Abstract
In maturity, motor skills depend on the corticospinal tract (CST) and brainstem pathways that together synapse on interneurons and motoneurons in the spinal cord. Descending signals to spinal neurons that mediate voluntary control can be distinguished from peripheral sensory signals, primarily for feedback control. These motor system circuits depend initially on developmental genetic mechanisms to establish their connections and neural activity- and use-dependent synaptic refinement during the early postnatal period to enable motor skills to develop. In this review we consider four key activity-dependent developmental mechanisms that provide insights into how the motor systems establish the proper connections for skilled movement control and how the same mechanisms also inform the mechanisms of motor impairments and developmental plasticity after corticospinal system injury: (1) synaptic competition between the CSTs from each hemisphere; (2) interactions between the CST and spinal cord neurons; (3) synaptic competition between the CST and proprioceptive sensory fibres; and (4) interactions between the developing corticospinal motor system and the rubrospinal tract (RuST). Our findings suggest that the corticospinal motor system effectively ‘oversees’ development of its subcortical targets through synaptic competition and trophic-like interactions and this has important implications for motor impairments after perinatal cortical stroke.
In maturity, motor skills depend on the corticospinal tract (CST), which contains the direct projection from the motor cortex to the spinal cord, as well as brainstem motor pathways.1 Although the ultimate target of these descending projections is the motoneuron, spinal interneurons also receive important input from the motor pathways and play key roles in coordinating muscle actions. Descending signals to motoneurons and interneurons mediating voluntary control can be distinguished from muscle and joint sensory signals that provide feedback for movement control. Whereas genetic mechanisms initially establish motor system connections, neural activity- and use-dependent processes refine these connections to enable motor skills to develop.2–4
To begin to understand the mechanisms by which refinement of motor circuits occurs, we have developed animal models in which we can (1) regulate corticospinal system activity; (2) examine precisely descending motor pathway and proprioceptive fibre connections; (3) determine key characteristics of spinal cord interneurons; and (4) map the functional motor representations in the brain. With these tools we can study activity-based developmental plasticity mechanisms, as well as the relationship of these mechanisms to early brain injury. We use cat and rodent models as they are species that share similarities and express differences with the human motor systems.1 Notably, unlike humans the cat and rodent CSTs do not have significant monosynaptic connections with motoneurons. The rubrospinal tract (RuST), a brainstem motor pathway, has not been studied in developing humans but may be smaller in mature humans.5 Most likely, the developmental mechanisms that we describe in animals also function in humans.
In this mini-review we focus on the corticospinal system because it is the principal motor system for skilled movements. Corticospinal system damage results in significant motor dysfunction. Damage during development produces weakness and paresis.6 Coordination of skilled movements becomes impaired, typically disproportionate to the motor strength impairment. Injury leads to additional impairments, including hyper-reflexia and spasticity. Weakness is likely owing primarily to the loss of the direct projections of the CST from motor cortex to spinal cord motor circuits. However, the other impairments are poorly understood and likely reflect additional factors than simply the loss of connections. They are better considered to be a gain of aberrant motor functions and may be due to new patterns of connections and function that emerge after injury.7 These aberrant motor functions can be especially debilitating in cerebral palsy.
We consider key mechanisms of activity-dependent developmental plasticity that provide insights into how the motor systems establish connections for skilled movement control, and how the same mechanisms also inform motor impairments after developmental injury. These mechanisms include synaptic competition between CST connections, CST regulation of downstream motor circuits in the spinal cord, and corticospinal system activity-dependent regulation of sensory and brainstem motor pathway development.
SYNAPTIC COMPETITION SHAPES ESTABLISHMENT OF CORTICOSPINAL MOTOR CIRCUITS
A common mechanism for establishing sensory system connections is initial development of excessive connections that are subsequently refined to a subset of connections that are stable throughout later development and maturity. An essential component of this refinement process is the activity of the neurons in the developing circuit. CST development is similar. We have shown that the refinement process is organized by the level of activity of the developing corticospinal systems.3
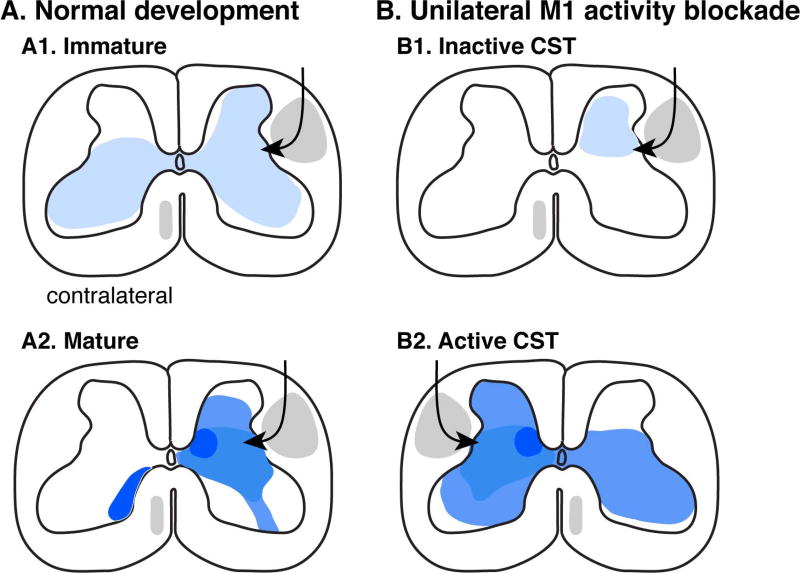
The CST, which originates primarily from motor cortex, but also premotor and somatic sensory cortex, has an early bilateral organization in cats and humans, based on anatomical tracing and electrophysiological assays.1,3,8 Using axonal tracing methods together with highly sensitive outcome measures, we have shown in a cat model that there are significant ipsilateral CST spinal projections that, combined with the dominant contralateral projection, lead to an immature bilateral spinal projection (Fig. 1a[a1]; immature CST projections are sparse, denoted by pale shading). Refinement of connections occurs by about 7 weeks of age (Fig. 1a[a2]), with a shift to a predominantly contralateral projection and regionally denser projections (denoted by regions of darker shading).9 The human corticospinal is similar. Using transcranial magnetic stimulation (TMS) in babies, contralateral and ipsilateral motor effects are remarkably similar,8,10 resulting in a bilateral corticospinal system early in development. In the typically developing human, TMS-evoked muscle responses on the contralateral side become stronger than those on the ipsilateral side after about 6 months of age.10
Figure 1.
Corticospinal tract (CST) development. (a) Normal development. Immature is less than 8 weeks of age in the cat model studied. CST projection density in the spinal cord is represented by shading darkness. (b) Development following unilateral motor cortex inactivation, which was produced by intracortical microinfusion of muscimol (γ-aminobutyric acid receptor A agonist). CST projections from the (b1) inactivated and (b2) non-inactivated sides are shown. Schematics based on prior studies.9,11
With a robust early bilateral substrate, circuit-level interactions between the two hemispheres can be abundant, not only via CST projections to the spinal cord, but also via cortical projections to the brainstem. One such particularly robust interaction during development is activity-dependent synaptic competition for establishment of connections with postsynaptic target neurons. More active CST neurons are more successful in establishing synaptic connections than less active neurons.3 We demonstrated this experimentally by pharmacologically inactivating the major source of the CST in motor cortex of one hemisphere during early development when the system is bilateral. Later in development and maturity, this results in a substantially reduced and spatially aberrant projection from the inactive side (Fig. 1b[b1]) and an overtaking by the active CST projection (Fig. 1b[b2]). Animals show unilateral motor impairments in skilled movements in this model.11 However, our findings show that a unilateral activity change in motor cortex affects development of the CST from both hemispheres; the active side develops an aberrant bilateral CST. Similarly, unilateral motor cortex injury in human babies impacts CST development from both the injured and intact hemispheres. It is well known that after unilateral perinatal motor cortex injury the intact motor cortex may develop and maintain a bilateral CST, evaluated using TMS.12 This is explained by our activity-based model. With substantial loss of CST projections due to an early developmental injury, spared CST axons on the injured side will be less effective in activating their targets in the spinal cord than the ipsilateral CST projection from the uninjured side (e.g. compare the right side of the spinal cord in Figs 1b[b1 and b2]). In our animal model, electrical stimulation of the damaged CST soon after injury helps restore connections and improves behavioural outcomes, consistent with enhancing synaptic competitiveness.3,13
CORTICOSPINAL ACTIVITY SHAPES DEVELOPMENT OF INTRINSIC SPINAL MOTOR CIRCUITS
So far, we have discussed how corticospinal system activity can help shape development of CST connections. Another type of interaction is between the CST and the downstream neural circuits that receive input from these descending projections. We have studied the role of corticospinal system activity in development of spinal cord interneurons.14 The CST makes monosynaptic connections directly with motoneurons in humans and some primate species, as well as synaptic contacts onto spinal interneurons that, in turn, synapse on motoneurons.1 One class of spinal interneuron that receives CST input and synapses on motoneurons uses acetylcholine as its transmitter (Fig. 2, inset). It expresses choline-acetyl transferase.15
Figure 2.
Cholinergic interneurons and corticospinal tract (CST) activity dependence. (a, b). Heat maps showing normal cholinergic interneuron density at 4 and 8 weeks of age. The local density is represented by a colour scale from low (blue) to high density (red). The colour scales are the same for both ages. Note that cholinergic neurons in the motor pools (black lines) are motoneurons, which do not show an age dependence. (c) Bar graph showing the effect of unilateral motor cortex inactivation between weeks 5 and 8. (d) Restoration of cholinergic interneuron density (laminae I–VII) with early, but not late, training intervention. Inset shows the pattern of connection between a subset of cholinergic premotor interneurons and motoneurons.14,18
As the CST develops during an early postnatal period there is approximately a sevenfold increase in the number of choline-acetyl transferase-containing interneurons (Fig. 2a,b; between 4 and 8 weeks of age). When the activity of the CST is reduced unilaterally during this period, only approximately 50% of the cholinergic interneurons in the spinal cord eventually develop (Fig. 2c). It is not known if the reduced number of cholinergic neurons reflects an activity-dependent neurotransmitter switch16 – more interneurons use a different neurotransmitter after corticospinal inactivation – or that a large number of cholinergic neurons fail to develop and die. In either case, without this source of cholinergic input to motoneurons, motor behaviours are not expressed normally.17
In our animal model of hemiplegic cerebral palsy,18 the reduced number of cholinergic interneurons can be restored by constraint-induced movement therapy (i.e. normal limb constraint and exclusive use of the impaired limb during skilled movement training; Fig. 2d, dark-blue bar), and this is associated with behavioural recovery.18 Importantly, constraint therapy was only effective in abrogating the interneuron reduction and motor impairment when the rehabilitation is applied immediately after injury. Delaying therapy by 1 month in this model prevented restoration of cholinergic interneuron number (Fig. 2d; light-blue bar) and prevents motor recovery. Extending the cat findings to humans, we hypothesize that intervention early in development (e.g. 6–12 months), when bilateral TMS-evoked limb responses are evoked in babies,10 may be more effective than later in development (5–10 years), when TMS evokes predominantly contralateral responses. Constraint alone was ineffective in restoring neuron numbers (Fig. 2d; grey bar) or behaviour, stressing the need for skill training. Promoting CST activity through motor cortex electrical stimulation, currently only tested in a mature rodent model, augments cholinergic synapses on motoneurons (Y. Jiang, unpublished observations). These findings point to spinal circuits as a novel therapeutic target for cerebral palsy in conjunction with behavioural or motor cortex stimulation therapy.
COMPETITIVE INTERACTIONS BETWEEN MUSCLE SENSORY FIBRES AND CST CONNECTIONS
The CST and muscle sensory fibres, which mediate proprioception and stretch reflexes, coordinate development of their spinal connections with those of the CST.19 Synaptic interactions also occur between these systems during development.20,21 Here we discuss a mature animal model to examine the strength and potential functional significance of muscle sensory fibre–CST interactions (Fig. 3). Normal CST and muscle sensory terminations are shown schematically (Fig. 3a; CST=blue; sensory=grey). Muscle sensory fibres were activated by muscle electrical stimulation.22 Muscle stimulation promoted outgrowth of muscle sensory fibres (Fig. 3b; note expanded sensory territory and denser connections) and this is associated with a reciprocal reduction in CST connections, by about 50% (lighter-blue shading22). Conversely, partial deafferentation produced a concomitant increase in CST connections (Fig. 3c; dark blue). The capacity for motor cortex stimulation to produce a muscle-evoked potential is diminished by about 50% after muscle electrical stimulation (Fig. 3e, dotted line) compared with normal controls, and doubled after partial deafferentation (red line).22 Finally, removal of CST axons by a brain lesion resulted in increased muscle sensory fibres in the spinal cord, similar to what we observe after muscle stimulation, and hyper-reflexia (Fig. 3d).23 These findings show robust reciprocal interactions between the CST and muscle sensory fibres that can be explained by competitive synaptic interactions.20,22 Studies are in progress to determine if there are similar CST and sensory fibre competitive interactions during development in the cat. If so, damage to the developing motor cortex, and the consequent loss of the CST, could lead to enhanced sensory fibre input to the spinal cord and altered sensory-motor integration. Activity-based interventions, similar to what we have used to promote CST spinal projections in development and maturity,13,24 could help restore the normal balance between the CST and sensory fibres after perinatal motor cortex lesion and ameliorate limb spasticity and tone-related impairments that hinder skill learning.
Figure 3.
Competitive interactions between the corticospinal tract (CST) and muscle sensory fibres in maturity. (a–d) Schematic representations of the distribution of CST projections (blue) and muscles sensory afferent fibres (grey) in the spinal cord. (a) Normal; (b) following 10 days of muscle electrical stimulation (extensor carpi radialis; 1 Hz; 6 h/day); (c) partial deafferentation produced by C4–C8 dorsal rootlets section; (d) CST lesion by unilateral medullary pyramid cut; (e) relationship between the intensity of motor cortex electrical stimulation and the muscle-evoked potential under three conditions: normal controls, after 10 days of muscle stimulation, and after partial deafferentation. Schematics based on data from prior studies.22,23
CORTICOSPINAL ACTIVITY SHAPES DEVELOPMENT OF A BRAINSTEM MOTOR PATHWAY
The developing corticospinal motor system also influences development of the rubrospinal system, a brainstem motor system comprised of the red nucleus and RuST, which has an anatomical organization of cervical spinal projections similar to that of the CST and is thought to function with the CST in skilled limb movements. The RuST develops spinal projections earlier than the CST and the red nucleus forelimb motor representation (or “homunculus”) develops earlier than the cortical motor cortex motor representation, especially for the distal joints.25 Later in development, as motor skills emerge, the CST and motor cortex motor representation exhibit further development while the RuST and red nucleus motor representation appear not to develop further. After development, the CST and RuST project to overlapping spinal territories (Fig. 4, inset). The rubrospinal system may be more important than the corticospinal system for control of early, possibly coarser, movements, but this dependency shifts to the corticospinal motor system later in development. This is another example of co-development of the corticospinal motor system and another motor system component.
Figure 4.
Activity-dependent interactions between the developing corticospinal and rubrospinal system. (a) Effect if unilateral motor cortex inactivation between postnatal weeks 5–7 on corticospinal tract (CST) development. Note that the motor cortex motor map is represented by the grey shading of cortex, with light grey denoting a sparse map and dark shading, a normal map. Spinal projections are shown below. (b) Effect of unilateral motor cortex inactivation on rubrospinal system development. The red nucleus motor map is represented by the darkness of red shading, with light pink denoting a sparse map and dark pink, a stronger map. The distribution of rubrospinal tract (RuST) projections are shown below. Light-pink shading denotes sparse RuST projections and dark pink, denser projections. The red nucleus motor maps and RuST projections are assessed at the end of the inactivation period. (c) Similar to (a) and (b), but at 1–2 months after the end of the cortical inactivation period, when the animals are adolescents. Note that the black lines represent the CST projections: bilateral and dense from the active side of cortex and contralateral and sparse from the inactive cortex. The inset shows the normal CST and RuST spinal projections. Schematics are based on original data from prior studies.9,11,25,26
Since the CST and RuST normally co-develop, we investigated if the two systems compete for control of spinal motor circuits, similar to what we have shown for the contralateral and ipsilateral CST projections and the CST and sensory fibres. We inactivated one motor cortex and asked if the ipsilateral and contralateral CSTs and RuST engage in an activity-dependent competition in establishing their connections with spinal motor circuits.26 Recall that early postnatal neural activity in motor cortex of one hemisphere, when the CST is establishing its spinal connections, produced CST and contralateral motor impairments (Fig. 4a; see also Fig. 1b). We studied the function and connections of the treated and untreated motor cortex/CST later in development, once activity returned. The affected motor cortex motor representation was sparse (denoted as the light-grey motor cortex on the brain drawing) – with few sites representing forelimb movements26 – and its CST failed to develop dense, strong spinal projections (Fig. 4a). As indicated earlier, the affected side showed skilled motor impairments. By contrast, the opposite motor cortex developed a normal motor representation (dark-grey motor cortex on brain drawing),27 a normal contralateral CST projection, as well as a dense ipsilateral spinal projection (Fig. 4a). In the example shown, the red nucleus and RuST would seem to have a competitive advantage during the period when the CST is less active. Indeed, while the CST remains inactivated the red nucleus motor representation was expanded relative to normal development (darker red colour; Fig. 4b) and the spinal projections were denser than with the active side, which had a sparse motor map and spinal projections. This is consistent with a competition between the CST and RuST for synaptic connections in the spinal cord.
However, this red nucleus/RuST asymmetry was short-lived. One to 2 months later the over-representation of the motor map and denser spinal projections became less than normal. Why was this short-term ‘win’ of the rubrospinal system not maintained? We propose that further development of the aberrant ipsilateral CST, together with enhanced sprouting of muscle sensory afferents on the affected side of the spinal cord, outcompete the RuST during the late phase of maturation. Many questions remain, including whether this new pattern of perturbed RuST projections is adaptive and helps restore function early after injury. Perhaps the contralateral-projecting RuST is better at controlling skilled limb movements than the sparse contralateral CST or denser ipsilateral CST? There may be a similar competition between the CST and other descending pathways. Motor dysfunction in cerebral palsy after localized cortical injury might reflect developmental changes in brainstem motor pathways produced indirectly because of a cortical injury.
CONCLUSION
The early-developing motor system is refined by activity, synaptic competition, and early motor experience.20,21,28 For the CST, activity-dependent interactions seem strongest during development, but they are also present in maturity.24 Factors other than competition may also be important mediators of developmental plasticity – such as cooperative interactions between inputs, which have been contrasted from competitive interactions under different developmental conditions20,21 – and of particular importance after injury, the availability of synaptic space. Synaptic interactions, especially competition, may be particularly important during early development when there is an overproduction of synaptic connections in the central nervous system and connections must be eliminated for circuit function.2
Our findings suggest that the corticospinal motor system effectively oversees development of its downstream subcortical targets through activity or trophic-like interactions and synaptic competition. This could have important implications for perinatal cortical stroke and traumatic injuries, where loss of CST projection from frontal motor areas to the spinal cord may deprive spinal target neurons of signals necessary for their development.1 Implementation of activity-based interventions, such as motor cortex stimulation and other neuromodulatory approaches, may be a way to overcome both the loss of connections, by driving CST axonal outgrowth, and to prevent the widespread reprogramming of motor system development that follows when cortical developmental regulation goes awry.
What this paper adds
Neural activity-dependent processes inform the brain and spinal cord response to injury.
The corticospinal motor system may ‘oversee’ development of its downstream subcortical targets through activity, trophic-like interactions, and synaptic competition.
Acknowledgments
Supported by NIH R01 NS036835 (JHM); NIH R01 NS064004 (JHM); CH Nielsen Foundation 261214 (JHM).
Abbreviations
- CST
Corticospinal tract
- RuST
Rubrospinal tract
- TMS
Transcranial magnetic stimulation
Footnotes
An early version of this paper was presented at the Early Intervention in Light of the ICF-CY Meeting held in Groningen, the Netherlands, 7–9 April 2016.
The authors have stated that they had no interests which might be perceived as posing a conflict or bias.
References
- 1.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 2.Gu Z, Serradj N, Ueno MY, et al. Skilled movements require non-apoptotic Bax/Bak pathway-mediated corticospinal circuit reorganization. Neuron. 2017;94:626–41. doi: 10.1016/j.neuron.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin J, Friel K, Salimi I, Chakrabarty S. Corticospinal development. In: Squire L, editor. Encyclopedia of Neuroscience. Oxford: Academic Press; 2009. pp. 302–14. [Google Scholar]
- 4.Gu Z, Kalambogias J, Yoshioka S, et al. Control of species-dependent cortico-motoneuronal connections underlying manual dexterity. Science. 2017;357:400–4. doi: 10.1126/science.aan3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang HS, Kwon HG, Hong JH, Hong CP, Jang SH. The rubrospinal tract in the human brain: diffusion tensor imaging study. Neurosci Lett. 2011;504:45–8. doi: 10.1016/j.neulet.2011.08.054. [DOI] [PubMed] [Google Scholar]
- 6.Sanger TD, Chen D, Delgado MR, Gaebler-Spira D, Hallett M, Mink JW. Definition and classification of negative motor signs in childhood. Pediatrics. 2006;118:2159–67. doi: 10.1542/peds.2005-3016. [DOI] [PubMed] [Google Scholar]
- 7.Sukal-Moulton T, Krosschell KJ, Gaebler-Spira DJ, Dewald JP. Motor impairment factors related to brain injury timing in early hemiparesis. Part I: expression of upper-extremity weakness. Neurorehabil Neural Repair. 2014;28:13–23. doi: 10.1177/1545968313500564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eyre JA, Smith M, Dabydeen L, et al. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann Neurol. 2007;62:493–503. doi: 10.1002/ana.21108. [DOI] [PubMed] [Google Scholar]
- 9.Martin JH, Kably B, Hacking A. Activity-dependent development of cortical axon terminations in the spinal cord and brain stem. Exp Brain Res. 1999;125:184–99. doi: 10.1007/s002210050673. [DOI] [PubMed] [Google Scholar]
- 10.Eyre JA, Miller S, Clowry GJ, Conway EA, Watts C. Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain. 2000;123:51–64. doi: 10.1093/brain/123.1.51. [DOI] [PubMed] [Google Scholar]
- 11.Friel KM, Drew T, Martin JH. Differential activity-dependent development of corticospinal control of movement and final limb position during visually-guided locomotion. J Neurophysiol. 2007;97:3396–406. doi: 10.1152/jn.00750.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–54. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- 13.Salimi I, Friel KM, Martin JH. Pyramidal tract stimulation restores normal corticospinal tract connections and visuomotor skill after early postnatal motor cortex activity blockade. J Neurosci. 2008;28:7426–34. doi: 10.1523/JNEUROSCI.1078-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakrabarty S, Shulman B, Martin JH. Activity-dependent codevelopment of the corticospinal system and target interneurons in the cervical spinal cord. J Neurosci. 2009;29:8816–27. doi: 10.1523/JNEUROSCI.0735-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A. 2007;104:2448–53. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spitzer NC. Activity-dependent neurotransmitter respecification. Nat Rev Neurosci. 2012;13:94–106. doi: 10.1038/nrn3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron. 2009;64:645–62. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friel K, Chakrabarty S, Kuo HC, Martin J. Using motor behavior during an early critical period to restore skilled limb movement after damage to the corticospinal system during development. J Neurosci. 2012;32:9265–76. doi: 10.1523/JNEUROSCI.1198-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakrabarty S, Martin JH. Co-development of proprioceptive afferents and the corticospinal tract within the cervical spinal cord. Eur J Neurosci. 2011;34:682–94. doi: 10.1111/j.1460-9568.2011.07798.x. [DOI] [PubMed] [Google Scholar]
- 20.Gibson CL, Arnott GA, Clowry GJ. Plasticity in the rat spinal cord seen in response to lesions to the motor cortex during development but not to lesions in maturity. Exp Neurol. 2000;166:422–34. doi: 10.1006/exnr.2000.7511. [DOI] [PubMed] [Google Scholar]
- 21.Clowry GJ, Davies BM, Upile NS, Gibson CL, Bradley PM. Spinal cord plasticity in response to unilateral inhibition of the rat motor cortex during development: changes to gene expression, muscle afferents and the ipsilateral corticospinal projection. Eur J Neurosci. 2004;20:2555–66. doi: 10.1111/j.1460-9568.2004.03713.x. [DOI] [PubMed] [Google Scholar]
- 22.Jiang YQ, Zaaimi B, Martin JH. Competition with primary sensory afferents drives remodeling of corticospinal axons in mature spinal motor circuits. J Neurosci. 2016;36:193–203. doi: 10.1523/JNEUROSCI.3441-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan AM, Chakrabarty S, Kimura H, Martin JH. Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia. J Neurosci. 2012;32:12896–908. doi: 10.1523/JNEUROSCI.6451-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmel JB, Berrol LJ, Brus-Ramer M, Martin JH. Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth. J Neurosci. 2010;30:10918–26. doi: 10.1523/JNEUROSCI.1435-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams PT, Kim S, Martin JH. Postnatal maturation of the red nucleus motor map depends on rubrospinal connections with forelimb motor pools. J Neurosci. 2014;34:4432–41. doi: 10.1523/JNEUROSCI.5332-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams PT, Martin JH. Motor cortex activity organizes the developing rubrospinal system. J Neurosci. 2015;35:13363–74. doi: 10.1523/JNEUROSCI.1719-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakrabarty S, Friel K, Martin JH. Activity-dependent plasticity improves M1 motor representation and corticospinal tract connectivity. J Neurophysiol. 2009;110:1283–93. doi: 10.1152/jn.91026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serradj N, Martin JH. Motor experience reprograms development of a genetically-altered bilateral corticospinal motor circuit. PLOS ONE. 2016;11:e0163775. doi: 10.1371/journal.pone.0163775. [DOI] [PMC free article] [PubMed] [Google Scholar]