Abstract
Myelin allows for the rapid and precise timing of action potential propagation along neuronal circuits and is essential for healthy auditory system function. In this review we discuss what is currently known about myelin in the auditory system with a focus on the timing of myelination during auditory system development, the role of myelin in supporting peripheral and central auditory circuit function, and how various myelin pathologies compromise auditory information processing. Additionally, in keeping with the increasing recognition that myelin is dynamic and is influenced by experience throughout life, we review the growing evidence that auditory sensory deprivation alters myelin along specific segments of the brain’s auditory circuit.
Introduction
The auditory system is responsible for processing a complex spectrum of acoustic information, such as sound pitch, intensity, and timing to create a faithful representation of the acoustic environment. Auditory processing involves a finely tuned interplay of events starting with the detection of sound by cochlear hair cells and ending at the cerebral cortex for higher order integration. A defining feature of the auditory system is its extraordinary temporal precision. For example, auditory circuits in the brainstem can detect microsecond-level differences in the arrival time of a sound between the two ears. Accordingly, the conduction speed along auditory circuits must be exquisitely well timed to ensure proper auditory function as even a slight loss of temporal precision can distort auditory perception. Myelin significantly increases conduction velocity along axons and is pivotal to the rapid and coordinated communication between neurons. Given the importance of timing in auditory circuitry, it is not surprising that myelin is vital to numerous auditory functions. Here, we provide a brief overview of the auditory system, highlighting key developments in the understanding of how myelin contributes to auditory function and how conditions that compromise myelin disrupt auditory processing.
The Auditory Pathway
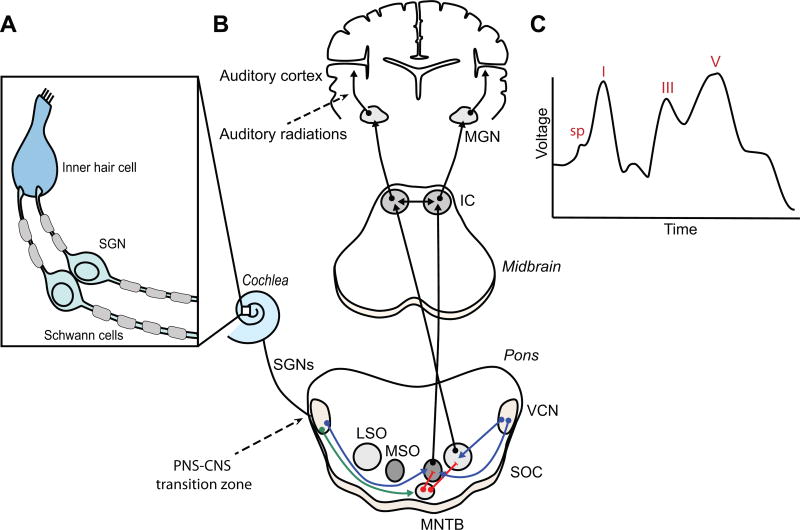
The auditory pathway (Figs. 1A, B) begins at the cochlea, where inner ear hair cells transduce sound waves into electrochemical signals that drive neurotransmitter release onto spiral ganglion neurons (SGNs), the primary sensory neurons that form the cochlear nerve. Action potentials then propagate along the cochlear nerve axons, which synapse onto neurons of the cochlear nucleus (CN) in the auditory brainstem. Neurons in the anteroventral cochlear nucleus (AVCN) respond to sound timing and intensity and project bilaterally to a collection of nuclei in the pons known as the superior olivary complex (SOC). These circuits then project to the inferior colliculus (IC) in the midbrain, which is a key point of integration of sound localization information. The IC in turn provides the main auditory input to the medial geniculate body (MGN) of the thalamus. The highest level of auditory processing begins at the auditory cortex, which receives input from the MGN and integrates auditory information with multiple association areas in the brain.
Figure 1. The Auditory Pathway.
A) Schematic representation of peripheral SGN myelination. In the auditory periphery, cochlear hair cells activate SGNs that project to the CN in the auditory brainstem. Oligodendrocyte myelination of SGN axons begins at the CNS-PNS transitional zone (arrow). B) Schematic representation of the central auditory pathway highlighting ITD and IID circuitry at the level of the pons. AVCN SBCs (blue) extend excitatory projections to the LSO and MSO in the SOC. AVCN GBCs (green) extend excitatory projections to the MNTB, which then send inhibitory projections (red) to the LSO and MSO. Auditory information then passes through several subsequent nuclei including the IC before reaching the MGN and projecting to the auditory cortex. C) Auditory system function can be measured using Auditory Brainstem Responses (ABR), a.k.a. Brainstem Auditory Evoked Responses (BAER). This method uses electrodes to detect evoked electrical potentials at various stages along the early auditory pathway, from the cochlea through the midbrain. The summating potential (SP) reflects activation of inner hair cells, peak I corresponds to evoked potentials in SGNs while ABR peaks III and IV correspond to the CN and IC, respectively. Fig.1B is adapted from (Cope et al., 2015). Fig. 1C is adapted from (Liberman et al., 2016).
AVCN: anteroventral cochlear nucleus; LSO: lateral superior olive; MSO: medial superior olive; GBC: globular bushy cell; SBC: spherical bushy cell; SOC: superior olivary complex; MNTB: medial nucleus of the trapezoid body; MGN: medial geniculate nucleus; SGNs: spiral ganglion neurons.
Different myelin sources in the auditory pathway: peripheral vs central
Different classes of glial cells produce myelin in the peripheral and central nervous systems, i.e. Schwann cells in the peripheral nervous system (PNS) and oligodendrocytes in the central nervous system (CNS). While the biophysical properties of myelin generated by each cell type are similar, these two cell types have different developmental origins (i.e. Schwann cells derive from the neural crest while oligodendrocytes derive from the subventricular zone); myelin produced by these cells differs in protein composition; their maturation is regulated by different mechanisms, and whereas each Schwann cell engages and myelinates only a single axon, an individual oligodendrocyte may myelinate as many as 60 axons (Nave and Werner, 2014).
The spiral ganglion contains two types of neurons. Type I cells are the most abundant (>90%), they innervate inner hair cells and have large diameter myelinated axons. Type II cells innervate outer hair cells and are unmyelinated. As type I SGN axons enter the CNS at the so-called CNS-PNS transitional zone (Fraher, 1992) (Fig. 1), their myelination changes from Schwann cell to oligodendrocyte. Histological examination of the rat cochlear nerve showed that the myelin along the axon segment between the SGN cell body and the transitional zone is comprised of peripheral myelin proteins while myelin between the transitional zone and brainstem is exclusively of the central type (Toesca, 1996). In rodents, myelin markers appear on peripheral and central projections of the cochlear nerve simultaneously (Knipper et al., 1998). Myelination of the CNS segment by oligodendrocytes is initiated in close proximity to the CNS-PNS transitional zone between postnatal days 7 and P8 and then extends centrally toward the brainstem (Wang et al., 2013).
The Timing of Auditory Pathway Myelination Parallels that of Auditory Function
The timeline of auditory system myelination mirrors key milestones in auditory functional development. Specifically, myelination along the cochlear nerve and within the auditory brainstem occurs near the onset of hearing. Additionally, in humans, higher order auditory centers such as the auditory cortex and associated white matter tracts undergo a pattern of myelination that is contemporaneous with language acquisition, suggesting an important interaction between auditory experience, auditory circuit maturation and myelination.
Peripheral Auditory Myelin Development
Humans respond to auditory stimuli in utero starting at 24–25 weeks of gestation (Birnholz and Benacerraf, 1983), but Schwann cells begin myelinating SGN axons at around the 15th fetal week, starting first along the distal axon segment and then progressing proximally (Moore and Linthicum, 2001). The pattern of human SGN myelination also follows the cochlea’s tonotopic gradient wherein the SGN axons that project to the basal turn of the cochlea are myelinated before axons projecting to the apical turn (Sanchez del Rey and Sanchez Fernandez, 2006). In the mouse cochlea, myelination by Schwann cells starts near birth at the cell body of SGNs and progresses to the central and peripheral processes between postnatal days 8 and 10, just days prior to the onset of hearing (Wang et al., 2013).
In humans, SGNs, like other bipolar sensory neurons, have myelinated axons but their cell bodies are covered by a thin rim of satellite cell cytoplasm (Liu et al., 2015; Tylstedt et al., 1997). Remarkably, SGN cell bodies in several mammalian species are myelinated. For example, SGN cell bodies in rats are covered by a thin and morphologically heterogeneous myelin sheath (Rosenbluth, 1962) and SGN cell body myelination is observed around the time of birth in the cat cochlea (Romand et al., 1980). The functional implications of cell body myelination and the mechanisms driving it remain unknown.
Central Auditory Myelin Development
In the human auditory brainstem, myelination coincides with hearing onset and occurs as early as the 26th fetal week (Moore et al., 1995). Myelin sheaths become visible by standard histological stains throughout the auditory brainstem and midbrain (CN, SOC and IC) beginning between fetal weeks 26 and 29 and increase in intensity up to 1 year of age (Moore et al., 1995). In contrast, studies using MRI to visualize auditory system myelin detect signal intensity changes that appear approximately 11 weeks later than those observed histologically. These changes in myelin can be seen by the 37th fetal week in the CN and SOC and are slightly delayed further up the auditory pathway appearing in the IC near the 38th fetal week (Sano et al., 2007). The delayed onset of myelin change by MRI measurements indicates possible functional maturation of the auditory brainstem after initial myelination of the neurons.
Relative to the auditory brainstem, myelination of structures higher in the auditory pathway occurs later and has a more protracted timeline. MRI detects changes in signal intensities indicative of increased myelination in the MGN beginning at 10 weeks of age while changes in subcortical white matter tracts associated with auditory processing such as the auditory radiations and the splenium of corpus callosum are detectable by week 24 (Sano et al., 2008). In the primary auditory cortex, myelin signal intensities change most rapidly up to about 1.5 years of age and then progress at a slower rate into adulthood (Su et al., 2008). Myelin changes in the primary auditory cortex slightly precede myelination of cortical regions and association white matter tracts involved in language ability (i.e. Brocas, Wernickes, the angular gyrus and the arcuate fasciculus) (Su et al., 2008). This sequential pattern of white matter development observed in humans is consistent with myelin staining observed in other primates. For example, in the prosimian primate galago (Otolemur garnetti), the IC and MGN are partially myelinated at birth and reach maturity by weaning age, but myelination in the auditory cortex is nearly absent at birth and does not reach adult-like levels until near puberty (Miller et al., 2013).
Similar to humans, myelination of the murine central auditory pathway also coincides with the onset of hearing, which takes place between postnatal days 10 and 12 (Ehret, 1976). Within the mouse brainstem, Olig2 positive oligodendrocyte progenitors can be seen in auditory nuclei at birth (Dinh et al., 2014), but the onset of myelin gene expression does not begin until about the second postnatal week and becomes detectable throughout the brainstem and auditory cortex during this time period (Hackett et al., 2015; Kolson et al., 2016). As myelin gene expression increases, myelin thickness and conduction velocity also increase in parts of the auditory brainstem, reaching adult-like levels by P35 (Sinclair et al., 2017). The timeline for CNS myelin development in the mouse is comparable to what is observed in other rodents. For instance, in the rat and gerbil SOC, myelin gene expression and myelin protein immunoreactivity increase during the second to third postnatal week (Ehmann et al., 2013; Hafidi et al., 1996; Saliu et al., 2014).
The Role of Myelin in Auditory System Function
Myelin and Cochlear Nerve Function
Cochlear nerve myelination is essential to peripheral auditory system function. In the postnatal mouse, SGNs must attain myelination before action potentials can be elicited (Anniko, 1983). Studies with myelin-deficient animals highlight the importance of myelin in cochlear nerve structure and function. Rodents with peripheral dysmyelination caused by either direct ablation of Schwann cells with diphtheria toxin at birth (Zhou et al., 1995a; Zhou et al., 1995b) or due to mutations in the peripheral myelin protein 22 (Pmp22) gene (bt hamster) (Naito et al., 1999, 2003) display remarkably similar auditory neuropathy; i.e. significant myelin and neuronal loss without affecting hair cell survival (Naito et al., 2003; Zhou et al., 1995b). The auditory neuropathy in these animals is reflected in elevated auditory brainstem response (ABR) thresholds, reduced ABR amplitudes and prolonged ABR latencies (Naito et al., 1999; Zhou et al., 1995a) (Refer to Fig. 1C for a description of ABR waveforms). Loss of other myelin proteins, proteolipid protein (PLP) and its homolog M6B, also results ABR threshold elevations in mice, although the myelin quality in the cochlear nerve was not assessed in these mutants (Werner et al., 2013). MIce lacking both PLP and M6B also present with delays in peaks II-V of the ABR wave form, consistent with CNS white matter alterations in patients with PLP mutations, i.e. Pelizaeus-Merzbacher disease. In addition, loss of the gap junction protein connexin 29 (Cx29) causes peripheral myelinopathy associated with a delay in the maturation of auditory thresholds and the prolongation of auditory latencies (Tang et al., 2006). Congenic Ly5.1 mice, which carry a specific allelic variant of protein tyrosine phosphatase receptor type C (PTPRC), also display remarkable myelin pathology in the cochlea and abnormal ABR thresholds and latencies (Jyothi et al., 2010). While abnormal ABR thresholds in myelin-deficit animals reflect neuronal dysfunction, the prolongation of ABR latencies in these animals is consistent with a critical role of myelination in nerve conduction velocity.
At the molecular and cellular level, myelination of SGNs is accompanied by clustering of voltage-gated sodium channels at the central and peripheral axonal initial segments and nodes of Ranvier flanking the bipolar SGN. These structures form voltage generators that respond to synaptic inputs from hair cells by generating and transmitting action potentials to the CN (Hossain et al., 2005). Recent studies indicate that the heminodes formed by the first Schwann cells close to the inner hair cells (IHCs) are the initial spike generators of SGNs (Kim and Rutherford, 2016; Rutherford et al., 2012; Wan and Corfas, 2017). The structural and molecular features of SGN heminodes mature after the onset of hearing in rats (Kim and Rutherford, 2016). Disruption of SGN heminodes significantly impairs the synchronized conduction of action potentials, as evidenced by reduced ABR wave 1 amplitudes and increased wave 1 latencies (Wan and Corfas, 2017). These studies indicate that myelination of peripheral axons of SGNs plays a critical role not only in action potential propagation but also in generation and integration of the initial sound-driven nerve spikes.
Myelin and Sound Localization
The ability to localize sounds is important for tracking threats or prey, understanding speech in noisy settings, and analyzing complex acoustic scenes. To localize sounds along the horizontal plane, birds and mammals compare the arrival times and intensities of sounds reaching the two ears (see review by Grothe and Pecka, 2014). Conceptually, these computations are simple, but in practice, localizing sounds with binaural cues requires extreme precision. Because of the speed of sound, interaural time differences (ITDs) for humans maximally reach ~700 μs, and humans are able to discriminate sound sources with ITDs that differ by as little as 10 – 20 µs (Klumpp and Eady, 1956). Similarly, humans can discriminate interaural intensity differences (IIDs) that differ by as little as 1 dB (Mills, 1960).
In mammals, the detection of ITDs and IIDs takes place in two specialized nuclei in the SOC: the medial superior olive (MSO) and the lateral superior olive (LSO). Neurons in these nuclei integrate synaptic input from pathways that originate at the two ears (Fig. 1B). To detect ITDs, MSO neurons compare the timing of excitatory synaptic input driven by spherical bushy cells (SBCs) in the ipsi- and contralateral cochlear nuclei (Cant and Casseday, 1986; Lindsey, 1975; Stotler, 1953; Warr, 1966). MSO neurons fire when these binaural inputs arrive within a narrow time window (Goldberg and Brown, 1969; Yin and Chan, 1990). LSO neurons detect IIDs by integrating excitatory input from ipsilateral SBCs (Cant and Casseday, 1986) with contralaterally driven inhibitory input. Inhibitory input to the LSO is provided by neurons in the medial nucleus of the trapezoid body (MNTB), which in turn are driven by globular bushy cells (GBCs) in the contralateral cochlear nucleus (Harrison and Warr, 1962; Spangler et al., 1985). Because of this circuit arrangement, sounds arriving with greater intensity at one ear will tend to excite neurons in the ipsilateral LSO and inhibit neurons in the contralateral LSO (Tollin, 2003). Importantly, the MNTB also modulates activity levels in the MSO by providing a temporally precise, contralaterally driven inhibitory input to MSO neurons (Banks and Smith, 1992; Spangler et al., 1985).
The circuitry needed for sound localization presents distinct anatomical challenges as axons projecting to the ipsilateral and contralateral SOC must travel varying distances but provide synaptic output with near simultaneous timing. Recent studies suggest that distinct patterns of myelination allow for the fine tuning of conduction velocities along neuronal circuits and provide insights into the mechanisms underlying sound localization circuitry. In the gerbil ITD pathway, axons from SBCs projecting to the contralateral MSO have longer internodes and larger axon diameters than their ipsilateral projections, which is predicted to raise conduction velocity along contralateral projections to help overcome a greater relative travel distance (Seidl and Rubel, 2016).
In both ITD and IID pathways, GBCs projecting to the contralateral MNTB are tonotopically arranged such that cells responding to high frequency sound target the medial MNTB; however, GBCs responsive to low frequency sound project to the lateral MNTB and therefore their axons must travel a greater relative distance (Guinan et al., 1972). In the gerbil, it was recently shown that the pattern of myelin along GBC axons is tuned to their site of termination such that axons responding to low frequency sounds have shorter internodes and larger diameter axons than their high frequency counterparts, the combination of which led to faster relative conduction velocity along low frequency axons (Ford et al., 2015).
As mentioned above, AVCN SBCs send direct excitatory projections to the MSO and LSO whereas GBCs directly excite the MNTB, which in turn provides inhibitory input to MSO and LSO (see Fig. 1). Thus, the inhibitory pathway involves an extra synapse and neuron relative to the excitatory pathway. One of the mysteries in the auditory brainstem has been how the inhibitory pathway overcomes these additional steps to provide inhibition that arrives at the same time or slightly before excitation (Brand et al., 2002; Grothe and Sanes, 1993; Roberts et al., 2013). It was recently found that the axons of GBCs have longer internodes and greater axon diameters than SBCs, which was predicted to result in faster action potential velocity in GBCs (Ford et al., 2015). This pattern of myelination may help the inhibitory pathway match the speed of the excitatory pathway despite the additional synaptic step.
Other species also appear to rely on differential patterns of myelination to regulate conduction velocity and timing along sound localization circuitry. Studies in the chick ITD pathway found that both internode distance and axon diameter are significantly greater along contralateral axon projections relative to ipsilateral projections (Seidl et al., 2010) and these differences correlate with divergent conduction velocities (Seidl et al., 2014; Seidl et al., 2010). Interestingly, a recent comparative study found that the myelin adaptations identified in the gerbil sound localization circuit are absent in the mouse, which do not possess similar ITD requirements (Stange-Marten et al., 2017). Thus, specializations in myelin may represent a key mechanism used to calibrate temporal processing in sound localization pathways.
Myelin Plasticity in the Central Auditory System
Myelin has proven to be much more dynamic than previously imagined and appears to be influenced by multiple forms of experience. Myelin plasticity is clearly apparent in the context of experiential deprivation, which causes regional specific myelin deficits. For example, periods of social deprivation reduce both myelin thickness and the number of internodes generated by each oligodendrocyte in the medial prefrontal cortex (Liu et al., 2012; Makinodan et al., 2012). Experience also influences myelin along pathways involved in primary sensory information processing. In the visual system, monocular deprivation reduces internode length along the optic nerve (Etxeberria et al., 2016). In the somatosensory system, loss of tactile sensory input through whisker trimming reduces myelin levels (Barrera et al., 2013) and oligodendrocyte progenitor cell survival in the mouse barrel cortex (Hill et al., 2014).
Myelin in the auditory pathway may be similarly affected by alterations in auditory sensory experience. Imaging studies of individuals with congenital deafness indicate that auditory deprivation is associated with myelin deficits in the auditory cortex and underlying white matter tracts. MRI-volumetric measurements of the brains of congenitally deaf adults showed reduced white matter to grey matter ratios in Heschl's gyrus and superior temporal gyrus relative to normal hearing adults (Emmorey et al., 2003; Hribar et al., 2014) and these myelin deficits appeared as early as infancy (Smith et al., 2011). Diffusor tensor imaging (DTI) also detected changes in the microstructural properties in white matter with deaf subjects showing decreased fractional anisotropy in the Heschl's gyrus and the surrounding temporal lobe (Hribar et al., 2014; Kim et al., 2009). Studies using mice suggested that age-related hearing loss may also affect auditory cortical myelin. Inbred C57BL/6 mice have a genetic predisposition to age associated deafness due to mutations in the cadherin 23 gene (Kane et al., 2012) and show aberrant myelin ultrastructure in the primary auditory cortex but not in the primary visual cortex (Tremblay et al., 2012).
There is also evidence that hearing impairment jeopardizes myelin develoment and maintenance along auditory brainstem circuitry. It was recently shown that elevating auditory thresholds via earplugging in mice during the time of hearing onset reduces myelin thickness along axons projecting to the MNTB (Sinclair et al., 2017). Moveover, earplugging reduces myelin thickness even in adult mice, suggesting that auditory experience is needed to sustain myelin along auditory brainstem circuitry throughout life and that the myelin along these circuits might be compromised by even partial hearing loss (Sinclair et al., 2017).
Auditory enrichment may also promote myelination in the brain. Several DTI studies detected increased white matter connectivity in the corpus callosum in individuals with extensive musical training during childhood. These increases correlated with training duration and the age at which training began (Bengtsson et al., 2005; Steele et al., 2013). Additionally, an age-related decline in myelin gene expression in the primary auditory cortex is partially reversed by auditory training via operant conditioning in aged rats and is associated with improved cortical circuit temporal processing (de Villers-Sidani et al., 2010). Thus, while insufficient auditory experience may diminish levels of myelin, certain forms of auditory exposure may promote and/or maintain myelin in the developing and aging brain.
How might auditory experience influence myelin in the auditory system? Neuronal activity has been shown to promote both oligodendrocyte progenitors cell proliferation and myelin formational along axons (Barres and Raff, 1993; Demerens et al., 1996; Gibson et al., 2014; Lee et al., 2016; Mensch et al., 2015; Wake et al., 2011). Activity may regulate myelin formation by inducing the release of various neuronally-derived factors that act on oligodendrocytes to promote myelin development. For example, glutamate released by neurons during heightened activity activates glutamate receptors on oligodendrocyte lineage cells to promote myelin protein synthesis (Wake et al., 2011). Activity may also raise levels of neuronally-derived ATP, which indirectly promotes myelination by inducing release of cytokine leukemia inhibitory factor (LIF) from astrocytes (Ishibashi et al., 2006). Neurons also express neurotrophic factors that promote myelination, such as neuregulin-1 (NRG1) and brain-derived neurotrophic factor (BDNF), (Taveggia et al., 2008; Xiao et al., 2010), and the effects of social deprivation on medial prefrontal cortex myelin appear to be mediated by a reduction in NRG1 expression (Makinodan et al., 2012). It is possible that elevated levels and/or patterns of neuronal activity during auditory experience promote oligodendrocyte differentiation and myelination. Consequently, altered or reduced neural activity during auditory deprivation may inadequately stimulate myelination along auditory circuitry.
Myelin Pathology in the Peripheral Auditory System
Given the essential role hair cells play in sound detection and their fragility relative to other cochlear cell types, our understanding of peripheral auditory system pathologies has primarily been driven by research into conditions that compromise cochlear hair cell function. Nonetheless, there is emerging evidence that the myelin along the cochlear nerve may also be damaged by both genetic mutations and environmental insults and that altered peripheral myelin impairs auditory function. Here we provide a summary of pathologies that affect myelin in the peripheral auditory system.
Peripheral Neuropathy
Myelin pathology is a common cause of peripheral auditory neuropathy in humans, which is defined as a peripheral hearing deficit that occurs in the presence of healthy cochlear hair cells. A dominant missense mutation in the peripheral myelin protein 22 (PMP22) gene was found to cause Charcot-Marie-Tooth disease with deafness (Kovach et al., 1999). Interestingly, the most prominent change in ABR recordings of these patients is the prolongation of ABR wave latencies, consistent with the role of PMP22 in Schwann cell SGN myelination. Another myelin gene, myelin protein zero (MPZ), was found to be mutated in a family with hereditary sensory motor neuropathy and deafness (Starr et al., 2003). Patients in this pedigree developed auditory neuropathy with reduced neural inputs, and post mortem analysis of one case showed a significant loss of SGNs and distal SGN fibers with the remaining fibers displaying apparent demyelination (Starr et al., 2003).
Myelin deficits may also underlie some forms of acquired hearing loss. Guillain-Barre syndrome (GBS) is a peripheral neuropathy caused by Schwann cell damage, possibly through an autoimmune mechanism. Although the incidence is relatively low, a portion of GBS patients show abnormal ABRs (Nelson et al., 1988; Ropper and Chiappa, 1986; Schiff et al., 1985; Takazawa et al., 2012; Ueda and Kuroiwa, 2008). Intriguingly, although hearing thresholds gradually recover in some patients, their ABR waveforms show persistent increases in interpeak latencies (Takazawa et al., 2012), suggestive of permanent myelin deficits in these patients. Our recent study using mouse models showed that when Schwann cells are lost, they regenerate and remyelinate SGNs, but heminodes remain disrupted and ABR latencies prolonged (Wan and Corfas, 2017). Thus, heminodal disruption may be the underlying cause of the auditory neuropathy observed in some GBS patients. In addition, animal studies showed that cochlear nerve demyelination and auditory neuropathy is also observed in hearing loss caused by lack of iron (Lee et al., 2012) or hyperbilirubinemia (Ye et al., 2012).
Acoustic Trauma
Acoustic trauma is primarily associated with cochlear hair cell loss, however, the myelin along the cochlear nerve also shows significant damage after prolonged noise exposure in humans (Rask-Andersen et al., 2000). Studies in animal models illustrate the susceptibility of cochlear myelin to damage by acoustic trauma. After prolonged noise exposure (100 dB SPL white noise at 12 hr/day for 30 days), myelin sheaths can detach from the type I SGNs, which account for approximately 95% of SGNs, and Schwann cells begin to develop intensely osmiophilic myelin bodies (Rossi et al., 1976). Intense noise (110 dB SPL 14.8 kHz single tone noise at 3 hr/day for 3 days) is sufficient to cause perinodal dysmyelination in the cochlea (Tagoe et al., 2014). Considering the rapid onset of noise-induced myelin damage, it is possible that the myelin sheath may be a primary target of noise overexposure.
Ototoxins
Ototoxic drugs, such as chemotherapeutic agents, result in sensorineural hearing loss by targeting the sensory hair cells. However, emerging evidence indicates that other cochlear cells (e.g. supporting cells) and structures (e.g. synapses and myelin sheath) may also be affected by these compounds. Platinum-based chemotherapeutic agents, including cisplatin and carboplatin, induce hearing loss and are linked to Schwann cell toxicity in other peripheral nerves (Carozzi et al., 2015). Within a week after cisplatin treatment in guinea pigs, SGN perikarya begin to shrink and myelin sheaths begin to swell causing myelin detachment (van Ruijven et al., 2004). Myelinopathy is also one of the primary features of carboplatin ototoxicity and is characterized by myelin loss within 1 day after carboplatin treatment in chinchillas (Ding et al., 2002).
Aging
Myelin pathology in the cochlea is one of the hallmarks of age-related hearing loss. In guinea pigs, the number of degenerating myelin sheaths of the cochlear nerve significantly increases at 6 months and peaks at 26.5 months (Hoeffding and Feldman, 1988). In mice, the myelin sheaths of the cochlear nerve loosen and unravel with age, which leads to demyelination of the perikarya and eventual loss of SGNs (Cohen et al., 1990). Similarly, a significant reduction in myelin basic protein (MBP) expression and loss of MBP+ cochlear nerve fibers are observed in older human cochleae (>60 years of age) (Xing et al., 2012).
Myelin Pathology in the Central Auditory System
As myelin regulates action potential conduction velocity and therefore the timing and connectivity of neuronal circuity, it is not surprising that neurological disorders associated with abnormal CNS myelin also exhibit impaired auditory system function. These conditions offer insight into the role of myelin in facilitating auditory information processing and illuminate the deleterious effects the loss of myelin may have on healthy auditory function.
Demyelinating Disease
Multiple sclerosis (MS) is a chronic autoimmune disease characterized by the focal destruction of myelin that can affect any region of the CNS including those responsible for hearing and auditory information processing. Acute demyelinating lesions can significantly slow conduction velocity in the affected area resulting in a host of cognitive and sensory defects. Comprehensive studies indicate that MS does not lead to chronically elevated hearing thresholds (Doty et al., 2012). Although sudden sensorineural hearing loss in cases of episodic demyelination is observed in a minority of MS patients, such hearing loss largely undergoes complete remission (Hellmann et al., 2011). These reports indicate that central auditory demyelination does not have significant impact on the sound detection and reception. However, relatively subtle deficits in temporal processing of auditory information appear to be associated with MS. Specifically, some MS patients exhibit increased ABR I–V peak latencies and diminished peak V amplitude (Chiappa et al., 1980). These ABR changes suggest compromised myelin along the auditory circuitry in the brainstem. MS patients can also display deficits in processing binaural stimuli and impaired detection of ITD and IIDs (Furst and Levine, 2015), which may reflect impaired conduction velocity along sound localization pathways.
Auditory Processing Disorders
Developmental abnormalities in CNS myelin also impair auditory function. Auditory processing disorder (APD) is a heterogeneous developmental disorder characterized by listening difficulties including deficits in attention, language comprehension and speech discrimination. A defining characteristic of APD is that symptoms appear to be due to defects in the central auditory system since patients exhibit normal cochlear and hearing thresholds. While most APD cases do not have a clear underlying cause, decreased temporal precision in action potential firing has been proposed as a candidate mechanism that could jeopardize the fidelity of auditory signal timing (Kopp-Scheinpflug and Tempel, 2015). In line with this hypothesis, APD patients display myelin abnormalities in multiple brain regions associated with auditory processing. DTI studies of children diagnosed with APD detected increased mean diffusion in the auditory radiations (Farah et al., 2014), and reduced myelin in these regions could disrupt thalamo-cortical communication. The same study also found decreased anisotropy in the prefrontal cortex and anterior cingulate (Farah et al., 2014), which are areas of the brain closely tied to attention and top-down information processing and which may underlie difficulties in attending to auditory stimuli in APD.
Abnormalities in CNS myelin might also contribute to the auditory dysfunction observed in other neurodevelopmental disorders. For example, hypersensitivity to sound and distorted auditory perception are well documented in individuals with autism spectrum disorder (ASD) (Gomes et al., 2008; O'Connor, 2012). ASD patients have also been found to exhibit both reduced temporal processing of auditory stimuli (Foss-Feig et al., 2017) and decreased white matter connectivity in multiple brain areas including the temporal lobes (Travers et al., 2012). Thus, the possibility that impairments in CNS myelin contribute to auditory processing deficits in ASD should be further explored.
Psychiatric Disorders
Abnormalities in CNS myelin are found in multiple psychiatric disorders including schizophrenia, bipolar disorder, and chronic depression (Fields, 2008), and these conditions are linked to deficits in auditory sensory processing (Kahkonen et al., 2007; McLachlan et al., 2013; Zenisek et al., 2015). One link between abnormal myelin and auditory dysfunction in mental illness is the pathophysiology of auditory verbal hallucinations (AVH), which is a common symptom of psychiatric illness. DTI studies of schizophrenia patients with AVH have found changes in connectivity along auditory fiber bundles and shown that these changes vary with the duration of AVH history (Hubl et al., 2004; Knochel et al., 2012; Mulert et al., 2012; Wigand et al., 2015). However, some of these studies found increases in the fractional anisotropy of auditory fiber bundles, while others found decreases. Thus, the precise nature of these myelin changes and how they might contribute to AVH are not understood
Future Directions
The temporally precise nature of the auditory system makes it uniquely suited to investigate the role of myelin in neural circuit behavior. The importance of myelin in audition is underscored by the fact that auditory circuit myelination coincides with the functional development of the auditory system and by findings that even subtle perturbations in myelin due to disease and developmental disorders can jeopardize hearing and auditory information processing. Despite this recognition, we are only beginning to appreciate how myelin contributes to auditory function and numerous questions are still waiting to be addressed. For instance, what mechanisms underlie deficits in myelin in hearing impaired individuals? Are the complex patterns of myelin in brainstem sound localization circuitry also influenced by experience? Moreover, beyond changes in conduction velocity, how might alterations in myelin affect other aspects of auditory information processing? Myelin deficits have been shown to alter neurotransmission in other areas of the brain (Roy et al., 2007). Do changes in myelin similarly alter neurotransmission within auditory circuitry? Overcoming these gaps in knowledge has the potential to significantly enhance our ability to treat both auditory disorders and myelin pathologies that impair auditory function.
References
- Anniko M. Early development and maturation of the spiral ganglion. Acta oto-laryngologica. 1983;95:263–276. doi: 10.3109/00016488309130943. [DOI] [PubMed] [Google Scholar]
- Banks MI, Smith PH. Intracellular recordings from neurobiotin-labeled cells in brain slices of the rat medial nucleus of the trapezoid body. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:2819–2837. doi: 10.1523/JNEUROSCI.12-07-02819.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera K, Chu P, Abramowitz J, Steger R, Ramos RL, Brumberg JC. Organization of myelin in the mouse somatosensory barrel cortex and the effects of sensory deprivation. Dev Neurobiol. 2013;73:297–314. doi: 10.1002/dneu.22060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Birnholz JC, Benacerraf BR. The development of human fetal hearing. Science. 1983;222:516–518. doi: 10.1126/science.6623091. [DOI] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–547. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- Cant NB, Casseday JH. Projections from the anteroventral cochlear nucleus to the lateral and medial superior olivary nuclei. J Comp Neurol. 1986;247:457–476. doi: 10.1002/cne.902470406. [DOI] [PubMed] [Google Scholar]
- Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neuroscience letters. 2015;596:90–107. doi: 10.1016/j.neulet.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Chiappa KH, Harrison JL, Brooks EB, Young RR. Brainstem auditory evoked responses in 200 patients with multiple sclerosis. Ann Neurol. 1980;7:135–143. doi: 10.1002/ana.410070208. [DOI] [PubMed] [Google Scholar]
- Cohen GM, Park JC, Grasso JS. Comparison of demyelination and neural degeneration in spiral and Scarpa's ganglia of C57BL/6 mice. Journal of electron microscopy technique. 1990;15:165–172. doi: 10.1002/jemt.1060150208. [DOI] [PubMed] [Google Scholar]
- Cope TE, Baguley DM, Griffiths TD. The functional anatomy of central auditory processing. Pract Neurol. 2015;15:302–308. doi: 10.1136/practneurol-2014-001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Alzghoul L, Zhou X, Simpson KL, Lin RC, Merzenich MM. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc Natl Acad Sci U S A. 2010;107:13900–13905. doi: 10.1073/pnas.1007885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, McFadden SL, Salvi RJ. Calpain immunoreactivity and morphological damage in chinchilla inner ears after carboplatin. Journal of the Association for Research in Otolaryngology : JARO. 2002;3:68–79. doi: 10.1007/s101620020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh ML, Koppel SJ, Korn MJ, Cramer KS. Distribution of glial cells in the auditory brainstem: normal development and effects of unilateral lesion. Neuroscience. 2014;278:237–252. doi: 10.1016/j.neuroscience.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Tourbier I, Davis S, Rotz J, Cuzzocreo JL, Treem J, Shephard N, Pham DL. Pure-tone auditory thresholds are not chronically elevated in multiple sclerosis. Behav Neurosci. 2012;126:314–324. doi: 10.1037/a0027046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann H, Hartwich H, Salzig C, Hartmann N, Clement-Ziza M, Ushakov K, Avraham KB, Bininda-Emonds OR, Hartmann AK, Lang P, Friauf E, Nothwang HG. Time-dependent gene expression analysis of the developing superior olivary complex. J Biol Chem. 2013;288:25865–25879. doi: 10.1074/jbc.M113.490508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus) J Am Audiol Soc. 1976;1:179–184. [PubMed] [Google Scholar]
- Emmorey K, Allen JS, Bruss J, Schenker N, Damasio H. A morphometric analysis of auditory brain regions in congenitally deaf adults. Proc Natl Acad Sci U S A. 2003;100:10049–10054. doi: 10.1073/pnas.1730169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etxeberria A, Hokanson KC, Dao DQ, Mayoral SR, Mei F, Redmond SA, Ullian EM, Chan JR. Dynamic Modulation of Myelination in Response to Visual Stimuli Alters Optic Nerve Conduction Velocity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:6937–6948. doi: 10.1523/JNEUROSCI.0908-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah R, Schmithorst VJ, Keith RW, Holland SK. Altered white matter microstructure underlies listening difficulties in children suspected of auditory processing disorders: a DTI study. Brain Behav. 2014;4:531–543. doi: 10.1002/brb3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MC, Alexandrova O, Cossell L, Stange-Marten A, Sinclair J, Kopp-Scheinpflug C, Pecka M, Attwell D, Grothe B. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nature communications. 2015;6:8073. doi: 10.1038/ncomms9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig JH, Schauder KB, Key AP, Wallace MT, Stone WL. Audition-specific temporal processing deficits associated with language function in children with autism spectrum disorder. Autism Res. 2017 doi: 10.1002/aur.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraher JP. The CNS-PNS transitional zone of the rat. Morphometric studies at cranial and spinal levels. Progress in neurobiology. 1992;38:261–316. doi: 10.1016/0301-0082(92)90022-7. [DOI] [PubMed] [Google Scholar]
- Furst M, Levine RA. Hearing disorders in multiple sclerosis. Handb Clin Neurol. 2015;129:649–665. doi: 10.1016/B978-0-444-62630-1.00036-6. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol. 1969;32:613–636. doi: 10.1152/jn.1969.32.4.613. [DOI] [PubMed] [Google Scholar]
- Gomes E, Pedroso FS, Wagner MB. Auditory hypersensitivity in the autistic spectrum disorder. Pro Fono. 2008;20:279–284. doi: 10.1590/s0104-56872008000400013. [DOI] [PubMed] [Google Scholar]
- Grothe B, Pecka M. The natural history of sound localization in mammals--a story of neuronal inhibition. Front Neural Circuits. 2014;8:116. doi: 10.3389/fncir.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe B, Sanes DH. Bilateral inhibition by glycinergic afferents in the medial superior olive. J Neurophysiol. 1993;69:1192–1196. doi: 10.1152/jn.1993.69.4.1192. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Guinan SS, Norris BE. Single Auditory Units in Superior Olivary Complex .2. Locations of Unit Categories and Tonotopic Organization. Int J Neurosci. 1972;4:147-&. [Google Scholar]
- Hackett TA, Guo Y, Clause A, Hackett NJ, Garbett K, Zhang P, Polley DB, Mirnics K. Transcriptional maturation of the mouse auditory forebrain. BMC Genomics. 2015;16:606. doi: 10.1186/s12864-015-1709-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafidi A, Katz JA, Sanes DH. Differential expression of MAG, MBP and L1 in the developing lateral superior olive. Brain Res. 1996;736:35–43. doi: 10.1016/0006-8993(96)00653-1. [DOI] [PubMed] [Google Scholar]
- Harrison JM, Warr WB. A study of the cochlear nuclei and ascending auditory pathways of the medulla. J Comp Neurol. 1962;119:341–379. doi: 10.1002/cne.901190306. [DOI] [PubMed] [Google Scholar]
- Hellmann MA, Steiner I, Mosberg-Galili R. Sudden sensorineural hearing loss in multiple sclerosis: clinical course and possible pathogenesis. Acta Neurol Scand. 2011;124:245–249. doi: 10.1111/j.1600-0404.2010.01463.x. [DOI] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Goncalves CM, Grutzendler J, Nishiyama A. Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat Neurosci. 2014;17:1518–1527. doi: 10.1038/nn.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffding V, Feldman ML. Changes with age in the morphology of the cochlear nerve in rats: light microscopy. J Comp Neurol. 1988;276:537–546. doi: 10.1002/cne.902760408. [DOI] [PubMed] [Google Scholar]
- Hossain WA, Antic SD, Yang Y, Rasband MN, Morest DK. Where is the spike generator of the cochlear nerve? Voltage-gated sodium channels in the mouse cochlea. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6857–6868. doi: 10.1523/JNEUROSCI.0123-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hribar M, Suput D, Carvalho AA, Battelino S, Vovk A. Structural alterations of brain grey and white matter in early deaf adults. Hearing research. 2014;318:1–10. doi: 10.1016/j.heares.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyothi V, Li M, Kilpatrick LA, Smythe N, LaRue AC, Zhou D, Schulte BA, Schmiedt RA, Lang H. Unmyelinated auditory type I spiral ganglion neurons in congenic Ly5.1 mice. J Comp Neurol. 2010;518:3254–3271. doi: 10.1002/cne.22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahkonen S, Yamashita H, Rytsala H, Suominen K, Ahveninen J, Isometsa E. Dysfunction in early auditory processing in major depressive disorder revealed by combined MEG and EEG. J Psychiatry Neurosci. 2007;32:316–322. [PMC free article] [PubMed] [Google Scholar]
- Kane KL, Longo-Guess CM, Gagnon LH, Ding D, Salvi RJ, Johnson KR. Genetic background effects on age-related hearing loss associated with Cdh23 variants in mice. Hearing research. 2012;283:80–88. doi: 10.1016/j.heares.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Park SY, Kim J, Lee DH, Park HJ. Alterations of white matter diffusion anisotropy in early deafness. Neuroreport. 2009;20:1032–1036. doi: 10.1097/WNR.0b013e32832e0cdd. [DOI] [PubMed] [Google Scholar]
- Kim KX, Rutherford MA. Maturation of NaV and KV Channel Topographies in the Auditory Nerve Spike Initiator before and after Developmental Onset of Hearing Function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:2111–2118. doi: 10.1523/JNEUROSCI.3437-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp RG, Eady HR. Some Measurements of Interaural Time Difference Thresholds. J Acoust Soc Am. 1956;28:859–860. [Google Scholar]
- Knipper M, Bandtlow C, Gestwa L, Kopschall I, Rohbock K, Wiechers B, Zenner HP, Zimmermann U. Thyroid hormone affects Schwann cell and oligodendrocyte gene expression at the glial transition zone of the VIIIth nerve prior to cochlea function. Development. 1998;125:3709–3718. doi: 10.1242/dev.125.18.3709. [DOI] [PubMed] [Google Scholar]
- Knochel C, Oertel-Knochel V, Schonmeyer R, Rotarska-Jagiela A, van de Ven V, Prvulovic D, Haenschel C, Uhlhaas P, Pantel J, Hampel H, Linden DE. Interhemispheric hypoconnectivity in schizophrenia: fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. Neuroimage. 2012;59:926–934. doi: 10.1016/j.neuroimage.2011.07.088. [DOI] [PubMed] [Google Scholar]
- Kolson DR, Wan J, Wu J, Dehoff M, Brandebura AN, Qian J, Mathers PH, Spirou GA. Temporal patterns of gene expression during calyx of held development. Dev Neurobiol. 2016;76:166–189. doi: 10.1002/dneu.22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Tempel BL. Decreased temporal precision of neuronal signaling as a candidate mechanism of auditory processing disorder. Hearing research. 2015;330:213–220. doi: 10.1016/j.heares.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach MJ, Lin JP, Boyadjiev S, Campbell K, Mazzeo L, Herman K, Rimer LA, Frank W, Llewellyn B, Jabs EW, Gelber D, Kimonis VE. A unique point mutation in the PMP22 gene is associated with Charcot-Marie-Tooth disease and deafness. American journal of human genetics. 1999;64:1580–1593. doi: 10.1086/302420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DL, Strathmann FG, Gelein R, Walton J, Mayer-Proschel M. Iron deficiency disrupts axon maturation of the developing auditory nerve. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:5010–5015. doi: 10.1523/JNEUROSCI.0526-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HU, Nag S, Blasiak A, Jin Y, Thakor N, Yang IH. Subcellular Optogenetic Stimulation for Activity-Dependent Myelination of Axons in a Novel Microfluidic Compartmentalized Platform. ACS Chem Neurosci. 2016;7:1317–1324. doi: 10.1021/acschemneuro.6b00157. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Epstein MJ, Cleveland SS, Wang H, Maison SF. Toward a Differential Diagnosis of Hidden Hearing Loss in Humans. PloS one. 2016;11:e0162726. doi: 10.1371/journal.pone.0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey BG. Fine structure and distribution of axon terminals from the cochlear nucleus on neurons in the medial superior olivary nucleus of the cat. J Comp Neurol. 1975;160:81–103. doi: 10.1002/cne.901600106. [DOI] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Edin F, Atturo F, Rieger G, Lowenheim H, Senn P, Blumer M, Schrott-Fischer A, Rask-Andersen H, Glueckert R. The pre- and post-somatic segments of the human type I spiral ganglion neurons--structural and functional considerations related to cochlear implantation. Neuroscience. 2015;284:470–482. doi: 10.1016/j.neuroscience.2014.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan NM, Phillips DS, Rossell SL, Wilson SJ. Auditory processing and hallucinations in schizophrenia. Schizophr Res. 2013;150:380–385. doi: 10.1016/j.schres.2013.08.039. [DOI] [PubMed] [Google Scholar]
- Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci. 2015;18:628–630. doi: 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Lackey EP, Hackett TA, Kaas JH. Development of myelination and cholinergic innervation in the central auditory system of a prosimian primate (Otolemur garnetti) J Comp Neurol. 2013;521:3804–3816. doi: 10.1002/cne.23379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AW. Lateralization of High-Frequency Tones. J Acoust Soc Am. 1960;32:132–134. [Google Scholar]
- Moore JK, Linthicum FH., Jr Myelination of the human auditory nerve: different time courses for Schwann cell and glial myelin. The Annals of otology, rhinology, and laryngology. 2001;110:655–661. doi: 10.1177/000348940111000711. [DOI] [PubMed] [Google Scholar]
- Moore JK, Perazzo LM, Braun A. Time course of axonal myelination in the human brainstem auditory pathway. Hearing research. 1995;87:21–31. doi: 10.1016/0378-5955(95)00073-d. [DOI] [PubMed] [Google Scholar]
- Mulert C, Kirsch V, Whitford TJ, Alvarado J, Pelavin P, McCarley RW, Kubicki M, Salisbury DF, Shenton ME. Hearing voices: a role of interhemispheric auditory connectivity? World J Biol Psychiatry. 2012;13:153–158. doi: 10.3109/15622975.2011.570789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito R, Murofushi T, Mizutani M, Kaga K. Auditory brainstem responses, electrocochleograms, and cochlear microphonics in the myelin deficient mutant hamster 'bt'. Hearing research. 1999;136:44–48. doi: 10.1016/s0378-5955(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Naito R, Murofushi T, Mizutani M, Kaga K. Myelin-deficiency in the cochlear nerve of the 'bt' mutant hamster. Hearing research. 2003;176:17–24. doi: 10.1016/s0378-5955(02)00550-6. [DOI] [PubMed] [Google Scholar]
- Nave KA, Werner HB. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- Nelson KR, Gilmore RL, Massey A. Acoustic nerve conduction abnormalities in Guillain-Barre syndrome. Neurology. 1988;38:1263–1266. doi: 10.1212/wnl.38.8.1263. [DOI] [PubMed] [Google Scholar]
- O'Connor K. Auditory processing in autism spectrum disorder: a review. Neurosci Biobehav Rev. 2012;36:836–854. doi: 10.1016/j.neubiorev.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Rask-Andersen H, Ekvall L, Scholtz A, Schrott-Fischer A. Structural/audiometric correlations in a human inner ear with noise-induced hearing loss. Hearing research. 2000;141:129–139. doi: 10.1016/s0378-5955(99)00216-6. [DOI] [PubMed] [Google Scholar]
- Roberts MT, Seeman SC, Golding NL. A mechanistic understanding of the role of feedforward inhibition in the mammalian sound localization circuitry. Neuron. 2013;78:923–935. doi: 10.1016/j.neuron.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romand R, Romand MR, Mulle C, Marty R. Early stages of myelination in the spiral ganglion cells of the kitten during development. Acta oto-laryngologica. 1980;90:391–397. doi: 10.3109/00016488009131740. [DOI] [PubMed] [Google Scholar]
- Ropper AH, Chiappa KH. Evoked potentials in Guillain-Barre syndrome. Neurology. 1986;36:587–590. doi: 10.1212/wnl.36.4.587. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. The fine structure of acoustic ganglia in the rat. The Journal of cell biology. 1962;12:329–359. doi: 10.1083/jcb.12.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G, Robecchi MG, Penna M. Effects of acoustic trauma on corti's ganglion. Acta oto-laryngologica. 1976;81:270–277. doi: 10.3109/00016487609119962. [DOI] [PubMed] [Google Scholar]
- Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, Benoit-Marand M, Chen C, Moore H, O'Donnell P, Brunner D, Corfas G. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci U S A. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford MA, Chapochnikov NM, Moser T. Spike encoding of neurotransmitter release timing by spiral ganglion neurons of the cochlea. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:4773–4789. doi: 10.1523/JNEUROSCI.4511-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliu A, Adise S, Xian S, Kudelska K, Rodriguez-Contreras A. Natural and lesion-induced decrease in cell proliferation in the medial nucleus of the trapezoid body during hearing development. J Comp Neurol. 2014;522:971–985. doi: 10.1002/cne.23473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez del Rey A, Sanchez Fernandez JM. Development of the human fetal cochlear nerve: a morphometric study. Hearing research. 2006;212:251. doi: 10.1016/j.heares.2005.11.001. author reply 252–253. [DOI] [PubMed] [Google Scholar]
- Sano M, Kaga K, Kuan CC, Ino K, Mima K. Early myelination patterns in the brainstem auditory nuclei and pathway: MRI evaluation study. Int J Pediatr Otorhinolaryngol. 2007;71:1105–1115. doi: 10.1016/j.ijporl.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Sano M, Kuan CC, Kaga K, Itoh K, Ino K, Mima K. Early myelination patterns in the central auditory pathway of the higher brain: MRI evaluation study. Int J Pediatr Otorhinolaryngol. 2008;72:1479–1486. doi: 10.1016/j.ijporl.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Schiff JA, Cracco RQ, Cracco JB. Brainstem auditory evoked potentials in Guillain-Barre syndrome. Neurology. 1985;35:771–773. doi: 10.1212/wnl.35.5.771. [DOI] [PubMed] [Google Scholar]
- Seidl AH, Rubel EW. Systematic and differential myelination of axon collaterals in the mammalian auditory brainstem. Glia. 2016;64:487–494. doi: 10.1002/glia.22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl AH, Rubel EW, Barria A. Differential conduction velocity regulation in ipsilateral and contralateral collaterals innervating brainstem coincidence detector neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:4914–4919. doi: 10.1523/JNEUROSCI.5460-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl AH, Rubel EW, Harris DM. Mechanisms for adjusting interaural time differences to achieve binaural coincidence detection. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:70–80. doi: 10.1523/JNEUROSCI.3464-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JL, Fischl MJ, Alexandrova O, Hess M, Grothe B, Leibold C, Kopp-Scheinpflug C. Sound-evoked activity influences myelination of brainstem axons in the trapezoid body. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2017 doi: 10.1523/JNEUROSCI.3728-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Mecoli MD, Altaye M, Komlos M, Maitra R, Eaton KP, Egelhoff JC, Holland SK. Morphometric differences in the Heschl's gyrus of hearing impaired and normal hearing infants. Cereb Cortex. 2011;21:991–998. doi: 10.1093/cercor/bhq164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler KM, Warr WB, Henkel CK. The projections of principal cells of the medial nucleus of the trapezoid body in the cat. J Comp Neurol. 1985;238:249–262. doi: 10.1002/cne.902380302. [DOI] [PubMed] [Google Scholar]
- Stange-Marten A, Nabel AL, Sinclair JL, Fischl M, Alexandrova O, Wohlfrom H, Kopp-Scheinpflug C, Pecka M, Grothe B. Input timing for spatial processing is precisely tuned via constant synaptic delays and myelination patterns in the auditory brainstem. Proc Natl Acad Sci U S A. 2017;114:E4851–E4858. doi: 10.1073/pnas.1702290114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A, Michalewski HJ, Zeng FG, Fujikawa-Brooks S, Linthicum F, Kim CS, Winnier D, Keats B. Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (Tyr145->Ser) Brain : a journal of neurology. 2003;126:1604–1619. doi: 10.1093/brain/awg156. [DOI] [PubMed] [Google Scholar]
- Steele CJ, Bailey JA, Zatorre RJ, Penhune VB. Early musical training and white-matter plasticity in the corpus callosum: evidence for a sensitive period. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:1282–1290. doi: 10.1523/JNEUROSCI.3578-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotler WA. An experimental study of the cells and connections of the superior olivary complex of the cat. J Comp Neurol. 1953;98:401–431. doi: 10.1002/cne.900980303. [DOI] [PubMed] [Google Scholar]
- Su P, Kuan CC, Kaga K, Sano M, Mima K. Myelination progression in language-correlated regions in brain of normal children determined by quantitative MRI assessment. Int J Pediatr Otorhinolaryngol. 2008;72:1751–1763. doi: 10.1016/j.ijporl.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Tagoe T, Barker M, Jones A, Allcock N, Hamann M. Auditory nerve perinodal dysmyelination in noise-induced hearing loss. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:2684–2688. doi: 10.1523/JNEUROSCI.3977-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa T, Ikeda K, Murata K, Kawase Y, Hirayama T, Ohtsu M, Harada H, Totani T, Sugiyama K, Kawabe K, Kano O, Iwasaki Y. Sudden deafness and facial diplegia in Guillain-Barre Syndrome: radiological depiction of facial and acoustic nerve lesions. Internal medicine. 2012;51:2433–2437. doi: 10.2169/internalmedicine.51.7737. [DOI] [PubMed] [Google Scholar]
- Tang W, Zhang Y, Chang Q, Ahmad S, Dahlke I, Yi H, Chen P, Paul DL, Lin X. Connexin29 is highly expressed in cochlear Schwann cells, and it is required for the normal development and function of the auditory nerve of mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:1991–1999. doi: 10.1523/JNEUROSCI.5055-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, Einheber S, Salzer JL. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56:284–293. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- Toesca A. Central and peripheral myelin in the rat cochlear and vestibular nerves. Neuroscience letters. 1996;221:21–24. doi: 10.1016/s0304-3940(96)13273-0. [DOI] [PubMed] [Google Scholar]
- Tollin DJ. The lateral superior olive: a functional role in sound source localization. Neuroscientist. 2003;9:127–143. doi: 10.1177/1073858403252228. [DOI] [PubMed] [Google Scholar]
- Travers BG, Adluru N, Ennis C, Tromp do PM, Destiche D, Doran S, Bigler ED, Lange N, Lainhart JE, Alexander AL. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res. 2012;5:289–313. doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Zettel ML, Ison JR, Allen PD, Majewska AK. Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices. Glia. 2012;60:541–558. doi: 10.1002/glia.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylstedt S, Kinnefors A, Rask-Andersen H. Neural interaction in the human spiral ganglion: a TEM study. Acta oto-laryngologica. 1997;117:505–512. doi: 10.3109/00016489709113429. [DOI] [PubMed] [Google Scholar]
- Ueda N, Kuroiwa Y. Sensorineural deafness in Guillain-Barre syndrome. Brain Nerve. 2008;60:1181–1186. [PubMed] [Google Scholar]
- van Ruijven MW, de Groot JC, Smoorenburg GF. Time sequence of degeneration pattern in the guinea pig cochlea during cisplatin administration. A quantitative histological study. Hearing research. 2004;197:44–54. doi: 10.1016/j.heares.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan G, Corfas G. Transient auditory nerve demyelination as a new mechanism for hidden hearing loss. Nature communications. 2017;8:14487. doi: 10.1038/ncomms14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang B, Jiang H, Zhang L, Liu D, Xiao X, Ma H, Luo X, Bojrab D, 2nd, Hu Z. Myelination of the postnatal mouse cochlear nerve at the peripheral-central nervous system transitional zone. Front Pediatr. 2013;1:43. doi: 10.3389/fped.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr WB. Fiber degeneration following lesions in the anterior ventral cochlear nucleus of the cat. Exp Neurol. 1966;14:453–474. doi: 10.1016/0014-4886(66)90130-0. [DOI] [PubMed] [Google Scholar]
- Werner HB, Kramer-Albers EM, Strenzke N, Saher G, Tenzer S, Ohno-Iwashita Y, De Monasterio-Schrader P, Mobius W, Moser T, Griffiths IR, Nave KA. A critical role for the cholesterol-associated proteolipids PLP and M6B in myelination of the central nervous system. Glia. 2013;61:567–586. doi: 10.1002/glia.22456. [DOI] [PubMed] [Google Scholar]
- Wigand M, Kubicki M, Clemm von Hohenberg C, Leicht G, Karch S, Eckbo R, Pelavin PE, Hawley K, Rujescu D, Bouix S, Shenton ME, Mulert C. Auditory verbal hallucinations and the interhemispheric auditory pathway in chronic schizophrenia. World J Biol Psychiatry. 2015;16:31–44. doi: 10.3109/15622975.2014.948063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Wong AW, Willingham MM, van den Buuse M, Kilpatrick TJ, Murray SS. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals. 2010;18:186–202. doi: 10.1159/000323170. [DOI] [PubMed] [Google Scholar]
- Xing Y, Samuvel DJ, Stevens SM, Dubno JR, Schulte BA, Lang H. Age-related changes of myelin basic protein in mouse and human auditory nerve. PloS one. 2012;7:e34500. doi: 10.1371/journal.pone.0034500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye HB, Shi HB, Wang J, Ding DL, Yu DZ, Chen ZN, Li CY, Zhang WT, Yin SK. Bilirubin induces auditory neuropathy in neonatal guinea pigs via auditory nerve fiber damage. Journal of neuroscience research. 2012;90:2201–2213. doi: 10.1002/jnr.23107. [DOI] [PubMed] [Google Scholar]
- Yin TC, Chan JC. Interaural time sensitivity in medial superior olive of cat. J Neurophysiol. 1990;64:465–488. doi: 10.1152/jn.1990.64.2.465. [DOI] [PubMed] [Google Scholar]
- Zenisek R, Thaler NS, Sutton GP, Ringdahl EN, Snyder JS, Allen DN. Auditory processing deficits in bipolar disorder with and without a history of psychotic features. Bipolar Disord. 2015;17:769–780. doi: 10.1111/bdi.12333. [DOI] [PubMed] [Google Scholar]
- Zhou R, Abbas PJ, Assouline JG. Electrically evoked auditory brainstem response in peripherally myelin-deficient mice. Hearing research. 1995a;88:98–106. doi: 10.1016/0378-5955(95)00105-d. [DOI] [PubMed] [Google Scholar]
- Zhou R, Assouline JG, Abbas PJ, Messing A, Gantz BJ. Anatomical and physiological measures of auditory system in mice with peripheral myelin deficiency. Hearing research. 1995b;88:87–97. doi: 10.1016/0378-5955(95)00104-c. [DOI] [PubMed] [Google Scholar]