Abstract
We report herein a process safety assessment of the iron-catalyzed direct olefin diazidation for the preparation of a broad range of synthetically valuable vicinal primary diamines. Differential scanning calorimetry analysis of the corresponding reagents, intermediates, and a list of representative diazide/diaminium salt products revealed that all of them are thermal stable at the reaction temperature. The drop weight test of the diazides suggested that they are moderately impact-sensitive. Guided by this assessment, an optimized olefin diazidation/diamination procedure has been developed which allows for the gram-scale diaminium salt synthesis without purification of the diazide intermediate.
Vicinal primary diamine moieties are present in numerous small-molecule pharmaceuticals and functional materials; therefore, extensive research efforts have been devoted to develop a general and selective olefin diamination method.1 Although significant progress has been achieved, it still remains challenging to directly convert unfunctionalized olefins, especially internal olefins, to vicinal primary diamines. Likewise, stereochemical control for diamination of unfunctionalized olefins has been difficult.1h,2 As an alternative approach, catalytic olefin diazidation has emerged with unique value because it provides a convenient approach to producing synthetically important vicinal primary diamines that are difficult to obtain with the existing olefin diamination methods. A variety of olefin diazidation methods have been reported; however, the existing chemical diazidation methods are predominantly tailored for certain limited types of olefins.3 Recently emerged electrochemical methods involve the usage of superstoichiometric amounts of NaN3 under acidic conditions, which render large-scale processes less desirable.4
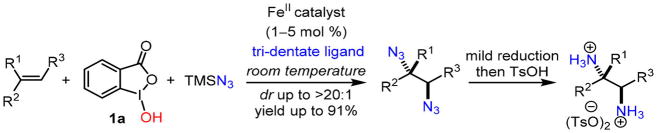
In 2015, we reported an iron-catalyzed direct diazidation method for a broad range of olefins, in which an iron catalyst readily activates stable TMSN3 in the presence of bench-stable benziodoxole 1a to achieve diastereoselective olefin diazidation (Scheme 1).5 This heterogeneous reaction occurs at room temperature with low catalyst loading, and it tolerates a wide variety of both unfunctionalized and highly functionalized olefins, including those that are incompatible with existing methods (Figure 1). Notably, the anti-selectivity for cyclic olefins can be modulated by iron catalysts (dr up to >20:1). Coupled with facile reduction, this method readily provides a wide variety of valuable vicinal primary diamines.
Scheme 1.
Iron-Catalyzed Direct Olefin Diazidation for Vicinal Primary Diamine Synthesis
Figure 1.
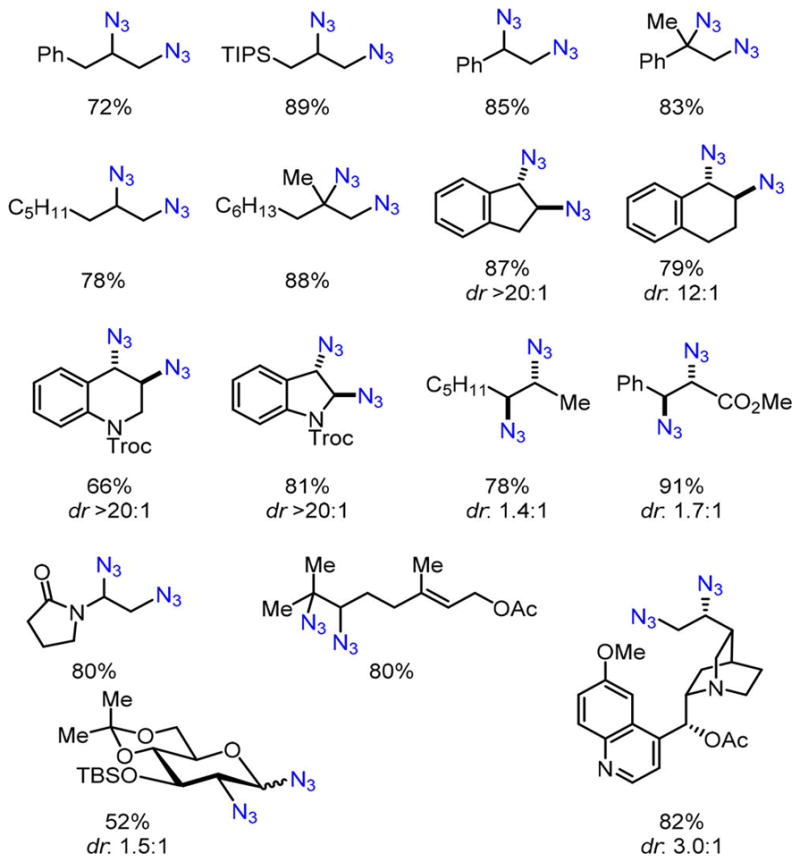
Representative vicinal diazides obtained from the iron-catalyzed olefin diazidation.
Organic azides, especially those with lower molecular weights, may present potential safety concerns for their handling, with regard to their thermo- and mechanical impact stabilities.6 To explore the feasibility of the iron-catalyzed olefin diazidation on an industrial scale and to develop a general and practical synthetic strategy to produce vicinal primary diamines, it is imperative to carry out chemical hazard assessment of the olefin diazidation process. A reactive chemical hazard assessment refers the identification and possibly quantification of dangerous energy release scenarios for a chemical process of interest. Differential scanning calorimetry (DSC) is one of the most commonly applied thermal stability testing methods for organic compounds,7 while the drop weight test (DWT) has been routinely applied to detect the sensitivity of a chemical toward mechanical impact. Herein, we describe our chemical safety studies and potential hazard assessment of the iron-catalyzed direct olefin diazidation, using both DSC and DWT analysis.
We select the Fe(OAc)2-catalyzed diastereoselective indene diazidation as a model reaction for safety analysis (Scheme 2). In this process, a readily available Fe(OAc)2–tridentate ligand L1 complex (5 mol %) activates stable TMSN3 (2.4 equiv) in the presence of bench-stable benziodoxole 1a (1.2 equiv), which rapidly converts indene (2) to trans-indene diazide 3 at room temperature in good yield and dr (86% yield, dr: 12:1). The crude reaction mixture can be readily reduced (PPh3) and protonated (with TsOH) to afford the trans-indene diaminium salt 4 in excellent yield (92% yield).
Scheme 2.
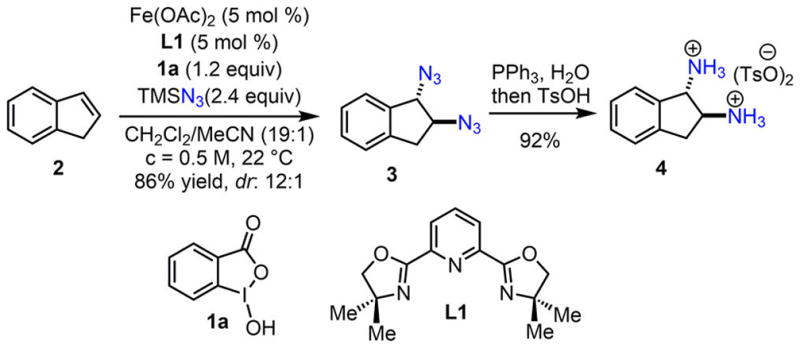
Iron-Catalyzed Procedure for Expedient Synthesis of trans-Indene Diaminium Salt
Our mechanistic studies have revealed a mechanistic working hypothesis that best corroborates the experimental data (Scheme 3). In the absence of an iron catalyst, benziodoxole 1a is stable toward TMSN3 at room temperature, and the vast majority of 1a is recovered after 24 h. Furthermore, only 1a can be recovered, and there is no accumulation of 1b when a reaction is quenched before it reaches full conversion. Therefore, we propose that benziodoxole 1a can be reversibly converted to azidoiodinane 1b.8 Since the solubilities of both 1a and 1b are less than 10 μM in the mixed solvent applied (CH2Cl2/MeCN [20:1]), the concentration of 1b should be less than 10 μM through the catalytic cycle, and most of the iodine(III) oxidants exist as 1a in its solid-state form. A second equivalent of TMSN3 may subsequently convert 1b to a transient iodine(III)–diazide species A. In the presence of an iron catalyst, it may reductively cleave the I–N3 bond of A, which presumably generates a high-valent iron species and an azido-radical. Reversible azido-radical addition to an olefin should afford a carbo-radical species B that is associated with the iron catalyst. It is likely that the high-valent iron species facilites the rate-determining oxidation of B through inner-sphere azido ligand transfer to afford the diazide.9 The major side product of this process is a silyl benzoate 5.
Scheme 3.
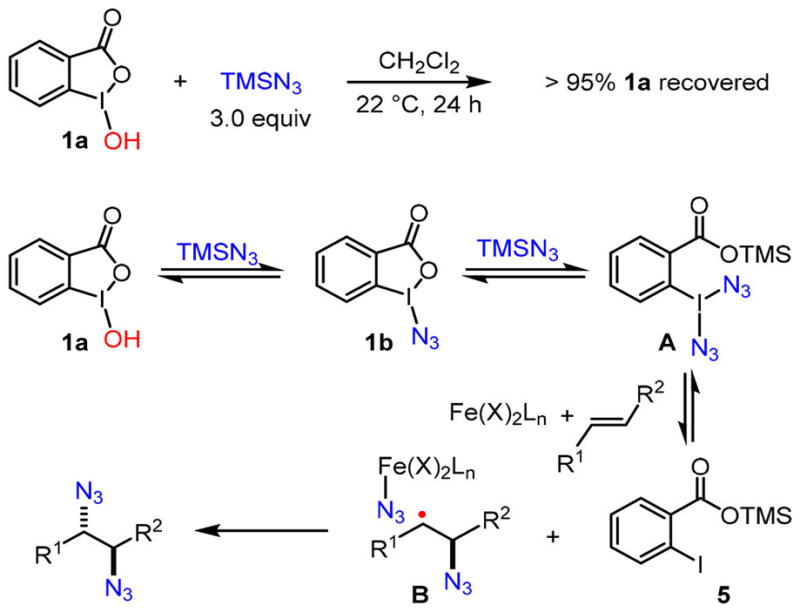
Mechanistic Analysis of the Iron-Catalyzed Olefin Diazidation
The process safety studies for the iron-catalyzed olefin diazidation were carried out with two major goals: (a) to design a safe and scalable process to support downstream route development and (b) to develop a safe and robust process to support long-term project needs. As our first step to explore the feasibility of applying this reaction on an industrial scale, we evaluated the thermal stability of all of the reagents and intermediates that are in sufficient concentrations under the reaction conditions using differential scanning calorimetry (DSC) (Figure 2).
Figure 2.
Differential scanning calorimetry (DSC) analysis of the reagents, products, and intermediates involved in the iron-catalyzed olefin diazidation/diamination.
We observed that both Fe(OAc)2 and tridentate ligand L1 are thermally stable and no exothermic event occurs up to 300 °C. TMSN3 starts to evaporate around 40 °C without triggering any decomposition. Notably, benziodoxole 1a, the stoichiometric oxidant applied in olefin diazidation, is also thermally stable: it starts to melt at 175 °C and does not decompose until 245 °C (Figure 2a). On the contrary, azidoiodinane 1b, a commonly used azido-group transfer reagent,8 appears to be much less stable: DSC analysis revealed its strong decomposition starting at 126 °C: this highly exothermic event is characteristic of a violent decomposition (ΔH = −417 kJ/mol) (Figure 2b). Our mechanistic studies revealed that most of the iodine(III) oxidants exist as 1a under the reaction condition and that 1b does not accumulate through the catalytic cycle; therefore, the thermal stability character of 1b is less of concern under the iron-catalyzed olefin diazidation condition.
We subsequently evaluated the thermal stabilities of both diazides and diaminium salts. Although indene diazide 3 decomposes with a high energy (ΔH = −239 kJ/mol), it does not occur until 156 °C, which allows for a convenient operating margin in carrying out the diazidation at room temperature (Figure 2c). To our pleasure, indene diaminium salt 4 is much more stable: neither decomposition nor exothermic event was observed up to 300 °C (Figure 2d).
Based upon these experiments, we further evaluated a range of representative diazides, including 2-azido-glycosyl azide 6, acetyl quinine diazide 7, N-Troc indole diazide 8, and geranyl acetate diazide 9 (Figure 3). All of them decompose at high temperatures, none of which occurs below 120 °C, at least 100 °C higher than the diazidation reaction temperature. Furthermore, their decomposition energy profiles are similar. Notably, all of them are liquid except for N-Troc indole diazide 8, which melts around 56 °C and decomposes around 140 °C.
Figure 3.
Functionalized vicinal diazides analyzed by DSC.
The observed thermal stabilities of the reagents, intermediates, and products in the iron-catalyzed olefin diazidation prompt us to further evaluate the mechanic impact sensitivities of these chemicals. The determination of the sensitivity to impact stimuli is one of the most important characteristics of energetic materials, and the fall hammer test (drop weight test) is designed to determine the sensitivity of potentially high explosive compounds. In this study, all samples were analyzed by dropping a 5 kg weight from the height of 0.8 m. Interestingly, although azidoiodinane 1b originates a strong detonation at the DWT, benziodoxole 1a, Fe(OAc)2, L1, and TMSN3 uniformly demonstrate negative results in the test. Moreover, all of the aforementioned diazides are moderately sensitive to the DTW: colorless liquid and foams turned dark black after the DWT. On the contrary, diaminium salt 4 is nonsensitive to DTW.
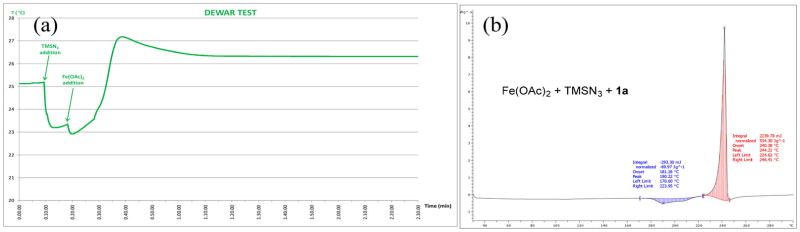
Since the Fe(OAc)2 catalyst may be converted to the iron-azide species in situ under the reaction condition and it may present a safety concern, we further investigated thermal stability of the reaction mixture. As a reasonable approximation to address the safety issues of putative iron-azide species, we studied thermodynamics of a blank reaction in the absence of olefins and ligands (Figure 4a). This control reaction was carried out in a quasi-adiabatic calorimeter glass-Dewar, which mimics the concentration of an iron-catalyzed diazidation reaction.
Figure 4.
(a) Dewar test of a “blank reaction”; (b) DSC analysis of the mixture from a runaway reaction in the absence of substrates and ligands.
We observed only a slight exothermic process (exp ΔTad = 1.9 °C; corrected by reagent temperature and Phi factor = 4.1 °C) when TMSN3 (7.27 g; 8.4 mL; 63.1 mmol; 4.0 equiv; 8 °C) was added in one-shot to the suspension of benziodoxole 1a (5.0 g; 18.9 mmol; 1.2 equiv) in anhydrous CH2Cl2 (24 mL, 25 °C). Subsequently, an Fe(OAc)2 catalyst suspension (137 mg; 0.79 mmol; 5 mol %) in anhydrous CH2Cl2/MeCN (6.4/ 1.6 mL) was added to the reaction mixture at once and the colorless suspension turned dark and a slight increase of temperature was recorded (exp ΔTad = 4.2 °C; corrected by reagent temperature and Phi factor = 4.0 °C). These experiments suggest that a risk of high-energy release in a runaway reaction is small (Figure 4a).
A sample of the blank-reaction mixture was concentrated in in vacuo under a nitrogen stream and subsequently submitted to DSC analysis (Figure 4b). The profile is very similar compared to the one of benziodoxole 1a, except that the onset temperate for decomposition is 220 °C (instead of 245 °C). This result corroborates that most of the iodine(III) oxidants exist as 1a under the reaction condition and that the amount of 1b is minimal. These experiments suggest that a blank reaction and any putative iron-azide species generated in situ are thermally stable.
Guided by these process safety studies of diazides and diaminium salts, we improved our original iron-catalyzed olefin diazidation workup procedure, such that purification of diazide intermediates can be excluded and the diaminium salts can be directly isolated through recrystallization. We selected a gram-scale indene diazidation–reduction sequence as a proof of principle for the safe large-scale procedure of the expedient synthesis of vicinal primary diamines from olefins (Scheme 4).
Scheme 4.
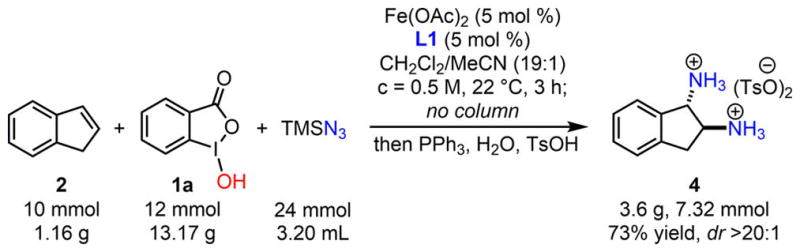
Iron-Catalyzed Gram-Scale Indene Diazidation/ Diamination Sequence that Excludes Chromatography Purification
In summary, we have carried out the process safety assessment of the iron-catalyzed olefin diazidation process through DSC and DWT of the corresponding reagents, intermediates, and representative diazide/diaminium salt products. These experiments suggest that all of the diazides investigated are sufficiently thermal stable at the reaction temperature; however, they are moderately impact-sensitive. DSC analysis of a blank reaction suggests that all of the reagents and reactive intermediates (with sufficient concentration) are thermally and impact-stable. It also corroborates that impact-sensitive azidoiodinane 1b is reversibly generated from 1a, and it does not build up under the reaction conditions. Guided by these process safety studies, we optimized the original iron-catalyzed olefin diazidation/diamination procedure, which allows for the gram-scale diastereoselective synthesis of indene diaminium salt without purification of the diazide intermediate.
EXPERIMENTAL SECTION
Safety Considerations
Standard precautions with regard to handling TMSN3 should be taken during reaction workup.
Differential Scanning Calorimetry (DSC)
The DSC measurements were performed in a Mettler 821e using 40 μL aluminum punctured crucibles under nitrogen atmosphere or 60 μL high pressure (gold plated) steel crucibles under air atmosphere. All measurements were carried out at a heating rate of 5 K/min.
Mechanical Sensitivity Test
The fall hammer test (drop hammer) was designed to determine the sensitivity of potentially high explosive compounds, and it was carried out by dropping a 5 kg weight from the height of 0.8 m.
A Procedure for the Iron-Catalyzed Indene Diazidation and Diamination
Fe(OAc)2 (86.5 mg, 0.5 mmol, 5 mol %), L1 (136.5 mg, 0.5 mmol, 5 mol %), and benziodoxole 1a (3.17 g, 12.0 mmol, 1.2 equiv) were mixed and suspended in anhydrous CH2Cl2 (19 mL) and MeCN (1.0 mL) under N2 at room temperature for 10 min before distilled indene 2 (1.16 mL, 10 mmol, 1.0 equiv) and freshly opened TMSN3 (3.20 mL, 24 mmol, 2.4 equiv) were subsequently added to the suspension. The mixture was stirred at room temperature for 3 h until 2 was fully consumed (monitored by TLC) and then quenched with saturated Na2CO3 solution (50 mL). The mixture was diluted with heptane (60 mL) and vigorously stirred for 10 min. The organic phase was separated from the aqueous phase, and the aqueous phase was further extracted with CH2Cl2 (30.0 mL); the combined organic phase was filtered through a short silica gel pad (diameter: 3.3 cm; length: 3.0 cm) and further eluted with a mixture of heptane/Et2O: 95:5 (30 mL). The resulting organic filtrate was transferred to a flask, and the mixed solvents were exchanged with THF (60 mL) in vacuo. TsOH·H2O (4.3 g, 22.5 mmol, 2.5 equiv) and H2O (0.81 mL, 45 mmol, 5 equiv) were subsequently added. The flask was cooled down to 0 °C under N2, and PPh3 (5.9 g, 22.5 mmol, 2.5 equiv) in THF (40 mL) was added dropwise. The reaction mixture was warmed up to room temperature and kept for stirring for additional 3.5 h. During this time, indene diaminium tosylate 4 gradually precipitated. The reaction was monitored by IR until the complete disappearance of the absorption of azido groups. Et2O (30 mL) was subsequently added, and the mixture was further stirred for 30 min. The slurry was filtered under reduced pressure, and the pale-white solid was washed with additional Et2O (30 mL) and dried in vacuo to furnish the desired product as a single diastereomer (3.6 g, 7.32 mmol, 73% combined yield, dr > 20:1).
(±)-(1R,2R)-2,3-Dihydro-1H-indene-1,2-diaminium Di-tosylate (4)
4 is a white solid. (mp 276–279 °C). IR νmax (neat)/cm−1: 3036 (s), 2920 (s), 1599 (m), 1437 (w), 1166 (s), 1119 (s), 1032 (s), 1009 (s), 812 (m), 723 (m), 679 (s). 1H NMR (400 MHz, DMSO-d6) δ 8.37 (brs, 6H), 7.58 (d, J = 7.7 Hz, 1H), 7.48 (d, J = 8.0 Hz, 4H), 7.45–7.34 (m, 3H), 7.12 (d, J = 7.9 Hz, 4H), 4.82 (d, J = 4.9 Hz, 1H), 3.99 (dd, J = 13.7, 5.6 Hz, 1H), 3.47 (dd, J = 16.8, 8.3 Hz, 1H), 3.01 (dd, J = 16.8, 5.9 Hz, 1H), 2.29 (s, 6H); 13C NMR (100 MHz, DMSO-d6) δ 144.9, 140.1, 138.3, 136.0, 130.0, 128.3, 127.8, 125.6, 125.2, 125.0, 58.5, 54.4, 35.5, 20.9; HRMS (ESI, m/z): calcd for C9H13N2+, [M + H+], 149.1073, found 149.1067.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institutes of Health (GM110382) and Alfred P. Sloan Foundation (FG-2015-65240).
H.X. is an Alfred P. Sloan Research Fellow. We thank Cheng-Liang Zhu for his assistance during manuscript preparation.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.oprd.7b00312.
Experimental details (PDF)
References
- 1.For selected references of olefin diamination reactions, see: Chong AO, Oshima K, Sharpless KB. J Am Chem Soc. 1977;99:3420.Bäckvall JE. Tetrahedron Lett. 1978;19:163.Becker PN, White MA, Bergman RG. J Am Chem Soc. 1980;102:5676.Li G, Wei HX, Kim SH, Carducci MD. Angew Chem, Int Ed. 2001;40:4277. doi: 10.1002/1521-3773(20011119)40:22<4277::AID-ANIE4277>3.0.CO;2-I.Bar GLJ, Lloyd-Jones GC, Booker-Milburn KI. J Am Chem Soc. 2005;127:7308. doi: 10.1021/ja051181d.Streuff J, Hövelmann CH, Nieger M, Muñiz K. J Am Chem Soc. 2005;127:14586. doi: 10.1021/ja055190y.Zabawa TP, Kasi D, Chemler SR. J Am Chem Soc. 2005;127:11250. doi: 10.1021/ja053335v.Du H, Yuan W, Zhao B, Shi Y. J Am Chem Soc. 2007;129:11688. doi: 10.1021/ja074698t.Zhao BG, Peng XG, Cui SL, Shi YA. J Am Chem Soc. 2010;132:11009. doi: 10.1021/ja103838d.Zhao B, Peng X, Zhu Y, Ramirez TA, Cornwall RG, Shi Y. J Am Chem Soc. 2011;133:20890. doi: 10.1021/ja207691a.Sibbald PA, Rosewall CF, Swartz RD, Michael FE. J Am Chem Soc. 2009;131:15945. doi: 10.1021/ja906915w.Iglesias Á, Pérez EG, Muñiz K. Angew Chem, Int Ed. 2010;49:8109. doi: 10.1002/anie.201003653.Olson DE, Su JY, Roberts DA, Du Bois J. J Am Chem Soc. 2014;136:13506. doi: 10.1021/ja506532h.
- 2.For selected references of enantioselective olefin diamination reactions, see ref 1h, and Du H, Zhao B, Yuan W, Shi Y. Org Lett. 2008;10:4231. doi: 10.1021/ol801605w.Huang DS, Wang HN, Xue FZ, Guan H, Li LJ, Peng XY, Shi Y. Org Lett. 2011;13:6350. doi: 10.1021/ol202527g.Röben C, Souto JA, González Y, Lishchynskyi A, Muñiz K. Angew Chem, Int Ed. 2011;50:9478. doi: 10.1002/anie.201103077.Muñiz K, Barreiro L, Romero RM, Martínez C. J Am Chem Soc. 2017;139:4354. doi: 10.1021/jacs.7b01443.Ingalls EL, Sibbald PA, Kaminsky W, Michael FE. J Am Chem Soc. 2013;135:8854. doi: 10.1021/ja4043406.
- 3.For selected examples of chemical olefin diazidation methods, see: Minisci F. Acc Chem Res. 1975;8:165.Fristad WE, Brandvold TA, Peterson JR, Thompson SR. J Org Chem. 1985;50:3647.Snider BB, Lin H. Synth Commun. 1998;28:1913.Moriarty RM, Khosrowshahi JS. Tetrahedron Lett. 1986;27:2809.Arimoto M, Yamaguchi H, Fujita E, Nagao Y, Ochiai M. Chem Pharm Bull. 1989;37:3221.Magnus P, Lacour J. J Am Chem Soc. 1992;114:767.Chung R, Yu E, Incarvito CD, Austin DJ. Org Lett. 2004;6:3881. doi: 10.1021/ol0490532.Sasaki T, Kanematsu K, Yukimoto Y. J Org Chem. 1972;37:890.Nocquet-Thibault S, Rayar A, Retailleau P, Cariou K, Dodd RH. Chem - Eur J. 2015;21:14205. doi: 10.1002/chem.201501782.Kamble DA, Karabal PU, Chouthaiwale PV, Sudalai A. Tetrahedron Lett. 2012;53:4195.Lu MZ, Wang CQ, Loh TP. Org Lett. 2015;17:6110. doi: 10.1021/acs.orglett.5b03130.Peng H, Yuan Z, Chen P, Liu G. Chin J Chem. 2017;35:876.
- 4.For selected examples of electrochemical olefin diazidation methods, see: Schäfer H. Angew Chem, Int Ed Engl. 1970;9:158.Fu N, Sauer GS, Saha A, Loo A, Lin S. Science. 2017;357:575. doi: 10.1126/science.aan6206.
- 5.Yuan YA, Lu DF, Chen YR, Xu H. Angew Chem, Int Ed. 2016;55:534. doi: 10.1002/anie.201507550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bräse S, Gil C, Knepper K, Zimmermann V. Angew Chem, Int Ed. 2005;44:5188. doi: 10.1002/anie.200400657. [DOI] [PubMed] [Google Scholar]
- 7.(a) Stoessel F, Ubrich O. J Therm Anal Calorim. 2001;64:61. [Google Scholar]; (b) Frurip DJ, Elwell T. Process Saf Prog. 2007;26:51. [Google Scholar]
- 8.Zhdankin VV, Krasutsky AP, Kuehl CJ, Simonsen AJ, Woodward JK, Mismash B, Bolz JT. J Am Chem Soc. 1996;118:5192. [Google Scholar]
- 9.For selected references of metal-mediated radical oxidation, see: Kharasch MS, Sosnovsky G. J Am Chem Soc. 1958;80:756.Kharasch MS, Sosnovsky G, Yang NC. J Am Chem Soc. 1959;81:5819.Kochi JK. Science. 1967;155:415. doi: 10.1126/science.155.3761.415.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.