Abstract
Impaired connectivity is proposed to underlie pathophysiology of schizophrenia. Existing studies on functional connectivity show inconsistent results. We examined functional connectivity in a clinically homogenous sample of 34 early course schizophrenia patients compared with/to 19 healthy controls using resting state functional magnetic resonance imaging (rsfMRI).
Mean duration of illness for schizophrenia patients was 4 ± 1.78 years. Following a comprehensive clinical assessment, rsfMRI data were acquired using a 3.0 T magnetic resonance imaging scanner, and analyzed using FSL version 5.01 software (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Compared to healthy controls, schizophrenia patients had significantly decreased functional connectivity in the left fronto-parietal network, lateral and medial visual network, motor network, default mode network and auditory network. Our data suggests significant functional hypoconnectivity in selected brain networks in early schizophrenia patients compared to controls. It is likely that the observed functional hypoconnectivity may be associated with features of schizophrenia other than those examined in this study. It is possible that hypoconnectivity is necessary but not sufficient to the clinical manifestation of schizophrenia. The examination of functional connectivity as a biomarker should be extended to a wider array of disease phenotypes to better understand its significance.
Keywords: Resting state networks, Early course schizophrenia, Auditory network, Motor network, Visual network
1. Introduction
Impaired neural connectivity is implicated in schizophrenia (Friston et al., 1995). Functional imaging has been extensively used to highlight such connectivity deficits. Schizophrenia patients and their relatives show aberrant functional connectivity in default mode networks (Whitfield-Gabrieli et al., 2009), like the medial prefrontal, cingulate and inferior parietal cortices, and regions regulating executive functions such as the dorsolateral prefrontal cortex (DLPFC) (Bhojraj et al., 2010).
Amongst novel functional imaging approaches, resting state functional MRI (rsfMRI) may help unravel some neurobiological abnormalities associated with neuropsychiatric disorders. Resting state fMRI (rsfMRI) is an imaging technique that measures Spontaneous Low-Frequency Fluctuations (SLFFs) in the blood oxygenation level dependent (BOLD) signal during the resting condition (Lee et al., 2013). RsfMRI experiments are less prone to multisite variability and uninfluenced by task performance with low signal-to-noise ratio leading to higher probability of acquiring more reliable data (Fox and Greicius, 2010). SLFF are postulated to reflect neural synchrony among brain regions. Since reporting of functional significance of SLFFs by Biswal et al. (Biswal et al., 1995), functional connectivity of the brain in the resting state has gained increasing attention. Variations in spontaneous brain activity reflected by these SLFFs can be considered a putative biomarker of schizophrenia (Zhou et al., 2007, 2010).
Impaired connectivity leading to dysfunctional information processing may underlie clinical symptoms of schizophrenia. Deranged communication among critical regions of the brain networks may underlie impairments in multiple cognitive domains, such as executive functions, working memory, language and speed of processing (Whitfield-Gabrieli et al., 2009). RsfMRI data may identify such altered functional connectivity. Although diffusion tensor imaging (DTI) can elegantly show the differences in anatomical connectivity, actual functional significance and flow of neural signals across the regions is difficult to infer. Prior studies showed impaired fronto-temporal and prefrontal commissural anatomical connectivity (Calhoun et al., 2008) (Venkataraman et al., 2012). Thus, impaired functional connectivity could illustrate a predictable pattern of synchronous activations that underlie clinical symptoms (Venkataraman et al., 2012).
Inconsistent data from existing rsfMRI studies may be due to the examination of a clinically non-homogenous sample of patients (Guo et al., 2014a, 2015; Ke et al., 2009; Lui et al., 2009; Nsisya et al., 2013; Yu et al., 2007; Zhou et al., 2010). Most authors agreed that there was altered functional connectivity in schizophrenia compared to healthy controls in many areas of the brain like default mode network (DMN), medial temporal and prefrontal regions as well as decreased interhemispheric connectivity. Examination of patients in the early course of the illness helps avoid some of the confounders such as the duration and course of illness. Our aim in this study was to examine altered functional connectivity in early course schizophrenia compared to healthy control subjects using rsfMRI. We hypothesize that alteration in resting state networks will appear in brain areas that control cognitive and mood functions deficient in schizophrenia.
2. Methods
2.1. Clinical assessments
Persons with early course schizophrenia seeking treatment at the Department of Psychiatry, Dr. Ram Manohar Lohia Hospital, New Delhi, India were enrolled through their treating clinicians. Eligible subjects with early course schizophrenia of both sexes between 18 and 50 years, and controls were explained about all study procedures and written informed consent was obtained. Controls were selected to match age and sex (highlighted in results).
The diagnosis was confirmed by administering a structured interview using the Hindi version of the Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger et al., 1994), which has been validated previously (Deshpande et al., 1998). The DIGS includes the Global Assessment Scale (GAS), the Schedule for Assessment of Positive Symptoms (SAPS), and the Schedule for Assessment of Negative Symptoms (SANS). The SANS and SAPS assess the severity of negative and positive symptoms on a six-point scale in the previous month (Andreasen and Olsen, 1982; Andreasen, 1989). Inter-rater reliability of scoring was 0.83–0.92. In addition to structured patient interview, collateral information was obtained through caregivers.
We defined early course schizophrenia as the illness duration of 7 years or less from the onset of first psychotic symptoms. The Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) was administered to rate the presence and severity of psychotic symptoms. Participants were required to have a score of 4 or more on one or more items of the PANSS, and be on a stable dose of antipsychotics for at least 1 month prior to experimental procedures. For the control group, comparable participants not suffering from any psychiatric illness were enrolled from similar neighborhoods.
Participants with a history of substance dependence in the past 6 months, excluding nicotine dependence, those with a history of or current medical/neurological illnesses, e.g. epilepsy were excluded. Pregnancy, mental retardation (DSM-IV) or any contraindication to MRI procedures, such as having pacemakers or metal fragments/shrapnel were the other exclusion criteria.
2.2. Imaging methods
Brain images were acquired using a 3 T whole-body MRI scanner (Magnetom Skyra, Siemens) while participants lay supine. Foam pads on either side of the head were used to minimize head movement. Participants were instructed not to move, and to lie quietly awake, with closed eyes and not engaged in any specific cognitive activity during the experiment. We collected high-resolution T1-weighted images using a magnetization prepared rapid acquisition gradient-echo (MPRAGE) sequence (TR = 1900 ms; TE = 2.07 ms; inversion time = 900 ms; flip angle (FA) = 9°; FOV = 256 × 256 mm; slice thickness = 1 mm; number of slices = 160). We acquired T2-weighted images [repetition time (TR) = 10,000 ms; TE = 100 ms; FA = 150°] using a dual-echo turbo spin-echo sequence in the axial plane, with a 256 × 256 matrix size, 220×220 mm field of view (FOV), 4.0 mm slice thickness, and 1.2 mm interslice gap to aid in anatomical identification and evaluation for any anatomical defects. We also collected functional brain volumes using an echo-planar T2*-weighted imaging sequence. Each volume consisted of 30 interleaved 5-mm thick slices without interslice gap (TE = 30 ms, TR = 2000 ms, FOV = 240 mm, flip angle = 90°, voxel size = 3.75 × 3.75 × 5 mm3). Total scanning time for fMRI was 6.83 min (205 brain volumes). Images were re-checked for quality at the end of every scan, and if any head movement was noticeable, the scan was repeated.
2.3. Plan of analysis
We used the Statistical Package for Social Sciences (SPSS v21) for assessment of demographic and clinical data. The numerical demographic data and characteristics of schizophrenia and control subjects were compared with independent samples t-tests, and categorical characteristics were compared using the Chi-square test. Statistical threshold values of p < 0.05 were considered a significant difference.
For resting state fMRI data analysis, we used FSL version 5.01 software (www.fmrib.ox.ac.uk). Firstly, standard preprocessing steps were followed for individual data. This step includes removal of the first 5 brain volumes, high-pass temporal filtering (100 s), motion correction, slice timing, brain-extraction and spatial smoothing (5 mm full width at half-maximum Gaussian kernel). Each fMRI volume was registered on individual structural scans and on the Montreal Regional Institute (MNI152) template (Jenkinson et al., 2002) using the FMRIB Linear Image Registration Tool (FLIRT). Finally, the registered fMRI sequences were temporally concatenated into a single four-dimensional dataset. The data set was decomposed into independent components, with a free estimation for the number of components. We selected components of interest by visual inspection based on previous literature (Beckmann et al., 2005; Damoiseaux et al., 2006) and the frequency spectra of the time courses of the components.
After these preprocessing steps, dual regression analysis was performed for a voxel-wise comparison of the resting functional connectivity for between-group analysis (Filippini et al., 2009). Spatial maps of the group independent component analysis (ICA) were used in general linear model to generate subject-specific versions of the spatial maps, and associated time-series, using dual regression into the subject’s 4D dataset. Thereafter, spatial maps of all subjects were collected into single 4-dimensional files for each original independent component.
Then we performed voxel-wise group difference analyses between subjects and controls using nonparametric permutation testing with 10,000 permutations (Nichols and Holmes, 2002). Finally, for multiple comparisons, we conducted a family-wise error (FWE) correction with a significance threshold of p < 0.05 (Smith and Nichols, 2009). We used the Harvard–Oxford cortical and subcortical atlases (Harvard Centre for Morphometric Analysis) to identify anatomical representations of clusters of the resulting probabilistic independent component analysis maps, which indicated significant differences between the two groups.
The correlation between clinical symptoms and occupation status with between-network resting state functional connectivity were also assessed using partial correlation. The statistical significance threshold was set at p < 0.05, FWE corrected.
3. Results
3.1. Sample characteristics
Demographic and head movement parameters of schizophrenia patients and controls are summarized in Tables 1 and 2. No significant differences in age (p = 0.280), gender (p = 1.0), and marital status (p = 0.401) were present. There is no significant difference between head movement parameters between patients and controls.
Table 1.
Socio-demographic profile of the schizophrenia and control sample.
| Schizophrenia n = 34 | Controls n = 19 | Chi square/t-test | p value | |
|---|---|---|---|---|
| Gender (M/F) | 22/12 | 12/7 | χ2 = 0.13 | 1.000 |
| Age (Mean ± Std. deviation) | 29.324 ± 7.078 | 31.526 ± 6.979 | t = 1.092 | 0.280 |
| Marital Status (Ever married/Never married) | 17/17 | 12/7 | χ2 = 0.852 | 0.401 |
| Years of School (Mean ± Std.deviation) | 10.794 ± 3.649 | 11.263 ± 3.997 | t = 0.434 | 0.666 |
| Occupation (Unemployed/Employed/Student) | 25/7/2 | 0/17/2 | χ2 = 27.091 | <0.001 |
| Head Motion (in mm) (Mean ± Std.deviation) | 0.13 ± 0.08 | 0.09 ± 0.08 | t = 1.744 | 0.09 |
Table 2.
Clinical profile of the schizophrenia sample (n = 34).
| Mean ± SD | RANGE | |
|---|---|---|
| Age at onset (in years) | 24.676 ± 7.23 | 18–44 |
| Duration of illness (in weeks) | 194.71 ± 93.28 | 50–442 |
| Current Episode Global Assessment Scale (GAS) score | 20.588 ± 5.716 | 7–41 |
| SANS total score | 31.74 ± 18.51 | 0–74 |
| Affective flattening | 2.50 ± 1.66 | 0–5 |
| Alogia | 1.94 ± 1.69 | 0–5 |
| Avolition/apathy | 3.35 ± 1.32 | 0–5 |
| Anhedonia/asociality | 3.37 ± 1.69 | 0–5 |
| Attention | 1.24 ± 1.54 | 0–4 |
| SAPS total score | 15.00 ± 10.55 | 0–51 |
| Hallucinations | 1.24 ± 1.54 | 0–5 |
| Delusions | 3.53 ± 1.69 | 0–9 |
| Bizarre behavior | 1.41 ± 1.62 | 0–5 |
| Formal thought disorder | 0.21 ± 0.84 | 0–4 |
Clinical profile of schizophrenia participants is tabulated in Table 2. Although schizophrenia participants experienced symptoms of psychosis (mean SAPS score=15 ± 10.55; mean SANS score = 31.74 ± 18.51) with a mean GAF score of 31.77 ± 9.22 in the past month, they were cooperative for structured clinical interview and fMRI (Table 2).
3.2. Resting State Networks
Seven resting state networks (RSNs) were identified from the group MELODIC output which included the right frontoparietal network, left frontoparietal network, default mode network (DMN), medial visual network, lateral visual network, motor network and auditory network (Supplementary figures 1–7).
Left Frontoparietal Network
Schizophrenia patients showed significantly decreased functional connectivity in the left frontoparietal network compared to control subjects in the brain regions including lateral occipital cortex, superior parietal lobule, angular gyrus, precuneus cortex and occipital pole (Fig. 1a and Table 3).
Fig. 1.
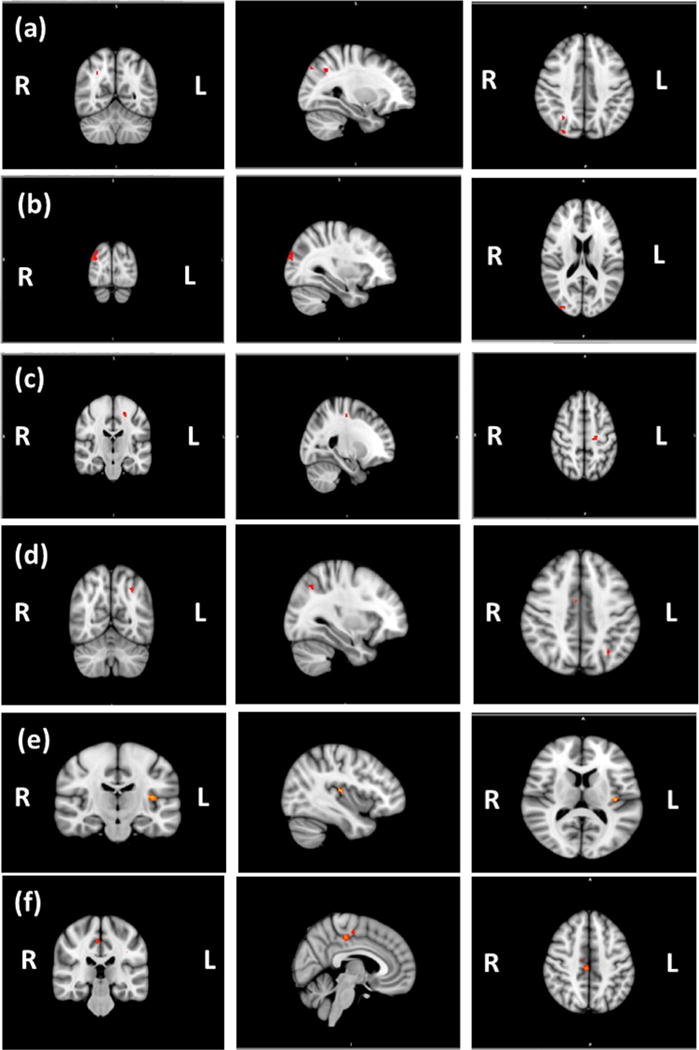
Decreased functional connectivity in brain regions of (a) Left Frontoparietal Network (b) Medial Visual Network, (c) Motor Network, (d) Lateral Visual Network, (e) Auditory Network and (f) Default Mode Network.
Table 3.
Summary of the differences detected in control vs schizophrenia patient group contrast.
| Hemisphere | Cluster voxels | MNI (x,y,z) | #Mean probability | |||||
|---|---|---|---|---|---|---|---|---|
| Right Frontoparietal Network | ||||||||
| Lateral Occipital Cortex (superior division) | 39.96 | |||||||
| Precuneus Cortex | 10.54 | |||||||
| Angular Gyrus | 1.61 | |||||||
| Superior Parietal Lobule | R | 82 | 30 | − 62 | 40 | 1.15 | ||
| Lateral Occipital Cortex (superior division) | 60 | 26 | − 82 | 40 | 61.22 | |||
| Occipital Pole | R | 1.42 | ||||||
| Lateral Visual Network | ||||||||
| Temporal Occipital Fusiform Cortex | 21.24 | |||||||
| Temporal Fusiform Cortex, posterior division | 14.02 | |||||||
| Inferior Temporal Gyrus, temporooccipital part | L | 231 | − 38 | − 46 | − 12 | 12.81 | ||
| Inferior Temporal Gyrus, posterior division | 5.14 | |||||||
| Cingulate Gyrus, anterior division | 42.87 | |||||||
| Juxtapositional Lobule Cortex (formerly Supplementary Motor Cortex) | R | 178 | 6 | −6 | 48 | 25.31 | ||
| Cingulate Gyrus, posterior division | 6.60 | |||||||
| Lateral Occipital Cortex, superior division | 44.65 | |||||||
| Superior Parietal Lobule | L | 93 | − 30 | − 62 | 44 | 7.53 | ||
| Angular Gyrus | 4.74 | |||||||
| Medial Visual Network | ||||||||
| Lateral Occipital Cortex (superior division) | R | 73 | 38 | − 26 | 20 | 41.32 | ||
| Occipital Pole | 23.45 | |||||||
| Cingulate Gyrus, posterior division | 63.44 | |||||||
| Precuneus Cortex | R | 29 | 6 | − 30 | − 36 | 1.58 | ||
| Motor Network | ||||||||
| Precentral Gyrus | L | 291 | − 30 | − 14 | 48 | 33.25 | ||
| Superior Frontal Gyrus | 14.22 | |||||||
| Postcentral Gyrus | 3.92 | |||||||
| Default Mode Network (DMN) | ||||||||
| Cingulate Gyrus, posterior division | 35.22 | |||||||
| Precentral Gyrus | 23.57 | |||||||
| Juxtapositional Lobule Cortex (formerly Supplementary Motor Cortex) | R | 692 | 6 | − 26 | 48 | 7.54 | ||
| Cingulate Gyrus, anterior division | 2.78 | |||||||
| Precuneus Cortex | 1.65 | |||||||
| Lateral Occipital Cortex, inferior division | 35 | |||||||
| Superior Temporal Gyrus, posterior division | 23.29 | |||||||
| Middle Temporal Gyrus, posterior division | L | 40 | − 38 | − 70 | 4 | 15.06 | ||
| Superior Temporal Gyrus, anterior division | 1.41 | |||||||
| Subcallosal Cortex | R | 18 | 2 | 22 | −16 | 72 | ||
| Auditory Network | ||||||||
| Insular Cortex | 35.64 | |||||||
| Heschl’s Gyrus (includes H1 and H2) | 14.86 | |||||||
| Central Opercular Cortex | L | 392 | − 38 | − 18 | 12 | 6.45 | ||
| Parietal Operculum Cortex | 1.78 | |||||||
Note:
p < 0.05 FWE corrected. MNI (x,y,z): coordinates of peak locations in the space of Montreal Neurological Institute (MNI).
Medial Visual Network
Significantly decreased connectivity within the medial visual network including lateral occipital cortex, occipital pole, cingulate gyrus and precuneus cortex was observed in schizophrenia (Fig. 1b and Table 3).
Motor Network
Decreased connectivity within the motor network among schizophrenia cases was seen in the precentral and postcentral gyri, and superior frontal gyrus (Fig. 1c and Table 3).
Lateral Visual Network
The regions of the lateral visual network in which the schizophrenia participants specifically showed significantly decreased temporal correlation compared to controls included temporal occipital cortices, inferior temporal gyrus, supplementary motor area, cingulate gyrus, lateral occipital cortex, angular gyrus, and superior parietal lobule (Fig. 1d and Table 3).
Auditory Network
Schizophrenia participants showed reduced connectivity among the insular cortex, the Heschl’s gyrus, the central opercular cortex, and parietal operculum cortex (Fig. 1e and Table 3).
Default Mode Network
In DMN, schizophrenia patients showed reduced connectivity in the cingulate gyrus, precentral gyrus, juxtapositional lobule cortex (formerly Supplementary Motor Cortex), precuneus cortex, lateral occipital cortices, temporal gyrus and subcallosal cortex (Fig. 1f and Table 3).
Relationship between functional connectivity and clinical variables
No significant correlation was found between occupation status and resting state function connectivity in any network. The decreased connectivity of the four networks did not significantly correlate with sub-syndrome or total scores on SANS and SAPS, duration of illness, age of onset or the GAF scores on repeating functional imaging analysis to correlate the SANS and SAPS sub scores and total scores.
4. Discussion
Our study reports decreased functional connectivity in six RSNs including left frontoparietal network, lateral visual network, medial visual network, motor network, DMN, and auditory network, in early course schizophrenia patients compared to healthy controls.
Our focus on patients in the early course of illness (mean illness duration 3.75 years) makes it more distinctive and may address possible confounding factors of chronicity and exposure to long-term psychotropic medications. Schizophrenia patients were experiencing moderately severe positive and more severe negative symptoms that may be related to unique findings reported here. Zhou (Zhou et al., 2007) examined almost similar patients but examined the dorsolateral prefrontal cortex connectivity by seeding this region-of-interest. They found reduced connectivity of the dorsolateral prefrontal cortex with the parietal, posterior cingulate, thalamus and striatum in schizophrenia patients. We used a whole-brain approach agnostic to any particular region-of-interest. This suggests that networks other than those involving the dorsolateral prefrontal cortex may still be associated with schizophrenia.
Implicated regions in our study concur with previous studies on the resting state alterations, which are manifestations of SLFF with schizophrenia providing evidence of their future usefulness as biomarkers of schizophrenia.
The frontoparietal networks are associated with higher order cognitive functions. Specifically, the left frontoparietal network displays a functional association with language processing, as well as visual working memory, attention and episodic memory retrieval (Coull et al., 1996; Ostby et al., 2011). Our findings indicated that the lateral occipital cortex, superior parietal lobule, angular gyrus, precuneus cortex and occipital pole showed decreased functional connectivity in schizophrenia patients compared with healthy controls. Various resting state fMRI studies have shown reduced cluster of activations in frontal and parietal nodes of functional networks in schizophrenia (Lynall et al., 2009; van den Heuvel and Hulshoff Pol, 2010). DTI studies have also reported anatomical disconnection in several brain regions in frontal and parietal lobe in schizophrenia patients (Mori et al., 2007; Kubicki et al., 2005). In line with these studies, the reduced functional connectivity in the left frontoparietal network is suggestive of possible deficits in cognitive functions associated with the schizophrenia.
Reduced connectivity in the lateral and medial visual networks was present, seen through significant alteration of the occipital cortex and associated regions and manifesting in both visual networks and motor network. Traditionally considered synonymous with visual processing, emerging evidence from functional and structural studies validates the subtle occipital lobe abnormalities including primary visual processing deficits in schizophrenia (Tohid et al., 2015). However, the directionality remains debated. Task-based fMRI studies revealed reduced activation in the fusiform, lentiform, and middle occipital gyri during emotion processing tasks (Kubicki et al., 2002, 2009). Argyelan (Argyelan et al., 2014) also found greater disconnectivity in the temporal-occipital fusiform cortex in schizophrenia compared to controls. Our findings support the involvement of primary visual processing abnormalities in schizophrenia. Thus, negative symptoms like apathy and affective blunting may reflect poor facial emotion processing linked to occipital cortex dysconnectivities (Kubicki et al., 2002). Further, hypoconnectivity of the right middle and superior temporal gyrus in first-episode schizophrenia subjects correlated with the severity of positive and negative symptoms (Lui et al., 2009).
Hypoconnectivity in the auditory network was notable because all participants, except two, reported auditory hallucinations during the course of their illness, although not while scanning. This hypoconnectivity did not correlate with hallucination sub-score on SAPS. A similar network encompassing the temporo-parietal junction, inferior frontal gyrus, anterior cingulate cortex (ACC), amygdala, and insula known to be associated with speech processing and verbal working memory was previously reported (Vercammen et al., 2010). Our network consisted of the same areas, along with the opercular cortex, postcentral gyrus and lateral occipital cortex, suggesting inner speech related problems in schizophrenia implicated in the neurobiology of auditory hallucinations.
We observed hypoconnectivity in the motor network. It comprised of different regions of the frontal cortex, considered the anatomical basis of executive and motivation-guided behavior (Calhoun et al., 2008). Welsh et al. (Welsh et al., 2010) reported significantly reduced thalamo-cortical connectivity in schizophrenia, which is hypothesized to be connected to the motor network. A study on 49 first episode, drug-naïve schizophrenia patients (Guo et al., 2014b) also implicated decreased inter-hemispheric coordination in the precentral gyrus, middle occipital gyrus and precuneus of the superior parietal lobule, which are components of our motor network as well. This could be suggestive of neurobiological changes underlying negative symptoms, considering the predominance of those symptoms in our sample.
We also found evidence for less functional integrity of the DMN in schizophrenia patients, relative to healthy controls. In DMN network, schizophrenia patients showed clusters of reduced resting state functional connectivity in the cingulate gyrus, precentral gyrus, Juxtapositional Lobule Cortex (formerly Supplementary Motor Cortex), precuneus cortex, lateral occipital cortices, temporal gyrus and subcallosal cortex. Default mode network (DMN) is the set of brain regions that are consistently more activated during resting condition and routinely exhibit a decrease in activity during task performance (Fox and Raichle, 2007). It is often described as a unitary, homogeneous system that is largely responsible for many aspects of internally-directed cognition, such as episodic memory, theory of mind, self-evaluation, and introspection (Sestieri et al., 2011). Our results are well supported by earlier resting state fMRI study that has shown altered functional connectivity in schizophrenia patients (Tang et al., 2013; Mingoia et al., 2012; Woodward et al., 2011; Garrity et al., 2007).
None of the networks correlated statistically significantly with severity/duration of illness or the functional status. Absence of correlation suggests that a wider array of clinical and demographic features need to be examined. In addition, the hypoconnectivity may be a consequence of treatment and course of the illness. This is supported by a recent study that reported increased network homogeneity in the posterior cingulate and cerebellum among first-episode medication-naïve schizophrenia patients correlated with positive and negative symptoms (Guo et al., 2014b). A longitudinal design could address such questions. Trends were noticed in the correlation of DMN and right fronto-parietal network with severity of psychopathology. A sample with adequate power could address such relationship with treatment.
The limitations of our study include a cross-sectional design and varying dose, duration and type of medication. However, early course of the illness among schizophrenia participants mitigate this to some extent. As nicotine use is a common comorbidity in schizophrenia with 48% prevalence in Indian studies (Vatss et al., 2012), for feasibility of recruitment nicotine dependence was not used as an exclusion criterion, and only few participants were nicotine dependent. Correlation with cognitive performance could be useful in future studies. We could not formally measure socioeconomic status of the participants which could be a limitation of the study but on occupation status, there was no significant difference between patients and controls.
The present study showed altered resting-state functional connectivity networks, including the left frontoparietal network, lateral and medial visual network, motor network, DMN and auditory network in early-course schizophrenia patients compared to controls. These network abnormalities did not correlate with positive/negative symptoms or illness duration suggesting that the network hypoconnectivities may not be sufficient for these symptoms.
Supplementary Material
Acknowledgments
This work was supported in part by the Stanley Medical Research Institute, USA (11T-007; PI: Dr. S.N. Deshpande), and R01TW008289 - Fogarty International Center (Dr. Triptish Bhatia).
We thank all psychiatrists at Department of Psychiatry, PGIMER, and Dr. R.M.L. Hospital for referring patients for the study. We also thank Dr. Satnam, Dr. Aishwarya, Dr. Mona, Sreelatha, Deepak, Ashita, Nupur, Hemant, Gyan and Dr. Popli for their support.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.pscychresns.2017.11.013.
Footnotes
Contributors
Aastha Sharma: Data acquisition, Review of literature, preparation of manuscript.
Arvind Kumar: Review of literature, manuscript editing.
Sadhana Singh: MRI data collection, analysis.
Triptish Bhatia: Concept, manuscript review and editing.
R. P. Beniwal: Design, manuscript editing, review.
Subhash Khushu: MRI review, analysis.
Konasale M. Prasad: Manuscript review and editing.
Smita N Deshpande: Concept, design, manuscript editing, review.
Conflict of interest
There is no conflict of interest reported by any of the authors.
References
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry. 1989;(Suppl 7):49–58. [PubMed] [Google Scholar]
- Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39(7):789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- Argyelan M, Ikuta T, DeRosse P, Braga RJ, Burdick KE, John M, et al. Resting-state fMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophr Bull. 2014;40(1):100–110. doi: 10.1093/schbul/sbt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Prasad KM, Eack SM, Francis AN, Montrose DM, Keshavan MS. Do inter-regional gray-matter volumetric correlations reflect altered functional connectivity in high-risk offspring of schizophrenia patients? Schizophr Res. 2010;118(1–3):62–68. doi: 10.1016/j.schres.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo ‐ planar mri. Magn Reson. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29(7):828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Frackowiak FS, Grasby PM. A fronot-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci Usa. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande SN, Mathur MN, Das SK, Bhatia T, Sharma S, Nimgaonkar VL. A Hindi version of the diagnostic interview for genetic studies. Schizophr Bull. 1998;24(3):489–493. doi: 10.1093/oxfordjournals.schbul.a033343. [DOI] [PubMed] [Google Scholar]
- Filippini N, Zarei M, Beckmann CF, Galluzzi S, Borsci G, Testa C, Bonetti M, et al. Regional atrophy of transcallosal prefrontal connections in cognitively normal APOE epsilon4 carriers. J Magn Reson Imaging. 2009;29(5):1021–1026. doi: 10.1002/jmri.21757. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Poline J, Frith CD, Heather J, Frackowiak RS. Spatial registration and normalization of images. Hum Brain Mapp. 1995:165–189. [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164(3):450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Guo W, Jiang J, Xiao C, Zhang Z, Zhang J, Yu L, et al. Decreased resting-state interhemispheric functional connectivity in unaffected siblings of schizophrenia patients. Schizophr Res. 2014a;152(1):170–175. doi: 10.1016/j.schres.2013.11.030. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Xiao C, Zhang Z, Yu M, Liu J, et al. Dissociation of anatomical and functional alterations of the default-mode network in first-episode, drug-naive schizophrenia. Clin Neurophysiol. 2015;126(12):2276–2281. doi: 10.1016/j.clinph.2015.01.025. [DOI] [PubMed] [Google Scholar]
- Guo W, Yao D, Jiang J, Su Q, Zhang Z, Zhang J, et al. Abnormal default-mode network homogeneity in first-episode, drug-naive schizophrenia at rest. Prog Neuropsychopharmacol Biol Psychiatry. 2014b;49:16–20. doi: 10.1016/j.pnpbp.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Ke X, Tang T, Hong S, Hang Y, Zou B, Li H, et al. White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain Res. 2009;1265:171–177. doi: 10.1016/j.brainres.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Niznikiewicz M, Connor E, Ungar L, Nestor P, Bouix S, Dreusicke M, Kikinis R, McCarley R, Shenton M. Relationship between white matter integrity, attention, and memory in schizophrenia: a diffusion tensor imaging study. Brain Imaging Behav. 2009;3(2):191–201. doi: 10.1007/s11682-009-9061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Park HJ, Westin CF, Nestor PG, Mulkern RV, Maier SE, et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;15:1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159(5):813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Smyser CD, Shimony JS. Resting-state fMRI: a review of methods and clinical applications. AJNR Am J Neuroradiol. 2013;34(10):1866–1872. doi: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Deng W, Huang X, Jiang L, Ma X, Chen H, et al. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166(2):196–205. doi: 10.1176/appi.ajp.2008.08020183. [DOI] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci: Off J Soc Neurosci. 2009;30(28):9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingoia G, Wagner G, Langbein K, et al. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr Res. 2012;138(2–3):143–149. doi: 10.1016/j.schres.2012.01.036. [DOI] [PubMed] [Google Scholar]
- Mori T, Ohnishi T, Hashimoto R, Nemoto K, Moriguchi Y, Noguchi H, et al. Progressive changes of white matter integrity in schizophrenia revealed by diffusion tensor imaging. Psychiatry Res. 2007;154:133–145. doi: 10.1016/j.pscychresns.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Nsisya TE, Wang Z, Tao H, Zhang H, Hu A, Guo S, et al. The diminished interhemispheric connectivity correlates with negative symptoms and cognitive impairment in first-episode schizophrenia. Schizophr Res. 2013;150(1):144–150. doi: 10.1016/j.schres.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch General Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. (discussion 863–864) [DOI] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AnM, Walhovd KB. Morphometry and connectivity of the fronto-parietal verbal working memory network in development. Neuropsychologia. 2011;49:3854–3862. doi: 10.1016/j.neuropsychologia.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the Default Mode Network: functional and topographic analyses. J Neurosci: Off J Soc Neurosci. 2011;31(12):4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Tang J, Liao Y, Song M. Aberrant default mode functional connectivity in early onset schizophrenia. PLoS One. 2013;8(7):e71061. doi: 10.1371/journal.pone.0071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohid H, Faizan M, Faizan U. Alterations of the occipital lobe in schizophrenia. Neurosci (Riyadh) 2015;20(3):213–e71224. doi: 10.17712/nsj.2015.3.20140757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatss S, Mehar H, Bhatia T, Richard J, Gur RC, Gur RE, et al. Patterns of tobacco consumption among indian men with schizophrenia compared to their male siblings. Psychiatry Investig. 2012;9(3):245–251. doi: 10.4306/pi.2012.9.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Venkataraman A, Whitford TJ, Westin CF, Golland P, Kubicki M. Whole brain resting state functional connectivity abnormalities in schizophrenia. Schizophr Res. 2012;139(1–3):7–12. doi: 10.1016/j.schres.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen A, Knegtering H, Liemburg EJ, den Boer JA, Aleman A. Functional connectivity of the temporo-parietal region in schizophrenia: effects of rTMS treatment of auditory hallucinations. J Psychiatr Res. 2010;44(11):725–731. doi: 10.1016/j.jpsychires.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36(4):713–722. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci Usa. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130(1–3):86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CE, Seltman H, Peskind ER, Galloway N, Zhou PX, Rosenthal E, et al. Comprehensive analysis of APOE and selected proximate markers for late-onset Alzheimer’s disease: patterns of linkage disequilibrium and disease/marker association. Genomics. 2007;89(6):655–665. doi: 10.1016/j.ygeno.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010;133(Pt 5):1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007;417(3):297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


