Abstract
The PI3K/AKT signaling pathway plays a critical role in the maintenance of equilibrium between cell survival and apoptosis. The Pik3ca gene is mutated in a range of human cancers. It has been found to be oncogenic, and mutations lead to constitutive activation of the PI3K/AKT pathway. The expression patterns of PIK3CA proteins in the uterus of mice during early pregnancy indicate that it may play a role in the regulation of glandular epithelial cells, which is required to support uterine receptivity. To further investigate the role of Pik3ca in uterine function, Pik3ca was conditionally ablated only in the PGR-positive cells (Pgrcre/+Pik3caf/f; Pik3cad/d). A defect of uterine gland development and decidualization led to subfertility observed in Pik3cad/d mice. Pik3cad/d mice showed significantly decreased uterine weight compared to Pik3caf/f mice. Interestingly, a significant decrease of gland numbers were detected in Pik3cad/d mice compared to control mice. In addition, we found a decrease of Foxa2 expression, which is a known uterine gland marker in Pik3cad/d mice. Furthermore, the excessive proliferation of endometrial epithelial cells was observed in Pik3cad/d mice. Our studies suggest that Pik3ca has a critical role in uterine gland development and female fertility.
Introduction
The endometrial glands are critical for embryo-uterus communication, and their secretions are essential for blastocyst survival, development, and implantation [1–3]. Adenogenesis, the uterine gland formation, is an important developmental process, which determines the receptivity of the adult uterus [3–5]. The organogenesis of mammals is usually established in utero. However, the mouse uterus develops from the Mullerian ducts during the fetal period, and then uterine specific histology is completed in the postnatal period. In mice, adenogenesis occurs after birth. The glands bud from the luminal epithelium. After the budding of the gland, it proceeds to grow into stromal tissue. The folding of luminal epithelium (LE) appears between birth and postnatal day 6 (PD 6), followed by the formation of glandular epithelium (GE) buds [6, 7]. Simultaneously, three layers of mesenchyme are segregated into radially oriented endometrial stroma and inner circular myometrium [8]. By PD 10 in mice, the uterine glands extend from the LE into surrounding endometrial stroma. The histology of the mouse uterus is organized similarly to the adult uterus by PD 15 [9, 10]. Abnormalities in uterine gland development and function result in infertility and miscarriages in human and animal models [11, 12]. The regulation of cell proliferation is involved in postnatal uterus development, as well as the regeneration of cycling endometrium [13]. Uterine luminal and glandular epithelium at the site of implantation should be regenerated after the parturition.
The PI3K/AKT signaling pathway is important for the maintenance of balance between cell survival and apoptosis [14, 15]. Phosphoinositide kinases (PIKs) are lipid kinases, which are categorized into three families: PI3Ks, PIP4Ks and PIP5Ks, according to the phosphorylated site on their carbohydrate. Among those, PI3Ks are further subcategorized into class I, II or III depending on their subunit structure. PI3K is a heterodimeric enzyme consisting of a catalytic subunit (p110) and a regulatory subunit (p85). The Pik3ca gene, located on chromosome 3q26.32, codes for the p110a catalytic subunit of PI3K. PI3Kα, which belongs to the class 1A of PI3Ks, has a catalytic domain encoded by Pik3ca. Mutations in Pik3ca may contribute to the alteration of the PI3K/AKT signaling pathway in several tumors [16]. The mutations are predominantly located in the helical (exon 9) and kinase (exon 20) domains. Several PI3K isoforms have been involved in human disease [16]. Pik3ca mutations are frequently detected in endometrial carcinomas [17]. Activation of PI3Kα-regulated signaling led to an increase in dependent or independent AKT pathways in endometrioid endometrial carcinomas [18]. Pik3ca mutations were found in 24–36% of endometrial cancer patients, which are associated with poor prognoses such as vascular invasion [19, 20].
Although the effect of Pik3ca has been elucidated in tumorigenesis, the role of Pik3ca in pregnancy is unknown. In order to evaluate the role of Pik3ca in the uterus, we have generated a mouse with Pik3ca gene expression that is ablated specifically within the PGR-expressing cells (Pgrcre/+ Pik3caflox/flox). We demonstrated the expression of Pik3ca mRNA during early pregnancy and the effect of Pik3ca conditional ablation on implantation and decidualization.
Materials and methods
Animals and tissue collection
All mouse experiments were carried out according to the protocol (MSU 11/16-192-00) approved by the Institutional Animal Care and Use Committee of Michigan State University. The surgical procedures have the potential for pain and/or distress post-surgery. Signs of major discomfort include difficulty rising, loss of appetite, and difficulty moving back and forth. To reduce the chances of pain and/or distress in the animals, Ketoprofen was administrated prior to surgery. Post-surgery animals were monitored for several days. Carbon dioxide (CO2) is used for euthanasia.
To assess female reproductive ability, 8-week-old Pik3cad/d mice (n = 6) and control female mice (n = 5) were individually mated to wild-type C57BL/3 mice with proven fertility. The numbers of litters and pups were tracked from each female mouse. Copulation was confirmed by vaginal plugs the following morning. The day that a vaginal plug was observed was considered day 0.5 of gestation (GD 0.5). The pregnancy tissues were obtained through the mating of wild-type C57BL/3 mice and Pik3cad/d, then uterine tissues were flash frozen at the time of dissection or fixed with 4% paraformaldehyde (vol/vol) and paraffin embedded.
For artificial decidualization, control and Pik3cad/d female mice (n = 3 per genotype) were ovariectomized under anesthesia at 6 weeks of age. After the duration of at least 2 weeks to eliminate the endogenous ovarian hormone completely, the mice were treated daily with subcutaneous injections (s.c.) of estrogen (E2) (100ng) for 3 days. The mice were treated daily with 1mg of progesterone (P4) s.c. and 6.7 ng of E2 s.c. for 3 days after undergoing 2 days of rest. The left horn of each mouse was mechanically scratched the full length of the anti-mesometrial lumen 6 hours after the third P4 and E2 injection, while the right horn was left unstimulated as an adjusted control. A 25 gauge sterile needle was inserted into the uterine lumen through the uterine wall near the tubal utero junction. The end of the needle was brushed against the antimesometrial uterine wall to traumatize the uterus of one uterine horn. Ketoprofen was administrated before the skin incision is made. Mice were anaesthetized with isoflurane gas by inhalation. To induce the maximal decidual reaction, the mice continued daily treatment with s.c. injections of 1 mg P4 and 6.7ng E2 for 5 days after the mechanical stimulation. The wet weights of the stimulated and control uterine horns of each mouse were measured. Uterine tissues were then collected from both horns and fixed in 4% paraformaldehyde (vol/vol) and paraffin embedded. To investigate the function of Pik3ca on uterine gland development, uteri of Pik3cad/d mice were collected for histologic evaluation at 20 days and 8 weeks of age.
Immunohistochemistry
Uterine sections were incubated with 10% normal goat serum in PBS for 2 hours. Sections were exposed to primary antibody anti-PIK3CA (CS-4252, Cell Signaling), anti-FOXA2 (WRAB-FOXA2, Seven Hills Bioreagents), anti-Ki67 (BD550609, BD Bioscience) in blocking solution overnight at 4°C. After secondary antibody (Vector Laboratories, Burlingame, CA) incubation, sections were treated with the horseradish peroxidase-conjugated streptavidin substrate. We detected positive signaling with the Vectastain Elite DAB kit (Vector Laboratories). Proliferation and the number of uterine glands was quantified by counting the number of glands and proliferation-positive cells in the uterine sections.
RNA isolation and quantitative real-time PCR
Total RNA was isolated from the uteri using a Qiagen RNeasy total RNA isolation kit (Qiagen, Valencia, CA, USA). The expression of Pik3ca, Foxa2, and Spink3 was quantified by real-time PCR TaqMan (Foxa2) and SYBR green (Pik3ca and Spink3) analysis using an Applied Biosystems StepOnePlus system. It was based upon the manufacturer’s instructions (Applied Biosystem, Foster City, CA, USA). Template cDNA was generated from 1 μg of total RNA using random hexamers and MMLV Reverse Transcriptase (Invitrogen Corp.). Standard curves were produced by serial dilution of a prepared sample of total RNA isolated from the mouse uteri. The results of real-time RT-PCR were normalized against pre-validated probes, primers, and 18S RNA using an ABI Universal Master mix reagent.
Western blot analysis
Isolation of uterine tissue and western blot were perfomed as described previously [21]. Briefly, total 20μg protein was loaded onto gels and transferred to membrane. The membrane was incubated with 0.5% (w/v) casein (Sigma Aldrich) for 3 hours and then treated with anti-FOXA2 (WRAB-FOXA2, Seven Hills Bioreagents). Total Actin (SC1616, Santa Cruz Biotechnology, Inc.) levels were used for loading controls.
Statistical analysis
For data that contained more than two groups, a parametric Tukey-Kramer one-way ANOVA was used to test the null hypothesis of group differences, followed by a Wilcoxon test. For data from two groups, an unpaired t-test was used. All data is presented as mean ± SEM. P < 0.05 was considered statistically significant. All statistical analysis was performed by Instat package from GraphPad (San Diego, CA, USA).
Results
The expression of Pik3ca during early pregnancy
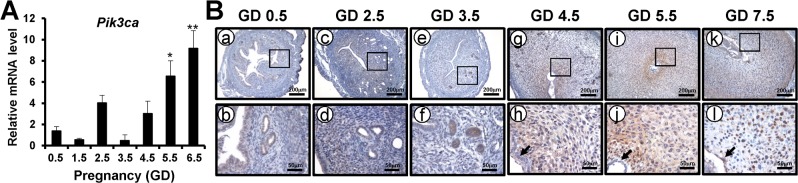
The expression of Pik3ca was examined in the uterus from gestational day (GD) 0.5 to GD 6.5 by real-time RT-PCR (Fig 1A). There was a peak in Pik3ca expression at GD 2.5 and then it sharply decreases at GD 3.5. The expression of Pik3ca gradually increased from GD 3.5 until GD 6.5. The expression of Pik3ca was significantly increased at the time of implantation and continues to increase as pregnancy progresses indicating an important role for Pik3ca in pregnancy. Next, we examined the spatial expression profile of PIK3CA protein during early pregnancy using immunohistochemistry (Fig 1B). The expression of PIK3CA proteins were weakly detected at the luminal epithelium, glandular epithelium, and stromal cells but not myometrium at GD 0.5 and GD 2.5. Interestingly, PIK3CA proteins were strongly expressed at the glandular epithelium of GD 3.5. After embryo implantation, the expression of PIK3CA was remarkably strong in decidual cells.
Fig 1. The expression of Pik3ca mRNA and protein from GD 0.5 to GD 7.5.
(A) The mRNA expression of Pik3ca was investigated in the uteri of C57BL/6 mice during early pregnancy. Pik3ca expression was significantly increased at GD 5.5 and GD 6.5. (A) The localization pattern of PIK3CA proteins was investigated through immunohistochemistry in uteri of C57BL/6 mice on GD 0.5 (a and b), GD 2.5 (c and d), GD 3.5 (e and f), GD 4.5 (g and h) GD 5.5 (i and j) and GD 7.5 (k and l). An arrow indicates an embryo. The results represent the mean ± SEM. **, p < 0.01; *, p < 0.05.
Ablation of Pik3ca in mouse uterus
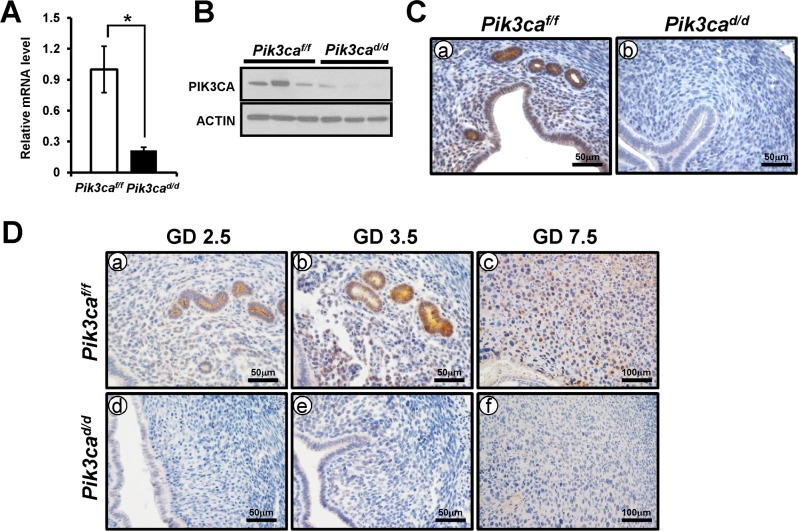
To investigate the function of Pik3ca in the uterus, we generated a mouse model with the uterine Pik3ca ablation in the PGR-expressing cells (Pgrcre/+ Pik3caf/f; Pik3cad/d) [22, 23]. Ablation of Pik3ca was confirmed in the uteri from 8 weeks old mutant Pik3cad/d mice by real time PCR, Western blot, and immunohistochemical analysis (Fig 2). Pik3ca mRNAs were significantly decreased in uteri of Pik3cad/d mice compared to Pik3caf/f mice. To assess the efficiency of ablation, the levels of PIK3CA proteins were examined during early pregnancy. Expression of PIK3CA proteins was not observed in the uterus of Pik3cad/d mice at GD 2.5, GD 3.5, and GD 7.5 (Fig 2D). These results confirm our successful ablation of Pik3ca in the uterus of Pik3ca d/d mice.
Fig 2. The generation of mouse with uterine specific Pik3ca ablation.
(A) The expression of Pik3ca mRNA was evaluated in uteri by real time PCR. The data represents the mean ± SEM. *, p < 0.05. PIK3CA protein expression was assessed by Western blot (B) and immunohistochemistry (C). Total RNA and protein were prepared from whole uteri. (D) The efficiency of PIK3CA loss was evaluated in uterus of Pik3cad/d mice at GD 2.5 (a and d), GD 3.5 (b and e), and GD 7.5 (c and f).
Pik3cad/d mice are subfertile due to impaired implantation
Female fertility was assessed by mating Pik3cad/d (n = 6) and control females (n = 5) with wild type males continuously for 6 months and follow up of the quantification of litters and pups from each female mouse. Pik3cad/d mice were subfertile with only 1.33 ± 0.49 litters/mouse compared with 4.20 ± 0.58 litters/mouse from control mice (Table 1). Control mice had an average 7.43± 0.43 pups/litter, whereas Pik3cad/d mice had 3.75±0.80 pups/litter. Pik3cad/d females produced significantly fewer pups per mouse (p<0.001), fewer litters per mouse (p<0.01), and fewer pups per litter (p<0.01) than the control females.
Table 1. Pik3cad/d mice were subfertile.
| Genotype | Number of Mice Tested |
Number of Litters |
Number of Pups |
Average of Pups/Litter |
Average Number of Litter/mouse |
|---|---|---|---|---|---|
| Pik3caf/f | 5 | 21 | 156 | 7.43 ± 0.43 | 4.20 ± 0.58 |
| Pik3cad/d | 6 | 8 | 30 | 3.75 ± 0.80 | 1.33 ± 0.49 |
To test for a possible ovarian defect, blastocysts generated by natural mating were collected at GD 3.5 by uterine flushing with M2 medium, and counted. The number of blastocysts from uteri of Pik3caf/f and Pik3cad/d mice was not different. Also, histological analysis of the Pik3cad/d ovary did not show any alterations in ovarian morphology (S1 Fig). These results show that ovarian morphology and functioning were not affected in the Pik3cad/d mice, suggesting that the fertility defect is primarily due to a uterine defect.
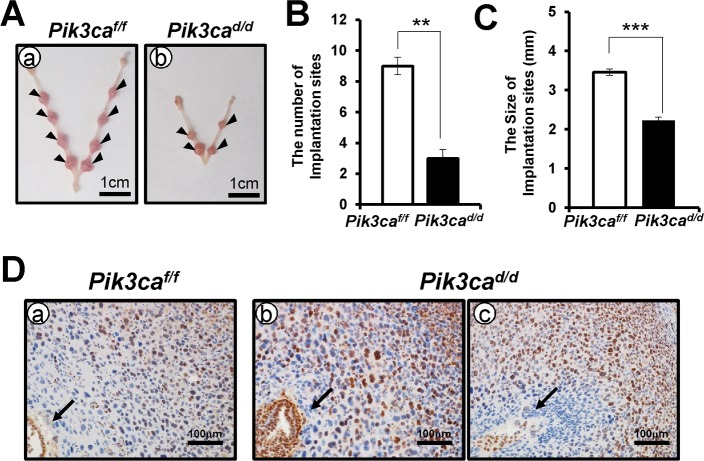
To investigate whether Pik3ca plays an essential role in implantation, female control and Pik3cad/d mice were mated with wild-type C57BL/3 mice and then the mice were examined at the morning of GD 7.5 to evaluate embryo implantation. A significant decrease in the number of implantation sites was observed in the Pik3cad/d mice (3.0 ± 0.6) compared with the control (9.0 ± 0.6) (Fig 3A and 3B). These results revealed that defect of implantation is one of the causes of the subfertility observed in Pik3cad/d mice
Fig 3. Pik3cad/d mice have impaired implantation.
(A) Gross anatomy of implantation sites in both the control (a) and Pik3cad/d uterus (b) at GD 7.5. (B) The number of implantation sites were recorded in the uteri. (C) The size of implantation sites were measured. (D) The proliferative cells were assessed in control and Pik3cad/d uterus at GD 7.5 by immunohistochemical staining using anti-Ki67 antibody. Arrow indicates the blastocyst. The data represents the mean ± SEM. ***, p < 0.001; **, p < 0.01.
In addition, the size of implantation sites was significantly decreased in Pik3cad/d mice (control vs mutant; 3.4 ± 0.2 vs 2.2 ± 0.1). (Fig 3C). However, the proliferation of stromal and decidual cells was not different in Pik3cad/d mice (57.11 ± 1.40%) compared with control mice (56.72 ± 2.51%) at GD 7.5. The average of implantation sites (3.50 ± 0.50) and the average of pups per litter in Pik3cad/d mice (3.75 ± 0.80) were not different. These results suggest that Pik3cad/d mice do not have any defect after implantation.
Aberrant proliferation of epithelial cells in Pik3cad/d mice at the pre-implantation stage
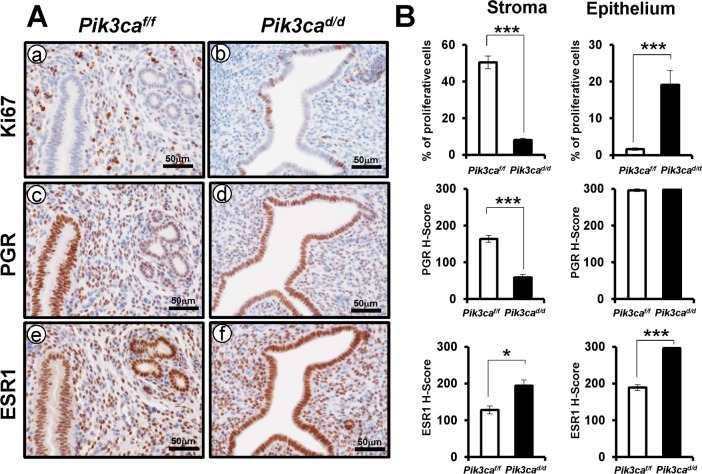
In the rodent uterus, E2 induces the proliferation of luminal and glandular epithelial cells at GD 1.5–2.5 [24]. Such cell proliferation is redirected from the epithelial to the stromal cell by P4 stimuli after GD 3.5 [25]. Stromal cells proliferate and differentiate to specialized maternal tissue referred to as the decidua that should be necessary to maintain pregnancy. To determine whether the proliferation of endometrial cells is dysregulated in the pre-implantation period by ablation of Pik3ca, we examined the change in the spatial distribution of proliferative marker Ki-67 in the uteri of Pik3cad/d mice at GD 3.5 using immunohistochemical staining (Fig 4A). The proliferation is significantly increased in the endometrial epithelial cells of Pik3cad/d mice compared with control mice. Furthermore, proliferation of endometrial stromal cells showed a significant decrease in Pik3cad/d mice compared to control (Fig 4B). This suggests that Pik3ca has an essential role in regulating proliferation of endometrial epithelial and stromal cells for successful implantation.
Fig 4. Proliferation of endometrial cells was dysregulated in the uteri of Pik3cad/d mice at the pre-implantation stage.
(A) Immunohistochemical analysis of Ki67 (a and b), PGR (c and d), and ESR1 (e and f) proteins at GD 3.5 in endometrium of control (a, c, and e) and Pik3cad/d (b, d, and f) mice. (B) Quantification of Ki67 positive cells and semi-quantitative analysis of PRG and ESR1 in uterine epithelial and stromal cells of control and Pik3cad/d mice. The results represent the mean ± SEM. ***, p < 0.001; *, p < 0.05.
Somatic mutations of the PIK3CA gene have been shown to activate AKT and induce oncogenic transformation in vitro and in vivo [26–28]. Therefore, we examined the levels of pAKT and total AKT in Pik3caf/f and Pik3cad/d mice using immunohistochemistry. However, the levels of pAKT and total AKT were not changed in Pik3cad/d mice compared to Pik3caf/f mice at GD 3.5 (S2 Fig).
Since an increase of epithelial proliferation is observed in Pik3cad/d mice, we further investigated whether Pik3ca ablation altered the expression of PGR and ESR1. Interestingly, stromal PGR expression was significantly reduced in Pik3cad/d mice compared to Pik3caf/f mice. We also observed an increase of ESR1 protein expression in the stroma and epithelium of the Pik3cad/d mice compared to the Pik3caf/f mice. These results suggest that Pik3ca loss causes dysregulation of PGR and ESR1 expression in the uterus.
Pik3cad/d mice exhibited a defect of decidualization
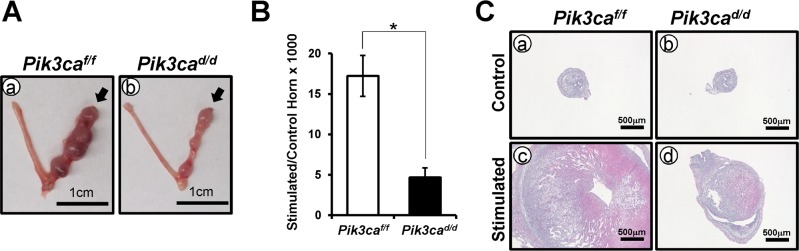
Implantation is an important step in the establishment of a pregnancy, where the blastocyst attaches to the endometrial epithelium and invades to the differentiated endometrial stromal cells; the decidual cell. To investigate whether the ablation of Pik3ca affects decidualization, control and Pik3cad/d mice underwent experimentally-induced decidualization after hormone treatment. Ovariectomized Pik3cad/d (n = 3) and control (n = 3) mice were treated with E2 and P4, and the uteri were stimulated by scratch to mimic embryo attachment and to induce decidualization [21]. The gross anatomy and histology of the Pik3cad/d uteri 5 days after having artificially induced decidualization revealed that the ratio of stimulated to unstimulated horn weight in mutant uteri was significantly decreased compared to control mice (Fig 5). The results demonstrate that Pik3cad/d mice exhibit a defect of decidualization.
Fig 5. Pik3cad/d mice exhibited a defect of decidualization.
(A) Gross anatomy of artificially induced decidualized uteri of control (a) and Pik3cad/d (b) mice. Arrow head indicates a stimulated horn. (B) There was a significant decrease in stimulated and unstimulated (control) horn weight ratio in Pik3cad/d mice as compared with control. The results represent the mean ± SEM. *, p < 0.05. (C) Histology of control (a and b) and stimulated (c and d) horn in control (a and c) and Pik3cad/d mice (b and d) at day 5.
Pik3cad/d mice exhibit a defect of uterine gland formation
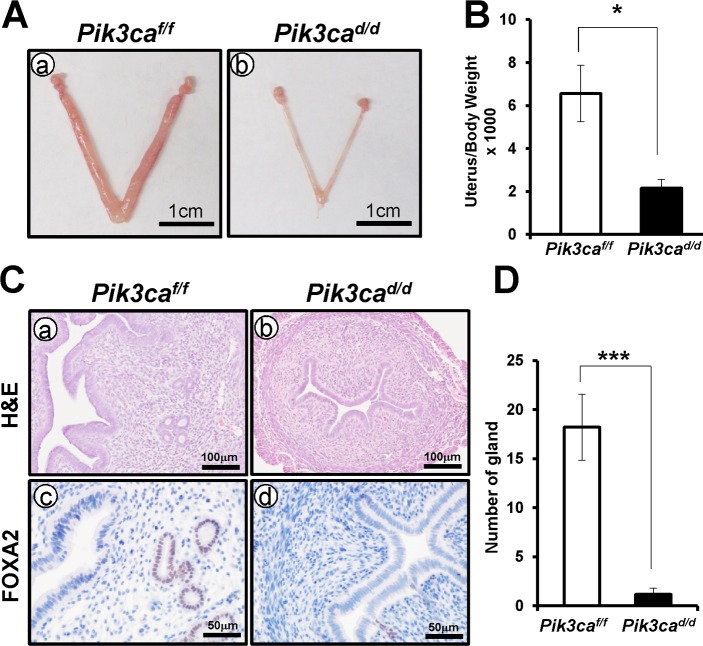
To investigate the cause of subfertility in Pik3cad/d mice, we next examined the uterine development in Pik3cad/d mice. Gross examination revealed that there was a significant decrease in the uterine size and weight in Pik3cad/d mice when compared to the control at 8 weeks of age (Fig 6A and 6B). Interestingly, we found a defect of uterine gland development in Pik3cad/d mice by histological analysis (Fig 6C). To validate uterine gland formation, we examined the expression of FOXA2, a uterine gland marker [29], by immunohistochemical analysis (Fig 6C). There was a significant reduction in the number of uterine glands in Pik3cad/d mice compared to control mice (Fig 6D).
Fig 6. The defect of gland formation in Pik3cad/d mice.
(A) Gross anatomy of 8-week-old control (a) and Pik3cad/d (b) mice. (B) There was a significant decrease in the ratio of uterine weight in Pik3cad/d mice. (C) Histology of the uterus of control (a) and Pik3cad/d (b) mice. The expression of FOXA2 in the uterus of control (c) and Pik3cad/d (d) mouse. (D) Quantification of FOXA2 positive endometrial glands in mouse uteri. The results represent the mean ± SEM. *, p < 0.05; ***, p < 0.001.
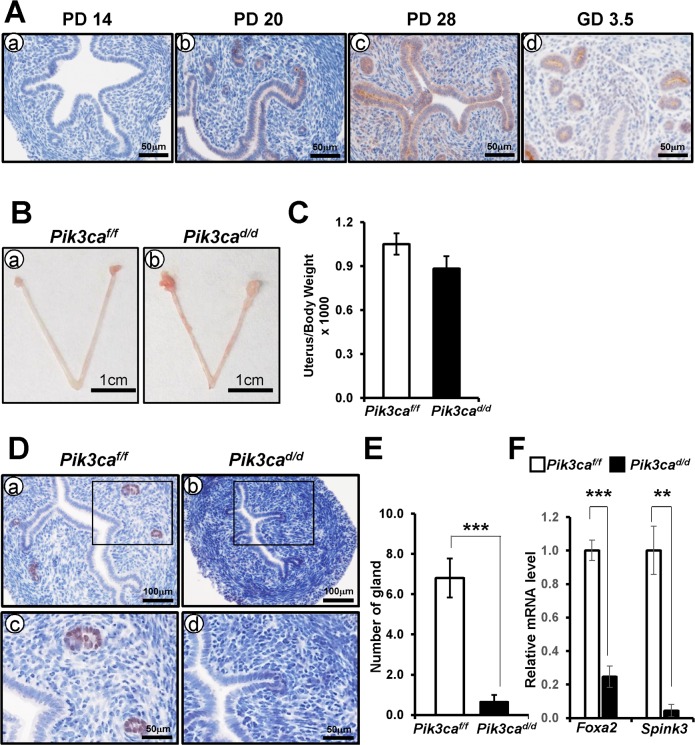
To better understand the role of PIK3CA in uterine gland, we examined the expression of PIK3CA during uterine gland development. As illustrated in Fig 7A, immunohistochemistry analysis revealed that PIK3CA positive cells were not observed on PD 14, but were observed by PD 21, only in some luminal and glandular epithelial cells. The expression of PIK3CA was increased and extended to all luminal and glandular epithelial cells by PD 28. To investigate the defect of glandular development in Pik3cad/d mice, we examined the uterine histology of peripubertal mice (PD 20). Murine uterine glands extend from the luminal epithelium into surrounding endometrial stroma by PD 10, and the histoarchitecture of the mouse uterus resembles that of the adult by PD15 [9, 10]. Therefore, PD 20 mice could have just completed uterine morphology. There was no difference in the size and weight of the uteri between the mice at PD 20 (Fig 7B and 7C). However, histological analysis and immunohistochemical analysis of FOXA2 showed that the number of endometrial glands was significantly decreased in Pik3cad/d mice compared to control mice at PD 20 (Fig 7D and 7E). Furthermore, glandular epithelium expressed genes Foxa2 and Spink3 were significantly decreased in uteri of Pik3cad/d mice at PD 20 (Fig 7F). These results suggest that Pik3ca plays an important role in uterine glandular development.
Fig 7. A defect of uterine gland development in Pik3cad/d mice at 20 days of age.
(A) The expression of PIK3CA was examined during uterine development. Immunohistochemical staining of PIK3CA in the uterus of PD 14 (a), PD 20 (b), and PD 28 (c). The uterus of GD 3.5 was used a positive control (d). (B) Gross anatomy of control (a) and Pik3cad/d (b) mice at PD 20. (C) The weight of uteri was not different between control and Pik3cad/d mice. (D) Immunohistochemical staining of FOXA2 in control (a and c) and Pik3cad/d (b and d) mice. (E) Quantification of FOXA2 positive endometrial glands in uteri of control and Pik3cad/d mice at PD 20. (F) The expression of Foxa2 and Spink3 in the uteri of control and Pik3cad/d mice. The results represent the mean ± SEM. ***, p < 0.001; **, p < 0.01.
Discussion
This study has revealed that Pik3ca has an important role in early pregnancy and uterine gland development. The expression of Pik3ca is significantly increased at the time of implantation and continues to increase as pregnancy progresses indicating a critical role for Pik3ca in pregnancy. PIK3CA protein had been strongly detected during decidualization. Pik3cad/d mice were subfertile due to impaired implantation and decidualization. Pik3cad/d mice showed a decrease of implantation sites and dysregulated proliferation at the time of pre-implantation, as well as impaired decidualization in Pik3cad/d mice.
Mutations of Pik3ca are frequently detected in endometrial cancer. When PI3Kα-mediated signaling is inappropriately activated, AKT-dependent or AKT-independent signaling is increased. The PI3K/AKT signaling pathway is a major regulator in maintaining the equilibrium between cell survival and apoptosis. PI3K, along with downstream effectors such as AKT1 and mammalian target of rapamycin (mTOR), have been regarded as a possible target for cancer therapy [16]. However, PI3K-AKT-mTOR pathway signaling works in many normal tissues as well as malignant cells. PI3K is common to the signaling pathways of a number of peripheral metabolic mediators, including insulin and leptin, which regulate both metabolism and reproduction [30]. Male mice of Pik3ca knockout in adipose tissue displayed delayed onset of puberty accompanied by a reduction in plasma LH levels and testicular weight [31]. We could not observed dysregulation of AKT and pAKT in Pik3cad/d mice. However, the lack of altered expression of total AKT and phospho-AKT at the preimplantation of Pik3cad/d mice (S2 Fig) does not rule out the possibility that Pik3ca regulates adenogenesis through PI3K/AKT signaling during the neonatal period, when glands are initially formed. Therefore, Future studies are needed to determine the potential role of complex interactions between Pik3ca and PI3K/AKT signaling to either initiate or maintain glandular identity.
The uterine glands are known to be derived from and remain connected to the luminal epithelium [12]. Prolactin and estradiol-17 beta may be involved in endometrial adenogenesis of neonatal ungulates [9, 12]. This could play a role in the regulation of human endometrial gland regeneration after menstruation. On the other hand, P4 exposure suppresses endometrial adenogenesis in the neonatal uterus by modulating expression of gland development related genes and cell proliferation [32]. Perinatal uterine development is a critical period for future reproductive health, and exposure to endocrine disruptors can have long term adverse consequences [33]. Mouse models in which Wnt5a, Wnt7a, and β-catenin (Ctnnb1) were ablated all displayed a lack of uterine glands [34–37]. PIK3CA mutations were associated with over-expression of several genes involved in the Wnt signaling pathway [38]. The reciprocal interaction between Pik3ca and Wnt signaling has been noted also in that Ctnnb1, a downstream effector of the Wnt pathway, can be promote by oncogenic PIK3CA [39]. Future studies are needed to determine the potential role of complex interactions between Wnt/β-Catenin and PIK3CA signaling to either initiate or maintain glandular identity.
We found that peripubertal uteri of Pik3cad/d mice showed decreased Foxa2 and Spink3 mRNA expression. Foxa2 is known as a critical gene in the formation of glands in the uterus. Foxa1 and Foxa2 are necessary for normal development of endoderm-derived organs [40–43]. In reproductive organs, the ovary does not express Foxa1 and Foxa2, and only glandular epithelium of the uterus expresses Foxa2 [44]. Foxa2 induces epithelial specific gland bud formation during postnatal uterine development. The uteri of adult FOXA2-deleted mice were morphologically normal and contained glands, whereas the uteri of neonatal FOXA2-deleted mice were completely aglandular [45, 46]. Foxa2 transcriptional factors are able to bind chromatin and induce chromatin decondensation, which facilitates the binding of other transcription factors, such as glucocorticoid, androgen, and estrogen receptor 1 [47].
Serine peptidase inhibitor, Kazal type 3 (Spink3) is a trypsin inhibitor, and also a growth factor that has a similar structure to epidermal growth factor (EGF), which could bind with epidermal growth factor receptor (EGFR) to promote cell proliferation [48]. Spink3 was likely to promote hepatocytes proliferation in liver regeneration through P38, PKC, JAK-STAT and AKT [49]. Spink3, which is derived from the uterine glands, is involved in endometrial decidualization and implantation [50]. In this study, decreased expression of Foxa2 and Spink3 mRNA in peripubertal period of Pik3cad/d mice might be involved in postnatal uterine gland formation.
Ovarian hormones stimulate uterine cell proliferation by various mechanisms, such as induction of growth factors, and paracrine signaling, and by direct regulation of cell cycle genes [51]. Tissue-specific knockout of the Pgr in the epithelial cells of mouse uteri resulted in the continued proliferation of epithelial cells in the absence of P4 action [52]. In addition, the proliferative switch from epithelial cell to stroma also occurred normally at GD 3.5 in mice. Stromal cell proliferation and differentiation are prerequisites to a receptive uterus for embryo implantation. Our results showed that the proliferation of stromal cells were significantly decreased in Pik3cad/d mice at GD 3.5 compared with control mice. This indicates that Pik3ca might have an important bearing on the regulation of stromal cell proliferation. In addition, Pik3ca mutant mice had the uterine phenotype of decreased endometrial glands. Our study supports that the disruption of uterine development during critical organizational periods can alter the functional capacity and embryotrophic potential of the adult uterus.
In summary, we have generated a mouse model with conditional ablation of Pik3ca in the uterus. Uterine-specific knockout of Pik3ca demonstrates a defect in uterine gland formation. The mutant mice were subfertile with impaired implantation and decidualization. This study supports that Pik3ca is an important gene for early pregnancy, and improves the understanding of possible effects of anticancer therapy targeting Pik3ca signaling on female reproduction.
Supporting information
Ovarian histology of Pik3caf/f (a) and Pik3cad/d (b) mice.
(PDF)
Immunohistochemical staining of total phospho-AKT (a and b) and AKT (c and d) in Pik3caf/f (a and c) and Pik3cad/d (b and d) mice.
(PDF)
Acknowledgments
We would like to thank Paige Wells for manuscript preparation.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by NICHD R01HD084478 (to J.W.J) and the National Research Foundation of Korea grant (NRF-2016R1A2B4007539) and the BK21 plus program of the Ministry of Education, Korea (to U.H.H).
References
- 1.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359(6390):76–9. Epub 1992/09/03. doi: 10.1038/359076a0 . [DOI] [PubMed] [Google Scholar]
- 2.Cooke PS, Spencer TE, Bartol FF, Hayashi K. Uterine glands: development, function and experimental model systems. Molecular human reproduction. 2013;19(9):547–58. doi: 10.1093/molehr/gat031 ; PubMed Central PMCID: PMC3749806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filant J, Spencer TE. Uterine glands: biological roles in conceptus implantation, uterine receptivity and decidualization. The International journal of developmental biology. 2014;58(2–4):107–16. Epub 2014/07/16. doi: 10.1387/ijdb.130344ts ; PubMed Central PMCID: PMCPMC4199233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spencer TE. Biological roles of uterine glands in pregnancy. Semin Reprod Med. 2014;32(5):346–57. Epub 2014/06/25. doi: 10.1055/s-0034-1376354 ; PubMed Central PMCID: PMCPMC4198167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelleher AM, Burns GW, Behura S, Wu G, Spencer TE. Uterine glands impact uterine receptivity, luminal fluid homeostasis and blastocyst implantation. Scientific reports. 2016;6:38078 Epub 2016/12/03. doi: 10.1038/srep38078 ; PubMed Central PMCID: PMCPMC5131473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Mullerian) epithelial differentiation. Developmental biology. 2001;240(1):194–211. doi: 10.1006/dbio.2001.0458 . [DOI] [PubMed] [Google Scholar]
- 7.Branham WS, Sheehan DM, Zehr DR, Ridlon E, Nelson CJ. The postnatal ontogeny of rat uterine glands and age-related effects of 17 beta-estradiol. Endocrinology. 1985;117(5):2229–37. doi: 10.1210/endo-117-5-2229 . [DOI] [PubMed] [Google Scholar]
- 8.Brody JR, Cunha GR. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: I. Normal development. The American journal of anatomy. 1989;186(1):1–20. doi: 10.1002/aja.1001860102 . [DOI] [PubMed] [Google Scholar]
- 9.Hu J, Gray CA, Spencer TE. Gene expression profiling of neonatal mouse uterine development. Biology of reproduction. 2004;70(6):1870–6. doi: 10.1095/biolreprod.103.026336 . [DOI] [PubMed] [Google Scholar]
- 10.Spencer TE, Dunlap KA, Filant J. Comparative developmental biology of the uterus: insights into mechanisms and developmental disruption. Molecular and cellular endocrinology. 2012;354(1–2):34–53. doi: 10.1016/j.mce.2011.09.035 . [DOI] [PubMed] [Google Scholar]
- 11.Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. The Journal of clinical endocrinology and metabolism. 2002;87(6):2954–9. doi: 10.1210/jcem.87.6.8563 . [DOI] [PubMed] [Google Scholar]
- 12.Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, et al. Developmental biology of uterine glands. Biology of reproduction. 2001;65(5):1311–23. . [DOI] [PubMed] [Google Scholar]
- 13.Cunha GR. Epithelial-stromal interactions in development of the urogenital tract. International review of cytology. 1976;47:137–94. . [DOI] [PubMed] [Google Scholar]
- 14.Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer research. 2004;64(21):7678–81. doi: 10.1158/0008-5472.CAN-04-2933 . [DOI] [PubMed] [Google Scholar]
- 15.Samuels Y, Diaz LA, Jr., Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer cell. 2005;7(6):561–73. doi: 10.1016/j.ccr.2005.05.014 . [DOI] [PubMed] [Google Scholar]
- 16.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554 doi: 10.1126/science.1096502 . [DOI] [PubMed] [Google Scholar]
- 17.Growdon WB, Roussel BN, Scialabba VL, Foster R, Dias-Santagata D, Iafrate AJ, et al. Tissue-specific signatures of activating PIK3CA and RAS mutations in carcinosarcomas of gynecologic origin. Gynecologic oncology. 2011;121(1):212–7. doi: 10.1016/j.ygyno.2010.11.039 . [DOI] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113 ; PubMed Central PMCID: PMC3704730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer research. 2005;65(23):10669–73. doi: 10.1158/0008-5472.CAN-05-2620 . [DOI] [PubMed] [Google Scholar]
- 20.Catasus L, Gallardo A, Cuatrecasas M, Prat J. PIK3CA mutations in the kinase domain (exon 20) of uterine endometrial adenocarcinomas are associated with adverse prognostic parameters. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21(2):131–9. doi: 10.1038/modpathol.3800992 . [DOI] [PubMed] [Google Scholar]
- 21.Kim TH, Yoo JY, Wang Z, Lydon JP, Khatri S, Hawkins SM, et al. ARID1A Is Essential for Endometrial Function during Early Pregnancy. PLoS genetics. 2015;11(9):e1005537 doi: 10.1371/journal.pgen.1005537 ; PubMed Central PMCID: PMC4574948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58–66. Epub 2005/02/01. doi: 10.1002/gene.20098 . [DOI] [PubMed] [Google Scholar]
- 23.Zhao JJ, Cheng H, Jia S, Wang L, Gjoerup OV, Mikami A, et al. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc Natl Acad Sci U S A. 2006;103(44):16296–300. Epub 2006/10/25. doi: 10.1073/pnas.0607899103 ; PubMed Central PMCID: PMCPMC1637576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finn CA, Martin L. Patterns of cell division in the mouse uterus during early pregnancy. The Journal of endocrinology. 1967;39(4):593–7. . [DOI] [PubMed] [Google Scholar]
- 25.Rider V, Psychoyos A. Inhibition of progesterone receptor function results in loss of basic fibroblast growth factor expression and stromal cell proliferation during uterine remodelling in the pregnant rat. The Journal of endocrinology. 1994;140(2):239–49. . [DOI] [PubMed] [Google Scholar]
- 26.Vogt PK, Hart JR, Gymnopoulos M, Jiang H, Kang S, Bader AG, et al. Phosphatidylinositol 3-kinase: the oncoprotein. Curr Top Microbiol Immunol. 2010;347:79–104. Epub 2010/06/29. doi: 10.1007/82_2010_80 ; PubMed Central PMCID: PMCPMC2955792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bader AG, Kang S, Vogt PK. Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc Natl Acad Sci U S A. 2006;103(5):1475–9. Epub 2006/01/25. doi: 10.1073/pnas.0510857103 ; PubMed Central PMCID: PMCPMC1360603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isakoff SJ, Engelman JA, Irie HY, Luo J, Brachmann SM, Pearline RV, et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer research. 2005;65(23):10992–1000. Epub 2005/12/03. doi: 10.1158/0008-5472.CAN-05-2612 . [DOI] [PubMed] [Google Scholar]
- 29.Kim TH, Franco HL, Jung SY, Qin J, Broaddus RR, Lydon JP, et al. The synergistic effect of Mig-6 and Pten ablation on endometrial cancer development and progression. Oncogene. 2010;29(26):3770–80. doi: 10.1038/onc.2010.126 ; PubMed Central PMCID: PMC4013686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acosta-Martinez M. PI3K: An Attractive Candidate for the Central Integration of Metabolism and Reproduction. Frontiers in endocrinology. 2011;2:110 doi: 10.3389/fendo.2011.00110 ; PubMed Central PMCID: PMC3356143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson VL, Negron AL, Reid I, Thomas JA, Yang L, Lin RZ, et al. Loss of PI3K p110alpha in the Adipose Tissue Results in Infertility and Delayed Puberty Onset in Male Mice. BioMed research international. 2017;2017:3756089 doi: 10.1155/2017/3756089 ; PubMed Central PMCID: PMC5357525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filant J, Zhou H, Spencer TE. Progesterone inhibits uterine gland development in the neonatal mouse uterus. Biology of reproduction. 2012;86(5):146, 1–9. doi: 10.1095/biolreprod.111.097089 ; PubMed Central PMCID: PMC3364926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartol FF, Wiley AA, Floyd JG, Ott TL, Bazer FW, Gray CA, et al. Uterine differentiation as a foundation for subsequent fertility. Journal of reproduction and fertility Supplement. 1999;54:287–302. . [PubMed] [Google Scholar]
- 34.Carta L, Sassoon D. Wnt7a is a suppressor of cell death in the female reproductive tract and is required for postnatal and estrogen-mediated growth. Biol Reprod. 2004;71(2):444–54. Epub 2004/04/09. doi: 10.1095/biolreprod.103.026534 biolreprod.103.026534 [pii]. . [DOI] [PubMed] [Google Scholar]
- 35.Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, et al. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene. 2008. Epub 2008/09/23. onc2008363 [pii] doi: 10.1038/onc.2008.363 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development. 2004;131(9):2061–72. Epub 2004/04/10. doi: 10.1242/dev.01090 [pii]. . [DOI] [PubMed] [Google Scholar]
- 37.Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development (Cambridge, England). 1998;125(16):3201–11. Epub 1998/07/22. . [DOI] [PubMed] [Google Scholar]
- 38.Cizkova M, Cizeron-Clairac G, Vacher S, Susini A, Andrieu C, Lidereau R, et al. Gene expression profiling reveals new aspects of PIK3CA mutation in ERalpha-positive breast cancer: major implication of the Wnt signaling pathway. PLoS One. 2010;5(12):e15647 Epub 2011/01/07. doi: 10.1371/journal.pone.0015647 ; PubMed Central PMCID: PMCPMC3012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardone L, Bardelli A, Avvedimento VE. Activation of beta-catenin by oncogenic PIK3CA and EGFR promotes resistance to glucose deprivation by inducing a strong antioxidant response. PLoS One. 2012;7(5):e37526 Epub 2012/06/05. doi: 10.1371/journal.pone.0037526 ; PubMed Central PMCID: PMCPMC3360841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bastidas-Ponce A, Roscioni SS, Burtscher I, Bader E, Sterr M, Bakhti M, et al. Foxa2 and Pdx1 cooperatively regulate postnatal maturation of pancreatic beta-cells. Molecular metabolism. 2017;6(6):524–34. doi: 10.1016/j.molmet.2017.03.007 ; PubMed Central PMCID: PMC5444078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding Y, Chang C, Niu Z, Dai K, Geng X, Li D, et al. Overexpression of transcription factor Foxa2 and Hnf1alpha induced rat bone mesenchymal stem cells into hepatocytes. Cytotechnology. 2016;68(5):2037–47. doi: 10.1007/s10616-016-9944-7 ; PubMed Central PMCID: PMC5023577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan H, Xu Y, Ikegami M, Stahlman MT, Kaestner KH, Ang SL, et al. Foxa2 is required for transition to air breathing at birth. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(40):14449–54. doi: 10.1073/pnas.0404424101 ; PubMed Central PMCID: PMC521955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao N, Ishii K, Mirosevich J, Kuwajima S, Oppenheimer SR, Roberts RL, et al. Forkhead box A1 regulates prostate ductal morphogenesis and promotes epithelial cell maturation. Development. 2005;132(15):3431–43. doi: 10.1242/dev.01917 . [DOI] [PubMed] [Google Scholar]
- 44.Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene expression patterns: GEP. 2004;5(2):193–208. doi: 10.1016/j.modgep.2004.08.006 . [DOI] [PubMed] [Google Scholar]
- 45.Kelleher AM, Peng W, Pru JK, Pru CA, DeMayo FJ, Spencer TE. Forkhead box a2 (FOXA2) is essential for uterine function and fertility. Proc Natl Acad Sci U S A. 2017;114(6):E1018–E26. Epub 2017/01/05. doi: 10.1073/pnas.1618433114 ; PubMed Central PMCID: PMCPMC5307455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeong JW, Kwak I, Lee KY, Kim TH, Large MJ, Stewart CL, et al. Foxa2 is essential for mouse endometrial gland development and fertility. Biology of reproduction. 2010;83(3):396–403. doi: 10.1095/biolreprod.109.083154 ; PubMed Central PMCID: PMC2924802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Molecular cell. 2002;9(2):279–89. . [DOI] [PubMed] [Google Scholar]
- 48.Schneider MR, Wolf E. The epidermal growth factor receptor and its ligands in female reproduction: insights from rodent models. Cytokine & growth factor reviews. 2008;19(2):173–81. doi: 10.1016/j.cytogfr.2008.01.003 . [DOI] [PubMed] [Google Scholar]
- 49.Chang CF, Yang J, Li XF, Zhao WM, Chen SS, Wang GP, et al. [SPINK3: A novel growth factor that promotes rat liver regeneration]. Molekuliarnaia biologiia. 2016;50(3):457–65. doi: 10.7868/S0026898416030058 . [DOI] [PubMed] [Google Scholar]
- 50.Duncan CG, Killela PJ, Payne CA, Lampson B, Chen WC, Liu J, et al. Integrated genomic analyses identify ERRFI1 and TACC3 as glioblastoma-targeted genes. Oncotarget. 2010;1(4):265–77. Epub 2010/11/30. 137 [pii]. doi: 10.18632/oncotarget.137 ; PubMed Central PMCID: PMC2992381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones SR, Kimler BF, Justice WM, Rider V. Transit of normal rat uterine stromal cells through G1 phase of the cell cycle requires progesterone-growth factor interactions. Endocrinology. 2000;141(2):637–48. doi: 10.1210/endo.141.2.7332 . [DOI] [PubMed] [Google Scholar]
- 52.Franco HL, Rubel CA, Large MJ, Wetendorf M, Fernandez-Valdivia R, Jeong JW, et al. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 2012;26(3):1218–27. Epub 2011/12/14. fj.11-193334 [pii] doi: 10.1096/fj.11-193334 ; PubMed Central PMCID: PMC3289500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ovarian histology of Pik3caf/f (a) and Pik3cad/d (b) mice.
(PDF)
Immunohistochemical staining of total phospho-AKT (a and b) and AKT (c and d) in Pik3caf/f (a and c) and Pik3cad/d (b and d) mice.
(PDF)
Data Availability Statement
All relevant data are within the paper.