Abstract
Species flower production and flowering phenology vary from year to year due to extrinsic factors. Inter-annual variability in flowering patterns may have important consequences for attractiveness to pollinators, and ultimately, plant reproductive output. To understand the consequences of flowering pattern variability, a community approach is necessary because pollinator flower choice is highly dependent on flower context. Our objectives were: 1) To quantify yearly variability in flower density and phenology; 2) To evaluate whether changes in flowering patterns result in significant changes in pollen/nectar composition. We monitored weekly flowering patterns in a Mediterranean scrubland community (23 species) over 8 years. Floral resource availability was estimated based on field measures of pollen and nectar production per flower. We analysed inter-annual variation in flowering phenology (duration and date of peak bloom) and flower production, and inter-annual and monthly variability in flower, pollen and nectar species composition. We also investigated potential phylogenetic effects on inter-annual variability of flowering patterns. We found dramatic variation in yearly flower production both at the species and community levels. There was also substantial variation in flowering phenology. Importantly, yearly fluctuations were far from synchronous across species, and resulted in significant changes in floral resources availability and composition at the community level. Changes were especially pronounced late in the season, at a time when flowers are scarce and pollinator visitation rates are particularly high. We discuss the consequences of our findings for pollinator visitation and plant reproductive success in the current scenario of climate change.
Introduction
Flower production and flowering phenology are species-specific traits that are strongly constrained by life form and phylogeny [1,2]. However, these traits are also influenced by extrinsic factors such as weather variables [3–7] and nutrient availability [8–10], and therefore show a certain level of variation across years [11–13]. Inter-annual variability in flowering patterns may have important consequences in terms of attractiveness to pollinators and flower predators, and ultimately affect plant reproductive output. For example, large floral displays (number of flowers per individual) (reviewed in [14]) and high flower densities [15–18] usually result in increased pollinator visitation rates. Yearly variability in flowering patterns may also have a strong impact on the reproductive success of flower-visiting insects. In situations in which a given species does not bloom profusely, flower visitors foraging on this plant take longer to gather food resources [19,20], and may be forced to switch to alternative, non-preferred flower species [21].
Interest in yearly variability in flowering time has increased in the current scenario of climate change, especially in relation to potential temporal mismatches between plants and their pollinators [7,22–27]. On the other hand, much less attention has been given to variation in flower production [12,13,22,28,29]. Most studies have focused on individual species and studies at the community level remain scarce. A community approach is essential to fully understand the consequences of variation in flowering patterns for interacting species such as pollinators, because flower choice by pollinators is highly dependent on floral context (abundance and composition of co-flowering species) [18,21,30,31]. If yearly changes in flowering patterns are pronounced and, most importantly, if they are not synchronous across species in the community, flower-visiting insects may encounter a different "flower landscape" from year to year. Such temporal variability could have important consequences for the structure of plant-pollinator interactions and pollination function. Alternatively, if changes in flowering patterns are small and/or all species in the community fluctuate in parallel, flower composition and interactions with pollinators may remain relatively consistent through time, a scenario that would favour specialization in plant-pollinator interactions [32].
In this study, we monitored flower production and flowering phenology in a coastal scrubland community (23 species) over 8 years. Our specific objectives are: 1) To quantify yearly variability in flowering patterns in terms of flower density and phenology. We are interested in establishing whether the various species in the community fluctuate synchronously or not. Our flower community is strongly seasonal [33,34]. For this reason, we are also interested in potential interactions between inter-annual and seasonal (intra-annual) variability; 2) To evaluate to what extent changes in flowering patterns result in significant changes in flower resource (pollen and nectar) availability and composition.
Methods
Study site
The study area is a Mediterranean scrubland located in the Garraf Natural Park, near Barcelona, NE Spain. Field work was conducted with permission of the Park’s administration. The study plot (~ 1ha; UTM: 409345.0, 4569737.5) is located 340 m above sea level and 1700 m from the coastline. The vegetation is dominated by Quercus coccifera, Pistacia lentiscus, Thymus vulgaris and Rosmarinus officinalis. The climate is characterized by warm dry summers and mild winters. Most precipitation occurs in autumn and spring. Mean annual temperature is 15.5°C and yearly rainfall is around 600 mm.
Flower counts
Data collection took place from March to June of years 2006 to 2014 (except 2010). Bloom becomes extremely scarce in July due to the severe summer drought. We used 6 permanent 50 x 1 m transects forming a grid. Distance between adjacent parallel transects was 20 m. Once a week, all open flowers of entomophilous species were counted in these transects. Flower life span was less than a week in all species. We analyze the data corresponding to the 23 most abundant species, representing 15 plant families, and amounting to 99.4% of the total number of flowers counted throughout the study.
We characterized the flowering pattern of each species based on the total number of flowers produced per hectare, year and transect (flowering density), the duration (in weeks) of the flowering period (flowering duration) and the date (week; week 1 = first week of March) of maximum flowering intensity (flowering peak). We do not analyze date of flowering onset because it was strongly correlated to flowering peak date every year (Pearson’s correlation, n = 23 species, r = 0.67–0.91; all p < 0.001), in addition, peak is a better measure for phenological comparisons [35]. Although in some years Rosmarinus officinalis started its bloom earlier than our flower-sampling campaign, we always captured most of the flowering period. Since sampling dates were not exactly the same each year, we use linear interpolations to work with the same days of the year (DOY) across the eight years. Nine species did not bloom at all in at least one of the eight years. For these species, flowering peak and flowering duration values are calculated excluding years of null flowering.
Nectar and pollen production
To understand whether fluctuations in flowering pattern resulted in temporal changes in floral resource composition we measured pollen (mm3) and nectar production per flower (mg of sugar in 24 h) in the 23 plant species. These measurements were taken in 2006 and 2007, depending on the species. On haphazardly chosen plants, we used nylon bags to enclose branches with mature buds. After 24 h we extracted and measured the nectar accumulated in individual flowers by using Drummond micropipettes of 0.25, 0.50 and 1 μl (samples size = 18–144 flowers per species; S1 Table). To measure flower pollen content, we selected between 10 and 15 flower buds per species. Flower buds were removed and kept in vials with 70% ethanol and taken to the laboratory. Each flower bud was dissected under a stereomicroscope and the number of anthers was counted. Then, three anthers per flower were removed, suspended in 2 ml of 70% ethanol and sonicated in a water bath for 2–4 minutes to dislodge pollen grains and 9 ml of isotonic solution was added. The number of pollen grains in the resulting suspension was then estimated using an electronic particle counter (Coulter Multisizer) with 200 μm aperture. From these data we obtained the total number of pollen grains produced per flower. The use of data from two years for the entire eight-year series assumes that intra-specific pollen and nectar production per flower was consistent throughout the duration of the study. Both pollen and nectar production per flower are known to vary at the species level from year to year [36–38]. However, because in our study inter-specific differences in pollen and nectar production per flower are as high as 276-fold and 178-fold, respectively (see Results), we assume that the relatively much smaller intra-specific yearly differences will not significantly alter our community level results. Flower density data and pollen/nectar production per flower were combined to obtain estimates of pollen and nectar production per ha.
Data analysis
Variability in flowering patterns
We used Linear Mixed-effect Models (LMMs) to analyze the effect of year, species and the interaction between year and species on flowering density, flowering peak and flowering duration. A significant year effect and lack of significant year x species interaction would indicate that all species in the community fluctuate more or less in synchrony. Transect was added as a random factor because the same transects were repeatedly sampled over the years. Eight species did not bloom at all in at least one of the eight years. For these species/years, flower density was 0 and flowering peak and flower duration could not be calculated. Consequently, these species were removed from the analyses. Flowering density was log-transformed and residuals were checked to satisfy normality and homoscedasticity assumptions. Analyses were performed with the lmer function of lme4 package [39] in R (version 3.3.3, R Core Team, Vienna (Austria), 2017, https://www.R-project.org).
To analyze yearly and seasonal variability in overall (sum of all species) flower density, we conducted a LMM with flower density as the dependent variable and year and month (March, April, May, June) as factors. The month x year interaction was also included in the model. Transect was added as a random factor. The same analysis was subsequently repeated with nectar and pollen production data. Flower, nectar and pollen data were log-transformed to achieve normality. Nectar and pollen results were very similar. For this reason, only nectar results are shown.
To analyse yearly and seasonal variation in flower composition we conducted permutational multivariate analyses of variance (PERMANOVA). The response variable was a Bray-Curtis dissimilarity matrix of flower composition (flower density of each species) between the different sampling events (combinations of years and months). Year, month and their interaction were included as predictors, and transects were selected as groups (strata) within which permutations were restricted. The analysis was executed with 9999 permutations, using Adonis function of vegan package [40] in R. To facilitate interpretation of the results, non-metric multidimensional scaling (NMDS) was performed based on flower composition Bray-Curtis distances using the metaMDS function of vegan package in R. Subsequently, equivalent PERMANOVA and NMDS analyses were conducted with nectar and pollen composition data. Again, because pollen and nectar results were very similar, we only show nectar results. All analyses were conducted without 10 species that had missing values in some transects during the entire season for the 8 years of sampling.
Phylogenetic constraints
To find out whether inter-annual flowering pattern variability was constrained by phylogeny, we conducted tests of phylogenetic signal for continuous variables based on Bloomberg’s K assuming Brownian motion character evolution [41]. We first built a phylogenetic tree of the 23 species with phylomatic V.3 [42]. Polytomies were resolved manually using timetree databases [43]. Then, we used the phylosig function of phytools R package [44] to detect phylogenetic signal in flowering density CV, flowering peak SD, and flowering duration SD. To estimate the significance of the observed phylogenetic signals, K values obtained with the real data were compared to K values obtained from randomizations (1000 trees in which species where shuffled across the tips of the phylogenetic tree) [41]. We found that variability in flower density, flowering peak and flowering duration was not constrained by phylogeny (S2 Table). That is, phylogenetically related species did not show similar propensity to variation (or lack thereof) in flowering patterns. Therefore, phylogeny was not accounted for in subsequent analyses.
Results
Variation in flowering phenology and density and consequences for floral resource availability
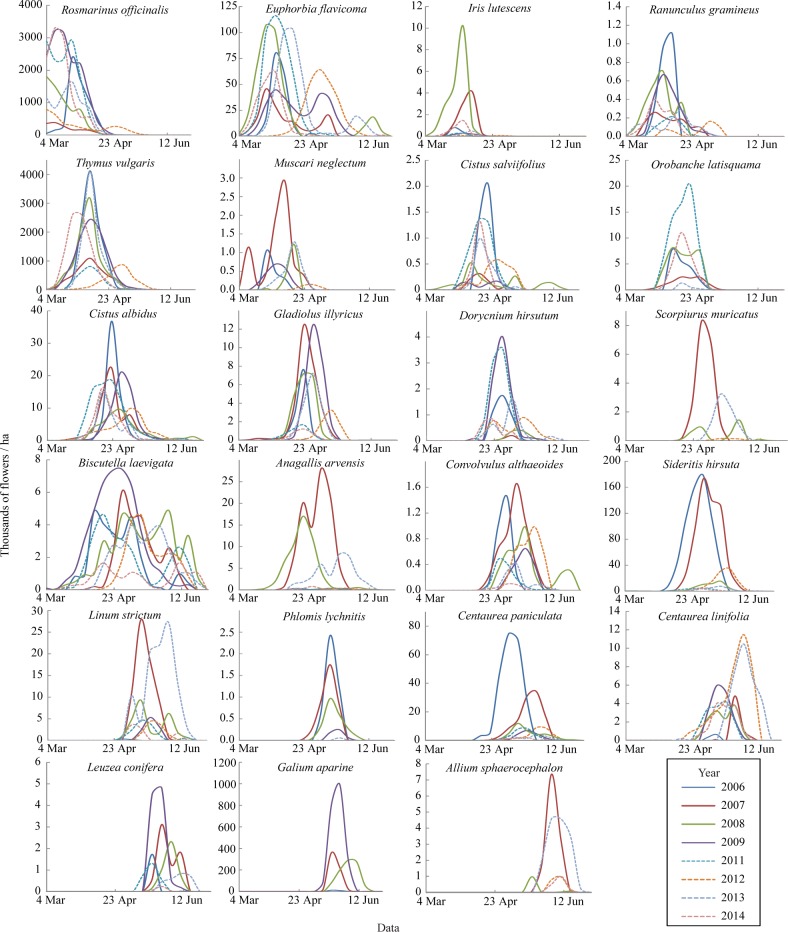
Yearly variation in flower density was strongly species-dependent (Fig 1). Some species (e.g., Cistus albidus, Euphorbia flavicoma) had relatively consistent flower density, while others (e.g., Scorpiurus muricatus, Galium aparine) fluctuated dramatically (flowering density CV > 1.6; S3 Table). Maximum differences (ratio between highest and lowest years) were 267-fold in Leuzea conifera and 135-fold in Linum strictum (Fig 1), and eight species did not bloom at all in one or more years. As for flowering phenology, some species showed important shifts in flowering peak date (e.g., Anagallis arvensis, Cistus salviifolius) while others were much more consistent (e.g., Linum strictum, Orobanche latisquama) (S3 Table, Fig 1). Different species also showed different levels of variation in flowering duration. For example, Scorpiurus muricatus and Iris lutescens were highly variable, while Euphorbia flavicoma and Biscutella laevigata were relatively consistent (S3 Table, Fig 1). In all species, the CV of flower density was higher than the CV of flowering duration (S3 Table). Differences across years in flower density, flowering peak and flowering duration of the 23 species were highly significant (Table 1). Importantly, the interaction year x species was also highly significant for the three variables (Table 1), indicating that yearly variation in flowering patterns was strongly asynchronous across species.
Fig 1. Yearly flowering curves of the 23 most abundant plant species of the Garraf community.
Ordered by timing of peak bloom. Note different scales on y-axis.
Table 1. Anova tables of the fitted LMMs analyzing yearly changes in mean flower density (log-transformed), flowering peak, and flowering duration of the 15 most abundant species of the Garraf community.
| Variable | Factor | DF | F | P |
|---|---|---|---|---|
| Flower density | Year | 7 | 4.6 | < 0.001 |
| Species | 14 | 197.0 | < 0.001 | |
| Year x Species | 98 | 2.8 | < 0.001 | |
| Flowering peak | Year | 7 | 31.9 | < 0.001 |
| Species | 14 | 243.0 | < 0.001 | |
| Year x Species | 98 | 4.1 | < 0.001 | |
| Flowering duration | Year | 7 | 20.5 | < 0.001 |
| Species | 14 | 72.0 | < 0.001 | |
| Year x Species | 98 | 3.3 | < 0.001 |
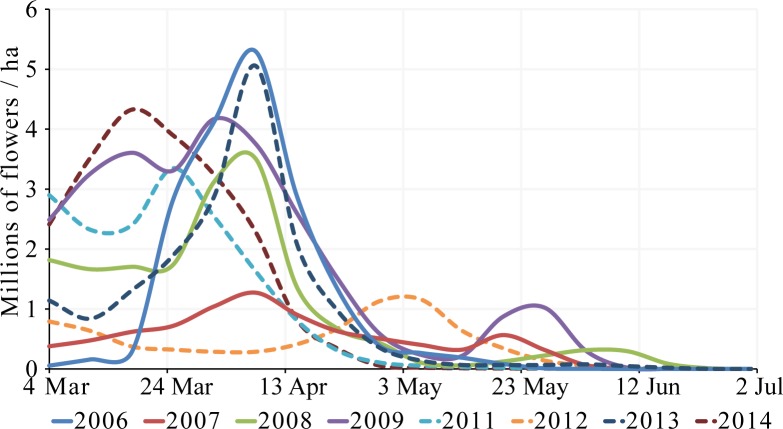
The community was largely dominated by two species, Rosmarinus officinalis and Thymus vulgaris, which together accounted for 91.7 ± 6.5% of the total (8-year) flower density. Because these two species showed important maximum yearly differences in flower density (10- and 4-fold, respectively), overall flower availability was highly variable across years (Fig 2, Table 2). In most years, total flower production ranged from 16 to 21 million flowers / ha (Fig 2). However, in 2007 and 2012 flower production was only ~ 8 million flowers / ha, and in 2009 it was ~ 28 million flowers / ha. The seasonal distribution of flower density also changed noticeably across years. In most years, flowering peaked in late March or early April, but in 2014 peak bloom occurred in mid-March, and in 2012 in early May (Fig 2).
Fig 2. Yearly overall (23 species) flowering curves of the Garraf community.
Each curve represents the mean value across transects (n = 6).
Table 2. Anova tables of the fitted LMMs analyzing yearly and monthly variation in overall (23 species) flower density and nectar availability in the Garraf community.
| Variable | Factor | DF | F | P |
|---|---|---|---|---|
| Flower density (flowers / ha) | Year | 7 | 11.9 | < 0.001 |
| Month | 3 | 235.7 | < 0.001 | |
| Year x Month | 21 | 10.5 | < 0.001 | |
| Nectar availability (sugar mg / ha) | Year | 7 | 17.9 | < 0.001 |
| Month | 3 | 756.4 | < 0.001 | |
| Year x Month | 21 | 17.6 | < 0.001 |
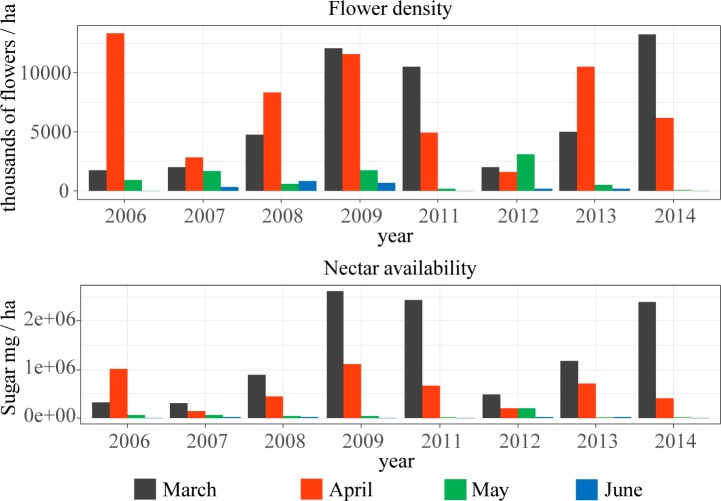
As expected given the strong seasonal component of the flower community, overall flower density also varied dramatically across months (Table 2). Of more interest is the highly significant year x month interaction, indicating that annual variation is far from uniform across seasons (Table 2, Fig 3). Importantly, changes in overall flower density had consequences for nectar availability, which also presented high inter- and intra-annual variability (Fig 3), and a highly significant year x month interaction (Table 2). Nectar availability was usually highest in March, but in 2006 maximum availability occurred in April (Fig 3).
Fig 3. Monthly distribution of flower density and nectar availability in the Garraf community across eight years.
Variation in flower composition and consequences for floral resource composition
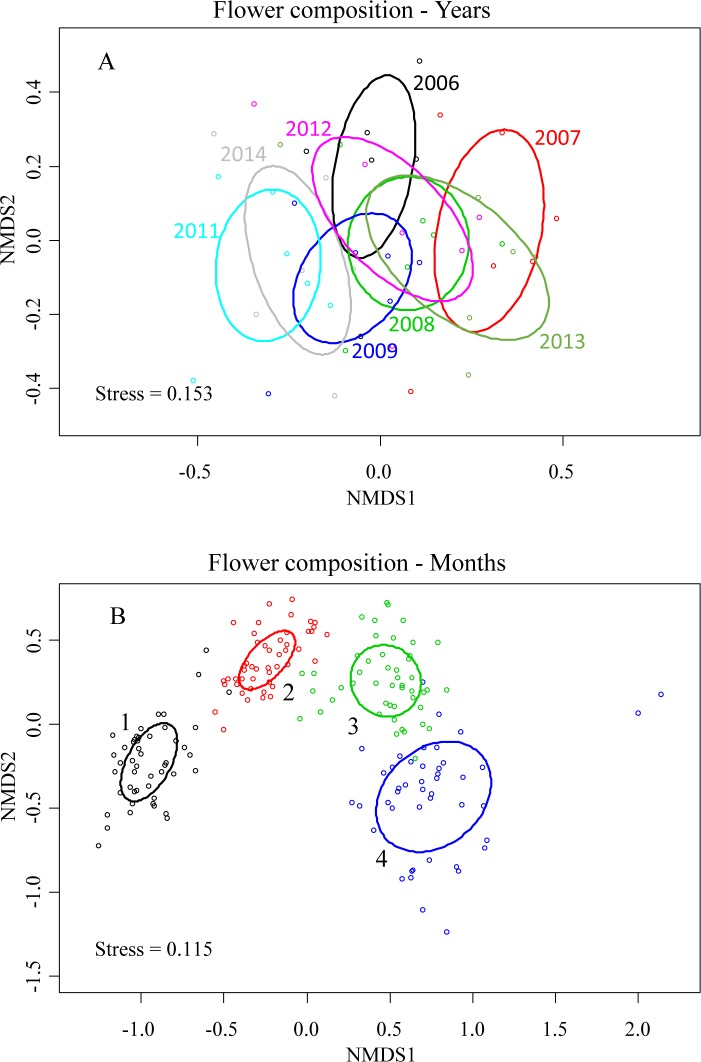
Flower composition differed significantly across years (Table 3, Fig 4A), although the factor year explained only a small part of the observed variability (9.2%). As mentioned, the Garraf flower community is largely dominated by two species, Rosmarinus officinalis and Thymus vulgaris that bloom profusely in early spring. Such dominance is likely to mask changes in flower composition involving non-dominant species. As expected, flower composition differed strongly across months (Table 3, Fig 4B), explaining 32.6% of the observed variability. More importantly, the year x month interaction was also strongly significant (Table 3), indicating that the expected pattern of seasonal variability was not consistent across years. Yearly variability in flower composition was greatest at the end of the flowering period (June; Fig 4B). Variability in nectar composition closely paralleled the results obtained with flower composition (Table 3). Interestingly, consecutive years often had contrasting nectar compositions (e.g., 2006 vs. 2007; 2013 vs. 2014).
Table 3. Results of PERMANOVAs analyzing yearly and monthly variation in flower and nectar composition in the Garraf community.
| Flower composition | DF | F | R2 | P |
|---|---|---|---|---|
| Year | 7 | 5.84 | 0.09 | < 0.001 |
| Month | 3 | 48.27 | 0.33 | < 0.001 |
| Year x Month | 21 | 4.70 | 0.22 | < 0.001 |
| Residuals | 160 | 0.36 | ||
| Total | 191 | 1 | ||
| Nectar composition | ||||
| Year | 7 | 6.73 | 0.085 | < 0.001 |
| Month | 3 | 72.32 | 0.394 | < 0.001 |
| Year x Month | 21 | 6.05 | 0.230 | < 0.001 |
| Residuals | 160 | 0.290 | ||
| Total | 191 | 1 |
Fig 4. Non-metric multidimensional scaling (NMDS) analysis describing yearly and monthly variation in flower composition.
Ellipses correspond to standard deviations of the sampling events of each grouping factor (years or months). 1: March; 2: April; 3: May; 4: June. Points on figure (A) represents transect–year values. Points on figure (B) represents each transect–month–year combination. The results for nectar composition were similar and are shown in S1 Fig.
Discussion
Our study demonstrates that flowering patterns change dramatically from year to year both at the species and community levels. At the species level, most variability is due to changes in flower density, rather than flowering phenology. Some studies have addressed the influence of environmental factors on flowering patterns (see [13] for a novel methodological approach). In general, these studies have shown flower production to be positively related to rainfall [4,22]. On the other hand, flowering phenology appears to be mostly regulated by temperature, with warmer temperatures advancing flowering periods [7,45–47].
Importantly, our study also demonstrates that yearly flowering fluctuations are by no means synchronized across species, resulting in significant yearly changes in flower composition, especially late in the season (June). Flower seasonal composition is important because most pollinator species in Garraf have short activity periods in relation to the overall flowering period of the community, and the the Garraf pollination network is structured in seasonal modules [33,48]. By June, flower density in the Garraf scrubland dramatically declines, and visitation rates (visits per flower and time unit) are very high [33,34]. At that time, it is not infrequent to see several pollinators foraging simultaneously on the same inflorescence. Two species blooming in June, Sideritis hirsuta and Galium aparine show marked fluctuations in flower density (Fig 1), resulting in drastic yearly changes in flower resource availability. Thus, June-active pollinators are exposed to floral resources that are both scarce and unreliable from year to year, a situation that is expected to hinder foraging specialization [32].
Changes in flower density and composition are known to affect pollinator flower choice and visitation rates, with potentially important consequences for plant reproductive success [16,49–51]. Changes in flower visitation rates have been shown to affect stigma pollen deposition, sometimes resulting in changes in seed-set [52,53]. Plant reproductive success is also likely to be affected by changes in pollinator composition. Different pollinator species differ in their pollinating efficiency both in terms of number of pollen grains delivered per visit [54–57], and the quality of the pollen deposited (e.g. levels of geitonogamy [54, 58,59]). Changes in flower composition may also influence indirect interactions among plant species competing for pollinators or facilitating pollinator visitation [15,60–63]. Because the effects of flower neighborhood on pollinator visitation are density-dependent (facilitation may turn into competition as the facilitating species becomes increasingly abundant) [15,63], shifts in flower composition may change the direction of these interactions.
Changes in pollen and nectar availability imply that pollinators are confronted with inconsistent floral resource landscapes from year to year, with potential ecological consequences for their fitness. In years with low flower densities, pollinators are forced to fly longer distances to gather pollen/nectar loads. These increased foraging costs result in slow nest provisioning rates and decreased offspring body size [19,64,65], ultimately leading to increased developmental and wintering mortality [19,66]. Long provisioning trips are also likely to result in increased parasitism by cleptoparasites and parasitoids that enter bee nests and lay their eggs while the nest founder is away foraging [67,68].
In the current scenario of climate change, the Mediterranean Basin is predicted to experience important temperature increases [69,70]. This is expected to advance the flowering time of most plant species [7,45–47]. Pollinators may be able to track these phenological changes if, as suggested by current evidence [26,71–74], they also respond to global warming by advancing their activity period. We know less about the potential effects of climate change on flower production. Increased temperature has been shown to have either positive or negative effects on flower production depending on the species [75]. However, the predicted increase in the occurrence of drought episodes in the Mediterranean Basin [69,70] is expected to increase the frequency of years with low floral resources [4,22]. Our study shows shifts in flowering intensity to be as least as important as shifts in flowering time, and to be highly asynchronous across species. For this reason, and given the irregular occurrence of drought episodes, we expect shifts in flowering density to have high negative effects on pollinators.
Supporting information
(PDF)
(PDF)
Species ordered by timing of flowering peak.
(PDF)
Ellipses correspond to standard deviations of the sampling events of each grouping factor (years or months). 1: March; 2: April; 3: May; 4: June. Points on figure (A) represents transect–year values. Points on figure (B) represents each transect–month–year combination.
(PDF)
(ZIP)
Acknowledgments
We are very grateful to several students that participated in the flower counts. We thank Roberto Molowny-Horas for his support in statistical analysis. We also thank two anonymous reviewers for their helpful comments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The Spanish MICINN supported this study through projects CGL2005-00491, CSD2008-0040 and CGL2009-12646.
References
- 1.Kochmer JP, Handel SN. Constraints and competition in the evolution of flowering phenology. Ecol Monogr. 1986;56: 303–325. [Google Scholar]
- 2.Petanidou T, Ellis WN, Margaris NS, Vokou D. Constraints on flowering phenology in a Phryganic (east Mediterranean shrub) community. Am J Bot. 1995;82: 607–620. [Google Scholar]
- 3.Peñuelas J, Filella I, Zhang X, Llorens L, Ogaya R, Lloret F, et al. Complex spatiotemporal phenological shifts as a response to rainfall changes. New Phytol. 2004;161: 837–846. [DOI] [PubMed] [Google Scholar]
- 4.Prieto P, Peñuelas J, Ogaya R, Estiarte M. Precipitation-dependent flowering of Globularia alypum and Erica multiflora in Mediterranean shrubland under experimental drought and warming, and its inter-annual variability. Ann Bot. 2008;102: 275–85. doi: 10.1093/aob/mcn090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crimmins TM, Crimmins MA, David Bertelsen C. Complex responses to climate drivers in onset of spring flowering across a semi-arid elevation gradient. J Ecol. Blackwell Publishing Ltd; 2010;98: 1042–1051. doi: 10.1111/j.1365-2745.2010.01696.x [Google Scholar]
- 6.Lesica P, Kittelson PM. Precipitation and temperature are associated with advanced flowering phenology in a semi-arid grassland. J Arid Environ. 2010;74: 1013–1017. http://dx.doi.org/10.1016/j.jaridenv.2010.02.002 [Google Scholar]
- 7.Gordo O, Sanz JJ. Impact of climate change on plant phenology in Mediterranean ecosystems. Glob Chang Biol. 2010;16: 1082–1106. doi: 10.1111/j.1365-2486.2009.02084.x [Google Scholar]
- 8.Sardans J, Rodà F, Peñuelas J. Effects of water and a nutrient pulse supply on Rosmarinus officinalis growth, nutrient content and flowering in the field. Environ Exp Bot. 2005;53: 1–11. doi: 10.1016/j.envexpbot.2004.02.007 [Google Scholar]
- 9.Albrectsen BR, Ericson L, Lundberg P. Nutrient addition extends flowering display, which gets tracked by seed predators, but not by their parasitoids. Oikos. Munksgaard; 2008;117: 473–480. doi: 10.1111/j.2008.0030–1299.16381.x [Google Scholar]
- 10.Petraglia A, Carbognani M, Tomaselli M. Effects of nutrient amendments on modular growth, flowering effort and reproduction of snowbed plants. Plant Ecol Divers. Taylor & Francis; 2013;6: 475–486. doi: 10.1080/17550874.2013.795628 [Google Scholar]
- 11.Arroyo J. Ritmos climaticos y de floración en matorrales del SW de España. Lagascalia. 1990;16: 25–50. [Google Scholar]
- 12.Law B, Mackowski C, Schoer L, Tweedie T. Flowering phenology of myrtaceous trees and their relation to climatic, environmental and disturbance variables in northern New South Wales. Austral Ecol. 2000;25: 160–178. doi: 10.1046/j.1442-9993.2000.01009.x [Google Scholar]
- 13.Chen Y-Y, Satake A, Sun I-F, Kosugi Y, Tani M, Numata S, et al. Species-specific flowering cues among general flowering Shorea species at the Pasoh Research Forest, Malaysia. J Ecol. 2017; 1–13. doi: 10.1111/1365-2745.12836 [Google Scholar]
- 14.Ohashi K, Yahara T. Behavioral responses of pollinators to variation in floral display size and their influences on the evolution of floral traits In: Chittka Lars, Thomson James D., editors. Cognitive Ecology of Pollination Animal Behaviour and Floral Evolution. Cambridge University Press; 2001. pp. 274–296. [Google Scholar]
- 15.Rathcke B. Competition and facilitation among plants for pollination In: L R, editor Pollination biology. New York: Academic publication; 1983. pp. 305–326. [Google Scholar]
- 16.Kunin WE. Population size and density in pollination: effects pollinator foraging and plant reproductive success in experimental arrays of Brassica kaber. J Ecol. 1997;85: 225–234. doi: 10.2307/2960653 [Google Scholar]
- 17.Hegland SJ, Boeke L. Relationships between the density and diversity of floral resources and flower visitor activity in a temperate grassland community. Ecol Entomol. 2006;31: 532–538. doi: 10.1111/j.1365-2311.2006.00812.x [Google Scholar]
- 18.Lázaro A, Lundgren R, Totland Ø. Co-flowering neighbors influence the diversity and identity of pollinator groups visiting plant species. Oikos. Blackwell Publishing Ltd; 2009;118: 691–702. doi: 10.1111/j.1600-0706.2008.17168.x [Google Scholar]
- 19.Bosch J. Production of undersized offspring in a solitary bee. Anim Behav. 2008;75: 809–816. doi: 10.1016/j.anbehav.2007.06.018 [Google Scholar]
- 20.Zurbuchen A, Cheesman S, Klaiber J, Müller A, Hein S, Dorn S. Long foraging distances impose high costs on offspring production in solitary bees. Anim Ecol. 2010;79: 674–81. doi: 10.1111/j.1365-2656.2010.01675.x [DOI] [PubMed] [Google Scholar]
- 21.Mitchell RJ, Flanagan RJ, Brown BJ, Waser NM, Karron JD. New frontiers in competition for pollination. Ann Bot. 2009;103: 1403–13. doi: 10.1093/aob/mcp062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inouye DW, Saavedra F, Lee-Yang W. Environmental influences on the phenology and abundance of flowering by Androsace septentrionalis (Primulaceae). Am J Bot. Botanical Society of America; 2003;90: 905–910. doi: 10.3732/ajb.90.6.905 [DOI] [PubMed] [Google Scholar]
- 23.Hegland SJ, Nielsen A, Lázaro A, Bjerknes A-L, Totland Ø. How does climate warming affect plant-pollinator interactions? Ecol Lett. Blackwell Publishing Ltd; 2009;12: 184–195. doi: 10.1111/j.1461-0248.2008.01269.x [DOI] [PubMed] [Google Scholar]
- 24.Forrest J, Miller-Rushing AJ. Toward a synthetic understanding of the role of phenology in ecology and evolution. Philos Trans R Soc B Biol Sci. 2010;365: 3101 LP–3112. doi: 10.1098/rstb.2010.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diez JM, Ibáñez I, Miller-Rushing AJ, Mazer SJ, Crimmins TM, Crimmins M a, et al. Forecasting phenology: from species variability to community patterns. Ecol Lett. 2012;15: 545–53. doi: 10.1111/j.1461-0248.2012.01765.x [DOI] [PubMed] [Google Scholar]
- 26.Bartomeus I, Ascher JS, Wagner D, Danforth BN, Colla S, Kornbluth S, et al. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc Natl Acad Sci. 2011;108: 20645–20649. doi: 10.1073/pnas.1115559108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rafferty NE, Caradonna PJ, Burkle L a, Iler AM, Bronstein JL. Phenological overlap of interacting species in a changing climate: an assessment of available approaches. Ecol Evol. 2013;3: 3183–93. doi: 10.1002/ece3.668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CaraDonna PJ, Iler AM, Inouye DW. Shifts in flowering phenology reshape a subalpine plant community. Proc Natl Acad Sci. 2014;111: 4916–4921. doi: 10.1073/pnas.1323073111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldridge G, Inouye DW, Forrest JRK, Barr WA, Miller-Rushing AJ. Emergence of a mid-season period of low floral resources in a montane meadow ecosystem associated with climate change. J Ecol. Blackwell Publishing Ltd; 2011;99: 905–913. doi: 10.1111/j.1365-2745.2011.01826.x [Google Scholar]
- 30.Lázaro A, Totland O. Population dependence in the interactions with neighbors for pollination: A field experiment with Taraxacum officinale. J Bot. 2010;97: 760–9. doi: 10.3732/ajb.0900263 [DOI] [PubMed] [Google Scholar]
- 31.Petanidou T, Kallimanis AS, Sgardelis SP, Mazaris AD, Pantis JD, Waser NM. Variable flowering phenology and pollinator use in a community suggest future phenological mismatch. Acta Oecologica. 2014;59: 104–111. doi: 10.1016/j.actao.2014.06.001 [Google Scholar]
- 32.Ebeling A, Klein A-M, Tscharntke T. Plant–flower visitor interaction webs: Temporal stability and pollinator specialization increases along an experimental plant diversity gradient. Basic Appl Ecol. 2011;12: 300–309. doi: 10.1016/j.baae.2011.04.005 [Google Scholar]
- 33.Bosch J, González AMM, Rodrigo A, Navarro D. Plant-pollinator networks: adding the pollinator’s perspective. Ecol Lett. 2009;12: 409–19. doi: 10.1111/j.1461-0248.2009.01296.x [DOI] [PubMed] [Google Scholar]
- 34.Filella I, Primante C, Llusià J, Martín González AM, Seco R, Farré-Armengol G, et al. Floral advertisement scent in a changing plant-pollinators market. Sci Rep. 2013;3: 3434 doi: 10.1038/srep03434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller-Rushing AJ, Inouye DW, Primack RB. How well do first flowering dates measure plant responses to climate change? The effects of population size and sampling frequency. J Ecol. Blackwell Publishing Ltd; 2008;96: 1289–1296. doi: 10.1111/j.1365-2745.2008.01436.x [Google Scholar]
- 36.Walker AK, Barnes DK, Furgala B. Genetic and environmental effects on quantity and quality of alfalfa nectar Crop Sci. Madison, WI: Crop Science Society of America; 1974;14: 235–238. doi: 10.2135/cropsci1974.0011183X001400020020x [Google Scholar]
- 37.Zywiec M, Delibes M, Fedriani JM. Microgeographical, inter-individual, and intra-individual variation in the flower characters of Iberian pear Pyrus bourgaeana (Rosaceae). Oecologia. Springer; 2012;169: 713–722. doi: 10.1007/s00442-011-2232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khanduri VP, Sharma CM, Kumar KS, Ghildiyal SK. Annual variation in flowering phenology, pollination, mating system, and pollen yield in two natural populations of Schima wallichii (DC.) Korth. Sci World J. 2013;2013: 1–11. Available: http://dx.doi.org/10.1155/2013/350157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bates D, Maechler M, Bolker B, Steven W. lme4: Linear mixed-effects models using Eigen and S4. 2014. Available: http://cran.r-project.org/package=lme4 [Google Scholar]
- 40.Oksanen AJ, Blanchet FG, Kindt R, Legen- P, Minchin PR, Hara RBO, et al. Package “vegan.” 2014. [Google Scholar]
- 41.Blomberg SP, Garland T, Ives AR. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution (N Y). 2003;57: 717–45. doi: 10.1111/j.0014-3820.2003.tb00285.x [DOI] [PubMed] [Google Scholar]
- 42.Cam W, Donoghue M. Phylomatic V3. 2012. [cited 7 Oct 2014]. Available: http://www.phylodiversity.net/phylomatic [Google Scholar]
- 43.Kumar S, Hedges SB. TimeTree2: species divergence times on the iPhone. Bioinformatics. 2011;27: 2023–4. doi: 10.1093/bioinformatics/btr315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Revell MLJ. Package “phytools”. 2016. Available: https://cran.r-project.org/web/packages/phytools/phytools.pdf [Google Scholar]
- 45.Diekmann M. Relationship between flowering phenology of perennial herbs and meteorological data in deciduous forests of Sweden. Can J Bot. NRC Research Press; 1996;74: 528–537. doi: 10.1139/b96-067 [Google Scholar]
- 46.Sparks TH, Jeffree EP, Jeffree CE. An examination of the relationship between flowering times and temperature at the national scale using long-term phenological records from the UK. Int J Biometeorol. United States; 2000;44: 82–87. doi: 10.1007/s004840000049 [DOI] [PubMed] [Google Scholar]
- 47.Sherry RA, Zhou X, Gu S, Arnone JA, Schimel DS, Verburg PS, et al. Divergence of reproductive phenology under climate warming. Proc Natl Acad Sci. 2007;104: 198–202. doi: 10.1073/pnas.0605642104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martín González AM, Allesina S, Rodrigo A, Bosch J. Drivers of compartmentalization in a Mediterranean pollination network. Oikos. Blackwell Publishing Ltd; 2012;121: 2001–2013. doi: 10.1111/j.1600-0706.2012.20279.x [Google Scholar]
- 49.Lázaro A, Jakobsson A, Totland Ø. How do pollinator visitation rate and seed set relate to species’ floral traits and community context? Oecologia. 2013;173: 881–93. doi: 10.1007/s00442-013-2652-5 [DOI] [PubMed] [Google Scholar]
- 50.Bruckman D, Campbell DR. Floral neighborhood influences pollinator assemblages and effective pollination in a native plant. Oecologia. 2014;176: 465–476. doi: 10.1007/s00442-014-3023-6 [DOI] [PubMed] [Google Scholar]
- 51.Schmid B, Nottebrock H, Esler KJ, Pagel J, Pauw A, Böhning-Gaese K, et al. Responses of nectar-feeding birds to floral resources at multiple spatial scales. Ecography. Blackwell Publishing Ltd; 2016;39: 619–629. doi: 10.1111/ecog.01621 [Google Scholar]
- 52.Engel EC, Irwin RE. Linking pollinator visitation rate and pollen receipt. Am J Bot. 2003;90: 1612–1618. doi: 10.3732/ajb.90.11.1612 [DOI] [PubMed] [Google Scholar]
- 53.Karron JD, Mitchell RJ, Bell JM. Multiple pollinator visits to Mimulus ringens (Phrymaceae) flowers increase mate number and seed set within fruits. Am J Bot. 2006;93: 1306–1312. doi: 10.3732/ajb.93.9.1306 [DOI] [PubMed] [Google Scholar]
- 54.Herrera CM. Componentes del flujo génico en Lavandula latifolia Medicus: polinización y dispersión de semillas. An del Jardín Botánico Madrid. 1987;44: 49–61. [Google Scholar]
- 55.Gómez JM, Zamora R. Generalization vs. specialization in the pollination system of Hormathophylla spinosa (Cruciferae). Ecology. Ecological Society of America; 1999;80: 796–805. doi: 10.2307/177018 [Google Scholar]
- 56.Olsen KM. Pollination effectiveness and pollinator importance in a population of Heterotheca subaxillaris (Asteraceae). Oecologia. Springer; 1997;109: 114–121. doi: 10.1007/PL00008811 [DOI] [PubMed] [Google Scholar]
- 57.Sahli HF, Conner JK. Characterizing ecological generalization in plant-pollination systems. Oecologia. Springer; 2006;148: 365–372. doi: 10.1007/s00442-006-0396-1 [DOI] [PubMed] [Google Scholar]
- 58.Schmitt J. Pollinator foraging behavior and gene dispersal in Senecio (Compositae). Evolution; 1980;34: 934–943. doi: 10.1111/j.1558-5646.1980.tb04031.x [DOI] [PubMed] [Google Scholar]
- 59.Arnan X, Escolà A, Rodrigo A, Bosch J. Female reproductive success in gynodioecious Thymus vulgaris: pollen versus nutrient limitation and pollinator foraging behaviour. Bot J Linn Soc. 2014;175: 395–408. doi: 10.1111/boj.12173 [Google Scholar]
- 60.Schemske DW. Floral convergence and pollinator sharing in two bee-pollinated tropical herbs. Ecology. 1981;62: 946–954. doi: 10.2307/1936993 [Google Scholar]
- 61.Moeller DA. Facilitative interactions among plants via shared pollinators. Ecology. 2004;85: 3289–3301. doi: 10.1890/03-0810 [Google Scholar]
- 62.Bell JM, Karron JD, Mitchell RJ. Interspecific competition for pollination lowers seed production and outcrossing in Mimulus ringens. Ecology. 2005;86: 762–771. doi: 10.1890/04-0694 [Google Scholar]
- 63.Ghazoul J. Floral diversity and the facilitation of pollination. J Ecol. 2006;94: 295–304. doi: 10.1111/j.1365-2745.2006.01098.x [Google Scholar]
- 64.Peterson AJH, Roitberg BD. Impact of resource levels on sex ratio and resource allocation in the solitary bee, Megachile rotundata. Environ Entomol. 2006;35: 1404–1410. Available: https://doi.org/10.1093/ee/35.5.1404 [Google Scholar]
- 65.Zurbuchen A, Landert L, Klaiber J, Müller A, Hein S, Dorn S. Maximum foraging ranges in solitary bees: only few individuals have the capability to cover long foraging distances. Biol Conserv. Elsevier Ltd; 2010;143: 669–676. doi: 10.1016/j.biocon.2009.12.003 [Google Scholar]
- 66.Bosch J, Kemp WP. Effect of pre-wintering and wintering temperature regimes on weight loss, survival, and emergence time in the mason bee Osmia cornuta (Hymenoptera: Megachilidae). Apidologie. 2004;35: 469–479. doi: 10.1051/apido [Google Scholar]
- 67.Goodell K. Food availability affects Osmia pumila (Hymenoptera: Megachilidae) foraging, reproduction, and brood parasitism. Oecologia. 2003;134: 518–27. doi: 10.1007/s00442-002-1159-2 [DOI] [PubMed] [Google Scholar]
- 68.Seidelmann K. Open-cell parasitism shapes maternal investment patterns in the Red Mason bee Osmia rufa. Behav Ecol. 2006;17: 839 doi: 10.1093/beheco/arl017 [Google Scholar]
- 69.Hoerling M, Eischeid J, Perlwitz J, Quan X, Zhang T, Pegion P. On the increased frequency of Mediterranean drought. J Clim. American Meteorological Society; 2011;25: 2146–2161. doi: 10.1175/JCLI-D-11-00296.1 [Google Scholar]
- 70.Giorgi F, Lionello P. Climate change projections for the Mediterranean region. Glob Planet Change. 2008;63: 90–104. doi: 10.1016/j.gloplacha.2007.09.005 [Google Scholar]
- 71.Bosch J, Kemp WP. Effect of wintering duration and temperature on survival and emergence time in males of the orchard pollinator Osmia lignaria (Hymenoptera: Megachilidae). Environ Entomol. Entomological Society of America; 2003;32: 711–716. doi: 10.1603/0046-225X-32.4.711 [Google Scholar]
- 72.Stefanescu C, Peñuelas J, Filella I. Effects of climatic change on the phenology of butterflies in the northwest Mediterranean Basin. Glob Chang Biol. Blackwell Science Ltd; 2003;9: 1494–1506. doi: 10.1046/j.1365-2486.2003.00682.x [Google Scholar]
- 73.Forrest J, Inouye DW, Thomson JD. Flowering phenology in subalpine meadows: does climate variation influence community co-flowering patterns? Ecology. 2010;91: 431–40. doi: 10.1890/09-0099.1 [DOI] [PubMed] [Google Scholar]
- 74.Sgolastra F, Bosch J, Molowny-Horas R, Maini S, Kemp WP. Effect of temperature regime on diapause intensity in an adult-wintering Hymenopteran with obligate diapause. J Insect Physiol. 2010;56: 185–194. doi: 10.1016/j.jinsphys.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 75.Scaven VL, Rafferty NE. Physiological effects of climate warming on flowering plants and insect pollinators and potential consequences for their interactions. Curr Zool. 2013;59: 418–426. doi: 10.1093/czoolo/59.3.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Species ordered by timing of flowering peak.
(PDF)
Ellipses correspond to standard deviations of the sampling events of each grouping factor (years or months). 1: March; 2: April; 3: May; 4: June. Points on figure (A) represents transect–year values. Points on figure (B) represents each transect–month–year combination.
(PDF)
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.