Abstract
Limbic white matter pathways link emotion, cognition, and behavior and are potentially malleable to the influences of traumatic events throughout development. However, the impact of interactions between childhood and later life trauma on limbic white matter pathways has yet to be examined. Here, we examined whether childhood maltreatment moderated the effect of combat exposure on diffusion tensor imaging measures within a sample of military veterans (N = 28). We examined five limbic tracts of interest: two components of the cingulum (cingulum, cingulate gyrus, and cingulum hippocampus [CGH]), the uncinate fasciculus, the fornix/stria terminalis, and the anterior limb of the internal capsule. Using effect sizes, clinically meaningful moderator effects were found only within the CGH. Greater combat exposure was associated with decreased CGH fractional anisotropy (overall structural integrity) and increased CGH radial diffusivity (perpendicular water diffusivity) among individuals with more severe childhood maltreatment. Our findings provide preliminary evidence of the moderating effect of childhood maltreatment on the relationship between combat exposure and CGH structural integrity. These differences in CGH structural integrity could have maladaptive implications for emotion and memory, as well as provide a potential mechanism by which childhood maltreatment induces vulnerability to later life trauma exposure.
A substantial literature has demonstrated that adverse and traumatic experiences in childhood are linked to differences in the function, volume, and structural integrity of regions and white matter pathways throughout the brain (Nemeroff, 2016; Teicher & Samson, 2016). Studies demonstrating childhood trauma-related differences in brain structure have spanned development (Cicchetti, 2016; Teicher & Samson, 2016) and have included nonpsychiatric (Baker et al., 2013; Dannlowski et al., 2012; Gorka, Hanson, Radtke, & Hariri, 2014) and psychiatric samples, including those with posttraumatic stress disorder (PTSD) and other comorbidities (Bremner et al., 1997; Carrion et al., 2001; De Bellis et al., 1999; Keding & Herringa, 2015; Stein, Koverola, Hanna, Torchia, & McClarty, 1997). Studies examining childhood trauma-related differences in white matter integrity among children and adults have generally observed decreases in the structural integrity of limbic white matter pathways, including the cingulum, uncinate fasciculus, and fornix (Choi, Jeong, Rohan, Polcari, & Teicher, 2009; Eluvathingal et al., 2006; Hanson et al., 2013).
Thus, a mounting literature has demonstrated limbic brain differences associated with childhood trauma. However, fewer studies have considered the broader context of early and later life trauma. Some studies of military veterans have considered both childhood trauma and later life trauma, namely, combat exposure. In a prior study with the current sample, we showed that both childhood trauma and combat exposure are independently associated with dorsal anterior cingulate activity in response to emotional faces (Herringa, Phillips, Fournier, Kronhaus, & Germain, 2013). Further, only two studies of military veterans have directly examined and found interaction effects between childhood trauma and combat exposure on gray matter volume. In these studies, combat exposure was associated with decreased amygdala and anterior cingulate cortex volume within individuals with a history of childhood trauma under the age of 13 (Kuo, Kaloupek, & Woodward, 2012; Woodward, Kuo, Schaer, Kaloupek, & Eliez, 2013). These findings suggest that childhood trauma may sensitize limbic brain regions to later trauma exposure in ways that could contribute to vulnerability to mental illness. However, the impact of interactions between childhood and later life trauma on the structural integrity of limbic white matter pathways has yet to be examined. To our knowledge, this is the first report to examine the moderating effect of childhood maltreatment on the relationship between combat exposure and the structural integrity of limbic white matter pathways in a military sample.
Limbic white matter pathways interconnect cortical and subcortical regions that link visceral states, emotion, cognition, and behavior (for review, see Catani, Dell’Acqua, & Thiebaut de Schotten, 2013). For example, the cingulum is a large, limbic white matter bundle involved in executive functions, emotional processing, and memory (Beckmann, Johansen-Berg, & Rushworth, 2009). The uncinate fasciculus is another large, limbic bundle involved in episodic memory and socioemotional processing (Von Der Heide, Skipper, Klobusicky, & Olson, 2013). These functions are supported by their anatomical organization; the cingulum and the uncinate fasciculus connect amygdala-related structures with corticofrontal structures, including the cingulate and orbitofrontal cortex, respectively (Catani & De Schotten, 2008; Heilbronner & Haber, 2014; Jones, Christiansen, Chapman, & Aggleton, 2013; Von Der Heide et al., 2013).
In addition to the cingulum and uncinate fasciculus, the fornix and stria terminalis are limbic white matter pathways that connect amygdalohippocampal regions and associated cortices with other subcortical regions, including the hypothalamus and bed nucleus of the stria terminalis (Adelmann, Deller, & Frotscher, 1996; De Olmos & Ingram, 1972; Dong, Petrovich, & Swanson, 2001). As such, these pathways are involved in memory, as well as homeostatic and neuroendocrine functions (Cragg & Hamlyn, 1959; Feldman & Conforti, 1980; Flood, Merbaum, & Morley, 1995; King, Rollins, Grundmann, & Olivier, 2003; Osborne, Sivakumaran, & Black, 1979; Packard, Hirsh, & White, 1989). Finally, the anterior thalamic projections, running through the anterior limb of the internal capsule, connect thalamic regions carrying visceral/sensory signals to the orbitofrontal cortex and cingulate (Axer & Keyserlingk, 2000; Catani et al., 2013).
These pathways have long-ranging developmental trajectories reaching peak structural integrity between the early 20s and mid-40s (Lebel et al., 2012), rendering them potentially malleable to the influences of traumatic events throughout development. Given the functional importance of these pathways, differences in their structural integrity may have important implications for physical and mental health outcomes known to be associated with childhood trauma (Danese et al., 2009). Thus, the present study of military veterans is focused on examining interactions between childhood maltreatment and combat exposure on the structural integrity of limbic white matter pathways. Structural integrity measures were derived from diffusion tensor imaging (DTI) and extracted from five tracts of interest (TOIs): two components of the cingulum (cingulum, cingulate gyrus [CGC] and cingulum, hippocampus [CGH]), the fornix/stria terminalis (FX/ST), the uncinate fasciculus (UNC), and the anterior limb of the internal capsule (ALIC). We hypothesized that childhood maltreatment may induce vulnerability to further trauma exposure within limbic white matter. This would be demonstrated by childhood maltreatment moderating the relationship between combat exposure and limbic white matter structural integrity within TOIs (i.e., decreased fractional anisotropy and/or increased radial diffusivity), above and beyond the effects of potential confounders, including depressive and posttraumatic stress symptoms (PTSS).
Method
Participants
The participants were 28 male combat veterans from Operations Enduring and Iraqi Freedom who were between 18 and 35 years old (mean age = 26.4, SD 2.6). This sample has been described previously (Birn, Patriat, Phillips, Germain, & Herringa, 2014; Herringa et al., 2013). Briefly, participants were originally recruited from the Pittsburgh area through public media advertisements from October 2010 to November 2011 to participate in ongoing studies. This sample was 89% White (n = 25); 2 participants were Black or African American, and 1 was multiracial. Of the 25 White participants, one was of Hispanic or Latino descent. Written informed consent was obtained from all participants after procedures were explained in accordance with the University of Pittsburgh Institutional Review Board.
Information regarding the participants’ military service was obtained via Department of Defense Form 214 documentation. Exclusion criteria included left-handedness, magnetic resonance imaging (MRI) contraindications, any medications within the previous 3 weeks (fluoxetine within the past 2 months), active substance abuse within the past month, suicidality, psychotic or bipolar disorder, or neurological disease, including treatment for any sequelae of traumatic brain injury (TBI) or concussive symptoms at the time of the study. TBI information was assessed using the Military Acute Concussion Evaluation (French, McCrea, & Baggett, 2008) and the Clinician-Administered PTSD Scale (CAPS; Blake et al., 1995), and none of the participants reported concussive symptoms at the time of enrollment.
Participants were assessed with the Structured Clinical Interview for DSM-IVAxis I Disorders (First, Spitzer, Gibbon, & Williams, 2002) and with the CAPS for past month PTSS. Eighteen participants met full criteria for PTSD as identified by the CAPS, using the F1/I2 criteria (Weathers, Keane, & Davidson, 2001). As determined by the Structured Clinical Interview for DSM-IV Axis I Disorders, three participants met criteria for current major depressive disorder and were free of other current comorbid psychiatric disorders. Additional assessments included the Beck Depression Inventory (Beck, Steer, & Carbin, 1988) for depressive symptoms, the Combat Exposure Scale (CES; Keane et al., 1989) for the severity of combat exposure, and the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 1994) for childhood maltreatment history. Participant characteristics for primary measures of interest are shown in Table 1 and correlations between primary measures of interest are shown in Table 2. All correlations were below r = .7, indicating that multicollinearity was not high among our primary measures of interest.
Table 1. Summary of participant characteristics.
(N=27) The CTQ total score corresponds to a minimal level of childhood maltreatment. The CES total score corresponds to a moderate level of combat exposure. The BDI total score corresponds to minimal depressive symptoms. Here, TBI was coded as 0, indicating no TBI or 1, indicating any past symptoms of TBI. (CTQ, Childhood Trauma Questionnaire; BDI, Beck Depression Inventory; CAPS, Clinician Administered PTSD Scale; TBI, Traumatic Brain Injury; CES, Combat Exposure Scale)
| Characteristic | Mean | Standard Deviation | Range |
|---|---|---|---|
| CTQ total score | 35.11 | 11.13 | 25–63 |
| CTQ, physical abuse | 7.37 | 2.72 | 5–14 |
| CTQ, emotional abuse | 7.19 | 3.90 | 5–20 |
| CTQ, physical neglect | 6.48 | 2.03 | 5–11 |
| CTQ, emotional neglect | 8.81 | 4.71 | 5–19 |
| CTQ, sexual abuse | 5.26 | 1.35 | 5–12 |
| CES total score | 17.37 | 9.97 | 0–35 |
| Age (years) | 26.22 | 2.52 | 22–32 |
| TBI | 0.41 | 0.50 | 0–1 |
| BDI total score | 8.81 | 7.61 | 0–35 |
| CAPS (past month) | 41.41 | 22.05 | 2–84 |
| CAPS (lifetime) | 60.74 | 25.69 | 15–107 |
Table 2. Correlations between primary variables (n=27).
Spearman’s rho values are presented. While some relationships between variables are significant, none represented high multicollinearity.
| Variable | CTQ total | CES | Age | TBI | CAPS (month) |
|---|---|---|---|---|---|
| CTQ total | -- | -- | -- | -- | -- |
| CES | −.167 | -- | -- | -- | -- |
| Age | .394* | −.340 | -- | -- | -- |
| TBI | .087 | .504** | −.184 | -- | -- |
| CAPS (month) | .417* | .470* | −.300 | .450* | -- |
| BDI | .347 | .155 | −.130 | .278 | .588** |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
The CTQ total score was used in the present study to examine cumulative adversity from all maltreatment subtypes as it includes physical, emotional, and sexual abuse (PA, EA, and SA) and physical and emotional neglect (PN and EN). Scores range from 25 to 125, with 25 indicating no maltreatment. CTQ total scores were examined first and the sub-scales were examined post hoc (described below in Data Analyses). Only one participant indicated a history of SA, therefore the SA subscale was not examined. The distribution of CTQ total and subscale scores is presented in Table 3.
Table 3. Proportion of Participants in Childhood Trauma Questionnaire Classification Groups (n=27).
The majority of our participants fell in the “none or minimal” range for all of the maltreatment subscales, with decreasing proportions as severity increased. For CTQ total, we considered scores of 25–36 none to minimal, 37–51 low to moderate, 52–68 moderate to severe, and >68 severe to extreme. The CTQ subscale classifications are defined by Bernstein and Fink, 1998.
| None or Minimal | Low to Moderate | Moderate to Severe | Severe to Extreme | |
|---|---|---|---|---|
| CTQ Total | 17 | 7 | 3 | 0 |
| Emotional Abuse | 21 | 3 | 1 | 2 |
| Physical Abuse | 17 | 6 | 1 | 3 |
| Emotional Neglect | 17 | 6 | 2 | 2 |
| Physical Neglect | 21 | 2 | 4 | 0 |
DTI acquisition
MRI was performed on a 3-Tesla Trio TIM whole-body MRI scanner (Siemens), equipped with a 12-channel phased-array head coil. DTI was performed using a pair of pulsed-gradient, spin-echo sequences with a single-shot echo-planar imaging readout. A parallel imaging algorithm (generalized autocalibrating partial-parallel acquisition) was applied during diffusion imaging to reduce echo-planar distortion. DTI parameters were 8400-ms time to repetition, 91-ms time to echo, 180° flip angle, 2×2 mm pixel size, 128×128 resolution (256 ×256 mm field of view), 64 2-mm thick slices with no gap, and 9 min 56 s total imaging time. Diffusion-sensitizing gradient encoding was applied in 68 uniform angular directions with a diffusion weighting of b = 1000 s/mm2. A reference image with no diffusion gradient (b = 0) was also acquired.
DTI processing and analysis
DTI data were first processed using the FSL Diffusion Tool-box (Version 2.0; http://www.fmrib.ox.ac.uk/fsl/fdt/index.html), which comprised correction for motion and eddy current distortions by affine registration to the reference image, removal of skull and nonbrain tissue, and calculation of diffusion parameters by fitting the diffusion images to a diffusion tensor model. The voxelwise eigenvalues λ1, λ2, and λ3 and the eigenvectors of the diffusion tensor were computed from each participant’s image. Fractional anisotropy (FA), the variance of diffusion rate, was computed from the eigenvalues by the following equation:
where axial diffusivity equals λ1, which is the largest eigenvalue reflecting water diffusivity parallel to the principle fiber direction, and radial diffusivity (RD) is the average of λ2 and λ3 corresponding to perpendicular water diffusivity (Hagmann et al., 2006). FA is the primary DTI measure to assess structural integrity, with larger FA values reflecting greater “structural integrity.”
The FSL tract-based spatial statistics toolbox (Version 1.2, http://www.fmrib.ox.ac.uk/fsl/tbss/index.html) was used to compute a skeleton-projected FA map for each participant following prior guidelines (Smith et al., 2007). For these analyses, FA images were eroded to remove likely outliers from the diffusion tensor-fitting step. FA images were then normalized to the 1×1×1 mm MNI152 stereotaxic space via the FSL FA template (FMRIB58_FA). This was done by combining two transformations: (a) a nonlinear registration of each participant’s FA image to the FMRIB59_FA template, and (b) an affine transformation of the template to MNI152 space. Next, a mean FA image of all participants was computed and “thinned” to create a skeleton, or a single surface line corresponding to the center of the tracts common to all participants. A threshold was then applied to the mean skeleton according to convention (Smith et al., 2007) to retain FA values above 0.2. Finally, each participant’s FA map was projected onto the mean FA skeleton by searching for the local center of the relevant white matter tract (i.e., the location of the local maximum of FA values). This was done to account for residual misalignments uncorrected by the nonlinear registration described above. After processing, each image was smoothed with a 2-mm3 Gaussian smoothing kernel.
Region of interest analyses
In order to examine whether childhood trauma and/or combat exposure predict DTI measures within specific TOIs, we used a 48-tract white-matter atlas (Mori, Wakana, Van Zijl, & Nagae-Poetscher, 2005; http://cmrm.med.jhmi.edu/). Of these 48 tracts, we selected five limbic TOIs: CGC, CGH, UNC, FX/ST, and ALIC (Figure 1). For each TOI, the mean of the FA and RD values across all voxels were extracted. We had no a priori hypotheses regarding laterality; thus, DTI values were averaged across hemispheres.
Figure 1.
Three-dimensional representation of tracts of interest. The cingulum, cingulate gyrus (CGC; maroon online), cingulum, hippocampus (CGH; red online), fornix/stria terminalis (FX/ST; blue online), uncinate fasciculus (UNC; green online), and anterior limb of the internal capsule (ALIC; yellow online) tracts of interest are displayed.
Data analyses
Our primary goal was to determine whether cumulative childhood maltreatment (the CTQ total score) moderated the effect of combat exposure (the CES score) on limbic white matter structural integrity, including DTI variables FA and RD within our five limbic TOIs (CGC, CGH, UNC, FX/ST, and ALIC). TOI axial diffusivity was also examined for exploratory purposes (not reported here). Age and neurological insults, such as TBI, are important determinants of white matter structural integrity (Kraus et al., 2007; Lebel et al., 2012; Shenton et al., 2012). Thus, all models adjusted for age and history of TBI. However, it is also important to consider the impact of childhood maltreatment and combat exposure above and beyond mood and anxiety symptoms (Korgaonkar et al., 2011; Lebel et al., 2012; Long et al., 2013; Shenton et al., 2012). Therefore, we also evaluated whether our findings remained after adjusting for depressive (assessed with the Beck Depression Inventory) and PTSS (assessed with the CAPS, past month), in addition to age and history of TBI.
In accordance with the American Statistical Association’s recent statement, this report focuses on effect sizes, which is the appropriate method to examine a small sample in order to emphasize the clinical (rather than statistical) significance of our findings (Wasserstein & Lazar, 2016). Our strategy was to first determine whether there was a clinically meaningful interactive effect of CTQ total and CES on each outcome after controlling for age and TBI. We defined a clinically meaningful interactive effect as one with at least a moderate η2 effect size (i.e., η2 ≥ 0.13; Levine & Hullett, 2002), with values of 0.02, 0.13, and 0.26 proposed as estimates of small, moderate, and large effect sizes, respectively (Cohen, Cohen, West, & Aiken, 2003). This effect size measure is equivalent to R2 change related to the interaction term in a multiple regression model including the interaction term, the main effects of CTQ and CES, and our a priori selected covariates of interest. If no clinically meaningful interactive effect was found, we removed the interaction term and evaluated whether the main effects of CTQ and/or CES were associated with at least moderate η2 effect sizes. For outcomes with clinically meaningful interaction or main effects, we explored which CTQ subscale may have been driving the effects (i.e., PA, EA, PN, or EN) and also whether the finding remained after adjusting for depressive symptoms and PTSS. For clinically meaningful moderator or main effects, we used multiple regression methods to further examine the relative contributions of each of the variables in the model. Finally, when we identified that CTQ (total or subscale) moderated the effects of CES on a DTI outcome within a TOI, we used simple slopes analysis (Aiken, West, & Reno, 1991) to further investigate the interactive relationship. For all simple slopes analyses, CTQ total or subscale values that indicate either “none” or “moderate” levels of maltreatment, based on defined severity classifications (Bernstein & Fink, 1998), were used in order to provide estimates of predicted structural integrity at that value (i.e., CTQ total: none = 25, moderate = 51; physical neglect: none = 5, moderate = 9).
We then evaluated whether childhood maltreatment moderated the effects of combat exposure on PTSS. We determined whether there was a clinically meaningful interactive effect of CTQ total and CES on PTSS after adjusting for age and past TBI. If no clinically meaningful interactive effect was found, we removed the interaction term and evaluated whether the main effects of CTQ and/or CES were associated with at least moderate η2 effect sizes. For outcomes with clinically meaningful main effects, we explored which CTQ subscale might be driving the effects and also investigated whether the finding remained after adjusting for depressive symptoms.
All continuous variables were standardized, and TBI history was coded as 0.5 (yes) versus −0.5 (no) for interpretability. One participant with an extreme CTQ total score was considered a statistical outlier (>3 SD from the mean) and was excluded from the analyses. However, for completeness and transparency, we also fit models including this outlier and summarize the results. Because of their small size, CGH RD values were multiplied by 10,000 in order to obtain values that would not be rounded to 0 using the statistical package, R.R Version 3.3.0 (used for all analyses).
Results
DTI outcomes
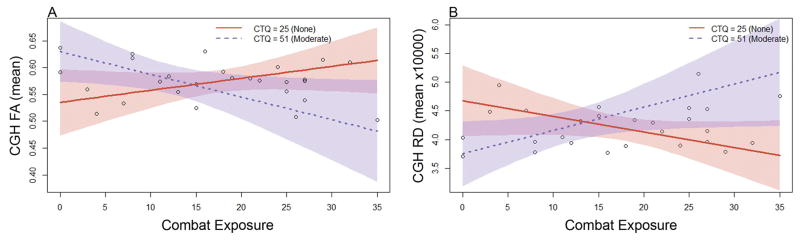
Using CTQ total and adjusting for age and past TBI, we found significant, clinically meaningful moderator effects on CGH FA (η2 = 0.168, p = .046) and RD (η2 = 0.181, p = .036). These effects, their consistency within CTQ subscales, and after adjusting for depressive symptoms and PTSS, are described in further detail below. (See Figure 2 for images of DTI measures within the CGH.)
Figure 2.
Skeletonized diffusion tensor imaging measures within cingulum, hippocampus. (Left) coronal, (middle) sagittal, and (right) axial sections display the cingulum, hippocampus (cyan online) tract of interest overlaid on skeletonized fractional anisotropy (violet online) and radial diffusivity (red online) maps.
Moderator effects within the CGH
CTQ total moderated the effect of combat exposure on mean FA within the CGH after adjusting for age and past TBI (η2 = 0.168, p = .046; Figure 3a). This moderation was also observed with PN (η2 = 0.257, p = .011), EN (η2 = 0.145, p = .066), and EA (η2 = 0.163, p = .051) subscales adjusting for age and past TBI. Simple slopes analysis indicated that at the “none” level of the CTQ total (CTQ = 25), the estimated simple slope of CES was β = 0.002 (SE = 0.002; t = 1.398, p = .178), corresponding to a correlation of r = .269. Conversely, at the “moderate” level of the CTQ total (CTQ = 51) the estimated simple slope of CES was β = −0.004 (SE = 0.002; t = −2.200, p =.040), corresponding to a correlation of r =−.402.
Figure 3.
Cumulative childhood maltreatment moderated the effect of combat exposure on cingulum, hippocampus structural integrity. Plots display (a) mean fractional anisotropy (FA) and (b) mean radial diffusivity (RD) extracted from the cingulum, hippocampus (CGH) tract of interest. Lines represent predicted FA and RD values at “none” (Childhood Trauma Questionnaire [CTQ] = 25, solid red line online) and “moderate” (CTQ =51, dashed blue line online) CTQ total scores, adjusting for age and past traumatic brain injury. Thus, higher levels of combat exposure accompanied by greater childhood maltreatment was associated with decreased CGH FA and increased CGH RD. Shaded regions depict 95% confidence intervals.
After including depressive symptoms and PTSS in the model (i.e., adjusting for age, past TBI, depressive symptoms, and PTSS), this moderation effect was observed only in the PN subscale (η2 = 0.188, p = .030; Figure 4a). At the none level of PN (PN = 5), the estimated simple slope of CES on CGH FA was β = 0.001 (SE = 0.001; t = 0.983, p =.339), corresponding to a correlation of r =.193. At moderate PN (PN = 9), the estimated simple slope of CES on CGH FA was β = −0.003 (SE = 0.001; t = −2.504, p = .022), corresponding to a correlation of r = −.448.
Figure 4.
Physical neglect moderated the effect of combat exposure on cingulum, hippocampus structural integrity. Plots display (a) mean fractional anisotropy (FA) and (b) mean radial diffusivity (RD) extracted from the cingulum, hippocampus (CGH) tract of interest. Lines represent predicted FA and RD values at “none” (physical neglect; PN = 5, solid red line online) and “moderate” (PN = 9, dashed blue line online) PN scores, adjusting for age, past traumatic brain injury, and depressive and posttraumatic stress symptoms. Thus, higher levels of combat accompanied by greater childhood PN was associated with decreased CGH FA and increased CGH RD. Shaded regions depict 95% confidence intervals.
CTQ total also moderated the effect of combat exposure on mean CGH RD after adjusting for age and past TBI (η2 = 0.181, p =.036; Figure 3b). This moderation was also observed in PN (η2 = 0.284, p = .006), EN (η2 = 0.144, p = .065), and EA (η2 = 0.139, p = .069) subscales when adjusting for age and past TBI. Simple slopes analysis indicated that at the none level of the CTQ total (CTQ = 25), the estimated simple slope of CES was β = −0.027 (SE = 0.016; t = −1.722, p = .101), corresponding to a correlation of r = −.326. Conversely, at the moderate level of the CTQ total (CTQ = 51), the estimated simple slope of CES was β = 0.041 (SE = 0.019; t = 2.125, p = .046), corresponding to a correlation of r = .391.
After including depressive symptoms and PTSS in the model, the moderation effect on CGH RD was observed only in the PN subscale (η2 =0.206, p = .021; Figure 4b). At the none level of PN (PN = 5), the estimated simple slope of CES on CGH RD was β =–0.020 (SE =0.014; t = −1.448, p = .165), corresponding to a correlation of r = −.278. At moderate PN (PN = 9), the estimated simple slope of CES on CGH RD was β = 0.028 (SE = 0.012; t = 2.251, p = .037), corresponding to a correlation of r = .411.
Examination of the full set of effects from multiple regression (Table 4) indicated that the interaction between CTQ total and CES consistently contributed to the largest increase in R2 (equivalent to η2) relative to the other covariates in the models. Finally, when the outlier was retained in the CTQ total CGH model after adjusting for age and past TBI, the moderator effects on FA and RD were stronger (CGH FA, η2 = 0.225, p = .011; CGH RD, η2 = 0.280, p = .003).
Table 4. Multiple Regression Results for Childhood Maltreatment and Combat Exposure Interaction Models.
The full set of effects from multiple regression indicated that the interaction between Childhood Trauma Questionnaire (CTQ) total and Combat Exposure Scale (CES) on cingulum, hippocampus (CGH) fractional anisotropy (FA) and radial diffusivity (RD) contributed to the largest increase in R2 (equivalent to η2) relative to the other covariates in the model. After including depressive and post-traumatic stress symptoms in the model, the moderation effect on CGH FA and RD was observed only in the physical neglect subscale. (TBI, Traumatic Brain Injury; BDI, Beck Depression Inventory; CAPS, Clinician Administered PTSD Scale)
| CGH (Mean FA) | |||||
|---|---|---|---|---|---|
| Scaled Variable | Beta | SE | t-statistic | p-value | eta-squared |
| CTQ total | −0.008 | 0.009 | −0.841 | 0.410 | 0.027 |
| CES | −0.003 | 0.009 | −0.316 | 0.755 | 0.004 |
| CES*CTQ | −0.028 | 0.013 | −2.119 | 0.046* | 0.168 |
| Age | 0.001 | 0.008 | 0.075 | 0.941 | 0.000 |
| TBI | −0.019 | 0.018 | −1.077 | 0.294 | 0.043 |
| CGH (Mean RD) | |||||
| Scaled Variable | Beta | SE | t-statistic | p-value | eta-squared |
| CTQ total | 0.110 | 0.089 | 1.228 | 0.233 | 0.054 |
| CES | −0.009 | 0.088 | −0.100 | 0.922 | 0.000 |
| CES*CTQ | 0.290 | 0.129 | 2.244 | 0.036* | 0.181 |
| Age | −0.003 | 0.079 | −0.038 | 0.970 | 0.000 |
| TBI | 0.298 | 0.177 | 1.690 | 0.106 | 0.103 |
| CGH (Mean FA) | |||||
| Scaled Variable | Beta | SE | t-statistic | p-value | eta-squared |
| Physical Neglect (PN) | −0.009 | 0.009 | −0.997 | 0.331 | 0.034 |
| CES | −0.003 | 0.010 | −0.282 | 0.781 | 0.003 |
| CES*PN | −0.023 | 0.010 | −2.341 | 0.030* | 0.188 |
| Age | 0.006 | 0.008 | 0.716 | 0.483 | 0.018 |
| TBI | −0.019 | 0.018 | −1.081 | 0.293 | 0.040 |
| BDI | −0.001 | 0.009 | −0.118 | 0.907 | 0.000 |
| CAPS (month) | 0.012 | 0.010 | 1.114 | 0.279 | 0.043 |
| CGH (Mean RD) | |||||
| Scaled Variable | Beta | SE | t-statistic | p-value | eta-squared |
| Physical Neglect (PN) | 0.110 | 0.092 | 1.198 | 0.246 | 0.047 |
| CES | −0.025 | 0.097 | −0.256 | 0.800 | 0.002 |
| CES*PN | 0.243 | 0.096 | 2.519 | 0.021* | 0.206 |
| Age | −0.051 | 0.079 | −0.641 | 0.530 | 0.013 |
| TBI | 0.272 | 0.173 | 1.574 | 0.132 | 0.080 |
| BDI | 0.027 | 0.090 | 0.301 | 0.767 | 0.003 |
| CAPS (month) | −0.084 | 0.101 | −0.827 | 0.419 | 0.022 |
PTSS outcomes
CTQ total did not moderate the effect of CES on PTSS after controlling for age and past TBI. However, there was a large main effect of CTQ total on PTSS after controlling for CES, age, and past TBI (η2 = 0.261, p = .002). This main effect was also observed with the PN (η2 = 0.236, p = .004), EN (η2 = 0.268, p = .002), and PA (η2 = 0.161, p = .022) sub-scales after controlling for CES, age, and past TBI. When depressive symptoms were added to the model, the main effect of CTQ total was still observed (η2 = 0.196, p = .005), as well as with the PN (η2 = 0.139, p = .021) and EN (η2 = 0.210, p = .003) subscales. (The full set of effects from multiple regression for both models are presented in Table 5.) Finally, when the outlier was included, the main CTQ total effect was still observed after adjusting for CES, age, and past TBI (η2 = 0.217, p = .005), as well as after adding depressive symptoms to the model (η2 = 0.139, p = .018).
Table 5. Multiple Regression Results for Post-traumatic Stress Symptom Outcomes.
A large effect of CTQ total on post-traumatic stress symptoms was observed after controlling for CES, age and past TBI, which remained at a moderate effect size after depressive symptoms were added to the model. (CTQ, Childhood Trauma Questionnaire; CES, Combat Exposure Scale; CAPS, Clinician Administered PTSD Scale; TBI, Traumatic Brain Injury; BDI, Beck Depression Inventory)
| Model 1: Post-traumatic Stress Symptoms (CAPS month) | |||||
|---|---|---|---|---|---|
| Scaled Variable | Beta | SE | t-statistic | p-value | eta-squared |
| CTQ total | 11.994 | 3.485 | 3.442 | 0.002* | 0.261 |
| CES | 8.477 | 3.861 | 2.196 | 0.039* | 0.106 |
| Age | −5.751 | 3.459 | −1.663 | 0.111 | 0.060 |
| TBI | 8.230 | 7.388 | 1.114 | 0.277 | 0.027 |
| Model 2: Post-traumatic Stress Symptoms (CAPS month) | |||||
| Scaled Variable | Beta | SE | t-statistic | p-value | eta-squared |
| CTQ total | 10.644 | 3.359 | 3.169 | 0.005* | 0.196 |
| CES | 7.513 | 3.674 | 2.045 | 0.054 | 0.082 |
| Age | −6.364 | 3.277 | −1.942 | 0.066 | 0.074 |
| TBI | 4.283 | 7.259 | 0.590 | 0.561 | 0.007 |
| +BDI | 6.452 | 3.335 | 1.935 | 0.067 | 0.073 |
Discussion
The present study extends the current literature by providing preliminary evidence that childhood and later life trauma interact to impact brain structural integrity, as measured by DTI. As limbic white matter pathways have a long developmental trajectory progressing into adulthood, the structural integrity of these pathways may be particularly malleable to the influence of traumatic experiences throughout the life course. Thus, among young adult, male combat veterans, we hypothesized that childhood maltreatment may increase vulnerability to later combat exposure and that this would be revealed by a moderating influence of childhood maltreatment on the relationship between combat exposure and the structural integrity of limbic white matter pathways, above and beyond the influences of potential confounders, including age and TBI. Demonstrated for the first time to our knowledge, cumulative childhood maltreatment (CTQ total, which includes physical, emotional, and sexual abuse and physical and emotional neglect) moderated the effect of combat exposure on the structural integrity of the hippocampal cingulum, such that, among those with high childhood maltreatment, greater combat exposure was associated with decreased FA and increased RD. The moderating effect of childhood maltreatment was unique to the CGH, suggesting specificity to the cingulum bundle. This moderating effect was even more robust when considering the physical neglect subscale, which remained after adjusting for depressive symptoms and PTSS.
Childhood trauma, combat exposure, and the structural integrity of the cingulum
The cingulum is a large bundle containing both association and projection fibers, the longest of which extend from the uncus and parahippocampal gyrus to subgenual areas of the cingulate gyrus (Jones et al., 2013; Nieuwenhuys, Voogd, & van Huijzen, 2008). Among other roles, the cingulum is implicated in autonomic functions, emotional processing and memory, as well as mood and anxiety disorders (Catani et al., 2013). The present study demonstrated that childhood trauma moderated the effect of combat exposure on the structural integrity of the CGH, such that thosewith high levels of childhood trauma and greater combat exposure display decreased FA and increased RD. RD has been shown to increase with demyelination and is canonically interpreted as such (Mori & Zhang, 2006; Song et al., 2002). Further, a more disorganized configuration of the axons within the CGH may increase the RD. Taken together, these findings suggest an overall decrease in structural integrity that may be driven by demyelination or disorganization of CGH axons (Johansen-Berg & Behrens, 2009). These differences in CGH structural integrity may have implications for vulnerability to mental illness, specific to its functions.
The parahippocampal retrosplenial network encompasses the CGH TOI used in the present study, which includes both the dorsal and the ventral posterior cingulate cortex (Catani et al., 2013; Vogt, 2005). The dorsal posterior cingulate cortex (PCC) plays a role in visuospatial orientation, potentially in orienting toward or away from innocuous or noxious stimuli (Vogt, 2005). Vogt describes the ventral PCC as the preprocessor of information regarding the self-relevance of emotional stimuli. The ventral PCC conveys this information to the subgenual anterior cingulate cortex, involved in autonomic regulation and emotional valence. A meta-analysis of functional studies demonstrates that posterior, retrosplenial clusters are also activated by memory tasks, with these same clusters having a high probability of hippocampal connections (Beckmann et al., 2009). A small proportion of cingulum fibers course through the entire tract (Jones et al., 2013); thus, decreased CGH structural integrity and/or demyelination could suggest alterations in the described posterior functions, as well as more anterior functions, such as autonomic and emotion regulation (Vogt, 2005), all of which have potentially maladaptive implications. Among children with a history of institutionalized care, who were likely neglected and/or deprived, decreased CGH FA was associated with worse neurocognitive performance in a test of spatial planning (Hanson et al., 2013). Our results also indicate that physical neglect (e.g., not being attended to with regard to food, clean clothes, medical attention, and/or safety) is the primary type of maltreatment driving the moderator effects of CTQ total on CGH FA and RD. When compared to physical abuse, physical neglect is associated with more severe cognitive deficits, as well as more internalizing and emotional problems (Hildyard & Wolfe, 2002), which may be due in part to trauma-related alterations in cingulum structural integrity. Further, previous research in both children and adults demonstrates that childhood trauma-related decreases in cingulum FA are associated with depressive symptoms or the development of psychiatric disorders later in life (Choi et al., 2009; Huang, Gundapuneedi, & Rao, 2012).
No main effects of childhood trauma on FA or RD within any other TOIs were revealed in the present study, which is inconsistent with previous reports that have shown childhood trauma-related decreases in cingulum FA among children and adults. Studies in children/adolescents who have experienced severe neglect or physical or sexual abuse have shown decreased FA in the cingulum compared to controls (Hanson et al., 2013; Huang et al., 2012). Young, healthy adults with a history of parental verbal abuse also show decreased cingulum FA (Choi et al., 2009). However, individuals with a history of childhood trauma display increased cingulum FA in a study that spanned a large age range and included individuals with major depressive disorder, potentially accounting for the difference in directionality (Ugwu, Amico, Carballedo, Fagan, & Frodl, 2014). The cingulum has the longest developmental trajectory among other major white matter bundles, reaching peak FA in the early 40s (Lebel et al., 2012). Thus, it may have the longest window within which both childhood and later life traumas, like combat exposure, could influence its structural integrity, as demonstrated in the present report. Our results support the hypothesis that childhood maltreatment may sensitize the CGH adversely to later combat exposure.
Childhood trauma, combat exposure, and PTSS
While there was no moderating effect of cumulative childhood maltreatment on the relationship between combat exposure and PTSS in our sample, there were statistically significant contributions of both childhood maltreatment and combat exposure to PTSS. However, only the effect of childhood maltreatment was above a moderate effect size and remained so after adjusting for depressive symptoms. Our findings are consistent with previous literature showing that greater childhood adversity increases risk for PTSD (Koenen, Moffitt, Poulton, Martin, & Caspi, 2007). Further, adults with higher levels of childhood adversity and recent major stressors show greater prevalence of PTSD, perhaps indicating a stress sensitization process (McLaughlin, Conron, Koenen, & Gilman, 2010). Further, in military samples, childhood adversity predicts nonresilient postdeployment trajectories of PTSS (Berntstein et al., 2012) and contributes to PTSS to a greater extent than combat exposure (Cabrera, Hoge, Bliese, Castro, & Messer, 2007). Thus, our findings support the hypothesis that childhood adversity shapes neural developmental trajectories, particularly cingulum structural integrity, in ways that may sensitize this and potentially other limbic pathways to later life stress or trauma.
Complex trauma intervention implications throughout the life course
Complex trauma is defined as trauma occurring throughout the life course with often prolonged traumas, such as combat exposure, accompanied by a developmental history of childhood maltreatment or adversity. Complex trauma often yields symptom complexity, which can include affect dysregulation, mal-adaptive coping strategies, impulsivity, self-injurious behavior, substance abuse, and/or comorbidity with other mood or anxiety disorders. This symptom complexity contributes to complex PTSD, developmental trauma disorder, or related conditions, which are more difficult to treat as the therapeutic response is often more modest and/or requires more prolonged, intensive treatment (for a review, see Briere & Scott, 2015).
Our finding that the interaction between childhood maltreatment and combat exposure is associated with decreased CGH structural integrity may implicate the cingulum as a target for prevention and/or intervention. Reduced CGH structural integrity may also represent a neural marker of complex trauma, potential vulnerability to subsequent trauma, and/or a neural predictor of intervention effectiveness. With the cingulum’s long developmental trajectory, our findings suggest a potential target of biological or psychosocial intervention that may be responsive even into adulthood. However, the extent to which treatment response to first-line pharmacological and cognitive behavioral treatments of PTSD is mediated by CGH integrity or treatment-induced changes in CGH integrity remains to be determined. Of particular relevance to our findings are interventions that may target cingulum-related functions, including “self-relevance” processing of emotional stimuli and emotional regulation (Vogt, 2005). For example, multitarget interventions including dialectical behavioral therapy and skill training in affect regulation, in which emotional coping skills are developed and emotional processing occurs (Briere & Scott, 2015), may act on cingulum structural integrity to enhance these capabilities and potentially augment first-line treatments.
Interventions specific to military populations that employ cognitive and emotion regulation strategies may also influence cingulum structural integrity. For example, the Battlemind program, implemented during or after deployment, includes psychological debriefings that facilitate cognitive and emotional processing of traumatic events proximal to adult trauma exposure. This program also incorporates training to cognitively reframe common postdeployment symptoms by addressing how necessary combat-related skills (e.g., maintaining tactical awareness) can naturally become problematic in civilian environments (e.g., hypervigilance; Adler, Bliese, McGurk, Hoge, & Castro, 2009; Adler, Castro, & McGurk, 2009; Castro, Adler, McGurk, & Bliese, 2012). Veterans with high combat exposure who engaged in the Battlemind program report fewer postdeployment posttraumatic stress and/or depressive symptoms compared to those who received control interventions (Adler, Bliese, et al., 2009; Castro et al., 2012). Thus, we speculate that the cingulum may be a contributing neural mechanism by which these interventions yield effective treatment responses. Such interventions may be particularly useful for individuals with a history of childhood maltreatment, who are presumably more biologically vulnerable to the effects of trauma exposure during combat. However, further study would be needed to examine this possibility.
There is evidence that interventions specifically targeting the cingulum may be effective. For example, therapeutically effective deep brain stimulation used to treat depression includes electrode placements involving various segments of the cingulum bundle (see Heilbronner & Haber, 2014, for review). Further, there is evidence that interventions effective in reducing symptoms enhance cingulum structural integrity. One study showed that electroconvulsive therapy used to treat depression increases FA within dorsal frontolimbic circuits that include the anterior cingulum (Lyden et al., 2014). Lower cingulum FA contributes to a prediction of nonremission with antidepressant medication treatment (Korgaonkar et al., 2015). Thus, cingulum structural integrity could also potentially be used as a personalized neural marker to predict treatment response.
Complementary or alternative approaches, such as meditation, may also be useful methods to support or enhance cingulum structural integrity. Luders, Clark, Narr, and Toga (2011) demonstrated that compared to age- and sex-matched controls, long-term meditation practitioners display greater structural integrity of major white matter bundles, including the CGH. Long-term meditation practitioners also display less age-related decline in CGC structural integrity (Luders et al., 2011). Further, brief mindfulness training that improves emotion regulation is associated with structural changes within the anterior cingulate cortex (Tang, Holzel, & Posner, 2015; Tang, Tang, & Posner, 2016). Thus, meditation could be used as a noninvasive intervention throughout the life course, particularly among individuals with a history of childhood maltreatment or adversity, to promote cingulum structural integrity and potentially ameliorate neural and psychological effects of subsequent trauma exposure. Further research into how the cingulum could be used as a neural marker of complex trauma and treatment efficacy, and a target for prevention/intervention, is necessary. In addition, research into how interventions may alter the cingulum’s developmental trajectory in potentially adaptive ways within maltreated, trauma-exposed, and complex trauma populations is of great interest.
Limitations of the present research
We note several limitations of the present report including a small sample, a cross-sectional study design, and the retrospective recall of childhood maltreatment and combat exposure. Further, as the sample is composed of all male military veterans, the results of the study may not be generalizable to females and other forms of later life trauma, aside from combat exposure.
The present findings are based on a small sample of 27 young adult combat veterans. Given this small sample size, we emphasized clinically meaningful effect sizes that reached at least moderate levels. In the American Statistical Society’s recent statement on p values, the authors note that p values do not measure the importance of a result and are highly influenced by sample size: a small p value can be achieved with a small effect if the sample size is large enough and a large effect may produce large p values if the sample size is small (Wasserstein & Lazar, 2016). The primary moderation effects described in the present report (i.e., CTQ total moderating the effect of CES on CGH FA and RD adjusting for age and TBI, and PN moderating the effect of CES on CGH FA and RD, adjusting for all covariates) were statistically significant, in addition to having moderate effect sizes; however, multiple tests were run to adequately examine these data. Thus, these findings are preliminary, not conclusive, and warrant further replication; however, the effect sizes suggest increased statistical significance with larger samples, and these findings provide an interesting and novel direction for future research, namely, examining interactions between multiple levels of trauma throughout the life span on neural outcomes, including limbic white matter structural integrity.
Although retrospective reporting of childhood maltreatment can be associated with recall bias, congruence between sibling retrospective reports of abuse, and prospectively collected court, clinic, and research records suggests reliability of such reports (Bifulco, Brown, & Harris, 1994; Bifulco, Brown, Lillie, & Jarvis, 1997; Hardt & Rutter, 2004). It is possible that individuals reporting more severe depressive symptoms and PTSS may report more childhood maltreatment. In our sample, CTQ total scores were moderately correlated with depressive (p = .076) and posttraumatic stress symptoms (p = .030, see Table 2). However, Scott, McLaughlin, Smith, and Ellis (2012) found no differences between prospective and retrospective reports of maltreatment history in the strength of the relationships between maltreatment history and mood or anxiety disorders). Ultimately, longitudinal research is necessary to distinguish the influences of genetic predispositions, prenatal exposures, childhood maltreatment, and later life traumas, as well as the importance of the developmental timing of traumatic experiences. Further analyses examining how the structural integrity of limbic tracts may mediate relationships between childhood maltreatment and/or combat exposure and mood and anxiety symptoms were not feasible in the current study, but are important to investigate in future research.
Summary
In summary, this report provides preliminary evidence of the moderating effect of childhood maltreatment on the relationship between combat exposure and CGH FA and RD, such that having high childhood maltreatment and high combat exposure was associated with decreased FA and increased RD, suggesting decreased myelination contributing to an overall decrease in structural integrity. These findings could potentially have maladaptive implications, particularly for the perception of emotional stimuli, emotional memory, as well as autonomic and emotional regulation. Overall, these differences in CGH structural integrity may contribute to increased risk for psychopathology, particularly with greater cumulative exposure to traumatic experiences across development. Further research is needed to discern changes within more neuroanatomically specific components of these limbic white matter pathways and how these changes may contribute differentially to neural, physiological, and psychological outcomes. Further, our findings suggest the cingulum as a potential target of interventions that may be effective even into adulthood, particularly for individuals with exposure to multiple levels of trauma throughout development.
Acknowledgments
The authors thank Benjamin Paul, Ashlee McKeon, Ryan Stocker, and Noelle Rode for their assistance with participant screening, MRI procedures, and data organization. This work was supported by the American Academy of Child & Adolescent Psychiatry (AACAP) Pilot Research Award (to R.J.H.); National Institute of Mental Health Grants MH102406-01 (to L.B.), MH096944 (to M.L.W.), MH080696 and MH083035 (to A.G.), and MH100267 (to R.J.H.); and Department of Defense Congressionally Directed Medical Research Program Grants PT073961 and PR054093 (to A.G.).
References
- Adelmann G, Deller T, Frotscher M. Organization of identified fiber tracts in the rat fimbria-fornix: An anterograde tracing and electron microscopic study. Anatomy and Embryology. 1996;193:481–493. doi: 10.1007/BF00185879. [DOI] [PubMed] [Google Scholar]
- Adler AB, Bliese PD, McGurk D, Hoge CW, Castro CA. Battlemind debriefing and battlemind training as early interventions with soldiers returning from Iraq: Randomization by platoon. Journal of Consulting and Clinical Psychology. 2009;77:928. doi: 10.1037/a0016877. [DOI] [PubMed] [Google Scholar]
- Adler AB, Castro CA, McGurk D. Time-driven battlemind psychological debriefing: A group-level early intervention in combat. Military Medicine. 2009;174:21. doi: 10.7205/milmed-d-00-2208. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG, Reno RR. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- Axer H, Keyserlingk DG. Mapping of fiber orientation in human internal capsule by means of polarized light and confocal scanning laser microscopy. Journal of Neuroscience Methods. 2000;94:165–175. doi: 10.1016/s0165-0270(99)00132-6. [DOI] [PubMed] [Google Scholar]
- Baker LM, Williams LM, Korgaonkar MS, Cohen RA, Heaps JM, Paul RH. Impact of early vs. late childhood early life stress on brain morphometrics. Brain imaging and Behavior. 2013;7:196–203. doi: 10.1007/s11682-012-9215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. Journal of Neuroscience. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire: A retrospective self-report: Manual. Orlando, FL: Psychological Corporation; 1998. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, … Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151:1132. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Berntsein D, Johannessen KB, Thomsen YD, Bertelsen M, Hoyle RH, Rubin DC. Peace and war: Trajectories of posttraumatic stress disorder symptoms before, during, and after military deployment in Afghanistan. Psychological Science. 2012;23:1557–1565. doi: 10.1177/0956797612457389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifulco A, Brown GW, Harris T. Childhood Experience of Care and Abuse (CECA): A retrospective interview measure. Journal of Child Psychology and Psychiatry. 1994;35:1419–1435. doi: 10.1111/j.1469-7610.1994.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown G, Lillie A, Jarvis J. Memories of childhood neglect and abuse: Corroboration in a series of sisters. Journal of Child Psychology and Psychiatry. 1997;38:365–374. doi: 10.1111/j.1469-7610.1997.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Birn RM, Patriat R, Phillips ML, Germain A, Herringa RJ. Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depression and Anxiety. 2014;31:880–892. doi: 10.1002/da.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, … Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—A preliminary report. Biological Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere J, Scott C. Complex trauma in adolescents and adults: Effects and treatment. Psychiatric Clinics of North America. 2015;38:515–527. doi: 10.1016/j.psc.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Cabrera OA, Hoge CW, Bliese PD, Castro CA, Messer SC. Childhood adversity and combat as predictors of depression and post-traumatic stress in deployed troops. American Journal of Preventive Medicine. 2007;33:77–82. doi: 10.1016/j.amepre.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- Castro CA, Adler AB, McGurk D, Bliese PD. Mental health training with soldiers four months after returning from Iraq: Randomization by platoon. Journal of Traumatic Stress. 2012;25:376–383. doi: 10.1002/jts.21721. [DOI] [PubMed] [Google Scholar]
- Catani M, De Schotten MT. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell’Acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neuroscience & Biobehavioral Reviews. 2013;37:1724–1737. doi: 10.1016/j.neubiorev.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological Psychiatry. 2009;65:227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. Socioemotional, personality, and biological development: Illustrations from a multilevel developmental psychopathology perspective on child maltreatment. Annual Review of Psychology. 2016;67:187–211. doi: 10.1146/annurev-psych-122414-033259. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3. Mahwah, NJ: Erlbaum; 2003. [Google Scholar]
- Cragg BG, Hamlyn LH. Histologic connections and electrical and autonomic responses evoked by stimulation of the dorsal fornix in the rabbit. Experimental Neurology. 1959;1:187–213. doi: 10.1016/0014-4886(59)90001-9. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington HL, Milne BJ, Polanczyk G, Pariante CM, … Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: Depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatrics and Adolescent Medicine. 2009;163:1135. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, … Kugel H. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, … Ryan ND. Developmental traumatology: Part II. Brain development. Biological Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- De Olmos JS, Ingram W. The projection field of the stria terminalis in the rat brain: An experimental study. Journal of Comparative Neurology. 1972;146:303–333. doi: 10.1002/cne.901460303. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Research Reviews. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhász C, Muzik O, Maqbool M, … Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics. 2006;117:2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N. Inhibition of adrenocortical responses following olfactory stimulation in rats with stria terminalis lesions. Neuroscience. 1980;5:1323–1329. doi: 10.1016/0306-4522(80)90204-3. [DOI] [PubMed] [Google Scholar]
- Flood JF, Merbaum MO, Morley JE. The memory enhancing effects of cholecystokinin octapeptide are dependent on an intact stria terminalis. Neurobiology of Learning and Memory. 1995;64:139–145. doi: 10.1006/nlme.1995.1053. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, research version, patient edition (SCID-I/P) New York: New York State Psychiatric Institute Biometrics Research; 2002. [Google Scholar]
- French L, McCrea M, Baggett M. The military acute concussion evaluation (MACE) Journal of Special Operations Medicine. 2008;8:68–77. [Google Scholar]
- Gorka AX, Hanson JL, Radtke SR, Hariri AR. Reduced hippocampal and medial prefrontal gray matter mediate the association between reported childhood maltreatment and trait anxiety in adulthood and predict sensitivity to future life stress. Biology of Mood and Anxiety Disorders. 2014;4:1–10. doi: 10.1186/2045-5380-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, Meuli R. Understanding diffusion MR imaging techniques: From scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics. 2006;26(Suppl 1):S205–S223. doi: 10.1148/rg.26si065510. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, Pollak SD. Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Development. 2013;84:1566–1578. doi: 10.1111/cdev.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. Journal of Child Psychology and Psychiatry. 2004;45:260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Heilbronner SR, Haber SN. Frontal cortical and subcortical projections provide a basis for segmenting the cingulum bundle: Implications for neuroimaging and psychiatric disorders. Journal of Neuroscience. 2014;34:10041–10054. doi: 10.1523/JNEUROSCI.5459-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Phillips ML, Fournier JC, Kronhaus DM, Germain A. Childhood and adult trauma both correlate with dorsal anterior cingulate activation to threat in combat veterans. Psychological Medicine. 2013;43:1533–1542. doi: 10.1017/S0033291712002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildyard KL, Wolfe DA. Child neglect: Developmental issues and outcomes. Child Abuse & Neglect. 2002;26:679–695. doi: 10.1016/S0145-2134(02)00341-1. [DOI] [PubMed] [Google Scholar]
- Huang H, Gundapuneedi T, Rao U. White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology. 2012;37:2693–2701. doi: 10.1038/npp.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TEJ. Diffusion MRI: From quantitative measurement to in-vivo neuroanatomy. 1. New York: Elsevier Science; 2009. [Google Scholar]
- Jones DK, Christiansen KF, Chapman RJ, Aggleton JP. Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: Implications for neuropsychological investigations. Neuropsychologia. 2013;51:67–78. doi: 10.1016/j.neuropsychologia.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, Mora CA. Clinical evaluation of a measure to assess combat exposure. Journal of Consulting and Clinical Psychology. 1989;1:53. [Google Scholar]
- Keding TJ, Herringa RJ. Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacology. 2015;40:537–545. doi: 10.1038/npp.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BM, Rollins BL, Grundmann SJ, Olivier LG. Excessive weight gains in female rats with transections of the stria terminalis. Physiology & Behavior. 2003;78:563–568. doi: 10.1016/S0031-9384(03)00042-8. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Poulton R, Martin J, Caspi A. Early childhood factors associated with the development of post-traumatic stress disorder: Results from a longitudinal birth cohort. Psychological Medicine. 2007;37:181–192. doi: 10.1017/S0033291706009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar MS, Grieve SM, Koslow SH, Gabrieli JDE, Gordon E, Williams LM. Loss of white matter integrity in major depressive disorder: Evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Human Brain Mapping. 2011;32:2161–2171. doi: 10.1002/hbm.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar MS, Rekshan W, Gordon E, Rush AJ, Williams LM, Blasey C, Grieve SM. Magnetic resonance imaging measures of brain structure to predict antidepressant treatment outcome in major depressive disorder. EBioMedicine. 2015;2:37–45. doi: 10.1016/j.ebiom.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain. 2007;130:2508. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Kuo JR, Kaloupek DG, Woodward SH. Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: A cross-sectional study. Archives of General Psychiatry. 2012;69:1080–1086. doi: 10.1001/archgenpsychiatry.2012.73. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Levine TR, Hullett CR. Eta squared, partial eta squared, and misreporting of effect size in communication research. Human Communication Research. 2002;28:612–625. [Google Scholar]
- Long Z, Duan X, Xie B, Du H, Li R, Xu Q, … Chen H. Altered brain structural connectivity in post-traumatic stress disorder: A diffusion tensor imaging tractography study. Journal of Affective Disorders. 2013;150:798–806. doi: 10.1016/j.jad.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Luders E, Clark K, Narr KL, Toga AW. Enhanced brain connectivity in long-term meditation practitioners. NeuroImage. 2011;57:1308–1316. doi: 10.1016/j.neuroimage.2011.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden H, Espinoza RT, Pirnia T, Clark K, Joshi SH, Leaver AM, … Narr KL. Electroconvulsive therapy mediates neuroplasticity of white matter microstructure in major depression. Translational Psychiatry. 2014;4:e380. doi: 10.1038/tp.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Conron KJ, Koenen KC, Gilman SE. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: A test of the stress sensitization hypothesis in a population-based sample of adults. Psychological Medicine. 2010;40:1647–1658. doi: 10.1017/S0033291709992121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PCM, Nagae-Poetscher LM. MRI atlas of human white matter. Amsterdam: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Paradise lost: The neurobiological and clinical consequences of child abuse and neglect. Neuron. 2016;89:892–909. doi: 10.1016/j.neuron.2016.01.019. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen C. The human central nervous system: A synopsis and atlas. 4. Berlin: Springer; 2008. [Google Scholar]
- Osborne B, Sivakumaran T, Black AH. Effects of fornix lesions on adrenocortical responses to changes in environmental stimulation. Behavioral and Neural Biology. 1979;25:227–241. doi: 10.1016/S0163-1047(79)90584-3. [DOI] [PubMed] [Google Scholar]
- Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: Evidence for multiple memory systems. Journal of Neuroscience. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, McLaughlin KA, Smith DAR, Ellis PM. Childhood maltreatment and DSM-IV adult mental disorders: Comparison of prospective and retrospective findings. British Journal of Psychiatry. 2012;200:469. doi: 10.1192/bjp.bp.111.103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, … Koerte I. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging and Behavior. 2012;6:137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, … Bartsch AJ. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nature Protocols. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychological Medicine. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Tang YY, Holzel BK, Posner MI. The neuroscience of mindfulness meditation. Nature Reviews Neuroscience. 2015;16:213–225. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- Tang YY, Tang R, Posner MI. Mindfulness meditation improves emotion regulation and reduces drug abuse. Drug and Alcohol Dependence. 2016;163(Suppl 1):S13–S18. doi: 10.1016/j.drugalcdep.2015.11.041. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA. Annual Research Review: Enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology and Psychiatry. 2016;57:241–266. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugwu ID, Amico F, Carballedo A, Fagan AJ, Frodl T. Childhood adversity, depression, age and gender effects on white matter microstructure: A DTI study. Brain Structure and Function. 2015;220:1997–2009. doi: 10.1007/s00429-014-0769-x. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain. 2013;136:1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstein RL, Lazar NA. The ASA’s statement on p-values: Context, process, and purpose. American Statistician. 2016 Advance online publication. [Google Scholar]
- Weathers FW, Keane TM, Davidson JRT. Clinician-Administered PTSD Scale: A review of the first ten years of research. Depression and Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Kuo JR, Schaer M, Kaloupek DG, Eliez S. Early adversity and combat exposure interact to influence anterior cingulate cortex volume in combat veterans. NeuroImage. 2013;2:670–674. doi: 10.1016/j.nicl.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]