Abstract
Circulating monocytes and tissue macrophages play complex roles in the pathogenesis of hypertension, a highly prevalent disease associated with catastrophic cardiovascular morbidity. In the vasculature and kidney, macrophage-derived reactive oxygen species (ROS) and inflammatory cytokines induce endothelial and epithelial dysfunction, respectively, resulting in vascular oxidative stress and impairment of sodium excretion. By contrast, VEGF-C-expressing macrophages in the skin can facilitate the removal of excess interstitial stores of sodium by stimulating lymphangiogenesis. Inappropriate activation of the renin-angiotensin system (RAS) contributes to essential hypertension in a majority of patients, and macrophages express the type 1 (AT1) receptor for angiotensin II (Ang II). While proinflammatory macrophages clearly contribute to RAS-dependent hypertension, activation of the AT1 receptor directly on macrophages suppresses their M1 polarization and limits tubular and interstitial damage to the kidney during hypertension. Thus, stimulating the macrophage AT1 receptor ameliorates the target organ damage and immune stimulation provoked by AT1 receptor activation in intrinsic renal and vascular cells. The proinflammatory cytokines TNF-α and IL-1β produced by M1 macrophages drive blood pressure elevation and consequent target organ damage. However, additional studies are needed to identify the tissues in which these cytokines act and the signaling pathways they stimulate during hypertension. Moreover, identifying the precise myeloid cell subsets that contribute to hypertension should guide the development of more precise immunomodulatory therapies for patients with persistent blood pressure elevation and progressive end-organ injury.
Keywords: Hypertension, Macrophage, Monocyte, Angiotensin II, Tumor necrosis factor, Interleukin 1
Introduction
Hypertension is a global pandemic, impacting more than one billion people. As uncontrolled hypertension leads to severe end-organ damage manifested as congestive heart failure (CHF), myocardial infarction (MI), stroke, and chronic kidney disease (CKD), it is the leading cause of preventable morbidity and mortality worldwide [30]. A systematic analysis of patient data from over 90 countries found that only 46.5% of individuals were aware of their hypertension, 36.9% were treated with anti-hypertensive medications, and 13.5% had their blood pressure adequately controlled. While these statistics varied somewhat by national income, with high-income countries having improved awareness, rates of treatment and control even in these richer nations were only 55.6 and 28.4%, respectively [71]. These data showing inadequate blood pressure control despite treatment with antihypertensive medications suggest that while hypertension is a multifactorial problem and social factors certainly play a role, novel therapeutic approaches will be necessary to reduce the global burden of hypertensive disease.
A promising path in the search for new drug targets involves investigating the role of the immune system in hypertension. The immune system has long been implicated in the pathogenesis of hypertension [69, 92, 119]. Pathologic assessments of kidney biopsy specimens have shown renal invasion of leukocytes in the setting of hypertension, with a positive correlation between the degree of lymphocyte aggregation and arteriolar nephrosclerosis [39, 102]. Further studies in rodent models have shown that, in addition to lymphocytes, other immune cells including myeloid cells also accumulate in the kidney during hypertension [111] and that the transfer of these immune cells from hypertensive animals could lead to the development of hypertension in previously normotensive animals [81]. Moreover, modulation of inflammatory cell function with immunosuppressive agents can blunt renin-angiotensin system (RAS)-induced hypertension [91] and reduce target organ damage in this setting [74].
One immune cell lineage that has demonstrated particular importance in the development of RAS-induced hypertension and subsequent end-organ damage is the macrophage. Macrophages are resident tissue phagocytes of myeloid descent. In addition to phagocytosis, these cells play a critical role in the innate immune system by secreting cytokines and chemokines and by recruiting and activating other immune cells. Through activation of type 1 receptors, angiotensin (Ang) II drives hemodynamic injury [85] and subsequent recruitment of monocytes and other inflammatory cells into the heart, vasculature, and kidney during hypertension [74, 82, 118]. After infiltration in tissues, monocytes can differentiate into one of at least two classically described phenotypes, M1 or M2 macrophages, based on a simplified paradigm [88]. M1 macrophages induce inflammation through the production of reactive oxygen species (ROS) along with proinflammatory cytokines, including interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α). M2 macrophages play an anti-inflammatory role and mediate tissue repair through the secretion of IL-10 and transforming growth factor-beta (TGF-β) [33, 100]. This paradigm has recently been updated to a more precise nomenclature based on specific activating agents [75], and we will discuss this newer nomenclature below as it pertains to the effects of macrophage subpopulations on blood pressure. Following their initial polarization, macrophages remain plastic and retain the ability to differentiate into other phenotypes with appropriate activation [8]. This review will discuss the role that macrophages play in RAS-induced hypertension and subsequent target organ damage.
Diverse roles for macrophages in hypertension
Macrophages are one of the major effector cells of the innate immune system and numerous studies point to their involvement in the pathogenesis of hypertension. Circulating monocytes from hypertensive patients have an enhanced proinflammatory phenotype compared to normotensive controls [86], and sera from these hypertensive patients contain elevated concentrations of injurious cytokines [25, 66, 84]. These inflammatory cytokines and ROS secreted by macrophages can elevate blood pressure by triggering vascular endothelial dysfunction with consequent impairment in renal sodium excretion [69]. As activated macrophages and monocytes express lysozyme M (LysM+), the LysM Cre mouse line has been utilized in conditional gene targeting strategies to study the independent role of macrophages in hypertension [17]. Other potential Cre models could be used for this purpose, including the colony-stimulating factor 1 receptor (CSF1r) Cre. However, as CSF-1 is critical to macrophage production, differentiation, and function, it is less specific than LysM to activated macrophages and may therefore be less specific for infiltrating macrophages in disease states such as hypertension [59]. Using LysM Cre mice crossed with mice expressing a cre-inducible diphtheria toxin (DTX) receptor (iDTR), Wenzel et al. selectively ablated LysM+ macrophages and monocytes through the administration of diphtheria toxin. In the chronic Ang II infusion model, depletion of these LysM-expressing cells limited blood pressure elevation, ameliorated vascular endothelial and smooth muscle dysfunction, and attenuated vascular ROS formation. As granulocytes can also express LysM, the investigators further demonstrated that transfer of wild-type (WT) CD11b+ monocytes and not WT Gr1+ neutrophils into their LysMiDTR mice restored Ang II-induced hypertension, vascular dysfunction, and ROS generation. [118]. Further work demonstrated that these LysM+ monocytes can also spur ROS production by uncoupling nitric oxide synthase (NOS) 3, culminating in further increases in vascular oxidative stress and hypertension [54].While recent studies have established that not all tissue macrophages are descendants of circulating blood monocytes, these monocytes do significantly contribute to tissue macrophage populations under inflammatory conditions [41]. Thus, the previously described experiments from Wenzel and colleagues suggest that macrophages play a key pathogenic role in RAS-induced hypertension and its sequelae.
In contrast to their pro-hypertensive actions in the vasculature, macrophages in the skin can limit the susceptibility to salt-sensitive hypertension by stimulating lymphangiogenesis [47, 93]. In the classical paradigm of salt-sensitive hypertension, inappropriate retention of sodium and water by the kidney permits an expansion in extracellular fluid volume (ECV) with consequent increases in cardiac output and hypertension per Ohm’s Law. Challenging the simplicity of this model, a team led by Titze found that an excess of sodium bound to proteoglycans in the skin and muscle correlates with prolonged hypertension and that dermal lymphatic vessels may regulate blood pressure by mobilizing these non-vascular sodium reservoirs [22, 32, 53, 112]. In response to osmotic stress, induction of the transcription factor tonicity-responsive enhancer-binding protein (TONEBP/NFAT5) in dermal macrophages upregulates their expression of vascular endothelial growth factor C (VEGF-C) [65]. In turn, local VEGF-C drives lymphangiogenesis and mobilization of sodium from the skin. Accordingly, deletion of TONEBP from macrophages in rodent models impairs VEGF-C generation and lymph vessel proliferation leading to blood pressure elevation on a high salt diet (HSD) [64, 120]. As TONEBP also mediates NOS2-dependent generation of NO in macrophages, and NO drives vasodilation and sodium excretion in the kidney, TONEBP in kidney macrophages could conceivably also combat salt sensitivity by limiting renal sodium re-absorption [43, 130]. Recent studies by Zhang et al. further illustrate that cyclooxygenase-2 (COX2) in macrophages can alleviate salt sensitivity not only by driving the production of natriuretic prostaglandins but also by promoting VEGF-C-dependent lymphangiogenesis [128].
Growing evidence also suggests that dietary salt can conversely influence macrophage polarization. Lipopolysaccharide (LPS) induces polarization of bone marrow-derived macrophages (BMDM) toward a proinflammatory phenotype, M(LPS). In culturing M(LPS) in high salt media, Jantsch et al. found that high salt concentration led to an exaggerated proinflammatory response evidenced by enhanced p38 mitogen-activated protein kinase (p38/MAPK)-dependent TONEBP/NFAT5 activation and a resultant increase in NO production via a type-2 nitric oxide synthase (Nos2)-dependent mechanism in these cells [43]. Moreover, high NaCl concentrations have also been shown to blunt IL-4, IL-13-dependent activation of macrophages toward an anti-inflammatory phenotype, M(IL-4 + IL-13), in vitro via impaired AKT/mTOR signaling and cellular metabolism [7]. Even in the absence of other external activators, high salt alone can increase proinflammatory gene expression and decrease anti-inflammatory gene expression in BMDM, resulting in a unique macrophage activation state, M(Na) [129]. Thus, sodium retention may elevate blood pressure not only by expanding intravascular volume but also by modulating macrophage phenotype to promote injury in the kidney and vasculature.
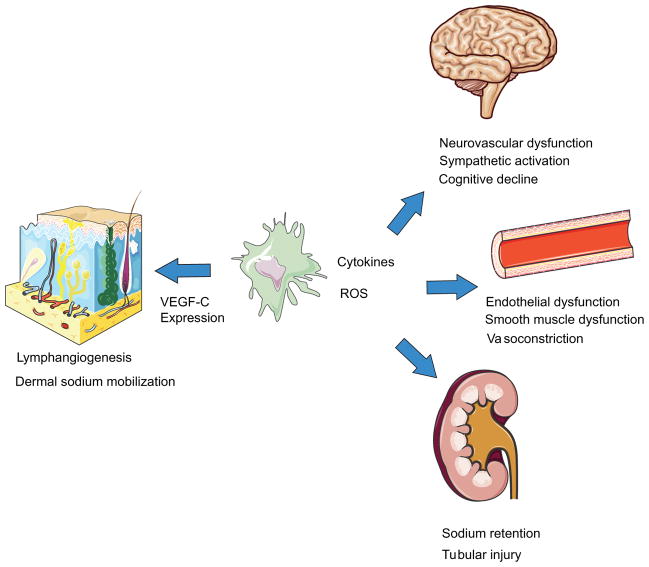
Macrophages also play a role in mediating hypertensive end-organ damage through blood pressure-independent mechanisms. Monocyte chemoattractant protein (MCP-1 or CCL2) is a chemokine that binds CC chemokine receptor 2 (CCR2) on monocytes, driving their recruitment into injured tissues [9, 10]. In the Ang II hypertension model, CCR2 deficiency limits the accumulation of macrophages in the kidney, attenuating local oxidative stress and renal fibrosis while preserving the glomerular filtration rate [60]. Mice deficient in the monocyte/macrophage chemotactic factor, macrophage colony-stimulating factor (m-CSF) [105, 115], due to the osteopetrotic mutation in the m-CSF gene (Op/Op) have monocytopenia and macrophage deficiency [61, 121, 122]. Similar to the CCL2 knockouts, Op/Op mice are partially protected from Ang II-induced endothelial dysfunction, vascular remodeling, and oxidative stress [24]. These findings have been confirmed in the deoxycorticosterone acetate (DOCA)-salt model [51]. Recent studies also demonstrate the importance of macrophages in hypertensive neuroinflammation. Santisteban et al. found that transplant of bone marrow from spontaneously hypertensive rats into nonhypertensive rats resulted in increased neuroinflammation and sympathetic nerve activation likely via CCL2-mediated extravasation of mononuclear cells into the CSF and subsequent microglial activation [38, 95, 97]. Furthermore, perivascular macrophages (PVMs) in the brain (CD206+CD45hiCDllb+) have been implicated in cognitive decline associated with hypertension. Through selective depletion of this cell population in mice with clodronate and use of two models of hypertension, Faraco and colleagues showed that PVMs were critical in hypertensive neurovascular dysfunction [27, 37]. Thus, macrophages play an important role in hypertension-induced damage to the kidney, vasculature, and brain (Fig. 1).
Fig. 1.
Summary of the roles of macrophages in hypertension. Through generation of inflammatory cytokines and reactive oxygen species, macrophages can directly impair vasculature endothelial and smooth muscle function leading to vasoconstriction and resultant hypertension. Similar processes in the renal vasculature impair sodium excretion by the kidney also resulting in blood pressure elevation. Macrophage-induced damage to the renal parenchyma can also instigate renal tubular injury and interstitial fibrosis seen in hypertension. In the brain, distinct macrophage populations increase hypertensive neuroinflammation and sympathetic neuron activation. Populations of macrophages in the brain also mediate neurovascular dysfunction and cognitive decline associated with hypertension. Through a different mechanism, dermal macrophages increase expression of VEGF-C in response to osmotic stress resulting increased lymphangiogenesis and lymphatic flow. These processes work to mobilize dermal sodium stores and may thereby serve as an important extra-renal regulator of sodium homeostasis and consequently blood pressure
Interaction between macrophages and the renin-angiotensin system
Through activation of the type 1 angiotensin (AT1) receptor, Ang II plays a central role in driving chronic blood pressure elevation via its capacity to augment global vascular tone and promote sodium retention in the kidney [36, 42, 80, 117]. However, AT1 receptor activation can also mediate hypertensive organ damage through blood pressure-independent mechanisms. For example, Müller et al. showed that AT1 receptor blockade with Valsartan alleviates hypertensive kidney and heart injury in transgenic rats with over-expression of human renin and angiotensinogen genes to a greater extent than blood pressure lowering with hydralazine, hydrochlorothiazide, and reserpine [70, 73]. In this model, the protective effects of AT1 receptor blockade accrued from inhibition of proinflammatory NF-κB signals, suggesting that AT1 receptor stimulation can propagate an inflammatory response independently of blood pressure elevation [72].
Global RAS activation triggers monocyte migration and activation. For example, chronic Ang II infusion drives persistent accumulation of macrophages in the renal interstitium leading to kidney fibrosis [82]. Systemic AT1 receptor ligation stimulates egress of monocytes from the spleen and their migration to vascular subendothelium, necessary steps in mediating vascular injury and the development of atherosclerotic lesions [49, 109]. Ang II promotes the differentiation of hematopoietic stem cells (HSCs) toward CCR2+ inflammatory monocytes and enhances monocyte production of cytokines and adhesion to endothelial cells [35]. Thus, at earlier stages of myeloid cell development, Ang II favors differentiation, mobilization, and activation of proinflammatory monocytes [48].
AT1 receptors are broadly expressed on immune cell populations [44, 77]. However, in several studies, AT1 receptor stimulation on these cells has dampened the RAS-induced inflammatory response in an apparent feedback mechanism [20, 125, 126]. In contrast to humans who have one AT1 isoform, rodents possess two AT1 receptor isoforms, AT1A and AT1B. AT1A receptors predominate in most tissues and have been considered the most homologous to the human AT1 receptor [13, 18]. Thus, initial work in this area utilized murine bone marrow chimeras lacking AT1A solely on immune cells. Tsukuba mice have human renin and angiotensinogen genes and therefore serve as a model of hypertension induced by chronic RAS stimulation. Tsukuba mouse chimeras lacking the AT1A receptor on bone marrow-derived cells have a preserved hypertensive response but more severe atherosclerosis in the aorta compared to wild-type transplant controls [46]. Using a chronic Ang II infusion model, our group found that Agtr1a−/− bone marrow chimeras have exacerbated blood pressure elevation, albuminuria, and accumulation of T cells and macrophages in the kidney compared to their WT counterparts [20]. These studies point to a protective role for the AT1 receptor on immune cells during hypertension.
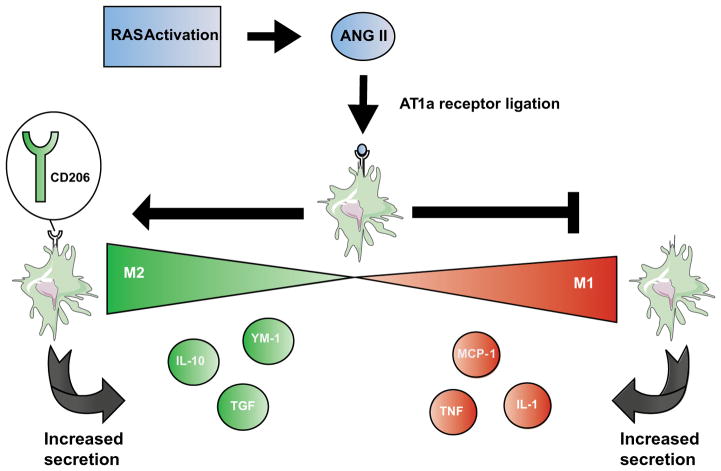
Several protective actions of the immune cell AT1 receptor that have been elucidated in murine bone marrow chimera studies can be ascribed directly to the AT1 receptor on macrophages. For example, Nishida and colleagues reported that Agtr1−/− bone marrow chimeras have more severe kidney fibrosis following unilateral ureteral obstruction (UUO), and that AT1 receptor deficiency or blockade on BMDMs impaired their phagocytic capacity [79]. The beneficial actions of the macrophage AT1 receptor could also accrue from effects on macrophage polarization. In this regard, peritoneal macrophages from Agtr1a−/− animals have enhanced expression of proinflammatory M1 phenotypic markers (MCP-1, TNF-α, IL-1β) and reduced markers of M2 activation (mannose receptor (CD206), Ym-1, TGF-β, IL-10, decoy IL-1R), suggesting that AT1 receptor activation on macrophages suppresses their M1 polarization [63]. Using a Cre-Lox conditional gene targeting strategy to delete the AT1A receptor selectively from LysM+ myeloid cells (“Macro KO”), we detected enhanced M1 polarization in AT1 receptor-deficient macrophages, leading to their enhanced secretion of TNF-α and IL-1β. These Macro KO animals lacking the macrophage AT1 receptor developed more severe kidney tubular damage and fibrosis following 4 weeks of Ang II-induced hypertension and in the UUO model [126]. Thus, AT1 receptor activation on macrophages does not have a major role in regulating blood pressure homeostasis, but does limit kidney damage and fibrosis during RAS activation by blunting M1 macrophage differentiation (Fig. 2). Although we were unable to detect AT1B expression in macrophages, other groups have detected this isoform in mononuclear cells and attributed autoimmune inflammation in the central nervous system to signaling via the AT1B receptor [106]. Future experiments should therefore consider utilizing AT1A/AT1B double knockout experiments to more precisely delineate the independent roles of the 2 AT1 receptor isoforms on macrophages in hypertension.
Fig. 2.
Effect of renin-angiotensin system activation on macrophage polarization. RAS activation triggers monocyte migration and activation. In the kidney, this process results in the accumulation of macrophages in the renal interstitium. AT1a receptor ligation on macrophages reduces expression of M1 phenotypic markers (MCP-1 (CCL-2), TNF-α, IL-1β), favoring expression of M2 phenotypic markers (Mannose receptor (CD206), TGF-beta, and IL-10). These alterations in gene expression have an anti-inflammatory effect and function to restrict damage to kidney tubules and interstitium instigated by RAS activation in the target organ
To reconcile the seemingly discrepant findings that global RAS activation generally induces inflammation whereas AT1 receptor activation directly on myeloid cells is largely anti-inflammatory, we postulate that AT1 receptor stimulation on macrophages may provide a feedback mechanism to temper the pathogenic effects of inappropriate RAS activation in the kidney, vasculature, and nervous system. Using a kidney cross-transplant model, we previously reported that kidney AT1 receptors instigate RAS-induced hypertension, cardiac hypertrophy, and kidney injury [19, 21]. Thus, we posit that activation of AT1 receptors in the kidney mediates target organ damage that, in turn, invokes secondary immune activation with consequent release of proinflammatory cytokines, including TNF-α and IL-1β, from mononuclear cells that infiltrate these injured tissues.
Role of M1 cytokines in hypertension and target organ damage
Several lines of evidence indicate that the prototypical M1 macrophage cytokines, TNF-α and IL-1β, can play an important role in blood pressure homeostasis. Serum levels of both cytokines are markedly elevated during hypotension in sepsis [31]. Furthermore, intravenous infusion of either TNF-α or IL-1β can trigger natriuresis and thereby reduce blood pressure [52, 94, 96, 113]. On the other hand, genetic deletion of TNF or the IL-1 receptor alleviates blood pressure elevation during RAS activation [34, 104, 127, 130]. In aggregate, these experiments suggest that the blood pressure-modulating effects of these cytokines may depend on their absolute levels and tissue localization. To better understand the specific roles of TNF and IL-1 in mediating hypertension and target organ damage, our lab and others have examined the tissue-specific actions of these cytokines.
Global RAS activation triggers expression of TNF-α in a variety of cell types including macrophages, T cells, mesangial cells, kidney epithelial cells, and mammalian heart cells [28, 45, 50]. After secretion, TNF mediates its effects through one of two receptors, TNFR1 and TNFR2, which although ubiquitously expressed, have tissue-dependent differences in relative density [11, 15]. Receptor activation, in turn, activates multiple signaling pathways, including mitogen-activated protein kinase (MAPK), TGF-β, NF-κB, and NADPH oxidase [50, 76, 103]. Activation of these receptors appears to have divergent effects on blood pressure [14, 15, 101]. However, experiments with TNF “knockout” animals illustrate that, in the setting of RAS activation, TNF potentiates blood pressure elevation by augmenting salt appetite through activity in the central nervous system (CNS) and stimulating sodium reabsorption in the kidney’s thick ascending limb (TAL) via NO synthase 3 suppression [87, 104]. Data from our group support these findings, as selective TNF deficiency in kidney generated via a murine cross-transplant approach attenuates the chronic hypertensive response [127]. TNF-dependent activation of NF-κB and NADPH oxidase also impairs vascular endothelial function by stimulating ROS generation and impairing NO production in the vasculature, processes that serve to enhance susceptibility to hypertensive stimuli [2, 57, 78, 108].
TNF mediates damage to the kidney and heart during hypertension, but whether macrophages are the source of the TNF that drives this injury is not clear. In rodents, TNF blockade or deficiency attenuates kidney injury during RAS-dependent hypertension [74, 127]. In this setting, TNF secreted by proinflammatory Th1 lymphocytes increases injury within the kidney glomerulus through a blood pressure-independent mechanism [125]. Moreover, an infusion of TNF is directly toxic to the glomerular endothelium [6], and induces renal vasoconstriction, increasing the risk for local ischemia. TNF plays a pathogenic role in kidney fibrosis, which could impair renal sodium excretion and exacerbate hypertension [67]. In the setting of acute kidney injury, others and we find that TNF produced by intrinsic kidney epithelial cells can mediate renal parenchymal damage, but the relative contributions of TNF produced by the kidney versus macrophages to hypertensive kidney injury have yet to be described [124, 131]. As in the kidney, TNF in the heart and vasculature mediates injury and pathologic remodeling in RAS-induced hypertension [3, 4, 12, 56, 76, 107]. Given the broad detrimental actions of TNF in the kidney and vasculature during hypertension, future studies will need to explore how to target this pathway in hypertensive patients while limiting excessive immunosuppression.
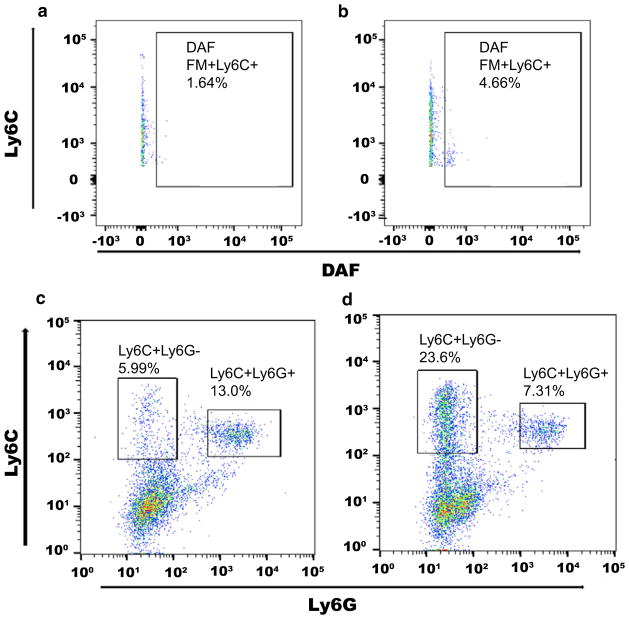
Analogous to the effects of TNF, several actions of IL-1 potentiate hypertension and hypertensive organ damage [116, 126]. In response to blood pressure elevation, components of the NLRP3 inflammasome are upregulated resulting in increased expression of IL-1β [55]. Deficiency of NLRP3 components leads to attenuation of the hypertensive response, highlighting the importance of IL-1β induction in mediating hypertension [99]. Upon activation, IL-1 may instigate hypertension through several tissue-specific mechanisms. In the CNS, IL-1 injection stimulates catecholamine release leading to systemic blood pressure elevation [83, 98, 110]. IL-1 can also induce vasoconstriction in the aorta and pulmonary vasculature [26, 114]. Recent studies from our lab reveal that mice deficient in the IL-1 receptor are partially protected from Ang II-induced blood pressure elevation. In our hands, IL-1 receptor activation suppresses the accumulation of NO-expressing macrophages in the kidney early in the course of RAS-dependent hypertension (Fig. 3), thereby easing the tonic inhibition of the NKCC2 sodium cotransporter by NO, with consequent renal salt retention. In this setting, IL-1 receptor stimulation appears to inhibit NOS2 expression from intra-renal macrophages leading to reduction of NO secretion, such that NO-depletion restores the hypertensive response of the IL-1 receptor-deficient animals [130]. Interestingly, in other contexts, IL-1 receptor activation on macrophages can actually enhance NO secretion [62]. Thus, further studies should clarify whether these discrepancies are due to context-specific actions of IL-1 on myeloid cells or to an indirect effect of IL-1 receptors on kidney parenchymal cells to influence the phenotype of infiltrating myeloid cells. The capacity of intrinsic renal cells to alter the polarization of infiltrating macrophages has been demonstrated in the setting of acute kidney injury [58].
Fig. 3.
Activation of IL-1R1 constrains the accumulation of nitric oxide (NO)-producing myeloid cells in the hypertensive kidney. Reproduced from Zhang et al. Cell Metab. 2016;23(2):360–368 [128]. a, b Enhanced production of nitric oxide by IL-1R1-deficient (KO)-activated macrophages infiltrating the kidney at day 7 of chronic Ang II infusion. Diaminofluorescein (DAF) staining for NO on intra-renal CD11b+ Ly6C+ myeloid cells from a WT and b IL-1R1 KO cohorts. c, d Shift away from Ly6C + Ly6G+ double-positive immature myeloid populations toward single Ly6C+ phenotype among intra-renal IL-1R1 KO-activated macrophages at day 7 of Ang II. Representative flow plots of Ly6C versus Ly6G staining among c WT and d IL-1R1 KO CD11b+ myeloid cells
Conclusion
Macrophages are clearly important in the pathogenesis of hypertension as are their classical proinflammatory cytokines TNF-α and IL-1β. From the evidence above, macrophages promote hypertension through the generation of these M1 cytokines and reactive oxygen species resulting in enhanced renal sodium retention and target organ damage. The presence of elevated levels of these cytokines in monocytes and the serum in human studies of hypertensive patients is consistent with this conclusion [5, 23, 25]. As broad immune suppression has been associated with blood pressure reduction in patients with rheumatologic disease, interventions to modulate macrophage function may represent a novel class of therapies for patients with recalcitrant hypertension and end-organ injury [40]. Nevertheless, these therapies are not without risk, as immunosuppression can impede tumor surveillance and permit reactivation of latent infection [29].
Targeting macrophage cytokines including TNF and IL-1 may yield therapeutic benefits once investigations further parse the relevant, tissue-specific signaling pathways activated by these mediators during hypertension. The discrepant effects of the two TNF receptors and apparent tissue-specific actions of the IL-1 receptor pose challenges in bringing blockade of these cytokines into the hypertension clinic, and human trials of TNF or IL-1 blockade for heart failure or atherosclerosis, respectively, have so far not proved conclusive [1, 16, 68, 89, 90]. However, the lack of a blood pressure signal in these trials should not preclude a careful study of cytokine antagonism for severe hypertension. To this point, selective inhibition of TNF with infliximab reduced continuous ambulatory blood pressure in a small trial for patients with hypertension and rheumatoid arthritis [123]. We therefore submit that blockade of macrophage functions or cytokines should be studied further particularly in hypertensive patients with evidence of M1 macrophage activation and markers of cardiac or renal damage. In these patients, the potential longer term benefits of immunomodulation may outweigh the immediate risks. In the meantime, pre-clinical studies should identify more precisely the myeloid cell subpopulations involved in the pathogenesis of hypertension and its complications in order to develop more incisive immunomodulatory therapies for this pandemic disease.
Acknowledgments
NIH grants DK087893, HL128355; Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Grant BX000893; Duke O’Brien Center for Kidney Research.
References
- 1.Abbate A, Kontos M, Grizzard J, Biondi-Zoccai G, Van Tassell B, Robati R, Roach L, Arena R, Roberts C, Varma A, Gelwix C, Salloum F, Hastillo A, Dinarello C, Vetrovec G VA Investigators. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction Virginia Commonwealth University Anakinra Remodeling Trial (VCU-ART) Pilot study. Am J Cardiol. 2010;105:1371–1377. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 2.Alonso J, Sánchez de Miguel L, Montón M, Casado S, López-Farré A. Endothelial cytosolic proteins bind to the 3′ untranslated region of endothelial nitric oxide synthase mRNA: regulation by tumor necrosis factor alpha. Mol Cell Biol. 1997;17:5719–5726. doi: 10.1128/mcb.17.10.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angel K, Provan S, Gulseth H, Mowinckel P, Kyien T, Atar D. Tumor necrosis factor-alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: a controlled study. Hypertension. 2010;55:333–338. doi: 10.1161/HYPERTENSIONAHA.109.143982. [DOI] [PubMed] [Google Scholar]
- 4.Baluk P, Yao L, Feng J, Romano T, Jung S, Schreiter J, Yan L, Shealy D, McDonald D. TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest. 2009;119:2954–2964. doi: 10.1172/JCI37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bautista L, Vera L, Arenas I, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-α) and essential hypertension. J Hum Hypertens. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 6.Bertani T, Abbate M, Zoja C, Corna D, Perico N, Ghezzi P, Remuzzi G. Tumor necrosis factor induces glomerular damage in the rabbit. Am J Pathol. 1989;134:419–430. [PMC free article] [PubMed] [Google Scholar]
- 7.Binger K, Gebhardt M, Heinig M, Rintisch C, Schroeder A, Neuhofer W, Hilgers K, Manzel A, Schwartz C, Kleinwietfeld M, Voelkl J, Schatz V, Linker R, Lang F, Voehringer D, Wright M, Hubner N, Dechend R, Jantsch J, Titze J, Müller D. High salt reduces the activation of IL-4 and IL-13 stimulated macrophages. J Clin Invest. 2015;125:4223–4238. doi: 10.1172/JCI80919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas S, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 9.Boring L, Gosling J, Chensue S, Kunkel S, Farese R, Jr, Broxmeyer H, Charo I. Impaired monocyte migration and reduced type 1 (Th1) cytokine repsonses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boring L, Gosling J, Cleary M, Charo I. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 11.Bruggeman L, Drawz O, Kahoud N, Lin K, Barisoni L, Nelson P. TNFR2 interposes the proliferative and NF-kappaB-mediated inflammatory response by podocytes to TNF-alpha. Lab Investig. 2011;91:413–425. doi: 10.1038/labinvest.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998;97:1375–1381. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 13.Burson J, Aguilera G, Gross K, Sigmund C. Differential expression of angiotensin receptor 1A and 1B in mouse. Am J Phys. 1994;267:E260–E267. doi: 10.1152/ajpendo.1994.267.2.E260. [DOI] [PubMed] [Google Scholar]
- 14.Castillo A, Islam M, Prieto M, Majid D. Tumor necrosis factor-receptor type 1, not type 2, mediates its acute responses in the kidney. Am J Physiol Renal Physiol. 2012;302:F1650–F1657. doi: 10.1152/ajprenal.00426.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Pedraza P, Hao S, Stier C, Ferreri N. TNFR1-deficient mice display altered blood pressure and renal responses to ANG II infusion. Am J Physiol Renal Physiol. 2010;299:F1141–F1150. doi: 10.1152/ajprenal.00344.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung E, Packer M, Lo K, Fasanmade A, Willerson J. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure. Circ Res. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 17.Cross M, Renkawitz R. Repetitive sequence involvement in the duplication and divergence of mouse lysozyme genes. EMBO J. 1990;9:1283–1288. doi: 10.1002/j.1460-2075.1990.tb08237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowley S, Tharaux P, Audoly L, Coffman T. Exploring type I angiotensin (AT1) receptor functions through gene targeting. Acta Physiol Scand. 2004;181:561–570. doi: 10.1111/j.1365-201X.2004.01331.x. [DOI] [PubMed] [Google Scholar]
- 19.Crowley S, Gurley S, Herrera M, Ruiz P, Griffiths R, Kumar A, Kim H, Smithies O, Le T, Coffman T. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowley S, Song Y, Sprung G, Griffiths R, Sparks M, Yan M, Burchette J, Howell D, Lin E, Okeiyi B, Stegbauer J, Yang Y, Tharaux P, Ruiz P. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension. 2010;55:99–108. doi: 10.1161/HYPERTENSIONAHA.109.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crowley S, Zhang J, Herrera M, Griffiths R, Ruiz P, Coffman T. Role of AT1 receptor-mediated salt retention in angiotensin II-dependent hypertension. Am J Physiol Renal Physiol. 2011;301:F1124–F1140. doi: 10.1152/ajprenal.00305.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahlmann A, Dörfelt K, Eicher F, Linz P, Kopp C, Mössinger I, Horn S, Büschges-Seraphin B, Wabel P, Hammon M, Cavallaro A, Eckardt K, Kotanko P, Levin N, Johannes B, Uder M, Luft F, Müller D, Titze J. Magnetic resonance-determined sodium removal from tissue stroes in hemodialysis patients. Kidney Int. 2015;87:434–441. doi: 10.1038/ki.2014.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalekos G, Elisaf M, Bairaktari E, Tsolas O, Siamopoulos K. Increased serum levels of interleukin-1beta in the systemic circualtion of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J Lab Clin Med. 1997;129:300–308. doi: 10.1016/s0022-2143(97)90178-5. [DOI] [PubMed] [Google Scholar]
- 24.De Ciuceis C, Amiri F, Brassard P, Endemann D, Touyz R, Schiffrin E. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage Colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol. 2005;25:2106–2113. doi: 10.1161/01.ATV.0000181743.28028.57. [DOI] [PubMed] [Google Scholar]
- 25.Dörffel Y, Lätsch C, Stuhlmüller B, Schreiber S, Scholze S, Burmester G, Scholze J. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension. 1999;34:113–117. doi: 10.1161/01.hyp.34.1.113. [DOI] [PubMed] [Google Scholar]
- 26.Dorrance A. Interleukin 1-beta (IL-1beta) enhances contractile responses in endothelium-denuded aorta from hypertensive, but not normotensive, rats. Vasc Pharmacol. 2007;47:160–165. doi: 10.1016/j.vph.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban M, Racchumi G, Murphy M, Van Rooijen N, Anrather J, Iadecola C. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. 2016;126:4674–4689. doi: 10.1172/JCI86950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreri N, Escalante B, Zhao Y, An S, McGiff J. Angiotensin II induces TNF production by the thick ascending limb: functional implications. Am J Phys. 1998;274:F148–F155. doi: 10.1152/ajprenal.1998.274.1.F148. [DOI] [PubMed] [Google Scholar]
- 29.Gardam M, Keystone E, Menzies R, Manners S, Skamene E, Long R, Vinh D. Anti-tumour necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. Lancet Infect Dis. 2003;3:148–155. doi: 10.1016/s1473-3099(03)00545-0. [DOI] [PubMed] [Google Scholar]
- 30.GBD. Risk factors collaborators (2015) global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupation, and metabolic risk factors or clusters of risk in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2013;386:2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girardin E, Grau G, Dayer J, Roux-Lombard P, Lambert P Group tJS. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988;319:397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- 32.Go W, Liu X, Roti M, Liu F, Ho S. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci U S A. 2004;101:10673–10678. doi: 10.1073/pnas.0403139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordan S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 34.Guzik T, Hoch N, Brown K, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hahn A, Jonas U, Buhler F, et al. Activation of human peripheral monocytes by angiotensin II. FEBS Lett. 1994;347:178–180. doi: 10.1016/0014-5793(94)00531-1. [DOI] [PubMed] [Google Scholar]
- 36.Hall J, Brands M, Henegar J. Angiotensin II and long-term arterial pressure regulation: the overriding dominance fo the kidney. J Am Soc Nephrol. 1999;10(suppl 12):S258–S265. [PubMed] [Google Scholar]
- 37.Harrison D, Guzik T. Macrophages come to mind as keys to cognitive decline. J Clin Invest. 2016;126:4393–4395. doi: 10.1172/JCI91277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hay M. The good and the bad: immune cells and hypertension. Circ Res. 2015;117:830–831. doi: 10.1161/CIRCRESAHA.115.307506. [DOI] [PubMed] [Google Scholar]
- 39.Heptinstall R. Renal biospies in hypertension. Br Heart J. 1954;16:133–141. doi: 10.1136/hrt.16.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herrera J, Ferrebuz A, MacGregor E, Rodríguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–S225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 41.Höfer T, Busch K, Klapproth K, Rodewald H. Fate mapping and quantitation of hematopoiesis in vivo. Annu Rev Immunol. 2016;34:449–478. doi: 10.1146/annurev-immunol-032414-112019. [DOI] [PubMed] [Google Scholar]
- 42.Ito M, Oliverio M, Mannon P, Best C, Maeda N, Smithies O, Coffman T. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci U S A. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jantsch J, Schatz V, Friedrich D, Schröeder A, Kopp C, Siegert I, Maronna A, Wendelborn D, Linz P, Binger K, Gebhardt M, Heinig M, Neubert P, Fischer F, Teufel S, David J, Neufert C, Cavallaro A, Rakova N, Küper C, Beck F, Neuhofer W, Muller D, Schuler G, Uder M, Bogdan C, Luft F, Titze J. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab. 2015;21:493–501. doi: 10.1016/j.cmet.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jurewicz M, McDermott D, Sechler J, Tinckam K, Takakura A, Carpenter C, Milford E, Abdi R. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol. 2007;18:1093–1102. doi: 10.1681/ASN.2006070707. [DOI] [PubMed] [Google Scholar]
- 45.Kalra D, Sivasubramanian N, Mann D. Angiotensin II induces tumor necrosis factor biosynthesis in the adult mammalian heart through a protein kinase C-dependent pathway. Circulation. 2002;105:2198–2205. doi: 10.1161/01.cir.0000015603.84788.47. [DOI] [PubMed] [Google Scholar]
- 46.Kato H, Ishida J, Nagano K, Honjo K, Sugaya T, Takeda N, Sugiyama F, Yagami K, Fujita T, Nangaku M, Fukamizu A. Deterioration of atherosclerosis in mice lacking angiotensin II type 1 a receptor in bone marrow-derived cells. Lab Investig. 2008;88:731–739. doi: 10.1038/labinvest.2008.42. [DOI] [PubMed] [Google Scholar]
- 47.Kerjaschki D. The crucial role of macrophages in lymphangiognesis. J Clin Invest. 2005;115:2316–2319. doi: 10.1172/JCI26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S, Zingler M, Harrison J, Scott E, Cogle C, Luo D, Raizada M. Angiotensin II regulation of proliferation, differentiation, and engraftment of hematopoietic stem cells. Hypertension. 2016;67:574–584. doi: 10.1161/HYPERTENSIONAHA.115.06474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kintscher U, Wakino S, Kim S, Fleck E, Hsueh W, Law R. Angiotensin II induces migration and Pyk2/paxillin phosphorylation of human monocytes. Hypertension. 2001;37:587–593. doi: 10.1161/01.hyp.37.2.587. [DOI] [PubMed] [Google Scholar]
- 50.Kleinbongard P, Heusch G, Schulz R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127:295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Ko E, Amiri F, Pandey N, Javeshghani D, Leibovitz E, Touyz R, Schiffrin E. Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am J Physiol Heart Circ Physiol. 2007;292:H1789–H1795. doi: 10.1152/ajpheart.01118.2006. [DOI] [PubMed] [Google Scholar]
- 52.Kohan D, Merli C, Simon E. Micropuncture localization of the natriuretic effect of interleukin 1. Am J Physiol Renal Fluid Electrolyte Physiol. 1989;256:F810–F813. doi: 10.1152/ajprenal.1989.256.5.F810. [DOI] [PubMed] [Google Scholar]
- 53.Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Müller D, Schmieder R, Cavallaro A, Eckardt K, Uder M, Luft F, Titze J. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61:635–640. doi: 10.1161/HYPERTENSIONAHA.111.00566. [DOI] [PubMed] [Google Scholar]
- 54.Kossmann S, Hu H, Steven S, Schönfelder T, Fraccarollo D, Mikhed Y, Brähler M, Knorr M, Brandt M, Karbach S, Becker C, Oelze M, Bauersachs J, Widder J, Münzel T, Daiber A, Wenzel P. Inflammatory monocytes determine endothelial nitric-oxide synthase uncoupling and nitro-oxidative stress induced by angiotensin II. J Biol Chem. 2014;289:27540–27550. doi: 10.1074/jbc.M114.604231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krishnan S, Dowling J, Ling Y, Diep H, Chan C, Ferens D, Kett M, Pinar A, Samuel C, Vinh A, Arumugam T, Hewitson T, Kemp-Harper B, Robertson A, Cooper M, Latz E, Mansell A, Sobey C, Drummond G. Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br J Pharmacol. 2016;173:752–765. doi: 10.1111/bph.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kubota T, McTiernan C, Frye C, et al. Dilated cardiomyop-athy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-α. Circ Res. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 57.Landry D, Couper L, Bryant S, Lindner V. Activation of the NFkappaB and I kappa B system in smooth muscle cells after rat arterial injury. Induction of vascular cell adhesion molecule-1 and monocyte chemoattractant protein-1. Am J Pathol. 1997;151:1085–1095. [PMC free article] [PubMed] [Google Scholar]
- 58.Lee S, Huen S, Nishio H, Nishio S, Lee H, Choi B, Ruhrberg C, Cantley L. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Chen K, Zhu L, Pollard J. Conditional deletion of the colony stimulating factor-1 receptor (c-fms proto-oncogene) in mice. Genesis. 2006;44:328–335. doi: 10.1002/dvg.20219. [DOI] [PubMed] [Google Scholar]
- 60.Liao T, Yang X, Liu Y, Shesely E, Cavasin M, Kuziel W, Pagano P, Carretero O. Role of inflammation in the development of renal damage and dysfunction in angiotensin II induced hypertension. Hypertension. 2008;52:256–263. doi: 10.1161/HYPERTENSIONAHA.108.112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lieschke G, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler K, Basu S, Zhan Y, Dunn A. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–1746. [PubMed] [Google Scholar]
- 62.Lima-Junior D, Costa D, Carregaro V, Cunha L, Silva A, Mineo T, Gutierrez F, Bellio M, Bortoluci K, Flavell R, Bozza M, Silva J, Zamboni D. Inflammasome-derived IL-1beta production induces nitric oxide-mediated resistance to Leishmania. Nat Med. 2013;19:909–915. doi: 10.1038/nm.3221. [DOI] [PubMed] [Google Scholar]
- 63.Ma L, Corsa B, Zhou J, Yang H, Li H, Tang Y, Babaey V, Major A, Linton M, Fazio S, Hunley T, Kon V, Fogo A. Angiotensin type 1 receptor modulates macrophages and renal injury in obesity. Am J Physiol Renal Physiol. 2011;300:F1203–F1213. doi: 10.1152/ajprenal.00468.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park J, Beck F, Müller D, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers K, Alitalo K, Eckardt K, Luft F, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 65.Machnik A, Dahlmann A, Kopp C, Goss J, Wagner H, van Rooijen N, Eckardt K, Müller D, Park J, Luft F, Kerjaschki D, Titze J. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension. 2010;55:755–761. doi: 10.1161/HYPERTENSIONAHA.109.143339. [DOI] [PubMed] [Google Scholar]
- 66.Madej A, Okopień B, Kowalski J, Haberka M, Herman Z. Plasma concentrations of adhesion molecules and chemokines in patients with essential hypertension. Pharmacol Rep. 2005;57:878–881. [PubMed] [Google Scholar]
- 67.Majid D. Tumor necrosis factor-α and kidney function: experimental findings in mice. Adv Exp Med Biol. 2011;691:471–480. doi: 10.1007/978-1-4419-6612-4_48. [DOI] [PubMed] [Google Scholar]
- 68.Mann D, McMurray J, Packer M, Swedberg K, Borer J, Colucci W, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, Veldhuisen D, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circ Res. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 69.McMaster W, Kirabo A, Madhur M, Harrison D. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116:1022–1033. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mervaala E, Muller D, Schmidt F, Park J, Gross V, Bader M, Breu V, Ganten D, Haller H, Luft F. Blood pressure-independent effects in rats with human renin and angiotensinogen genes. Hypertension. 2000;35:587–594. doi: 10.1161/01.hyp.35.2.587. [DOI] [PubMed] [Google Scholar]
- 71.Mills K, Bundy J, Kelly T, Reed J, Kearney P, Reynolds K, Chen J, Jiang H. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muller D, Dechend R, Mervaala E, Park J, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft F. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000a;35:193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- 73.Muller D, Mervaala E, Dechend R, Fiebeler A, Park J, Schmidt F, Theuer J, Breu V, Mackman N, Luther T, Schneider W, Gulba D, Ganten D, Haller H, Luft F. Angiotensin II (AT(1)) receptor blockade reduces vascular tissue factor in angiotensin II-induced cardiac vasculopathy. Am J Pathol. 2000b;157:111–122. doi: 10.1016/S0002-9440(10)64523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muller D, Shagdarsuren E, Park J, et al. Immunosuppresive treatment protects against angiotensin II-induced renal damage. Am J Pathol. 2002;161:1679. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murray P, Allen J, BIswas S, Fisher E, Gilroy D, Goerdt S, Gordon S, Hamilton J, Ivashkiv L, Lawrence T, Locati M, Mantovani A, Martinez F, Mege J, Mosser D, Natoli G, Saeij J, Schultze J, Shirey K, Sica A, Suttles J, Udalova I, van Ginderarchter J, Vogel S, Wynn T. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, et al. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation. 1998;98:794–799. doi: 10.1161/01.cir.98.8.794. [DOI] [PubMed] [Google Scholar]
- 77.Nataraj C, Oliverio M, Mannon R, Mannon P, Audoly L, Amuchastegui C, Ruiz P, Smithies O, Coffman T. Angiotensin II regulates cellular immune responses through a calcineurin-dependent pathway. J Clin Invest. 1999;104:1693–1701. doi: 10.1172/JCI7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neumann P, Gertzberg N, Johnson A. TNF-alpha induces a decrease in eNOS promoter activity. Am J Physiol Lung Cell Mol Physiol. 2004;286:L452–L459. doi: 10.1152/ajplung.00378.2002. [DOI] [PubMed] [Google Scholar]
- 79.Nishida M, Fujinaka H, Matsusaka T, Price J, Kon V, Fogo A, Davidson J, Linton M, Fazio S, Homma T, Yoshida H, Ichikawa I. Absence of angiotensin II type 1 receptor in bone-marrow derived cells is detrimental in the evolution of renal fibrosis. J Clin Invest. 2002;110:1859–1868. doi: 10.1172/JCI15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oliverio M, Best C, Smithies O, Coffman T. Regulation of sodium balance and blood pressure by the AT(1A) receptor for angiotensin II. Hypertension. 2000;35:550–554. doi: 10.1161/01.hyp.35.2.550. [DOI] [PubMed] [Google Scholar]
- 81.Olsen F. Transfer of arterial hypertension by spelnic cells from DOCA-salt hypertensive and renal hypertensive rats to nor-motensive recipients. Acta Pathol Microbiol Scand C. 1980;88:1–5. doi: 10.1111/j.1699-0463.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 82.Ozawa Y, Kobori H, Suzaki Y, Navar L. Sustained renal interstitial macrophage infiltration follwing chronic angiotensin II infusions. Am J Physiol Renal Physiol. 2007;292:F330–F339. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palazzolo D, Quadri S. Interleukin-1 stimulates catechol-amine release from the hypothalamus. Life Sci. 1990;47:2105–2109. doi: 10.1016/0024-3205(90)90309-f. [DOI] [PubMed] [Google Scholar]
- 84.Palomo I, Marín P, Alarcón M, Gubelin G, Viñambre X, Mora E, Icaza G. Patients with essential hypertension present higher levels of sE-selectin and sVCAM-1 than normotensive volunteers. Clin Exp Hypertens. 2003;25:517–523. doi: 10.1081/ceh-120025335. [DOI] [PubMed] [Google Scholar]
- 85.Papaharalambus C, Griendling K. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med. 2007;17:48–54. doi: 10.1016/j.tcm.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parissis J, Korovesis S, Giaziatzoglou E, Kalivas P, Katritisis D. Plasma profiles of peripheral monocyte-related inflammatory markers in patients with arterial hypertension. Correlations with plasma endothelin-1. Int J Cardiol. 2002;83:13–21. doi: 10.1016/s0167-5273(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 87.Ramseyer V, Garvin J. Tumor necrosis factor-α: regulation of renal function and blood pressure. Am J Physiol Renal Physiol. 2013;304:F1231–F1242. doi: 10.1152/ajprenal.00557.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ricardo S, van Goor H, Eddy A. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ridker P, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 90.Ridker P, Howard C, Walter V, Everett B, Libby P, Hensen J, Thuren T CPI Group. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 91.Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordan K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson R. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int. 2001;59:2222–2232. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 92.Rudemiller N, Crowley S. Interactions between the immune and the renin-angiotensin systems in hypertension. Hypertension. 2016;68:289–296. doi: 10.1161/HYPERTENSIONAHA.116.06591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schoppmann S, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schreiner G, Kohan D. Regulation of renal transport processes and hemodynamics by macrophages and lymphocytes. Am J Physiol Renal Fluid Electrolyte Physiol. 1990;258:F761–F767. doi: 10.1152/ajprenal.1990.258.4.F761. [DOI] [PubMed] [Google Scholar]
- 95.Shah K, Shi P, Giani J, Janjulia T, Bernstein E, Li Y, Zhao T, Harrison D, Bernstein K, Shen X. Myeloid suppressor cells accumulate and regulate blood pressure in hypertension. Circ Res. 2015;117:858–869. doi: 10.1161/CIRCRESAHA.115.306539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shahid M, Francis J, Majid D. Tumor necrosis factor-induces renal vasoconstriction as well as natriuresis in mice. Am J Physiol Renal Physiol. 2008;295:F1836–F1844. doi: 10.1152/ajprenal.90297.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shen X, Li Y, Li L, Shah K, Bernstein K, Lyden P, Shi P. Microglia participate in neurogenic regulation of hypertension. Hypertension. 2015;66:309–316. doi: 10.1161/HYPERTENSIONAHA.115.05333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shi P, Diez-Freire C, Jun J, Qi Y, Katovich M, Li Q, Sriramula S, Francis J, Sumners C, Raizada M. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shirasuna K, Karasawa T, Usui F, Kobayashi M, Komada T, Kimura H, Kawashima A, Ohkuchi A, Taniguchi S, Takahashi M. NLRP3 deficiency improves angiotensin II-induced hypertension but not fetal growth restriction during pregnancy. Endocrinology. 2015;156:4281–4292. doi: 10.1210/en.2015-1408. [DOI] [PubMed] [Google Scholar]
- 100.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh P, Bahrami L, Castillo A, Majid D. TNF-α type 2 receptor mediates renal inflammatory response to chronic angiotensin II administration with high salt intake in mice. Am J Physiol Renal Physiol. 2013;304:991–999. doi: 10.1152/ajprenal.00525.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sommers S, Relman A, Smithwick R. Histologic studies of kidney biopsy specimens from patients with hypertension. Am J Pathol. 1958;34:685–715. [PMC free article] [PubMed] [Google Scholar]
- 103.Sriramula S, Francis J. Tumor necrosis factor-alpha is essential for angiotensin II-induced ventricular remodeling: role for oxidative stress. PLoS One. 2015;10:e0138372. doi: 10.1371/journal.pone.0138372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sriramula S, Haque M, Majid D, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects of salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stanley E, Guilbert L, Tushinski R, Bartelmez S. CSF-1-a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21:151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- 106.Stegbauer J, Lee D, Seubert S, Ellrichmann G, Manzel A, Kvakan H, Muller D, Gaupp S, Rump L, Gold R, Linker R. Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proc Natl Acad Sci U S A. 2009;106:14942–14947. doi: 10.1073/pnas.0903602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun M, Chen M, Dawood F, Zurawska U, Li J, Parker T, Kassiri Z, Kirshenbaum L, Arnold M, Khokha R, Liu P. Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation. 2007;115:1398–1407. doi: 10.1161/CIRCULATIONAHA.106.643585. [DOI] [PubMed] [Google Scholar]
- 108.Sun H, Zeng D, Li R, Pang R, Yang H, Hu Y, Zhang Q, Jiang Y, Huang L, Tang Y, Yan G, Zhou J. Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension. 2012;60:1407–1414. doi: 10.1161/HYPERTENSIONAHA.112.197301. [DOI] [PubMed] [Google Scholar]
- 109.Swirski F, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo J, Kohler R, Chudnovskiy A, Waterman P, Aikawa E, Mempel T, Libby P, Weissleder R, Pittet M. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takahashi H, Nishimura M, Sakamoto M, Ikegaki I, Nakanishi T, Yoshimura M. Effects of interleukin-1 beta on blood pressure, sympathetic nerve activity, and pituitary endocrine functions in anesthetized rats. Am J Hypertens. 1992;5:224–229. doi: 10.1093/ajh/5.4.224. [DOI] [PubMed] [Google Scholar]
- 111.Takebayashi S. Ultrastructual studies on glomerular lesions in experimental hypertension. Acta Pathol Jpn. 1969;19:179–200. doi: 10.1111/j.1440-1827.1969.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 112.Titze J. Sodium balance is not just a renal affair. Curr Opin Nephrol Hypertens. 2014;23:101–105. doi: 10.1097/01.mnh.0000441151.55320.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Lanschot J, Mealy K, Jacobs D, Evans D, Wilmore D. Splenectomy attenuates the inappropriate diuresis associated with tumor necrosis factor administration. Surg Gynecol Obstet. 1991;172:293–297. [PubMed] [Google Scholar]
- 114.Voelkel N, Tuder R, Bridges J, Arend W. Interleukin-1 receptor antagonist treatment reduces pulmonary hypertension generated in rats by monocrotaline. Am J Respir Cell Mol Biol. 1994;11:664–675. doi: 10.1165/ajrcmb.11.6.7946395. [DOI] [PubMed] [Google Scholar]
- 115.Wang J, Griffin J, Rambaldi A, Chen Z, Mantovani A. Macrophage growth factor CSF-1 stimulates human monocyte production of interferon, tumor necrosis factor, and colony stimulating activity. J Immunol. 1986;137:2281–2285. [PubMed] [Google Scholar]
- 116.Wang Y, Li Y, Wu Y, Jia L, Wang J, Xie B, Hui M, Du J. 5TNF-α and IL-1β neutralization ameliorates angiotensin II-induced cardiac damage in male mice. Endocrinology. 2014;155:2677–2687. doi: 10.1210/en.2013-2065. [DOI] [PubMed] [Google Scholar]
- 117.Weir M, Dzau V. The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am J Hypertens. 1999;12:205S–213S. doi: 10.1016/s0895-7061(99)00103-x. [DOI] [PubMed] [Google Scholar]
- 118.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach S, Schwenk M, Yogev N, Schulz E, et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 119.Wenzel U, Turner J, Krebs C, Kurts C, Harrison D, Ehmke H. Immune mechanisms in arterial hypertension. J Am Soc Nephrol. 2016;27:677–686. doi: 10.1681/ASN.2015050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wiig H, Schröeder A, Neuhofer W, Jantsch J, Kopp C, Karlsen T, Boschmann M, Goss J, Bry M, Rakova N, Dahlmann A, Brenner S, Tenstad O, Nurmi H, Mervaala E, Wagner H, Beck F, Müller D, Kerjaschki D, Luft F, Harrison D, Alitalo K, Titze J. Immune cells control skin lymphatic electrolye homeostasis and blood pressure. J Clin Invest. 2013;123:2803–2815. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wiktor-Jedrzejczak W, Bartocci A, Ferrante A, Jr, Ahmed-Ansari A, Sell K, Pollard J, ERS Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz L, Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 123.Yoshida S, Takeuchi T, Kotani T, et al. Infliximab, a TNF-α inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J Hum Hypertens. 2014;28:165–169. doi: 10.1038/jhh.2013.80. [DOI] [PubMed] [Google Scholar]
- 124.Zhang B, Ramesh G, Norbury C, Reeves W. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-α produced by renal parenchymal cells. Kidney Int. 2007;72:37–44. doi: 10.1038/sj.ki.5002242. [DOI] [PubMed] [Google Scholar]
- 125.Zhang J, Patel M, Song Y, Griffiths R, Burchette J, Ruiz P, Sparks M, Yan M, Howell D, Gomez J, Spurney R, Coffman T, Crowley S. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. J Am Heart Assoc. 2012;110:1604–1617. doi: 10.1161/CIRCRESAHA.111.261768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang J, Patel M, Griffiths R, Dolber P, Ruiz P, Sparks M, Stegbauer J, Jin H, Gomez J, Buckley A, Lefler W, Chen D, Crowley S. Type 1 angiotensin receptors on macrophages ameliorate IL-1 receptor-mediated kidney fibrosis. J Clin Invest. 2014a;124:2198–2203. doi: 10.1172/JCI61368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang J, Patel M, Griffiths R, Mao A, Song Y, Karlovich N, Sparks M, Jin H, Wu M, Lin E, Crowley S. Tumor necrosis factor-α produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension. 2014b;64:1275–1281. doi: 10.1161/HYPERTENSIONAHA.114.03863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang M, Yao B, Wang Y, Yang S, Wang S, Fan X, Harris R. Inhibition of cyclooxygenase-2 in hematopoietic cells results in salt-sensitive hypertension. J Clin Invest. 2015a;125:4281–4294. doi: 10.1172/JCI81550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang W, Zheng X, Du L, Sun J, Shen Z, Shi C, Sun S, Zhang Z, Chen X, Qin M, Liu X, Tao J, Jia L, Fan H, Zhou B, Yu Y, Ying H, Hui L, Liu X, Yi X, Liu X, Zhang L, Duan S. High salt primes a specific activation state of macrophages, M(Na) Cell Res. 2015b;25:893–910. doi: 10.1038/cr.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang J, Rudemiller N, Patel M, Karlovich N, Wu M, McDonough A, Griffiths R, Sparks M, Jeffs A, Crowley S. Interleukin-1 receptor activation potentiates salt reabsorption in angiotensin II-induced hypertension via the NKCC2 Co-transporter in the nephron. Cell Metab. 2016a;23:360–368. doi: 10.1016/j.cmet.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang J, Rudemiller N, Patel M, Wei Q, Karlovich N, Jeffs A, Wu M, Sparks M, Privratsky J, Herrera M, Gurley S, Nedospasov S, Crowley S. Competing actions of type 1 angiotensin receptors on T lymphocytes and kidney epithelium during Cisplatin-induced acute kidney injury. J Am Soc Nephrol. 2016b;27:2257–2264. doi: 10.1681/ASN.2015060683. [DOI] [PMC free article] [PubMed] [Google Scholar]