed if possible. Although the researcher emphasized the importance of collecting a sample as close to wake-up as possible, parents were told that sampling within the first 30 min of wake-up was acceptable. For the bedtime collection, parents were told to collect the sample as close to bedtime as possible. Parents collected saliva samples by putting the end of a dental cotton roll in the child’s mouth. Flavored beverage crystals (only a few crystals) were provided to aide in sampling. Flavored crystals only minimally affect cortisol with being analyzed with radioimmunoassay (Gordon, Peloso, Auker, & Dozier, 2005; Talge, Donzella, Kryzer, Gierens, & Gunnar, 2005). The parent was instructed to wet the cotton roll in the child’s mouth, dip the cotton into a cup with the flavored crystals, and return the cotton back into the child’s mouth until the cotton was soaking wet. Given their age, parents often reported that children took an active role in collecting the samples themselves, by holding the cotton swab, chewing on it, and dipping it into the flavored beverage crystals. Parents placed the cotton roll into a pre-labeled vial and recorded the date and time of sampling. Parents stored the vials in their freezers until the research staff retrieved the samples, either during another visit to the home or at the next follow-up laboratory visit. Parents reported collecting wake-up samples between 5:30 a.m. and 12:00 p.m. (M = 8:03 a.m., Median = 7:59 a.m., SD = 1:05). Parents reported collecting bedtime samples between 5:45 p.m. and 12:45 a.m. (M = 9:05 p.m., Median = 9:00 p.m., SD = 1:03). Descriptive statistics of sampling times are presented in Table 2. To ensure guidelines were followed, a binder was given to the families with full sampling instructions (including directions and accompanying photographs). Research staff instructed parents to delay sampling if children were sick and to not have the child eat or drink anything or brush their teeth within the 30 min prior to sampling.
Table 2.
Descriptive Statistics of Cortisol Samples
| Time of Sample
|
Cortisol Value (in ug/dl)
|
Log-Transformed Cortisol Value
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | M (SD) | Min | Max | M (SD) | Min | Max | M (SD) | Min | Max | |
| High risk (n = 53) | ||||||||||
| Wake—Day 1 | 45 | 7:52 (1:00) | 5:30 | 10:12 | .21 (.20) | .01 | .85 | −.90 (.49) | −2.05 | −.07 |
| Wake—Day 2 | 45 | 8:03 (1:04) | 5:30 | 11:00 | .18 (.16) | .00 | .70 | −.97 (.56) | −3.00 | −.16 |
| Wake—Day 3 | 42 | 8:02 (1:07) | 5:30 | 10:34 | .27 (.17) | .00 | .69 | −1.00 (.48) | −2.40 | −.16 |
| Bed—Day 1 | 46 | 8:50 (1:04) | 6:00 | 11:34 | .13 (.13) | .01 | .47 | −1.10 (.46) | −2.10 | −.33 |
| Bed—Day 2 | 47 | 9:01 (0:52) | 7:19 | 11:00 | .16 (.16) | .00 | .69 | −.96 (.47) | −2.10 | −.16 |
| Bed—Day 3 | 46 | 8:57 (0:57) | 6:30 | 11:36 | .17 (.18) | .02 | .83 | −.96 (.43) | −1.80 | −.08 |
| Low risk (n = 41) | ||||||||||
| Wake—Day 1 | 37 | 8:07 (1:15) | 5:50 | 10:42 | .26 (.23) | .03 | 1.00 | −.74 (.37) | −1.60 | .00 |
| Wake—Day 2 | 38 | 8:15 (1:19) | 5:45 | 12:00 | .20 (.10) | .02 | .46 | −.77 (.29) | −1.62 | −.34 |
| Wake—Day 3 | 38 | 7:59 (1:12) | 5:40 | 10:30 | .23 (.16) | .02 | .68 | −.76 (.36) | −1.74 | −.17 |
| Bed—Day 1 | 36 | 9:32 (1:02) | 7:30 | 12:16 | .07 (.12) | .00 | .51 | −1.53 (.60) | −2.40 | −.29 |
| Bed—Day 2 | 38 | 9:16 (1:08) | 5:45 | 12:00 | .09 (.14) | .00 | .56 | −1.46 (.65) | −2.70 | −.25 |
| Bed—Day 3 | 34 | 9:33 (1:22) | 7:00 | 12:45 | .12 (.15) | .00 | .62 | −1.31 (.63) | −2.40 | −.21 |
Saliva samples were stored in a freezer at −20°C prior to assay procedures. Samples were assayed using a high-sensitivity salivary cortisol enzyme immunoassay kit (Salimetrics, LLC, State College, Pennsylvania). All samples from a child were assayed in duplicate on the same plate to minimize variability. Following procedures commonly used in previous studies (Fisher, Stoolmiller, Gunnar, & Burraston, 2007), cortisol values that were implausible (>2.0) and those that fell 3 SDs above the mean were considered outliers and excluded from analyses. Of 564 possible samples (i.e., 94 participants with up to 6 samples each), 21 outliers were removed and 51 samples were missing due to an inadequate volume of saliva or because no sample was taken, representing 12.7% of the data. The majority of participants had all six samples included in analyses (n = 65; 69.1%), with others having five samples (n = 7; 7.4%), four samples, (n = 8; 8.5%), three samples (n = 9; 9.6%), two samples (n = 3; 3.2%), or one sample (n = 2; 2.1%) included. When separated by group, low-risk comparison children were missing 10.2% of possible samples, and CPS-referred children were missing 14.8% of possible samples. There were no significant group differences in number of total samples or in the likelihood of missing any particular sample.
Child Behavior Checklist
Parents completed the Child Behavior Checklist (CBCL/1.5–5; Achenbach & Rescorla, 2000), a parent-report questionnaire of children’s behavioral and emotional problems. At the home visit, the researcher read the questionnaire to the parent and recorded the responses. Reading the questionnaire was expected to minimize reporting errors associated with poor reading ability or difficulty understanding. The CBCL/1.5–5 includes 99 items that describe specific behavioral, emotional, and social problems that are seen in preschool children. Parents were instructed to rate each item as 0 = Not true, 1 = Somewhat or sometimes true, or 2 = Very true or often true of the child, based on their observations from the preceding two months. The CBCL is a widely used tool for measuring children’s behavior problems, and has good psychometric properties (Achenbach & Rescorla, 2000). Given our interest in externalizing behavior problems, we used the subscale for Externalizing Behavior (number of items in factor = 24), which demonstrated good internal reliability for this sample (Cronbach’s α = .92).
Involvement With CPS and Income-To-Needs Ratio
Two risk factors were included as predictors in primary analyses. First, involvement with CPS reflected group membership and was based on whether the child was referred following CPS involvement (coded as 1) or recruited from the community with no history of CPS involvement according to parent-report (coded as 0). Second, we computed an income-to-needs ratio for each family based on the household income and federal standards for poverty level based on family size. Scores above 1 indicate that families are living above the poverty line, whereas scores below 1 indicated that families are living below the poverty line. The continuous variable was used in analyses (See Tab. 1 for descriptive statistics).
Data Analytic Approach
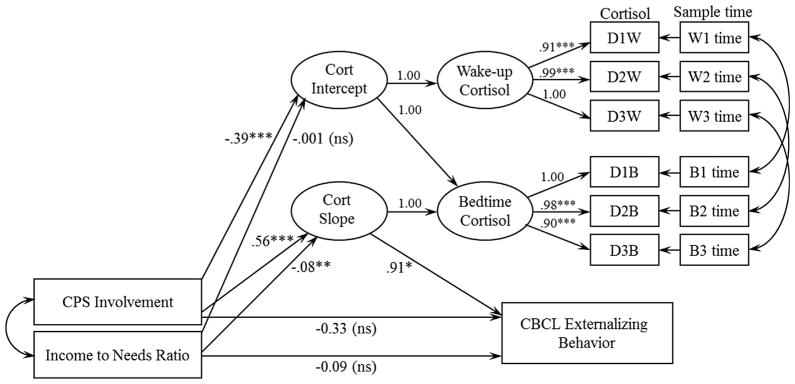
We used MPlus 7.11 (Muthen & Muthen, 1998–2013) for primary analyses. The full model is depicted in Figure 1. Wake-up and bedtime cortisol were defined as latent variables, each with three indicators (i.e., log-transformed cortisol values from Days 1, 2, and 3). Sample time was included as a time-varying covariate by regressing each observed cortisol sample variable on its respective time of sample collection (in hours since the average sample time of all participants). Residuals of observed cortisol indicators from the same days were allowed to correlate. Following procedures used previously, the pattern of diurnal cortisol change was modeled as a latent change score (Kertes, Gunnar, Madsen, & Long, 2008; McArdle & Hamagami, 2001). By setting the regression pathways as depicted, the cortisol intercept reflects the average wake-up cortisol level, and the cortisol slope (i.e., latent change score) reflects the change in cortisol across the day (i.e., Bedtime—Wake-up). In order to examine whether diurnal cortisol explained the association between involvement with CPS and externalizing behavior and/or between income and externalizing behavior, the cortisol slope and standardized externalizing behavior score were regressed onto involvement with CPS (0 = low-risk comparison group, 1 = CPS-referred group) and the income-to-needs ratio, and the indirect effects of the pathways (i.e., CPS involvement/income on externalizing behavior via cortisol slope) were estimated. Child age, gender, and intervention group were included as a covariate (reasons described below). The model was estimated using maximum likelihood estimation and absolute model fit was assessed with the χ2 to df ratio (χ2/df), the root mean square error of approximation (RMSEA), the comparative fit index (CFI), and the Tucker-Lewis index (TLI).
FIGURE 1.
Path diagram with unstandardized coefficients for full model of involvement with CPS and income predicting externalizing behavior via blunted diurnal cortisol. D1W-D3W and D1B-D3B represent log-transformed wake-up cortisol indicators and bedtime cortisol indicators for Days 1 through 3, respectively. W1time—W3time and B1time—B3time represent the sample collection times (as hours from average collection time) for wake-up and bedtime samples, respectively. Covariates (described in text and presented in Tab. 3) are not depicted. Model fit statistics indicated good fit: χ2/df = 1.20, RMSEA = .05, CFI = 0.95, TLI = .93. *p <.05, **p<.01, ***p <.001.
RESULTS
Preliminary Analyses
Bivariate correlations for study variables are presented in Table 3. We first examined whether groups (i.e., CPS-referred vs. low-risk comparison) differed on demographic characteristics. There was a significant association between group and income-to-needs ratio, with low-risk comparison parents having a higher income-to-needs ratio than CPS-referred parents, t (92) = 5.13, p <.001. There were no significant group differences in child age, t(92) = 1.66, p >.05, or minority group status (coded as 0 for non-minority, 1 for minority), χ2 = 1.22, p >.05. There was a significant group difference in gender distribution, with a higher proportion of males to females in the CPS-referred group than the low-risk group, χ2 = 4.49, p <.05. Given group differences in child gender, it was included as a covariate in analyses.
Table 3.
Bivariate Correlations Between Study Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. Gender | ||||||||
| 2. Minority | −.04 | |||||||
| 3. Involvement with CPS | −.23* | .11 | ||||||
| 4. Income-to-needs ratio | .14 | −.27** | −.48*** | |||||
| 5. Age | .20 | .06 | −.17 | .05 | ||||
| 6. Avg AM cort | −.03 | −.02 | −.22* | .17 | −.11 | |||
| 7. Avg PM cort | −.09 | .12 | .36*** | −.38*** | −.09 | .36*** | ||
| 8. Avg PM–AM difference | −.10 | .13 | .51*** | −.50*** | .02 | −.40*** | .71*** | |
| 9. Externalizing behavior | −.21* | .14 | .31** | −.35** | −.19 | −.21* | .23* | .36*** |
| Effect | Estimate | SE | Est/SE | p | 95%CI
|
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Path model: Time-varying covariates | ||||||
| D1W cort ON D1W time | −.12 | .03 | −3.42 | .001 | −.18 | −.05 |
| D2W cort ON D2W time | −.04 | .04 | −1.10 | .271 | −.11 | .03 |
| D3W cort ON D3W time | .003 | .03 | .09 | .925 | −.06 | .06 |
| D1B cort ON D1B time | −0.10 | .04 | −2.36 | .018 | −.19 | −.02 |
| D2B cort ON D2B time | −0.02 | .04 | −.49 | .621 | −.10 | .06 |
| D3B cort ON D3B time | −0.03 | .02 | −1.59 | .112 | −.06 | .01 |
Note: Gender: 0 (male), 1 (female); Minority: 0 (non-minority), 1 (minority). Averages of log-transformed cortisol values used for correlations, and simple difference score [PM-AM] for diurnal decline.
p <.05,
p <.01,
p <.001
Before conducting primary analyses, we examined the direct association between each predictor (i.e., involvement with CPS and income-to-needs ratio) and externalizing behavior. Indeed, CPS-referred children demonstrated higher levels of externalizing behavior (sum of raw scores across externalizing items) than low-risk comparison children, t(92) = 3.03, p = .003, 95%CI [1.95, 9.36]. CPS-referred children had an average Externalizing Behavior T score of 53.9 (SD = 11.55), whereas low-risk comparison children had an average Externalizing Behavior T score of 46.8 (SD = 10.46). On the CBCL, a T score above 70 is considered to fall in the clinical range (>98th percentile). Within the low-risk comparison group, one child (2.4%) scored in the clinical range. Within the CPS-referred group, six children (11.3%) scored in the clinical range. There was also a significant correlation between income-to-needs ratio and externalizing behavior, r = −.35, p = .001, with children with a higher income-to-needs ratio showing lower levels of externalizing behavior than children with a lower income-to-needs ratio. Given the association between involvement with CPS and income-to-needs ratio, we entered them simultaneously in primary analyses to examine each predictor’s unique effect taking into account the other.
Among CPS-referred children, there was no effect of parenting intervention group on externalizing behavior, t(51) = .13, p >.05. Consistent with what has been reported previously for the larger randomized clinical trial (reference removed for peer review), intervention group had an effect on average log-transformed wakeup cortisol, t(51) = 3.34, p <.01, and on the average wake-up to bedtime change in cortisol computed as a simple difference score, t(51) = 2.03, p <.05. Specifically, children who received the ABC intervention showed higher wake-up cortisol, and a steeper decline in cortisol across the day. Thus, participation in the ABC intervention was included as a covariate in analyses, along with gender and child age.
Primary Analyses
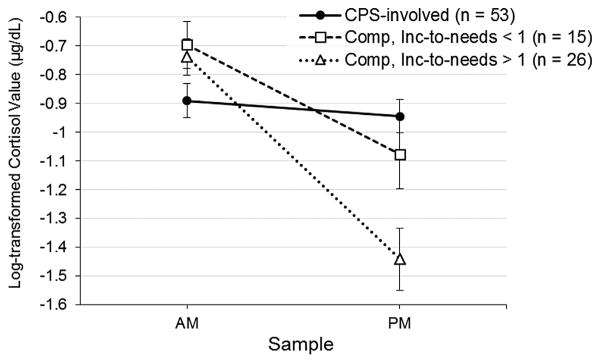
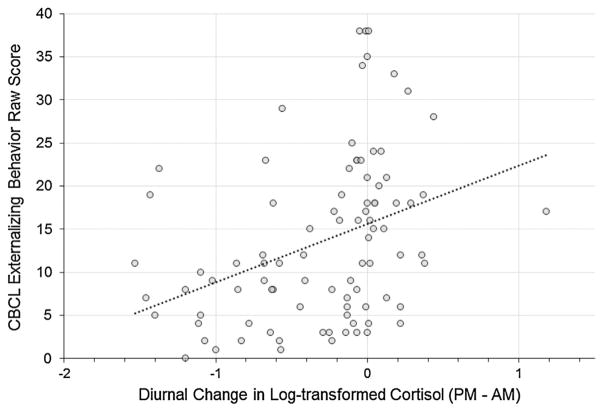
The model fit statistics demonstrated good fit to the data. The relative χ2 (i.e., ratio of χ2 to degrees of freedom) fell in the 2–1 range, which indicates acceptable fit, χ2/df = 1.20 (Byrne, 1989; Carmines & McIver, 1981). Similarly, the RMSEA = .05, CFI = .95, and TLI = .93 met criteria for good fit between the estimated model and observed data, based on respective cutoffs of ≤.08 (for RMSEA) and ≥.90 (for CFI and TLI) (Browne & Cudeck, 1993; Chen, Sousa, & West, 2005). Figure 1 shows the model with unstandardized coefficients and significance levels, with detailed information about estimated parameters presented in Table 4. Involvement with CPS (vs. lack of CPS involvement in low-risk comparison group) was associated with more blunted (i.e., less negative) cortisol slopes (b = .56, p <.001) (see Fig. 2). Income-to-needs ratio was inversely associated cortisol slopes (b = −.08, p = .002, such that children with lower income-to-needs ratios had more blunted cortisol slopes than children with higher income-to-needs ratios (see Fig. 2). Thus, each risk factor uniquely accounted for variance in cortisol slopes. Further, there was a positive association between cortisol slope and externalizing behavior (b = .91, p = .021), demonstrating that more blunted cortisol was associated with higher externalizing behavior; the positive coefficient indicated that as cortisol slopes became more blunted (i.e., less negative), externalizing behavior increased (see Fig. 3). Notably, the indirect effect of involvement with CPS on externalizing behavior through the diurnal cortisol slope was significant (b = .51, p = .013). The indirect effect of income-to-needs ratio on externalizing behavior through the diurnal cortisol slope was not significant (b = −.07, p = .064). When the cortisol slope was included, the direct effects of involvement with CPS and poverty on externalizing behavior were not statistically significant. Results for the indirect effects also held when we ran the model excluding one sibling from each of the four sibling pairs (for CPS involvement, b = .50, p = .035; for income-to-needs ratio, b = −.08, p = .063).
Table 4.
Model estimated Parameters for Full Model
| Effect | Unstandardized Estimate | SE | Est/SE | p | 95%CI
|
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Measurement model factor loadings | ||||||
| Wake-up cort | ||||||
| Day 1 | .91 | .05 | 17.76 | .000 | .81 | 1.01 |
| Day 2 | .99 | .05 | 20.09 | .000 | .90 | 1.09 |
| Day 3 | 1.00 | – | – | – | – | – |
| Bedtime cort | ||||||
| Day 1 | 1.00 | – | – | – | – | – |
| Day 2 | .98 | .04 | 25.23 | .000 | .90 | 1.05 |
| Day 3 | .90 | .04 | 23.10 | .000 | .83 | .98 |
| Path model: Direct effects | ||||||
| Cortisol slope ON | ||||||
| Involvement with CPS | .56 | .11 | 5.10 | .000 | .34 | .77 |
| Income-to-needs ratio | −.08 | .03 | −3.14 | .002 | −.13 | −.03 |
| Covariate: Child age | .12 | .08 | 1.58 | .114 | −.06 | .18 |
| Covariate: Intervention | −.11 | .11 | −1.01 | .314 | −.32 | .10 |
| Covariate: Female | −.01 | .07 | −.12 | .906 | −.14 | .10 |
| Cortisol intercept ON | ||||||
| Involvement with CPS | −.39 | .10 | −3.85 | .001 | −.58 | −.19 |
| Income-to-needs ratio | −.001 | .02 | −.06 | .952 | −.05 | .05 |
| Covariate: Child age | −.14 | .06 | −2.54 | .011 | −.25 | −.03 |
| Covariate: Intervention | .32 | .10 | 3.22 | .001 | .13 | .52 |
| Covariate: Female | −.02 | .06 | −.34 | .734 | −.14 | .10 |
| CBCL Externalizing ON | ||||||
| Cortisol slope | .91 | .39 | 2.31 | .021 | .14 | 1.68 |
| Involvement with CPS | −.33 | .32 | −1.01 | .312 | −.96 | .31 |
| Income-to-needs ratio | −.09 | .07 | −1.35 | .178 | −.21 | .04 |
| Covariate: Child age | −.32 | .14 | −2.30 | .021 | −.58 | −.05 |
| Covariate: Intervention | .15 | .24 | .62 | .534 | −.32 | .62 |
| Covariate: Female | −.13 | .15 | −.88 | .378 | −.42 | .16 |
| Path model: Indirect effects | ||||||
| CPS to Ext via Cort slope | .51 | .24 | 2.13 | .033 | .04 | .97 |
| Income to Ext via Cort slope | −.07 | .04 | −1.85 | .064 | −.15 | .004 |
FIGURE 2.
Diurnal cortisol slopes (plotted using average log-transformed wake-up [AM] cortisol values and average log-transformed bedtime [PM] cortisol values) for CPS-referred children and low-risk comparison children with no history of CPS involvement. Low-risk comparison children are further split into groups based on income-to-needs ratios less than 1 and greater than 1.
FIGURE 3.
Association between diurnal cortisol slopes (plotted using a simple difference score between average log-transformed wake-up [AM] cortisol values and average log-transformed bedtime [PM] cortisol values) and CBCL externalizing behavior scores. For diurnal change in cortisol, values less than 0 indicate a decline in cortisol across the day, whereas values greater than 0 indicate a rise in cortisol across the day.
We further explored the association between each risk indicator and wake-up and bedtime cortisol levels, given that blunted cortisol patterns could have been driven by low wake-up levels, high bedtime levels, or both. Bivariate correlations suggested that the income-to-needs ratio was specifically related to bedtime cortisol only, whereas involvement with CPS was related to both wake-up and bedtime cortisol (see Tab. 3). However, these analyses did not control for the influence of the other risk factor. Thus, the full structural model allowed us to examine each predictor’s association with the cortisol intercept, accounting for the other risk factor. Involvement with CPS was significantly associated with wake-up cortisol (b = −.39, p = .001), whereas income-to-needs ratio was not (b = −.001, p = .95). We reran the model after recentering the cortisol intercept to represent the bedtime cortisol level. Income-to-needs ratio was significantly association with bedtime cortisol (b = −.08, p = .003), whereas involvement with CPS was not (b = .18, p = .12). Thus, although both risk predictors were associated with blunted diurnal cortisol patterns, it appears that they may have unique influences on wakeup versus bedtime samples.
There was significant variability in morning sampling time, which could have interfered with accurately capturing true morning cortisol levels (if sampling time reflected noncompliance, rather than a true late awakening). Our full model controlled for this variability by including sampling time as a covariate. However, we also wanted to address this potential issue of late morning sampling more directly. First, we conducted bivariate correlations between morning sample collection time and cortisol levels. Sampling time was not significantly correlated with morning cortisol levels on Day 1 (r = −.14, p >.05), Day 2 (r = −.12, p >.05), or Day 3 (r = −.11, p >.05). Second, we examined whether the findings from the full model held if samples collected more than 1 SD after the average morning sampling time were excluded from analyses. We excluded samples collected after 9:05 a.m. on Day 1 (n = 17), 9:17 a.m. on Day 2 (n = 18), and 9:12 a.m. on Day 3 (n = 16). Results for the indirect effects of risk factor on externalizing behavior through diurnal cortisol slope were similar when excluding these late morning samples (for involvement with CPS, b = .45, p = .052; for income-to-needs ratio, b = −.07, p = .084).
DISCUSSION
We found that involvement with CPS had an indirect effect on externalizing behavior through blunted diurnal cortisol (i.e., a less steep cortisol decline across the day). Although poverty (i.e., income-to-needs ratio) was also associated with blunted cortisol rhythms, the indirect effect of poverty on externalizing behavior was nonsignificant. Our findings provide further support for neurobiological models of antisocial behavior (Susman, 2006; van Goozen, Fairchild, Snoek, & Harold, 2007), by identifying cortisol dysregulation as a potential mechanism linking early adversity to externalizing behavior in early childhood.
The association between the early risk indicators studied here (i.e., history of involvement with CPS and poverty) and blunted cortisol may be a result of exposure to chronic stress or cumulative risk more broadly (Dowd, Simanek, & Aiello, 2009). In studies of cumulative risk, dichotomized risk indicators (e.g., composite of risk indicators such as maternal depression, single parent status, low parent education) are added together to create one score, which is often found to be a stronger predictor of problematic outcomes than any particular risk factor alone (Sameroff, Seifer, Zax, & Barocas, 1987; Slopen, Koenen, & Kubzansky, 2014). In line with a cumulative risk model, Zalewski, Lengua, Kiff et al. (2012) found trend-level evidence of an indirect effect of low income predicting low morning cortisol through cumulative risk exposure in early childhood. These findings of disrupted HPA axis functioning as a result of cumulative risk may reflect “allostatic load,” which refers to the physiological toll resulting from an overload of stressful experiences (McEwen, 2004). Poor parenting quality may be another mechanism by which poverty or involvement with CPS is linked to cortisol dysregulation. In early childhood, low maternal sensitivity (e.g., low warmth, lack of responsiveness, intrusiveness, negativity) has been found to predict low morning cortisol and blunted diurnal patterns (Pendry & Adam, 2007; Roisman et al., 2009; Zalewski, Lengua, Kiff et al., 2012). In future research exploring links between early adversity and cortisol dysregulation, it will be useful to examine exposure to chronic stress (i.e., cumulative risk) as well as adverse caregiving as possible mechanisms.
The present study included a subset of children from a randomized clinical trial of a parenting intervention that aimed to enhance sensitivity among CPS-involved families. Although the parenting intervention demonstrated positive effects on cortisol regulation (i.e., higher wake-up cortisol and steeper decline) compared to a control condition, the parenting intervention did not influence children’s externalizing behavior. The reason for this null finding is unclear. In future studies, it will be important to examine whether the ABC intervention demonstrates effects on children’s externalizing behavior using the full sample, at other time-points (e.g., school-age assessments), and using other measures (e.g., observational assessments). If there are indeed effects of ABC on externalizing behavior, it will be important to examine whether changes to specific parenting behaviors (e.g., nurturance, following the lead) mediate any associations between ABC and externalizing behavior, and whether other factors moderate the effectiveness of ABC on reducing externalizing behavior. Such analyses, whether significant or nonsignificant, may further inform our understanding of the pathways examined in the present study.
There are multiple possibilities about why blunted cortisol may be associated with externalizing behavior. Susman (2006) described a psychobiological model of persistent antisocial behavior, proposing that stress in early development leads to an attenuation of physiological reactivity resulting in low basal cortisol levels, in addition to disruptions to other neurobiological systems. Due to low levels of physiological arousal, children with low cortisol may seek out stimulation and may be relatively unresponsive to the negative consequences of acting out (Alink et al., 2008; Gunnar & Vazquez, 2001; Heim, Ehlert, & Hellhammer, 2000; Shirtcliff, Granger, Booth, & Johnson, 2005; Zuckerman, 1979). Additionally, cortisol dysregulation may negatively impact developing brain regions that are critical in self-regulation and executive functioning. Zalewski, Lengua, Fisher et al. (2012) found that low cortisol was associated with lower effortful control (i.e., the ability to inhibit a dominant response). Effortful control engages the prefrontal cortex, an area of the brain known to be affected by early life stress through chronic activation of the HPA axis (Teicher et al., 2003; van Harmelen et al., 2010). It is important to keep in mind that there are likely bidirectional associations between biology and behavior, such that cortisol dysregulation and behavioral dysregulation mutually influence each other across development. For example, children’s behavior problems in early childhood have been found to elicit more insensitive parenting (Wang, Christ, Mills-Koonce, Garrett-Peterss, & Cox, 2013). Thus, children with externalizing behavior problems may provoke responses from their environments (e.g., parents, teachers, peers) that further interfere with physiological regulation.
Several limitations should be noted when interpreting the results of our study. First, cortisol measurements and externalizing behavior were measured concurrently. A better test of mediation would involve measuring cortisol dysregulation prior to the outcome assessment of problem behavior. Even better, a longitudinal design with cortisol and behavior measured across multiple time points would further allow for examining the direction of effects, such as whether changes to biology proceed changes to behavior or vice versa. We expect that biological regulation and behavioral control develop together over time, and are further shaped by environmental factors (e.g., stress, parenting). Second, we had limited information about the reasons that children in the CPS-referred group were identified by child protective services. Given that we lacked detailed histories, we were unable to examine more nuanced questions about the associations between specific early adverse experiences (e.g., type of maltreatment, substance exposure) and the measured outcomes. Additionally, we relied on parent-report to confirm lack of involvement with CPS for the comparison sample; we did not have objective information (e.g., check of CPS records) to ensure the accuracy of their report. Third, we relied on a parent-report measure of externalizing behavior, which is potentially problematic given that parents from clinical samples (e.g., abusive single-parent families) may be more biased in their reporting on child behavior problems than low-risk parents (Glaser, Kronsnoble, & Forkner, 1997). Future studies could benefit from the inclusion of observational measures of disruptive behavior as well as teacher reports; additionally, examining these associations in a clinical population (i.e., children referred for behavior problems) would further extend the conclusions we can draw. Additionally, externalizing behavior was relatively low in general in our sample. It is possible that effects would be stronger in a clinical sample of children. Regardless, our findings suggest that even variability in externalizing behavior within the typical range relates to risk factors and dysregulation HPA axis functioning in expected ways.
Finally, there were several limitations with regard to the procedures for salivary sampling that should be noted. First, we only included parent-reported sampling time as a covariate for cortisol values in analyses. Thus, we did not account for variability in actual wake time (which could have been best assessed objectively via actigraphy) nor in the latency from waking to sample collection. Both wake-up time and latency to sampling have been shown to influence cortisol levels (Dockray, Bhattacharyya, Molloy, & Steptoe, 2008; Edwards, Evans, Hucklebridge, & Chow, 2001). Second, we did not use electronic monitoring devices of parent compliance in order to ensure that atypical cortisol levels were not an artifact of poor compliance (Kudielka, Broderick, & Kirschbaum, 2003). Although we used multiple aids to help with parents follow the procedures (e.g., instructions with photos, diary with questions and reminders about illness and eating/drinking), we did not control for a number of potential variables that can influence cortisol levels, such as child care attendance on sampling days, medication use, or quality of sleep.
Despite these limitations, our study adds to the literature regarding the effects of early adversity on externalizing behavior through physiological dysregulation. Given that childhood adversity is associated with long-term mental and physical health problems (Heim, Shugard, Craighead, & Nemeroff, 2010), we consider the identification of early risk indicators, such as cortisol dysregulation, to be a critical step for the field. Identifying associations between physiological and behavioral dysregulation in early childhood may help to appropriately target prevention and intervention efforts for high-risk children.
References
- Achenbach TM, Rescorla L. Manual for the ASEBA school-age forms & profiles: An integrated system of multi-informant assessment. Burlington, VT: ASEBA; 2000. [Google Scholar]
- Ackerman BP, Brown ED, Izard CE. The relations between persistent poverty and contextual risk and children’s behavior in elementary school. Developmental Psychology. 2004;40:367–377. doi: 10.1037/0012-1649.40.3.367. [DOI] [PubMed] [Google Scholar]
- Alink LR, Van IJzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in children and adolescents: Mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Developmental Psychobiology. 2008;50:427–450. doi: 10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- Baer JC, Martinez CD. Child maltreatment and insecure attachment: A meta-analysis. Journal of Reproductive and Infant Psychology. 2006;24:187–197. doi: 10.1080/02646830600821231. [DOI] [Google Scholar]
- Bernard K, Butzin-Dozier Z, Rittenhouse J, Dozier M. Cortisol production patterns in young children living with birth parents vs children placed in foster care following involvement of child protective services. Archives of Pediatrics and Adolescent Medicine. 2010;164:438–443. doi: 10.1001/archpediatrics.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SL, Heitkemper MM. Morningness-eveningness and early-morning salivary cortisol levels. Biological Psychiatry. 1991;32:181–192. doi: 10.1016/j.psychres.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Blair C, Berry D, Mills-Koonce R, Granger D. Cumulative effects of early poverty on cortisol in young children: Moderation by autonomic nervous system activity. Psychoneuroendocrinology. 2013;38:2666–2675. doi: 10.1037/t07209-000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KS, Long JS, editors. Testing structural equation models. Beverly Hills, CA: Sage Publications; 1993. pp. 136–162. [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Development and Psychopathology. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J, Gunnar MR, Pears KC, Fisher PA. Early adverse care, stress neurobiology, and prevention science: Lessons learned. Prevention Science. 2013;14:247–256. doi: 10.1007/s11121-012-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne BM. A primer of LISREL: Basic applications and programming for confirmatory factor analytic models. New York: Springer-Verlag; 1989. [Google Scholar]
- Campbell KA, Thomas AM, Cook LJ, Keenan HT. Longitudinal experiences of children remaining at home after a first-time investigation for suspected maltreatment. The Journal of Pediatrics. 2012;161:340–347. doi: 10.1016/j.jpeds.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Dragomir C, Earls F, Farrell M, Macovei O, Nystrom P, Sparling J. Cortisol regulation in home-reared and institutionalized Romanian children. Society of Neuroscience Abstracts. 1995;218:12. [Google Scholar]
- Carmines EG, McIver JP. Analyzing models with unobserved variables. In: Bohrnstedt GW, Borgatta EF, editors. Social measurement: Current issues. Beverly Hills: Sage Publications; 1981. pp. 65–115. [Google Scholar]
- Chen FF, Sousa KH, West SG. Teacher’s corner: Testing measurement invariance of second-order factor models. Structural Equation Modeling. 2005;12:471–492. doi: 10.1207/s15328007sem1203_7. [DOI] [Google Scholar]
- Cicchetti D, Toth SL. Child maltreatment. Annual Review of Clinical Psychology. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- Dockray S, Bhattacharyya MR, Molloy GJ, Steptoe A. The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology. 2008;33:77–82. doi: 10.1016/j.psyneuen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Simanek AM, Aiello AE. Socioeconomic status, cortisol, and allostatic load: A review of the literature. International Journal of Epidemiology. 2009:1–13. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M the Infant Caregiver Project. Unpublished manuscript. University of Delaware, Department of Psychology; 2006. Attachment and biobehavioral catch-up intervention. [Google Scholar]
- Drake B, Jonson-Reid M, Way I, Chung S. Substantiation and recidivism. Child Maltreatment. 2003;8:248–260. doi: 10.1177/1077559503258930. [DOI] [PubMed] [Google Scholar]
- Edwards S, Evans P, Hucklebridge F, Chow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology. 2001;26:613–622. doi: 10.1016/S0306-4530(01)00015-4. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer D. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Glaser BA, Kronsnoble KM, Forkner CBW. Parents and teachers as raters of children’s problem behaviors. Child & Family Behavior Therapy. 1997;19:1–13. doi: 10.1300/J019v19n04_01. [DOI] [Google Scholar]
- Godinet MT, Li F, Berg T. Early childhood maltreatment and trajectories of behavioral problems: Exploring gender and racial differences. Child Abuse & Neglect. 2013;38:544–556. doi: 10.1016/j.chiabu.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Peloso E, Auker A, Dozier M. Effect of flavored beverage crystals on salivary cortisol enzyme-immunoreactive assay measurements. Developmental Psychobiology. 2005;47:189–195. doi: 10.1002/dev.20081. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/S0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;12:515–538. doi: 10.1017/S0954579401003066. [DOI] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Developmental Psychobiology. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hofer M. Hidden regulators in attachment, separation and loss. Monographs of the Society for Research in Child Development. 1994;59:192–207. doi: 10.1111/j.1540-5834.1994.tb01285.x. [DOI] [PubMed] [Google Scholar]
- Hofer M. Psychobiological roots of early attachment. Current Directions in Psychological Science. 2006;15:84–88. [Google Scholar]
- Hussey JM, Marshall JM, English DJ, Knight ED, Lau AS, Dubowitz H, Kotch J. Defining maltreatment according to substantiation: Distinction without a difference? Child Abuse and Neglect. 2005;29:479–492. doi: 10.1016/j.chiabu.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Gunnar MR, Madsen NJ, Long JD. Early deprivation and home basal cortisol levels: A study of internationally adopted children. Development and Psychopathology. 2008;20:473–491. doi: 10.1017/S0954579408000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosomatic Medicine. 2003;65:313–319. doi: 10.1097/01.PSY0000058374.50240.BF. [DOI] [PubMed] [Google Scholar]
- Kohl PL, Jonson-Reid M, Drake B. Time to leave substantiation behind: Findings from a national probability study. Child Maltreatment. 2009;14:17–26. doi: 10.1177/1077559508326030. [DOI] [PubMed] [Google Scholar]
- Kotch JB, Lewis T, Hussey JM, English D, Thompson R, Litrownik AJ, … Dubowitz H. Importance of early neglect for childhood aggression. Pediatrics. 2008;121:725–731. doi: 10.1542/peds.2006-3622. [DOI] [PubMed] [Google Scholar]
- Manly J, Kim J, Rogosch F, Cicchetti D. Dimensions of child maltreatment and children’s adjustment: Contributions of developmental timing and subtype. Development and Psychopathology. 2001;13:759–782. [PubMed] [Google Scholar]
- Martin CG, Kim HK, Bruce J, Fisher PA. Child diurnal cortisol rhythms, parenting quality, and externalizing behaviors in preadolescence. Psychoneuroendocrinology. 2014;40:170–180. doi: 10.1016/j.psyneuen.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F. Linear dynamic analyses of incomplete longitudinal data. In: Collins LM, Sayer A, editors. New methods for the analysis of change. Washington, DC: American Psychological Association; 2001. pp. 137–176. [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mills R, Scott J, Alati R, O’Callaghan M, Najman JM, Strathearn L. Child maltreatment and adolescent mental health problems in a large birth cohort. Child Abuse & Neglect. 2013;37:292–302. doi: 10.1016/j.chiabu.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss E, Dubois-Comtois K, Cyr C, Tarabulsy GM, St-Laurent D, Bernier A. Efficacy of a home-visiting intervention aimed at improving maternal sensitivity, child attachment, and behavioral outcomes for maltreated children: A randomized clinical trial. Developmental Psychopathology. 2011;23:195–210. doi: 10.1017/S0954579410000738. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus user’s guide. 5. Los Angeles, CA: Muthen & Muthen; 1998. [Google Scholar]
- Pendry P, Adam EK. Associations between parents’ marital functioning, maternal parenting quality, maternal emotion, and child cortisol levels. International Journal of Behavioral Development. 2007;31:218–231. doi: 10.1177/0165025407074634. [DOI] [Google Scholar]
- Plybon LE, Kliewer W. Neighborhood types and externalizing behavior in urban school-age children: Tests of direct, mediated, and moderated effects. Journal of Child and Family Studies. 2001;10:419–437. doi: 10.1023/A:1016781611114. [DOI] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. doi: 10.1037//0033-2909.128.2.230. [DOI] [PubMed] [Google Scholar]
- Roisman GI, Susmann E, Barnett-Walker K, Booth-LaForce C, Owen MT, Belsky J … NICHD Early Child Care Research Network. Early family and child-care antecedents of awakening cortisol levels in adolescence. Child Development. 2009;80:907–920. doi: 10.1111/j.1467-8624.2009.01305.x. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ, Seifer R, Zax M, Barocas R. Early indicators of developmental risk: The Rochester Longitudinal Study. Schizophrenia Bulletin. 1987;13:383–393. doi: 10.1093/schbul/13.3.383. [DOI] [PubMed] [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Helhammer DH, Frederenko I, Rohleder N, Schürmeyer TH, Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Science. 1999;64:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17:167–184. doi: 10.1017/S0954579405050091. [DOI] [PubMed] [Google Scholar]
- Slopen N, Fitzmaurice G, Williams DR, Gilman SE. Poverty, food insecurity, and the behavior for childhood internalizing and externalizing disorders. Journal of the American Academy of Child Psychiatry. 2010;49:444–452. doi: 10.1097/00004583-201005000-00005. [DOI] [PubMed] [Google Scholar]
- Slopen N, Koenen KC, Kubzansky LD. Cumulative adversity in childhood and emergent risk factors for long-term health. Journal of Pediatrics. 2014;164:631–638. doi: 10.1016/j.jpeds.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Talge NM, Donzella B, Kryzer EM, Gierens A, Gunnar MR. It’s not that bad: Error introduced by oral stimulants in salivary cortisol. Developmental Psychobiology. 2005;47:369–376. doi: 10.1002/dev.20097. doi: org.proxy.nss.udel.edu/10.1002/dev.20097. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Andersen CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioral Reviews. 2003;27:33–44. doi: 10.1016/S0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Teisl M, Cicchetti D. Physical abuse, cognitive and emotional processes, and aggressive/disruptive behavior problems. Social Development. 2008;17:1–23. doi: 10.1111/j.1467-9507.2007.00412.x. [DOI] [Google Scholar]
- Tremblay RE, Nagin DS, Sequin JR, Zoccolillo M, Zelazo PD, Pérusse D, … Japel C. Physical aggression during early childhood: Trajectories and predictors. Pediatrics. 2004;114:43–50. doi: 10.1542/peds.114.1.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentacosta CJ, Hyde LW, Shaw DS, Dishion TJ, Gardner F, Wilson M. The relations among cumulative risk, parenting, and behavior problems during early childhood. Journal of Child Psychology and Psychiatry. 2008;49:1211–1219. doi: 10.1111/j.1469-7610.2008.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Goozen SHM, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133:149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- van Harmelen AL, van Tol MJ, van der Wee NJA, Veltman DJ, Aleman A, Spinhoven P, … Elzinga BM. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biological Psychiatry. 2010;68:832–838. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Wang F, Christ SL, Mills-Koonce WR, Garrett-Peters P, Cox MJ. Association between maternal sensitivity and externalizing behavior from preschool to preadolescence. Journal of Applied Developmental Psychology. 2013;34:89–100. doi: 10.1016/j.appdev.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: Relations with napping and effortful control. Developmental Psychobiology. 2004;45:125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- Winberg J. Mother and newborn baby: Mutual regulation of physiology and behavior— A selective review. Developmental Psychobiology. 2005;47:217–229. doi: 10.1002/dev.20094. [DOI] [PubMed] [Google Scholar]
- Woodruff K, Lee B. Identifying and predicting problem behavior trajectories among pre-school children investigated for child abuse and neglect. Child Abuse & Neglect. 2011;35:491–503. doi: 10.1016/j.chiabu.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Zalewski M, Lengua LJ, Fisher PA, Trancik A, Bush NR, Meltzoff AN. Poverty and single parenting: Relations with preschoolers’ cortisol and effortful control. Infant and Child Development. 2012;21:537–554. doi: 10.1002/icd.1759. [DOI] [Google Scholar]
- Zalewski M, Lengua L, Kiff C, Fisher P. Understanding the relation of low income to HPA-axis functioning in preschool children: Cumulative family risk and parenting as pathways to disruptions in cortisol. Child Psychiatry Hum Dev. 2012;43:924–942. doi: 10.1007/s10578-012-0304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking: Beyond the optimal level of arousal. Hillsdale, NJ: Erlbaum Associates; 1979. [Google Scholar]