Abstract
The role of the cervicovaginal (CV) microbiome in regulating cervical function during pregnancy is poorly understood. Gardnerella vaginalis (G. vaginalis) is the most common bacteria associated with the diagnosis of bacterial vaginosis (BV). While BV has been associated with preterm birth (PTB), clinical trials targeting BV do not decrease PTB rates. It remains unknown if G. vaginalis is capable of triggering molecular, biomechanical and cellular events that could lead to PTB. The objective of this study was to determine if cervicovaginal colonization with G. vaginalis, in pregnant mice, induced cervical remodeling and modified cervical function. CD-1 timed-pregnant mice received a 5X108 CFU/mL intravaginal inoculation of G. vaginalis or control on embryonic day 12 (E12) and E13. On E15, the mice were sacrificed and cervicovaginal fluid (CVF), amniotic fluid (AF), cervix, uterus, placentas and fetal membranes (FM) were collected. Genomic DNA was isolated from the CVF, placenta, uterus and FM and QPCR was performed to confirm colonization. IL-6 was measured in the CVF and AF and soluble e-cadherin (seCAD) was assessed in the CVF by ELISA. RNA was extracted from the cervices to evaluate IL-10, IL-8, IL-1β, TNF-α, Tff-1, SPINK-5, HAS-1 and LOX expression via QPCR. Mucicarmine and trichrome staining was used to assess cervical mucin and collagen. Biomechanical properties of the cervix were studied using quasi-static tensile load-to-failure biomechanical tests. G. vaginalis successfully colonized the CV space. This colonization induced immune responses (increased IL-6 levels in CVF and AF, increased mRNA expression of cervical cytokines), altered the epithelial barrier (increased seCAD in the CVF), induced cervical remodeling (increased mucin production, altered collagen) and altered cervical biomechanical properties (a decrease in biomechanical modulus and an increase in maximum strain). The ability of G. vaginalis to induce these molecular, immune, cellular and biomechanical changes suggests that this bacterium may play a pathogenic role in premature cervical remodeling leading to PTB.
Introduction
Preterm birth (PTB) is the leading cause of perinatal morbidity and mortality worldwide. In the United States, one in nine babies is born prematurely and over 11 million PTB cases were reported last year [1, 2]. Premature babies have a higher incidence of developing medical complications [3] resulting in a financial cost of over 26 billion dollars a year in the United States alone [1]. Despite ongoing research, there are no effective strategies to predict or prevent the majority of preterm births. Recently, studies focusing on the causes of PTB have shown that women with bacterial vaginosis (BV) are at higher risk for spontaneous PTB (sPTB) [4–6]. BV is the most common genital tract infection affecting women worldwide [7]. This disease is characterized by a polymicrobial imbalance, or dysbiosis, of the natural microflora of the cervicovaginal (CV) space. While studies have shown an association between BV and PTB, [8] clinical trials targeting treatment of BV have failed to show differences in PTB rates [9–11]. The human microbiome project has provided valuable information about the diverse bacteria subspecies that make up a BV-like state [12, 13]. CV microflora mainly composed of Lactobacilli subspecies (spp.) is considered to be associated with a healthy CV space [8, 10, 14–24] while the lack of these species is integral to the diagnosis of BV and is considered a marker of an unhealthy CV space. Many BV cases are characterized by a decrease of Lactobacillus subspecies (spp.) and an increase in biofilms that may include Mobilincus spp., Mycoplasma hominis, Atopobium vaginae, Bacterioides spp. and Prevotellla spp. and Gardnerella vaginalis (G. vaginalis) [25, 26]. The conflicting data regarding BV and sPTB may be due, in part, to the role and/or pathogenicity of the different organisms that may compose the clinical diagnosis of BV [27, 28]. Since G. vaginalis is predominantly found in most cases of BV [29–31], the ability of this bacterium to induce cellular and molecular changes in the CV space, during pregnancy, is of scientific and clinical interest [27].
Recent studies have shown that disruption of the cervical epithelial barrier appears to be an important primary step critical to the initiation of cervical remodeling [32–34]. Cervical remodeling is a process that starts weeks, if not months, prior to parturition [32, 33, 35]. During this cervical remodeling process, cervical tissue undergoes robust changes at the molecular, histological and biomechanical levels to allow for delivery of a fetus [33, 36]. A clinical study attempting to identify biomarkers for cervical epithelial remodeling found increased levels of soluble epithelial-cadherin (seCAD) within the CV space [37]. seCAD, a soluble byproduct of proteolytic cleavage of e-cadherin (a member of the adherens junction complex), which may act as a physiologically relevant biomarker capable of predicting sPTB.[37]. Additionally, there is enhanced expression of several genes in the mouse cervix near delivery. These genes are responsible for cervical distensibility (Hyaluronan, HA) [38], collagen disorganization (lysyl oxidase or LOX) [39, 40], and initiate changes to the cervical extracellular matrix (trefoil factor I (Tff-1) and a Serine protein inhibitor Kasal 5 (SPINK-5)) [35, 41, 42]. Histology of the mouse cervix has revealed evidence of collagen rearrangement which is consistent with previous studies showing that the stiffness of the cervix decreases as parturition approaches [32]. Concordantly, cervical biomechanical studies in the mouse have provided valuable information to understanding how important structural mechanical properties, such as cervical load and stiffness, change at different embryonic stages [33, 43]. Recently, Barnum et al evaluated the intrinsic material properties of the mouse cervix during pregnancy [43]. This study provides evidence that alterations to the biomechanical properties of the cervix are likely part of the process of cervical remodeling.
While some of the processes governing cervical remodeling at term have been revealed, how specific bacteria, associated with BV, modify these processes have not been studied. Therefore, the objective of this study was to determine if specific BV-associated bacteria can be pathogenic during pregnancy and induce premature cervical remodeling. We hypothesize that G. vaginalis colonization of the CV space alters cervical function, contributes to dysfunction of the cervical epithelial barrier and, consequently, initiates cervical remodeling. We created a humanized pregnant mouse model of G. vaginalis colonization to determine if G. vaginalis colonization altered the local immune response, induced cervical remodeling and/or altered cervical biomechanics. Our results provide evidence that G. vaginalis colonization of the CV space leads to inflammation, cervical remodeling and altered cervical biomechanics.
Materials and methods
Animals
CD-1 timed-pregnant mice were purchased from Charles River Laboratories (Wilmington, MA). We considered E0 as mating day and E1 was determined based on presence of copulatory plug. Animals were shipped on day 10 after mating, and housed individually in our facilities. These animals were acclimated for 4 days before performing experiments. All the experiments were performed in accordance with the National Institutes of Health Guidelines on Laboratory Animals and with approval from the University of Pennsylvania’s Institutional Animal Care and Use Committee (IACUC #:805513).
Bacterial cultivation
Gardnerella vaginalis was purchased from the ATCC depository (ATCC# 14019) and grown anaerobically at 37°C with 5% CO2 in Tryptic Soy Broth (TSB) (Becton, Dickinson and Company, Sparks MD, USA) or Tryptic Soy Agar (TSA) (Becton, Dickinson and Company, Sparks MD, USA) supplemented with 5% horse serum (Gibco, Thermo Fisher Scientific). Efficient bacteria growth was measured and quantified by colony forming unit (CFU) assays. Bacteria were centrifuged twice to remove the growth media and the final pellet was resuspended in sterile filtered sugar water (10% fructose, 10% maltose, 10% glucose in sterile H2O (Sigma-Aldrich, Saint Louis MO, USA)) for use in animal experiments. This sugar water was used as the control in our animal trials.
Cervicovaginal colonization with G. vaginalis
We created a pregnant mouse model of G. vaginalis colonization as follows. CD-1 embryonic day 12 (E12) timed-pregnant mice were anaesthetized with isoflurane and five cervicovaginal lavages were performed with 100 μL of sterile PBS prior to control treatment or bacterial inoculation. Bacterial doses were determined using published data in a non-pregnant mouse model [44], and then recapitulating similar G. vaginalis loads in pregnant mice. The animals then received an intravaginal inoculation of G. vaginalis by inserting a sterile pipette tip and injecting 50 μL of 5X108 CFU/ml or sugar water. The inoculations were performed on E12 and repeated on E13 for both the G. vaginalis and the control group. This time point was chosen to mimic a change in the cervicovaginal microflora early in pregnancy. Immediately post-inoculation, each animal was positioned in dorsal decubitus under isoflurane anesthesia for 3 minutes and 100% pure petroleum jelly (Vaseline, Unilever USA) was added with a sterile swab to ensure the inoculum would remain within the cervicovaginal space. Animals were observed for 48 hours and specimens were collected on E15.
Using this protocol, we performed five separate trials. The first three trials were performed to 1) determine the preterm birth rate and 2) to collect fluids/tissues for assessment of inflammation, cervical remodeling and bacterial colonization. A fourth trial was performed to assess the effects of higher CFU dose, where we increase the bacteria load to 5X1010 CFU/mL. A final trial was performed to collect whole cervices for biomechanical testing (N = 12 animals in each experimental group) and cervical histology (N = 4 animals in each experimental group). In the first trial, on E15, a subset of the G. vaginalis (N = 12) and control (N = 8) inoculated animals were sacrificed to collect tissues for downstream assays (described below). The remaining animals (G. vaginalis, N = 4 and control, N = 4) were monitored for preterm birth and allowed to deliver to record pup weight and size. Preterm birth was defined as delivery prior to E18. Around 24 hours post-delivery, we counted and weighed individual pups in each litter. For the remaining trials, dams were sacrificed on E15 and the following specimens were collected from both G. vaginalis (N = 10 per trial) and control (N = 8–12 per trial) groups: cervicovaginal fluid (CVF), amniotic fluid (AF), cervix, lower uterus, placentas and fetal membranes. To assess for active colonization of G. vaginalis, on E15, immediately following CVF collection (N = 12), 50 μL of CVF was spread on Tryptic Soy Agar supplemented with 5% rabbit blood and incubated for 48 hours under the conditions mentioned above.
Tissue and specimen collection
From the first three animal trials, on E15 CVF, AF, cervix, lower uterus, placenta and fetal membranes were collected. CVF was collected by gently rinsing the cervicovaginal space (pipetting in and out seven times) with 100 μL of sterile PBS. The washes were pooled together into one sterile tube for each dam. AF was collected by aspirating the fluid out of the fetal sacs with a 19 gauge needle. AF from all pups per dam were collected and pooled into one tube and spun at 1,500 rpm for 5 minutes at 4°C. The AF supernatants were stored at -80°C until needed. The cervix was dissected away from the vagina and the lower uterus and was collected after removing the bladder, adipose tissue and rectum. A total of four placentas and their respective fetal membranes were collected from the four fetuses closest to the cervix. The cervices, lower uterus, placentas and fetal membranes were collected and flash frozen in liquid nitrogen and stored at -80°C until needed for downstream assays.
From the fourth animal trial, cervices were collected and placed in 10 mL of 4% formalin, stored for 48–72 hours and used for tissue histology and staining as described below. For biomechanical testing, a second cohort of mice from the same animal trial were sacrificed and stored at -20°C and cervical tissues were harvested at the time of testing as described below.
Genomic DNA isolation and QPCR
Genomic DNA (gDNA) was isolated and purified from the CVF with the ZR fecal MiniPrep DNA extraction kit (Zymo Research, Irvine, CA, USA). To purify gDNA from placenta, uterus and fetal membranes we used the DNeasy Blood and Tissue mini column DNA extraction kit (Qiagen, Germantown, MD, USA) following the manufacture’s protocol. To quantify the amount of G. vaginalis gDNA, we used a 16S specific probe to this bacterium (Applied Biosystems, Foster City, CA, USA). gDNA from the CVF, fetal membranes, uterus and placenta was quantified by QPCR to determine tissue specific colonization. A standard curve was created from serially diluted gDNA from G. vaginalis to quantify the amplification. This standard curve was used for relative quantification of G. vaginalis abundance using the 7900HT Real-Time PCR System (Applied Biosystems). The results were analyzed using the RQ manager software v2.4 (Applied Biosystems).
ELISA assays
Amniotic fluid was used for measurement of IL-6. Cervicovaginal fluid was used for measurement of IL-6 and soluble E-cadherin (seCAD) using commercially available ELISA assay kits following the manufacturer’s protocol (R&D Systems, Minneapolis, MN, USA).
RNA isolation from cervix
To isolate RNA from the previously collected cervices, we placed the cervix in a round bottom 2.0 mL Eppendorf tube with TRIzol (Invitrogen, Thermo-Fisher Scientific). The cervices were then mechanically homogenized with stainless steel beads (5mm, Qiagen) at a frequency of 30 Hz/sec for 10 minutes in a TissueLyser II (Qiagen) and underwent phenol-chloroform extraction. RNA concentration was determined via a NanoDrop 2000 Spectrophotometer (Nanodrop™ Rockland, DE) prior to the generation of cDNA.
cDNA generation and QPCR
cDNA was generated from 1 μg of isolated RNA from cervical tissue using the high capacity cDNA reverse transcription kit (Applied Biosystems, Thermo-Fisher Scientific). QPCR was performed on the 7900HT Real-Time PCR System (Applied Biosystems) using the TaqMan Universal PCR Master Mix (Applied Biosystems) according to the manufacturers’ protocols. The standard curve method was used for relative expression quantification using the RQ manager software v2.4 (Applied Biosystems). In TaqMan QPCR assays, the relative abundance of the target of interest was divided by the relative abundance of 18S in each sample to generate a standardized abundance for the target transcript of interest. All mRNA primers were purchased from Applied Biosystems: IL-10, IL-8, IL-1β, TNF-α, Tff-1, SPINK-5, HAS-1, LOX and 18S (TaqMan gene expression assays, Applied Biosystems, Thermo-Fisher).
Trichrome and mucicarmine assay
At E15, post G. vaginalis inoculation, the cervices were harvested as noted above, and placed in formalin for 48–72 hours and paraffin embedded. The cervices were sectioned (10 μm) and mounted onto glass microscope slides. These sections were stained using hematoxylin and eosin (H&E) (ScyTech, Logan Utah, USA), trichrome and mucicarmine staining kits (Abcam, Cambridge, MA, USA), following the manufacturer’s instructions and as previously reported [45]. Pictures were taken with a Nikon Eclipse microscope (Nikon Instruments Inc., NY, USA) with a 1394 color digital camera (Scion corp. Model 1310, NY, USA), and Image J software (Version 1.34s Wayne Rasband, Java 1.5.0_19) was used to analyze the pictures.
Biomechanical testing
Biomechanical testing was performed using methods previously described [43]. At the time of testing, female reproductive tissues were carefully harvested, removing all musculature and surrounding soft tissue, and hydrated in phosphate buffered saline (PBS) (N = 12 animals of each experimental group). Orientation of the cervix was noted to ensure consistency throughout the biomechanical experiments. The cervix was dissected free of any extra soft tissue and the uterus and vagina were carefully removed. The cervix was laid flat to expose the lumen. The ends were affixed between two pieces of sandpaper for gripping, such that a uniaxial tensile load on the grips would simulate dilation of the cervical canal (loading occurred perpendicular to the proximal-distal direction). The prepared sample was continually immersed in PBS until the start of mechanical testing. A custom laser device was used to measure the cross sectional area at a minimum of two locations, which took less than 60 seconds [46]. The cervix was then placed in custom fixtures to grip it at both ends. The cervix was then tested under uniaxial tension using an Instron 5848 testing system (Instron Corp., Norwood, MA). The testing protocol consisted of a preload of 0.005N followed by a hold of 5 minutes and then a ramp to failure at a rate of 1mm/minute. The entire test was performed in a saline bath at room temperature. The location of failure was recorded for each sample. Samples were excluded from further analysis if failure did not occur within the mid-substance of the tissue.
Statistical analysis
Statistical analyses were performed for all experiments with the GraphPad Prism Software (Version 4.0, La Jolla, CA, USA). For data that were normally distributed, an unpaired t-test was used. If data were not normally distributed, then the unpaired t-test with Welch’s correction was used. For biomechanical testing, t-test analyses were used to compare between groups for mechanical parameters. One-way ANOVA with Bonferroni-corrected post hoc tests were used to evaluate differences in fiber re-alignment. P<0.05 was considered to be statistically significant. P<0.1 was considered to be a trend.
Results
G. vaginalis colonization of the CV space of timed-pregnant CD-1 mice
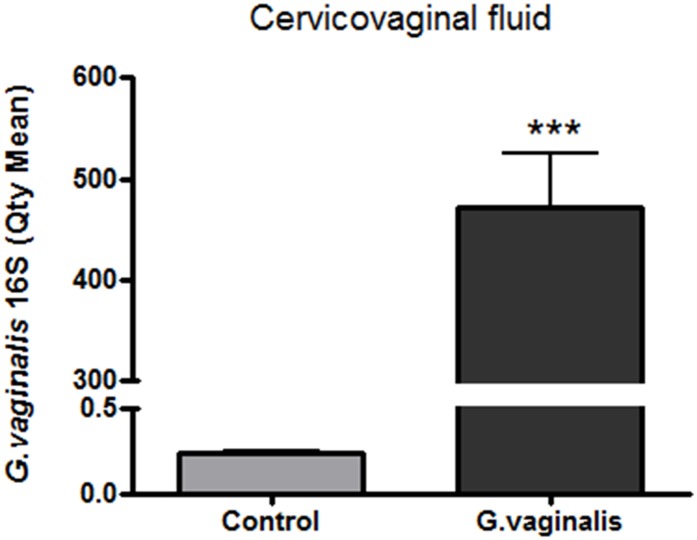
G. vaginalis successfully colonized the CV space (Fig 1, p<0.0001) and was not detected in the uterus or placentas (S1 Methods; S1–S3 Figs). The most effective colonization was achieved using a single dose of 5x108 CFU/mL of bacteria inoculated into the CV space for two consecutive days. In this study, we confirmed that live G. vaginalis was present in the CV space 48 hours after first inoculation (S4 Fig). Importantly, G. vaginalis colonization did not affect the litter size or the pup weight (S5 Fig). Animals treated with 5x108 CFU/mL G. vaginalis had a PTB rate that ranged from 0 to 20 percent in three independent experiments (3 out of 12, 1 out of 10 and 0 out of 12 animals) delivered before E18,which is our metric standard to define PTB (data not shown). The CV space of the animals treated with 5x1010 CFU/mL G. vaginalis were adequately colonized (S6 Fig) and had a PTB rate of 0 percent (0 out of 10) (data not shown).
Fig 1. G. vaginalis colonization of the CV space of timed-pregnant CD-1 mice.
Quantification of the 16S gene of G. vaginalis in the CVF of animals inoculated with 5X108 CFU/mL, was performed via qPCR using a specific G. vaginalis 16S probe. Graphs shows the average quantity mean detected by qPCR of N = 8 Control and N = 12 G. vaginalis group. T-test analyses with Welch’s correction between these groups was performed (***p<0.0001). Values are mean ± SD.
Elevated inflammation in the cervix of G. vaginalis colonized animals
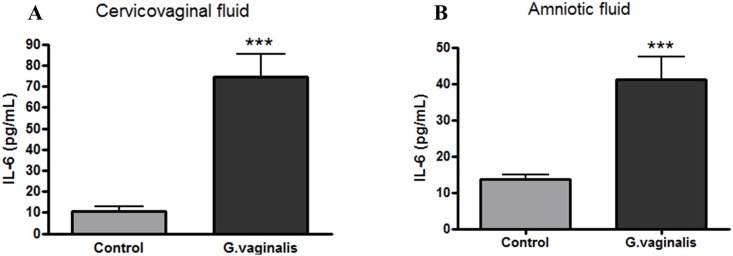
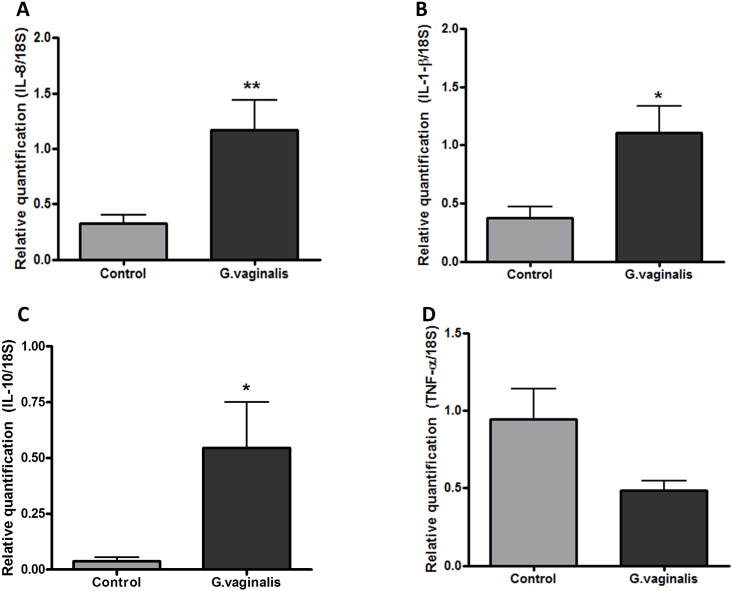
In order to determine if G. vaginalis colonization of the CV space results in a local inflammatory response, we assessed IL-6 protein levels as a marker of generalized inflammation. IL-6 was significantly increased in the CVF of animals inoculated with G. vaginalis in comparison to the samples in the control group (Fig 2A, p = 0.0007). We also observed a significant increase in IL-6 in the amniotic fluid (Fig 2B, p = 0.0008) of animals colonized by G. vaginalis, despite the absence of ascending bacteria into the fetal membranes, placenta or uterus. Additionally, we quantified the gene expression of other known pro-inflammatory cytokines and chemokines in the cervix including TNF-α, IL-10, IL1β and IL-8. Cervical gene expression of IL-8 (p = 0.0055), IL-1β (p = 0.0120) and IL-10 (p = 0.0140) were significantly enhanced (Fig 3A, 3B and 3C respectively). TNF-α was not significantly altered (Fig 3D, p = 0.0842).
Fig 2. G. vaginalis increases levels of IL-6 in the cervicovaginal space and amniotic fluid.
Levels of IL-6 in the CVF (A) and in the AF (B) were measured via ELISA. T-test analysis with Welch’s correction was performed to determine statistical significance between two groups (***p = 0.0007 in the CVF and ***p = 0.0008 in the AF analysis) (N = 8 Control and N = 12 G. vaginalis group). Values are mean ± SD.
Fig 3. Increased gene expression of IL-1β, IL-8 and IL-10 in the cervix of G. vaginalis colonized animals.
Gene expression levels of IL-8 (A), IL-1-β (B), IL-10 (C), and TNF-α (D) were measured by QPCR. T-test with Welch’s correction was performed in each group (N = 8 Control and N = 11 G. vaginalis group) (**IL-8 (p = 0.0055),*IL-1-β (p = 0.0120) and *IL-10 (p = 0.0140)). Values are mean ± SD.
Colonization of the CV space with G. vaginalis induces cervical remodeling
As we have shown previously that increased seCAD is a molecular marker of cervical epithelial barrier disruption, [47] we assessed if seCAD was altered in this model. Levels of seCAD were significantly increased in dams colonized with G. vaginalis compared to controls (Fig 4, p<0.0001). In addition to seCAD, we measured the gene expression of LOX, HAS-2, Tff-1 and SPINK-5 which have all been previously reported to be involved in cervical remodeling [48]. We observed increased expression of Tff-1 (Table 1, p = 0.026), whereas the gene expression levels of SPINK-5 (Table 1, p = 0.080), HAS-2 (Table 1, p = 0.076) and LOX (Table 1, p = 0.3625) were not significantly different.
Fig 4. G. vaginalis increases soluble E-cadherin in the CV space.
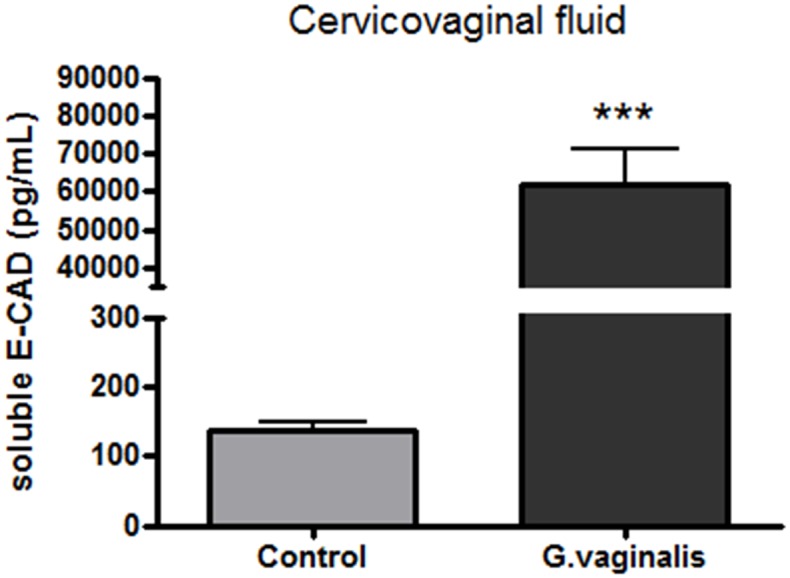
Levels of soluble E-cadherin were measured by ELISA. T-test analysis with Welch’s correction was calculated for significance of protein expression between the groups (***p<0.0001). Values are mean ± SD.
Table 1. Increased expression of Tff-1 in the cervix of G. vaginalis colonized animals.
| Protein | Fold-change | p-values |
|---|---|---|
| Tff-1 | 3* | p = 0.03 |
| SPINK-5 | 1 | p = 0.08 |
| HAS-2 | 1 | p = 0.08 |
| LOX | -1 | p = 0.36 |
The expression levels of proteins associated with cervical remodeling Tff-1, SPINK-5, HAS-2, and LOX were measured by qPCR. T-test with Welch’s correction test was performed between each group (N = 8 Control and N = 11 G. vaginalis group). Asterisk indicates statistical significance with a p-value <0.05.
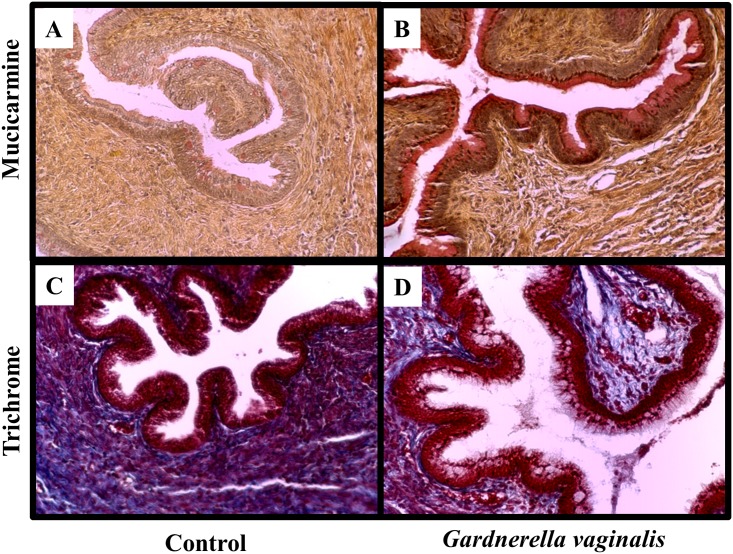
Prior work has demonstrated that histological changes within the cervix are consistent with cervical remodeling [49, 50]. Mucicarmine and trichrome stainings were performed to assess for the presence of mucin and collagen in cervices of G. vaginalis colonized compared to control animals (Fig 5). We observed increased expression of mucin in the cervices from dams colonized with G. vaginalis compared to controls, consistent with cervical ripening (Fig 5A and 5B). Additionally, we observed a dispersion of collagen fibers in animals colonized with G. vaginalis in comparison to animals treated with sugar water (Fig 5C and 5D).
Fig 5. G. vaginalis colonization increased expression of mucin and decreased collagen dispersion within cervical tissues.
Representative cervical sections from Control (A, C) or G. vaginalis (B, D) treated animlas (N = 4 in each group). Cervices were stained with mucicarmine (A, B) for analysis of mucin production while trichrome stain (C, D) shows collagen dispersion. Pictures were taken at a 10X magnification. Trichrome stains collagen blue, muscle fibers red and nuclei black-blue. Mucicarmine stains mucin pink/red, the nuclei blue and any other tissue component yellow.
G. vaginalis colonization alters the cervical biomechanics
Using previously described [43] techniques and metrics, we found that colonization with G. vaginalis demonstrated a decrease in modulus (Fig 6E, p< 0.05) as well as an increase in maximum strain (Fig 6F, p< 0.05) but no change in tissue cross-sectional area (Fig 6A), maximum load (Fig 6B), stiffness (Fig 6C, p<0.1) or maximum stress (Fig 6D). No difference in collagen fiber re-alignment was observed during cervical mechanical testing between groups (S1 Methods; S7 Fig).
Fig 6. Mechanical properties of G. vaginalis colonized cervices.
Area (A), Max Load (B), Stiffness (C), Max Stress (D), Modulus (E), and Max Strain (F) are shown for both control and G. vaginalis colonized cervices. All data is presented as means with standard deviations and significance noted at p< 0.05, (n = 10–11 in each group).
Discussion
As bacterial infection is thought to be one of the predominate factors associated with spontaneous PTB [1, 2] this study has provided novel insight into the role of cervicovaginal (CV) bacteria in the CV space and, specifically, on cervical function. The results from this study suggest that G. vaginalis, the most common bacteria associated with BV [4, 5], has the ability to initiate cervical remodeling through multiple biological, molecular and biomechanical mechanisms. In this study, we successfully generated a novel pregnant mouse model with a CV space colonized with G. vaginalis. This new model allowed us to study the effects of this bacterium within the CV space and its association with premature cervical remodeling. This study provides evidence that G. vaginalis colonization, localized to the CV space, was able to alter cervical function through multiple biological mechanisms including activating a cervical immune response, initiating cervical remodeling and modifying the material biomechanical properties of the cervix. Therefore, these results suggest that the presence of G. vaginalis within the CV space during pregnancy has the ability to directly alter many of the biological mechanisms regulating cervical remodeling and, hence, could contribute to the pathogenesis of PTB.
A previous study investigating the effects of G. vaginalis in the CV space of a non-pregnant mouse model showed that G. vaginalis was able to replicate within the CV space and ascend into the uterine horns [44]. Additionally, this same study showed that CV colonization of G. vaginalis resulted in many of the hallmark symptoms typically associated with clinical BV such as increased sialidase activity and vaginal epithelial exfoliation similar to phenotypes observed in clue cells in BV cases [44]. Similarly, in our G. vaginalis colonized pregnant mouse model, we observed high levels of G. vaginalis 16S in the CV space 48 hours post inoculation. It is important to point out that G. vaginalis inoculated into the CV space remained alive 48 hours post inoculation as evidenced by the growth of G. vaginalis colonies from CVF lavages (S6 Fig). G. vaginalis 16S was not detected within the uterus, placenta or fetal membranes suggesting that, in our model, G. vaginalis primarily colonizes the CV space with no ascension into the uterus. In contrast, it has been shown that G. vaginalis is capable of ascending into the uterus of a non-pregnant mouse model [44]. The ability of G. vaginalis to ascend into the uterus in the non-pregnant model but not in our pregnant model is biologically important. Due to the presence of increased cervicovaginal mucus known to be associated with pregnancy, it is biologically plausible to suggest that the presence of increased cervical mucus (and its associated mucosal immunity) may play a significant role in preventing the ascension of G. vaginalis into the uterus. Indeed, it has been observed that non pregnant mice have ascending bacteria, but this does not occur in pregnant animals as we have demonstrated. The inability of bacteria to ascend in the pregnant state may be due to expression changes of toll-like receptors and anti-microbial peptides (eg mucin) on the cervix during pregnancy [51]. The absence of detectable bacteria in the uterus correlates with the fact that G. vaginalis colonization did not affect litter size or pup weight (S4 Fig). To our knowledge this study is the first to show G. vaginalis colonization in pregnant mice.
The varied PTB rate animals treated with G. vaginalis may be attributed to the out-bred genetic background of our mouse model which inherently adds to a variable outcome and response to the colonization of G. vaginalis. Studies showing varying associations between BV treatment and PTB rates [4] may suggest that, in human pregnancy, G. vaginalis alone may not be sufficient to cause sPTB. Instead, G. vaginalis in combination with other BV-associated (or other non-BV) bacteria may be needed in order to initiate a more consistent preterm birth phenotype. Additionally, it is possible that the exposure time of G. vaginalis in the CV space could have significant effects on the cervix. In our model the animals were only exposed to G. vaginalis beginning on embryonic day 12, for a maximum time ranging from 48 hours to 5 days. Therefore, we cannot rule out the duration of exposure to G. vaginalis as being a contributing factor to the pathogenesis of preterm birth. Finally, it is important to note that the mouse epithelium is keratinized in contrast to the human, therefore G. vaginalis colonization and adherence might be different in our mouse model as compared to the human population.
The increased expression of IL-6 in the CVF of animals colonized with G. vaginalis indicated that the presence of this bacterium was able to initiate a localized immune response within the CV space. Interestingly, even in the absence of ascending bacteria, elevated IL-6 in the AF of animals inoculated with G. vaginalis suggests that increased cytokines localized to the CV space might have the ability to further activate an inflammatory response within the uterine cavity. Furthermore, when we analyzed the gene expression of these cytokines/chemokines in the cervix of G. vaginalis colonized animals, we observed an increase in PTB-associated cytokines [3, 52] such as IL-8, IL-10 and IL-1β. In a reported non-pregnant model, G. vaginalis colonization showed no histological inflammation [44], however cytokine and chemokine expression levels were not assessed after CV infection. Therefore, our results provide pertinent information about the inflammatory pathways induced by G. vaginalis colonization of the CV space during pregnancy. Our study is the first to show that G. vaginalis has the ability to induce an inflammatory response within the CV space of pregnant mice and it is biologically plausible that this inflammation may be capable of altering cervical function and integrity.
Previous work from our laboratory has demonstrated that an inflammatory insult leads to breakdown of the cervical epithelial barrier [47]. These observations are also present in other mucosal epithelial tissues such as the gut [53–59]. In the gut, inflammation leads to the activation of matrix metalloproteases (MMPs) that lead to disruption of the epithelial junctional proteins including epithelial-cadherin (e-cadherin) [53]. Specifically, MMPs and other serine proteases cleave the extracellular domain of e-cadherin resulting in the release of soluble e-cadherin (seCAD) into the extracellular spaces. Thus, in the presence of G. vaginalis colonization, increased seCAD in the CVF indicates a breakdown of the adherens junctions within the cervical epithelial barrier, as we have demonstrated in in vitro studies with cervical epithelial cells [47].Therefore, in our pregnant animal model, the fact that G. vaginalis colonization leads to increased levels of seCAD suggests that inflammation within the CV space has the ability to initiate the breakdown of the cervical epithelial barrier leading to cervical remodeling.
Based on these results, we aimed to further demonstrate that colonization of the CV space with G. vaginalis results in cervical remodeling. Previous work has demonstrated numerous molecular markers associated with cervical ripening and remodeling in different embryonic stages of mouse pregnancy [32]. To confirm cervical remodeling, we assessed LOX, Tff-1 and SPINK-5 gene expression as they have been reported to be associated with cervical ripening and dilation [32, 35]. Interestingly, increases in Tff-1 have been previously associated with increased internalization of e-cadherin, a process that occurs upon cleavage of the extracellular domain of e-cadherin resulting in an elevation of seCAD, as was observed in this study [60]. Thus, increased Tff-1 gene expression levels in the cervix along with the significantly elevated levels of seCAD in the CVF, provide evidence that G. vaginalis has the ability to alter the process of cervical remodeling. There are characteristic histological changes of the mouse cervix indicative of cervical remodeling; especially as parturition approaches there is evidence of collagen rearrangement and a decrease in cervical stiffness [32]. In addition to the observed changes in gene expression, mucicarmine and trichrome staining showed an increase in mucin expression as well as a dispersion of collagen fibers in the cervix of animals colonized with G. vaginalis providing additional evidence of cervical remodeling. While it is unknown if the alterations in histological cervical remodeling or an activated immune response occurs first, it is interesting to note, that increased mucin production has been linked to activation of the innate immune system as a response to bacterial infection [61]. Therefore, it is plausible to hypothesize that mucin is increased, in part, through the host’s biological mechanisms to protect the cervical epithelial cells from G. vaginalis infection. These results agree with previous studies showing an activated inflammatory response causes an increase in histological mucin expression and collagen dispersion [45, 50, 62]. While the exact pathological mechanisms leading to histological cervical remodeling remain unclear, the results from this study provide evidence that G. vaginalis has the ability to increase cervical remodeling.
Since both molecular and histological differences were observed in the cervix due to colonization of the CV space with G. vaginalis, cervical biomechanical parameters were also assessed. There was a significant decrease in tissue modulus of cervices colonized with G. vaginalis, when compared with the controls, indicating a change in its inherent material response. Concomitantly, a trend towards decreased stiffness of the cervical tissue points towards a structural (size) increase. This indicates a clear differential mechanical response of the murine cervix to colonization with G. vaginalis. Further, we have previously observed similar material and structural changes in the normal pregnant cervix immediately prior to parturition (E18.5). Interestingly, although the modulus values fall to similar levels as those observed in the normal E18.5 cervices, stiffness of the G. vaginalis samples remains appreciably higher. This finding suggests that some of the mechanical mechanisms contributing to cervical remodeling are different in animals colonized with G. vaginalis opposed to term parturition [36]. It is important to note that some of the observed mechanical changes such as modulus and stiffness in the G. vaginalis cervices could indicate more rapid cervical remodeling. In our model, colonization of the CV space with G. vaginalis provides evidence that cervical softening is occurring faster/earlier in comparison to our control group, as well as, in normal gestation [36, 40].
One limitation of this animal model is that, unlike the human, mice are quadrupedal not bipedal. The load of pregnancy would be divergently distributed in the mouse compared to the human and could affect cervical biomechanics. Despite this limitation, in the future it is imperative to continue to define how both structural and material mechanical properties of the cervix work in tandem with other biological and mechanical factors to regulate both term and preterm birth.
We did not observe drastic differences in collagen fiber re-alignment between cervices colonized with G. vaginalis versus control (S5 Fig.) indicating that the decrease in stiffness and modulus of cervical tissue in the G. vaginalis samples was not due to any reorganization of the load-bearing response of cervical collagen fibers. However, other factors such as collagen cross-linking, density of collagen fibers, or change in collagen fiber diameter could also explain the observed decrease in tissue mechanical properties [36, 63]. Both the dispersed collagen observed histologically combined with the increase of mucin expression could indicate other mechanisms of cervical remodeling through activation of immunological pathways.
Collectively, the results of this study show that colonization of G. vaginalis in the cervicovaginal space of pregnant mice has the ability to significantly alter cervical function. By evaluating multiple biological mechanisms known to be associated with the cervical remodeling process, we demonstrated that colonization of G. vaginalis in the cervicovaginal space can induce local inflammation, damage the cervical epithelial barrier, initiate cervical remodeling and alter the biomechanical characteristics of the cervix. The ability of G. vaginalis to induce these molecular, immune and cellular changes suggests that this bacterium could play a mechanistic role in sPTB in which cervical remodeling is the initiating event. Additionally, this study demonstrates the feasibility of mimicking the human CV microbiota in a pregnant mouse model. As shown in a recent study, G. vaginalis colonization has implications for other lower genitourinary tract conditions, such as the case of recurrent E.coli infections in the bladder [64]. By elucidating the mechanisms by which G. vaginalis alters the epithelium, underlying tissue and immune responses in the CV space, we might provide increased understanding of conditions associated with G. vaginalis such as HIV [65–70], UTI [64], recurrent pregnancy loss and preterm birth [71]. These findings have broader implications. An increased understanding of the role of the cervicovaginal microbiome and how they might mitigate or modify molecular, biomechanical and immune function in the CV space will be essential to developing future therapeutic options for preventing sPTB.
Supporting information
gDNA from the CVF was used with a G. vaginalis specific primer set to amplify G. vaginalis via PCR. PCR reactions were run on a 1% agarose gel with ethidium bromide and exposed to UV light to capture DNA bands. G. vaginalis positive bands were expected at an amplicon of 206 bp. As a positive control we included a PCR sample of gDNA isolated directly from G. vaginalis cultures. To determine the PCR product sizes we included wells with 1Kb and 100 bp ladders on each side of the gel.
(TIF)
gDNA from the uterus was used with a G. vaginalis specific primer set to amplify G. vaginalis via PCR. PCR reactions were run on a 1% agarose gel with ethidium bromide and exposed to UV light to capture DNA bands. G. vaginalis positive bands were expected at an amplicon of 206 bp. As a positive control we included a PCR sample of gDNA isolated directly from G. vaginalis cultures. To determine the PCR product sizes we included wells with 1Kb and 100 bp ladders on each side of the gel.
(TIF)
gDNA from the placenta was used with a G. vaginalis specific primer set to amplify G. vaginalis via PCR. PCR reactions were run on a 1% agarose gel with ethidium bromide and exposed to UV light to capture DNA bands. G. vaginalis positive bands were expected at an amplicon of 206 bp. As a positive control we included a PCR sample with gDNA isolated directly from G. vaginalis cultures. To determine the PCR product sizes we included wells with 1Kb and 100 bp ladders on each side of the gel.
(TIF)
Tryptic Soy Agar plates supplemented with 5% defibrillated rabbit blood were inoculated with 50μL of CVF collected from mice 48 hours post-inoculation and incubated for 72 hours in an anaerobic jar at 37°C and 5% CO2. After incubation, the numbers of colonies were counted on each plate.
(TIF)
Dams treated with sugar water or 5X108 CFU/mL of G. vaginalis were allowed to deliver (N = 8 in each group). The individual pup weights (A) and the number of pups per litter (B) were recorded. T-test with Mann-Whitney nonparametric correction analysis was performed to determine statistical significance between the two groups (pup weight: p = 0.8785 and Litter size: p = 0.6454). Values are mean ± SD.
(TIF)
Quantification of the 16S gene of G. vaginalis in the CVF of animals inoculated with 5X1010 CFU/mL was performed via qPCR using a specific G. vaginalis 16S probe. Graphs shows the average quantity mean detected by qPCR of N = 10 Control and N = 10 G. vaginalis group. T-test analyses with Welch’s correction between these groups was performed (p = 0.0002). Values are mean ± SD.
(TIF)
Representative plots of polarized light analysis at toe, end of toe, 45% of maximum load, and 90% of maximum load. The control group is shown on the left (A) and G. vaginalis colonized cervices are shown on the right (B). Lines represent significance of p< 0.05 (n = 10–11).
(TIF)
(PDF)
Acknowledgments
We would like to thank the Children’s Hospital of Philadelphia Pathology core, especially Socrates Agrio and Elizabeth A. Tomeski. This paper was supported in part by the March of Dimes Prematurity Research Center at the University of Pennsylvania (22-FY17-890), The Maternity and Child Health Research Center at the University of Pennsylvania and the Penn Center for Musculoskeletal Disorders (P30 AR069619).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This paper was supported in part by the March of Dimes Prematurity Research Center at the University of Pennsylvania (22-FY17-890) (M. A. Elovitz), The Maternal and Child Health Research Center at the University of Pennsylvania and the Penn Center for Musculoskeletal Disorders (P30 AR069619) (L. J. Soslowsky). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.In: Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. The National Academies Collection: Reports funded by National Institutes of Health. Washington (DC)2007. [PubMed]
- 2.2016 Premature Birth Report Card. http://www.marchofdimes.org/materials/premature-birth-report-card-united-states.pdf2016.
- 3.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–5. doi: 10.1126/science.1251816 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brabant G. [Bacterial vaginosis and spontaneous preterm birth]. Journal de gynecologie, obstetrique et biologie de la reproduction. 2016;45(10):1247–60. doi: 10.1016/j.jgyn.2016.09.014 . [DOI] [PubMed] [Google Scholar]
- 5.Luong ML, Libman M, Dahhou M, Chen MF, Kahn SR, Goulet L, et al. Vaginal douching, bacterial vaginosis, and spontaneous preterm birth. Journal of obstetrics and gynaecology Canada: JOGC = Journal d’obstetrique et gynecologie du Canada: JOGC. 2010;32(4):313–20. doi: 10.1016/S1701-2163(16)34474-7 . [DOI] [PubMed] [Google Scholar]
- 6.Thorsen P, Vogel I, Olsen J, Jeune B, Westergaard JG, Jacobsson B, et al. Bacterial vaginosis in early pregnancy is associated with low birth weight and small for gestational age, but not with spontaneous preterm birth: a population-based study on Danish women. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2006;19(1):1–7. doi: 10.1080/14767050500361604 . [DOI] [PubMed] [Google Scholar]
- 7.Gilbert NM, Lewis WG, Lewis AL. Clinical Features of Bacterial Vaginosis in a Murine Model of Vaginal Infection with Gardnerella vaginalis. PloS one. 2013;8(3). doi: 10.1371/journal.pone.0059539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson DB, Hanlon A, Hassan S, Britto J, Geifman-Holtzman O, Haggerty C, et al. Preterm labor and bacterial vaginosis-associated bacteria among urban women. Journal of perinatal medicine. 2009;37(2):130–4. doi: 10.1515/JPM.2009.026 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abramovici A, Lobashevsky E, Cliver SP, Edwards RK, Hauth JC, Biggio JR. Quantitative Polymerase Chain Reaction to Assess Response to Treatment of Bacterial Vaginosis and Risk of Preterm Birth. American journal of perinatology. 2015;32(12):1119–25. doi: 10.1055/s-0035-1549294 . [DOI] [PubMed] [Google Scholar]
- 10.Reid G, Bocking A. The potential for probiotics to prevent bacterial vaginosis and preterm labor. American journal of obstetrics and gynecology. 2003;189(4):1202–8. . [DOI] [PubMed] [Google Scholar]
- 11.Brocklehurst P, Gordon A, Heatley E, Milan SJ. Antibiotics for treating bacterial vaginosis in pregnancy. The Cochrane database of systematic reviews. 2013;(1):CD000262 doi: 10.1002/14651858.CD000262.pub4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PloS one. 2012;7(6):e37818 doi: 10.1371/journal.pone.0037818 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson DB, Rockwell LC, Prioleau MD, Goetzl L. The role of the bacterial microbiota on reproductive and pregnancy health. Anaerobe. 2016;42:67–73. doi: 10.1016/j.anaerobe.2016.09.001 . [DOI] [PubMed] [Google Scholar]
- 14.Breshears LM, Edwards VL, Ravel J, Peterson ML. Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC microbiology. 2015;15:276 doi: 10.1186/s12866-015-0608-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Bieda J, et al. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome. 2014;2:18 doi: 10.1186/2049-2618-2-18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma B, Forney LJ, Ravel J. Vaginal microbiome: rethinking health and disease. Annual review of microbiology. 2012;66:371–89. doi: 10.1146/annurev-micro-092611-150157 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai G, Gajer P, Nandy M, Ma B, Yang H, Sakamoto J, et al. Comparison of storage conditions for human vaginal microbiome studies. PloS one. 2012;7(5):e36934 doi: 10.1371/journal.pone.0036934 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences of the United States of America. 2011;108 Suppl 1:4680–7. doi: 10.1073/pnas.1002611107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mielczarek E, Blaszkowska J. Trichomonas vaginalis: pathogenicity and potential role in human reproductive failure. Infection. 2016;44(4):447–58. doi: 10.1007/s15010-015-0860-0 . [DOI] [PubMed] [Google Scholar]
- 20.Burgmeier C, Dreyhaupt J, Schier F. Gender-related differences of inguinal hernia and asymptomatic patent processus vaginalis in term and preterm infants. Journal of pediatric surgery. 2015;50(3):478–80. doi: 10.1016/j.jpedsurg.2014.08.015 . [DOI] [PubMed] [Google Scholar]
- 21.Holst E, Goffeng AR, Andersch B. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. Journal of clinical microbiology. 1994;32(1):176–86. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimberlin DF, Andrews WW. Bacterial vaginosis: association with adverse pregnancy outcome. Seminars in perinatology. 1998;22(4):242–50. . [DOI] [PubMed] [Google Scholar]
- 23.Martinez de Tejada B, Coll O, de Flores M, Hillier SL, Landers DV. [Prevalence of bacterial vaginosis in an obstetric population of Barcelona]. Medicina clinica. 1998;110(6):201–4. . [PubMed] [Google Scholar]
- 24.McDonald HM, O’Loughlin JA, Vigneswaran R, Jolley PT, Harvey JA, Bof A, et al. Impact of metronidazole therapy on preterm birth in women with bacterial vaginosis flora (Gardnerella vaginalis): a randomised, placebo controlled trial. British journal of obstetrics and gynaecology. 1997;104(12):1391–7. . [DOI] [PubMed] [Google Scholar]
- 25.Robinson LS, Perry J, Lek S, Wollam A, Sodergren E, Weinstock G, et al. Genome Sequences of 15 Gardnerella vaginalis Strains Isolated from the Vaginas of Women with and without Bacterial Vaginosis. Genome announcements. 2016;4(5). doi: 10.1128/genomeA.00879-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. Journal of clinical microbiology. 2007;45(10):3270–6. doi: 10.1128/JCM.01272-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(35):11060–5. doi: 10.1073/pnas.1502875112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callahan BJ, DiGiulio DB, Goltsman DSA, Sun CL, Costello EK, Jeganathan P, et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(37):9966–71. doi: 10.1073/pnas.1705899114 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilbert DW, Schuyler JA, Adelson ME, Mordechai E, Sobel JD, Gygax SE. Gardnerella vaginalis population dynamics in bacterial vaginosis. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2017. doi: 10.1007/s10096-017-2933-8 . [DOI] [PubMed] [Google Scholar]
- 30.Vodstrcil LA, Twin J, Garland SM, Fairley CK, Hocking JS, Law MG, et al. The influence of sexual activity on the vaginal microbiota and Gardnerella vaginalis clade diversity in young women. PloS one. 2017;12(2):e0171856 doi: 10.1371/journal.pone.0171856 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardy L, Jespers V, Van den Bulck M, Buyze J, Mwambarangwe L, Musengamana V, et al. The presence of the putative Gardnerella vaginalis sialidase A gene in vaginal specimens is associated with bacterial vaginosis biofilm. PloS one. 2017;12(2):e0172522 doi: 10.1371/journal.pone.0172522 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nallasamy S, Mahendroo M. Distinct Roles of Cervical Epithelia and Stroma in Pregnancy and Parturition. Seminars in reproductive medicine. 2017;35(2):190–200. doi: 10.1055/s-0037-1599091 . [DOI] [PubMed] [Google Scholar]
- 33.Nallasamy S, Yoshida K, Akins M, Myers K, Iozzo R, Mahendroo M. Steroid Hormones Are Key Modulators of Tissue Mechanical Function via Regulation of Collagen and Elastic Fibers. Endocrinology. 2017;158(4):950–62. doi: 10.1210/en.2016-1930 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anton L, DeVine A, Sierra LJ, Brown AG, Elovitz MA. miR-143 and miR-145 disrupt the cervical epithelial barrier through dysregulation of cell adhesion, apoptosis and proliferation. Scientific reports. 2017;7(1):3020 doi: 10.1038/s41598-017-03217-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction. 2007;134(2):327–40. doi: 10.1530/REP-07-0032 . [DOI] [PubMed] [Google Scholar]
- 36.Yoshida K, Mahendroo M, Vink J, Wapner R, Myers K. Material properties of mouse cervical tissue in normal gestation. Acta biomaterialia. 2016;36:195–209. doi: 10.1016/j.actbio.2016.03.005 . [DOI] [PubMed] [Google Scholar]
- 37.Bastek JA, Hirshberg A, Chandrasekaran S, Owen CM, Heiser LM, Araujo BA, et al. Biomarkers and cervical length to predict spontaneous preterm birth in asymptomatic high-risk women. Obstetrics and gynecology. 2013;122(2 Pt 1):283–9. doi: 10.1097/AOG.0b013e31829ab714 . [DOI] [PubMed] [Google Scholar]
- 38.Straach KJ, Shelton JM, Richardson JA, Hascall VC, Mahendroo MS. Regulation of hyaluronan expression during cervical ripening. Glycobiology. 2005;15(1):55–65. doi: 10.1093/glycob/cwh137 . [DOI] [PubMed] [Google Scholar]
- 39.Akins ML, Luby-Phelps K, Bank RA, Mahendroo M. Cervical softening during pregnancy: regulated changes in collagen cross-linking and composition of matricellular proteins in the mouse. Biology of reproduction. 2011;84(5):1053–62. doi: 10.1095/biolreprod.110.089599 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida K, Jiang H, Kim M, Vink J, Cremers S, Paik D, et al. Quantitative evaluation of collagen crosslinks and corresponding tensile mechanical properties in mouse cervical tissue during normal pregnancy. PloS one. 2014;9(11):e112391 doi: 10.1371/journal.pone.0112391 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timmons BC, Mahendroo M. Processes regulating cervical ripening differ from cervical dilation and postpartum repair: insights from gene expression studies. Reproductive sciences. 2007;14(8 Suppl):53–62. doi: 10.1177/1933719107309587 . [DOI] [PubMed] [Google Scholar]
- 42.Timmons BC, Mitchell SM, Gilpin C, Mahendroo MS. Dynamic changes in the cervical epithelial tight junction complex and differentiation occur during cervical ripening and parturition. Endocrinology. 2007;148(3):1278–87. doi: 10.1210/en.2006-0851 . [DOI] [PubMed] [Google Scholar]
- 43.Barnum CE, Fey JL, Weiss SN, Barila G, Brown AG, Connizzo BK, et al. Tensile Mechanical Properties and Dynamic Collagen Fiber Re-Alignment of the Murine Cervix are Dramatically Altered Throughout Pregnancy. Journal of biomechanical engineering. 2017;139(6). doi: 10.1115/1.4036473 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbert NM, Lewis WG, Lewis AL. Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PloS one. 2013;8(3):e59539 doi: 10.1371/journal.pone.0059539 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yellon SM, Ebner CA, Elovitz MA. Medroxyprogesterone acetate modulates remodeling, immune cell census, and nerve fibers in the cervix of a mouse model for inflammation-induced preterm birth. Reproductive sciences. 2009;16(3):257–64. doi: 10.1177/1933719108325757 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Favata M, Beredjiklian PK, Zgonis MH, Beason DP, Crombleholme TM, Jawad AF, et al. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2006;24(11):2124–32. doi: 10.1002/jor.20271 . [DOI] [PubMed] [Google Scholar]
- 47.Nold C, Anton L, Brown A, Elovitz M. Inflammation promotes a cytokine response and disrupts the cervical epithelial barrier: a possible mechanism of premature cervical remodeling and preterm birth. American journal of obstetrics and gynecology. 2012;206(3):208 e1–7. doi: 10.1016/j.ajog.2011.12.036 . [DOI] [PubMed] [Google Scholar]
- 48.Akgul Y, Word RA, Ensign LM, Yamaguchi Y, Lydon J, Hanes J, et al. Hyaluronan in cervical epithelia protects against infection-mediated preterm birth. The Journal of clinical investigation. 2014;124(12):5481–9. doi: 10.1172/JCI78765 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez JM, Xu H, Chai J, Ofori E, Elovitz MA. Preterm and term cervical ripening in CD1 Mice (Mus musculus): similar or divergent molecular mechanisms? Biology of reproduction. 2009;81(6):1226–32. doi: 10.1095/biolreprod.108.075309 . [DOI] [PubMed] [Google Scholar]
- 50.Elovitz MA, Gonzalez J. Medroxyprogesterone acetate modulates the immune response in the uterus, cervix and placenta in a mouse model of preterm birth. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2008;21(4):223–30. doi: 10.1080/14767050801923680 . [DOI] [PubMed] [Google Scholar]
- 51.Racicot K, Cardenas I, Wunsche V, Aldo P, Guller S, Means RE, et al. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. Journal of immunology. 2013;191(2):934–41. doi: 10.4049/jimmunol.1300661 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Seminars in reproductive medicine. 2007;25(1):21–39. doi: 10.1055/s-2006-956773 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lechuga S, Ivanov AI. Disruption of the epithelial barrier during intestinal inflammation: Quest for new molecules and mechanisms. Biochimica et biophysica acta. 2017;1864(7):1183–94. doi: 10.1016/j.bbamcr.2017.03.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflammatory bowel diseases. 2009;15(1):100–13. doi: 10.1002/ibd.20539 . [DOI] [PubMed] [Google Scholar]
- 55.Barmeyer C, Erko I, Awad K, Fromm A, Bojarski C, Meissner S, et al. Epithelial barrier dysfunction in lymphocytic colitis through cytokine-dependent internalization of claudin-5 and -8. Journal of gastroenterology. 2017. doi: 10.1007/s00535-017-1309-2 . [DOI] [PubMed] [Google Scholar]
- 56.Barmeyer C, Schulzke JD, Fromm M. Claudin-related intestinal diseases. Seminars in cell & developmental biology. 2015;42:30–8. doi: 10.1016/j.semcdb.2015.05.006 . [DOI] [PubMed] [Google Scholar]
- 57.Luettig J, Rosenthal R, Barmeyer C, Schulzke JD. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue barriers. 2015;3(1–2):e977176 doi: 10.4161/21688370.2014.977176 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Frontiers in immunology. 2013;4:280 doi: 10.3389/fimmu.2013.00280 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annual review of physiology. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Durer U, Hartig R, Bang S, Thim L, Hoffmann W. TFF3 and EGF induce different migration patterns of intestinal epithelial cells in vitro and trigger increased internalization of E-cadherin. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2007;20(5):329–46. doi: 10.1159/000107519 . [DOI] [PubMed] [Google Scholar]
- 61.Cornick S, Tawiah A, Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue barriers. 2015;3(1–2):e982426 doi: 10.4161/21688370.2014.982426 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elovitz M, Wang Z. Medroxyprogesterone acetate, but not progesterone, protects against inflammation-induced parturition and intrauterine fetal demise. American journal of obstetrics and gynecology. 2004;190(3):693–701. doi: 10.1016/j.ajog.2003.10.693 . [DOI] [PubMed] [Google Scholar]
- 63.Timmons BC, Reese J, Socrate S, Ehinger N, Paria BC, Milne GL, et al. Prostaglandins are essential for cervical ripening in LPS-mediated preterm birth but not term or antiprogestin-driven preterm ripening. Endocrinology. 2014;155(1):287–98. doi: 10.1210/en.2013-1304 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gilbert NM, O’Brien VP, Lewis AL. Transient microbiota exposures activate dormant Escherichia coli infection in the bladder and drive severe outcomes of recurrent disease. PLoS pathogens. 2017;13(3):e1006238 doi: 10.1371/journal.ppat.1006238 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sha BE, Chen HY, Wang QJ, Zariffard MR, Cohen MH, Spear GT. Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women. Journal of clinical microbiology. 2005;43(9):4607–12. doi: 10.1128/JCM.43.9.4607-4612.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sha BE, Zariffard MR, Wang QJ, Chen HY, Bremer J, Cohen MH, et al. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. The Journal of infectious diseases. 2005;191(1):25–32. doi: 10.1086/426394 . [DOI] [PubMed] [Google Scholar]
- 67.Cohn JA, Hashemi FB, Camarca M, Kong F, Xu J, Beckner SK, et al. HIV-inducing factor in cervicovaginal secretions is associated with bacterial vaginosis in HIV-1-infected women. Journal of acquired immune deficiency syndromes. 2005;39(3):340–6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al-Harthi L, Roebuck KA, Olinger GG, Landay A, Sha BE, Hashemi FB, et al. Bacterial vaginosis-associated microflora isolated from the female genital tract activates HIV-1 expression. Journal of acquired immune deficiency syndromes. 1999;21(3):194–202. . [DOI] [PubMed] [Google Scholar]
- 69.Al-Harthi L, Spear GT, Hashemi FB, Landay A, Sha BE, Roebuck KA. A human immunodeficiency virus (HIV)-inducing factor from the female genital tract activates HIV-1 gene expression through the kappaB enhancer. The Journal of infectious diseases. 1998;178(5):1343–51. . [DOI] [PubMed] [Google Scholar]
- 70.St John EP, Zariffard MR, Martinson JA, Simoes JA, Landay AL, Spear GT. Effect of mucosal fluid from women with bacterial vaginosis on HIV trans-infection mediated by dendritic cells. Virology. 2009;385(1):22–7. doi: 10.1016/j.virol.2008.08.031 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Isik G, Demirezen S, Donmez HG, Beksac MS. Bacterial vaginosis in association with spontaneous abortion and recurrent pregnancy losses. Journal of cytology. 2016;33(3):135–40. doi: 10.4103/0970-9371.188050 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gDNA from the CVF was used with a G. vaginalis specific primer set to amplify G. vaginalis via PCR. PCR reactions were run on a 1% agarose gel with ethidium bromide and exposed to UV light to capture DNA bands. G. vaginalis positive bands were expected at an amplicon of 206 bp. As a positive control we included a PCR sample of gDNA isolated directly from G. vaginalis cultures. To determine the PCR product sizes we included wells with 1Kb and 100 bp ladders on each side of the gel.
(TIF)
gDNA from the uterus was used with a G. vaginalis specific primer set to amplify G. vaginalis via PCR. PCR reactions were run on a 1% agarose gel with ethidium bromide and exposed to UV light to capture DNA bands. G. vaginalis positive bands were expected at an amplicon of 206 bp. As a positive control we included a PCR sample of gDNA isolated directly from G. vaginalis cultures. To determine the PCR product sizes we included wells with 1Kb and 100 bp ladders on each side of the gel.
(TIF)
gDNA from the placenta was used with a G. vaginalis specific primer set to amplify G. vaginalis via PCR. PCR reactions were run on a 1% agarose gel with ethidium bromide and exposed to UV light to capture DNA bands. G. vaginalis positive bands were expected at an amplicon of 206 bp. As a positive control we included a PCR sample with gDNA isolated directly from G. vaginalis cultures. To determine the PCR product sizes we included wells with 1Kb and 100 bp ladders on each side of the gel.
(TIF)
Tryptic Soy Agar plates supplemented with 5% defibrillated rabbit blood were inoculated with 50μL of CVF collected from mice 48 hours post-inoculation and incubated for 72 hours in an anaerobic jar at 37°C and 5% CO2. After incubation, the numbers of colonies were counted on each plate.
(TIF)
Dams treated with sugar water or 5X108 CFU/mL of G. vaginalis were allowed to deliver (N = 8 in each group). The individual pup weights (A) and the number of pups per litter (B) were recorded. T-test with Mann-Whitney nonparametric correction analysis was performed to determine statistical significance between the two groups (pup weight: p = 0.8785 and Litter size: p = 0.6454). Values are mean ± SD.
(TIF)
Quantification of the 16S gene of G. vaginalis in the CVF of animals inoculated with 5X1010 CFU/mL was performed via qPCR using a specific G. vaginalis 16S probe. Graphs shows the average quantity mean detected by qPCR of N = 10 Control and N = 10 G. vaginalis group. T-test analyses with Welch’s correction between these groups was performed (p = 0.0002). Values are mean ± SD.
(TIF)
Representative plots of polarized light analysis at toe, end of toe, 45% of maximum load, and 90% of maximum load. The control group is shown on the left (A) and G. vaginalis colonized cervices are shown on the right (B). Lines represent significance of p< 0.05 (n = 10–11).
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.