Abstract
Schistosomes are blood-dwelling trematodes with global impact on human and animal health. Because medical treatment is currently based on a single drug, praziquantel, there is urgent need for the development of alternative control strategies. The Schistosoma mansoni genome project provides a platform to study and connect the genetic repertoire of schistosomes to specific biological functions essential for successful parasitism. G protein–coupled receptors (GPCRs) form the largest superfamily of transmembrane receptors throughout the Eumetazoan phyla, including platyhelminths. Due to their involvement in diverse biological processes, their pharmacological importance, and proven druggability, GPCRs are promising targets for new anthelmintics. However, to identify candidate receptors, a more detailed understanding of the roles of GPCR signalling in schistosome biology is essential. An updated phylogenetic analysis of the S. mansoni GPCR genome (GPCRome) is presented, facilitated by updated genome data that allowed a more precise annotation of GPCRs. Additionally, we review the current knowledge on GPCR signalling in this parasite and provide new insights into the potential roles of GPCRs in schistosome reproduction based on the findings of a recent tissue-specific transcriptomic study in paired and unpaired S. mansoni. According to the current analysis, GPCRs contribute to gonad-specific functions but also to nongonad, pairing-dependent processes. The latter may regulate gonad-unrelated functions during the multifaceted male–female interaction. Finally, we compare the schistosome GPCRome to that of another parasitic trematode, Fasciola, and discuss the importance of GPCRs to basic and applied research. Phylogenetic analyses display GPCR diversity in free-living and parasitic platyhelminths and suggest diverse functions in schistosomes. Although their roles need to be substantiated by functional studies in the future, the data support the selection of GPCR candidates for basic and applied studies, invigorating the exploitation of this important receptor class for drug discovery against schistosomes but also other trematodes.
Introduction
GPCRs form the largest known superfamily of transmembrane receptors in the Eumetazoa. They are involved in diverse biological processes, including growth, differentiation, neuronal signalling, olfaction, metabolism, and reproduction by interacting with different ligands such as neuropeptides, hormones, neurotransmitters, gases, volatile compounds, and biogenic amines. The importance of GPCRs is further reflected by their medical relevance because 30% to 50% of all pharmaceutical compounds target GPCRs and GPCR-mediated signalling pathways [1–4].
GPCRs possess seven alpha helices spanning the plasma membrane in a serpentine manner. While the N-terminus and extracellular loops are involved in ligand binding, the cytosolic parts and the C-terminus interact with downstream partners. Classically, GPCRs are defined as ligand-activated guanine nucleotide exchange factors (GEFs) for heterotrimeric guanine nucleotide-binding (G) proteins that transmit signals intracellularly by interacting with different effector molecules. This mostly results in the modulation of second messenger concentrations, which in turn provoke cellular responses [5]. In addition to this traditional view, research activities have led to a tremendous increase in knowledge on the versatility of GPCR signalling. Among other functions, GPCRs activate G protein–independent signalling pathways through adaptor proteins like arrestins [6,7], and they form homo- and hetero-oligomers or receptor mosaics consisting of three or more protomers [8,9]. Furthermore, GPCRs can cooperate with different membrane proteins such as integrins and receptor tyrosine kinases (RTKs) [10–13]. This functional flexibility, combined with the use of different downstream effector molecules such as signal transmitters, has fundamental consequences; it impacts receptor function and physiology, offering a platform for the diversification of signalling processes, regulation, crosstalk, internalization, trafficking, and GPCR pharmacology [9].
Several classification systems have been developed to divide the GPCR superfamily into subclasses. According to sequence similarities, the A–F system (used here) splits GPCRs into six main classes, in which class A (rhodopsin-like receptors), class B (secretin/adhesion receptors), class C (glutamate receptors), and class F (frizzled receptors) are the main classes in the Eumetazoa [5,14–17]. Among these, class A comprises the majority of all known GPCRs [16,18]. Additionally, some lineage-specific GPCR classes have been identified, such as the nematode chemosensory receptors [19] or insect gustatory receptors (which is still under debate [20]).
While much information on GPCR signalling exists for model organisms and higher vertebrates, our knowledge of platyhelminth GPCRs is still fragmentary. This invertebrate phylum includes free-living and parasitic flatworms like blood flukes of the genus Schistosoma. Schistosomes cause schistosomiasis, an infectious disease with tremendous impact on human health and socioeconomic development worldwide. Schistosomiasis is endemic in 76 countries, mainly in the developing regions of Africa, Asia, and America with over 230 million infected people [21]. A vaccine is not available, and controlling schistosomiasis relies on a single drug, praziquantel. Due to the prospect of emerging resistance, there is an urgent need to find alternative treatment strategies [22–24]. Understanding the biology of this parasite is central to the identification of candidate genes and/or proteins, which may serve as new targets for drug or vaccine development.
Because egg production is essential for life cycle completion and for triggering the pathological consequences of schistosomiasis [25], the unique reproduction biology of schistosomes is of particular interest [26,27]. As an exception among trematodes, schistosomes have evolved separate sexes. Adult male and female worms live constantly paired, a prerequisite for the development of the female gonads [26,27]. Pairing-inexperienced females (sF) are sexually immature and possess stem cell–like precursor vitelline cells and a small ovary containing stem cell–like precursor oocytes, the oogonia. Upon pairing, differentiation processes are induced, leading to the maturation of the ovary and vitellarium that characterizes a sexually mature female (bF). In contrast with females, pairing-inexperienced males (sM) possess testes with differentiated spermatocytes and exhibit no morphological differences from pairing-experienced males (bM) [28–31]. Nevertheless, pairing also induces changes in male gene expression [32–34].
Sequencing of the S. mansoni genome provided the basis for a variety of in silico analyses [35,36]. Among others, bioinformatics unravelled GPCRs as the largest superfamily of transmembrane receptors, and all major subfamilies were represented, including a platyhelminth-specific rhodopsin subfamily [37,38]. Although these findings emphasize the importance of GPCR signalling in schistosomes, only a few GPCRs have been functionally characterized. Most of these respond to classical biogenic amines and neurotransmitters like dopamine, serotonin, histamine, and acetylcholine. Using RNA interference (RNAi) or pharmacological antagonism, GPCR functions were associated with muscular activity in larval or adult worms [39–42]. Only a few studies linked schistosome GPCRs to other functions such as gametogenesis and embryogenesis [43]. Nevertheless, the diversity of GPCR genes in S. mansoni suggests a broad spectrum of different functions, potentially including reproduction. This hypothesis is supported by studies of the planarian Schmidtea mediterranea in which neuropeptide GPCRs with key roles in reproductive development were identified [44].
An updated phylogenetic analysis of the S. mansoni GPCRome
An updated analysis of the S. mansoni GPCR complement confirmed many patterns originally deduced from the initial description of the genome [37]. There remain 115 putative GPCRs with three or more predicted transmembrane domains (TMs), two less than originally suggested. Importantly, each receptor included here is linked to a gene model validated by previous whole transcriptome RNA sequencing (RNA-seq) experiments [36], indicating remarkable congruence with the original analysis that at the time had very few expressed sequence tags (ESTs) available. Using the new gene models, we were able to more precisely annotate some of these genes (S1 Table). Specifically, we reduced the subset of class A GPCRs, added one receptor to both class B and class C, and maintained the original count of class F receptors. Two receptors (Smp_049330, Smp_170350) escaped classification into any of the GPCR classes [17], both of which contain a Lung_7-TM domain (pfam06814) and one of which shows similarity to GPR107, an intracellular signalling receptor that localizes to the trans-Golgi network [45].
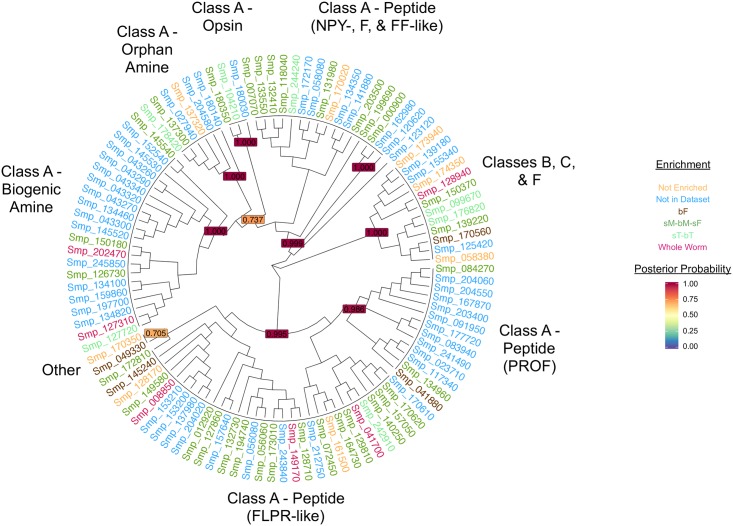
We analysed the phylogeny of 105 of these putative GPCRs, only including those that had more than four predicted TMs in order to infer the highest confidence topology (Fig 1). The tree is rooted between class A and classes B, C, and F. The topology mimics phylogenies inferred from other organisms, showing that the class A aminergic receptors, which include orphan amines, biogenic amines, and opsins, evolved from a common, peptide receptor-like ancestor [46]. The putative peptidergic receptors split into three highly supported clades—one containing receptors similar to Neuropeptide Y (NPY), Neuropeptide F (NPF), and Neuropeptide FF (NPFF) GPCRs, one containing receptors similar to FMRFamide-like Peptide GPCRs (FLPRs), and a flatworm-specific clade containing GPCRs originally designated the Platyhelminth-Specific Rhodopsin-like Orphan-Family (PROF). The Lung_7-TM domain receptors were found to be most nearly related to the FLPRs. The PROF family has so far defied annotation, though some have suggested it shows similarity to an ancient family of chemoreceptors, the nematode Srw family [19,44]. However, unlike the Caenorhabditis elegans Srw family, of which 90% are concentrated on the same chromosome [47], the PROF orthologs of S. mansoni are spread throughout the genome (S1 Table).
Fig 1. Phylogenetic analysis of S. mansoni GPCR genes.
A Bayesian tree of putative S. mansoni GPCRs was inferred with the software tool MrBayes3.2 [92]. The Tree is rooted between class A and classes B, C, F, and others. Broad subclassifications are indicated, each corresponding to a highly supported node. Gene IDs are coloured according to transcriptomic enrichment. bF, pairing-experienced (bisex) females; bM, pairing-experienced (bisex) males; bT, testes from bM; FLPR, FMRFamide-like Peptide GPCR; GPCR, G protein–coupled receptor; PROF, Platyhelminth-Specific Rhodopsin-like Orphan-Family; sF, pairing-inexperienced (single-sex) females; sM, pairing-inexperienced (single-sex) males; sT, testes from sM.
Transcriptomic data reveal new insights into GPCR function
Based on progress in organ isolation from schistosomes [43,48], a comparative RNA-seq analysis on paired versus unpaired S. mansoni and their gonads recently unravelled sex-, tissue-, and pairing-dependent transcription patterns [32]. These data revealed that approximately 60% of the GPCR genes were expressed in adult S. mansoni, covering all classes of the phylogenetic analyses. With respect to the complex life cycle of schistosomes, which includes different larval stages, it was expected that part of the GPCRome would not or only weakly be expressed in adults. Indeed, several missing GPCRs were linked to functions in the larval stages like the miracidium [49]. Additionally, transcriptome data obtained by a former RNA-seq study [36] indicate that most of the missing 47 GPCRs are less abundantly transcribed in adult worms compared with other life stages (S1 Fig). In addition, few GPCRs already functionally characterized in adults were also absent from the transcriptome data of Lu et al. [32] due to transcript levels below threshold. These included the amine receptors SmGPR-1 (Smp_043260), SmGPR-2 (Smp_043340), and SmGPR-3 (Smp_043290) that were shown to be expressed in the nervous system of adult worms [42,50,51]. In general, GPCRs are low-abundantly expressed in adult S. mansoni compared with other gene families, which is in accordance with findings from other organisms [52]. Focusing on GPCRs in adult S. mansoni, most exhibited sex-/pairing- and/or tissue-preferential transcription. These findings add to existing knowledge allowing the categorization of GPCRs into specific functional groups (Fig 2).
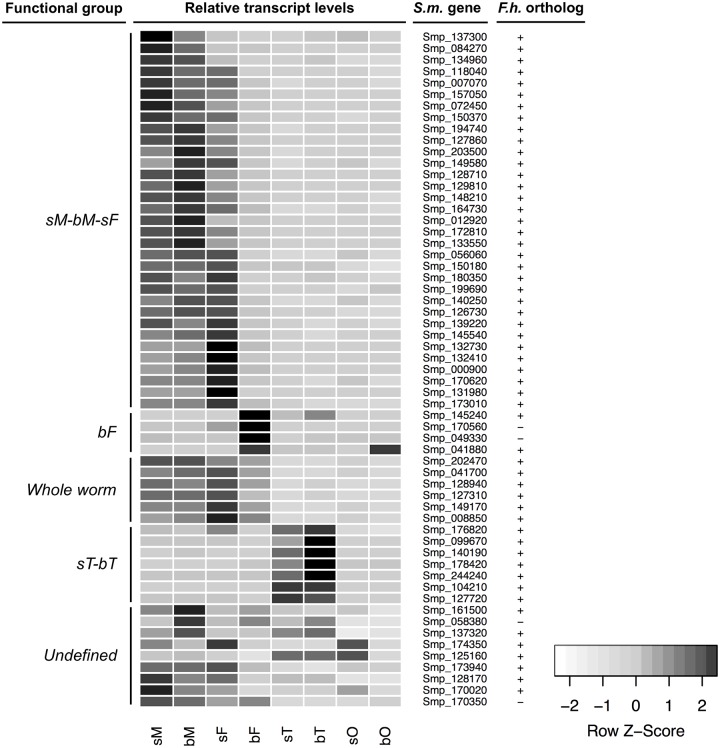
Fig 2. Hierarchical clustering of GPCR genes expressed in adult S. mansoni.
Shown are functional groups to which GPCRs were assigned according to their relative transcript levels in adult S. mansoni obtained by a recent RNA-seq study from Lu et al. [32]. The heat map shows relative gene expression in all samples, which was calculated by the Z-score method implemented in the heatmap.2 function of the gplots package. Darker color indicates higher expression across all samples, and lighter color indicates lower expression levels (Color Key). The sM-bM-sF group contains GPCR genes with a transcription bias towards males and pairing-inexperienced females, while the “whole worm” group displays a balanced transcription rate among both sexes independently of pairing. Instead of this, the bF and sT-bT groups contain GPCRs that are preferentially transcribed in reproductive organs, namely testis, ovary, or vitellarium. S.m. gene provides Smp numbers for the listed GPCRs of S. mansoni. Existing/missing orthologs in F. hepatica (F. h.) are indicated by +/- (see S1 Table for details). sM, pairing-inexperienced (single-sex) males; bM, pairing-experienced (bisex) males; sF, pairing-inexperienced (single-sex) females; bF, pairing-experienced (bisex) females; sT, testes from sM; bT, testes from bM; sO, ovaries from sF; bO, ovaries from bF.
Expression of GPCRs in nongonad tissues
Most GPCRs detected in the RNA-seq analysis were found to be mainly transcribed in nongonad tissues of both sexes. This included deorphanized neurotransmitter-activated GPCRs like the serotonin receptor Sm5THR (Smp_126730) that is expressed throughout the nervous system of adults and was shown to be involved in the control of muscle activity using RNAi [40]. The dopamine receptor SmD2 (Smp_127310) is expressed in the in subtegumental muscles and the muscular lining of the intestine [53]. SmGluR (Smp_128940) is phylogenetically related to metabotropic glutamate receptors and responsive to glutamate in ligand-binding assays. Immunolocalization revealed its expression throughout the nervous system of adult worms and along the female reproductive duct, including the oviduct, ootype, and uterus, whereas no expression was detected in the ovary and vitellarium [54]. Remarkably, for the majority of GPCRs expressed in nongonad tissue, a pairing-influenced transcript occurrence was observed, supporting recent data on the importance of neuronal processes in schistosome male–female interaction [32,33,55]. Moreover, because the transcript levels of most of these GPCRs showed a bias towards sM, bM, and sF (sM-bM-sF group; Fig 2), the data are in agreement with previous findings that the transcriptome of the sF seems to be more closely related to the male than to the bF [32]. These similarities in GPCR expression suggest that biological processes like locomotion or partner attraction have similar importance in sM, bM, and sF. Within this group, approximately 30% of the GPCRs dominate in one of the three subgroups (Fig 2), respectively. The GPCRs with the highest transcript levels in sF may contribute to processes in male attraction and/or repressing female maturation until pairing. In turn, highest transcript levels in sM suggest functions in locomotion and female attraction and/or perception. Those with the highest transcript levels in bM may indicate a higher need for neuronal processes associated with pairing and clasping processes and increased muscle activity for the successive female transport. Furthermore, the occurrence of GPCRs with sex-specific expression corresponds to findings from C. elegans and Drosophila melanogaster, for which it was shown that the nervous system regulates reproductive processes such as mating behavior, insemination, and fecundity in a sex-specific manner [56–62].
Expression of GPCRs in the reproductive organs
Surprisingly, only a minority of GPCRs detected in the RNA-seq analysis exhibited a gonad-preferential expression in S. mansoni. Upon pairing, the schistosome female streamlines its biology towards reproduction, which is also reflected by a remarkable shift in gene expression [32,34]. However, only four GPCRs, all so far functionally uncharacterized in S. mansoni, showed a significant transcription bias towards bF (Smp_049330, Smp_145240, Smp_170560, Smp_041880; bF group; Fig 2). This indicates an abundant expression in the vitellarium, which is also supported by an independent study exploring subtranscriptomes of the bF [63]. The vitellarium is the most prevalent tissue in bF, producing S4 vitellocytes for the synthesis of composite eggs [27]. One candidate of this group (Smp_041880) represents the only GPCR with a significant tissue-preferential transcript profile in the ovary. Smp_041880 codes for an ortholog of a potential allatostatin receptor recently highlighted as important for reproductive development in Schistosoma japonicum females [55]; allatostatin family neuropeptides are insect and crustacean hormones involved in the generation of juvenile hormone, feeding, and reproduction [64]. Nevertheless, due to our phylogenetic analysis, Smp_041880 is grouped into the PROF subfamily of class A GPCRs, suggesting that flatworm-specific molecules might serve as natural ligands of this receptor. The other members of the bF group were phylogenetically classified as FLPR (Smp_145240), class B (secretin/adhesion) GPCR (Smp_170560), a class known to be regulated by peptide hormones [65], or they escaped classification (Smp_049330).
Seven GPCRs exhibited a testis-biased transcription (sT-bT group; Fig 2), including two class-B (secretin/adhesion) GPCRs (Smp_176820, Smp_099670) and an opsin receptor (Smp_104210). The latter displays testis-specific expression, which appears to contrast with the established light and circadian rhythm–associated functions of opsins [66,67]. However, within the vertebrate opsin family are neuropsins, which have been localized in eye, brain, spinal cord, and testes, although their functions are unknown [68]. The remaining GPCRs of this group can be connected to amine (Smp_127720, Smp_178420) and neuropeptide signalling (Smp_242910, Smp_244240), most notable as neuropeptides play a conserved role in reproduction in different model organisms, including S. mediterranea [44,69] and D. melanogaster [70–72]. In S. mansoni, the transcription profile of neuropeptides points to their participation in sex-specific processes in adults [32]. Remarkably, four GPCRs of this group (sT-bT) show higher transcript levels upon pairing in the testis, supporting previous data showing that molecular changes occur in the gonads of males after pairing [32,33,43]. This may reflect an increased sperm production upon mating.
An outstanding role might be fulfilled by a frizzled ortholog (Smp_174350) that appears to be highly expressed in the ovary of sF. Previous studies revealed that Wnt proteins bind to receptors of the Frizzled family, and they are involved in the control of various types of stem cells, acting as niche factors maintaining stem cells in a self-renewing state [73]. Because immature oocytes, including stem cell–like oogonia, predominate in the ovary of sF [27,28], it is tempting to hypothesize that Smp_174350 may play a role in a Wnt signalling pathway important for the maintenance of the immature state of the oocytes. In agreement with this hypothesis, one of the dishevelled orthologs in S. mansoni (Smp_020300.1) revealed a similar transcript profile [74]; dishevelled proteins are involved in canonical and noncanonical Wnt pathways [75,76].
A glance at liver fluke GPCRs
Temperate (F. hepatica) and tropical (F. gigantica) liver fluke species have a profound impact on livestock animals globally [77,78] as well as represent a zoonotic threat to human health [79]. The recent publication of the F. hepatica genome [80] has facilitated comparative analyses of its GPCR complement, although tissue-specific expression data are not yet available for this species or other liver flukes. Comparisons of GPCR complements between the hermaphroditic liver fluke and dioecious schistosomes could allow interpretation of conserved and species-specific GPCR functions given the distinct biology of these trematode lineages. Using hidden Markov models (HMMs) trained against the originally published S. mansoni and S. mediterranea GPCR complements, 147 GPCRs were identified in the F. hepatica genome. These comprise 136 rhodopsin-like (class A), two adhesion (class B), and three metabotropic glutamate receptors (class C), with five frizzled and a single smoothened GPCR representing class F. Phylogenetically, the class A receptors comprise 40 aminergic receptors, two photo-activated opsins, and 94 putative peptide receptors. While many class A GPCRs have readily identifiable orthologs in model systems permitting the assignment of putative ligands, they also include groups lacking obvious nonflatworm orthologs that appear to be expanded within flatworm lineages. The latter includes six putative PROF1 receptors showing highest identities with existing PROF1s from S. mansoni, S. mediterranea, and Echinococcus multilocularis. At least two other clades appear to be expanded within the Echinostomatoidea lineage; phylogenetic reconstructions of these receptor clades suggest the absence of obvious nonflatworm orthologs (personal communication from P. McVeigh and A. Maule). A first comparison of both GPCRomes revealed that most schistosome GPCRs share orthologs with F. hepatica, while six GPCRs turned out to be S. mansoni-specific. Overall, 55 of the 59 GPCRs shown to be transcribed in adult schistosomes display homology to F. hepatica genes. According to transcriptome data available for different life stages of this parasite [80], most of those orthologs are transcribed in adult liver flukes as well.
Because there is a lack of tissue-specific analyses for F. hepatica, it seemed reasonable to take a closer look at GPCRs with a gonad-preferential transcription in S. mansoni. In total, nine of the eleven S. mansoni GPCRs categorized to the bF and sT-bT groups have orthologs in F. hepatica, but for only seven of them was transcription proven for the adult stage. While the detection of two testis-preferentially–transcribed GPCRs might have failed due to the absence of gonad-specific transcriptome data in liver flukes, both orthologs of the bF group are among the most abundantly transcribed GPCRs in adult F. hepatica and female S. mansoni. Because of the relevance of the vitellarium and the ovary in the egg-laying stages, this might indicate a common function of these GPCRs in both parasites. In combination with the transcription of at least five orthologs of the S. mansoni sT-bT group in adult liver flukes, these findings provide first evidence for conserved GPCR signalling pathways controlling gametogenesis and vitellogenesis in different parasitic flatworm species (Fig 2; S1 Table; S2 Fig).
GPCRs as potential drug targets
Besides transmitter-activated ion channels [81,82], GPCRs are a focus for research activities centered on helminth neurochemistry and the identification of druggable targets for the development of new anthelmintics [37,41,83–85]. Their role as privileged candidates originates from functional studies and drug-modelling approaches that have been well established for this receptor class. GPCRs reveal a large diversity among species and can bind distinct ligands, which allows the development of tailor-made compounds that reduce the possibility of host toxicity [86–91]. Among the candidate GPCRs in S. mansoni is the serotonergic GPCR (Sm5HTR; Smp_126730) [91], a member of the sM-bM-sF group, which is predominantly transcribed in males independent of pairing [32,74]. In bF, its transcription was found to be reduced by about 60% compared with sF. Through pharmacological profiling, new ligands and chemical series have been found, which are selective for Sm5HTR over Hs5HTR7, the closest human GPCR ortholog. The identified compounds, such as nuciferine, showed efficacy against adult worms and schistosomula in vitro as well as in vivo, and evidence was obtained that Sm5HTR was irreversibly inactivated [90,91]. These results demonstrate the potential for schistosome GPCRs as targets for new anthelmintics.
Conclusions
Schistosomes as well as other trematodes have a tremendous impact on global health and socioeconomic development. In the face of emerging resistance against commonly used therapeutics, alternative drug targets are needed to support the development of next-generation anthelmintics. To this end, basic research has the challenging task of connecting the improving genome data with biological functions to identify key pathways and molecules essential to parasite biology. With respect to their fundamental role throughout the Eumetazoa and their pharmacological importance, GPCRs represent promising candidate targets for parasite control. Phylogenetic analyses display their diversity in free-living and parasitic platyhelminths and suggest diverse functions in schistosomes. Whilst these receptors have been shown to play a role in neuronal processes and locomotion in adults and larval stages of S. mansoni, tissue-specific transcriptome analyses have provided new insights into their participation in its unique reproduction biology. These new resources not only support the identification of GPCRs with gonad-specific expression profiles but also reveal that the majority of GPCRs have a nongonad but pairing-dependent expression profile. While GPCRs of the first group might play roles in gametogenesis, vitellogenesis, and embryogenesis, the latter may contribute to the complex male–female interaction. Beyond this, orthologs of some of these GPCRs can be found in the genome of the liver fluke F. hepatica, opening the possibility of studying conserved GPCR signalling pathways in trematode reproduction as well as how GPCRs may differentially function in trematodes with divergent sexual characteristics. Although these preliminary findings need to be substantiated by functional studies in the future, the data support the selection of candidate receptors for basic and applied studies, invigorating the exploitation of this important receptor class for the discovery of drugs to control schistosomes and other trematodes.
Supporting information
GPCR, G protein–coupled receptor.
(XLSX)
GPCR, G protein–coupled receptor.
(PDF)
GPCR, G protein–coupled receptor.
(PDF)
Acknowledgments
This review article is dedicated to the memory of our wonderful and inspiring colleague Paula Ribeiro, who recently passed away. Paula was involved in the conceptual design of this article and part of the authors’ team before the health situation rendered finishing her section impossible.
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft (DFG), grant GR1549/7-3 (CGG), and the Wellcome Trust, grants 098051 (MB) and 107475/Z/15/Z (CGG, MB; FUGI). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chung KY. Structural Aspects of GPCR-G Protein Coupling. Toxicol Res. 2013;29: 149–155. doi: 10.5487/TR.2013.29.3.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miao Y, McCammon JA. G-protein coupled receptors: advances in simulation and drug discovery. Curr Opin Struct Biol. 2016;41: 83–89. doi: 10.1016/j.sbi.2016.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5: 993–996. doi: 10.1038/nrd2199 [DOI] [PubMed] [Google Scholar]
- 4.Bar-Shavit R, Maoz M, Kancharla A, Nag J, Agranovich D, Grisaru-Granovsky S, et al. G protein-coupled receptors in cancer. Int J Mol Sci. 2016;17: 1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luttrell LM. Reviews in molecular biology and biotechnology: transmembrane signaling by G protein-coupled receptors. Mol Biotechnol. 2008;39: 239–264. doi: 10.1007/s12033-008-9031-1 [DOI] [PubMed] [Google Scholar]
- 6.Lefkowitz RJ. Arrestins come of age. Prog Mol Biol Transl Sci. 2013. Pp. 3–18. [DOI] [PubMed] [Google Scholar]
- 7.Bologna Z, Teoh J-P, Bayoumi AS, Tang Y, Kim I-M. Biased G protein-coupled receptor signaling: new player in modulating physiology and pathology. Biomol Ther. 2017;25: 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gahbauer S, Böckmann RA. Membrane-mediated oligomerization of G protein coupled receptors and its implications for gpcr function. Front Physiol. 2016;7: 494 doi: 10.3389/fphys.2016.00494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farran B. An update on the physiological and therapeutic relevance of GPCR oligomers. Pharmacol Res. 2017;117: 303–327. doi: 10.1016/j.phrs.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 10.Woodside DG. Dancing with multiple partners. Sci STKE. 2002;2002: e14. [DOI] [PubMed] [Google Scholar]
- 11.El Zein N, D’Hondt S, Sariban E. Crosstalks between the receptors tyrosine kinase EGFR and trka and the GPCR, FPR, in human monocytes are essential for receptors-mediated cell activation. Cell Signal. 2010;22: 1437–1447. doi: 10.1016/j.cellsig.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 12.Pyne NJ, Pyne S. Receptor tyrosine kinase-G-protein-coupled receptor signalling platforms: out of the shadow? Trends Pharmacol Sci. 2011;32: 443–450. doi: 10.1016/j.tips.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 13.Cattaneo F, Guerra G, Parisi M, De Marinis M, Tafuri D, Cinelli M, et al. Cell-surface receptors transactivation mediated by g protein-coupled receptors. Int J Mol Sci. 2014;15: 19700–19728. doi: 10.3390/ijms151119700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolakowski LF Jr. GCRDb: a G-protein-coupled receptor database. Receptors Channels. 1994;2: 1–7. [PubMed] [Google Scholar]
- 15.Fredriksson R, Lagerström MC, Lundin L-G, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63: 1256–1272. doi: 10.1124/mol.63.6.1256 [DOI] [PubMed] [Google Scholar]
- 16.Krishnan A, Almén MS, Fredriksson R, Schiöth HB. The origin of GPCRs: identification of mammalian like Rhodopsin, Adhesion, Glutamate and Frizzled GPCRs in fungi. PLoS ONE. 2012;7: e29817 doi: 10.1371/journal.pone.0029817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isberg V, Mordalski S, Munk C, Rataj K, Harpsøe K, Hauser AS, et al. GPCRdb: an information system for G protein-coupled receptors. Nucleic Acids Res. 2016;45: 2936–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann L, Palczewski K. The G protein-coupled receptor rhodopsin: a historical perspective. Methods Mol Biol. 2015;1271: 3–18. doi: 10.1007/978-1-4939-2330-4_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnan A, Almén MS, Fredriksson R, Schiöth HB. Insights into the origin of nematode chemosensory GPCRs: putative orthologs of the Srw family are found across several phyla of protostomes. PLoS ONE. 2014;9: e93048 doi: 10.1371/journal.pone.0093048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nordstrom KJV, Sallman Almen M, Edstam MM, Fredriksson R, Schioth HB. Independent hhsearch, Needleman-Wunsch-based, and motif analyses reveal the overall hierarchy for most of the G protein-coupled receptor families. Mol Biol Evol. 2011;28: 2471–2480. doi: 10.1093/molbev/msr061 [DOI] [PubMed] [Google Scholar]
- 21.Colley DG, Bustinduy AL, Evan Secor W, King CH. Human schistosomiasis. Lancet. 2014;383: 2253–2264. doi: 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Wang L, Liang Y-S. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol Res. 2012;111: 1871–1877. doi: 10.1007/s00436-012-3151-z [DOI] [PubMed] [Google Scholar]
- 23.Cioli D, Pica-Mattoccia L, Basso A, Guidi A. Schistosomiasis control: praziquantel forever? Mol Biochem Parasitol. 2014;195: 23–29. doi: 10.1016/j.molbiopara.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 24.Tebeje BM, Harvie M, You H, Loukas A, McManus DP. Schistosomiasis vaccines: where do we stand? Parasit Vectors. 2016;9: 528 doi: 10.1186/s13071-016-1799-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olveda DU, Olveda RM, McManus DP, Cai P, Chau TNP, Lam AK, et al. The chronic enteropathogenic disease schistosomiasis. Int J Infect Dis. 2014;28: 193–203. doi: 10.1016/j.ijid.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 26.LoVerde PT. Presidential address. Sex and schistosomes: an interesting biological interplay with control implications. J Parasitol. 2002;88: 3–13. doi: 10.1645/0022-3395(2002)088[0003:PASASA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 27.Beckmann S, Quack T, Burmeister C, Buro C, Long T, Dissous C, et al. Schistosoma mansoni: signal transduction processes during the development of the reproductive organs. Parasitology. 2010;137: 497–520. doi: 10.1017/S0031182010000053 [DOI] [PubMed] [Google Scholar]
- 28.Popiel I, Basch PF. Reproductive development of female Schistosoma mansoni (Digenea: Schistosomatidae) following bisexual pairing of worms and worm segments. J Exp Zool. 1984;232: 141–150. doi: 10.1002/jez.1402320117 [DOI] [PubMed] [Google Scholar]
- 29.Kunz W. Schistosome male-female interaction: induction of germ-cell differentiation. Trends Parasitol. 2001;17: 227–231. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann KF. An historical and genomic view of schistosome conjugal biology with emphasis on sex-specific gene expression. Parasitology. 2004;128 Suppl 1: S11–22. [DOI] [PubMed] [Google Scholar]
- 31.Quack T, Beckmann S, Grevelding CG. Schistosomiasis and the molecular biology of the male-female interaction of S. mansoni. Berl Munch Tierarztl Wochenschr. 2006;119: 365–372. [PubMed] [Google Scholar]
- 32.Lu Z, Sessler F, Holroyd N, Hahnel S, Quack T, Berriman M, et al. Schistosome sex matters: a deep view into gonad-specific and pairing-dependent transcriptomes reveals a complex gender interplay. Sci Rep. 2016;6: 31150 doi: 10.1038/srep31150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leutner S, Oliveira KC, Rotter B, Beckmann S, Buro C, Hahnel S, et al. Combinatory microarray and supersage analyses identify pairing-dependently transcribed genes in Schistosoma mansoni males, including follistatin. PLoS Negl Trop Dis. 2013;7: e2532 doi: 10.1371/journal.pntd.0002532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzpatrick JM, Hoffmann KF. Dioecious Schistosoma mansoni express divergent gene repertoires regulated by pairing. Int J Parasitol. 2006;36: 1081–1089. doi: 10.1016/j.ijpara.2006.06.007 [DOI] [PubMed] [Google Scholar]
- 35.Berriman M, Haas BJ, LoVerde PT, Alan Wilson R, Dillon GP, Cerqueira GC, et al. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460: 352–358. doi: 10.1038/nature08160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Protasio AV, Tsai IJ, Babbage A, Nichol S, Hunt M, Aslett MA, et al. A systematically improved high quality genome and transcriptome of the human blood fluke Schistosoma mansoni. PLoS Negl Trop Dis. 2012;6: e1455 doi: 10.1371/journal.pntd.0001455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zamanian M, Kimber MJ, McVeigh P, Carlson SA, Maule AG, Day TA. The repertoire of G protein-coupled receptors in the human parasite Schistosoma mansoni and the model organism Schmidtea mediterranea. BMC Genomics. 2011;12: 596 doi: 10.1186/1471-2164-12-596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campos TDL, Young ND, Korhonen PK, Hall RS, Mangiola S, Lonie A, et al. Identification of G protein-coupled receptors in Schistosoma haematobium and S. mansoni by comparative genomics. Parasit Vectors. 2014;7: 242 doi: 10.1186/1756-3305-7-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macdonald K, Kimber MJ, Day TA, Ribeiro P. A constitutively active G protein-coupled acetylcholine receptor regulates motility of larval Schistosoma mansoni. Mol Biochem Parasitol. 2015;202: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patocka N, Sharma N, Rashid M, Ribeiro P. Serotonin signaling in Schistosoma mansoni: a serotonin-activated G protein-coupled receptor controls parasite movement. PLoS Pathog. 2014;10: e1003878 doi: 10.1371/journal.ppat.1003878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribeiro P, Gupta V, El-Sakkary N. Biogenic amines and the control of neuromuscular signaling in schistosomes. Invert Neurosci. 2012;12: 13–28. doi: 10.1007/s10158-012-0132-y [DOI] [PubMed] [Google Scholar]
- 42.El-Shehabi F, Taman A, Moali LS, El-Sakkary N, Ribeiro P. A novel G protein-coupled receptor of Schistosoma mansoni (smGPR-3) Is activated by dopamine and is widely expressed in the nervous system. PLoS Negl Trop Dis. 2012;6: e1523 doi: 10.1371/journal.pntd.0001523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hahnel S, Quack T, Parker-Manuel SJ, Lu Z, Vanderstraete M, Morel M, et al. Gonad RNA-specific qRT-PCR analyses identify genes with potential functions in schistosome reproduction such as SmFZ1 and SmFGFRs. Front Genet. 2014;5: 170 doi: 10.3389/fgene.2014.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saberi A, Jamal A, Beets I, Schoofs L, Newmark PA. GPCRs direct germline development and somatic gonad function in planarians. PLoS Biol. 2016;14: e1002457 doi: 10.1371/journal.pbio.1002457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tafesse FG, Guimaraes CP, Maruyama T, Carette JE, Lory S, Brummelkamp TR, et al. GPR107, a G-protein-coupled receptor essential for intoxication by Pseudomonas aeruginosa exotoxin A, localizes to the Golgi and is cleaved by furin. J Biol Chem. 2014;289: 24005–24018. doi: 10.1074/jbc.M114.589275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf S, Grünewald S. Sequence, structure and ligand binding evolution of rhodopsin-like G protein-coupled receptors: a crystal structure-based phylogenetic analysis. PLoS ONE. 2015;10: e0123533 doi: 10.1371/journal.pone.0123533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas JH, Robertson HM. The Caenorhabditis chemoreceptor gene families. BMC Biol. 2008;6: 42 doi: 10.1186/1741-7007-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hahnel S, Lu Z, Wilson RA, Grevelding CG, Quack T. Whole-organ isolation approach as a basis for tissue-specific analyses in Schistosoma mansoni. PLoS Negl Trop Dis. 2013;7: e2336 doi: 10.1371/journal.pntd.0002336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang D, Zhao M, Wang T, mcmanus DP, Cummins SF. GPCR and IR genes in Schistosoma mansoni miracidia. Parasit Vectors. 2016;9: 563 doi: 10.1186/s13071-016-1837-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El-Shehabi F, Vermeire JJ, Yoshino TP, Ribeiro P. Developmental expression analysis and immunolocalization of a biogenic amine receptor in Schistosoma mansoni. Exp Parasitol. 2009;122: 17–27. doi: 10.1016/j.exppara.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Shehabi F, Ribeiro P. Histamine signalling in Schistosoma mansoni: immunolocalisation and characterisation of a new histamine-responsive receptor (smGPR-2). Int J Parasitol. 2010;40: 1395–1406. doi: 10.1016/j.ijpara.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 52.Schiöth HB, Nordström KJV, Fredriksson R. Mining the gene repertoire and ESTs for G protein-coupled receptors with evolutionary perspective. Acta Physiol. 2007;190: 21–31. [DOI] [PubMed] [Google Scholar]
- 53.Taman A, Ribeiro P. Investigation of a dopamine receptor in Schistosoma mansoni: functional studies and immunolocalization. Mol Biochem Parasitol. 2009;168: 24–33. doi: 10.1016/j.molbiopara.2009.06.003 [DOI] [PubMed] [Google Scholar]
- 54.Taman A, Ribeiro P. Glutamate-mediated signaling in Schistosoma mansoni: a novel glutamate receptor is expressed in neurons and the female reproductive tract. Mol Biochem Parasitol. 2011;176: 42–50. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Yu Y, Shen H, Qing T, Zheng Y, Li Q, et al. Dynamic transcriptomes identify biogenic amines and insect-like hormonal regulation for mediating reproduction in Schistosoma japonicum. Nat Commun. 2017;8: 14693 doi: 10.1038/ncomms14693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barr M. Male mating behavior Wormbook. 2006; doi: 10.1895/wormbook.1.78.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Portman DS. Genetic control of sex differences in C. elegans neurobiology and behavior. Advances in Genetics. 2007;59: 1–37. doi: 10.1016/S0065-2660(07)59001-2 [DOI] [PubMed] [Google Scholar]
- 58.LeBoeuf B, Correa P, Jee C, García LR. Caenorhabditis elegans male sensory-motor neurons and dopaminergic support cells couple ejaculation and post-ejaculatory behaviors. Elife. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fagan KA, Portman DS. Sexual modulation of neural circuits and behavior in Caenorhabditis elegans. Semin Cell Dev Biol. 2014;33: 3–9. doi: 10.1016/j.semcdb.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferveur J-F. Drosophila female courtship and mating behaviors: sensory signals, genes, neural structures and evolution. Curr Opin Neurobiol. 2010;20: 764–769. doi: 10.1016/j.conb.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 61.Stowers L, Logan DW. Sexual dimorphism in olfactory signaling. Curr Opin Neurobiol. 2010;20: 770–775. doi: 10.1016/j.conb.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamamoto D, Sato K, Koganezawa M. Neuroethology of male courtship in Drosophila: from the gene to behavior. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2014;200: 251–264. doi: 10.1007/s00359-014-0891-5 [DOI] [PubMed] [Google Scholar]
- 63.Wang J, Collins JJ 3rd. Identification of new markers for the Schistosoma mansoni vitelline lineage. Int J Parasitol. 2016;46: 405–410. doi: 10.1016/j.ijpara.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stay B, Tobe SS. The role of allatostatins in juvenile hormone synthesis in insects and crustaceans. Annu Rev Entomol. 2007;52: 277–299. doi: 10.1146/annurev.ento.51.110104.151050 [DOI] [PubMed] [Google Scholar]
- 65.Hollenstein K, de Graaf C, Bortolato A, Wang M-W, Marshall FH, Stevens RC. Insights into the structure of class B GPCRs. Trends Pharmacol Sci. 2014;35: 12–22. doi: 10.1016/j.tips.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arendt D. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306: 869–871. doi: 10.1126/science.1099955 [DOI] [PubMed] [Google Scholar]
- 67.Williams DL. Light and the evolution of vision. Eye. 2016;30: 173–178. doi: 10.1038/eye.2015.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Terakita A. The opsins. Genome Biol. 2005;6: 213 doi: 10.1186/gb-2005-6-3-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collins JJ 3rd, Hou X, Romanova EV, Lambrus BG, Miller CM, Saberi A, et al. Genome-wide analyses reveal a role for peptide hormones in planarian germline development. PLoS Biol. 2010;8: e1000509 doi: 10.1371/journal.pbio.1000509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tayler TD, Pacheco DA, Hergarden AC, Murthy M, Anderson DJ. A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila. Proc Natl Acad Sci U S A. 2012;109: 20697–20702. doi: 10.1073/pnas.1218246109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim WJ, Jan LY, Jan YN. A PDF/NPF neuropeptide signaling circuitry of male Drosophila melanogaster controls rival-induced prolonged mating. Neuron. 2013;80: 1190–1205. doi: 10.1016/j.neuron.2013.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hussain A, Üçpunar HK, Zhang M, Loschek LF, Grunwald Kadow IC. Neuropeptides Modulate Female Chemosensory Processing upon Mating in Drosophila. PLoS Biol. 2016;14: e1002455 doi: 10.1371/journal.pbio.1002455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nusse R, Fuerer C, Ching W, Harnish K, Logan C, Zeng A, et al. Wnt signaling and stem cell control. Cold Spring Harb Symp Quant Biol. 2008;73: 59–66. doi: 10.1101/sqb.2008.73.035 [DOI] [PubMed] [Google Scholar]
- 74.Lu Z, Sessler F, Holroyd N, Hahnel S, Quack T, Berriman M, et al. A gene expression atlas of adult Schistosoma mansoni and their gonads. Sci Data 2017; 22,4: 170118 doi: 10.1038/sdata.2017.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katoh M, Katoh M. WNT Signaling Pathway and Stem Cell Signaling Network. Clin Cancer Res. 2007;13: 4042–4045. doi: 10.1158/1078-0432.CCR-06-2316 [DOI] [PubMed] [Google Scholar]
- 76.Gao C, Chen Y-G. Dishevelled: The hub of Wnt signaling. Cell Signal. 2010;22: 717–727. doi: 10.1016/j.cellsig.2009.11.021 [DOI] [PubMed] [Google Scholar]
- 77.Mehmood K, Zhang H, Sabir AJ, Abbas RZ, Ijaz M, Durrani AZ, et al. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microb Pathog. 2017;109: 253–262. doi: 10.1016/j.micpath.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 78.Cwiklinski K, O’Neill SM, Donnelly S, Dalton JP. A prospective view of animal and human Fasciolosis. Parasite Immunol. 2016;38: 558–568. doi: 10.1111/pim.12343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tolan RW. Fascioliasis due to Fasciola hepatica and Fasciola gigantica infection: an update on this “neglected” neglected tropical disease. Lab Med. 2011;42: 107–116. [Google Scholar]
- 80.Cwiklinski K, Dalton JP, Dufresne PJ, La Course J, Williams DJ, Hodgkinson J, et al. The Fasciola hepatica genome: gene duplication and polymorphism reveals adaptation to the host environment and the capacity for rapid evolution. Genome Biol. 2015;16: 71 doi: 10.1186/s13059-015-0632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holden-Dye L, Walker RJ. Anthelmintic drugs Wormbook. 2007; 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolstenholme AJ. Ion channels and receptor as targets for the control of parasitic nematodes. Int J Parasitol Drugs Drug Resist. 2011;1: 2–13. doi: 10.1016/j.ijpddr.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ribeiro P, Geary TG. Neuronal signaling in schistosomes: current status and prospects for post genomics. Can J Zool. 2010;88: 1–22. [Google Scholar]
- 84.Ribeiro P, El-Shehabi F, Patocka N. Classical transmitters and their receptors in flatworms. Parasitology. 2005;131 Suppl: S19–40. [DOI] [PubMed] [Google Scholar]
- 85.Komuniecki RW, Hobson RJ, Rex EB, Hapiak VM, Komuniecki PR. Biogenic amine receptors in parasitic nematodes: what can be learned from Caenorhabditis elegans? Mol Biochem Parasitol. 2004;137: 1–11. doi: 10.1016/j.molbiopara.2004.05.010 [DOI] [PubMed] [Google Scholar]
- 86.Rosano C, Ponassi M, Santolla MF, Pisano A, Felli L, Vivacqua A, et al. Macromolecular modelling and docking simulations for the discovery of selective GPER ligands. AAPS J. 2016;18: 41–46. doi: 10.1208/s12248-015-9844-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Couvineau A, Laburthe M. VPAC receptors: structure, molecular pharmacology and interaction with accessory proteins. Br J Pharmacol. 2012;166: 42–50. doi: 10.1111/j.1476-5381.2011.01676.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scholten DJ, Canals M, Maussang D, Roumen L, Smit MJ, Wijtmans M, et al. Pharmacological modulation of chemokine receptor function. Br J Pharmacol. 2012;165: 1617–1643. doi: 10.1111/j.1476-5381.2011.01551.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Michino M, Abola E, GPCR Dock 2008 participants, Brooks CL 3rd, Dixon JS, Moult J, et al. Community-wide assessment of GPCR structure modelling and ligand docking: GPCR Dock 2008. Nat Rev Drug Discov. 2009;8: 455–463. doi: 10.1038/nrd2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chan JD, Acharya S, Day TA, Marchant JS. Pharmacological profiling an abundantly expressed schistosome serotonergic GPCR identifies nuciferine as a potent antagonist. Int J Parasitol Drugs Drug Resist. 2016;6: 364–370. doi: 10.1016/j.ijpddr.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chan JD, McCorvy JD, Acharya S, Johns ME, Day TA, Roth BL, et al. A miniaturized screen of a Schistosoma mansoni serotonergic g protein-coupled receptor identifies novel classes of parasite-selective inhibitors. PLoS Pathog. 2016;12: e1005651 doi: 10.1371/journal.ppat.1005651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, et al. Mrbayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61: 539–542. doi: 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GPCR, G protein–coupled receptor.
(XLSX)
GPCR, G protein–coupled receptor.
(PDF)
GPCR, G protein–coupled receptor.
(PDF)