Abstract
A mathematical model is formulated for diffusive countercurrent exchange of oxygen between paired arterioles and venules. A closed form solution of the problem is obtained by linearizing the nonlinear oxyhemoglobin dissociation curve at the inlet PO2 in the vessel. The closed form solution is compared with the corresponding numerical solution of the nonlinear problem. Under normal conditions, longitudinal gradients of venular PO2 are found to be small. Examples are presented where the model predicts significant gradients of venular PO2 when the blood flow rate in the venule is several times smaller than that in the arteriole.
INTRODUCTION
One of the main functions of the blood is to supply adequate amounts of oxygen (O2) to the tissues. Until recently, O2 was believed to be exchanged exclusively at the level of the blood capillaries, but a number of investigators have demonstrated experimentally that oxygen tension (PO2) and hemoglobin saturation with oxygen (SO2) decrease gradually along the arteriolar network [2, 12, 13, 7, 3, 8, 19]. Mathematical models suggest that observed longitudinal gradients in oxygen tension (PO2) are qualitatively consistent with a diffusional mechanism of O2 transport from arterioles [15, 17]. Therefore, the site of O2 transport may not be restricted to capillaries, as was previously assumed, but includes precapillary vessels. However, it is not known at present how the oxygen lost from the arterioles is distributed or how universal this phenomenon is in different tissues and different physiologic states.
Even less information is available on the participation of venules in O2 exchange, although the question of countercurrent exchange has been discussed extensively in the literature [6]. There are numerous observations in many tissues that larger arterioles and venules run side by side, parallel to each other in countercurrent fashion. These vessels are henceforth termed paired. Pittman and Duling [14] and Swain and Pittman [19] observed that O2 may be gained by the venular blood as it proceeds from smaller to larger vessels. The question of the magnitude of countercurrent exchange has an important implication: if this effect is significant, then the oxygen tension (PO2) in the venous blood cannot be considered an accurate measure of either end-capillary or tissue PO2.The assumption that the venous PO2 is an indicator of tissue PO2 is commonly made in interpretation of whole organ experiments. Another mechanism that would lead to dissociation of the venous and capillary PO2 levels has to do with the kinetics of O2-hemoglobin binding [5].
Popel and Gross [15] formulated a mathematical model of O2 diffusion from noncapillary vessels to tissue and computed longitudinal PO2 gradients in a simplified vascular network using the characteristics of the hamster check pouch microcirculation. Roth and Wade [17] presented a compartmental model of organ circulation. In their model several generations of vessels are represented by vascular compartments; in addition, there is a tissue compartment and a connective tissue compartment. Each vascular compartment can exchange O2 with one of the tissue compartments. For the purpose of calculation of exchange coefficients, each noncapillary vessel is modeled as a cylinder surrounded by two concentric layers of finite thickness representing the vascular wall and a tissue. Piiper et al. [11] presented estimates of the effect of countercurrent exchange of oxygen between arterioles and venules using essentially a compartmental model. They concluded that the effect should be much smaller for O2 than for inert gases, due to the chemical binding of oxygen to hemoglobin.
The aim of the present study is to formulate a model of O2 transport between paired arterioles and venules. The model of Popel and Gross [15] is extended, and the O2 fluxes between the arterioles, tissue, and venules are calculated. A systematic analysis of arteriovenous O2 exchange in physiological situations will be presented elsewhere.
MATHEMATICAL MODEL
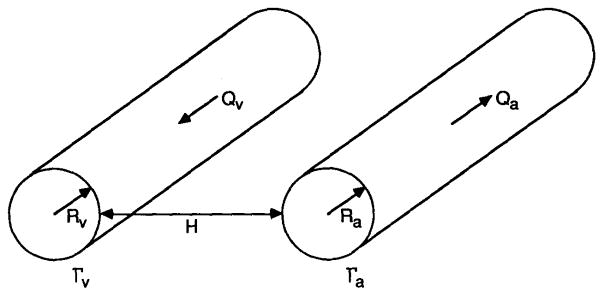
Consider a pair of circular parallel unbranched vessels of radii Ra and Rv at a distance H apart (Figure 1). The subscripts a and v refer to arteriolar and venular segments, respectively.
Fig. 1.
Schematic diagram of the model.
The problem of simulating O2 transport in the vicinity of the arteriolar and venular vessels, taking into account the specific local geometry and hemodynamics of the capillary bed, does not seem to be mathematically tractable at present. Also, the necessary experimental information on the capillary geometry in relation to arteriolar and venular vessels is not available. Popel and Gross [15] have treated this problem by formulating a phenomenological model for the O2 transport around the arteriolar vessel. The formulation is based on the following requirements: (a) the model should not include the heterogeneities of PO2 at the scale of single capillaries and cells; (b) the model should be concerned with “large scale” diffusional processes in the vicinity of sources or sinks of oxygen such as arteriolar and venular vessels and the external boundary of the tissue; (c) far from such sources or sinks, the model should give an average PO2 level, denoted as P∞, which is solely determined by capillary-tissue O2 exchange. The PO2 distribution in the extravascular space satisfying the above requirements is governed by the diffusion equation:
| (1) |
where P is the oxygen tension in the tissue, lt is the penetration depth, and P∞ is a given oxygen tension at “infinity.” We consider the diffusion only in the x-y plane perpendicular to the arteriole-venule axes; diffusion parallel to the axes, in the z direction, is neglected. The parameter lt characterizes the depth of penetration of oxygen from a “large scale” source or sink, e.g., arteriole, venule, or tissue surface; in the model the value of this parameter has to be specified. In the absence of the macroscopic oxygen tension gradients, equation (1) yields P = P∞; thus P∞ is the mean oxygen tension determined by the capillary-tissue exchange.
Equation (1) is subject to the boundary conditions
| (2) |
and
| (3) |
Here Pa and Pv are the PO2’s on the surface of the arteriole and the venule, respectively. We ignore the effect of the vascular wall.
The diffusive fluxes of O2 from the arteriole and venule are
| (4) |
where n is the unit outward normal to the arteriolar or venular surfaces, D is the diffusion coefficient, and α is the solubility coefficient of O2 in the tissue. The integrals in (4) are taken over the corresponding circuits, Γa and Γv, which are the intersections of the arteriole and venule with a plane z = const.
DIFFUSION CONDUCTANCES
Equation (1) and the boundary conditions (2)–(4) are linear; thus, the flux of oxygen from the arteriole, Ja, can be expressed as a linear combination of oxygen tensions Pa, Pv, and P∞. There is also a constraint that the flux must vanish when all three oxygen tensions are equal (Pa = Pv = P∞). From these conditions it follows that flux can be expressed as
| (5) |
where F1 and F2 are coefficients that depend only on the vascular geometry, (i.e. on Ra, Rv, and H) and the penetration depth lt. Similarly, the net flux from the venule, Jv, is
| (6) |
where G1 and G2 are geometrical coefficients.
It can be shown that G1 = −F2. Therefore, the second term in Equation (5) represents the amount of oxygen transferred from the arteriole to the venule, whereas the first term represents the amount transferred from the arteriole to the surrounding tissue less the amount transferred to the venule. The two terms in Equation (6) are interpreted accordingly. Hence counter-current exchange of oxygen is quantitatively expressed by the terms F2(Pa − Pv) = −G1(Pa − Pv) in Equations (5) and (6).
The coefficients F1, F2, G1, and G2 can be determined numerically for each set of parameters Ra, Rv, H, and lt. The details of the numerical procedure are given in the Appendix.
OXYGEN TRANSPORT IN THE BLOOD
Oxygen is transported in the blood by convective motion of red blood cells and plasma in both longitudinal and radial directions and by molecular diffusion. It is known that at higher rates of shear the transport can be significantly augmented, the effect being attributed to pseudorandom radial motion of red blood cells [1, 20]. Recently, Ellsworth and Pittman [4] measured oxygen hemoglobin saturation profiles in arterioles and venules and found that the profiles in some cases are nonuniform and asymmetric; the sources of nonuniformity or asymmetry have not been identified, but upstream vascular bifurcations and noncircular vessel shapes were implicated. To describe these effects, a careful analysis of O2 distribution in the vascular lumen is necessary. For the purpose of the present analysis we introduce “mixing cup” PO2 in the blood, Pb; i.e., we assume that O2 flow through the vessel cross section is given by the expression
where Q is the volumetric blood flow rate, αb is the oxygen solubility coefficient in blood, C is the oxygen-carrying capacity of the blood, and S is the fractional saturation of hemoglobin with O2. It is assumed that the chemical reaction between hemoglobin and oxygen is in equilibrium, which yields the relationship S(Pb).
Oxygen balance in a vessel can be expressed in the form
| (7) |
where J is the O2 flux from the vessel per unit length.
Using Equations (5), (6), and (7), the O2 transport in a paired arteriole and venule is given by the following simultaneous differential equations:
| (8) |
| (9) |
where the variables with subscripts a and v, respectively, pertain to the arteriolar and venular vessels. Φ is proportional to the slope of the oxyhemoglobin dissociation curve (ODC):
| (10) |
The equation (8) and (9) are subject to the boundary conditions, which are specified at the respective inlets of the vessels:
| (11) |
| (12) |
where L is the length of each vessel.
ANALYSIS
The saturation function for the ODC is nonlinear. Thus, Equations (8) and (9) with the boundary conditions (11)–(12) form a nonlinear system of differential equations. This system can be solved numerically. In the analysis of O2 transport in arterioles [15], it was found that the PO2 falls almost linearly in a given vessel. The experimental studies [14, 19] suggest that there are no large variations in the O2 saturation in a single vessel. Hence we obtain an analytical solution of the problem by approximating the slope of the ODC along the length of the vessel as that determined at the entry point. Thus, the variables Φ for the arteriolar and venular vessels are given by
| (13) |
and
| (14) |
Any form of the equation can be used to represent the ODC in the present model. We use the Hill equation:
| (15) |
where P50 is the partial pressure of O2 at 50% saturation and n is the Hill parameter. Note that the Hill equation is accurate in the range of hemoglobin saturation S = 0.2–0.8 and is not valid at low values of PO2.
Introducing dimensionless variables z* = z/lt, L* = L/lt, and using (13) and (14), we recast Equations (8) and (9):
| (16) |
and
| (17) |
in which
| (18) |
The boundary condition (12) becomes
| (19) |
Equations (16) and (17) are linear. The following two cases are considered:
Case 1: No countercurrent exchange
When there is no countercurrent exchange between the paired arteriole and venule, F2 = 0 and G1 = 0. From (18), f2 = 0 and g1 = 0. In this case, the solution of Equations (16) and (17) with the boundary conditions (11) and (19) is
| (20) |
Case 2: With countercurrent exchange
The solution of (16) and (17) with the boundary conditions (11) and (19) is given by
| (21) |
where
| (22) |
in which
| (23) |
It can be easily verified that the solution (21) with countercurrent exchange tends toward that without countercurrent exchange when f2 = 0 and g1 = 0.
PARAMETERS OF THE MODEL
For the computation of PO2 in the blood flowing through the arteriole and venule, we have chosen the parameters corresponding to the hamster cheek pouch microcirculation: arteriolar diameter Da = 60 μm and length L = 0.75 cm [9]; arteriolar velocity v = 0.25 cm/s [18]. The penetration depth lt is 100 μm, the same as was assumed in the previous theoretical study on O2 transport in the arteriolar networks [15]. The values of Hill’s parameters are P50 = 26.0 mmHg and n = 2.55, and the diffusion and solubility coefficients D = 1.5 × 10−5 cm2/s and α = αb ≈ 3 × 10−5 (cm3 O2)/cm3 mmHg [15]. Unless otherwise specified, the following values of the parameters are assumed: C = 0.2 (cm3 O2)/(cm3 blood), P∞ = 8 mmHg, H = 25 μm, Qv = Qa, , and Dv = 90 μm.
RESULTS AND DISCUSSION
The coefficients F1, F2, G1 and G2 depend only on the geometrical factors and the penetration depth. Once these coefficients are known, the diffusive flux from the arteriole and venule can be computed from the relations (5) and (6).
First we show the results of numerical solution, and then we compare them with the analytical solution. In Table 1 the diffusion conductance coefficients are presented for different values of vessel diameters; the coefficients decrease as the vessel diameter decreases. In Table 2 the diffusion conductance coefficients are presented for different values of the distance H between the arteriole and venule. For the computation, the diameters of the paired arteriole and venule are chosen as Da = 60 μm and Dv = 90 μm. The coefficients F2 and G1, which determine the countercurrent exchange, increase as the distance between the paired vessels decreases. If H = 1000 μm, there is no diffusional interaction between the arteriole and venule, and the venules do not affect the transport of O2 from the arterioles to the surrounding tissue. Popel and Gross [15] have derived an expression for the diffusion conductance for the arteriole without countercurrent exchange, based on the phenomenological model (1). The diffusion conductances computed from the present model for large values of H coincide, within the 1% accuracy of our numerical solution, with those obtained from the model of Popel and Gross [15].
TABLE 1.
Diffusion Conductance Coefficients for the Paired Arterioles and Venules Separated by H = 100 μma
| Da (μm) | Dv (μm) | F1 | F2 | G1 | G2 |
|---|---|---|---|---|---|
| 800 | 1200 | 25.57 | 3.03 | −3.03 | 36.7 |
| 120 | 160 | 5.48 | 1.05 | −1.05 | 6.84 |
| 60 | 90 | 3.59 | 0.73 | −0.73 | 4.50 |
| 40 | 40 | 2.99 | 0.51 | −0.51 | 2.99 |
| 7 | 10 | 1.61 | 0.22 | −0.22 | 1.81 |
Penetration depth lt = 100 μm.
TABLE 2.
Diffusion Conductance Coefficients for the Paired Arteriole and Venule with Variation of the Distance H between Thema
| H (μm) | F1 | F2 | G1 | G2 |
|---|---|---|---|---|
| 25 | 2.89 | 2.68 | −2.68 | 4.09 |
| 50 | 3.18 | 1.55 | −1.55 | 4.33 |
| 100 | 3.59 | 0.73 | −0.73 | 4.50 |
| 150 | 3.85 | 0.38 | −0.38 | 4.94 |
| 250 | 4.08 | 0.12 | −0.12 | 5.17 |
| 500 | 4.19 | 0.74 ×10−2 | −0.74×10−2 | 5.28 |
| 1000 | 4.21 | 0.39×10−4 | −0.39×10−4 | 5.30 |
| 2000 | 4.21 | 0.2×10−4 | −0.2×10−4 | 5.30 |
Da = 60 μm, Dv = 90 μm, lt = 100 μm.
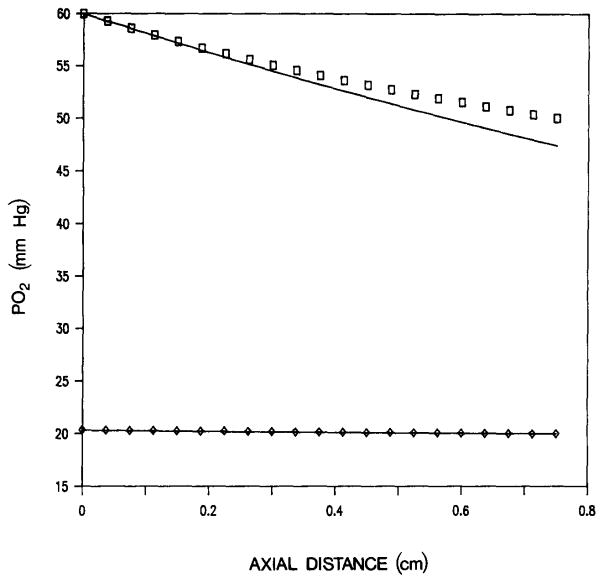
In the analytical calculations, we have approximated the slope of the oxyhemoglobin dissociation curve [Equation (10)] by the value determined at the inlet of the vessel. The equations then become linear, which makes possible a closed form analytical solution (21). Equations (8) and (9) with nonlinear slope have also been solved numerically. Figure 2 shows the PO2 in an arteriolar-venular pair computed from the analytical solution (21) and also numerically using the nonlinear dissociation curve. There is reasonably good agreement between the solutions except for a discrepancy toward the outlet in the arteriole. This difference reflects the change in the slope of the oxygen dissociation curve in the range of oxygen tensions 60 to 49 mmHg. The error introduced by the linearization of the ODC may become substantial when the solution is applied to vascular networks, where the error from individual vascular segments can accumulate. In this case, the numerical solution should be used.
Fig. 2.
Axial PO2 distribution in an arteriolar (upper curve) and a venular (lower curve) vessel. Analytical solution (solid curve), and numerical solutions for arteriole (□) and venule (◇).
Table 3 shows the effect of the distance H between the paired arteriole and venule on their outlet PO2. The outlet PO2 in the venule increases with decreasing distance H. At H = 1000 μm, PO2 corresponds to that of no countercurrent exchange. The diffusion conductance coefficients F2 and G1, proportional to the diffusive flux from the arteriole to the venule, increase significantly with decreasing H (Table 2), but not enough to significantly affect the venular PO2 (Table 3). Because the change in venular PO2 from inlet to outlet is so small, it appears that there is no countercurrent exchange. This may not be the case, however. To estimate the contribution of counter-current exchange, we average oxygen fluxes (5) and (6) over the length of the vessel:
| (24) |
| (25) |
TABLE 3.
Effect of the distance H between the Paired Arteriole and Venule on Their Outlet PO2a
| H (μm) | Outlet PO2 (mmHg) | |
|---|---|---|
|
| ||
| Arteriole | Venule | |
| 1000 | 48.9 | 19.5 |
| 250 | 49.0 | 19.5 |
| 100 | 49.1 | 19.7 |
| 50 | 48.6 | 20.0 |
| 25 | 47.4 | 20.3 |
| 10 | 44.5 | 20.9 |
Da = 60 μm, Dv = 90 μm, Qv = Qa, . H = 1000 μm corresponds to no countercurrent exchange.
An overbar denotes spatial averaging. The longitudinal distributions of arteriolar and venular PO2are nearly linear within a vessel (Figure 2); thus, we can replace average values Pa and Pv with the corresponding arithmetic averages of inlet and outlet values. For example, consider the case H = 50 μm. From Table 3 we obtain p̄a = 54.3 mmHg, p̄v = 20.0 mmHg. Taking the values of the diffusion conductance coefficients from Table 2, we obtain for the fluxes (24) and (25)
Thus about 27% of the total oxygen flux from the arteriole (53.2/200.4) is transferred to the venule. The total flux from the venule is approximately zero, since the amount received from the arteriole (−53.2) is nearly balanced by the amount transferred to the surrounding tissue (52.0). For H = 25 μm, according to Table 3, p̄a = 53.7 mmHg and p̄v = 20.2 mmHg; hence
In this case, 41% of the total flux from the arteriole is transferred to the venule, and the amount received by the venule (−89.8) is larger than the amount transferred to the surrounding tissue (49.9); as a result, the venule is gaining oxygen. In the case of a larger separation between the arteriole and venule, H = 100 μm, the values from Table 2 and 3 yield p̄a = 54.6 mmHg, p̄v = 19.9 mmHg, and
The flux from the arteriole into the venule (−25.3) is smaller than the flux from the venule to the surrounding tissue; thus there is a net loss of oxygen from the venule.
From this analysis we conclude that venular PO2 can remain constant while a substantial amount of oxygen is received from the paired arteriole, i.e. in the presence of countercurrent exchange, if the venule loses an equal amount of oxygen to the surrounding tissue. Thus, the magnitude of arteriovenular countercurrent exchange should be judged not by the venular longitudinal PO2 profile, but by the relative contribution of the four terms in the relationships (24) and (25).
It should also be kept in mind that due to nonlinearity of the oxyhemoglobin dissociation curve (15), equal changes in arteriolar and venular PO2 correspond to unequal changes in hemoglobin saturation; e.g., a change ΔPO2 = 1 mmHg at PO2 = 45 mmHg corresponds to ΔSO2 = 0.9%, whereas at PO2 = 20 mmHg it corresponds to ΔSO2 =1.5%. Thus, even small changes in venular PO2 may correspond to substantial changes in hemoglobin saturation.
Table 4 shows that the PO2 at the venular outlet increases as the blood flow rate in the venule decreases. The venular SO2 rises by 7.5% when its blood flow rate is reduced to 10% of the arteriolar blood flow rate. The ratio of venular to arteriolar diameter does not have appreciable effect on venular PO2 (Table 5). The difference in the PO2 between the outlet and inlet of the venule decreases as the inlet venular PO2 increases (Table 6). Also, Table 7 shows that the outlet venular PO2 decreases as the inlet PO2 in the arteriole decreases.
TABLE 4.
PO2 at the Outlet of the Paired Arteriole and Venule with Variation of the Ratio of Blood Flow Rates (Qv/Qa)a
| Qv/Qa | Outlet PO2 (mmHg) | |
|---|---|---|
|
| ||
| Arteriole | Venule | |
| 1 | 417.4 | 20.3 |
| 0.5 | 47.4 | 20.6 |
| 0.25 | 47.5 | 21.2 |
| 0.1 | 47.5 | 22.7 |
H = 25 μm, .
TABLE 5.
PO2 at the Outlet of the Paired Arteriole and Venule with Variation of the Ratio of Their Diameters, Dv/Daa
| Dv/Da | Outlet PO2 (mmHg) | |
|---|---|---|
|
| ||
| Arteriole | Venule | |
| 1 | 47.5 | 20.4 |
| 1.2 | 47.5 | 20.4 |
| 1.5 | 47.4 | 20.3 |
H = 25 μm, Da = 60 μm.
TABLE 6.
Effect of Inlet Venular PO2 on the Outlet PO2 in the Paired Arteriole and Venulea
|
|
Outlet PO2 (mmHg) | ||
|---|---|---|---|
|
| |||
| Arteriole | Venule | ||
| 30 | 48.7 | 29.7 | |
| 20 | 47.4 | 20.3 | |
| 15 | 46.8 | 15.6 | |
.
TABLE 7.
Effect of Inlet Arteriolar PO2 on the Outlet PO2 in the Paired Arteriole and Venulea
|
|
Outlet PO2 (mmHg) | ||
|---|---|---|---|
|
| |||
| Arteriole | Venule | ||
| 70 | 47.8 | 20.4 | |
| 60 | 47.4 | 20.3 | |
| 50 | 43.7 | 20.2 | |
.
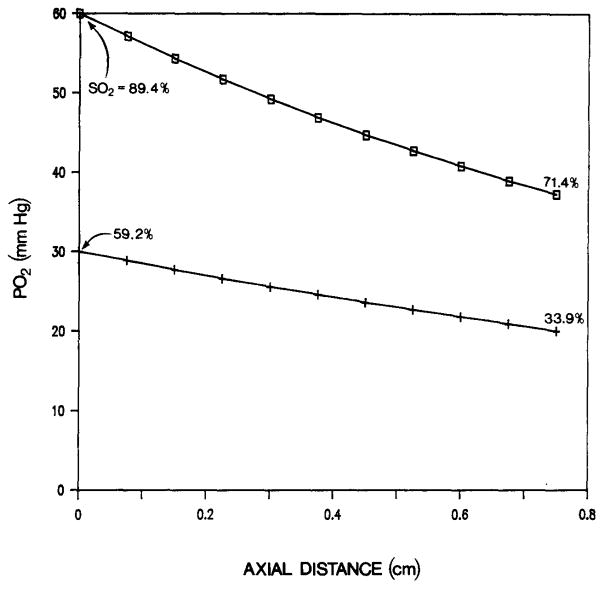
The following example shows that under certain conditions venular PO2 can significantly increase due to countercurrent exchange. We take Da = 60 μm, Dv = 90 μm, L = 0.75 cm, C = 0.1 (cm3 O2)/(cm3 blood) (a low value of C can be a result of anemia), v = 0.25 cm/s, Qv = Qa/10, P∞ = 12 mmHg, and H = 10 μm. For these parameters, the venous PO2 increases from 20 mmHg at the inlet to 30 mmHg at the outlet (Figure 3). The venous O2 saturation increases from 33.9 to 59.2%, i.e., by about 25.3%, as a result of countercurrent exchange.
Fig. 3.
Axial PO2 distribution in arteriolar (□) and venular (+) vessels. The numbers at the end points of the curve are the values of the O2 saturation. Low flow in the venule, Qv = Qa/10.
CONCLUSIONS
We have formulated a mathematical model for the O2 transport between a paired arteriole and venule. To obtain an analytical solution to the problem, we evaluated the nonlinear O2 dissociation curve at the inlet of the vessel. Using this approximation, we were able to obtain the solution in closed form. Reasonably good agreement is found between the exact solution of the linearized system and the numerical solution of the corresponding nonlinear system, but the error can accumulate if the solution is applied to vascular networks. We have found that the diffusive countercurrent exchange can contribute significantly to the total flux of oxygen from the venule, even though the effect on venular PO2 may be small. However, there are cases where the model predicts significant rise of venular PO2.
It is also possible that oxygen that diffuses from arterioles is carried to the venules convectively by the capillaries. This mechanism is not included in the present model.
Acknowledgments
This work was supported by NIH grant HL18292 and was carried out while one of the authors (M. S.) was on leave from the Indian Institute of Technology, Delhi. The authors wish to thank Ms. Brenda Pope for typing the manuscript.
APPENDIX. COMPUTATION OF THE DIFFUSION CONDUCTANCE COEFFICIENTS
Consider a plane z = const perpendicular to the vessel axes. In this plane we introduce the bipolar coordinates (ξ, θ) [10]:
| (A1) |
with scale factors
| (A2) |
where (± a, 0) are the coordinates of the foci which will be expressed below.
The radii of the vessels are expressed as
| (A3) |
and the coordinates of their centers (vessel axes) are
| (A4) |
Notice that ξa > 0 and ξv < 0.
The distance R between the centers of the vessels is
| (A5) |
From (A3), (A4), and (A5), the focus coordinate a is expressed as:
| (A6) |
The transformation (Al) maps the region outside the circles onto a rectangular region in the ξ-θ plane. The boundaries Γa and Γv are mapped to lines ξ = ξa and ξ = ξv; θ varies from 0 to 2π.
Equation (1) and the boundary conditions (2) become
| (A7) |
| (A8) |
We introduce the periodic boundary condition for θ:
| (A9) |
Using the transformed coordinates, we obtain
| (A10) |
| (A11) |
Equation (A7) with boundary conditions (A8) and (A9) is solved numerically by the finite difference method. The second derivatives are approximated by the central difference operator with second order accuracy. The resulting system of algebraic equations is solved by the successive overrelaxation method [16].
The derivatives at the boundary are computed from three points to retain the second order accuracy. Finally, the integrals (A10) and (A11) are computed numerically using Simpson’s rule [16].
Once and are computed, the parameters F1 and F2 are calculated as
| (A12) |
and
| (A13) |
G1 and G2 are obtained in a similar way.
References
- 1.Diller TE, Mikic BB, Drinker PA. Shear-induced augmentation of oxygen transfer in blood. J Biomech Engrg. 1980;102:67–72. doi: 10.1115/1.3138201. [DOI] [PubMed] [Google Scholar]
- 2.Duling BR, Berne RM. Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ Res. 1970;27:669–678. doi: 10.1161/01.res.27.5.669. [DOI] [PubMed] [Google Scholar]
- 3.Duling BR, Kuschinsky W, Wahl M. Measurements of the perivascular PO2 in the vicinity of the pial vessels of the cat. Pflügers Arch. 1979;383:29–34. doi: 10.1007/BF00584471. [DOI] [PubMed] [Google Scholar]
- 4.Ellsworth ML, Pittman RN. Evaluation of photometric methods for quantifying convective mass transport in microvessels. Amer J Physiol. 1986;251:H869–H879. doi: 10.1152/ajpheart.1986.251.4.H869. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez G. The rate of oxygen release and its effect on capillary O2, tension—a mathematical analysis. Respir Physiol. 1986;63:79–96. doi: 10.1016/0034-5687(86)90032-0. [DOI] [PubMed] [Google Scholar]
- 6.Harris PD. Movement of oxygen in skeletal muscle. News Physiol Sci. 1986;1:147–149. [Google Scholar]
- 7.Ivanov KP, Derii AN, Samoilov MO, Semenov DG. Diffusion of oxygen from the smallest arteries of the brain. Dokl Akud Nauk SSSR. 1979;244:1509–1511. [PubMed] [Google Scholar]
- 8.Ivanov KP, Derry AN, Vovenko EP, Samoilov MO, Seminov DG. Direct measurements of oxygen tension at the surface of arterioles, capillaries and venules of the cerebral cortex. Pflügers Arch. 1982;393:118–120. doi: 10.1007/BF00582403. [DOI] [PubMed] [Google Scholar]
- 9.Joyner WL, Davis MJ, Gilmore JP. Intravascular pressure distribution and dimensional analysis of microvessels in hamsters with renovascular hypertension. Microvasc Res. 1981;22:190–198. doi: 10.1016/0026-2862(81)90088-1. [DOI] [PubMed] [Google Scholar]
- 10.Morse PM, Feshbach H. Methods of Theoretical Physics, Part II. McGraw-Hill; New York: 1953. p. 1210. [Google Scholar]
- 11.Piiper J, Meyer M, Scheid P. Dual role of diffusion in tissue gas exchange: Blood-tissue equilibration and diffusion shunt. Respir Physiol. 1984;56:131–144. doi: 10.1016/0034-5687(84)90099-9. [DOI] [PubMed] [Google Scholar]
- 12.Pittman RN, Duling BR. Measurements of percent oxyhemoglobin in the microvasculature. J Appl Physiol. 1975;38:321–327. doi: 10.1152/jappl.1975.38.2.321. [DOI] [PubMed] [Google Scholar]
- 13.Pittman RN, Duling BR. Effect of altered carbon dioxide tension on hemoglobin oxygenation in hamster cheek pouch microvessels. Microvasc Res. 1977;13:211–224. doi: 10.1016/0026-2862(77)90086-3. [DOI] [PubMed] [Google Scholar]
- 14.Pittman RN, Duling BR. The determination of oxygen availability in the microcirculation. In: Jobsis FF, editor. Oxygen and Physiological Function. Professional Information Library; Dallas, Tex: 1977. pp. 133–147. [Google Scholar]
- 15.Popel AS, Gross JF. Analysis of oxygen diffusion from arteriolar networks. Amer J Physiol. 1979;237:H681–H689. doi: 10.1152/ajpheart.1979.237.6.H681. [DOI] [PubMed] [Google Scholar]
- 16.Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical Recipies. Cambridge U. P; Cambridge: 1986. [Google Scholar]
- 17.Roth AC, Wade K. The effects of transmural transport in the microcirculation: A two gas species model. Microvasc Res. 1986;32:64–83. doi: 10.1016/0026-2862(86)90044-0. [DOI] [PubMed] [Google Scholar]
- 18.Sarelius IH, Duling BR. Direct measurement of microvessel hematocrit, red cell flux, velocity and transit time. Amer J Physiol. 1982;243:H1018–1026. doi: 10.1152/ajpheart.1982.243.6.H1018. [DOI] [PubMed] [Google Scholar]
- 19.Swain DP, Pittman RN. Oxygen exchange in the microcirculation of hamster striated muscle (Abstract) Microvasc Res. 1984;27:266. [Google Scholar]
- 20.Zander R, Schmid-Schonbein H. Influence of intracellular convection on the oxygen release by human erythrocytes. Pflügers Arch. 1972;335:58–73. doi: 10.1007/BF00586935. [DOI] [PubMed] [Google Scholar]