Abstract
Background
Primary cutaneous B-cell lymphomas (PCBCL) are frequently misdiagnosed and a biopsy is needed to attain the correct diagnosis.
Objective
To characterize the dermoscopic features of PCBCL.
Methods
In this retrospective observational study we analyzed the pathology reports of 172 newly diagnosed PCBCL for the initial clinical differential diagnosis. The dermoscopic images of 58 PCBCL were evaluated for dermoscopic features. Two dermoscopy experts, who were blinded to the diagnosis and the study objective, evaluated images from 17 cases for a dermoscopic differential diagnosis.
Results
Of 172 biopsy-proven PCBCL lesions, cutaneous lymphoma was suspected by the clinician in 16.3%; the leading diagnosis was basal cell carcinoma in 17.4%, and other skin neoplasms in 21%. Studying 58 PCBCL dermoscopic images, we most frequently identified salmon-colored background/area (79.3%) and prominent blood vessels (77.6%), mostly of serpentine (linear-irregular) morphology (67.2%). Dermoscopic features did not differ significantly by subtype or location. Blinded evaluation by dermoscopy experts raised a wide differential diagnosis including PCBCL, arthropod bite, basal cell carcinoma, amelanotic melanoma and scar/keloid.
Conclusions
Two dermoscopic features, salmon-colored area/background and serpentine vessels, are frequently seen in PCBCL lesions. These characteristic dermoscopic features, although not specific, can suggest a possible diagnosis of PCBCL.
Introduction
Primary cutaneous B-cell lymphomas (PCBCLs) are B-cell phenotype skin lymphomas that originate in the skin, and have no extracutaneous disease at the time of diagnosis. PCBCLs are classified into three main types: primary cutaneous marginal zone lymphoma (PCMZL), primary cutaneous follicle center lymphoma (PCFCL), and primary cutaneous diffuse large B-cell lymphoma – leg type (PCDLBCL-LT). While the first two types are recognized as indolent lymphomas, PCDLBCL-LT has a more aggressive behavior1.
Classically, PCMZL is characterized by red to violaceous papules, plaques, or nodules, frequently solitary, on the trunk or extremities in patients in their 50s; PCFCL presents as pink to violaceous papules or nodules, mainly on the head, in patients in their 60s; and PCDLBCL-LT typically presents as red to bluish infiltrated plaques, nodules or tumors on the lower extremities in elderly patients over 75 years old2. PCBCLs are frequently misdiagnosed as inflammatory and infectious lesions or as other cutaneous neoplasms.
Recently, case reports3,4 and small case series5 have suggested that dermoscopy may help to augment the clinical recognition of PCBCLs. We aimed to characterize the dermoscopic features of PCBCL, stratified by subtype and anatomic location in order to better appraise the role of dermoscopy in PCBCL diagnosis.
Methods
We performed a retrospective search for all patients with biopsy-proven PCBCLs that were referred to Memorial Sloan Kettering Cancer Center (MSKCC) between the years 1992–2016. The study was approved by the Institutional Review Board.
We identified 172 PCBCLs patients (99 PCMZL, 43 PCFCL, 30 PCDLBCL) for whom the initial diagnostic skin biopsy was reviewed at MSKCC and whose pathology report included the clinical differential diagnosis. For the dermoscopic evaluation, we retrospectively identified all PCBCLs lesions that were dermoscopically photographed prior to biopsy. Clinical and dermoscopic images were retrospectively retrieved from our imaging database (Vectra™, Canfield Imaging Systems, Fairfield, NJ, U.S.A.). We identified high-quality dermoscopic images of 58 PCBCL lesions from 51 patients. We excluded lesions with low quality images (e.g. blur, over-exposure, excessive pressure resulting in skin blanching), and lesions that were imaged after biopsy had already been performed, to avoid confusion with scarring and inflammatory changes related to wound healing. Dermoscopic features evaluated for each lesion included color, vessel morphology (as defined by Kittler et al.6) and presence of scale and ulceration. Two dermoscopy experts (A.S. and R.B.) who were blinded to the study objectives and diagnoses, evaluated 17 dermoscopy images of PCBCL lesions with characteristic dermoscopic features and, for each lesion, chose their dermoscopic differential diagnosis from a given list.
Relative frequencies were used to describe the differential diagnoses and dermoscopic features, and were compared by PCBCL subtype and anatomic location using Chi square test. All analyses were performed using IBM SPSS Statistics software (version 24).
Results
Analyzing the clinical differential diagnosis in the pathology requisition slip for 172 newly diagnosed PCBCL biopsy-proven lesions, cutaneous lymphoma was suspected by the clinician in 28 cases (16.3%). Overall, skin malignancies were suspected in 94 lesions (54.7%); the leading diagnosis was basal cell carcinoma (BCC) in 30 cases (17.4%); other skin neoplasms including squamous cell carcinoma, dermatofibrosarcoma protuberans, cutaneous metastasis, and melanoma were suspected less frequently. Non-neoplastic conditions were suspected in some cases, including cyst (21.5%), granulomatous processes (15.7%), and infectious disease (4.7%). The differential diagnosis by PCBCL subtype and anatomic site is shown (Table 1). A low index of suspicion for skin lymphoma was seen regardless of PCBCL subtype and site; however, BCC was considered in the differential diagnosis significantly more frequently for PCFCL lesions (30.2%, p<0.05) and in lesions arising on the head and neck regions (31.1%, p<0.01).
Table 1.
Clinical differential diagnosis for primary cutaneous B-cell lymphoma (PCBCL) lesions according to subtype and anatomic location.
| Suspected clinical diagnosis | All PCBCL (n=172) | PCBCL subtype | P-value* | Anatomic location | P-value* | ||||
|---|---|---|---|---|---|---|---|---|---|
| PCMZL (n=99) | PCFCL (n=43) | PCDLBCL (n=30) | Head and neck (n=45) | Trunk (n=56) | Extremities (n=71) | ||||
| Cutaneous lymhpoma | 28 (16.3%) | 15 (15.2%) | 7 (16.3%) | 6 (20.0%) | 8 (17.8%) | 9 (16.1%) | 11 (15.5%) | ||
| Basal cell carcinoma | 30 (17.4%) | 11 (11.1%) | 13 (30.2%) | 6 (20.0%) | <0.05 | 14 (31.1%) | 12 (21.4%) | 4 (5.6%) | <0.01 |
| Squamous cell carcinoma | 10 (5.8%) | 1 (1.0%) | 6 (14.0%) | 3 (10.0%) | <0.01 | 4 (8.9%) | 2 (3.6%) | 4 (5.6%) | |
| Metastatsis | 6 (3.5%) | 1 (1.0%) | 3 (7.0%) | 2 (6.7%) | 1 (2.2%) | 3 (5.4%) | 2 (2.8%) | ||
| Dermatofibrosarcoma protuberans | 6 (3.5%) | 2 (2.0%) | 3 (7.0%) | 1 (3.3%) | 2 (4.4%) | 1 (1.8%) | 3 (4.2%) | ||
| Melanoma | 4 (2.3%) | 1 (1.0%) | 0 (0%) | 3 (10.0%) | <0.01 | 0 (0.0%) | 1 (1.8%) | 3 (4.2%) | |
| Other neoplasia/neoplastic process not specified | 32 (18.6%) | 15 (15.2%) | 10 (23.3%) | 7 (23.3%) | 10 (22.2%) | 9 (16.1%) | 13 (18.3%) | ||
| All skin malignancies | 94 (54.7%) | 41 (41.4%) | 31 (72.1%) | 22 (73.3%) | <0.001 | 31 (68.9%) | 32 (57.1%) | 31 (43.7%) | <0.05 |
| Cyst | 37 (21.5%) | 23 (23.2%) | 9 (20.9%) | 5 (16.7%) | 12 (26.7%) | 14 (25.0%) | 11 (15.5%) | ||
| Granulomatous process (Sarcoidosis, Granuloma annulare) | 27 (15.7%) | 17 (17.2%) | 5 (11.6%) | 5 (16.7%) | 6 (13.3%) | 7 (12.5%) | 14 (19.7%) | ||
P-value are mentioned if <0.05
PCBCL = primary cutaneous B-cell lymphoma; PCMZL = primary cutaneous marginal zone lymphoma; PCFCL = primary cutaneous follicle center lymphoma; PCDLBCL = primary cutaneous diffuse large B-cell lymphoma
We studied the dermoscopic images of 58 PCBCL lesions (Table 2, Figure 1). All lesions were non-pigmented. Most lesions showed salmon-pink or yellow-to-orange-colored background/areas (n=46, 79.3%) and prominent blood vessels (n=45, 77.6%), with serpentine (linear-irregular) being the most frequently-seen vascular morphology (n=39, 67.2%). The combination of salmon color and serpentine vessels was seen in 55% of all lesions while 5 cases showed neither (8.6%).
Table 2.
Dermoscopic characteristics of primary cutaneous B-cell lymphoma (PCBCL) lesions according to subtype and anatomic location.
| Dermoscopic feature | All PCBCL (n=58) | PCBCL subtype | Anatomic location | |||||
|---|---|---|---|---|---|---|---|---|
| PCMZL (n=31) | PCFCL (n=14) | Indolent PCBCL (n=10) | PCDLBCL (n=3) | Head and neck (n=8) | Trunk (n=32) | Extremities (n=18) | ||
| Blood vessels | 45 (77.6%) | 24 (77.4%) | 10 (71.4%) | 9 (90%) | 2 (66.7%) | 8 (100.0%) | 25 (78.1%) | 12 (66.7%) |
| Serpentine vessels | 39 (67.2%) | 21 (67.7%) | 8 (57.1%) | 9 (90.0%) | 1 (33.3%) | 7 (87.5%) | 21 (65.6%) | 11 (61.1%) |
| Arborizing vessels | 4 (6.9%) | 1 (3.2%) | 2 (14.3%) | 0 (0.0%) | 1 (33.3%) | 1 (12.5%) | 3 (9.4%) | 0 (0.0%) |
| Dotted vessels | 1 (1.7%) | 1 (3.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.6%) |
| Polymorphous vessels | 1 (1.7%) | 1 (3.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (3.1%) | 0 (0.0%) |
| Scale | 6 (10.3%) | 2 (6.5%) | 3 (21.4%) | 0 (0.0%) | 1 (33.3%) | 0 (0.0%) | 4 (12.5%) | 2 (11.1%) |
| Ulceration | 4 (6.9%) | 4 (12.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (6.3%) | 2 (11.1%) |
| Salmon colored background/area | 46 (79.3%) | 25 (80.6%) | 11 (78.6%) | 7 (70.0%) | 3 (100.0%) | 6 (75%) | 27 (84.4%) | 13 (72.2%) |
PCBCL = primary cutaneous B-cell lymphoma; PCMZL = primary cutaneous marginal zone lymphoma; PCFCL = primary cutaneous follicle center lymphoma; PCDLBCL = primary cutaneous diffuse large B-cell lymphoma
Figure 1. Primary cutaneous B-Cell lymphomas. Dermoscopic features.
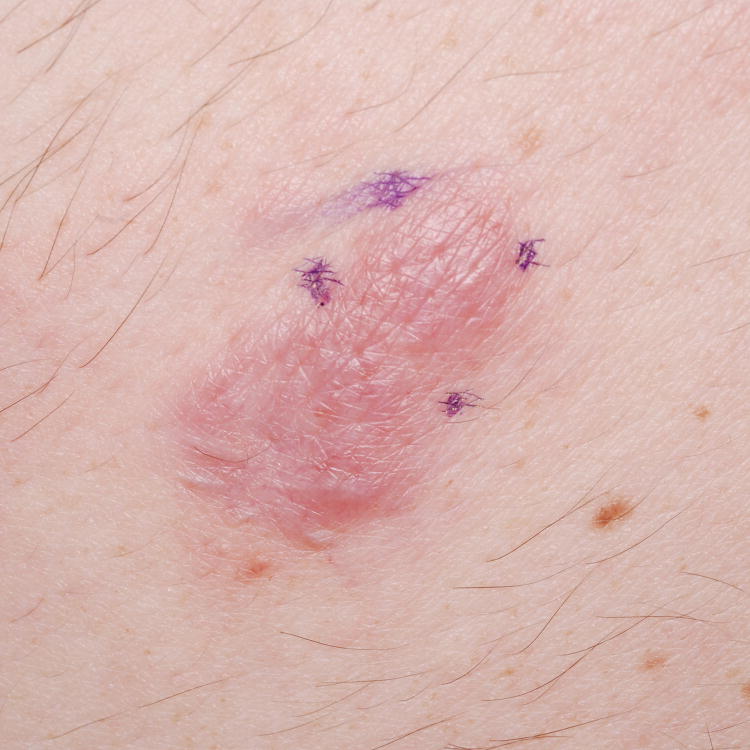
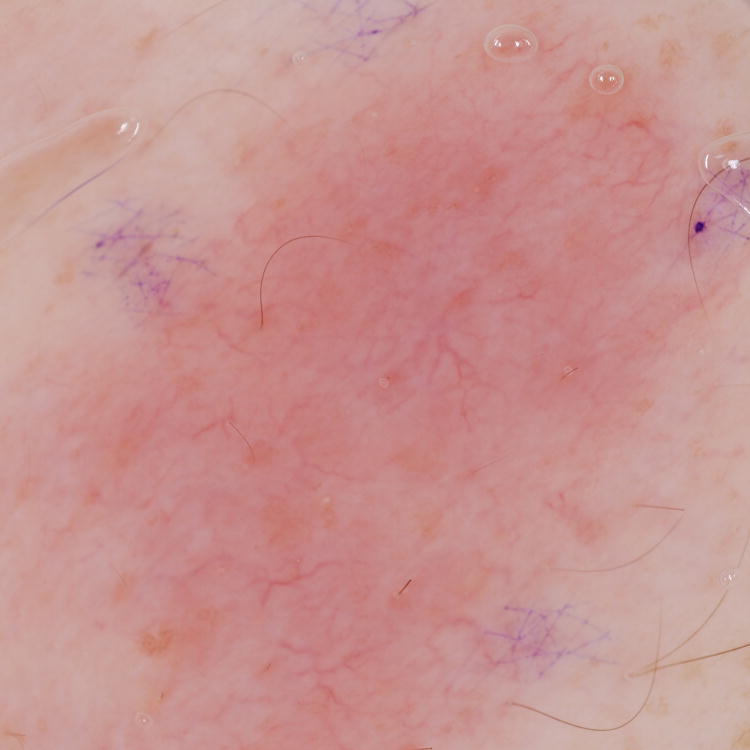
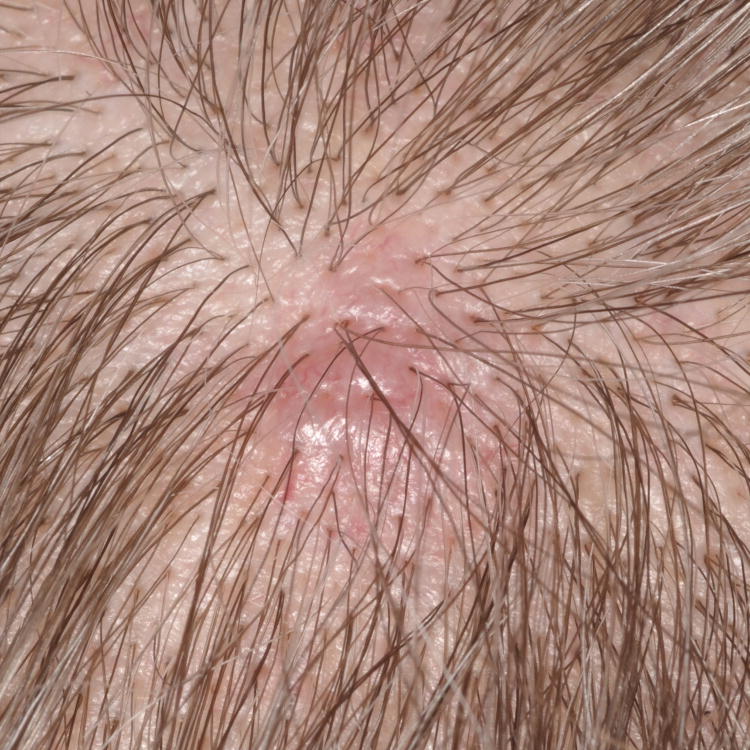
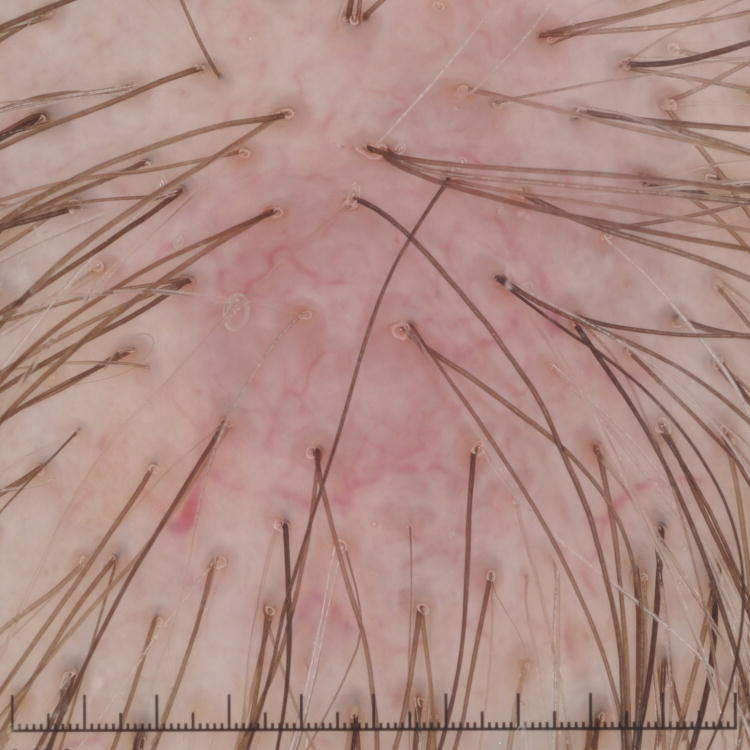
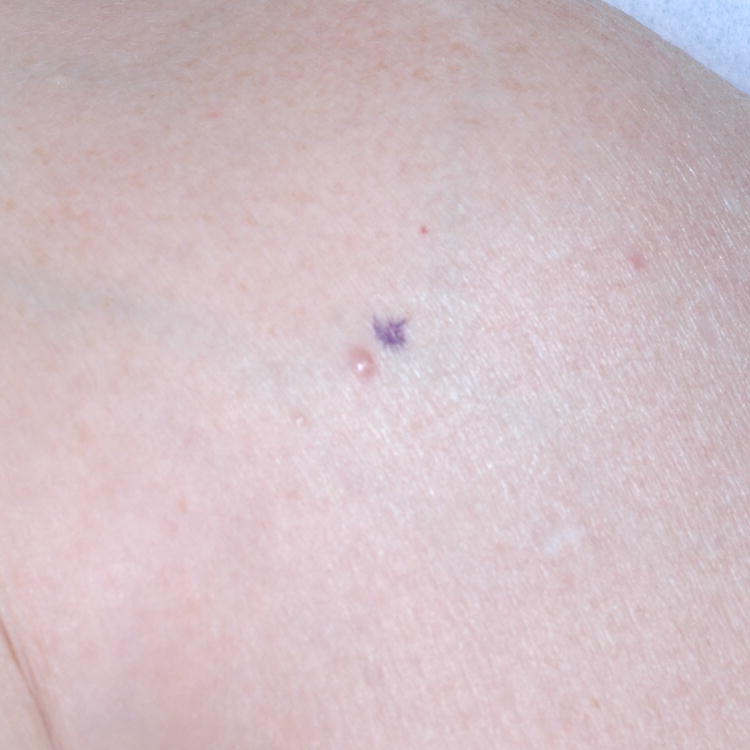
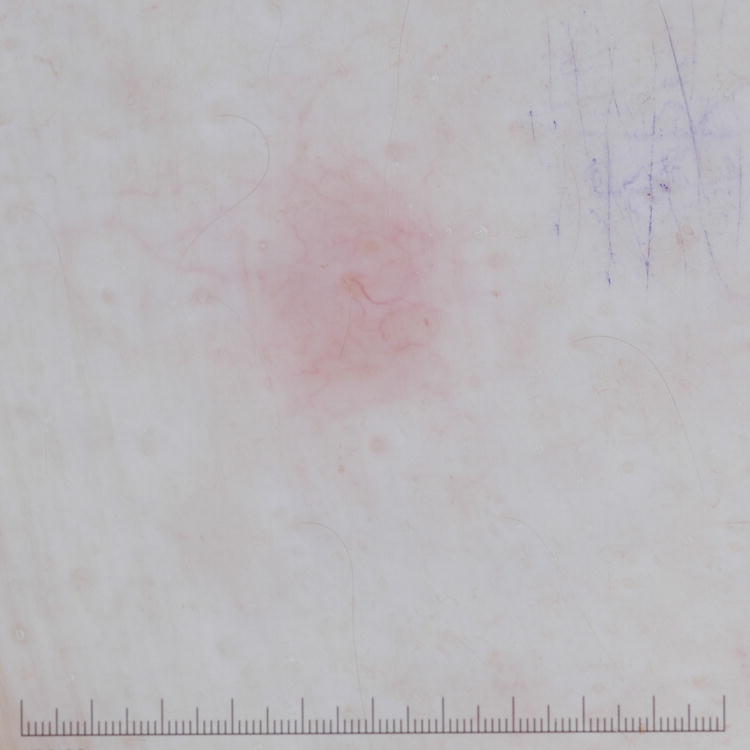
A, Primary cutaneous marginal zone lymphoma presenting as a plaque located on the back. B, Dermoscopic examination reveals salmon-pink-to-orange background with numerous fine, out-of-focus serpentine vessels that are absent from the peri-lesional skin (original magnification, ×10). C, Scalp nodule of primary cutaneous follicle center lymphoma. D, Dermoscopic image showing serpentine vessels within salmon-pink homogeneous area (original magnification, ×10). E, Primary cutaneous follicle center lymphoma presenting as a shiny pink papule on the shoulder. F, Dermoscopic examination reveals few fine, out-of-focus serpentine vessels with salmon-pink to yellowish homogeneous area (original magnification, ×10).
Two blinded dermoscopy experts evaluated the differential diagnosis, based on dermoscopic images alone, for 17 of the study lesions. They included CBCL in the differential diagnosis in 70.6% (12/17 lesions), while other common diagnoses mentioned by at least one of the experts were arthropod bite (58.8%), BCC (52.9%), amelanotic melanoma (47.1%) and scar/keloid (47.1%). The experts disagreed on 29.9% of the suggested differential diagnoses.
Discussion
The presentation of cutaneous lymphomas in general and of PCBCLs in particular can be non-specific and a biopsy is essential for a definitive diagnosis. In our study, skin lymphoma was clinically-suspected in only 16.3% of PCBCL biopsy-proven lesions, while misdiagnosis as other skin malignancies, mainly BCC, was common.
Few studies evaluated the diagnostic value of dermoscopy in cutaneous lymphoma. Characteristic dermoscopic patterns of early-stage mycosis fungoides (MF) consisting of fine short linear vessels and orange-yellowish patchy areas7 or dotted vessels8 were described. In lymphomatoid papulosis, a pattern of tortuous irregular vessels radiating from the center was reported in early lesions9. The dermoscopic features that were reported for 10 PCBCL lesions included: white circles, salmon-colored background/area, scales, arborizing vessels or a polymorphous vascular pattern5. It was suggested that those dermoscopic features may improve the clinical recognition of PCBCLs5.
Studying the dermoscopic features in 58 PCBCL lesions, we identified in the majority of cases salmon-colored background/area and prominent blood vessels, most frequently serpentine vessels. Orange or salmon color was previously-reported in MF7 and in PCBCL5; this hue might be due to the dense dermal lymphocytic infiltrate along with the increased vascular flow in cutaneous lymphomas. The increased vascularity in PCBCL is attributed to the angiogenesis that accompanies the neoplastic process.10 We found no significant differences in dermoscopic characteristics per subtype or anatomic location; other vascular morphologies, scaling and ulceration were rarely seen. PCBCL can present as subcutaneous nodules, and as expected, such deep-seated lesions display merely dull dermoscopic features, mostly homogeneous pink areas with no vessels. Although salmon-colored background and serpentine vessels can suggest the diagnosis of CBCL, they are not specific and can raise a wide differential diagnosis of malignant and inflammatory conditions, as was demonstrated in the blinded evaluation by dermoscopy experts. Serpentine vessels are found in other skin malignancies, such as superficial BCC and melanoma.6 Dermoscopic features should be evaluated cautiously to avoid overlooking other skin malignancies in PCBCL patients, such as collision between PCFCL and BCC4.
While dermoscopy offers a bridge between the naked-eye examination and the histopathological appearance, cutaneous lymphoma is diagnosed on a cellular level using histopathology, immunohistochemistry and molecular studies. Therefore, dermoscopy may serve as an ancillary tool in PCBCL; however, it cannot be diagnostic.
In conclusion, salmon-colored area/background and serpentine vessels are frequently-seen dermoscopic features of PCBCL. Recognizing these dermoscopic features can assist in the initial diagnosis of PCBCL and in assessment of PCBCL recurrence. However, these dermoscopic findings are not specific and should be integrated with the clinical findings.
Acknowledgments
Funding sources: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. Dr Shamir Geller is a recipient of a supplemental grant from the American Physicians and Friends For Medicine in Israel (APF).
The above funders were not involved in the study design, data collection, data analysis, manuscript preparation or publication decisions.
Footnotes
Conflict of interest disclosures: None.
References
- 1.Wilcox RA. Cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90(1):73–76. doi: 10.1002/ajh.23863. [DOI] [PubMed] [Google Scholar]
- 2.Suarez AL, Pulitzer M, Horwitz S, Moskowitz A, Querfeld C, Myskowski PL. Primary cutaneous B-cell lymphomas: part I. Clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69(3):329.e321–313. doi: 10.1016/j.jaad.2013.06.012. quiz 341–322. [DOI] [PubMed] [Google Scholar]
- 3.Piccolo V, Mascolo M, Russo T, Staibano S, Argenziano G. Dermoscopy of primary cutaneous B-cell lymphoma (PCBCL) J Am Acad Dermatol. 2016;75(4):e137–139. doi: 10.1016/j.jaad.2016.02.1217. [DOI] [PubMed] [Google Scholar]
- 4.Mascolo M, Pagliuca F, Costa C, Scalvenzi M. Deceitful clustered papules on the scalp of a middle-aged woman. Dermatol Pract Concept. 2017;7(1):67–69. doi: 10.5826/dpc.0701a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mascolo M, Piccolo V, Argenziano G, et al. Dermoscopy Pattern, Histopathology and Immunophenotype of Primary Cutaneous B-Cell Lymphoma Presenting as a Solitary Skin Nodule. Dermatology. 2016;232(2):203–207. doi: 10.1159/000442251. [DOI] [PubMed] [Google Scholar]
- 6.Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: Results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74(6):1093–1106. doi: 10.1016/j.jaad.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lallas A, Apalla Z, Lefaki I, et al. Dermoscopy of early stage mycosis fungoides. J Eur Acad Dermatol Venereol. 2013;27(5):617–621. doi: 10.1111/j.1468-3083.2012.04499.x. [DOI] [PubMed] [Google Scholar]
- 8.Bosseila M, Sayed Sayed K, El-Din Sayed SS, Abd El Monaem NA. Evaluation of Angiogenesis in Early Mycosis Fungoides Patients: Dermoscopic and Immunohistochemical Study. Dermatology. 2015;231(1):82–86. doi: 10.1159/000382124. [DOI] [PubMed] [Google Scholar]
- 9.Moura FN, Thomas L, Balme B, Dalle S. Dermoscopy of lymphomatoid papulosis. Arch Dermatol. 2009;145(8):966–967. doi: 10.1001/archdermatol.2009.167. [DOI] [PubMed] [Google Scholar]
- 10.Schaerer L, Schmid MH, Mueller B, Dummer RG, Burg G, Kempf W. Angiogenesis in cutaneous lymphoproliferative disorders: microvessel density discriminates between cutaneous B-cell lymphomas and B-cell pseudolymphomas. Am J Dermatopathol. 2000;22(2):140–143. doi: 10.1097/00000372-200004000-00009. [DOI] [PubMed] [Google Scholar]


