Abstract
The resurrection plant Reaumuria soongorica is widespread across Asia, southern Europe, and North Africa and is considered to be a constructive keystone species in desert ecosystems, but the impacts of climate change on this species in desert ecosystems are unclear. Here, the morphological responses of R. soongorica to changes in rainfall quantity (30% reduction and 30% increase in rainfall quantity) and interval (50% longer drought interval between rainfall events) were tested. Stage‐specific changes in growth were monitored by sampling at the beginning, middle, and end of the growing season. Reduced rainfall decreased the aboveground and total biomass, while additional precipitation generally advanced R. soongorica growth and biomass accumulation. An increased interval between rainfall events resulted in an increase in root biomass in the middle of the growing season, followed by a decrease toward the end. The response to the combination of increased rainfall quantity and interval was similar to the response to increased interval alone, suggesting that the effects of changes in rainfall patterns exert a greater influence than increased rainfall quantity. Thus, despite the short duration of this experiment, consequences of changes in rainfall regime on seedling growth were observed. In particular, a prolonged rainfall interval shortened the growth period, suggesting that climate change‐induced rainfall variability may have significant effects on the structure and functioning of desert ecosystems.
Keywords: climate change, desert ecosystems, growth season, rainfall regime, Reaumuria soongorica
1. INTRODUCTION
The Badain Jaran Desert climate has changed tremendously over the past 50 years, particularly in terms of rainfall patterns (Ning et al., 2011). Climate change models forecast reductions in annual precipitation and an increased prevalence of extreme events in the region, including longer intervals between rainfall events and fewer rainy days (Yue et al., 2013). Changes in climate affect species in different ways, the consequences of which may not be easy to determine. Previous studies have shown that changes in climate can alter species growth, biomass and morphology (Markesteijn & Poorter, 2009; Root et al., 2003; Wellstein & Cianfaglione, 2014); for example, changes in rainfall were found to cause pronounced mortality in the widespread desert moss Syntrichia caninervis (Reed, Coe, & Sparks, 2012). Water is the most important factor limiting the existence and distribution of desert communities. Additionally, some studies have demonstrated that shifts in the rainfall regime (such as changes in timing and duration) might be more important for ecosystems than changes in the quantity of annual rainfall (Reed et al., 2012; Weltzin et al., 2009). Indeed, the effects of climate change on water availability are likely to have widespread consequences for desert ecosystems.
While many plant species are able to survive in arid regions in desert ecosystems, only a few contribute to the majority of the biomass. In the Gobi and Badain Jaran Deserts, the desiccation‐tolerant halophytic desert shrub species Reaumuria soongorica (Pall.) Maxim. (Tamaricaceae) is a typical constructive xerophytic species, and it is widespread in desert ecosystems (Zhao, Hai, & Ungar, 2002). In the arid and semiarid Gobi Desert, R. soongorica is a constructive and dominant species in the community (Peixi, Chen, & Yan, 2008); it plays an important role in sustaining desert ecological stability (He et al., 2015) and may be considered a keystone species (Ma, Chen, Xia, Wang, & Chen, 2007). Therefore, R. soongorica can serve as an indicator of Gobi Desert community trends. This subshrub exhibits various anatomical, morphological, and metabolic adaptations to severe desiccation, including entering a state of dormancy upon dehydration followed by revival when water becomes available (Liu, Wang, et al., 2007). For survival when faced with water deficit, R. soongorica starch and nonstructural carbohydrate reserves increase (Xu et al., 2010), and its stems are a crucial organ (Liu, Zhang, Li, & Wang, 2007).
Changes in rainfall are symptoms of climate change. A recent study reported that altered rainfall trends, including declining rainfall quantity and increasing extreme rainfall events, are likely to increase in the future (Yue et al., 2013). Such effects will likely impact the growth characteristics of the species occurring in the Gobi. For example, shrubs showed increased growth with increasing precipitation variability, while grasses showed the opposite response (Gherardi & Sala, 2015a). While drought and decreased rainfall have been found to restrict the production of aboveground biomass (Holub, 2002; Lane, Coffin, & Lauenroth, 2000), the data on belowground biomass are less definitive. Furthermore, the majority of studies have been performed in grasslands, and few data exist with respect to desert ecosystems. Therefore, it is necessary to focus on desert ecosystems, as arid and mesic regions have differential responses to changes in precipitation (Sala, Gherardi, & Peters, 2015).
In this experiment, we tested the effects of changes in rainfall regime on R. soongorica growth in the Badain Jaran Desert. Based on the above information, we predicted that changes in the timing of rainfall events are likely to be especially impactful to the growth of R. soongorica. With reference to the predicted and observed changes in rainfall in the Badain Jaran Desert, we tested the response of R. soongorica growth to the effects of changes in rainfall quantity and timing at three stages during the growth period as well as the interactive effects of changes in rainfall regime and growth period sampling time.
2. METHODS
2.1. Study site
This study was conducted in a typical Gobi Desert ecosystem located in the southeast of the Badain Jaran Desert (39°24′N, 100°07′E), the third largest desert by area (47,000 km2) in China (Dong, Wang, & Wang, 2004). The study site is characterized by a temperate continental climate, the typical features of which are warm summers and long, cold winters. The average annual rainfall is nearly 117 mm and is concentrated largely between June and September, which constitutes the growing season. The annual mean wind speed is 3.2 m/s, and the maximum wind speed is 213.2 m/s. The annual mean air temperature is 7.6°C, while the annual mean surface geotemperature is 13.61°C, and the mean annual evaporation rate over the last 30 years was 2,390 mm (Li, Liu, Liu, Liu, & Niu, 2013). The vegetation of this region is dominated by a few drought‐resistant plants, including Nitraria sphaerocarpa, Salsola passerina, Suaeda glauca, and Artemisia scoparia, to name a few. The total plant community cover varies between 6% and 11% (Li et al., 2013).
2.2. Experimental design
The experiments began on 1 June 2014. A simple rain gauge was installed at the study site to manually measure the rainfall quantity by collecting rainwater when a rainfall event finished. We adjusted and recorded the rainfall based on the information provided by the device. The experiment included six treatment groups based on two factors: (1) rainfall quantity and (2) interval between rainfall events (Figure 1). We administered water over the top of the plants (similar to rain) using a watering can. The six treatments were as follows:
Reduced quantity (R−, I). Rainout shelters were used to shield the natural rainfall. In this treatment, 70% of the naturally occurring rainfall was immediately applied to the plots (within 12 hr of a rainfall event). This treatment reduced the rainfall quantity without altering the timing of the rainfall events.
Natural quantity and interval (R, I). No rainout shelters were used in this treatment, so no interference to the natural rainfall occurred. The plants were exposed to the naturally occurring rainfall regime, and the rainfall quantity and rainfall time were unaltered.
Increased quantity (R+, I). This treatment also included no rainout shelters, and additional water was applied based on the recorded rainfall quantity. The plants in this treatment were subject to the total naturally occurring rainfall, which was applied immediately, and they were also irrigated to increase the rainfall volume by 30%.
Decreased quantity and increased interval (R−, I+). Rainout shelters were constructed to shield the natural rainfall. The rainfall intervals were lengthened by 50%, and 70% of the natural rainfall quantity was applied in this treatment.
Increased interval (R, I+). Rainout shelters were used to shield the natural rainfall. The rainfall intervals were lengthened by 50%, but water in an amount equivalent to that of natural rainfall was applied.
Increased quantity and increased interval (R+, I+). Rainout shelters were used to shield the natural rainfall. The rainfall intervals were lengthened by 50%, and 130% of the quantity of natural rainfall was applied.
Figure 1.
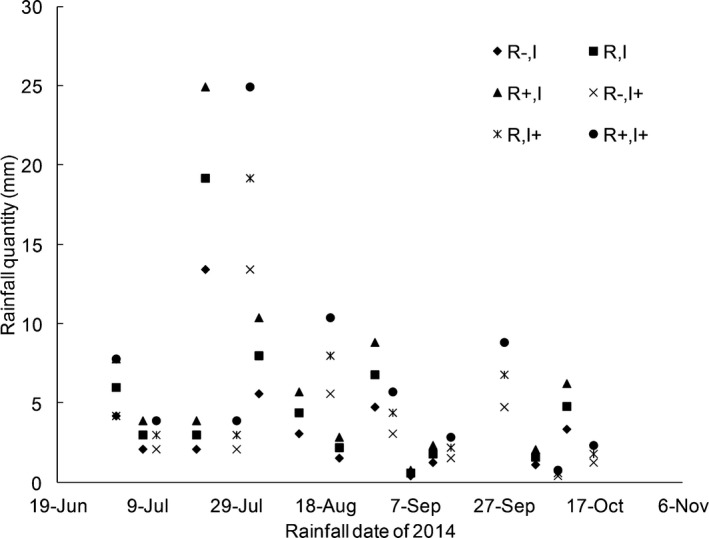
Rainfall quantity and intervals associated with each treatment. R indicates the natural rainfall quantity, R− indicates 70% of the natural rainfall quantity, and R+ indicates 130% of the natural rainfall quantity. It is same as following figures. I indicates the natural rainfall interval between two rainfall event, I+ indicates 1.5 times of the natural rainfall interval since last rainfall
Based on historical rainfall data, the rainfall quantities of the wettest and driest years were within ±30% of the average over the past 50 years, and a 50% increase in the length of the interval between rainfall events can be expected according to the current climate change predictions in this region (IPCC, 2014). For these reasons, we chose to use a 30% alteration in rainfall quantity and 50% increase in the length of the dry period in our experiment. The same parameters have generally been used in previous related studies (Fay, Carlisle, Knapp, Blair, & Collins, 2000; Harper, Blair, Fay, Knapp, & Carlisle, 2005).
2.3. Plot construction
Eighteen plots in total were randomly demarcated across approximately 12,000 m2 at the study site. Each plot measured 1 × 1 m (this area ensures that more than four 1‐year‐old seedlings can grow without inhibition) and was situated at least 2 m away from the other plots. We ensured that four 1‐year‐old seedlings were growing within every plot and determined that the seedlings were relatively healthy (based on leaf color, crown width, and plant surface integrity). Over 72 seedlings served as controls. Each plot was subjected to one of the six treatments, resulting in three replicates per treatment condition. Each sampled seedling was randomly selected from each plot, and 18 seedlings were harvested at once. As the roots of R. soongorica occur mainly in the shallow soil layer, the plots were surrounded by iron straps measuring 50 cm (40 cm beneath the soil, 10 cm above the ground) to eliminate the effects of water infiltration.
A 30‐cm stake was installed in the center of each plot, while a 15‐cm stake was placed in each corner. During rainfall, plastic sheeting (0.1 mm in thickness and 1.4 × 1.4 m in size) was used to construct a rainout shelter. The plastic sheeting was transparent and did not affect illumination. As there was plenty of space under the plastic sheeting, there was no greenhouse effect in the plots. These simple rainout shelters covered 1.3 × 1.3 m in area. The plastic sheeting was only applied when rainfall was expected to occur based on weather forecasts and experience. The treatments R, I and R+, I remained unsheltered for the entire duration of the experiment.
2.4. Sampling method
To evaluate the effects of changes in rainfall regime on R. soongorica seedlings over the growing season, we sampled the plots on 9 August, 17 September, and 22 October 2014, which represented the early, middle, and end of the growing season, respectively. As a rule of thumb, the seedlings adjust to the new environment after being transplanted for approximately 30 days. The first sampling event occurred after the rainfall experienced by the plants had been manipulated for 70 days; thereafter, we ensured that each sampling interval was greater than 30 days to allow the plants to adjust to their respective watering regimes before sampling. In addition, before we started the experiment, a time zero sample was measured to compare conveniently (see Table S1). We sampled the plots three times; during each sampling event, we harvested 18 saplings (1 plant from each plot), with 54 saplings assessed in total. We carefully excavated the complete root system using a traditional method known as the skeleton method (Böhm, 1979). The main root length and plant height were then measured using a tape measure, basal diameter was measured using a vernier caliper, and the plants were transported back to the laboratory. Soil adhering to the seedlings was brushed off, and the aboveground and belowground parts of the plants were separated in preparation for root analysis (total root length, root surface area and root volume) using Epson scanners and WinRHIZO Basic 2008a. The two sections were separately placed in an oven at 105°C for 2 hr and then maintained at 80°C for 48 hr, after which the biomass was weighed using an analytical balance (aboveground biomass and belowground biomass). Root‐shoot ratio and total biomass were calculated based on the aboveground and belowground biomass.
2.5. Analyses
We were interested in measuring the effects of changes in rainfall regime and growth period sampling time on biomass and growth indicators. First, the three precipitation treatments (30% increase, natural, 30% decrease) were assessed using one‐way ANOVA, and the two interval treatments (ambient, 50% increase) were also assessed using ANOVA. The analyses were conducted separately for each time period. We focused on plant height, root length, total biomass, aboveground biomass, and belowground biomass for these tests. Then, we assessed the influence of quantity × interval on the treatments using two‐way ANOVA and the same growth indicators as well as root‐shoot ratio, root volume, root surface area, and basal diameter. Additionally, we assessed whether these effects differed across sampling times (early, middle, or late growing season) using three‐way ANOVA (quantity × interval × timing). Bootstrapped p‐values were used when the data violated ANOVA assumptions. Tukey's HSD (honest significant difference) tests were used to determine which sample means differed from each other. All analyses were conducted using SPSS 21.0 (Thomey et al., 2011).
3. RESULTS
3.1. Rainfall regime
In total, 12 rainfall events occurred during the experiment (from July to October), altogether accounting for 64.0 mm of ambient rainfall. The prolonged timing treatment reduced the number of events (10), resulting in a total rainfall quantity of 57.6 mm (Figure 1). Over nearly 50 years, rainfall in the area has exhibited a trend of fluctuating decrease, with the 2014 growing season being drier (64.0 mm) than the average over the last three decades for the same period (96.4 mm). Below‐average rainfall also characterized the growing seasons of 2013 (70.6 mm) and 2015 (60.6 mm).
3.2. Morphological changes
The one‐way ANOVA showed significant differences in the morphology of R. soongorica across the different parts of the growing seasons (Figure 2; Table 1). In general, the increased rainfall interval decreased the main root length and plant height during the early and late stages of the growth period; however, the opposite effect was found during the middle of the growth period. Under the ambient intervals, the main root length and plant height increased significantly with increasing rainfall throughout the entire growth period (Figure 2). However, the different treatments remarkably influenced the main root length and plant growth.
Figure 2.
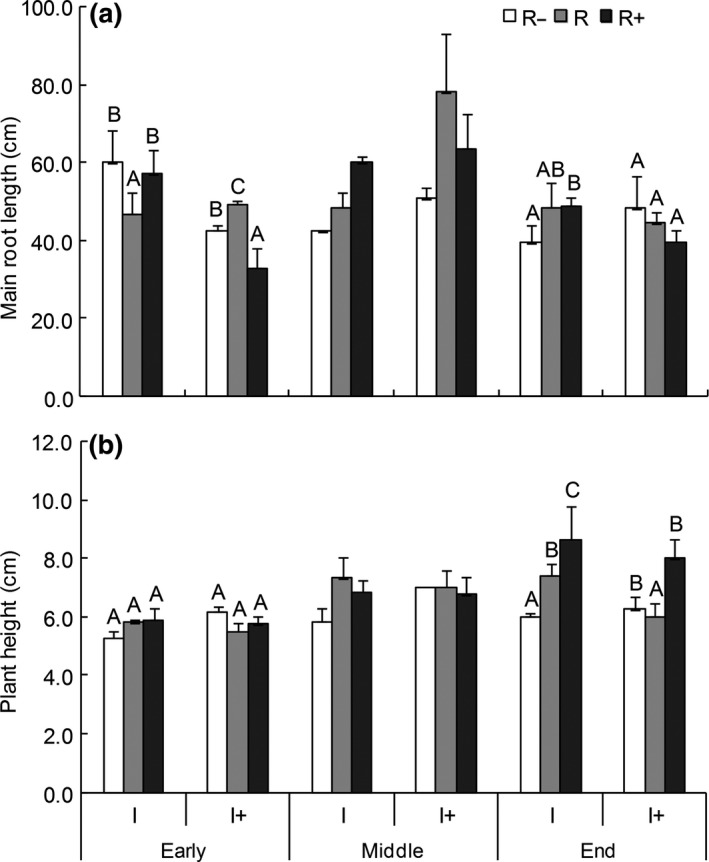
Dynamics of the main root length (a) and plant height (b) of Reaumuria soongorica seedlings under different rainfall volumes across the growing season. For all plots, different upper‐case letters are significantly different (p < .05) based on single‐factor analysis of variance (ANOVA) under the same rainfall interval. Means ± SE (n = 3)
Table 1.
Results (F‐values) based on three‐way ANOVA of the effects of rainfall quantity, rainfall interval, and sampling time on the main root length, plant height, basal diameter, aboveground biomass, belowground biomass, total biomass, and root‐shoot ratio of Reaumuria soongorica seedlings
| Q | I | T | Q × I | Q × T | I × T | Q × I × T | |
|---|---|---|---|---|---|---|---|
| Main root length | 1.85 | 0.33 | 7.87** | 4.10* | 2.02 | 7.85*** | 1.07 |
| Plant height | 3.70* | 0.08 | 11.54*** | 0.64 | 4.21** | 1.26 | 1.07 |
| Basal diameter | 2.42 | 3.81 | 3.37* | 2.04 | 1.41 | 4.92* | 1.47 |
| Total root length | 0.86 | 1.70 | 3.76* | 0.88 | 0.89 | 5.16* | 0.61 |
| Root surface area | 0.86 | 0.96 | 3.30* | 1.61 | 1.09 | 4.70* | 0.39 |
| Root volume | 0.86 | 0.38 | 2.59 | 2.27 | 1.12 | 3.34* | 0.46 |
| Aboveground biomass | 0.85 | 5.95* | 0.41 | 5.92** | 1.94 | 9.85*** | 2.84* |
| Belowground biomass | 1.39 | 1.20 | 5.66** | 1.76 | 2.04 | 6.38** | 0.96 |
| Root‐shoot ratio | 1.31 | 2.61 | 3.14 | 1.00 | 0.22 | 3.17 | 2.46 |
| Total biomass | 1.06 | 4.82* | 1.89 | 4.55* | 2.37 | 9.84*** | 2.26 |
The different levels of probability considered are ***p < .001, **p < .01, *p < .05. Q, rainfall quantity; I, rainfall interval; T, the time of sampling. Replicate number = 3.
Changes in interval alone did not significantly affect the morphology of R. soongorica, while the growth period significantly impacted several of the morphological indexes. The effect of rainfall quantity (Q) × rainfall interval (I) on root length was significant, while that of rainfall quantity (Q) × sampling time (T) significantly affected plant height. The effect of rainfall interval (I) × sampling time (T) significantly impacted most of the morphological indexes, such as main root length, plant height, basal diameter (Fig. S1), total root length, root surface area (Fig. S2), and root volume (Fig. S3). However, the interaction among the three factors (Q × I × T) had no significant effect on the morphological indexes of R. soongorica (Table 1).
The main root length under ambient rainfall quantity and prolonged rainfall duration was highest (78.0 cm), exceeding 70 cm by the middle of the growth season, and the maximum main root length was approached across all treatments by the end of the growth season (Figure 2a). Plant height increased significantly with increased rainfall. At the early stage of the growth season, there were no obvious distinctions in plant height across any of the treatments. However, by the end of the growth season, the heights had all increased, and the differences between most of the treatments were significant (Figure 2b).
3.3. Biomass accumulation and allocation
All three factors (rainfall quantity, interval and timing) significantly influenced biomass (Table 1). Under ambient rainfall intervals, the aboveground biomass, belowground biomass, and total biomass accumulated with time or increases in rainfall quantity (Table 1, Figure 3). By the middle of the growth season, all indicators of biomass had significantly increased with increasing precipitation under the ambient rainfall intervals. By the end of the growth season, the total biomass and aboveground biomass had decreased significantly only under the prolonged rainfall intervals (Figure 3). Over the entire season, rainfall quantity had no significant effect on aboveground or belowground biomass, while rainfall interval significantly impacted the aboveground and total biomass (p < .05; Table 1). Underground biomass significantly differed among the sampled growth stages (p < .01). However, rainfall quantity exerted a greater impact on biomass during the early growth season, while rainfall interval had the greatest effect on biomass in the late growth season (Figure 3).
Figure 3.
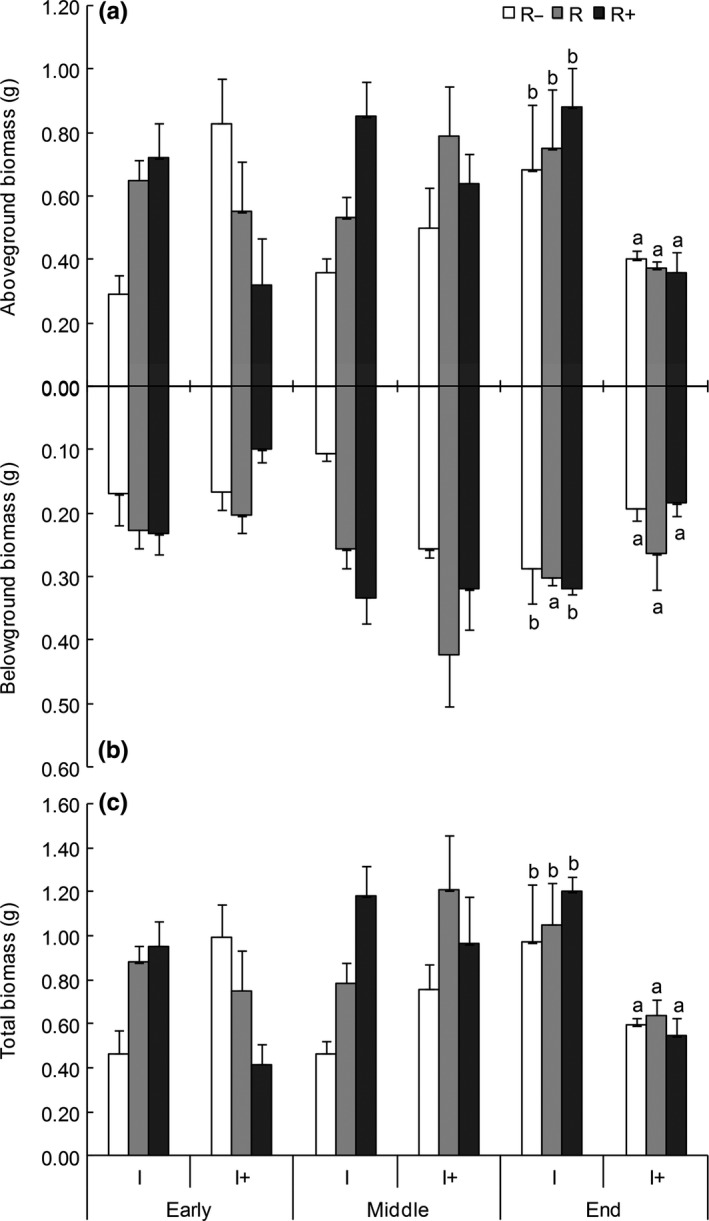
Dynamics of the aboveground biomass (a), belowground biomass (b), total biomass (c) of Reaumuria soongorica seedlings under different rainfall volumes across the growing season. For all plots, different lower‐case letters are significantly different (p < .05) based on single‐factor analysis of variance (ANOVA) under the same rainfall interval. Means ± SE (n = 3)
The rainfall quantity (Q) × rainfall interval (I) significantly affected the aboveground and total biomass, while rainfall quantity (Q) × sampling time (T) had no significant effect on biomass. The rainfall interval (I) × sampling time (T) effect was significant for aboveground, belowground, and total biomass. The interaction among the three factors (Q × I × T) had a significant effect on aboveground biomass. Furthermore, there was no significant effect of each factor alone or interactively on the root‐shoot ratio (Table 1; Fig. S4).
4. DISCUSSION
Moisture is the primary limiting factor in arid and semiarid areas, with rainfall constituting the primary water source for plants in these regions. Global climate change is gradually altering rainfall regimes, with a higher frequency of extreme events (including prolonged drought intervals and shorter, more intense rainfall periods) predicted for the future (Diffenbaugh & Giorgi, 2012). Many studies have demonstrated that increased precipitation results in the promotion of growth and the accumulation of biomass, while prolonging the interval between rainfall events has the opposite effect (Ansley, Boutton, & Jacoby, 2014; Fay et al., 2000). Some studies have shown that the interaction between rainfall quantity and rainfall interval is highly variable. However, most studies have involved only sampling once during the growing season and did not assess changes at different stages during the growing season. Furthermore, the majority of studies have focused on grassland ecosystems, while few have examined arid and semiarid environments.
We investigated the effects of changes in rainfall regime (rainfall quantity and interval) on growth and biomass indexes in R. soongorica in the early, middle, and late growing season as well as the interactions among these three factors. Water uptake is one of the main root functions (Gregory, 2006), with root systems first experiencing changes in water availability. A study by Steudle (2000) showed that roots and shoots differ in resistance; during water stress, the roots will deliver information to the shoot. Our study shows that the root length greatly differed across treatments during the early growth season but not at the end (Figure 2), while the opposite trend was observed in the stems (plant height). These findings suggest that the root may respond more quickly to rainfall regime changes than the stem. Furthermore, the biomass (aboveground, belowground, and total) greatly decreased when the interval between rainfall events was extended (Figure 3). Therefore, we infer that a prolonged rainfall interval may lead to a shortened growing season. The effects of changes in precipitation quantity and interval on all indexes remarkably differed at the different stages, with the exception of the root‐shoot ratio (Ansley et al., 2014; Holub, 2002).
4.1. Additional precipitation advances growth and biomass accumulation
As R. soongorica occurs in arid desert regions, it is likely to be sensitive to variation in water availability. Rainfall indirectly affects plant growth by affecting the soil moisture content. Thus, it may be necessary to more deeply study the association between rainfall and soil moisture content in the future studies. Fortunately, the results of previous studies addressing this association have been consistent: Rainfall increases the mean soil moisture content (Hovenden, Newton, & Wills, 2014; Thomey et al., 2011; Wilcox, Fischer, Muscha, Petersen, & Knapp, 2015). In our study, the altered rainfall regime did not change the root‐shoot ratio, and it has been suggested that such results occur because R. soongorica is able to adapt to its surroundings (Fay, Kaufman, Nippert, Carlisle, & Harper, 2008; Wu, Zhou, Zheng, Li, & Tang, 2014). However, increased precipitation advanced plant growth and biomass accumulation. Biomass has generally been found to increase along with increased rainfall in arid and semiarid areas, as water stress is the limiting factor in these environments (Kardol et al., 2010; Weiss, Gutzler, Coonrod, & Dahm, 2004), but some studies have suggested that additional precipitation does not influence or may decrease biomass (Fay et al., 2000; White, Campbell, Kemp, & Hunt, 2000). The possible reason for this discrepancy is that the water gradients did not differ enough to reflect the differences.
Under the increased rainfall quantity treatment, the main root length (Figure 2a) and aboveground biomass (Figure 3a) decreased, but the differences were not significant. The small R. soongorica seedlings grew better under ambient than increased precipitation, suggesting that this plant is not suited for conditions involving an overabundance of water, as shown in another study that focused more on the effects of precipitation and showed a hump‐shaped response (Gherardi & Sala, 2015b). The effect of the interaction between rainfall quantity and interval was irregular in this study, which may be attributed to the fact that the prolonged rainfall interval altered the growth period.
4.2. Growth period is shortened by prolonged rainfall intervals
Recent studies (Hovenden et al., 2014) have emphasized the importance of seasonal rainfall on ecosystems, as rainfall exerts different effects during different seasons. However, many of these studies have used grassland ecosystems as case studies, while studies regarding desert ecosystems are lacking. Furthermore, previous studies have not investigated the effects of differences in rainfall intervals during the growing season. Our study revealed that plants at different growth stages do not respond to prolonged rainfall intervals in the same manner.
Figure 2 indicates that the main root length significantly differed across all treatments in the early growth period, with the differences decreasing toward the end of the growth season, while plant height increased across the different growth periods. Based on these observations, we believe that the roots were better able to respond to the changes in the rainfall regime and adapt to the new environment than the stems (Ropars et al., 2017). As shown in Figure 2a (early season), there appear to be no adaptive response to high rainfall levels. However, it is unsurprising that the roots of this plant rotted under high rainfall conditions, as it is a resurrection plant and is therefore highly adapted to drought conditions. During the prolonged rainfall interval treatments, the biomass measures evaluated in this experiment decreased more significantly at the end of growth season compared to those at the middle of the season (Figure 2). As found by Roux, Mcgeoch, Nyakatya, and Chown (2005), we observed that the seedlings in the prolonged rainfall interval treatments shed their leaves earlier and that sections of the roots had begun to rot (although the plants were still alive). Previous studies (Liu, Wang, et al., 2007) have indicated that R. soongorica has a protective mechanism to reduce water loss through leaf abscission in environments lacking water. This reaction occurred under the drought conditions, suggesting that the plants had begun to enter a state of dormancy that would extend until the next rainfall season, thus constituting a strategy to enhance the survival rate during winter. It seems apparent that the growth period had been shortened by the prolonged rainfall interval.
The desiccation‐tolerant R. soongorica can survive water contents of more than 5% (Xu et al., 2010) or 15 days of desiccation (Liu, Zhang, et al., 2007). Irregular rainfall patterns stimulate adaptation to the water‐stressed conditions of desert ecosystems. Thus, plant morphology is expected to change in accordance with rainfall during the growing season, as observed in the changes in root length, plant height, aboveground biomass, belowground biomass, and total biomass in this study. While R. soongorica was able to persist when the quantity of rainfall was increased or decreased by 30%, it significantly suffered when the dry interval length was extended by 50%. Thus, we believe that the effects of changes in rainfall patterns, such as increased drought intervals, could be more severe than increases or decreases in rainfall on this species. In addition, it is necessary for future studies to clarify the consequences of the priming effect of extreme rainfall and possible lag effects in the plant. Changes in rainfall regime associated with climate change are thus likely to be important factors influencing the response of vegetation in the Gobi Desert. Understanding the interactions among these factors remains a serious challenge for predicting ecosystem responses to climate change.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Zhengzhong Zhang involved in the acquisition, analysis, and interpretation of data for the work, drafting the work, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved. Lishan Shan involved in revising it critically for important intellectual content. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved, and final approval of the version to be published. Yi Li involved in acquisition of funding. Substantial contributions to the conception and design of the work, revising it critically for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity and any part of the work are appropriately investigated and resolved.
Supporting information
ACKNOWLEDGMENTS
We thank the Linze National Field Station for Farmland Ecosystem, Chinese Academy of Sciences, for providing climate data, the reviewers and editors for valuable suggestions that significantly improved this manuscript and Wiley for its linguistic assistance during the revision of this manuscript. This work was supported by the National Natural Science Foundation of China (41361100, 31560135, 31360205 and 41461044) and the Gansu Science and Technology Support Program (1604FKCA088).
Zhang Z, Shan L, Li Y. Prolonged dry periods between rainfall events shorten the growth period of the resurrection plant Reaumuria soongorica . Ecol Evol. 2018;8:920–927. https://doi.org/10.1002/ece3.3614
Funding Information
This work was supported by the National Natural Science Foundation of China (41361100, 31560135, 31360205 and 41461044) and the Gansu Science and Technology Support Program (1604FKCA088)
REFERENCES
- Ansley, R. J. , Boutton, T. W. , & Jacoby, P. W. (2014). Root biomass and distribution patterns in a semi‐arid mesquite savanna, responses to long‐term rainfall manipulation. Rangeland Ecology & Management, 67, 206–218. https://doi.org/10.2111/REM-D-13-00119.1 [Google Scholar]
- Böhm, W. (1979). Methods of studying root systems. Berlin Heidelberg, Germany/New York, NY, USA: Springer‐Verlag; https://doi.org/10.1007/978-3-642-67282-8 [Google Scholar]
- Diffenbaugh, N. S. , & Giorgi, F. (2012). Climate change hotspots in the cmip5 global climate model ensemble. Climatic Change, 114, 813 https://doi.org/10.1007/s10584-012-0570-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Z. , Wang, T. , & Wang, X. (2004). Geomorphology of the megadunes in the Badain Jaran Desert. Geomorphology, 60, 191–203. https://doi.org/10.1016/j.geomorph.2003.07.023 [Google Scholar]
- Fay, P. A. , Carlisle, J. D. , Knapp, A. K. , Blair, J. M. , & Collins, S. L. (2000). Altering rainfall timing and quantity in a mesic grassland ecosystem: Design and performance of rainfall manipulation shelters. Ecosystems, 3, 308–319. https://doi.org/10.1007/s100210000028 [Google Scholar]
- Fay, P. A. , Kaufman, D. M. , Nippert, J. B. , Carlisle, J. D. , & Harper, C. W. (2008). Changes in grassland ecosystem function due to extreme rainfall events, implications for responses to climate change. Global Change Biology, 14, 1600–1608. https://doi.org/10.1111/j.1365-2486.2008.01605.x [Google Scholar]
- Gherardi, L. A. , & Sala, O. E. (2015a). Enhanced precipitation variability decreases grass‐ and increases shrub‐productivity. Proceedings of the National Academy of Sciences of the United States of America, 112, 12735–12740. https://doi.org/10.1073/pnas.1506433112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi, L. A. , & Sala, O. E. (2015b). Enhanced interannual precipitation variability increases plant functional diversity that in turn ameliorates negative impact on productivity. Ecology Letters, 18, 1293–1300. https://doi.org/10.1111/ele.12523 [DOI] [PubMed] [Google Scholar]
- Gregory, P. J. (2006). Plant roots: Growth, activity and interaction with soils. Blackwell Publishing Ltd, Oxford, UK. [Google Scholar]
- Harper, C. W. , Blair, J. M. , Fay, P. A. , Knapp, A. K. , & Carlisle, J. D. (2005). Increased rainfall variability and reduced rainfall amount decreases soil CO2 flux in a grassland ecosystem. Global Change Biology, 11, 322–334. https://doi.org/10.1111/j.1365-2486.2005.00899.x [Google Scholar]
- He, M. , Zhang, K. , Tan, H. , Hu, R. , Su, J. , Wang, J. , … Li, X. (2015). Nutrient levels within leaves, stems, and roots of the xeric species Reaumuria soongorica in relation to geographical, climatic, and soil conditions. Ecology & Evolution, 5, 1494–1503. https://doi.org/10.1002/ece3.1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holub, P. (2002). The expansion of Calamagrostis epigejos into alluvial meadows: Comparison of aboveground biomass in relation to water regimes. Ekologia Bratislava, 21, 27–37. [Google Scholar]
- Hovenden, M. J. , Newton, P. C. , & Wills, K. E. (2014). Seasonal not annual rainfall determines grassland biomass response to carbon dioxide. Nature, 511, 583–586. https://doi.org/10.1038/nature13281 [DOI] [PubMed] [Google Scholar]
- IPCC (2014). Climate change 2014: Synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri R. K. & Meyer L. A. (Eds.)], Geneva, Switzerland, 27, 408. [Google Scholar]
- Kardol, P. , Campany, C. E. , Souza, L. , Norby, R. J. , Weltzin, J. F. , & Classen, A. T. (2010). Climate change effects on plant biomass alter dominance patterns and community evenness in an experimental old‐field ecosystem. Global Change Biology, 16, 2676–2687. https://doi.org/10.1111/j.1365-2486.2010.02162.x [Google Scholar]
- Lane, D. R. , Coffin, D. P. , & Lauenroth, W. K. (2000). Changes in grassland canopy structure across a precipitation gradient. Journal of Vegetation Science, 11, 359–368. https://doi.org/10.2307/3236628 [Google Scholar]
- Li, F. R. , Liu, J. L. , Liu, C. A. , Liu, Q.‐J. , & Niu, R.‐X. (2013). Shrubs and species identity effects on the distribution and diversity of ground‐dwelling arthropods in a Gobi desert. Journal of Insect Conservation, 17, 319–331. https://doi.org/10.1007/s10841-012-9512-1 [Google Scholar]
- Liu, Y. B. , Wang, G. , Liu, J. , Zhao, X. , Tan, H. J. , & Li, X. R. (2007). Anatomical, morphological and metabolic acclimation in the resurrection plant Reaumuria soongorica during dehydration and rehydration. Journal of Arid Environments, 70, 183–194. https://doi.org/10.1016/j.jaridenv.2006.12.020 [Google Scholar]
- Liu, Y. B. , Zhang, T. G. , Li, X. R. , & Wang, G. (2007). Protective mechanism of desiccation tolerance in Reaumuria soongorica: Leaf abscission and sucrose accumulation in the stem. Science in China Series C: Life sciences, 50, 15–21. https://doi.org/10.1007/s11427-007-0002-8 [DOI] [PubMed] [Google Scholar]
- Ma, J. Y. , Chen, K. , Xia, D. S. , Wang, G. , & Chen, F.‐H. (2007). Variation in foliar stable carbon isotope among populations of a desert plant, Reaumuria soongorica (Pall.) Maxim. in different environments. Journal of Arid Environments, 69, 365–374. https://doi.org/10.1016/j.jaridenv.2006.11.002 [Google Scholar]
- Markesteijn, L. , & Poorter, L. (2009). Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought‐ and shade‐tolerance. Journal of Ecology, 97, 311–325. https://doi.org/10.1111/j.1365-2745.2008.01466.x [Google Scholar]
- Ning, M. A. , Wang, N. A. , Zhu, J. F. , Chen, X. L. , Chen, H. B. , & Dong, C. Y. (2011). Climate change around the Badain Jaran desert in recent 50 years. Journal of Desert Research, 31, 1541–1547. [Google Scholar]
- Reed, S. C. , Coe, K. K. , & Sparks, J. P. (2012). Changes to dryland rainfall result in rapid moss mortality and altered soil fertility. Nature Climate Change, 2012, 752–755. https://doi.org/10.1038/nclimate1596 [Google Scholar]
- Root, T. L. , Price, J. T. , Hall, K. R. , Schneider, S. H. , Rosenzweig, C. , & Alan Pounds, J. (2003). Fingerprints of global warming on wild animals and plants. Nature, 421, 57–60. https://doi.org/10.1038/nature01333 [DOI] [PubMed] [Google Scholar]
- Ropars, P. , Angers‐Blondin, S. , Gagnon, M. , Myers‐Smith, I. H. , Lévesque, E. , & Boudreau, S. (2017). Different parts, different stories, climate sensitivity of growth is stronger in root collars versus stems in tundra shrubs. Global Change Biology, 23, 3281–3291. https://doi.org/10.1111/gcb.13631 [DOI] [PubMed] [Google Scholar]
- Roux, P. C. L. , Mcgeoch, M. A. , Nyakatya, M. J. , & Chown, S. L. (2005). Effects of a short‐term climate change experiment on a sub‐Antarctic keystone plant species. Global Change Biology, 11, 1628–1639. https://doi.org/10.1111/j.1365-2486.2005.001022.x [Google Scholar]
- Sala, O. E. , Gherardi, L. A. , & Peters, D. P. C. (2015). Enhanced precipitation variability effects on water losses and ecosystem functioning: Differential response of arid and mesic regions. Climatic Change, 131, 213–227. https://doi.org/10.1007/s10584-015-1389-z [Google Scholar]
- Steudle, E. (2000). Water uptake by roots: Effects of water deficit. Journal of Experimental Botany, 51, 1531–1542. https://doi.org/10.1093/jexbot/51.350.1531 [DOI] [PubMed] [Google Scholar]
- Su, P. X. , Chen, H. , & Yan, Q. (2008). Plant community characteristics and their relationships with climate in the Hexi Corridor region of northwestern China. Frontiers of Forestry in China, 3, 393–400. https://doi.org/10.1007/s11461-008-0064-1 [Google Scholar]
- Thomey, M. L. , Collins, S. L. , Vargas, R. , Johnson, J. E. , Brown, R. F. , Natvig, D. O. , & Friggens, M. T. (2011). Effect of precipitation variability on net primary production and soil respiration in a Chihuahuan Desert grassland. Global Change Biology, 17, 1505–1515. https://doi.org/10.1111/j.1365-2486.2010.02363.x [Google Scholar]
- Weiss, J. L. , Gutzler, D. S. , Coonrod, J. E. A. , & Dahm, C. N. (2004). Long‐term vegetation monitoring with NDVI in a diverse semi‐arid setting, central New Mexico, USA. Journal of Arid Environments, 58, 249–272. https://doi.org/10.1016/j.jaridenv.2003.07.001 [Google Scholar]
- Wellstein, C. , & Cianfaglione, K. (2014). Impact of extreme drought and warming on survival and growth characteristics of different provenences of juvenile quercus pubescens willd. Folia Geobotanica, 49, 31–47. https://doi.org/10.1007/s12224-013-9186-9 [Google Scholar]
- Weltzin, J. F. , Loik, M. E. , Schwinning, S. , Williams, D. G. , Fay, P. A. , Haddad, B. M. , … Zak, J. C. (2009). Assessing the response of terrestrial ecosystems to potential changes in precipitation. BioScience, 53, 941–952. https://doi.org/10.1641/0006-3568(2003)053[0941:atrote]2.0.co;2 [Google Scholar]
- White, T. A. , Campbell, B. D. , Kemp, P. D. , & Hunt, C. L. (2000). Sensitivity of three grassland communities to simulated extreme temperature and rainfall events. Global Change Biology, 6, 671–684. https://doi.org/10.1046/j.1365-2486.2000.00344.x [Google Scholar]
- Wilcox, K. R. , Fischer, J. C. , Muscha, J. M. , Petersen, M. K. , & Knapp, A. K. (2015). Contrasting above‐ and belowground sensitivity of three great plains grasslands to altered rainfall regimes. Global Change Biology, 21, 335–344. https://doi.org/10.1111/gcb.12673 [DOI] [PubMed] [Google Scholar]
- Wu, Y. , Zhou, H. , Zheng, X. J. , Li, Y. , & Tang, L.‐S. (2014). Seasonal changes in the water use strategies of three co‐occurring desert shrubs. Hydrological Processes, 28, 6265–6275. https://doi.org/10.1002/hyp.v28.26 [Google Scholar]
- Xu, D. , Su, P. , Zhang, R. , Li, H. , Zhao, L. , & Wang, G. (2010). Photosynthetic parameters and carbon reserves of a resurrection plant Reaumuria soongorica during dehydration and rehydration. Plant Growth Regulation, 60, 83–190. https://doi.org/10.1007/s10725-009-9440-6 [Google Scholar]
- Yue, T. X. , Zhao, N. , Ramsey, R. D. , Wang, C.‐L. , Fan, Z.‐M. , Chen, C.‐F. , … Li, B.‐L. (2013). Climate change trend in china, with improved accuracy. Climatic Change, 120, 137–151. https://doi.org/10.1007/s10584-013-0785-5 [Google Scholar]
- Zhao, K. , Hai, F. , & Ungar, I. A. (2002). Survey of halophyte species in china. Plant Science, 163, 491–498. https://doi.org/10.1016/s0168-9452(02)00160-7 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


