Abstract
Compared to men, women disproportionally experience alcohol-related organ damage, including brain damage, and while men remain more likely to drink and to drink heavily, there is cause for concern because women are beginning to narrow the gender gap in alcohol use disorders (AUD). The hippocampus is a brain region that is particularly vulnerable to alcohol damage, due to cell loss and decreased neurogenesis. In the present study, we examined sex-differences in hippocampal damage following binge alcohol. Consistent with our prior findings, we found a significant binge-induced decrement in dentate gyrus (DG) granule neurons in the female DG. However, in the present study, we found no significant decrement in granule neurons in the male DG. We show that the decrease in granule neurons in females is associated with both spatial navigation impairments and decreased expression of trophic support molecules. Finally, we show that post-binge exercise is associated with an increase in trophic support and repopulation of the granule neuron layer in the female hippocampus. We conclude that sex differences in alcohol-induced hippocampal damage are due in part to a paucity of trophic support and plasticity-related signaling in females.
Keywords: alcohol binge, sex differences, neurodegeneration, cognitive deficits, trophic support, neurorestoration
Introduction
Although not traditionally classified as a neurodegenerative disease due to its preventable nature, excessive consumption of alcohol results in significant brain volume loss and cognitive impairment (Pfefferbaum et al., 1992, Sullivan et al., 2000, Crews and Nixon, 2009, de la Monte and Kril, 2014). Fronto-temporal regions, including the hippocampus, are particularly vulnerable (Sullivan et al., 1995, Agartz et al., 1999, Beresford et al., 2006). The effects of alcohol on hippocampal neurogenesis (including disruption of the ability of neural stem cells to form colonies, proliferate, differentiate, and survive) contribute to the neuropathological and cognitive consequences of alcohol (Nixon and Crews, 2002, Crews and Braun, 2003, Tateno et al., 2005). The mechanisms of alcohol-induced cell death and inhibition of cell birth and survival are complex but appear to involve proinflammatory cytokines, oxidative stress, and the loss of trophic factors, mechanisms that overlap with many neurodegenerative conditions (Crews, 2008, Crews and Nixon, 2009).
Like many diseases, alcoholism affects women and men differently. Decades of data show that, compared to men, women disproportionately experience alcohol-related health problems, including damage to the heart and liver (Erol and Karpyak, 2015), as well as a significantly higher mortality rate (Smith and Weisner, 2000, Walter et al., 2005). Mounting evidence indicates that they are also more likely than men to suffer alcohol-induced brain damage, including larger reductions in hippocampal volume (Agartz et al., 1999, Hommer et al., 2001, Agartz et al., 2003, Schweinsburg et al., 2003, Mann et al., 2005, Mancinelli et al., 2007, Sharrett-Field et al., 2013). And while men remain more likely to drink and to drink heavily (Dwyer-Lindgren et al., 2015, Erol and Karpyak, 2015), it is concerning that women are beginning to narrow the gender gap in prevalence of alcohol use disorders (AUD) (Holmila and Raitasalo, 2005, Wilsnack et al., 2013, Erol and Karpyak, 2015).
Rodent models replicate the pattern of brain damage seen in AUD (Collins et al., 1996, Obernier et al., 2002a, Obernier et al., 2002b, Crews and Nixon, 2009), and provide a means by which to systematically evaluate sex differences in extent of damage as well as underlying mechanisms. One goal of the present study was to evaluate sex differences in binge alcohol-induced brain damage and cognitive dysfunction using a well-established model (Majchrowicz, 1975, Collins et al., 1996) that reproduces the level of intoxication and neurodegeneration seen in human AUD (Collins et al., 1996, Corso et al., 1998, Obernier et al., 2002b, Crews et al., 2004, Hamelink et al., 2005). Using this model, we have previously shown a significant decrease in granule neurons in the hippocampal dentate gyrus (DG) of female rats (Leasure and Nixon, 2010, Maynard and Leasure, 2013), but whether the male hippocampus is similarly affected remains unknown.
Pre-clinical studies are beginning to elucidate mechanisms underlying female brain susceptibility to alcohol, and recent reports implicate inflammatory cytokines, aberrant gliosis, and glucocorticoids (Alfonso-Loeches et al., 2013, Wilhelm et al., 2015a, Wilhelm et al., 2015b). We have shown that the binge-induced decrease in DG granule neurons in the female brain persists for at least 5 weeks into abstinence, unless animals exercise (Maynard and Leasure, 2013). Exercise-driven restoration of the granule cell layer suggests that binge alcohol may damage the female brain in part by inducing a lasting decrement in trophic support, as one of the best documented effects of exercise is increased availability of trophic molecules in the brain (Gomez-Pinilla et al., 1997, Vaynman et al., 2004b, a, Llorens-Martin et al., 2008). Therefore, the second goal of the present study was to evaluate sex differences in trophic factor expression in the hippocampus after binge alcohol. Finally, we also determined whether exercise-driven restoration of the female DG was associated with increased expression of trophic factors.
We hypothesized that females would show a more severe binge-induced decrement in DG granule neurons and accompanying spatial navigation impairment, compared to males. We also hypothesized that females would have a more marked binge-induced decline in trophic support, including decreases in IGF-1, BDNF, their receptors (IGFR and TrkB, respectively) as well as cyclic AMP response element-binding protein (CREB), a downstream target of BDNF that in turn regulates BDNF transcription (see (Ehrlich and Josselyn, 2016) for review). Finally, we hypothesized that exercise-driven restoration of the binge-depleted granule cell population in the DG would be associated with increased expression of trophic molecules, their receptors, and CREB.
Materials and Methods
Alcohol Treatment
All applicable international, national and institutional guidelines for the care and use of animals were followed and all experimental procedures were approved by the University of Houston Institutional Animal Care and Use Committee (protocol number 14-013). A total of 173 (106 female, 67 male) Long-Evans rats (Harlan Sprague Dawley, Indianapolis, IN, USA), aged nine weeks were used across all experiments. Upon arrival, females weighed 175–200g and males 275–300g. Rats were group housed by sex in clear Plexiglas cages and given 1 week to acclimate to vivarium conditions, which includes ad libitum rat chow and water and a reversed light/dark cycle (lights off at 9:00/on at 21:00). Prior to beginning the experiments, all rats were tamed by gentle handling to acclimate them to the experimenters and gavage. Alcohol was administered via intragastric gavage according to a previously established paradigm modified from Majchrowicz (1975). This model was chosen because it mimics the high blood alcohol levels of binge-pattern drinkers (Tomsovic, 1974, Hunt, 1993) and for its well-documented neurodegeneration profile (Obernier et al., 2002b, Kelso et al., 2011). During binge exposure, food was removed from all animals but water was always available. Rats were gavaged with alcohol diet (25% alcohol w/v in vanilla Ensure™; Abbot Laboratories, Columbus, OH) or isocaloric control diet (Dextrose w/vanilla Ensure™) every 8 hours for 4 days, starting on the first day of the experiment (12 doses total). The initial dose for each animal was 5g/kg and caused significant intoxication; further doses were then determined based on a 6-point behavioral intoxication scale: 0, normal rat; 1, hypoactive; 2, ataxia; 3, ataxia with dragging abdomen and/or delayed righting reflex; 4, loss of righting reflex; 5, loss of eye blink reflex. Each score corresponds to a dose of ethanol between 0 and 5 g/kg, such that the greater the observed behavioral intoxication, the smaller the subsequent dose. This method maintains consistent intoxication relevant to AUD while avoiding mortality (Knapp and Crews, 1999, Nixon and Crews, 2002). Control animals received isocaloric diet at the average volume given to the alcohol group of the same sex to control for gavage experience and caloric intake (Gillette-Guyonnet and Vellas, 2008, Loncarevic-Vasiljkovic et al., 2012). Blood alcohol concentration (BAC) was determined from blood samples taken 90 minutes after the morning dose on day 3 (Leasure and Nixon, 2010, Maynard and Leasure, 2013) from either the tail vein or saphenous vein. For an additional 12 animals, blood samples were collected via saphenous vein 90 minutes after the third, fifth, and seventh doses of alcohol to track BAC progression during the binge and determine potential differences between doses and sexes. Samples were centrifuged, and then stored at −20°C until analysis. Serum was extracted and BAC determined using an AMI Analyzer based on external standards (Analox, Waltham, MA). Withdrawal symptoms began approximately 10 hours after the final dose, and were scored every half hour (Penland et al., 2001) until they subsided, approximately 27 hours after the last dose.
Behavioral Tests
Exploratory behavior in the open field
The open field test is commonly used to assess general locomotor activity levels and anxiety-like behavior in rodents (Prut and Belzung, 2003). Four and a half days after the last dose of alcohol each animal was tested in an open field, which consisted of a plywood box with a white plastic bottom divided into 25 equally-sized squares (each 15 × 15 cm) as previously reported (Leasure and Jones, 2008). Each animal was placed in the center of the open field and video recorded for 5 minutes. Locomotor activity was analyzed using an automated tracking system (Noldus, Amsterdam, Netherlands) which determined distance traveled and percentage of time spent in the center of the arena.
Spatial navigation in the Morris water maze
The Morris water maze (MWM) is widely used to study spatial learning and memory and is useful for detection of damage to cortical regions and the hippocampus (D’Hooge and De Deyn, 2001). The apparatus used consisted of a circular, galvanized steel pool, 167 cm in diameter and 76 cm deep as previously reported (Leasure and Jones, 2008). The pool was located in a room with many extramaze cues on the walls surrounding the pool (such as posters, electrical outlets, etc). The pool was filled with water that reached 2cm above the platform surface (12 cm diameter) and then made opaque with white non-toxic paint. Water was allowed to attain room temperature before testing began. Twenty-four hours after the open field task (6 days post binge) rats began MWM training, using a difficult two-trial per day paradigm that has been shown to detect subtle differences in performance (van Praag et al., 1999b, Vaynman and Gomez-Pinilla, 2005). The platform was placed in the northwest quadrant and remained there throughout spatial learning, but the release point changed each day. Each rat was given two trials per day for six consecutive days. Each trial had a 60s ceiling and a 60s inter-trial interval. Rats were placed into the water facing the wall, from one of four randomly chosen release points, and allowed 60s to find the hidden platform. Once the animal reached the platform, it was allowed to remain on the platform for approximately 10s. If the animal failed to reach the platform within the trial ceiling, it was gently guided there by the experimenter and placed on the platform for 10s. The animal was then removed from the water, dried off, and placed in its home cage to await its next trial. Pool water was agitated between animals and trials to prevent utilization of olfactory cues. Escape latency, swim speed, and path length were recorded and analyzed by an automated tracking system (Noldus, Amsterdam, Netherlands). Following six days of spatial reference memory testing, the animals were tested in a reversal learning task in order to assess binge-induced perseverative behavior, as previously described (Obernier et al., 2002b). The submerged platform was moved to the southeast corner (opposite quadrant from learning) and the animals were given an additional four trials. During reversal learning, the release point for an animal changed for each trial. Length of time and number of entries into the former platform quadrant were recorded and analyzed.
Voluntary Exercise
Consistent with our prior findings, in the current study female, but not male rats demonstrated a binge-induced reduction of dentate gyrus granule neurons. We have previously shown that four weeks of voluntary exercise reverses this loss in females (Maynard and Leasure, 2013). Therefore, in the current study, we only included female rats in our exercise experiments. On the seventh day following the last dose of alcohol, female rats in exercise groups were given access to voluntary exercise wheels for a maximum of four hours daily for one week, two weeks, or three weeks, beginning at the onset of their dark cycle (9:00 AM). Exercise began on the seventh post-binge day because binge alcohol exposure damages the brain, and the initial seven days following brain injury is a vulnerable period during which increased activity can exacerbate damage and limit recovery (Humm et al., 1998, Griesbach et al., 2004). In order to precisely monitor distance travelled, rats were removed from home cages and placed into individual running wheels equipped with counters. During the exercise period, animals had access to food and water ad libitum. After exercise, animals were returned to their home cages in the vivarium. Sedentary animals remained in their home cages in the vivarium during this period.
Histological Procedures
Perfusion and tissue sectioning
Rats were overdosed with anesthetic 8 hours after the last dose of alcohol and then intracardially perfused with saline, followed by 4% paraformaldehyde until the neck was stiff. Brains were dissected out and post-fixed overnight, then stored in 30% sucrose at 4°C. A freezing sliding microtome (Leica, Bannockburn, IL) was used to collect serial coronal sections (50 μm), which were stored in 96-well microtiter plates at −20°C in cryoprotectant.
Immunohistochemistry
To assess cell proliferation, every sixth serial section was processed for Ki67 using our standard immunohistochemistry (IHC) protocol (Maynard and Leasure, 2013). Free-floating sections were rinsed with 0.1 M tris-buffered saline (TBS) three times at room temperature for 10 minutes each. To quench endogenous peroxidases, sections were then treated for 30 minutes at room temperature in 0.6% hydrogen peroxide, followed by three 10-minute washes in TBS. Next, sections were treated with a 30 minute citrate buffer antigen retrieval step at 80° C, and then allowed to return to room temperature for 5 minutes. Following three additional TBS washes, sections were blocked for 60 minutes in 3% normal donkey serum (Sigma-Aldrich, St. Louis, MO, USA), followed by incubation at 4° C for 72 hours in primary antibody (rabbit anti-Ki67, Vector Laboratories, Burlingame, CA; 1:1800). After two TBS rinses for 15 minutes each and 15 minutes blocking in 3% normal donkey serum, sections were incubated overnight at room temperature in secondary antibody (biotinylated donkey anti-rabbit, Jackson ImmunoResearch Laboratories, West Grove, PA, USA; 1:250). Next, sections were rinsed three times in TBS for 10 minutes each, treated for 60 minutes in avidin-biotin complex (ABC, Vector Labs, Burlingame, CA, USA) and then rinsed three times in TBS for 10 minutes each. Sections reacted and were visualized with diaminobenzidine (DAB) and then rinsed four times in TBS for 10 minutes each, before being mounted onto gelatinized slides and allowed to dry overnight. Once dried, all brain sections were counterstained with Methyl Green, cleared in xylene, and coverslipped using Permount. Slides were coded such that the investigators performing cell counts were blind to experimental condition. The same slides were also used for quantification of dentate gyrus granule neurons using the methyl green Nissl counterstain.
Fluorojade B and TUNEL staining
Fluorojade B (FJB) staining of degenerating cells was conducted following published methods (Schmued and Hopkins, 2000, Leasure and Nixon, 2010). Every twelfth section from Bregma −1.80 μm and ending at Bregma −6.04 (Paxinos and Watson, 2009) was mounted onto Superfrost Plus ® slides (Fisher Scientific, Waltham, MA) and left to dry overnight. Slides were processed through graded alcohols, rinsed in distilled water, and then incubated in 0.06% potassium permanganate for 10 minutes on a shaker. Slides were then washed in distilled water for one minute before being incubated in FJB and DAPI (41-6-diamidino-2-phenylindole counterstaining to visualize nucleic material) in the dark for 20 minutes on a shaker. After three additional washes in distilled water, sections were air dried and then coverslipped in Cytoseal 40 ® (Thermo Fisher Scientific, Waltham, Massachusetts). Slides were then coded to ensure that cell counting was performed blind to experimental condition. It has been previously shown that cell death due to binge is necrotic in nature, not apoptotic (Obernier et al., 2002a; Morris et al., 2010), however these studies were conducted in males. Therefore, the terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) assay was selected to investigate whether cell death due to binge in females was apoptotic, because it is designed to detect apoptotic cells that undergo extensive DNA degradation during the late stages of apoptosis (Kyrylkova et al., 2012). TUNEL staining was performed using the DeadEnd™ Colorimetric TUNEL kit (Promega, Madison, Wisconsin). Briefly, tissue was dried overnight on Superfrost Plus ® slides (Fisher Scientific, Waltham, MA), then immersed in NaCl, phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde, and immersed again in PBS. The tissue was then permeabilized using Proteinase K for 30 minutes and then rinsed in PBS. The tissue was then re-fixed in 4% paraformaldehyde and immersed in PBS. The slides were then covered with equilibrium buffer and then incubated with recombinant terminal deoxynucleotidyl transferase (rTdT) reaction mixture for 1 hour. The rTdT reaction was terminated using a 2x saline-sodium citrate buffer (SSC) solution. The slides were then immersed in PBS, blocked in 0.3% hydrogen peroxide, and immersed again in PBS. Then the tissue was incubated in Streptavidin HRP for 30 minutes and then rinsed using PBS. The tissue then incubated for 10 minutes in DAB and then rinsed in deionized water. The slides were allowed to dry and then coverslipped using Permount (Fisher) as the mounting medium. The positive control was generated using DNase I (Promega) to induce DNA fragmentation.
Cell quantification
The number of remaining neurons in the dentate gyrus, was determined by unbiased stereology using the optical fractionator method applied via an automated system (StereoInvestigator, MicroBrightField, VT, USA). Using a Nikon Eclipse 80i upright microscope, the granule cell layer of the DG was traced using the 10x objective and cells were counted with two-dimensional counting frames using a 100x oil objective. The average mounted section thickness was approximately 37μm, thus top and bottom guard zones were set at 5μm each, for an optical dissector height of 27μm. Granule neurons were counted in every twelfth section in a single hemisphere beginning at the earliest emergence of the DG at Bregma −1.88 μm and ending at Bregma −6.04 (Paxinos and Watson, 2009). This resulted in 5–6 sections per brain. The counting frame size was 40 × 40 μm and the grid size was 200 × 200 μm. Coefficients of error (CE) were calculated using the method of Gunderson and were less than 0.05 for all groups (Gundersen et al., 1999).
To quantify Ki67-positive (Ki67+) cells in the dentate gyrus, the granule cell layer (GCL) and subgranular zone (SGZ) of the DG were traced using the 10x objective and cells counted using a 40x oil objective. Each labeled soma in the granule cell layer and subgranular zone (defined as zero to two cell bodies from the inner molecular layer) was counted in every sixth section from Bregma −1.88 μm and ending at Bregma −6.04 (Paxinos and Watson, 2009), using a 40 x oil objective. This resulted in 10–12 sections per brain.
Quantification of FJB-positive (FJB+) cells was conducted on a Nikon Eclipse 80i upright microscope fitted with epifluorescence including 405λ and 488λ cubes (for DAPI and FJB detection respectively). The dentate gyrus was traced at 405 using a 10x objective and then the cube switched to 488 for FJB detection. FJB+ cells were counted in one hemisphere of the hippocampal dentate gyrus (combined GCL and SGZ).
Western blotting
Eight hours following the last dose of alcohol rats were overdosed with anesthetic and observed for loss of toe pinch reflex as an indicator of anesthetic depth. Each animal was then decapitated, brains quickly removed and the hippocampi were dissected and separated into dorsal and ventral halves. Tissue was then flash frozen using dry ice and stored at −80°C. The dorsal hippocampus was homogenized in lysis buffer and centrifuged in order to isolate protein from the lysate. The dorsal hippocampus was chosen because it is associated with spatial navigation function (Bannerman et al., 2002, Bannerman et al., 2003), and a focus of this project was sex-specific impairment in spatial performance due to binge alcohol. The supernatant was taken and stored for further use. A sample of this was used for quantification of protein concentration via BCA protein assay (Thermo Fisher Scientific, Waltham, MA). Protein concentration was then adjusted with a portion of sample in loading buffer to 1 μg/μL for all samples and heated at 70°C for 10 min to denature proteins.
For each Western blot, 20 μl of each sample (standardized to 20 μg total protein content) was separated by molecular weight via gel electrophoresis and transferred to polyvinylidene (PVDF) membranes. Following transfer, membranes were washed six times for 10 min each in Tris-buffered saline with Tween-20 (TBST). Membranes were then incubated for 1h at room temperature I a blocking solution consisting of 5% powdered nonfat milk in TBST. Primary antibodies (rabbit host) were added to the blocking solution and membranes were transferred to 4°C, where they were incubated overnight. For BDNF and IGF-1, the primary antibody was from Santa Cruz Biotechnology (Dallas, TX) and used at a dilution of 1:100. For TrkB, IGFR, CREB and pCREB the primary antibodies were from Cell Signaling Technology (Danvers, MA) and used at a dilution of 1:1,000. Primary antibodies for either COX IV or GAPDH (Cell Signaling Technology, 1:1,000 and GeneTex, Irvine, CA, 1:10,000 respectively) were included on each blot as a positive loading control for basal protein expression, depending on molecular weight of target proteins. Membranes were then washed six times for 10 minutes each in TBST and incubated in a solution of 5% skim milk in TBST containing secondary antibody (goat anti-rabbit, 1:10,000) conjugated against horseradish peroxidase (HRP) that was optimized for use with the C-Digit blot scanner. Incubation lasted 1h at room temperature, after which membranes were washed a final six times in TBST for 10 minutes each. Visualization of proteins was achieved by chemiluminescence using a Li-Cor C-Digit blot scanner (Li-Cor Biosciences, Lincoln, NE) and luminol substrate from the same manufacturer. Band intensity was quantified using the accompanying software (Image Studio Digits ver 4.0). Background, signal and midtone levels were adjusted for each blot for fidelity in reporting, and a region of interest containing each band was drawn with minimal background included. The expression of a protein was then weighted by the corresponding intensity of the loading control in the same lane. This weight was obtained by dividing each loading control by the mean intensity of all loading controls within the same blot in order to account for any possible variability between protein concentrations in individual lanes. The mean of the weighted intensity values from all treatment groups were normalized against this average (percent of control). This was done out of necessity due to the large number of animals, which prevented simultaneously running every animal on a single blot, and the inability to compare data from separate blots directly.
Statistical analyses
Data were analyzed using SPSS Statistics 17.0 (IBM SPSS Statistics, IL, USA). Dose, BAC, and Western blotting data were analyzed using unpaired 2-tailed Student’s t tests. Because the intoxication and withdrawal scales are non-linear, these data were analyzed using Kruskal-Wallis. Neuroanatomical data and open field data were analyzed using 2-way ANOVA with Sex and Diet as between group variables. Water maze data were analyzed using three-way mixed model ANOVA, with Sex and Diet as between subjects variables and Training Day as the repeated measure. Where applicable, Bonferroni-corrected post hoc comparisons were made. All data are reported as means ± standard error of the mean and p values deemed statistically significant if < 0.05.
Results
Binge alcohol-induced brain damage
Mean intoxication score, alcohol dose, BAC, withdrawal score and peak withdrawal scores did not differ between males and females (see Table 1). Nonetheless, as shown in Figure 1, binge alcohol treatment significantly decreased the number of remaining granule neurons in the dentate gyrus of females, but not males. A two-way ANOVA revealed significant main effects of Sex [F(1,24) = 19.98, p < 0.0001] and Diet [F(1,24) = 5.02, p = 0.035], as well as a significant Sex x Diet interaction [F(1,24) = 4.51, p = 0.044]. To control for the possibility that neuron loss in the DG was linked to stage of estrous, remaining granule neurons were quantified in a separate group of females (n = 5) that began binge exposure during diestrous (determined by vaginal cytology). The mean number of remaining granule neurons in this group was 803,859 ± 6,772. One-way ANOVA was significant [F(2,17) = 7.96, p = 0.004] and post hoc analysis indicated that diestrous females had significantly fewer remaining granule neurons compared to controls [p = 0.014], but were not different from the binged females reported above.
Table 1. Intoxication, dose, blood alcohol concentration, and withdrawal score data for males and females.
Mean intoxication score, dose of alcohol received, blood alcohol concentration for males and females during the 4 days (12 doses) of binge alcohol exposure across all experiments. Mean withdrawal scores (average score during withdrawal monitoring) and mean peak withdrawal scores (average of highest score during withdrawal monitoring) for males and females starting 10 hours and ending 27 hours after the last dose of alcohol.
| Sex | Intox. behavior | p | Dose(g/kg/day) | p | BAC(mg/dl) | p | Mean Withd. | p | Peak Withd. | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Female | 2.1 ± .04 | .12 | 8.8 ± .13 | .07 | 245 ± 9.3 | .75 | 1.8 ± .05 | .17 | 2.9 ± .06 | .93 |
| Male | 2.3 ± .04 | 8.4 ± .13 | 240 ± 16 | 1.6 ± .10 | 2.9 ± .14 |
Figure 1. Sex-dependent effect of binge alcohol on the dentate gyrus.
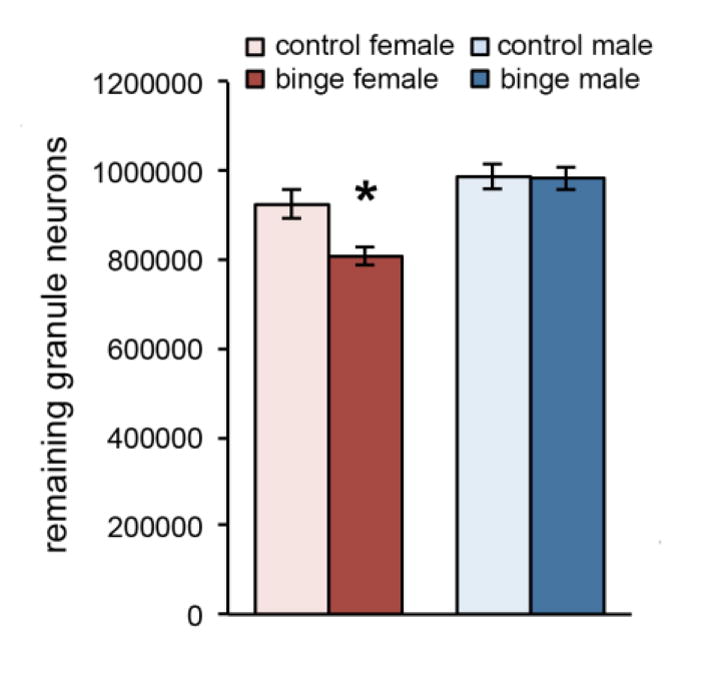
Binge alcohol significantly decreased the number of remaining granule neurons in the dentate gyrus of female rats, but not males. *p < 0.05 significantly different from control.
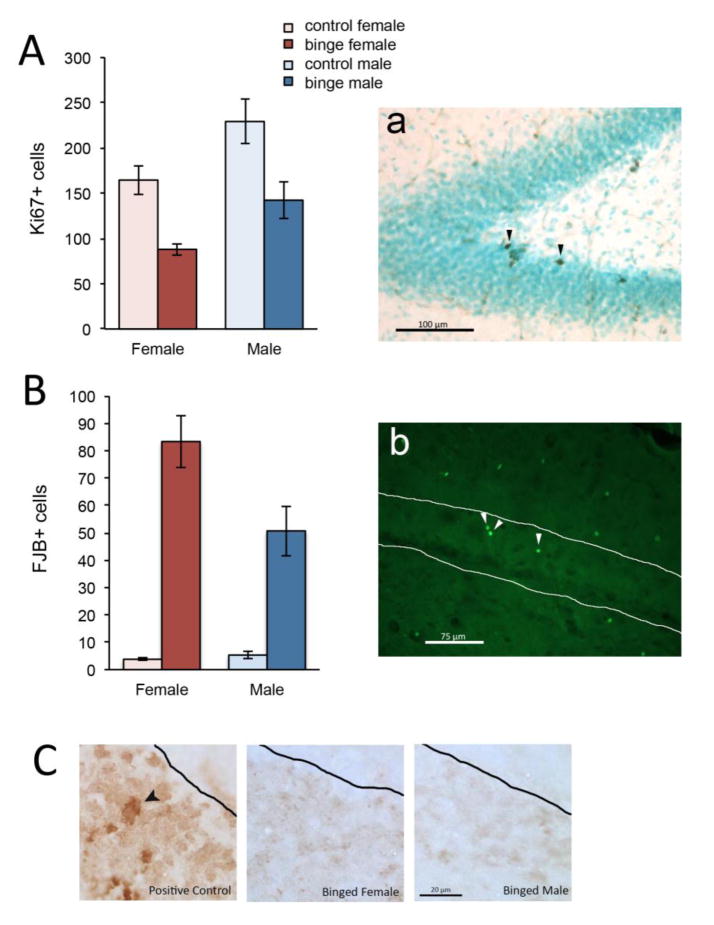
Cell proliferation and cell death in the dentate gyrus after binge alcohol
We next determined whether the binge-induced decrement in granule neurons in female rats was due to inhibition of cell birth, induction of cell death, or both. We used Ki67 labeling to assess cell birth (see Figure 2A). Two-way ANOVA revealed significant main effects of Sex [F(1,23) = 11.80, p = 0.002] and Diet [F(1,23) = 22.26, p < 0.0001] but no significant Sex x Diet interaction [F(1,23) = 0.090, p = 0.767]. Thus, binge resulted in a significant decrease in the number of proliferating cells in both sexes compared to same-sex controls (47% decrease in females and 38% in males). The significant main effect of Sex indicated that males on average had more Ki67+ cells than females, regardless of binge exposure. The 47% binge-induced decrease of Ki67+ cells in females was not significantly different than the 38% decrease in males [t(13) = .907, p = .381]. To assess cell death, we used FJB, a marker of degenerating neurons, whether apoptotic or necrotic (Schmued and Hopkins, 2000). Two-way ANOVA revealed significant main effects of Sex [F(1,23) = 4.33, p = 0.049] and Diet [F(1,23) = 68.71, p < 0.0001] and a Sex x Diet interaction [F(1,23) = 5.101, p = 0.034], indicating that binge increased cell death more severely in females than males (see Figure 2B). It has previously been reported that cell death due to binge is necrotic in nature, not apoptotic (Obernier et al., 2002a, Morris et al., 2010), but these studies were conducted in males. To investigate the possibility that granule neurons in the female dentate gyrus may show apoptosis after binge (whereas males do not), we performed TUNEL staining (Kyrylkova et al., 2012) in representative male and female brains. While we saw TUNEL+ cells in the positive control, there was none apparent in the binged male or female brains (see Figure 2C). While this analysis does not definitively determine the nature of cell death after binge alcohol, it provides evidence that the mechanism is not different between the sexes.
Figure 2. Binge alcohol decreased cell proliferation and increased cell death in the dentate gyrus.
Cell proliferation was significantly decreased by binge alcohol in both sexes (A; arrowheads in panel a indicate Ki67+ cells). Cell death (B) was increased by binge alcohol in the dentate gyrus of both sexes (FJB+ cells indicated in panel b by arrowheads). Cell death in the dentate gyrus did not appear to be apoptotic in nature, as we found virtually no TUNEL+ cells in binged animals (C; left panel shows positive control with arrowhead pointing to TUNEL+ cell).
Open field behavior and spatial navigation in the Morris water maze
To assess anxiety-like behavior, as well as nonspecific motor function and exploratory behavior, we used the open field test five days post binge (see Figure 3A). Two-way ANOVA revealed no significant main effect of Sex [F(1,40) = 2.36, p = 0.13] or Diet [F(1,40) = 0.23, p = 0.63] and no significant interaction [F(1,40) = 0.046, p = 0.831] for percent time spent in the center of the open field. A similar pattern was observed for distance traveled (Sex [F(1,40) = 2.16, p = 0.74]; Diet [F(1,40) = 1.82, p = 0.19]; Sex x Diet [F(1,40) = 0.046, p = 0.831]). These results indicate there were no sex differences or lingering effects of binge on exploratory behavior.
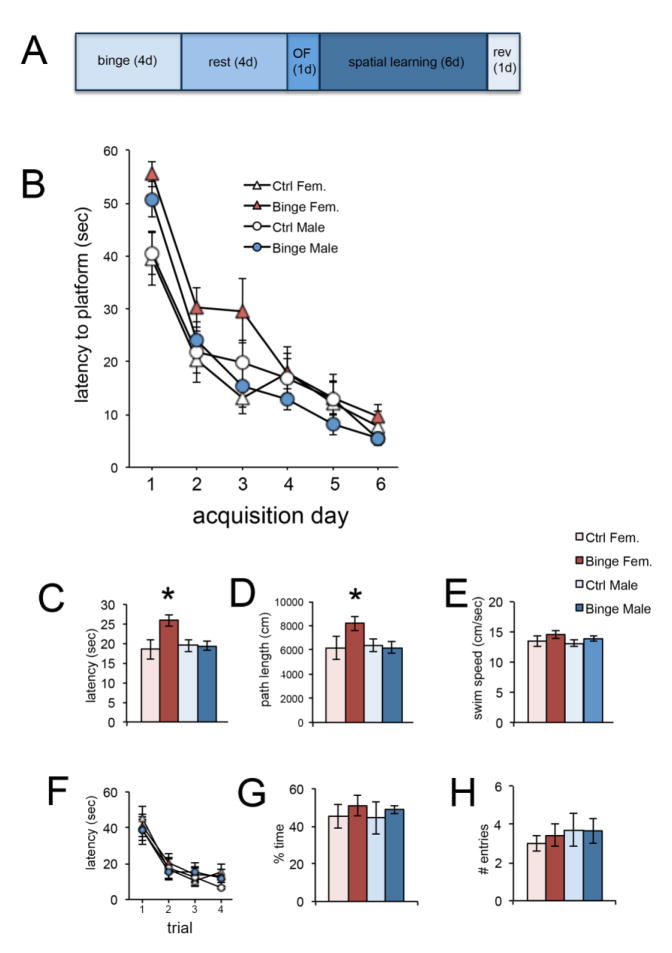
Figure 3. Sex-dependent effect of binge alcohol on spatial navigation.
Panel A shows the timeline of behavioral analysis following binge alcohol. Binge alcohol had a significant, sex-dependent effect on acquisition of the spatial task. All groups ultimately learned the platform location, but binged males and females were initially slower to find it compared to same-sex controls (B). Binged males more rapidly achieved control performance, compared to binged females. The significant Sex x Diet interaction is apparent for both latency (C) and path length (D), with binged females being significantly different from all other groups. There was no difference between groups for swim speed (E). There were no differences between groups for reversal learning (F–H). *p < 0.05 significantly different from all other groups.
Acquisition of the spatial reference memory task was measured by escape latency, path length to the platform, and swim speed. The average of each animal’s two daily trials was used for analysis. A three-way mixed model ANOVA revealed for escape latency significant main effects of Training Day [F(5,200) = 60.75, p < 0.05] and Diet [F(1,40) = 4.76, p < 0.05] and significant Sex x Diet [F(1,40) = 4.96, p < 0.05] and Diet x Training Day [F(5,200) = 2.67, p < 0.05] interactions (see Figure 3B). Thus, all groups improved performance over time, and Diet, when averaged across time, had a significant, sex-dependent effect. Bonferroni-corrected post hoc comparisons showed that binged females had significantly longer escape latencies compared to control females (p = 0.003) and binged males (p = 0.07) (see Figure 3C). For path length, there was a significant main effect of Training Day [F(5,200) = 90.36, p < 0.05] and significant Sex x Diet [F(1,40) = 4.97, p < 0.05] and Diet x Training Day [F(5,200) = 2.56, p < 0.05] interactions. Thus, all groups decreased path length over time, and Diet, when averaged across time, had a significant, sex-dependent effect. Bonferroni-corrected post hoc comparisons showed that binged females had significantly longer path lengths compared to control females (p = 0.006) and binged males (p = 0.014) (see Figure 3D). For swim speed, there was a significant main effect of Training Day [F(5,200) = 59.31, p < 0.05] and a significant Diet x Training Day [F(5,200) = 3.84, p < 0.05] interaction. Binge alcohol had no effect on performance in the reversal learning task, in which the submerged platform was moved to the opposite quadrant used in the reference memory task. Number of entries into and time spent in the original platform quadrant during the first trial were measured as a sign of perseverative behavior. ANOVA revealed a significant effect of Trial [F(3,120) = 41.165, p < .05] indicating that all animals decreased escape latency over the course of the trials. There were no significant main effects of Sex [F(1,40) = .490, p = .488] or Diet [F(1,40) = .048, p = .829] or significant interactions [F(1,40) = .033, p = .856] indicating that binge exposure had no effect on learning the new location of the platform (see Figure 3F). Also, there were no significant effects for average latency, path distance, or average velocity. Binge exposure had no effect on perseverative behavior during the first trial of the reversal learning task. ANOVA revealed no significant effects of Sex [F(1,40) = .063, p = .803], [F(1,40) = .542, p = .466] or Diet [F(1,40) = .767, p = .386], [F(1,40) = .088, p = .769] for percent of time spent in the original platform quadrant or number of entries into the original quadrant (see Figure 3G and H).
Protein expression during and after binge alcohol
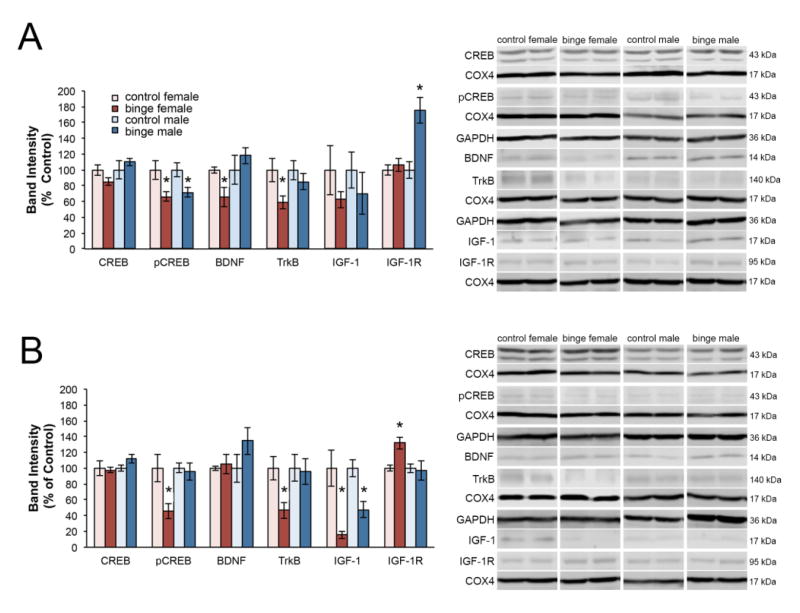
Expression of proteins associated with trophic support were assessed after 24 (Figure 4A) and 48 (Figure 4B) hours of binge exposure, and then 8 hours after the full four-day binge (Figure 5) using two-tailed Student’s t-tests comparing normalized control to normalized binge exposed for each protein of interest within sex. After 24 hours of binge exposure, females had a significant decrease in pCREB expression [t(7) = 2.909, p = .027], BDNF expression [t(4.798) = 2.609, p = .050], and expression of the high affinity BDNF receptor TrkB [t(7) = 2.592, p = .036]. On the other hand, 24 hours of binge exposure in males only resulted in a significant decrease in pCREB [t(7) = 2.828, p < .025] and a significant increase in expression of the IGF-1 receptor, IGF-1R [t(7) = −3.642, p < .008].
Figure 4. Sex differences in protein expression during binge exposure.
Expression of proteins associated with trophic support was assessed after 24 (A) and 48 (B) hours of binge exposure, Binge alcohol caused a decrease in pCREB, BDNF and trkB at 24 hours in females, whereas males showed a decrease in pCREB only. Binged males showed an increase in IGFR at 24 hours, but binged females did not show this until 48 hours, at which point IGF-1 was downregulated in binged animals of both sexes. At 48 hours, binged males had normalized expression of pCREB, but binged females did not. *p < 0.05 significantly different from same sex control.
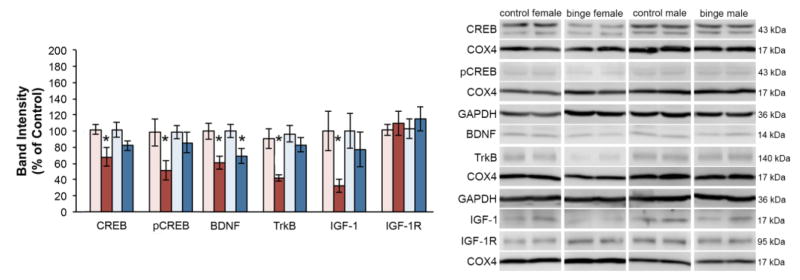
Figure 5. Sex differences in protein expression after binge exposure.
Expression of proteins associated with trophic support was assessed 8 hours after the end of binge exposure. Binged females continue to show a decrease in pCREB and at this point CREB is decreased as well. They also show decreased BDNF as well as the trkB receptor and IGF-1. In contrast, binged males only show a decrease in BDNF. *p < 0.05 significantly different from same sex control.
Following 48 hours of binge exposure, females had a significant decrease in pCREB expression [t(7) = 2.886, p = .023], TrkB expression [t(7) = 3.069, p = .018], IGF-1 expression [t(7) = 4.080, p = .005] and a significant increase in IGF-1R expression [t(7) = −3.441, p = .011]. In contrast to females, 48 hours of binge exposure in males only resulted in a significant decrease in IGF-1 expression [t(7) = 3.471, p = .010].
When assessed 8 hours after a full 4 day binge, females had a significant decrease in the protein expression of CREB [t(9) = 2.669, p = .026], pCREB [t(7) = 2.385, p = .049], BDNF [t(7) = 2.899, p = .023], TrkB [t(9) = 3.397, p = .008], and IGF-1 [t(6) = 2.628, p < .039]. However, a full 4 days of binge exposure in males only resulted in a significant decrease in BDNF expression [t(7) = 2.489, p = .042].
Exercise effects on the dentate gyrus after binge alcohol damage
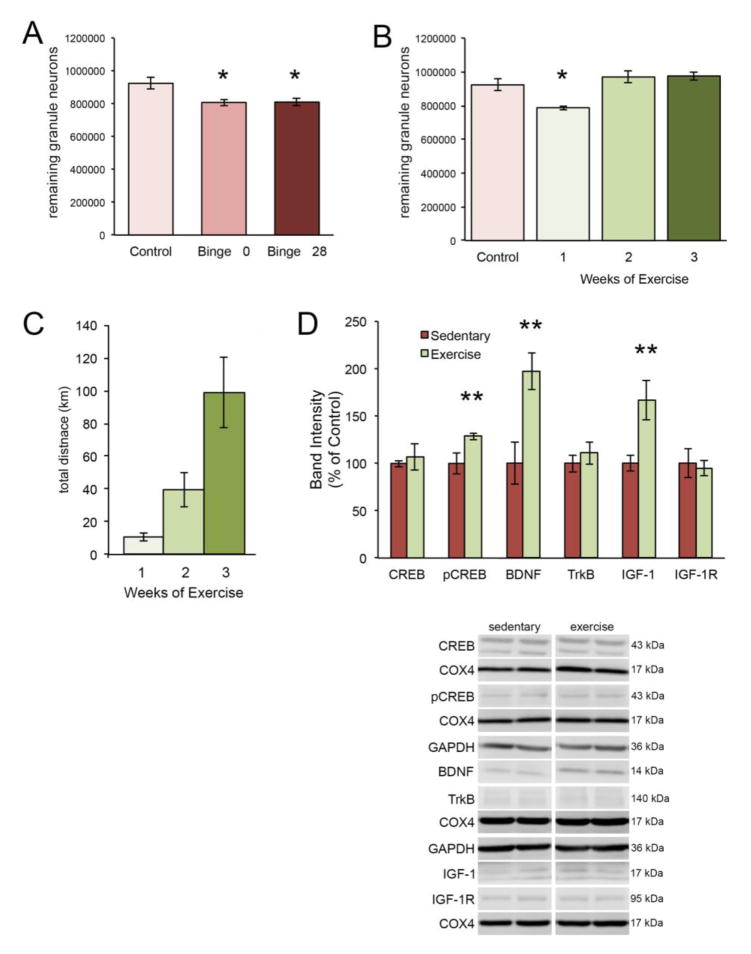
Only female rats demonstrated a significant binge-induced reduction of dentate granule neurons, therefore only female animals were included in exercise experiments. To show that the effects of binge alcohol on dentate gyrus granule neurons in the female is lasting, we compared control and binged animals described above to a group of rats sacrificed 4 weeks after binge exposure. One way ANOVA was significant [F(2,18) = 6.598, p < .05] and post hoc analysis showed that animals sacrificed either 8 hours (p = .010) or 4 weeks (p = .044) after binge had significantly fewer remaining dentate gyrus granule neurons compared to non-binged controls (see Figure 6A). As we have previously shown that post-binge exercise is associated with repopulation of the binge-damaged dentate gyrus (Maynard and Leasure, 2013), we allowed binged animals 1, 2 or 3 weeks of access to exercise wheels. There were no significant differences in average distance run during the first [F(2,20) = .310, p = .737] or second week of exercise [t(8) = .615, p = .615]. However, a one-way ANOVA for average distance run across the entire experiment revealed that animals that exercised for three weeks ran significantly more per day than animals that only exercised for one week [F(2,20) = 4.198, p < .05] likely because they had two extra weeks of access to exercise wheels (not shown). No differences were found however in average distance run between animals that had access for one week and two weeks (p = 0.10). In line with this, all animals increased distance run over the course of the experiment [F(6,120) = 8.357, p < .05]. As expected, more weeks of access to exercise wheels resulted in greater total distances run [F(2,20) = 10.805, p < .05] (Figure 6C). One-way ANOVA for the effect of exercise on remaining granule neurons after binge exposure revealed a significant effect of Time [F(3,21) = 9.69, p < .05]. Planned post hoc tests compared the number of remaining granule neurons after exercise compared to control animals revealed that two (p = 1.00) and three (1.00) weeks of exercise were associated with recovery from binge-induced granule cell loss but not one week of exercise (p < .05) when compared to control animals (see Figure 6B). We found no significant correlation between average distance run and remaining granule neurons (r = .38), however there was a significant correlation between total distance run and remaining granule neurons (r = .51). These results indicate that between seven and fourteen days of exercise are necessary to repopulate the DG following binge exposure.
Figure 6. Exercise-driven repair is associated with increased expression of plasticity-related proteins.
The binge-induced decrement in remaining granule neurons in the dentate gyrus of the female hippocampus is detectable immediately following binge (Binge 0) as well as 4 weeks later (Binge 28) (A). However, if animals exercise for at least 2 weeks post-binge, the number of granule neurons is normalized (B). Panel C depicts the total distance covered by animals that ran for 1, 2 or 3 weeks, beginning after 7 days of abstinence. One week of post-binge exercise increased the expression of pCREB, BDNF and IGF-1 (D). *p < 0.05 significantly different from control; **p < 0.05 significantly different from sedentary.
Changes in protein expression in binged female rats following exercise
Expression of proteins associated with trophic support was analyzed using Student’s t-tests comparing normalized protein expression in female binge-exposed sedentary animals to normalized protein expression in binge-exposed females that exercised for one week. Compared to binge-exposed sedentary animals, one week of exercise was associated with a significant increase in BDNF expression [t(8) = −3.299, p = .011] and IGF-1 expression [t(7) = −2.697, p = .031], but no difference in the expression of their respective receptors, TrkB [t(8) = −1.558, p = .163] and IGF-1R [t(8) = .297, p = .775] (see Figure 6D). Additionally, one week of exercise was associated with a significant increase in pCREB expression [t(8) = −2.638, p = .034] but no increase in CREB expression [t(8) = −.428, p = .682].
Discussion
In the present study, we directly compared the effects of a well-established model of AUD on the hippocampus of male and female rats. Consistent with our prior findings (Maynard and Leasure, 2013), the present results show that there is an immediate (present 8 hours post-binge) and prolonged (apparent for at least 4 weeks) decrease in DG granule neurons in female rats due to binge alcohol. Also consistent with our prior findings (Maynard and Leasure, 2013), we show that post-binge exercise leads to repopulation of the depleted DG. We extend those findings in the present report by showing that the binge-induced decrease in granule neurons is sex-dependent and associated with spatial navigation impairment. We also show that the decrease in granule neurons is associated with decreased trophic support and that exercise-driven restoration of the binge-depleted DG is accompanied by upregulation of trophic support and plasticity-related signaling. This is, to our knowledge, the first report of a sex difference in hippocampal damage, associated cognitive impairment and trophic factor expression in an animal model of an AUD.
Sex differences in ethanol pharmacokinetics in rodents have been reported (Middaugh et al., 1992, Rivier, 1993, Crippens et al., 1999), although these are typically assessed after only a single dose, and age and strain influence whether or not sex differences are observed (Collins et al., 1975, Erickson, 1984, Seitz et al., 1992). In the present study, we found no significant differences in BAC or dose of alcohol administered between males and females over the course of binge exposure. Nonetheless, our data show that binge alcohol significantly decreased the number of remaining DG granule neurons in females, but not males. In regions of the brain that do not show adult neurogenesis, a decrease in cell number due to exposure to a neurotoxin (such as alcohol) is most likely due to cell loss. In the dentate gyrus of the hippocampus, however, decreased neurogenesis is also a possibility, and binge alcohol has been shown to decrease neurogenesis (Nixon and Crews, 2002, Nixon, 2006). We therefore examined both cell loss and cell genesis in the DG of binged male and female rats. Cell death after binge alcohol has previously been found in male rats, and shown to be necrotic, with FJB+ (but not TUNEL+) cells present in vulnerable regions (Obernier et al., 2002a, Morris et al., 2010, Kelso et al., 2011). Consistent with these reports, we found FJB+ (but not TUNEL+) cells in the DG of binged animals of both sexes. There was, however, a significant sex difference in the number of FJB+ cells, with females more severely affected. While this analysis does not definitively determine the nature of cell death after binge alcohol, it provides evidence that the mechanism is not different between the sexes. Moreover, we found that binge alcohol suppressed cell proliferation in both males and females. Both sexes showed a significant decrease in Ki67 labeling due to binge alcohol, however, female rats showed a higher (although not significantly different) percent decline. Moreover, there was a main effect of Sex on Ki67+ cells, indicating that male rats had more cell proliferation under basal conditions. Collectively, these results suggest that a lower baseline level of cell proliferation in females, combined with a sex difference in binge-induced cell death (with females more affected) contribute importantly to the selective vulnerability of the female DG to binge alcohol damage.
Binged females were also more cognitively affected than binged males when tested in the Morris water maze, a task previously shown to be affected by the integrity of the DG (van Praag et al., 1999a, Rola et al., 2004). When averaged over time, binged females took longer to find the platform and had longer path lengths compared to all other groups. Importantly, there was no difference between groups for swim speed, so the inferior performance of the binged females was not due to swimming slower than animals in the other groups. It is also important to note that the binged females, although delayed in acquiring the task, eventually learned it. Moreover, after having learned the platform location, none of the animals had difficulty adjusting to its new location during the reversal learning task, thus, there was no indication of perseveration or behavioral inflexibility. Reversal learning impairment has previously been shown in male rats following binge alcohol (Obernier et al., 2002b). Possibly the superior spatial navigation ability of pigmented rats such as Long-Evans (compared to albino strains (Harker and Whishaw, 2002)) accounts for the discrepancy.
We have previously shown that the decrement in DG granule neurons in the female hippocampus following binge alcohol is chronic, unless animals exercise (Maynard and Leasure, 2013). Given that exercise enhances available brain neurotrophic factors (Gomez-Pinilla et al., 1997, Vaynman et al., 2004b, a, Vaynman et al., 2006, Munive et al., 2016), we reasoned that binge alcohol may induce a persistent dearth of trophic support molecules in the female brain. To examine this, we used Western blotting to assess expression of BDNF, IGF-1 and their associated receptors, as well as the transcription factor CREB and its phosphorylated form, pCREB, 24 and 48 hours into the binge, and then at the end of binge exposure. Our results support the hypothesis that compared to males, females have a much more pronounced binge-induced decline in trophic support. Females showed decreased expression of BDNF and the TrkB receptor after 24 hours of binge exposure (see Figure 4A), and these decreases were also present at the end of the binge (see Figure 5). In contrast, males are slower to decrease expression of BDNF, and never show a decrease in TrkB. Males show an immediate upregulation of IGF-1R, which may help to buffer their brains against a transient binge-induced decrease in IGF-1, which was found 48 hours into binge exposure. Females, on the other hand, did not upregulate expression of IGF-1R until 48 hours, which may have made them more vulnerable to decreased IGF-1, which they demonstrated beginning after 48 hours of binge exposure. Finally, females show immediate and persistent downregulation of pCREB, whereas for males this decrease is only seen at 24 hours. Collectively, these results are consistent with the idea that binge alcohol selectively and persistently decreases trophic support and downstream signaling in the female brain.
Our data show that by the end of binge exposure, female rats have downregulated expression of BDNF, TrkB, and IGF-1, indicating a severe dearth of trophic support. In addition, they show downregulation of both CREB and its phosphorylated form, pCREB. These losses very likely underlie the significant decrease in granule neurons found only in binged females. CREB promotes neuroprotection by regulating the transcription of other pro-survival factors to protect neurons from excitotoxicity and apoptosis (Lonze and Ginty, 2002, Mantamadiotis et al., 2002) and is critical for proliferation, differentiation, survival, and maturation of all types of cells, including new neurons in the rodent hippocampus (Nakagawa et al., 2002, Fujioka et al., 2004). Our data are consistent with previous findings that alcohol has profound effects on BDNF expression that appear to be dependent on the alcohol dose and brain region, with a pattern towards reduced BDNF with higher chronic doses (Stevenson et al., 2009, Moonat et al., 2010) that corresponds with alcohol-induced neurodegeneration (Davis, 2008) and reduced survival of new DG granule neurons (Nixon and Crews, 2002, Herrera et al., 2003). Moreover, alcohol reduces signaling through the BDNF-CREB feedback loop, resulting in a loss of trophic support (Zou & Crews, 2006).
In further support of this hypothesis, we found that exercise-driven repopulation of the granule neuron layer was associated with an increase of trophic support. Female rats either remained sedentary after binge alcohol or exercised for 1, 2 or 3 weeks, beginning 7 days after the end of binge. We found that 1 week of exercise was not sufficient to repopulate the granule neuron layer, but that with 2 or 3 weeks of exercise, the number of granule neurons was not different from control. We reasoned that if 2 weeks of exercise were sufficient to repopulate the granule neuron layer, changes in trophic support should be visible after 1 week. Accordingly, we examined protein expression in binged female rats that had exercised for 1 week. Our results show significant increases in expression of BDNF, IGF-1 and pCREB. Thus, post-binge exercise is associated with an increase in trophic support and repopulation of the granule neuron layer. In male rats, abstinence after prolonged alcohol exposure is associated with an initial increase in BDNF levels, which eventually normalize (Somkuwar et al., 2016). It is possible that this normalization of BDNF does not naturally occur in females in the absence of an intervention, such as exercise.
It is not apparent from our data whether it is the amount of exercise (in terms of distance or length of time) that is essential for repair, or whether a single week of exercise would be sufficient and repopulation would be evident if animals were sacrificed 1 week later. What is apparent from our data is that 3 weeks of exercise is no more effective than 2 weeks at restoring the granule cell layer, and that once repopulated, the granule layer does not continue to increase in size with additional exercise. Exercise is being used as a supplementary treatment for AUD as a means by which to target co-morbid disorders such as anxiety and depression (Giesen et al., 2015, Stoutenberg et al., 2016). The exercise-driven enhancement of trophic support in the alcohol-damaged female brain supports the use of exercise as a treatment for AUD, as it may help to restore damaged tissue as well as enhance affect.
In summary, we provide evidence that the female dentate gyrus is selectively vulnerable to binge alcohol damage. After binge alcohol, cell death was apparent in both sexes, but females showed a significant decrement in dentate gyrus granule neurons, whereas males did not. Females also showed a greater decrease in cell birth, compared to males. Associated with this was a spatial learning impairment and a more rapid and lasting binge-induced decrease in trophic support, although this was reversible with exercise during abstinence. Our results support the growing body of clinical evidence suggesting that the female brain is selectively vulnerable to alcohol and provide a model by which to study underlying mechanisms.
Acknowledgments
This study was supported by National Institute on Alcohol Abuse and Alcoholism R21 AA 021260. We thank Dr. Shaefali Rodgers for a critical reading of the manuscript.
Funding: This study was supported by National Institute on Alcohol Abuse and Alcoholism R21 AA 021260
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- Agartz I, Brag S, Franck J, Hammarberg A, Okugawa G, Svinhufvud K, Bergman H. MR volumetry during acute alcohol withdrawal and abstinence: a descriptive study. Alcohol and alcoholism. 2003;38:71–78. doi: 10.1093/alcalc/agg020. [DOI] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Archives of general psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M, Guerri C. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology. 2013;311:27–34. doi: 10.1016/j.tox.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behavioral neuroscience. 2002;116:884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behavioural brain research. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, Liu D, Shen D, Davatzikos C. Hippocampus volume loss due to chronic heavy drinking. Alcoholism, clinical and experimental research. 2006;30:1866–1870. doi: 10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Collins AC, Yeager TN, Lebsack ME, Panter SS. Variations in alcohol metabolism: influence of sex and age. Pharmacology, biochemistry, and behavior. 1975;3:973–978. doi: 10.1016/0091-3057(75)90004-0. [DOI] [PubMed] [Google Scholar]
- Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcoholism, clinical and experimental research. 1996;20:284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Corso TD, Mostafa HM, Collins MA, Neafsey EJ. Brain neuronal degeneration caused by episodic alcohol intoxication in rats: effects of nimodipine, 6,7-dinitro-quinoxaline-2,3-dione, and MK-801. Alcoholism, clinical and experimental research. 1998;22:217–224. [PubMed] [Google Scholar]
- Crews FT. Alcohol-related neurodegeneration and recovery: mechanisms from animal models. Alcohol research & health: the journal of the National Institute on Alcohol Abuse and Alcoholism. 2008;31:377–388. [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ. Binge ethanol treatment causes greater brain damage in alcohol-preferring P rats than in alcohol-nonpreferring NP rats. Alcoholism, clinical and experimental research. 2003;27:1075–1082. doi: 10.1097/01.ALC.0000075826.35688.0D. [DOI] [PubMed] [Google Scholar]
- Crews FT, Collins MA, Dlugos C, Littleton J, Wilkins L, Neafsey EJ, Pentney R, Snell LD, Tabakoff B, Zou J, Noronha A. Alcohol-induced neurodegeneration: when, where and why? Alcoholism, clinical and experimental research. 2004;28:350–364. doi: 10.1097/01.alc.0000113416.65546.01. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol and alcoholism. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippens D, White ML, George MA, Jaworski JN, Brunner LJ, Lancaster FE, Gonzales RA. Gender differences in blood levels, but not brain levels, of ethanol in rats. Alcoholism, clinical and experimental research. 1999;23:414–420. [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Davis MI. Ethanol-BDNF interactions: still more questions than answers. Pharmacol Ther. 2008;118:36–57. doi: 10.1016/j.pharmthera.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Kril JJ. Human alcohol-related neuropathology. Acta Neuropathol. 2014;127:71–90. doi: 10.1007/s00401-013-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer-Lindgren L, Flaxman AD, Ng M, Hansen GM, Murray CJ, Mokdad AH. Drinking Patterns in US Counties From 2002 to 2012. American journal of public health. 2015;105:1120–1127. doi: 10.2105/AJPH.2014.302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich DE, Josselyn SA. Plasticity-related genes in brain development and amygdala-dependent learning. Genes, brain, and behavior. 2016;15:125–143. doi: 10.1111/gbb.12255. [DOI] [PubMed] [Google Scholar]
- Erickson CK. Ethanol clearance in nine inbred rat strains. Alcoholism, clinical and experimental research. 1984;8:491–494. doi: 10.1111/j.1530-0277.1984.tb05710.x. [DOI] [PubMed] [Google Scholar]
- Erol A, Karpyak VM. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug and alcohol dependence. 2015 doi: 10.1016/j.drugalcdep.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Fujioka A, Duman RS. Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:319–328. doi: 10.1523/JNEUROSCI.1065.03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesen ES, Deimel H, Bloch W. Clinical exercise interventions in alcohol use disorders: a systematic review. Journal of substance abuse treatment. 2015;52:1–9. doi: 10.1016/j.jsat.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Gillette-Guyonnet S, Vellas B. Caloric restriction and brain function. Current opinion in clinical nutrition and metabolic care. 2008;11:686–692. doi: 10.1097/MCO.0b013e328313968f. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Dao L, So V. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain research. 1997;764:1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain research. 2004;1016:154–162. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. Journal of microscopy. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. The Journal of pharmacology and experimental therapeutics. 2005;314:780–788. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker KT, Whishaw IQ. Place and matching-to-place spatial learning affected by rat inbreeding (Dark-Agouti, Fischer 344) and albinism (Wistar, Sprague-Dawley) but not domestication (wild rat vs. Long-Evans, Fischer-Norway) Behavioural brain research. 2002;134:467–477. doi: 10.1016/s0166-4328(02)00083-9. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmila M, Raitasalo K. Gender differences in drinking: why do they still exist? Addiction. 2005;100:1763–1769. doi: 10.1111/j.1360-0443.2005.01249.x. [DOI] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R. Evidence for a gender-related effect of alcoholism on brain volumes. The American journal of psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Humm JL, Kozlowski DA, James DC, Gotts JE, Schallert T. Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain research. 1998;783:286–292. doi: 10.1016/s0006-8993(97)01356-5. [DOI] [PubMed] [Google Scholar]
- Hunt WA. Are binge drinkers more at risk of developing brain damage? Alcohol. 1993;10:559–561. doi: 10.1016/0741-8329(93)90083-z. [DOI] [PubMed] [Google Scholar]
- Kelso ML, Liput DJ, Eaves DW, Nixon K. Upregulated vimentin suggests new areas of neurodegeneration in a model of an alcohol use disorder. Neuroscience. 2011;197:381–393. doi: 10.1016/j.neuroscience.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcoholism, clinical and experimental research. 1999;23:633–643. [PubMed] [Google Scholar]
- Kyrylkova K, Kyryachenko S, Leid M, Kioussi C. Detection of apoptosis by TUNEL assay. Methods Mol Biol. 2012;887:41–47. doi: 10.1007/978-1-61779-860-3_5. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156:456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Nixon K. Exercise neuroprotection in a rat model of binge alcohol consumption. Alcoholism, clinical and experimental research. 2010;34:404–414. doi: 10.1111/j.1530-0277.2009.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Martin M, Torres-Aleman I, Trejo JL. Growth factors as mediators of exercise actions on the brain. Neuromolecular medicine. 2008;10:99–107. doi: 10.1007/s12017-008-8026-1. [DOI] [PubMed] [Google Scholar]
- Loncarevic-Vasiljkovic N, Pesic V, Todorovic S, Popic J, Smiljanic K, Milanovic D, Ruzdijic S, Kanazir S. Caloric restriction suppresses microglial activation and prevents neuroapoptosis following cortical injury in rats. PloS one. 2012;7:e37215. doi: 10.1371/journal.pone.0037215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Mancinelli R, Binetti R, Ceccanti M. Woman, alcohol and environment: Emerging risks for health. Neuroscience and biobehavioral reviews. 2007;31:246–253. doi: 10.1016/j.neubiorev.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcoholism, clinical and experimental research. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A, Tronche F, Kellendonk C, Gau D, Kapfhammer J, Otto C, Schmid W, Schutz G. Disruption of CREB function in brain leads to neurodegeneration. Nature genetics. 2002;31:47–54. doi: 10.1038/ng882. [DOI] [PubMed] [Google Scholar]
- Maynard ME, Leasure JL. Exercise enhances hippocampal recovery following binge ethanol exposure. PloS one. 2013;8:e76644. doi: 10.1371/journal.pone.0076644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middaugh LD, Frackelton WF, Boggan WO, Onofrio A, Shepherd CL. Gender differences in the effects of ethanol on C57BL/6 mice. Alcohol. 1992;9:257–260. doi: 10.1016/0741-8329(92)90062-f. [DOI] [PubMed] [Google Scholar]
- Moonat S, Starkman BG, Sakharkar A, Pandey SC. Neuroscience of alcoholism: molecular and cellular mechanisms. Cellular and molecular life sciences: CMLS. 2010;67:73–88. doi: 10.1007/s00018-009-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010;20:596–607. doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munive V, Santi A, Torres-Aleman I. A Concerted Action Of Estradiol And Insulin Like Growth Factor I Underlies Sex Differences In Mood Regulation By Exercise. Sci Rep. 2016;6:25969. doi: 10.1038/srep25969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman RS. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:9868–9876. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–295. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. Journal of neurochemistry. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcoholism, clinical and experimental research. 2002a;26:547–557. [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacology, biochemistry, and behavior. 2002b;72:521–532. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. London: Elsevier Academic; 2009. [Google Scholar]
- Penland S, Hoplight B, Obernier J, Crews FT. Effects of nicotine on ethanol dependence and brain damage. Alcohol. 2001;24:45–54. doi: 10.1016/s0741-8329(01)00142-2. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcoholism, clinical and experimental research. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European journal of pharmacology. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rivier C. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcoholism, clinical and experimental research. 1993;17:854–859. doi: 10.1111/j.1530-0277.1993.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Experimental neurology. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain research. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schweinsburg BC, Alhassoon OM, Taylor MJ, Gonzalez R, Videen JS, Brown GG, Patterson TL, Grant I. Effects of alcoholism and gender on brain metabolism. The American journal of psychiatry. 2003;160:1180–1183. doi: 10.1176/appi.ajp.160.6.1180. [DOI] [PubMed] [Google Scholar]
- Seitz HK, Xu Y, Simanowski UA, Osswald B. Effect of age and gender on in vivo ethanol elimination, hepatic alcohol dehydrogenase activity, and NAD+ availability in F344 rats. Res Exp Med (Berl) 1992;192:205–212. doi: 10.1007/BF02576276. [DOI] [PubMed] [Google Scholar]
- Sharrett-Field L, Butler TR, Reynolds AR, Berry JN, Prendergast MA. Sex differences in neuroadaptation to alcohol and withdrawal neurotoxicity. Pflugers Arch. 2013;465:643–654. doi: 10.1007/s00424-013-1266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WB, Weisner C. Women and alcohol problems: a critical analysis of the literature and unanswered questions. Alcoholism, clinical and experimental research. 2000;24:1320–1321. [PubMed] [Google Scholar]
- Somkuwar SS, Fannon MJ, Staples MC, Zamora-Martinez ER, Navarro AI, Kim A, Quigley JA, Edwards S, Mandyam CD. Alcohol dependence-induced regulation of the proliferation and survival of adult brain progenitors is associated with altered BDNF-TrkB signaling. Brain Struct Funct. 2016;221:4319–4335. doi: 10.1007/s00429-015-1163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JR, Schroeder JP, Nixon K, Besheer J, Crews FT, Hodge CW. Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:1209–1222. doi: 10.1038/npp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoutenberg M, Rethorst CD, Lawson O, Read JP. Exercise training - A beneficial intervention in the treatment of alcohol use disorders? Drug and alcohol dependence. 2016;160:2–11. doi: 10.1016/j.drugalcdep.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcoholism, clinical and experimental research. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism, clinical and experimental research. 2000;24:611–621. [PubMed] [Google Scholar]
- Tateno M, Ukai W, Yamamoto M, Hashimoto E, Ikeda H, Saito T. The effect of ethanol on cell fate determination of neural stem cells. Alcoholism, clinical and experimental research. 2005;29:225s–229s. doi: 10.1097/01.alc.0000190658.56149.d4. [DOI] [PubMed] [Google Scholar]
- Tomsovic M. “Binge” and continuous drinkers. Characteristics and treatment follow-up. Quarterly journal of studies on alcohol. 1974;35:558–564. [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999a;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature neuroscience. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabilitation and neural repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res. 2004a;76:356–362. doi: 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. The European journal of neuroscience. 2004b;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain research. 2006;1070:124–130. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Walter H, Dvorak A, Gutierrez K, Zitterl W, Lesch OM. Gender differences: does alcohol affect females more than males? Neuropsychopharmacol Hung. 2005;7:78–82. [PubMed] [Google Scholar]
- Wilhelm CJ, Hashimoto JG, Roberts ML, Bloom SH, Andrew MR, Wiren KM. Astrocyte Dysfunction Induced by Alcohol in Females but Not Males. Brain Pathol. 2015a doi: 10.1111/bpa.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Hashimoto JG, Roberts ML, Bloom SH, Beard DK, Wiren KM. Females uniquely vulnerable to alcohol-induced neurotoxicity show altered glucocorticoid signaling. Brain research. 2015b;1601:102–116. doi: 10.1016/j.brainres.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsnack SC, Wilsnack RW, Kantor LW. Focus on: women and the costs of alcohol use. Alcohol Res. 2013;35:219–228. doi: 10.35946/arcr.v35.2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Crews F. CREB and NF-kappaB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cellular and molecular neurobiology. 2006;26:385–405. doi: 10.1007/s10571-006-9045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]