Abstract
Purpose of review
Cardiometabolic diseases increasingly afflict our aging, dysmetabolic citizenry. Complex signals regulating low density lipoprotein receptor-related protein (LRP) and frizzled protein family members – the plasma membrane receptors for the cadre of Wnt polypeptide morphogens – contribute to the control of cardiovascular homeostasis.
Recent findings
Both canonical (β-catenin-dependent) and noncanonical (β-catenin-independent) Wnt signaling programs control vascular smooth muscle cell (VSM) phenotypic modulation in cardiometabolic disease. LRP6 limits VSM proliferation, reduces arteriosclerotic transcriptional reprogramming, and preserves insulin sensitivity while LRP5 restrains foam cell formation. Adipose, skeletal muscle, macrophages and VSM have emerged as important sources of circulating Wnt ligands that are dynamically regulated during the prediabetes-diabetes transition with cardiometabolic consequences. Platelets release Dkk1, an LRP5/LRP6 inhibitor that induces endothelial inflammation and the prosclerotic endothelial-mesenchymal transition. By contrast, inhibitory secreted frizzled-related proteins shape the Wnt signaling milieu to limit myocardial inflammation with ischemia-reperfusion injury. VSM sclerostin, an inhibitor of canonical Wnt signaling in bone, restrains remodeling that predisposes to aneurysm formation, and is down-regulated in aneurysmal vessels by epigenetic methylation.
Summary
Components of the Wnt signaling cascade represent novel targets for pharmacological intervention in cardiometabolic disease. Conversely, strategies targeting the Wnt signaling cascade for other therapeutic purposes will have cardiovascular consequences that must be delineated to establish clinically useful pharmacokinetic – pharmacodynamic relationships.
Keywords: Wnt, Low density lipoprotein receptor-related protein, Sclerostin, Frizzled, Dickkopf
Introduction
1.0. Wnt signaling and lipoprotein receptor-related proteins: An overview
1.1. Lipoprotein receptor-related receptors, Wnt signaling and molecular genetics of cardiometabolic disease
Lipoprotein receptors facilitate diverse signaling and transport systems well beyond their namesake. Lipoprotein levels and ratios were identified as key predictors of cardiovascular disease as early as the 1950s, but it was unknown how these lipoproteins were regulated to convey cardiometabolic risk [1]. Early clues came from patients with familial hypercholesterolemia (FH), who exhibited early coronary atherosclerosis and elevated serum LDL cholesterol regardless of diet [2]. While normal fibroblasts bound and internalized LDL, shutting off cholesterol synthesis within the cell, fibroblasts isolated from FH homozygotes neither bound nor internalized LDL, and did not reduce cholesterol synthesis [3]. Elucidating the molecular genetics of FH in cardiometabolic disease revealed the fundamentals of receptor-mediated endocytosis [4].
Since the early studies of the LDL receptor (LDLR), many other related receptors that bind lipoproteins have been identified – and once again, their detailed study is yielding fundamental insights[5]. While investigation initially focused upon cholesterol transport, the roles of the LDL receptor-related proteins in embryonic development, Wnt signaling, and adult homeostasis have increasingly occupied centre stage. Low density lipoprotein receptor-related proteins (LRPs) are a group of transmembrane receptors involved in apolipoprotein binding, Wnt signaling, and human diseases [6, 7]. LRP - 5 and - 6 (LRP5 and LRP6) are two such receptors with specific patient mutations leading to metabolic dysregulation, elevated serum LDL and triglycerides (TGs), atherosclerosis, and high or low bone mass phenotypes [8–10]. LRP6 can form a complex with LDLR, facilitating receptor-mediated endocytosis of LDL particles [10, 11], and also directly bind apolipoprotein E-containing lipoproteins. However, LRP5 and LRP6 also heterodimerize with the Frizzled (Fzd) family of G protein-coupled receptors and bind Wnt ligands via 4 cysteine rich extracellular [12] to propagate canonical Wnt signals (see below) [13, 14]. While they share similar patterns of expression, significant sequence homology and substantial functional redundancy in development and adult bone, the Lrp5 and Lrp6 genes are not functionally identical [15–18]. Homozygous deletion of Lrp6 is embryonic lethal, while mice lacking Lrp5 survive through adulthood [19]. Although Wnt ligands provide important morphogenetic signals during embryonic development, more recent work has demonstrated significance during adult homeostasis [20].
Nineteen different Wnt ligands and 10 members of the Fzd family of transmembrane G-protein-coupled receptors have been identified in humans [21]. Wnt - bound Fzd receptors competitively interact with co-receptors such as LRP5, LRP6, ROR2 and receptor-like tyrosine kinase (RYK) – and downstream signals vary dependent upon these Wnt-regulated interactions [18, 22–24]. Wnt ligands have classically been categorized as canonical versus noncanonical based upon beta-catenin activation (see below), though this categorization has limitations (see below). The task of assigning specific functions to any one Wnt, Wnt receptor or co-receptor becomes daunting in the absence of genetic evidence [25]. However, human and murine molecular genetics has revealed a uniquely important role for LRP6 in cardiometabolic disease [26–28]. Wnt signaling exemplifies context dependence in biology, relevant to personalized medicine, with identical inputs eliciting opposing outputs in different cell types and/or metabolic contexts [29]. Combinatorial complexity is necessary for development and homeostasis, but stymies attempts to rigidly assign a Wnt “code” in simple terms with respect to specific ligands. Nevertheless, it is both historically and practically helpful to organize receptor signaling regulated by Wnts into canonical vs. noncanonical programs when considering the cardiometabolic actions of LRP6 and other Wnt modulators (Figure 1). We provide a highly abridged overview to orient the reader.
Figure 1. A highly abridged overview of canonical and noncanonical Wnt signaling.
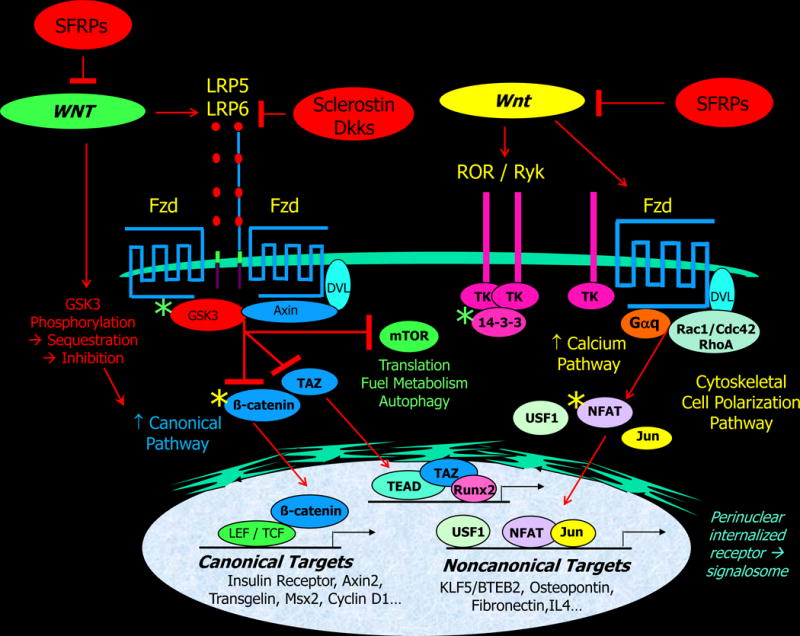
Frizzled (Fzd) Wnt receptors form ligand-dependent heterodimers with the LDL receptor related proteins LRP5 and LRP6 to convey canonical signals conveyed by nuclear β-catenin. Since GSK3 also inhibits Taz and mTOR, canonical Wnt activation is often associated with upregulation of these pathways. Inhibitory proteins of the sclerostin and Dickkopf (Dkk) families directly bind to LRP5/6. Other Wnt ligand-dependent Fzd receptor complexes signal via pathways that do not require LRPs and β-catenin; G proteins of the Rho/Rac/cdc42 and Gαq families, and intracellular calcium and cytoskeletal signals, utilize transcription factors of the NFAT, USF, and Jun/AP1 to convey these noncanonical signals. Dishevelled (Dvl) platform proteins contribute to both canonical and noncanonical programs. The ROR/Ryk family of receptor tyrosine kinases (TK) can signal within the Fzd cell polarity pathway or via 14-3-3 platform proteins. The secreted frizzled related proteins (SFRPs) bind Wnt ligands as faux (decoy) receptors, and inhibit both canonical and noncanonical signals. Green asterix, phosphorylation increased with Wnt signaling; yellow asterisk, phosphorylation decreased with Wnt signaling.
1.2. Canonical vs. noncanonical Wnt signals
Wnt ligands are polypeptides of approximately 350 residues, and are hydrophobic owing to post-translational serine fatty acid O-acylation with palmitoleic acid. Fatty acylation is essential for secretion, frizzled co-receptor binding and signaling [30–33]. Wnt ligands generate diverse signals in target cells, broadly categorized into canonical and noncanonical signaling modes. Canonical signaling denotes Wnt activation of the transcriptional co-activator, β-catenin, ultimately leading to the upregulation of target genes by the family of TCF/LEF (T cell factor/lymphoid enhancer factor) transcription factors [34]. However, β-catenin positively and negatively regulates a broad range of nuclear transcriptional responses. Under basal conditions, the kinase GSK3 phosphorylates β-catenin and fosters binding to the proteins Axin and APC, thereby assembling a β-catenin degradation complex [35, 36]. This state retains suppression of target genes by the LEF/TCF family of transcription factors and the transcriptional repressor, Groucho [21]. As canonical programs are activated, co-receptors LRP5/LRP6 and Fzd [37] recruit and polymerize Dishevelled signaling platform proteins on the Fzd receptor. This complex then directs GSK3 inhibition via phosphorylation and sequestration[38] which prevents β-catenin degradation [39, 40]. GSK3 sequestration liberates β-catenin, allowing its translocation to the nucleus (Figure 1). Nuclear β-catenin displaces Groucho repressors from target promoters, activating canonical genomic targets [37]. Since GSK3 also inhibits mTOR (mammalian target of rapamycin) activation [41] and TAZ (transcriptional co-activator with PDZ-binding motif) [42], activation of canonical Wnt signaling also upregulates these signaling pathways (Figure 1).
Noncanonical signaling encompasses the impressively broad range of β-catenin-independent responses elicited by Wnt ligands [43]. This category includes the planar cell polarity (PCP) pathway controlling cell orientation and cytoskeletal function, the Wnt/calcium pathway controlling calcium release from the endoplasmic reticulum, the Jun N-terminal kinase and mTOR signaling relays, and a few instances in which Wnt ligands have been shown to affect cells independent of β-catenin by other means [44–46] including the TAZ pathway mentioned above [47]. There is significant overlap and regulatory cross-talk between Wnt these signaling pathways [48].
The Wnt/calcium pathway is particularly relevant to adult metabolic and cardiovascular health [43, 49, 50], since calcium-regulated transcription factors control vascular smooth muscle (VSM) phenotypic modulation[51]. In this pathway, Wnt ligands engage Fzd co-receptor complexes without LRP5 or LRP6, leading to small G-protein activation, plasma membrane phospholipase-mediated liberation of inositol-1,4,5-trisphosphate, and calcium release from the endoplasmic reticulum [52–54]. Increased cytosolic calcium levels stimulate Ca2+/calmodulin dependent enzymes, including calmodulin-dependent protein kinase II (CamKII) and calcineurin [55]. The Ser/Thr phosphatase calcineurin dephosphorylates the cytoplasmic pool of nuclear factor associated with T-cells (NFAT), a family of transcription regulators controlling inflammation and immunity [56] and VSM gene expression [51]. De-phosphorylation enables nuclear import of NFAT and activation of its target genes [57]. Of note, calcium-dependent CamKII activation triggers a separate cascade that phosphorylates and inactivates TCF/LEF transcription [58]. Thus, CamKII reciprocally inhibits canonical β-catenin-LEF/TCF activation with the upregulation of noncanonical Wnt/calcium signals [59].
The ROR2/ROR1/Ryk family of receptor tyrosine kinases also bind Wnt ligands to form heterodimers with Fzd family members in the planar cell polarity pathway [48, 60], or alternatively homodimerize to elicit G-protein mediated cell migration [61] and other responses via tyrosine phosphorylation of 14-3-3 platforms[62] (Figure 1). However, Aaronson and colleagues highlighted that LRP5/6 and ROR/Ryk family members fundamentally compete for common Fzd co-receptors to support canonical vs. noncanonical signals, respectively [48]. Thus, LRP5 and LRP6 fine-tune the relative activation of canonical and noncanonical Wnt signals in a cell-autonomous fashion (Figure 2; and see below).
Figure 2. LRP6 finely tunes the balance of signals via canonical and noncanonical pathways in cardiovascular disease.
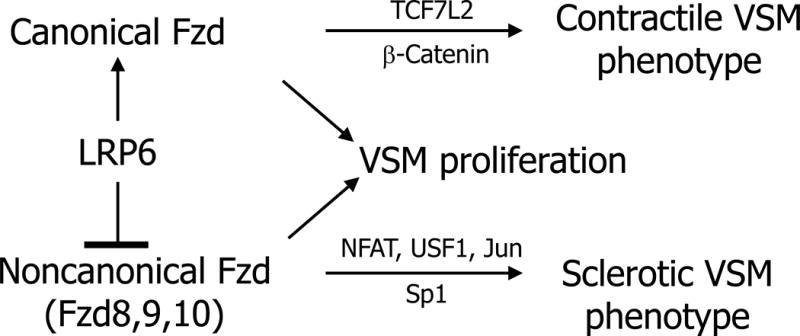
Mani and colleagues first identified hypomorphic LRP6 alleles exert dominant effects that convey precocious atherosclerosis with metabolic syndrome. Knockin LRP6 (R611C) mice and conditional knockout of LRP6 in VSM demonstrate that LRP6 stabilizes the VSM phenotype by inhibiting noncanonical Wnt signals. Fzd8 and Fzd9/Fzd10 appear to mediate osteofibrogenic signals. Canonical LRP6 signals also enhance insulin receptor expression and signaling in peripheral tissues and suppress hepatic lipoprotein biogenesis. See text Section 2 for discussion and details.
2.0. Wnt Signaling in Cardiometabolic Disease
2.1. Wnt signals as regulators of the dysmetabolic milieu driving cardiovascular disease
Given the evolutionary relationships between the LRPs and the LDL receptor, it is not surprising that the LRP/Wnt signaling cascade would play a role in metabolic homeostasis. In 2007, Mani and colleagues reported on a private mutation (rare and present in one family), LRP6 (R611C), that caused precocious coronary artery disease with metabolic syndrome and osteoporosis in a family of Iranian ancestry [28]. While this autosomal dominant variant does impair LDL cholesterol clearance, it also hampers canonical Wnt signaling required for TCF7L2-dependent transcriptional support of insulin receptor expression in peripheral tissues [63]. As a proof-of principle, Mani’s group went on to show that augmenting canonical Wnt signaling programs in LRP6 (611C/611C) homozygous mutant mice with recombinant Wnt3a injections reversed the combined dyslipidemia [64]. These studies revealed that hepatic de novo lipogenesis and apoB-containing lipoprotein secretion is held in check by canonical Wnt signals via LRP6 [64]. Since the insulin resistance and dyslipidemia of metabolic syndrome are clear contributors to cardiovascular disease risk [65], LRP6-dependent Wnt signaling tone globally mitigates cardiometabolic risk [28].
Likewise, LRP5 also plays a role in cardiometabolic risk. Badimon’s group demonstrated that LRP5 inhibits aortic macrophage infiltration and inflammatory cytokine production in mice fed diets that induce hypercholesterolemia [66]. The mechanism is not completely understood, but may relate to LRP5-dependent promotion of an anti-inflammatory macrophage phenotype [67].
Recently, Kozinski et al showed that high fat diabetogenic diets alter the ratio of circulating Wnt3a and Wnt4; specifically, upon progression to frank diabetes, Wnt3a levels fall while Wnt4 levels rise[68], eliciting a program that results in dysfunctional pancreatic islet cell function. While sources of circulating Wnts have yet to be robustly established in vivo, adipose and skeletal muscle[68] and VSM[27] can all produce Wnt4. In addition to inhibiting Wnt3a-induced pancreatic beta-cell insulin secretion[68], Wnt4 stimulates VSM proliferation and promote arterial intimal thickening[69] as relevant to the cardiovascular disease of diabesity. Importantly, in very recent data, George and colleagues have identified matricellular proteins of the CCN family (Cysteine-rich protein 61/Connective tissue growth factor/Nephroblastoma overexpressed gene) as down-stream mediators of Wnt-initiated VSM migration during neointima formation[70, 71].
2.2. Wnt signals as mediators of cardiovascular disease in response to the dysmetabolic milieu
LRP6 is also highly expressed in VSM, and recent studies have focused upon Wnt signaling in this cell type in cardiovascular disease[27, 72]. In addition to providing structure, ductility, and contractility, VSMs participate in local signal relays via endocrine/paracrine cues. VSM has prodigious capacity for phenotypic modulation[73]. Early descriptions focused upon contractile and synthetic phenotypes, the latter characterized by myofibroblast-like, high-level fibrillar collagen production. Subsequent studies established capacity for VSM osteochondral trans-differentiation that contributes to vascular calcification [73], and the ability to adopt macrophage-like foam cell phenotypes with cholesterol loading[74, 75]. More recently, Majesky et al showed that with de-differentiation VSM can generate adventitial Sca1+ vascular progenitors – a population that also has osteochondrogenic potential [76] – and the Yamanaka factor Klf4 is one important factor for this phenotypic plasticity [77]. Thus, inhibiting VSM plasticity with stabilization of the mature contractile phenotype holds great promise for mitigating arteriosclerosis of conduit vessels in response to dysmetabolic states.
VSM phenotypic modulation normally follows mechanical vessel injury and inflammation [78–80]. As a normal function of wound repair and innate immunity, Klf4 and NFAT are activated to promote phenotypic plasticity[81–83], losing features of contractile VSM and enabling trans-differentiation to resemble other cell types in the mesenchymal lineage [84, 85]. NFAT family members are Rel-domain transcription factors that dimerize with leucine zipper proteins and other Rel-domain proteins to control gene expression[86]. NFATs are critical components of noncanonical Wnt/calcium signals that regulate stem cell phenotypes [87] and VSM differentiation[51]. While the specific members of the NFAT family that mediate phenotypic switching in VSM have yet to be determined[51], NFATc1[88–91], NFATc3 and NFATc4[92] are likely to be important.
In studies of LRP6-deficient VSM, our laboratory identified that noncanonical Wnt signaling programs involving NFAT and USF1 were upregulated, activating arteriosclerotic osteogenic programs in vivo and in vitro [27]. This VSM-to-osteogenic phenotypic modulation was inhibited by a chemical antagonist that blocked cdc42/rac1 G-protein signaling downstream of Fzd10 but upstream of USF1 and NFAT activation by protein arginine methylation relays[27]. Mani’s group simultaneously identified that LRP6 (R611C) was hypomorphic for inhibition of VSM plasticity, arising in part due to enhanced Sp1 transcriptional activation of platelet-derived growth factor signaling[26]. Since (a) β-catenin/TCF7L2 interactions sustain the native, contractile VSM phenotype[26] including transgelin/SM22 expression[93]; and (b) noncanonical Wnt signaling inhibits beta-catenin actions[94], the data from our lab[27] converges with that of Mani’s [26] to establish the cell-autonomous role for LRP6 in maintaining the mature VSM phenotype via restraint of noncanonical programs (Figure 2). Of note, similar regulatory circuits likely function during development [97]. Thus, augmenting VSM noncanonical Wnt signals results in VSM plasticity, de-differentiation and an arteriosclerotic tissue phenotype [27] – thereby increasing myocardial workload, impairing Windkessel physiology necessary for smooth distal tissue perfusion and causing cardiovascular dysfunction[98, 99].
A number of inhibitors of Wnt signaling exist that function to shape cardiovascular health and disease. Secreted frizzled-related proteins (SFRPs) contain a cysteine-rich domain homologous to the Wnt binding sites of the membrane-bound Fzd G-protein coupled receptors [100]. Thus, SFRPs can limit Wnt signaling by binding and sequestering Wnt ligands as faux receptors, and inactivation of specific SFRP genes can enable constitutive Wnt actions [101–103]. This family of Wnt antagonists reduces endothelial and VSM cell proliferation in vitro and in vivo [104], with therapeutically relevant cardiovascular consequences. Overexpression of SFRP1 reduces myocardial infarction size in mice and improves cardiac function [105]. Similarly, SFRP5 inhibits myocardial inflammation and injury in a preclinical ischemia/reperfusion model[106]. Importantly, while SFRP family members can inhibit both canonical and noncanonical signaling, it appears that SFRP5 preferentially inhibits noncanonical signaling in proinflammatory cellular contexts [64, 107]. Of note, however, SFRP2 exhibits a more nuanced cardiovascular response, promoting myocardial stem cell survival and repair with ischemia[108], likely by shaping canonical [109] and planar cell polarity[110] signals that mitigate fibrosis[109, 111]. Additionally, during development, SFRP2 can bias noncanonical Wnt signaling via ROR2 in lieu of Fzd7 by stabilizing Wnt5a-ROR2 complexes[112]. By contrast, SFRP1 appears to be a pure antagonist[113]. A better understanding of how the SFRPs differentially shape the balance, duration, and extent of Wnt ligand is needed.
Two other types of Wnt signaling inhibitors, Dickkopf and sclerostin, antagonize function by binding to the LRP co-receptors. During development, Dickkopf and sclerostin family members play roles overlapping yet quite distinct from the SFRPs[114, 115]. The vertebrate Dickkopf (Dkk) proteins Dkk1, Dkk2, and Dkk4 are LRP5/LRP6 ligands that antagonize Wnt binding and activation of canonical programs[116]. Dkk1, Dkk2, Dkk3, and Dkk4 are canonical antagonists; however, Dkk3 can also indirectly activate canonical programs[117] and Dkk4 can indirectly activate noncanonical c-Jun signaling[118]. The precise molecular mechanisms whereby Dkk3 and Dkk4 elicits these latter surprising actions are as yet unknown, but may involve Dkk actions through their Kremen receptors that regulate LRP5/6 receptor trafficking[119]. Dkk1 is readily measurable in the circulation, is released by activated platelets [120] and induces secretion of inflammatory cytokines by adjacent endothelium[121]. As such, Dkk1 can promote a prosclerotic endothelial-mesenchymal transition[122]. Circulating levels of Dkk1 are elevated patients afflicted with acute ischemic stroke[123] and symptomatic aortic stenosis[124], and may ultimately prove to be a clinically useful biomarker.
Sclerostin is another canonical Wnt signaling inhibitor that binds LRP4, LRP5, and LRP6[125]. Production by osteocytes in bone participates in a negative feedback loop – a servo mechanism that restrains excessive canonical Wnt responses in the skeleton [125]. Unlike the Dkks, however, sclerostin does not appear to compete for Wnt ligand binding. Intriguingly, sclerostin is also expressed in aortic VSM[126], is upregulated during arteriosclerotic calcification[126], and limits vascular remodeling that predispose to aneurysm formation[127]. Epigenomic methylation of the sclerostin gene down-regulates its expression in those VSM residing within areas of human aortic aneurysms, and mice transgenic for sclerostin are resistant to angiotensin-induced aneurysm[127]. These latter data suggest local regulation of sclerostin actions with arterial remodeling, and are particularly important to consider since anti-sclerostin antibodies with prolonged pharmacokinetics are in development to treat osteoporosis[128, 129].
Oxidized LDL (oxLDL) upregulates Wnt ligands in multiple cell types including cells of the monocyte/macrophage lineage[130]. Recent data suggests that upregulation of Abca1 by Wnt5a in RAW264.7 myeloid cells following oxLDL treatment reduces lipid accumulation via enhanced reverse cholesterol transport[131]. Similarly, Boucher, Herz, and colleagues have shown that Wnt5a upregulates Abcg1 and inhibits 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase and synthetase in mouse embryonic fibroblasts, thereby limiting the intracellular accumulation of cholesterol[132]. Boucher has hypothesized that while Wnt5a induction may initially provide an adaptive mechanism to limit intracellular cholesterol accumulation downstream of LRP1, with time the increased Wnt5a tone may promote cardiovascular calcification[132, 133]. This intriguing notion adds to accumulating data indicating that atheroma formation (atherosis) and arterial sclerosis (fibrosis, calcification, arterial stiffness) must be independently assessed to fully capture the impact of Wnt signaling and its modulation in atherosclerotic disease[134–136].
3.0 The cardiometabolic opportunities and challenges for pharmacotherapies targeting the Wnt signaling cascade
As is evident, human and murine molecular genetics converge to indicate the important role for Wnt/LRP signaling in cardiometabolic health [26–28]. Wnt signaling plays a major role in the progression of heart disease, in terms of both metabolic alterations (insulin sensitivity) and cardiovascular remodeling and structural changes (fibrosis, sclerosis, atheroma formation, smooth muscle cell proliferation, hypertrophy) [137]. While there is great interest in identifying small-molecule modulators of Wnt signaling to treat multiple diseases, no compound has been identified with sufficient efficacy and specificity for use in humans [138–140]. However, a select few molecules have shown promise by targeting Wnt secretion or turnover of the β-catenin destruction complex. For example, GNF-6231 is an inhibitor of porcupine, the endoplasmic reticulum protein that is required for Wnt palmitoylation. GNF-6231 inhibits the secretion of both canonical and noncanonical Wnt ligands, and transient porcupine inhibitioin limits pro-fibrotic myocardial injury and enhances recovery in preclinical models of myocardial infarction[141]. Similarly, antagonists of tankyrase – a poly ADP-ribose polymerase that reduces the canonical pathway inhibitor Axin (Figure 1) – increase Axin levels, reduce canonical Wnt signaling and mechanical injury-induced neointima formation[142]. These data indicate that some novel small molecules in development for cancer therapies might be repurposed for certain cardiovascular diseases.
However, it becomes apparent that some of the most promising Wnt pathway modulators may biologicals – viz., recombinant proteins or neutralizing antibodies [143]. The SFRPs or engineered mimetics are particularly attractive, since they possess intrinsic capacities to productively shape canonical and noncanonical programs in response to complex ligand milieus[106, 110]. In certain settings, e.g. dilated cardiomyopathy [117], specific ligands such as Dkk3 with novel signaling profiles could hold therapeutic promise as well. However, the extent to which Dkk actions depend solely upon modulation of Wnt/LRP signaling versus actions via its Kremen receptors [116] has yet to be determined. Because Wnt ligands can heterodimerize to create unique biological responses[144], any therapies implementing recombinant Wnt proteins will ultimately have to consider this complexity – along with any untoward impact on occult malignancy. The extracellular domains of LRPs themselves are also biologically active, and enzymatic cleavage of LRP6 extracellular domain yields a constitutively active intracellular canonical signal [145, 146]. The released intracellular domain is constitutively active independent of Fzd co-receptors, binding to GSK3 in the destruction complex and relieving β-catenin inhibition [145]. On the cell surface, LRP6 extracellular domain inhibits noncanonical Wnt signaling [97], potentially forming complexes with Fzd proteins that preclude association with noncanonical coreceptors like ROR2. The soluble LRP extracellular domain also binds DKK1 and potentially mitigates its actions [147].
Antibodies directed against specific extracellular components of the Wnt regulatory cascade are in clinical development. Inhibitors of both Dkk1 and sclerostin have been developed to promote fracture repair and treat osteoporosis, respectively [148]. However, the prolonged pharmacokinetics afforded by inhibitory antibodies may exert unexpected and untoward responses given the dynamic, homeostatic interactions between Wnt activators and inhibitors. For example, prolonged exposure to Dkk1 stabilizes LRP6 protein accumulation until Kremen2 engages Dkk1[149], and continuous Dkk1 exposure can result in “rebound” canonical signals in vitro [150]. Conversely, disease-dependent anatomical differences in VSM sclerostin epigenetic silencing may significantly shape the arterial dose-response relationship to antibody-mediated sclerostin inhibition [127, 136]. The extent to which this occurs in vivo has yet to be established, but points to how either sustained activation or inhibition of Wnt signaling programs may elicit time-dependent yet mechanism-based “toxicities” due to poorly established pharmacokinetic-pharmacodynamic relationships. Thus, the cardiovascular consequences must be studied in detail for therapeutic approaches modulating Wnt signaling as pharmacotherapy for any indication – cancer, skeletal health, regenerative medicine, etc. – in addition to strategies targeting these pathways for cardiometabolic benefit.
4.0 Conclusion
Human and murine molecular genetics clearly identify the contributions of Wnt/LRP signaling to cardiometabolic health and homeostasis and cardiovascular disease. Successful modulation of Wnt pathways for any therapeutic indication will require a better understanding of how ligand-receptor complexes regulate context-dependent intracellular relays – including mediators other than β-catenin – within the cardiovascular system.
Summary.
Dysregulated Wnt signaling is a particularly damaging feature of cardiovascular inflammation, altering cellular plasticity, intracellular cholesterol accumulation, and osteofibrotic responses to metabolic or mechanical injury.
Human and murine molecular genetics have firmly established the important role of the Wnt co-receptor LRP6 in the biology of cardiometabolic health and disease.
Several components of the overall Wnt signaling cascade are attractive targets for therapeutic intervention, achieved either by small molecule inhibitors or by biologicals that mimic or modulate components of the extracellular regulatory machinery.
However, like most endocrine systems, Wnt signaling can be either deleterious or beneficial; this is dependent upon cell type, metabolic context, and stage-specific contributions of canonical vs. noncanonical relays to disease biology.
Thus, the pharmacokinetic-pharmacodynamic responses of any Wnt pathway therapeutic – including duration and intensity of modulation – must be carefully determined to optimize clinical benefits while mitigating mechanism-based deleterious responses.
Acknowledgments
Supported by NIH Grants HL069229 and HL114806 to D.A.T., and the Jean D. Wilson Center for Biomedical Research.
Financial support and sponsorship
This work was supported by NIH grants HL069220 and HL114806 to D.A.T., and Jean D. Wilson Center for Biomedical Research
Footnotes
Conflicts of interest
None.
References
- 1.Gordon T, Kannel WB, Castelli WP, Dawber TR. Lipoproteins, cardiovascular disease, and death: The framingham study. Archives of Internal Medicine. 1981;141:1128–1131. [PubMed] [Google Scholar]
- 2.Rader DJ, Cohen J, Hobbs HH. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. Journal of Clinical Investigation. 2003;111:1795–1803. doi: 10.1172/JCI18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein JL, Brown MS. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. The Journal of biological chemistry. 1974;249:5153–5162. [PubMed] [Google Scholar]
- 4.Brown MS, Goldstein JL. Receptor-mediated control of cholesterol metabolism. Science (New York, NY) 1976;191:150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- 5.Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annual review of biochemistry. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 6.Beisiegel U, Weber W, Ihrke G, et al. The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature. 1989;341:162–164. doi: 10.1038/341162a0. [DOI] [PubMed] [Google Scholar]
- 7.Etique N, Verzeaux L, Dedieu S, Emonard H. LRP-1: a checkpoint for the extracellular matrix proteolysis. BioMed research international. 2013;2013:152163. doi: 10.1155/2013/152163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams BO, Insogna KL. Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24:171–178. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keramati AR, Singh R, Lin A, et al. Wild-type LRP6 inhibits, whereas atherosclerosis-linked LRP6R611C increases PDGF-dependent vascular smooth muscle cell proliferation. Proceedings of the National Academy of Sciences. 2011;108:1914–1918. doi: 10.1073/pnas.1019443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye ZJ, Go GW, Singh R, et al. LRP6 protein regulates low density lipoprotein (LDL) receptor-mediated LDL uptake. The Journal of biological chemistry. 2012;287:1335–1344. doi: 10.1074/jbc.M111.295287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W, Mani S, Davis NR, et al. Mutation in EGFP Domain of LDL Receptor-Related Protein 6 Impairs Cellular LDL Clearance. Circulation Research. 2008;103:1280–1288. doi: 10.1161/CIRCRESAHA.108.183863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goel S, Chin EN, Fakhraldeen SA, et al. Both LRP5 and LRP6 receptors are required to respond to physiological Wnt ligands in mammary epithelial cells and fibroblasts. The Journal of biological chemistry. 2012;287:16454–16466. doi: 10.1074/jbc.M112.362137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schweizer L, Varmus H. Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors. BMC cell biology. 2003;4:4. doi: 10.1186/1471-2121-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Go G-W. Low-Density Lipoprotein Receptor-Related Protein 6 (LRP6) Is a Novel Nutritional Therapeutic Target for Hyperlipidemia, Non-Alcoholic Fatty Liver Disease, and Atherosclerosis. Nutrients. 2015;7:4453–4464. doi: 10.3390/nu7064453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joeng KS, Schumacher CA, Zylstra-Diegel CR, et al. Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Developmental biology. 2011;359:222–229. doi: 10.1016/j.ydbio.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riddle RC, Diegel CR, Leslie JM, et al. Lrp5 and Lrp6 Exert Overlapping Functions in Osteoblasts during Postnatal Bone Acquisition. PLOS ONE. 2013;8:e63323. doi: 10.1371/journal.pone.0063323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- 18.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 19.Pinson KI, Brennan J, Monkley S, et al. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 20.Nusse R. Wnt signaling in disease and in development. Cell research. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Science signaling. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- 23.Minami Y, Oishi I, Endo M, Nishita M. Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases. Developmental dynamics : an official publication of the American Association of Anatomists. 2010;239:1–15. doi: 10.1002/dvdy.21991. [DOI] [PubMed] [Google Scholar]
- 24.Fradkin LG, Dura JM, Noordermeer JN. Ryks: new partners for Wnts in the developing and regenerating nervous system. Trends in neurosciences. 2010;33:84–92. doi: 10.1016/j.tins.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 25.van Amerongen R, Fuerer C, Mizutani M, Nusse R. Wnt5a can both activate and repress Wnt/beta-catenin signaling during mouse embryonic development. Developmental biology. 2012;369:101–114. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **26.Srivastava R, Zhang J, Go GW, et al. Impaired LRP6-TCF7L2 Activity Enhances Smooth Muscle Cell Plasticity and Causes Coronary Artery Disease. Cell Rep. 2015;13:746–759. doi: 10.1016/j.celrep.2015.09.028. Demonstrates that vascular smooth muscle cell LRP6 restrains noncanonical Wnt signals that promote vascular smooth muscle cell proliferation and phenotypic plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **27.Cheng S-L, Ramachandran B, Behrmann A, et al. Vascular Smooth Muscle LRP6 Limits Arteriosclerotic Calcification in Diabetic LDLR−/− Mice by Restraining Noncanonical Wnt Signals. Circulation research. 2015;117:142–156. doi: 10.1161/CIRCRESAHA.117.306712. Demonstrates that vascular smooth muscle cell LRP6 restrains noncanonical Wnt signals that promote vascular calcification and arteriosclerotic vessel stiffening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mani A, Radhakrishnan J, Wang H, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science (New York, NY) 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niehrs C. The complex world of WNT receptor signalling. Nature reviews Molecular cell biology. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 30.Willert K, Brown JD, Danenberg E, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 31.Nusse R. Wnts and Hedgehogs: lipid-modified proteins and similarities in signaling mechanisms at the cell surface. Development. 2003;130:5297–5305. doi: 10.1242/dev.00821. [DOI] [PubMed] [Google Scholar]
- 32.Steinhauer J, Treisman JE. Lipid-modified morphogens: functions of fats. Current opinion in genetics & development. 2009;19:308–314. doi: 10.1016/j.gde.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janda CY, Waghray D, Levin AM, et al. Structural basis of Wnt recognition by Frizzled. Science (New York, NY) 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda H, Lyle S, Lazar AJF, et al. Human sebaceous tumors harbor inactivating mutations in LEF1. Nat Med. 2006;12:395–397. doi: 10.1038/nm1386. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda S, Kishida M, Matsuura Y, et al. GSK-3beta-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin. Oncogene. 2000;19:537–545. doi: 10.1038/sj.onc.1203359. [DOI] [PubMed] [Google Scholar]
- 36.Stamos JL, Weis WI. The β-Catenin Destruction Complex. Cold Spring Harbor Perspectives in Biology. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annual review of cell and developmental biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 38.Metcalfe C, Bienz M. Inhibition of GSK3 by Wnt signalling–two contrasting models. J Cell Sci. 2011;124:3537–3544. doi: 10.1242/jcs.091991. [DOI] [PubMed] [Google Scholar]
- 39.Schwarz-Romond T, Metcalfe C, Bienz M. Dynamic recruitment of axin by Dishevelled protein assemblies. Journal of Cell Science. 2007;120:2402–2412. doi: 10.1242/jcs.002956. [DOI] [PubMed] [Google Scholar]
- 40.Yanagawa S, van Leeuwen F, Wodarz A, et al. The dishevelled protein is modified by wingless signaling in Drosophila. Genes & development. 1995;9:1087–1097. doi: 10.1101/gad.9.9.1087. [DOI] [PubMed] [Google Scholar]
- 41.Vigneron F, Dos Santos P, Lemoine S, et al. GSK-3beta at the crossroads in the signalling of heart preconditioning: implication of mTOR and Wnt pathways. Cardiovasc Res. 2011;90:49–56. doi: 10.1093/cvr/cvr002. [DOI] [PubMed] [Google Scholar]
- 42.Azzolin L, Zanconato F, Bresolin S, et al. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 43.Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 44.Ho HY, Susman MW, Bikoff JB, et al. Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4044–4051. doi: 10.1073/pnas.1200421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oishi I, Suzuki H, Onishi N, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes to cells : devoted to molecular & cellular mechanisms. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Mlodzik M. Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt) Annual review of cell and developmental biology. 2015;31:623–646. doi: 10.1146/annurev-cellbio-100814-125315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park HW, Kim YC, Yu B, et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grumolato L, Liu G, Mong P, et al. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes & development. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hunter JJ, Chien KR. Signaling Pathways for Cardiac Hypertrophy and Failure. New England Journal of Medicine. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 50.Luo J, Chen J, Deng Z-L, et al. Wnt signaling and human diseases: what are the therapeutic implications? Lab Invest. 2007;87:97–103. doi: 10.1038/labinvest.3700509. [DOI] [PubMed] [Google Scholar]
- 51.Kudryavtseva O, Aalkjaer C, Matchkov VV. Vascular smooth muscle cell phenotype is defined by Ca2+-dependent transcription factors. FEBS J. 2013;280:5488–5499. doi: 10.1111/febs.12414. [DOI] [PubMed] [Google Scholar]
- 52.Green JL, Kuntz SG, Sternberg PW. Ror receptor tyrosine kinases: orphans no more. Trends in cell biology. 2008;18:536–544. doi: 10.1016/j.tcb.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. The Journal of biological chemistry. 2000;275:12701–12711. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- 54.Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- 55.Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 57.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 58.Ishitani T, Ninomiya-Tsuji J, Nagai S-I, et al. The TAK1-NLK-MAPK-related pathway antagonizes signalling between [beta]-catenin and transcription factor TCF. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- 59.De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta biochimica et biophysica Sinica. 2011;43:745–756. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- 60.Stricker S, Rauschenberger V, Schambony A. ROR-Family Receptor Tyrosine Kinases. Curr Top Dev Biol. 2017;123:105–142. doi: 10.1016/bs.ctdb.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Yu J, Chen L, Cui B, et al. Wnt5a induces ROR1/ROR2 heterooligomerization to enhance leukemia chemotaxis and proliferation. J Clin Invest. 2016;126:585–598. doi: 10.1172/JCI83535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, Ross JF, Bodine PV, Billiard J. Homodimerization of Ror2 tyrosine kinase receptor induces 14-3-3(beta) phosphorylation and promotes osteoblast differentiation and bone formation. Mol Endocrinol. 2007;21:3050–3061. doi: 10.1210/me.2007-0323. [DOI] [PubMed] [Google Scholar]
- 63.Singh R, De Aguiar RB, Naik S, et al. LRP6 enhances glucose metabolism by promoting TCF7L2-dependent insulin receptor expression and IGF receptor stabilization in humans. Cell Metab. 2013;17:197–209. doi: 10.1016/j.cmet.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Catalán V, Gómez-Ambrosi J, Rodríguez A, et al. Activation of Noncanonical Wnt Signaling Through WNT5A in Visceral Adipose Tissue of Obese Subjects Is Related to Inflammation. The Journal of Clinical Endocrinology & Metabolism. 2014;99:E1407–E1417. doi: 10.1210/jc.2014-1191. [DOI] [PubMed] [Google Scholar]
- 65.Wong ND, Nelson JC, Granston T, et al. Metabolic syndrome, diabetes, and incidence and progression of coronary calcium: the Multiethnic Study of Atherosclerosis study. JACC Cardiovasc Imaging. 2012;5:358–366. doi: 10.1016/j.jcmg.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *66.Borrell-Pages M, Romero JC, Badimon L. LRP5 deficiency down-regulates Wnt signalling and promotes aortic lipid infiltration in hypercholesterolaemic mice. J Cell Mol Med. 2015;19:770–777. doi: 10.1111/jcmm.12396. Delineates the important role for LRP5 in cells of the monocyte/macrophage lineage. Canonical signaling via LRP5 limits aortic lipid accumulation and leukocyte – mediated inflammation in mice challenged with cholesterol-rich diets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borrell-Pages M, Romero JC, Crespo J, et al. LRP5 associates with specific subsets of macrophages: Molecular and functional effects. J Mol Cell Cardiol. 2016;90:146–156. doi: 10.1016/j.yjmcc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- **68.Kozinski K, Jazurek M, Dobrzyn P, et al. Adipose- and muscle-derived Wnts trigger pancreatic beta-cell adaptation to systemic insulin resistance. Sci Rep. 2016;6:31553. doi: 10.1038/srep31553. Highlights the role for specific Wnt ligands as circulating endocrine cues relevant to the pathobiology of metabolic syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsaousi A, Williams H, Lyon CA, et al. Wnt4/beta-catenin signaling induces VSMC proliferation and is associated with intimal thickening. Circ Res. 2011;108:427–436. doi: 10.1161/CIRCRESAHA.110.233999. [DOI] [PubMed] [Google Scholar]
- **70.Williams H, Mill CA, Monk BA, et al. Wnt2 and WISP-1/CCN4 Induce Intimal Thickening via Promotion of Smooth Muscle Cell Migration. Arterioscler Thromb Vasc Biol. 2016;36:1417–1424. doi: 10.1161/ATVBAHA.116.307626. Establishes the role of CCN matricellular proteins as key mediators of Wnt-induced neointima formation. [DOI] [PubMed] [Google Scholar]
- 71.Mill C, Monk BA, Williams H, et al. Wnt5a-induced Wnt1-inducible secreted protein-1 suppresses vascular smooth muscle cell apoptosis induced by oxidative stress. Arterioscler Thromb Vasc Biol. 2014;34:2449–2456. doi: 10.1161/ATVBAHA.114.303922. [DOI] [PubMed] [Google Scholar]
- 72.Srivastava R, Zhang J, Go G-W, et al. Impaired LRP6 TCF7L 2 Activity Enhances Smooth Muscle Cell Plasticity and Causes Coronary Artery Disease. Cell Reports. 13:746–759. doi: 10.1016/j.celrep.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nguyen AT, Gomez D, Bell RD, et al. Smooth muscle cell plasticity: fact or fiction? Circ Res. 2013;112:17–22. doi: 10.1161/CIRCRESAHA.112.281048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feil S, Fehrenbacher B, Lukowski R, et al. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 75.Vengrenyuk Y, Nishi H, Long X, et al. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol. 2015;35:535–546. doi: 10.1161/ATVBAHA.114.304029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Majesky MW, Horita H, Ostriker A, et al. Differentiated Smooth Muscle Cells Generate a Subpopulation of Resident Vascular Progenitor Cells in the Adventitia Regulated by Klf4. Circ Res. 2017;120:296–311. doi: 10.1161/CIRCRESAHA.116.309322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.King KE, Iyemere VP, Weissberg PL, Shanahan CM. Kruppel-like factor 4 (KLF4/GKLF) is a target of bone morphogenetic proteins and transforming growth factor beta 1 in the regulation of vascular smooth muscle cell phenotype. The Journal of biological chemistry. 2003;278:11661–11669. doi: 10.1074/jbc.M211337200. [DOI] [PubMed] [Google Scholar]
- 78.Kershaw EE, Flier JS. Adipose Tissue as an Endocrine Organ. The Journal of Clinical Endocrinology & Metabolism. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 79.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological reviews. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 80.Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annual review of physiology. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- 81.Lee MY, Garvey SM, Baras AS, et al. Integrative genomics identifies DSCR1 (RCAN1) as a novel NFAT-dependent mediator of phenotypic modulation in vascular smooth muscle cells. Hum Mol Genet. 2010;19:468–479. doi: 10.1093/hmg/ddp511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shiny A, Regin B, Mohan V, Balasubramanyam M. Coordinated augmentation of NFAT and NOD signaling mediates proliferative VSMC phenotype switch under hyperinsulinemia. Atherosclerosis. 2016;246:257–266. doi: 10.1016/j.atherosclerosis.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Lighthouse JK, Small EM. Transcriptional control of cardiac fibroblast plasticity. J Mol Cell Cardiol. 2016;91:52–60. doi: 10.1016/j.yjmcc.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iyemere VP, Proudfoot D, Weissberg PL, Shanahan CM. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. Journal of Internal Medicine. 2006;260:192–210. doi: 10.1111/j.1365-2796.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- 85.Shanahan CM, Cary NR, Salisbury JR, et al. Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 86.Muller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10:645–656. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- 87.Sugimura R, Li L. Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth Defects Research Part C: Embryo Today: Reviews. 2010;90:243–256. doi: 10.1002/bdrc.20195. [DOI] [PubMed] [Google Scholar]
- 88.Karpurapu M, Wang D, Singh NK, et al. NFATc1 targets cyclin A in the regulation of vascular smooth muscle cell multiplication during restenosis. The Journal of biological chemistry. 2008;283:26577–26590. doi: 10.1074/jbc.M800423200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kundumani-Sridharan V, Singh NK, Kumar S, et al. Nuclear factor of activated T cells c1 mediates p21-activated kinase 1 activation in the modulation of chemokine-induced human aortic smooth muscle cell F-actin stress fiber formation, migration, and proliferation and injury-induced vascular wall remodeling. The Journal of biological chemistry. 2013;288:22150–22162. doi: 10.1074/jbc.M113.454082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kundumani-Sridharan V, Van Quyen D, Subramani J, et al. Novel interactions between NFATc1 (Nuclear Factor of Activated T cells c1) and STAT-3 (Signal Transducer and Activator of Transcription-3) mediate G protein-coupled receptor agonist, thrombin-induced biphasic expression of cyclin D1, with first phase influencing cell migration and second phase directing cell proliferation. The Journal of biological chemistry. 2012;287:22463–22482. doi: 10.1074/jbc.M112.362996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh NK, Kundumani-Sridharan V, Kumar S, et al. Protein kinase N1 is a novel substrate of NFATc1-mediated cyclin D1-CDK6 activity and modulates vascular smooth muscle cell division and migration leading to inward blood vessel wall remodeling. The Journal of biological chemistry. 2012;287:36291–36304. doi: 10.1074/jbc.M112.361220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Graef IA, Chen F, Chen L, et al. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105:863–875. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 93.Shafer SL, Towler DA. Transcriptional regulation of SM22alpha by Wnt3a: convergence with TGFbeta(1)/Smad signaling at a novel regulatory element. J Mol Cell Cardiol. 2009;46:621–635. doi: 10.1016/j.yjmcc.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ishitani T, Kishida S, Hyodo-Miura J, et al. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23:131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beljaars L, Daliri S, Dijkhuizen C, et al. WNT-5A regulates TGF-beta-related activities in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2017;312:G219–G227. doi: 10.1152/ajpgi.00160.2016. [DOI] [PubMed] [Google Scholar]
- 96.Spanjer AI, Baarsma HA, Oostenbrink LM, et al. TGF-beta-induced profibrotic signaling is regulated in part by the WNT receptor Frizzled-8. FASEB J. 2016;30:1823–1835. doi: 10.1096/fj.201500129. [DOI] [PubMed] [Google Scholar]
- 97.Bryja V, Andersson ER, Schambony A, et al. The extracellular domain of Lrp5/6 inhibits noncanonical Wnt signaling in vivo. Molecular biology of the cell. 2009;20:924–936. doi: 10.1091/mbc.E08-07-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fok H, Cruickshank JK. Future Treatment of Hypertension: Shifting the Focus from Blood Pressure Lowering to Arterial Stiffness Modulation? Curr Hypertens Rep. 2015;17:67. doi: 10.1007/s11906-015-0569-6. [DOI] [PubMed] [Google Scholar]
- 99.Westerhof N, Lankhaar JW, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput. 2009;47:131–141. doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 100.Ladher RK, Church VL, Allen S, et al. Cloning and Expression of the Wnt Antagonists Sfrp-2 and Frzb during Chick Development. Developmental biology. 2000;218:183–198. doi: 10.1006/dbio.1999.9586. [DOI] [PubMed] [Google Scholar]
- 101.He J, Sheng T, Stelter AA, et al. Suppressing Wnt signaling by the hedgehog pathway through sFRP-1. The Journal of biological chemistry. 2006;281:35598–35602. doi: 10.1074/jbc.C600200200. [DOI] [PubMed] [Google Scholar]
- 102.Suzuki H, Watkins DN, Jair KW, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nature genetics. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 103.Oshima T, Abe M, Asano J, et al. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106:3160–3165. doi: 10.1182/blood-2004-12-4940. [DOI] [PubMed] [Google Scholar]
- 104.Ezan J, Leroux L, Barandon L, et al. FrzA/sFRP-1, a secreted antagonist of the Wnt-Frizzled pathway, controls vascular cell proliferation in vitro and in vivo. Cardiovascular Research. 2004;63:731–738. doi: 10.1016/j.cardiores.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 105.Barandon L, Couffinhal T, Ezan J, et al. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation. 2003;108:2282–2289. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
- 106.Nakamura K, Sano S, Fuster JJ, et al. Secreted Frizzled-related Protein 5 Diminishes Cardiac Inflammation and Protects the Heart from Ischemia/Reperfusion Injury. The Journal of biological chemistry. 2016;291:2566–2575. doi: 10.1074/jbc.M115.693937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kongkham PN, Northcott PA, Croul SE, et al. The SFRP family of WNT inhibitors function as novel tumor suppressor genes epigenetically silenced in medulloblastoma. Oncogene. 2010;29:3017–3024. doi: 10.1038/onc.2010.32. [DOI] [PubMed] [Google Scholar]
- 108.Mirotsou M, Zhang Z, Deb A, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin H, Angeli M, Chung KJ, et al. sFRP2 activates Wnt/beta-catenin signaling in cardiac fibroblasts: differential roles in cell growth, energy metabolism, and extracellular matrix remodeling. Am J Physiol Cell Physiol. 2016;311:C710–C719. doi: 10.1152/ajpcell.00137.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schmeckpeper J, Verma A, Yin L, et al. Inhibition of Wnt6 by Sfrp2 regulates adult cardiac progenitor cell differentiation by differential modulation of Wnt pathways. J Mol Cell Cardiol. 2015;85:215–225. doi: 10.1016/j.yjmcc.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mastri M, Shah Z, Hsieh K, et al. Secreted Frizzled-related protein 2 as a target in antifibrotic therapeutic intervention. Am J Physiol Cell Physiol. 2014;306:C531–539. doi: 10.1152/ajpcell.00238.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brinkmann EM, Mattes B, Kumar R, et al. Secreted Frizzled-related Protein 2 (sFRP2) Redirects Non-canonical Wnt Signaling from Fz7 to Ror2 during Vertebrate Gastrulation. The Journal of biological chemistry. 2016;291:13730–13742. doi: 10.1074/jbc.M116.733766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Billiard J, Way DS, Seestaller-Wehr LM, et al. The orphan receptor tyrosine kinase Ror2 modulates canonical Wnt signaling in osteoblastic cells. Mol Endocrinol. 2005;19:90–101. doi: 10.1210/me.2004-0153. [DOI] [PubMed] [Google Scholar]
- 114.Satoh W, Matsuyama M, Takemura H, et al. Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/beta-catenin and the planar cell polarity pathways during early trunk formation in mouse. Genesis. 2008;46:92–103. doi: 10.1002/dvg.20369. [DOI] [PubMed] [Google Scholar]
- 115.Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 116.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 117.Lu D, Bao D, Dong W, et al. Dkk3 prevents familial dilated cardiomyopathy development through Wnt pathway. Lab Invest. 2016;96:239–248. doi: 10.1038/labinvest.2015.145. [DOI] [PubMed] [Google Scholar]
- 118.Hirata H, Hinoda Y, Majid S, et al. DICKKOPF-4 activates the noncanonical c-Jun-NH2 kinase signaling pathway while inhibiting the Wnt-canonical pathway in human renal cell carcinoma. Cancer. 2011;117:1649–1660. doi: 10.1002/cncr.25666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Feng Q, Gao N. Keeping Wnt signalosome in check by vesicular traffic. J Cell Physiol. 2015;230:1170–1180. doi: 10.1002/jcp.24853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Voorzanger-Rousselot N, Goehrig D, Facon T, et al. Platelet is a major contributor to circulating levels of Dickkopf-1: clinical implications in patients with multiple myeloma. Br J Haematol. 2009;145:264–266. doi: 10.1111/j.1365-2141.2009.07587.x. [DOI] [PubMed] [Google Scholar]
- 121.Ueland T, Otterdal K, Lekva T, et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1228–1234. doi: 10.1161/ATVBAHA.109.189761. [DOI] [PubMed] [Google Scholar]
- 122.Cheng SL, Shao JS, Behrmann A, et al. Dkk1 and MSX2-Wnt7b signaling reciprocally regulate the endothelial-mesenchymal transition in aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1679–1689. doi: 10.1161/ATVBAHA.113.300647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seifert-Held T, Pekar T, Gattringer T, et al. Circulating Dickkopf-1 in acute ischemic stroke and clinically stable cerebrovascular disease. Atherosclerosis. 2011;218:233–237. doi: 10.1016/j.atherosclerosis.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 124.Askevold ET, Gullestad L, Aakhus S, et al. Secreted Wnt modulators in symptomatic aortic stenosis. J Am Heart Assoc. 2012;1:e002261. doi: 10.1161/JAHA.112.002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burgers TA, Williams BO. Regulation of Wnt/beta-catenin signaling within and from osteocytes. Bone. 2013;54:244–249. doi: 10.1016/j.bone.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shao JS, Cheng SL, Pingsterhaus JM, et al. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ***127.Krishna SM, Seto SW, Jose RJ, et al. Wnt Signaling Pathway Inhibitor Sclerostin Inhibits Angiotensin II-Induced Aortic Aneurysm and Atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37:553–566. doi: 10.1161/ATVBAHA.116.308723. Demonstrates the important role for sclerostin, an inhibitory ligand for LRP5 and LRP6, in limiting aortic aneurysmal remodeling and dilatation. In addition to evidence obtained using murine disease models, this manuscript identifies that vascular smooth muscle sclerostic expression is down-regulated in human aortic aneurysm via epigenetic gene silencing. [DOI] [PubMed] [Google Scholar]
- 128.Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med. 2016;375:1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 129.Keaveny TM, Crittenden DB, Bolognese MA, et al. Greater Gains in Spine and Hip Strength for Romosozumab Compared to Teriparatide in Postmenopausal Women With Low Bone Mass. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017 doi: 10.1002/jbmr.3176. [DOI] [PubMed] [Google Scholar]
- 130.Bhatt PM, Lewis CJ, House DL, et al. Increased Wnt5a mRNA Expression in Advanced Atherosclerotic Lesions, and Oxidized LDL Treated Human Monocyte-Derived Macrophages. Open Circ Vasc J. 2012;5:1–7. doi: 10.2174/1877382601205010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Qin L, Hu R, Zhu N, et al. The novel role and underlying mechanism of Wnt5a in regulating cellular cholesterol accumulation. Clin Exp Pharmacol Physiol. 2014;41:671–678. doi: 10.1111/1440-1681.12258. [DOI] [PubMed] [Google Scholar]
- 132.El Asmar Z, Terrand J, Jenty M, et al. Convergent Signaling Pathways Controlled by LRP1 (Receptor-related Protein 1) Cytoplasmic and Extracellular Domains Limit Cellular Cholesterol Accumulation. The Journal of biological chemistry. 2016;291:5116–5127. doi: 10.1074/jbc.M116.714485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Woldt E, Terrand J, Mlih M, et al. The nuclear hormone receptor PPARgamma counteracts vascular calcification by inhibiting Wnt5a signalling in vascular smooth muscle cells. Nat Commun. 2012;3:1077. doi: 10.1038/ncomms2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stabley JN, Towler DA. Arterial Calcification in Diabetes Mellitus: Preclinical Models and Translational Implications. Arterioscler Thromb Vasc Biol. 2017;37:205–217. doi: 10.1161/ATVBAHA.116.306258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Towler DA. Commonalities Between Vasculature and Bone: An Osseocentric View of Arteriosclerosis. Circulation. 2017;135:320–322. doi: 10.1161/CIRCULATIONAHA.116.022562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Towler DA. “Osteotropic” Wnt/LRP Signals: High-Wire Artists in a Balancing Act Regulating Aortic Structure and Function. Arterioscler Thromb Vasc Biol. 2017;37:392–395. doi: 10.1161/ATVBAHA.116.308915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hermans KC, Blankesteijn WM. Wnt Signaling in Cardiac Disease. Comprehensive Physiology. 2015;5:1183–1209. doi: 10.1002/cphy.c140060. [DOI] [PubMed] [Google Scholar]
- 138.Willems E, Spiering S, Davidovics H, et al. Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circ Res. 2011;109:360–364. doi: 10.1161/CIRCRESAHA.111.249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Q, Major MB, Takanashi S, et al. Small-molecule synergist of the Wnt/beta-catenin signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7444–7448. doi: 10.1073/pnas.0702136104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saraswati S, Alfaro MP, Thorne CA, et al. Pyrvinium, a Potent Small Molecule Wnt Inhibitor, Promotes Wound Repair and Post-MI Cardiac Remodeling. PLOS ONE. 2010;5:e15521. doi: 10.1371/journal.pone.0015521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bastakoty D, Saraswati S, Joshi P, et al. Temporary, Systemic Inhibition of the WNT/beta-Catenin Pathway promotes Regenerative Cardiac Repair following Myocardial Infarct. Cell Stem Cells Regen Med. 2016;2 doi: 10.16966/2472-6990.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen L, Zhuang J, Singh S, et al. XAV939 Inhibits Intima Formation by Decreasing Vascular Smooth Muscle Cell Proliferation and Migration Through Blocking Wnt Signaling. J Cardiovasc Pharmacol. 2016;68:414–424. doi: 10.1097/FJC.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 143.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 144.Cha SW, Tadjuidje E, White J, et al. Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr Biol. 2009;19:1573–1580. doi: 10.1016/j.cub.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 145.Mi K, Johnson GVW. Regulated proteolytic processing of LRP6 results in release of its intracellular domain. Journal of Neurochemistry. 2007;101:517–529. doi: 10.1111/j.1471-4159.2007.04447.x. [DOI] [PubMed] [Google Scholar]
- 146.Brennan K, Gonzalez-Sancho JM, Castelo-Soccio LA, et al. Truncated mutants of the putative Wnt receptor LRP6/Arrow can stabilize beta-catenin independently of Frizzled proteins. Oncogene. 2004;23:4873–4884. doi: 10.1038/sj.onc.1207642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheng Z, Biechele T, Wei Z, et al. Crystal structures of the extracellular domain of LRP6 and its complex with DKK1. Nat Struct Mol Biol. 2011;18:1204–1210. doi: 10.1038/nsmb.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ke HZ, Richards WG, Li X, Ominsky MS. Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev. 2012;33:747–783. doi: 10.1210/er.2011-1060. [DOI] [PubMed] [Google Scholar]
- 149.Li Y, Lu W, King TD, et al. Dkk1 stabilizes Wnt co-receptor LRP6: implication for Wnt ligand-induced LRP6 down-regulation. PLoS One. 2010;5:e11014. doi: 10.1371/journal.pone.0011014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rai M, Walthall JM, Hu J, Hatzopoulos AK. Continuous antagonism by Dkk1 counter activates canonical Wnt signaling and promotes cardiomyocyte differentiation of embryonic stem cells. Stem Cells Dev. 2012;21:54–66. doi: 10.1089/scd.2011.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]


