Abstract
There is no standard approach to measuring GI symptoms in individuals with ASD, despite postulated interactions. The objectives of this study were to 1) describe the range of GI symptom ascertainment approaches in studies of ASD, 2) describe the range of prevalence estimates across studies, and 3) assess associations between ascertainment approach and prevalence estimates. Studies published from 1/1/1980 to 1/31/2017 were collected via PubMed. Eligibility included studies with at least ten individuals with ASD that measured GI symptoms or conditions. We excluded review and hypothesis papers. We extracted information on study design, GI symptom ascertainment method, demographics, and ASD diagnostic criteria. From a subset of studies, we extracted GI symptom estimates. Out of a possible 386 titles, 144 were included. The prevalence range for constipation was 4.3-45.5% (median 22%), for diarrhea was 2.3-75.6% (median 13.0%), and for any or more than one symptom was 4.2-96.8% (median 46.8%). GI symptoms differed significantly by age of individuals, primary goal of study, study design, study sample, and who reported symptoms (p<0.05). Due to small sample size, we were not able to test for associations between every GI symptom and study characteristic of interest, or examine associations between GI symptoms and intellectual or verbal disability. Studies used a broad range of methods to ascertain GI symptoms in ASD. GI symptoms varied widely across these studies, with significant differences by study characteristics. Our findings highlight the need for a reliable, valid GI assessment tool to be used consistently across studies of ASD.
Lay Summary
We reviewed studies having to do with autism spectrum disorder and the gastrointestinal system, dating back to 1980. We found that the median prevalence of constipation was 22.2%, diarrhea 13.0%, and any symptom 46.8%. All symptoms had a wide range of estimates across studies. GI symptoms were associated with characteristics of the study, including who measured the GI symptoms. We call for the development of a reliable and valid GI questionnaire for studies of ASD.
Keywords: Co-morbid conditions, Exposure assessment / Exposomics, Psychometrics
Introduction
In his seminal paper first describing autism, Leo Kanner noted that 6 of the 10 children with autism “presented severe feeding difficulty from the beginning of life” (Kanner, 1943). Since then, many studies have explored the association between autism spectrum disorder (ASD), gastrointestinal (GI) symptoms, and diet (Goodwin et al., 1971; Panksepp, 1979; D'Eufemia et al., 1996; Bresnahan et al., 2015). The argument that individuals with ASD have a non-healthy gut stems from the apparent increased prevalence of GI symptoms, as cited widely in the literature since Kanner's 1943 paper (Galli-Carminati et al., 2006; Mouridsen et al., 2010; Mazurek et al., 2013; Chaidez et al., 2014). For example, a study published in 2014 reported that 13% of their ASD sample had current frequent diarrhea, compared to 6.1% in the developmental delay group and 1.6% in the typically developing group (Chaidez et al., 2014). Other symptoms were elevated in ASD as well, though they were not statistically significantly different across groups. Although five questionnaires (Autism Treatment Evaluation Checklist (ATEC) subscale, Autism Treatment Network (ATN) Gastrointestinal Symptom Inventory, Childhood Autism Risks from Genetics and Environment (CHARGE) Gastrointestinal History Questionnaire, Parental Concerns Questionnaire, and ATN Diagnoses and Problems form) that query GI symptoms have been designed for the ASD population, to our knowledge none have been formally assessed for validity (Buie et al., 2010). Therefore, the true prevalence of various GI symptoms in this population is unknown.
Epidemiologists and experts in pediatric gastroenterology have recognized many limitations in the interpretation of published studies on the epidemiology of GI problems among children with ASD. In a report from a 2009 symposium of the North American Society of Pediatric Gastroenterology and its follow-up workshop, Coury et al. noted the very wide range of prevalence estimates of GI disorders in ASD, and recognized several methodological limitations among the studies (Coury et al., 2012). These included retrospective study design and inappropriate control groups, which potentially lead to measurement error and bias; enrollment of groups of children with ASD that are clinically heterogeneous, which generates wide estimates of symptom prevalence; bias incase selection, which may make the sample appear to have more or less symptoms than the underlying target population; as well as reliance on parent report of symptoms, which may provide incomplete or biased information, especially when symptoms are not defined precisely.
Buie et al. published a consensus report from a multidisciplinary panel on the evaluation and treatment of GI disorders and symptoms among people with ASD (Buie et al., 2010) noting the wide variability among prevalence estimates, and recommended the use of “validated instruments and outcome measures”. The authors further noted that GI symptoms may contribute to problem behaviors among people with ASD, and that the problem behaviors may complicate the clinical recognition of GI disorders.
Ascertaining accurate estimates of GI symptoms in children is a challenging issue in general. For example, while the Rome II criteria for functional constipation required at least 2 weeks of a defined type of stool “for the majority of stools” or another type of stool “2 or more times per week” (Rasquin-Weber et al., 1999), the North American Society of Gastroenterology and Nutrition (NASPGHAN) defined constipation in terms of the timing of and the distress caused by defecation, present for at least 2 weeks (Tabbers et al., 2014). Van den Berg et al. found that while some studies used these recognized, albeit disparate, criteria, other studies created their own criteria. They postulated that “it is unlikely that parents have comparable notions regarding constipation or about what constitutes normal bowel habits in children,” and that both these sources of heterogeneity (the variety of definitions and the variability of parental perceptions) contributed to the wide range of prevalence observed in their review of the literature (Van Den Berg et al., 2006).
However, these measurement challenges are compounded when assessing GI symptoms among children with social and communication difficulties, such as those with ASD. Even among neurotypical children, there can be low concordance between child and parent on intensity of pain/discomfort (children tend to rate pain/discomfort as more severe), pain during bowel movements, holding bowel movements, and heartburn, among others.(Caplan et al., 2005) This low concordance may be more pronounced in younger age groups. Because studies of ASD usually rely on proxy respondents, it is likely that frequency estimates of GI symptoms and pain are biased in this population, and may be more biased among children in the younger age groups or with more severe autism. Other populations with neurodevelopmental disabilities such as cerebral palsy (CP) have experienced similar challenges in assessing the presence of GI symptoms. Early studies in CP used their own questionnaires and for children the questionnaires were filled out by parents (Del Giudice et al., 1999; Sullivan et al., 2000). A recent study among adults with CP used a combination of questionnaires in a two-step process (Marciniak et al., 2015), specifically a questionnaire based on Rome III criteria and, if the criteria for constipation were met, then the Patient Assessment of Constipation-Symptom Scale; this study had the advantage of asking adult participants for information about their own bowel habits. For this review, we focused on ASD, since this population has been a primary target of complementary alternative therapies that aim to heal the gut and prevent or treat ASD, as well as research attempting to prove vaccines cause ASD through a damaged gastrointestinal system. All of this research rests on having an accurate measure of gastrointestinal symptoms. The implications of not having a reliable and valid assessment tool for GI symptoms are especially critical for the ASD population given the vulnerability of this population to potentially unsafe, ineffective interventions.
It is important to better understand the range of GI measurement approaches and their influence on symptom prevalence estimates in order to: a) accurately capture the prevalence of various GI symptoms, b) assess the safety and effectiveness of interventions on GI symptoms, and c) understand risk factors for and trajectories of ASD.
The aims of this study were to 1) describe the range of approaches to ascertaining GI symptoms and conditions in studies of ASD since 1980, 2) describe the range of estimates of prevalence across studies, and 3) assess how the variation in measurement approach is associated with GI symptom prevalence estimates. Lastly, we outline the critical components needed in a GI questionnaire, and hope that this review will provide insight that will help in the creation or modification of a reliable and valid tool for measuring GI symptoms among individuals with ASD.
Methods
We carried out a literature review; not a formal systematic review. However, we followed many of the best practices of PRISMA guidelines including explicit search criteria, inclusion/exclusion criteria, standardized extraction of the same fields, and resolution of extraction discrepancies across two reviewers. These are described in detail below.
Search Criteria
PubMed was used to find all studies published from 1/1/1980 to 1/31/2017, with a title including “Autism”, “Autistic”, “ASD”, “Pervasive Development*”, or “Asperger*”, and with Medical Subject Headings terms “Gastrointestinal Disease” or “Signs and Symptoms, Digestive”, or one of the following terms in the text: “gastrointestinal”, “gastric”, “gastritis”, “gut”, “GI”, “intestine*”. Reviews and articles not written in English were excluded. This search returned 386 studies. The asterisk (*) returns words that begin with the word truncated by the asterisk.
Exclusion Criteria, Information Extracted
From these 386 studies, exclusion criteria were: a) having fewer than 10 diagnosed “case” participants (ASD, PDD, etc.) (n=38); b) not ascertaining GI symptoms or diagnoses (n=66); c) animal studies (n=32); d) containing no data (n=3); e) including Andrew Wakefieldas a coauthor (n=11); and f) review articles, hypothesis papers, meta-analyses, narratives, editorials and mathematical model papers (n=92). Andrew Wakefield et al.'s 1998 Lancet paper (Wakefield et al., 1998), which suggested the measles, mumps, rubella vaccine predisposes to behavioral regression, was retracted in 2010 due to incorrect elements of the paper as well as ethical violations. It was later discovered that Wakefield et al. had conducted fraud by picking data that agreed with their hypothesis (Rao et al., 2011). The claims made in the original paper continue to have lasting effects on vaccination rates (Flaherty, 2011). For these reasons, we chose to omit studies on which he was a coauthor. As shown in Figure 1, 144 studies remained [Supplementary Table 1].
Figure 1. Exclusion Flowchart, from Initial 386 Studies to Final Sample of 144 Studies.
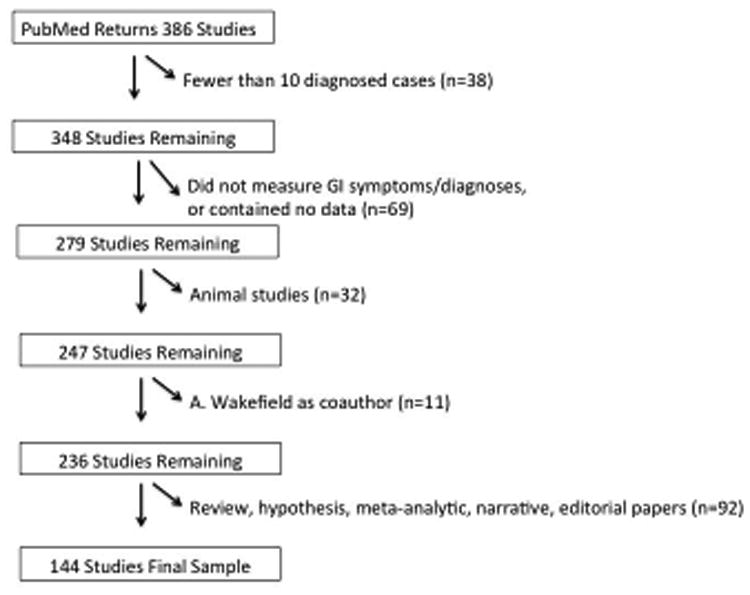
Figure 1 depicts the exclusion flow chart, beginning with 386 studies that were returned from PubMed. After applying our exclusion criteria, 144 studies remained in the final sample of studies we reviewed.
Information was extracted from each of 144 studies by one of two authors (CH & CN). Both authors examined a subset of the studies, and questions regarding any study were resolved together. Information extracted included study design, demographic information, ASD diagnostic criteria, and characteristics of data-collecting methods.
Prevalence Estimate Subset
Of the 144 studies, a subset was identified that contained prevalence estimates, in order to summarize GI symptom prevalence estimates across studies. Studies were excluded if: entry into the study, or the sampling frame, was based on the presence or absence of GI symptoms/disorders (including GI-related medical comorbidities) (n=20); the study included/excluded participants based on diet (n=2); the study was experimental (n=26); or the study did not report GI symptom/diagnosis estimates (n=12). The flowchart shows these exclusions (Figure 2).
Figure 2. Exclusion Flowchart, Resulting in the Prevalence Estimate Subset of 84 Studies.
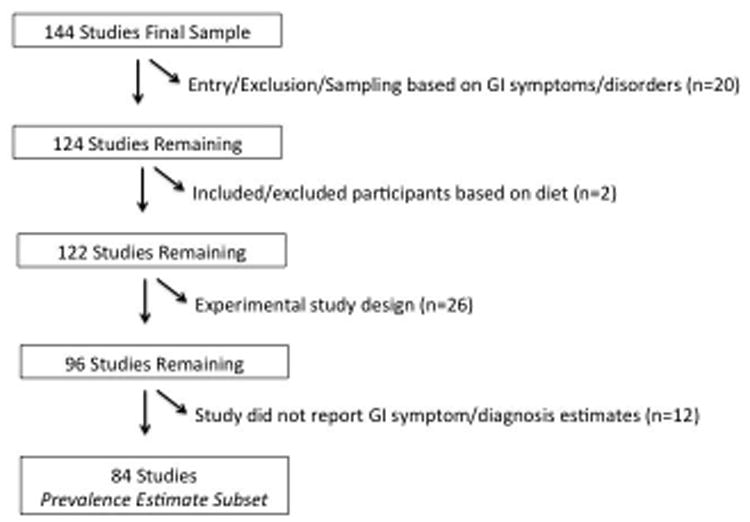
Figure 2 depicts the exclusion flow chart, beginning with the 144 studies that we reviewed. After applying our exclusion criteria, 84 studies remained in the final sample of studies from which we summarized the prevalence estimates of GI symptoms.
From the 84 studies that qualified to be included in the prevalence estimate subset, we extracted proportions of various GI symptoms and noted whether GI symptoms were assessed at more than one time point in the study. We summarized the proportions across studies for diarrhea, constipation, abdominal pain/discomfort, nausea/vomiting, bloating/flatulence, reflux, soiling/incontinence, and difficulty/pain while stooling. We also summarized non-specific (did not refer to a specific symptom) or aggregate (referred to more than one symptom) variables, such as “gut complaints”, “any symptom”, or “had either diarrhea or constipation”. For studies in which there were subgroups, such as children with/without language regression, or when there was more than one time point, we took the average across these subgroups. Studies that used scores or mean number of symptoms, rather than percentages, were excluded when calculating the summary prevalence measure.
We assessed relationships between GI symptom proportions and various study characteristics using ANOVA tests. We estimated the association between the mean proportion for each GI symptom and each study characteristics of interest (age, geographic region, publication year, diagnostic category, primary goal of study, type of study sample, study design, who reported the symptoms, and type of questionnaire). P-values less than 0.05 were considered statistically significant. All statistical analyses were performed using RStudio Version 0.98.1091 (R Developement Core Team, 2015) (Team, 2014).
Results
Characteristics of Studies
Study characteristics are summarized in Table 1. The number of study participants ranged from 10 to 4927 individuals (median 73). The source of study participants was clinic-based in 57 studies, population-based in 18 studies, both clinic- and population-based in 1 study, enriched-risk in 2 studies, and in 66 studies, insufficient information was provided to determine the source population.
Table 1. Characteristics of Studies and Demographic Information (n=144 studies).
Table 1 summarizes the study and sample characteristics of the 144 studies that were reviewed.
| Variables | Median (range) or Count (%) | |
|---|---|---|
| Sample Size | 73 (10-4927) | |
| Type of study sample | Clinic-based | 57 (40.0) |
| Population-based | 18 (12.5) | |
| Clinic-based & Population-based | 1 (0.7) | |
| Enriched-risk | 2 (1.4) | |
| Other, or insufficient description | 66 (46.0) | |
| Study Design | Experimental | 25 (17.4) |
| Cross-sectional | 33 (22.9) | |
| Cross-sectional with comparison group | 15 (10.4) | |
| Case-control | 62 (43.1) | |
| Cohort | 6 (4.2) | |
| Mixed designs | 3 (2.1) | |
| Primary goal of study | Obtaining estimate of GI symptoms/comparing across outcomes | 24 (16.7) |
| Associating GI symptoms with other variable | 51 (35.4) | |
| Obtaining estimate of GI symptoms/comparing across outcomes and Associating GI symptoms with other variable | 3 (2.1) | |
| Intervention effect on outcomes | 24 (16.7) | |
| Other | 42 (29.2) | |
| Region of Sample | Africa | 2 (1.4) |
| Asia | 10 (6.9) | |
| Europe | 22 (15.3) | |
| Oceania | 8 (5.6) | |
| South America | 1 (0.7) | |
| United Kingdom | 12 (8.3) | |
| US/Canada | 89 (61.8) | |
| Race/Ethnicity (among US/Canada, UK studies) | All white, majority white | 46 (45.5) |
| Mostly Latino/Hispanic | 3 (3.0) | |
| Not specified | 49 (48.5) | |
| No distributions provided | 3 (3.0) | |
| Age categories, median of means (years) | <2 | 0 (0) |
| 2-5 | 9 (10.3) | |
| 5-12 | 68 (78.2) | |
| 13-18 | 5 (5.7) | |
| 18+ | 5 (5.7) | |
| missing | 57 (39.6) | |
| Diagnostic Categories | DSM III-Ra | 1 (0.01) |
| DSM IVa | 73 (50.7) | |
| DSM Va | 6 (4.2) | |
| ICD-9 (but no DSM code) | 5 (3.5) | |
| ICD-10 (but no DSM code) | 11 (7.6) | |
| ICD-9 and ICD-10, but no DSM code | 1 (0.01) | |
| No DSM or ICD code reported, but used ADI-R and/or ADOS | 23 (16.0) | |
| No DSM or ICD code, or ADI-R and/or ADOS, but used Medical Record | 1 (0.01) | |
| Other, Not Specified | 23 (16.0) | |
These studies may or may not have included ICD codes as well.
There were 62 case-control studies, 48 cross-sectional studies, (15 of which included a comparison group), 25 experimental studies, 6 cohort studies, and 3 with mixed study designs. One hundred and sixteen studies were observational in nature, 25 were experimental, and 3 had both observational and experimental components.
The primary goals of the studies were to: 1) assess the relationships of GI symptoms and conditions with any other variable (n=51); 2) compare GI symptom estimates among groups of participants (e.g. ASD, Developmental Delay, PDD-NOS) (n=24); 3) assess outcomes of experimental treatments (n=24); and 4) other primary goal (n=42). Three studies had both primary goals of comparing prevalence estimates as well assessing associations between GI symptoms/conditions and other variables.
Demographic Information
Of the 144 studies, 61.8% were conducted in the United States or Canada, 15.3% in Europe, 6.9% in Asia, with a smaller proportion in the United Kingdom (8.3%), Oceania (5.6%), Africa (1.4%), and South America (0.7%) (Table 1). Of the 101 studies in the US, Canada, or UK, over half (51.5%) did not report the race/ethnicity composition of their study sample. Among the 49 studies that did report race/ethnicity, 94.0% of them included exclusively white participants or a very small proportion of participants were non-white. The ages of participants ranged from <1 to 64 years old. The median age within studies ranged from 4.5 to 36 years, and the median age (median of medians) across studies was 6.7 years (Table 1). Of the 87 studies that reported age, nine (10.3%) had a median of means between the ages of 2-5 years, 68 studies (78.2%) between ages 6-12 years, 5 studies (5.7%) between the ages of 13-18 years, and 5 studies (5.7%) ages 18 or older.
Diagnostic Classification of ASD Individuals
Studies used a variety of methods to diagnose ASD. Most studies either relied on prior assessment of ASD by a practitioner (sometimes with written verification) or assessment with the ADI-R, ADOS, or Mullen. Some studies relied on parent interviews as well as observation by a clinical team involving psychologists, psychiatrists, and/or developmental pediatricians.
A variety of diagnostic classifications were used among studies. Most studies required that participants meet DSM-IV criteria for Autistic Disorder, with or without including Asperger's Disorder or PDD-NOS (n=73, 50.7%), or DSM-V criteria for Autism Spectrum Disorder (n=6, 4.2%), and one earlier study used DSM-III-R (0.01%). These studies may or may not have used an ICD code in addition to DSM codes. Sixteen studies (11.1%) used an ICD code but did not use a DSM code. In addition, twenty-three (16.0%) studies did not report the specific DSM or ICD diagnosis but did use the ADI-R or ADOS. Twenty-three studies (16.0%) did not use ADI-R or ADOS and did not report a DSM or ICD diagnosis (Table 1). One study relied on medical records for diagnosis without specifying DSM or ICD category (0.7%).
Methods of Ascertainment of GI Symptoms and Conditions
Studies used the following methods to assess GI symptoms or diagnoses: 83 of 144 studies used questionnaires administered exclusively to parents or caregivers; 3 used questionnaires administered to either parents/caregivers or teachers; 6 used questionnaires administered to parents/caregivers or to the individuals with ASD themselves (Table 2). Only 18 studies used both questionnaires administered to parents/caregivers and also information from a professional medical source (8 from physicians and 10 from medical records). Of the 8 studies that involved a physician in the ascertainment of GI symptoms, only one mentioned that the physician was a gastroenterology specialist (Gorrindo et al., 2012). Medical record studies may have depended on GI specialists, but often who diagnosed the medical condition was not specified. Of the 8 studies that involved a physician, 3 mentioned that some participants also underwent endoscopic procedures. Two of these studies described that a portion of participants had undergone endoscopy and/or colonscopy in their initial GI evaluation, independent of the study. In one study (Ming et al., 2008), about 17% of individuals underwent endoscopic procedures (half of which showed pathologic results), and in the other study (Kang et al., 2014) 15% underwent both an endoscopy and colonscopy as well as a biopsy of GI mucosa. The third study (Gorrindo et al., 2012), also the one that incorporated a GI specialist, stated that endoscopic procedures were performed when clinically relevant, as part of the study. Thirty-give percent (35%) of children with ASD and GI dysfunction underwent an esophagogastroduodenoscopy, 7.5% received a flexible sigmoidoscopy, and 10% received a colonscopy.
Table 2. Measurement Approaches Across Studies.
Table 2 summarizes the measurement approaches across 144 studies. The respondent of GI symptoms/diagnoses, whether the study used symptom diaries, a questionnaire, or medical records, and what questionnaire was used is shown
| Respondent of GI Symptoms/Diagnoses | N=144 Studiesb | N=84 Studiesb,c |
|---|---|---|
| Parent/caregiver questionnaires only | 83 | 52 |
| Medical Records | 15 | 13 |
| Parent/caregiver and medical records | 10 | 7 |
| Parent/caregiver and physician | 8 | 6 |
| Parent/caregiver or self | 6 | 0 |
| Parent/caregiver or teacher | 3 | 0 |
| Claims data | 1 | 1 |
| Respondent unclear, not specified | 18 | 5 |
| Measurement Approach | ||
| Daily or weekly symptom diaries | 9 | 0 |
| Medical Records | 16 | 13 |
| Questionnaire | 119 | 68 |
| Questionnaire | ||
| Custom design for study | 65 | 44 |
| Custom design for study, informed by literature | 3 | 2 |
| Based on Existing Instrument | 51 | 22 |
| Rome III | 12 | 3 |
| The Gastrointestinal Severity Index (Schneider) | 5 | 2 |
| Rome II | 4 | 3 |
| Autism Treatment Network (ATN)- The Gastrointestinal Symptom Inventory | 3 | 3 |
| Bowel Symptom Questionnaire (Smith) | 3 | 2 |
| Childhood Autism Risks from Genetics and Environment (CHARGE) Gastrointestinal History Questionnaire (GIH) | 2 | 2 |
| GI Symptom Questionnaire (Chandler) | 2 | 2 |
| Global Behavior Rating Scale | 2 | 0 |
| Secretin Outcome Survey | 2 | 0 |
| Child Behavior Checklist (CBCL) | 1 | 1 |
| Gastrointestinal Symptom Rating Scale (GSRS) (Chisholm) | 2 | 1 |
| Lower Urinary Tract Symptoms (LUTS) | 1 | 1 |
| Modified GI symptom severity index questionnaire | 1 | 1 |
| Parental Concerns Questionnaire & ATN Diagnoses and Problems & Clinician Form, & Health and Mental Health History | 1 | 1 |
| ATEC subscale | 1 | 0 |
| Autism Treatment Evaluation Checklist (ATEC) subscale & Global Impressions Survey | 1 | 0 |
| Global Behavior Rating Scale & Additional Rating Scale | 1 | 0 |
| Global Impressions Survey | 1 | 0 |
| Irritable Bowel Syndrome (IBS) Global Improvement Scale | 1 | 0 |
| National Health Interview Survey (NHIS) questionnaire | 1 | 0 |
| Rome III & their own approach | 1 | 0 |
| Safety Monitoring Uniform Report Form | 1 | 0 |
| Safety Monitoring Uniform Report Form & Global Impressions Survey | 1 | 0 |
| Side Effects Review Form | 1 | 0 |
Categories are not mutually exclusive.
Eighty-four studies from the total set of 144 studies were used to summarize prevalence estimates of GI symptoms across the studies.
Fifteen studies used medical records exclusively. One study used claims data. Eighteen studies (12.5%) did not clearly specify who reported the GI symptoms or diagnoses (Table 2); 9 of these 18 studies used a questionnaire, although who filled out the questionnaire was not clear.
Of the 119 studies that used a questionnaire, 68 created their own instrument (i.e. a questionnaire never used before in other studies), while 51 studies used or modified an existing instrument. Three of the 68 studies that used their own approach noted that their method was informed by the literature, in particular the consensus report on GI disorders in people with ASD by Buie et al (Buie et al., 2010). The questionnaires that were used the most often were the Rome II (n=4) or III criteria (n=12), The Gastrointestinal Severity Index (Schneider) (Schneider et al., 2006) (n=5), the Autism Treatment Network Gastrointestinal Symptom Inventory (Mazurek et al., 2013) (n=3) and the Bowel Symptom Questionnaire (Smith) (Smith et al., 2009) (n=3) (Table 2). Symptom diaries (concurrent daily or weekly observations) were used in 9 studies. Supplementary Table 2 lists which studies used which questionnaires.
GI Symptoms and Conditions Ascertained in the Studies
The following symptoms and/or diagnoses were measured in the 144 studies (Table 3): constipation/chronic constipation (n=94), diarrhea/chronic diarrhea (n=95), abdominal pain or discomfort (n=66), nausea or vomiting (n=53), stool qualities or patterns (frequency, color, smell, presence of mucus) (n=51), bloating, gas, or flatulence (n=47), reflux or heartburn (n=41), food selectivity issues or allergies (n=36), soiling, incontinence or bedwetting (n=25), pain or difficulty having a bowel movement (n=20), and colic (n=4). Seventy-five studies measured a non-specific GI symptom or a variable that was a combination of several symptoms, such as constipation, diarrhea, or abdominal bloating. Twenty-three of those seventy-five studies reported only the category of non-specific/aggregate symptoms, while the other 52 reported specific symptoms as well. The variables that were analyzed most commonly for an association with GI symptoms/conditions were the diagnostic outcome group of the study (e.g. ASD/Developmental Delay/PDD-NOS) (n=46), behavioral, psychological, and IQ variables (n=28), and demographic variables (n=13) (Supplementary Table 3).
Table 3. Symptoms Ascertained Across Studies (N=144).
Table 3 summarizes the number of studies that ascertained specific symptoms across 144 studies.
| Symptom | Number of Studies (%)b |
|---|---|
| Constipation | 89 (61.8) |
| Chronic/Persistent Constipation | 5 (3.5) |
| Diarrhea | 86 (59.7) |
| Chronic/Persistent Diarrhea | 9 (6.3) |
| Abdominal pain or discomfort | 66 (45.8) |
| Nausea or vomiting | 53 (36.8) |
| Stool qualities or patterns | 51 (35.4) |
| Bloating, gas, or flatulence | 47 (32.6) |
| Reflux or heartburn | 41 (28.5) |
| Food sensitivities, eating issues | 36 (25.0) |
| Incontinence or bedwetting | 25 (17.4) |
| Pain or difficulty having bowel movement | 20 (13.9) |
| Colic | 4 (2.8) |
| Non-specific GI symptom/Aggregate of symptoms with or without specific symptoms | 75 (52.1) |
| Only non-specific GI symptom/Aggregate of symptoms | 23 (16.0) |
Categories are not mutually exclusive.
Prevalence Estimate Subset
The distribution of measurement approaches in the subset of 84 studies is summarized in Table 2. Sixty-eight of the 84 studies used questionnaires, 13 used medical records, and none used symptom diaries.
The symptoms with the highest median prevalence proportion across studies were “any GI symptom/aggregate of symptoms” (46.8%), constipation (22.0%), chronic/persistent constipation (19.7%), diarrhea (13.0%), chronic/persistent diarrhea (16.2%), and abdominal pain or discomfort (14.0%) (Table 4). The ranges of prevalence proportions were quite wide. Among the 62 studies that reported results on a category of “any” GI symptom, the range was 4.2 to 96.8% of participants. For constipation (n=32), the range was 4.3 to 45.5%, and for diarrhea (n=29), the range was 2.3 to 75.6%.
Table 4. Prevalence Proportions of GI Symptoms/Conditions Among 84 Studies.
Table 4 summarizes the prevalence proportions of specific gastrointestinal symptoms in 84 studies.
| Symptom | Median (Range) | Number of Studies Contributing to this Estimate; Reported Measuring Symptoms |
|---|---|---|
| Any GI symptom/Aggregate of symptoms | 46.8 (4.2, 96.8) | 62; 64 |
| Constipation | 22.0 (4.3, 45.5) | 32; 53 |
| Chronic/Persistent Constipation | 19.7 (8.8, 38.5) | 6; 6 |
| Diarrhea | 13 (2.3, 75.6) | 29; 51 |
| Chronic/Persistent Diarrhea | 16.2 (7.1, 37.0) | 9; 9 |
| Abdominal Pain/Discomfort | 14 (2.1, 46.6) | 20; 35 |
| Nausea or Vomiting | 6.1 (1.3, 21.5) | 13; 24 |
| Stool qualities (frequency, color, small, mucus) | -- | -- |
| Bloating, Flatulence, Gas | 12.5 (0, 55.2) | 14; 24 |
| Reflux, heartburn, acidic stomach, GERD, spitting up | 7.4 (0, 21.5) | 11; 21 |
| Food selectivity/sensitivities, allergies | -- | -- |
| Soiling, incontinence, bedwetting | 12.5 (2.3, 24.0) | 5; 12 |
| Difficult with bowel movements, pain/straining while stooling | 6.2 (6.2, 6.2) | 1; 10 |
A number of symptom prevalence proportions varied significantly by study characteristics. There were multiple differences in symptom proportions depending on who reported the symptoms (Figure 3). Proportions of diarrhea, abdominal pain/discomfort, nausea/vomiting, and bloating/flatulence/gas, all different significantly by respondent type (p<0.05; Figure 3). Symptoms had the highest prevalence proportion in studies that did not specify the respondent of symptoms, with the exception of the reflux symptoms, which did not have studies in this category. Reflux symptoms were highest in studies that used medical records or claims data.
Figure 3. GI Symptom Prevalence Estimates, Across Reporter of Symptoms (N=84).
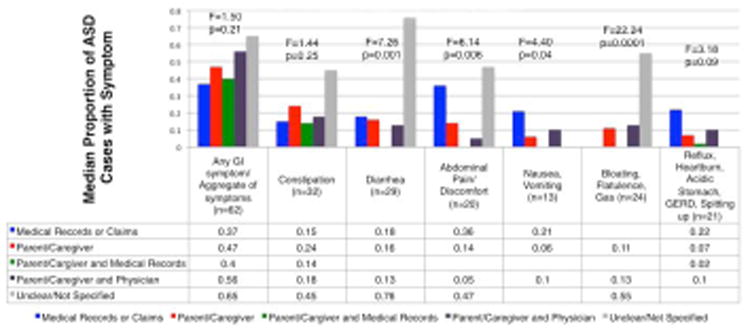
Figure 3 summarizes the associations between specific GI symptoms and the respondent of GI symptoms in 84 studies. GI symptom proportions are the median prevalence proportions across 84 possible studies. The number of studies contributing to the estimate is shown in the horizontal axis. An ANOVA test was carried out for each symptom to test for differences in estimates across types of respondent. P-values are indicated below the F-values.
Notably, both abdominal pain/discomfort and reflux proportions differed across age groups, with the highest proportions for both symptoms being in studies in which the mean age range was 13-18 (p<0.1, p<0.005, respectively, data not shown). Soiling/incontinence proportions different by the primary goal of the study; studies with the primary goal of comparing GI symptom proportions across outcome groups had the highest proportions (p<0.05, data not shown). Chronic or persistent constipation proportions differed significantly across study design types, with the highest proportions in observational studies with a comparison group (p<0.05, data not shown). Prevalence proportions of constipation differed significantly across types of study samples, with clinic-based and enriched-risk samples having the highest proportions (p<0.03, Table S3).
No symptom prevalence proportions differed significantly (p<0.05) or had a discernable association with diagnostic category, geographic region, publication year, or by the type of questionnaire (e.g. ATN, Rome criteria, Child Behavior Checklist). We were unable to examine associations with other variables such as intellectual disability or verbal ability due to the scarcity of proportions specific to these subgroups.
Discussion and Conclusion
Key Findings
In this review of 144 studies published 1980-2017, a broad range of approaches to ascertaining the presence of GI symptoms and conditions was observed. Most studies relied on questionnaires given to parents or caregivers, some used medical records, and a few used concurrent observations such as symptom and stool diaries or results of clinical diagnostic examinations. Of the 84 studies used in the calculation of GI prevalence estimates, only one obtained information directly from the participants and none used observational symptom diaries. Some studies were based on population samples while others recruited participants from clinical settings. Among the 116 observational studies, 82 studies (71%) included a comparison group. Future studies measuring GI symptoms in ASD should continue to use a comparison group in order to determine the relative risk magnitude of various GI symptoms.
Importantly, we found that over half of the studies that used a questionnaire used their own approach, while the rest used an instrument that had been previously developed. Among the existing instruments, only five (ATEC subscale, ATN Gastrointestinal Symptom Inventory, CHARGE GIH questionnaire, Parental Concerns Questionnaire, and ATN Diagnoses and Problems form) were designed with individuals with ASD in mind, and none have been formally validated. This diversity in measurement approaches makes it difficult to derive an accurate epidemiologic estimate of GI symptoms and an understanding of the risk factors that contribute to ASD and GI symptoms. The symptoms that were most commonly ascertained across the studies reviewed here were constipation, diarrhea, abdominal pain or discomfort, and nausea or vomiting. Twenty-three (16.0%) of the studies only reported a non-specific or aggregate GI variable, making it difficult to specify the particular symptoms that are reported in this population.
Not surprisingly, a wide range of proportions of symptom prevalences was observed. Notably, the proportion of non-specific/aggregate GI symptoms ranged from 4.2 to 96.8% of participants. Yet, more specific symptoms also had a wide range of proportions. For example, diarrhea, which is arguably one of the easier symptoms to detect or measure, still had a range of 2.3 to 75.6% across studies. It is not clear if these results are specific to ASD or reflect a broader group of central nervous system disorders. Some research suggests that individuals with ASD may experience a higher prevalence of GI symptoms than other developmental delay groups, however, the lack of precision and validity around measuring GI symptoms makes it difficult to confirm this (Chaidez et al., 2014). Importantly, current data may represent an under-estimate of GI symptoms and disorders, because behaviors that are reflective of GI distress (aggression, disruptive behaviors, self-injury) may be interpreted as features of ASD and therefore may go uninvestigated.
Ascertaining the presence of even one of the more common GI symptoms, functional constipation (that is, constipation not due to anatomic structural disorders), is a challenging problem, even among children who do not have developmental disorders. As noted in a 2006 review by van den Berg et al., the need to rely on reporting by parents, the lack of standardization of the definition of specific symptoms, as well as the reliance by physicians on parental interpretation, all contribute to the problem of inaccurate symptom measurement (Van Den Berg et al., 2006).
In support of this, we found that many GI symptom prevalence estimates were significantly associated with study characteristics. Diarrhea, abdominal pain/discomfort, nausea/vomiting, and bloating/flatulence/gas differed significantly by respondent type, soiling/incontinence differed by the primary goal of the study, and constipation differed significantly across study design types as well as type of study sample.
Our review has important limitations. First, we used a single database (PubMed), so studies that measured GI symptoms in ASD, but were published in journals not available through PubMed, are not reflected in this review. Secondly, since many studies did not specify the prevalence of GI symptoms in subgroups of their populations, such as individuals with an intellectual disability or who are non-verbal, we were unable to determine how GI symptoms differed across these groups. Despite these limitations, this review, and previous ones, highlight the need for standardized, validated tools to assess GI symptoms among people with ASD. There are a few notable examples of questionnaires that do assess GI symptoms and have been designed for ASD, yet each has current drawbacks, and none of the GI-specific sections have been validated. We briefly summarize three of these questionnaires and describe their strengths and limitations.
Strengths and Limitations of Current GI Symptom Questionnaires for ASD populations
The Autism Treatment Evaluation Checklist (ATEC) subscale (Autism Research Institute, 2016), developed by Bernard Rimland and Stephan M. Edelson at the Autism Research Institute, is a one-page form to be filled out by parents, teachers, or caregivers. The four subsections of the scale are 1) Speech/Language Communication, 2) Sociability, 3) Sensory/ Cognitive Awareness, and 4) Health/Physical/Behavior. The ATEC provides subscale scores and a total score, used to monitor how the child is doing over time, or following an intervention. The Health/Physical/Behavior section consists of 25 items, which the individual rates as Not a problem; Minor Problem; Moderate Problem; Serious Problem. The items that have to do with the gastrointestinal system are: bed-wetting; wets pants/diapers; soils pants/diapers; diarrhea; constipation; eats too much/too little; extremely limited diet (Rimland et al., 2000; Autism Research Institute, 2016). The strengths of the ATEC subscale are that it covers multiple constructs of ASD through its four subsections, it allows for a severity rating, it includes items on GI-related issues such as diet, bed-wetting and incontinence, and it has been used in a number of studies. Further, the ATEC total and sub-scale scores, including the health/physical/behavior subscale, have demonstrated good reliability and validity (Magiati et al., 2011; Geier et al., 2013). However, diarrhea, constipation, and incontinence/bed-wetting make up only 5 of the 25 items in this subscale, limiting its usefulness for assessing the breadth of GI symptoms and distress in this population. Further, no definitions of symptoms are provided.
The Childhood Autism Risks from Genetics and Environment (CHARGE) Gastrointestinal History Questionnaire is a parent-administered questionnaire. The questionnaire includes 10 Likert scale items on current gastrointestinal symptoms, food allergies, and dietary restrictions (0=never; 1=rarely; 2=sometimes; 3=frequently; 4=always). The symptoms assessed are abdominal pain, gaseousness/bloating, diarrhea, constipation, pain on stooling, vomiting, sensitivity to foods, difficulty swallowing, blood in stool, and blood in vomit, as well as four yes/no questions about presence of food allergies, diet restrictions, food dislikes, and whether the child has ever received a GI diagnosis. In addition, the form includes open-ended questions for parents to list food allergies, reasons for diet or food restrictions, and what GI condition(s) have been diagnosed (Hansen et al., 2008; Chaidez et al., 2014). The strengths of this questionnaire are that it assesses many GI symptoms, provides information on frequency, has many questions on mealtime behaviors and diet, and allows parents/caregivers to provide qualitative data. The major limitation of this questionnaire is that it doesn't incorporate behavioral symptoms that may reflect GI distress.
The Autism Treatment Network Gastrointestinal Symptom Inventory is an especially useful questionnaire (Autism Network, 2005). It is comprised of 35 items, plus additional items if a participant exhibits certain symptomatology, totaling 77 items. Parents fill out the inventory, which includes questions about presence, duration, and nature of a number of GI symptoms. For each item, parents are asked “has your child experienced any of the following gastrointestinal (tummy) symptoms?”, to which they can reply with “Yes”, “No”, or “Unsure”. For symptoms that are present, parents can also specify the duration of the symptoms (<3 months, 3-5 months, 6-11 months, 1 year or longer). This inventory is scored to provide binary variables for individual symptoms, any GI symptoms, and can provide the total number of GI symptoms experienced. In addition, the inventory allows for branching into specific types of symptoms (abdominal pain, abnormal bowel movements, reflux, and food insensitivity), which allows for the estimation of these symptom categories. Unfortunately, the tool has not been validated (Mazurek et al., 2013; Mazefsky et al., 2014; Abdelrahman et al., 2015). The strengths of this questionnaire are that it provides information on duration of symptoms, assesses a number of GI symptoms, and queries behaviors that might be reflective of GI distress. The limitation of this questionnaire is that mealtime behaviors or dietary practices are not explored to any great degree despite their potential role in the expression of GI symptoms.
Recommendations for Creation and Psychometric Testing of GI Questionnaire
To our knowledge, this is the first literature review to assess GI symptoms in studies of ASD reaching back to 1980 and to examine study design features, particularly approaches to measurement of GI symptoms, for associations with GI symptom prevalence estimates. This study builds on McElhanon et al.'s meta-analysis of 15 studies which found that studies relied primarily on parental reports or chart reviews, and used a wide variety of definitions of GI symptoms (McElhanon et al., 2014). They too recommended the development of “a standardized measure focusing on GI issues among children with ASD” and the use of a toileting diary of visual observations. Our review also confirms Dalton et al.'s hypothesis that the wide variability in prevalence estimates in ASD may be due to factors including the definition and type of GI symptoms, the sample of children, and the method by which the symptoms are investigated (Dalton et al., 2014).
A standardized method for the ascertainment of GI symptoms and conditions must be designed for people with ASD, in order not only to have reliable and valid prevalence estimates but also to compare GI symptom estimates with various risk factors, and to evaluate the outcome of trials or interventions. We recommend that an expert panel of clinicians, researchers, and individuals with ASD or their families be consulted to construct an item pool of symptoms and behaviors that are prevalent, meaningful, and relevant to the ASD population. The questionnaire should include not only GI symptoms but also stool patterns and qualities, mealtime and dietary behaviors, and behaviors that might be indicative of GI distress. In individuals who have difficulties communicating, behaviors or co-morbid conditions such as self-injury, aggression, chewing on non-edibles, putting pressure on the abdomen, sleep disruption or fragmentation, and any other unusual behaviors may be an indication of GI distress (Buie et al., 2010). We refer the reader to the Consensus Report by Buie et al. for more vocal and motor behaviors and changes to overall state that may be indicative of abdominal pain or discomfort in people with ASD (Buie et al., 2010). Not querying these behaviors could lead to an individual with ASD being incorrectly classified as not having a GI disorder or being in pain. This is relevant in research as well as clinical settings, where a patient's report of symptoms or pain typically precedes seeking care. Assessing for behaviors that might reflect GI distress as part of the clinical diagnostic process may help identify individuals in need of treatment. Although these behaviors and co-morbid conditions alone may not allow a questionnaire to be able to correctly diagnose a specific GI disorder such as reflux, Crohn's disease, ulcers, or tight rectal sphincter, it may indicate the need for a more comprehensive evaluation including endoscopy or colonoscopy when clinically indicated. Questions about GI symptoms should also include definitions, and information about duration, frequency, and severity. If possible, direct observations and prospective diaries of stool patterns, symptoms, and behaviors should be incorporated. After constructing the item pool, the GI questionnaire should be tested in a pilot sample of parent/caregiver/self-reporters for reliability and validity. We recommend factor analysis also be carried out to determine how individual items map onto constructs. These results, as well as feedback from participants, should be used to revise the questionnaire before administering to a second, independent sample of individuals. Although the types of reliability and validity that can be assessed will vary by study, we suggest that internal consistency, test-retest reliability, content validity, criterion-validity, construct validity, and exploratory and confirmatory factor analyses are important analyses to consider when psychometrically testing this questionnaire. Importantly, no GI questionnaire based on parent/caregiver/self-report data will be able to diagnose specific disorders. However, A GI questionnaire represents a rapid, feasible way to assess symptoms, and could be combined with physician evaluation and diagnostic tests when relevant to a specific condition of interest in particular settings.
In the absence of a standardized approach to measuring GI symptoms, current reports should at the minimum state explicitly which questionnaire was used, who filled out the questionnaire, the ages of participants, study design, and other pertinent characteristics. We found that 12.5% of studies did not specify who reported symptoms, and 46% of studies did not describe their study sample with enough detail to know if it was a clinic-based sample, population-based sample, or enriched-risk sample. This is especially problematic given that studies which did not specify who reported the GI symptoms tended to have significantly higher proportions of diarrhea, abdominal pain/discomfort, bloating, as well as non-specific GI complaints.
Another troubling finding is that among studies in the US, Canada, and UK, 48% did not specify the race/ethnicity distributions of their sample, and of those that did, 94% were comprised of all or almost all white individuals. This highlights the dearth of racial or ethnic diversity among participants in ASD research studies and begs for a greater focus on underrepresented populations. Similarly, the vast majority of studies did not report any information on socioeconomic status, making it difficult to assess how this impacts a family's ability to access GI specialists for diagnosis and treatment. This also reinforces the need for a more diverse sampling of individuals, greater reporting of socioeconomic variables, and an examination of how care is influenced by factors such as race, education, and income.
In summary, we found that ASD studies used a broad array of approaches to measuring the presence of GI symptoms and conditions, that most replied on parent or caregiver reports, and that the prevalence of GI symptom estimates across studies was very wide. Further, GI symptoms estimates were significantly associated with study characteristics such as respondent type, goal of the study, study design, type of study sample, and age of the participants. The current state of measuring GI symptoms in ASD hinders our ability to judge how symptoms vary over time and with other factors such as diet, sensory aversions, medication, psychological factors including anxiety or depression, the development of the child, and the various interventions that are explored. This review highlights the lack of consensus regarding frequency and subgrouping of GI symptoms in ASD. We argue that ASD individuals, their families, and the research community in general, would benefit from a standardized and valid approach to assessing GI symptoms in ASD. Psychometric testing of a GI questionnaire is an important first step in establishing the epidemiology of GI symptoms in individuals with ASD. If a reliable and valid open-access questionnaire were available to researchers, GI estimates across studies would be more comparable. By designing a questionnaire that has an item pool informed by an expert panel, that includes behaviors that could be indicative of GI distress (aggression, self-injury, pushing abdomen, etc.), and that undergoes psychometric testing and revision, we think GI estimates will be more precise, accurate, and the items queried will be of interest to researchers, clinicians, and individuals or families with ASD. The consistent and accurate measurement of specific symptoms is crucial to understanding the role that the gut plays in the expression and course of ASD.
Supplementary Material
Acknowledgments
This research was supported by the NIMH Psychiatric Epidemiology Training Program grant 5T32MH014592-39 (C.H.)
We thank Public Health Informationist Donna Hesson for her help with developing the search criteria.
Abbreviations
- ASD
Autism Spectrum Disorder
- ATEC
Autism Treatment Evaluation Checklist
- ATN
Autism Treatment Network
- CBCBL
Child Behavior Checklist
- CHARGE
Childhood Autism Risks from Genetics and Environment
- CP
Cerebral Palsy
- GI
Gastrointestinal
- GIH
Gastrointestinal History Questionnaire
- GSRS
Gastrointestinal Symptom Rating Scale
- IBS
Irritable Bowel Syndrome (IBS)
- LUTS
Lower Urinary Tract Symptoms
- NASPHAN
North American Society of Gastroenterology and Nutrition
- NHIS
National Health Interview Survey
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- Abdelrahman HM, Sherief LM, Alghobashy AA, Salam SMA, Hashim HM, Fattah NRA, et al. Research in developmental disabilities. Vol. 36. Elsevier; 2015. Association of 5-HT2A receptor gene polymorphisms with gastrointestinal disorders in Egyptian children with autistic disorder; pp. 485–490. [DOI] [PubMed] [Google Scholar]
- Autism Network. GI symptom inventory questionnaire, vers. 3.0. New York, NY: Autism Speaks; 2005. [Google Scholar]
- Autism Research Institute. Autism Treatment Evaluation Checklist (ATEC) [Accessed: 8 January 2017];2016 Available at: https://www.autism.com/ind_atec.
- Van Den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: A systematic review. American Journal of Gastroenterology. 2006:2401–2409. doi: 10.1111/j.1572-0241.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- Bresnahan M, Hornig M, Schultz AF, Gunnes N, Hirtz D, Lie KK, et al. Association of Maternal Report of Infant and Toddler Gastrointestinal Symptoms With Autism: Evidence From a Prospective Birth Cohort. JAMA psychiatry. 2015;10032(5):1–9. doi: 10.1001/jamapsychiatry.2014.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buie T, Campbell DB, Fuchs GJ, Furuta GT, Levy J, Vandewater J, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;25 Suppl(January):S1–S18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- Caplan A, Walker L, Rasquin A. Development and preliminary validation of the questionnaire on pediatric gastrointestinal symptoms to assess functional gastrointestinal disorders in children and adolescents. Journal of pediatric gastroenterology and nutrition. 2005;41(3):296–304. doi: 10.1097/01.mpg.0000172748.64103.33. [DOI] [PubMed] [Google Scholar]
- Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. Journal of Autism and Developmental Disorders. 2014;44(5):1117–1127. doi: 10.1007/s10803-013-1973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coury DL, Ashwood P, Fasano a, Fuchs G, Geraghty M, Kaul a, et al. Gastrointestinal Conditions in Children With Autism Spectrum Disorder: Developing a Research Agenda. Pediatrics. 2012;130(Supplement):S160–S168. doi: 10.1542/peds.2012-0900N. [DOI] [PubMed] [Google Scholar]
- D'Eufemia P, Celli M, Finocchiaro R, Pacifico L, Viozzi L, Zaccagnini M, et al. Abnormal intestinal permeability in children with autism. Acta paediatrica. 1996;85(9):1076–1079. doi: 10.1111/j.1651-2227.1996.tb14220.x. [DOI] [PubMed] [Google Scholar]
- Dalton N, Chandler S, Turner C, Charman T, Pickles A, Loucas T, et al. Gut permeability in autism spectrum disorders. Autism Research. 2014;7(3):305–313. doi: 10.1002/aur.1350. [DOI] [PubMed] [Google Scholar]
- Flaherty DK. The vaccine-autism connection: A public health crisis caused by unethical medical practices and fraudulent science. Annals of Pharmacotherapy. 2011;45(10):1302–1304. doi: 10.1345/aph.1Q318. [DOI] [PubMed] [Google Scholar]
- Galli-Carminati G, Chauvet I, Deriaz N. Prevalence of gastrointestinal disorders in adult clients with pervasive developmental disorders. Journal of Intellectual Disability Research. 2006;50(10):711–718. doi: 10.1111/j.1365-2788.2006.00833.x. [DOI] [PubMed] [Google Scholar]
- Geier DA, Kern JK, Geier MR. Journal of mental health research in intellectual disabilities. 4. Vol. 6. Taylor & Francis; 2013. A comparison of the Autism Treatment Evaluation Checklist (ATEC) and the Childhood Autism Rating Scale (CARS) for the quantitative evaluation of autism; pp. 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice E, Staiano A, Capano G, Romano A, Florimonte L, Miele E, et al. Brain and Development. 5. Vol. 21. Elsevier; 1999. Gastrointestinal manifestations in children with cerebral palsy; pp. 307–311. [DOI] [PubMed] [Google Scholar]
- Goodwin MS, Goodwin TC, Cowen MA. Malabsorption and cerebral dysfunction: A multivariate and comparative study of autistic children. Journal of Autism and Childhood Schizophrenia. 1971;1(1):48–62. doi: 10.1007/BF01537742. [DOI] [PubMed] [Google Scholar]
- Gorrindo P, Williams KC, Lee EB, Walker LS, McGrew SG, Levitt P. Gastrointestinal dysfunction in autism: Parental report, clinical evaluation, and associated factors. Autism Research. 2012;5(2):101–108. doi: 10.1002/aur.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RL, Ozonoff S, Krakowiak P, Angkustsiri K, Jones C, Deprey LJ, et al. Ambulatory Pediatrics. 1. Vol. 8. Elsevier; 2008. Regression in autism: prevalence and associated factors in the CHARGE Study; pp. 25–31. [DOI] [PubMed] [Google Scholar]
- Kang V, Wagner GC, Ming X. Autism Research. 4. Vol. 7. Wiley Online Library; 2014. Gastrointestinal dysfunction in children with autism spectrum disorders; pp. 501–506. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943:217–250. doi: 10.1105/tpc.11.5.949. [DOI] [PubMed] [Google Scholar]
- Magiati I, Moss J, Yates R, Charman T, Howlin P. Journal of Intellectual Disability Research. 3. Vol. 55. Wiley Online Library; 2011. Is the Autism Treatment Evaluation Checklist a useful tool for monitoring progress in children with autism spectrum disorders? pp. 302–312. [DOI] [PubMed] [Google Scholar]
- Marciniak CM, Lee J, Jesselson M, Gaebler-Spira D. Archives of physical medicine and rehabilitation. 12. Vol. 96. Elsevier; 2015. Cross-Sectional Study of Bowel Symptoms in Adults With Cerebral Palsy: Prevalence and Impact on Quality of Life; pp. 2176–2183. [DOI] [PubMed] [Google Scholar]
- Mazefsky CA, Schreiber DR, Olino TM, Minshew NJ. The association between emotional and behavioral problems and gastrointestinal symptoms among children with high-functioning autism. Autism : the international journal of research and practice. 2014;18(5):493–501. doi: 10.1177/1362361313485164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek MO, Vasa RA, Kalb LG, Kanne SM, Rosenberg D, Keefer A, et al. Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. Journal of Abnormal Child Psychology. 2013;41(1):165–176. doi: 10.1007/s10802-012-9668-x. [DOI] [PubMed] [Google Scholar]
- McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Meta-analysis. Pediatrics. 2014:peds.2013–3995. doi: 10.1542/peds.2013-3995. [DOI] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Chaaban J, Zimmerman-Bier B, Wagner GC. Journal of Child Neurology. 1. Vol. 23. SAGE Publications; 2008. Autism spectrum disorders: concurrent clinical disorders; pp. 6–13. [DOI] [PubMed] [Google Scholar]
- Mouridsen SE, Rich B, Isager T. A longitudinal study of gastrointestinal diseases in individuals diagnosed with infantile autism as children. Child: Care, Health and Development. 2010;36(3):437–443. doi: 10.1111/j.1365-2214.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- Panksepp J. A neurochemical theory of autism. Trends in Neurosciences. 1979;2(C)(79):174–177. 90071–7. doi: 10.1016/0166-2236. [DOI] [Google Scholar]
- R Developement Core. Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2015;1:409. doi: 10.1007/978-3-540-74686-7. [DOI] [Google Scholar]
- Rao TSS, Andrade C. The MMR vaccine and autism: Sensation, refutation, retraction, and fraud. Indian journal of psychiatry, Medknow Publications. 2011;53(2):95. doi: 10.4103/0019-5545.82529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasquin-Weber A, Hyman PE, Cucchiara S, Fleisher DR, Hyams JS, Milla PJ, et al. Childhood functional gastrointestinal disorders. Gut. 1999;45 Suppl 2(Suppl II):II60–8. doi: 10.1136/gut.45.2008.ii60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimland B, Edelson SM. Autism treatment evaluation checklist (ATEC) 2000 Oct 23;:2006. Retrieved. [Google Scholar]
- Schneider CK, Melmed RD, Barstow LE, Enriquez FJ, Ranger-Moore J, Ostrem JA. Oral human immunoglobulin for children with autism and gastrointestinal dysfunction: A prospective, open-label study. Journal of Autism and Developmental Disorders. 2006;36(8):1053–1064. doi: 10.1007/s10803-006-0141-y. [DOI] [PubMed] [Google Scholar]
- Smith Ra, Farnworth H, Wright B, Allgar V. Are there more bowel symptoms in children with autism compared to normal children and children with other developmental and neurological disorders?: A case control study. Autism : the international journal of research and practice. 2009;13(4):343–355. doi: 10.1177/1362361309106418. [DOI] [PubMed] [Google Scholar]
- Sullivan PB, Lambert B, Rose M, Ford-Adams M, Johnson A, Griffiths P. Developmental Medicine & Child Neurology. 10. Vol. 42. Cambridge Univ Press; 2000. Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study; pp. 674–680. [DOI] [PubMed] [Google Scholar]
- Tabbers MM, DiLorenzo C, Berger MY, Faure C, Langendam MW, Nurko S, et al. Journal of Pediatric Gastroenterology and Nutrition. 2. Vol. 58. LWW; 2014. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN; pp. 258–274. [DOI] [PubMed] [Google Scholar]
- Team, R. C. R Foundation for Statistical Computing. Vienna, Austria: 2014. R: A language and environment for statistical computing; p. 2013. [Google Scholar]
- Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351(9103):637–641. doi: 10.1016/s0140-6736(97)11096-0. Retracted article. See vol 363, pg 750, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


