Abstract
Objective
Depressive symptoms are common among older adults with obesity and diabetes. Nonetheless, the mechanisms for this association are not clear, but may involve changes in the insulin cascade signaling. We aimed to investigate the association, and potential mediators, between obesity, insulin resistance, and depressive symptoms among older adults from a homogenous cohort of Mexican-Americans.
Methods
We included a total of 500 Mexican-American older adults assessed in the Cameron County Health Study. We evaluated depressive symptoms using the Center for Epidemiologic Survey Depression Scale (CES-D). Central obesity was definied by waist circumference. Insulin resistance was evaluated by the HOMA-IR index. We estimated the association between obesity, insulin resistance, and depressive symptoms by carrying out univariate and multivariate regression analyses.
Results
In unadjusted regression analysis, HOMA-IR (unstandardized β=0.31±0.12, p=0.007), waist circumference (unstandardized β=0.066±0.0.028, p=0.017), and Hb1Ac levels (unstandardized β=0.52±0.24, p=0.03) were significantly associated with CES-D scores. The association of HOMA-IR and CES-D remained statistically significant after controlling for socio-demographic and clinical variables in multivariate analysis (unstandardized β=0.28±0.11, p=0.01).
Conclusion
Our results suggest that depressive symptoms are associated with insulin resistance in older Mexican-American adults. In addition, poorer glucose control and obesity are important mediators of this relationship. Additional studies are needed to evaluate whether interventions that increase insulin sensitivity can also reduce depressive symptoms in this population.
Keywords: elderly, late-life depression, insulin resistance, Hispanic, population-based study
Introduction
Depressive symptoms are common in patients with cardio-metabolic disorders, including diabetes, obesity, and metabolic syndrome. Recent studies showed that the presence of diabetes significantly increases the risk of depressive symptoms by 15 to 50% in the general population, and specifically among older adults (Hasan et al., 2015; Mezuk et al., 2008; Valkanova et al., 2013). Obesity and the diagnosis of metabolic syndrome are also associated with 40 to 50% higher risk of depression compared to subjects without metabolic syndrome (Pan et al., 2012; Ruas et al., 2016, Olvera et al., 2015). The mechanisms by which cardio-metabolic disorders increase the risk of depression are not clear, but may involve intrinsic biological changes and psychological responses to the disease. Recent studies showed that abnormalities in pro-inflammatory markers and cerebrovascular disease are related to depression in these patients (Nousen et al., 2013). In addition, our group showed in a recent study that awareness of the diagnosis of a diabetes and the related psychological impact play a major role in the development of depressive symptoms among the Hispanic population (Olvera et al., 2016).
Insulin resistance is one of the key pathophysiological features of diabetes (Zaccardi 2016). In addition, obese individuals show increased insulin resistance (Lee 2014). Preclinical and clinical studies also suggest that insulin resistance and the activation of intracellular signaling cascades related to the insulin action are associated with depression in young and older adults (Moulton et al., 2015; Diniz et al., 2015; Diniz et al., 2016; Mendes-Silva et al., 2016). As insulin resistance can be directly evaluated using the HOMA-IR (Homeostatic model assessment – insulin resistance) index, past studies investigated whether insulin resistance is associated with major depression in the general population. Everson-Rose (2004) showed that depressive symptoms were associated with higher HOMA-IR values in middle-aged women. The results were independent of participants’ race, but were not statistically significant after adjustment for central adiposity. In another population-based study of older adult males, HOMA-IR was associated with higher prevalent depressive symptoms and predicted incident depressive symptoms independently of clinical and demographic variables (Ford et al., 2014). However, another study did not show a significant association between HOMA-IR and depressive symptoms (Lawlor, et al., 2005).
Mexican-Americans shows a high prevalence of cardio-metabolic disorders, as well as of depressive disorders (Fisher-Hoch et al., 2010; Olvera et al., 2015). However, there is few information about the association between obesity, insulin resistance and depressive symptoms among older Mexican-Americans. The aim of this study is to investigate the association, and potential mediators, between between obesity, insulin resistance and depressive symptoms from a homogenous cohort of Mexican-Americans.
Methods
Sample
This is a population-based study in which participants were recruited from randomly selected households and invited to participate in the Cameron County Hispanic Cohort (CCHC, Fisher-Hoch et al. 2010). Households were randomly selected based on the 2000 census tract data in the city of Brownsville, Texas, situated on the US–Mexico border. All selected households were visited, and all occupants over the age of 18 years invited to participate. A total of 3778 subjects were enrolled at the baseline assessment. The mean age of the cohort at baseline assessment was 43.9±16.6 years old. A total of 631 subjects were 60 years and older and 500 completed the Center for Epidemiologic Studies – Depression (CES-D) scale for the assessment of depressive symtpoms. We included only those subjects with complete CES-D in the current analysis. There was no significant differences in sociodemographic characteristics between the subjects who were included vs. excluded from the analysis.
This cohort is predominantly Mexican American (>98%). Extensive family, socioeconomic, educational, and personal medical histories, as well as physical activity and dietary histories, were obtained using a directed questionnaire. Surveys and data collection were conducted in the participants’ language of preference (Spanish or English) by bilingual research nurses and field workers.
Measures
All clinical measures were taken during the study interview by trained research assistants using standardized research interview and questionnaires. Socioeconomic status/demographic characteristics: Socioeconomic and demographic characteristics assessed included age (in years), gender, years of education, annual income, marital and retirement status.
Anthropometric characteristics: BMI was calculated based on weight and height measurements (kg/m2). Weight was measured without shoes to the nearest 0.2 kg using a portable electronic scale, and height was measured to the nearest 0.2 cm using a stadiometer. Waist circumference was measured with the participant in a standing position, at the level of the umbilicus to the nearest 0.2 cm.
Depressive symptoms: The Center for Epidemiological Studies Depression (CES-D) questionnaire is a 20-item self-report measure of depressive symptoms over the past week [Radloff et al., 1977]. Items are rated on a 4-point scale, and total scores range from 0 to 60. Greater scores suggest greater depressive symptoms. Clinical and laboratory variables: Data about past and current medical illness, drinking and smoking habits, list of current medication use, and the systolic and diastolic blood pressure.
Blood specimens were taken and aliquots immediately stored at −70 °C for a range of clinical and experimental assays. Blood glucose measurement was performed on site, HbA1c was measured by high-performance liquid chromatography and stored specimens were sent in batches to a Clinical Laboratory Improvement Amendments (CLIA)-approved clinical laboratory for clinical chemistries, including the measure of HDL-cholesterol, LDL-cholesterol and triglycerides levels. Insulin level was measured by ELISA.
We used the homeostasis model assessment equation to determine insulin resistance (HOMA-IR = glucose (mg/dL)/18 × insulin (mU/L)/22.5) (Matthews et al., 1985).
Statistical analysis
We carried out univariate and multivariate linear regression analyses to evaluate the association between depressive symptoms and HOMA-IR in Hispanic older adults. We carried out univariate regression analysis with CES-D scores as the outcome variable to select the predictor variables to be included in the final multivariate regression model to avoid the risk of model overfitting. The criterion for selection of the predictor variable was those with p-value below 0.1 in the univariate regression analysis. The final multivariate regression model included 3 blocks of predictor variables: sociodemographic variables, clinical variables, metabolic variables. We excluded the predictor variables insulin level and fasting glucose blood level from the multivariate model because the HOMA-IR is a function of these two variables, to avoid the risk of circularity and collinearity in the analysis. Likewise, since waist circumference and BMI showed a high collinearity, we included only waist circumference in the analyses since it is a direct measure of central adiposity.
Results
The sociodemographic and clinical characteristics of this sample are shown table 1. Among cardio-metabolic variables, HOMA-IR (unstandardized β=0.31±0.12, p=0.007), waist circumference (unstandardized β=0.066±0.0.028, p=0.017), and Hb1Ac levels (unstandardized β=0.52±0.24, p=0.03) were significantly associated with CES-D scores. CES-D scores were not statistically significant associated with tryglicerides, HDL-C and LDL-C levels, diastolic and systolic blood pressure (Table 2). The association of HOMA-IR and CES-D remained statistically significant after controlling for socio-demographic and clinical variables in multivariate analysis (unstandardized β=0.28±0.11, p=0.01). However, the association between waist circumference and CES-D scores was not statistically significant after controlling for socio-demographic and clinical variables (unstandardized β=0.05±0.03, p=0.08).
Table 1.
Descriptive analysis of the subjects included in this study.
| N | Mean ± SD | ||
|---|---|---|---|
| Gender | Male | 173 | |
| Female | 327 | ||
| Socioeconomic strata | Lower strata (<P25) | 133 | |
| Intermediate strata (P25 – P75) | 283 | ||
| Higher strata (>P75) | 84 | ||
| Marital status | Single / never married | 39 | |
| Married | 291 | ||
| Divorced / Separated | 58 | ||
| Widowed | 112 | ||
| Retired | Yes | 269 | |
| No | 227 | ||
| Years of education | 8.8 ± 6.0 | ||
| Heart attack | Yes | 26 | |
| No | 474 | ||
| Angina | Yes | 27 | |
| No | 473 | ||
| Stroke | Yes | 23 | |
| No | 477 | ||
| Drink | Never | 319 | |
| Sometimes | 120 | ||
| Often | 61 | ||
| Smoke | Yes | 164 | |
| No | 336 | ||
| Endarterectomy | Yes | 6 | |
| No | 494 | ||
| Body Mass Index | 31.0 ± 6.5 | ||
| CES-D scores | 19 ± 9 | ||
| Waist circumference (cm) | 105.3 ± 14.8 | ||
| Hb1Ac (%) | 6.34 ± 1.71 | ||
| Triglycerides | 163.4 ± 96.9 | ||
| HDL-cholesterol | 48.7 ± 12.9 | ||
| LDL-cholesterol | 105.4 ± 34.9 | ||
| Insulin level (pg/mL) | 13.1 ± 8.7 | ||
| Systolic blood pressure (mmHg) | 128.8 ± 19.0 | ||
| Diastolic blood pressure (mmHg) | 70.9 ± 10.8 | ||
| Fasting glucose level (mg/dL) | 120.2 ± 44.1 | ||
| HOMA-IR | 3.97 ± 3.63 | ||
Table 2.
Univariate analysis for the association between CES-D (outcome variable) and selected predictor variables.
| Outcome variable: CES-S scores | Unstandardized β | F(1,499) | p-value |
|---|---|---|---|
| HOMA-IR | 0.31±0.11 | 7.34 | 0.007 |
| Waist circumference (cm) | 0.31±0.121 | 5.75 | 0.017 |
| Hb1Ac | 0.07±0.0.03 | 4.67 | 0.03 |
| Triglycerides | 0.003±0.004 | 0.38 | 0.66 |
| HDL-cholesterol | −0.06±0.03 | 3.35 | 0.08 |
| LDL-cholesterol | −0.005±0.01 | 0.17 | 0.68 |
| Systolic blood pressure (mmHg) | −0.02±0.02 | 0.85 | 0.35 |
| Diastolic blood pressure (mmHg) | −0.07±0.04 | 3.37 | 0.07 |
To further explore the relationship between metabolic variables and CES-D, we carried out additional mediational analyses. We used the SPSS macro PROCESS v2.16 (available at http://processmacro.org/index.html) for the mediation analyses. The figure 1 shows the mediation pathways for each variable. In the mediation analysis, we included only metabolic variables that were significantly associated with CES-D scores in the univariate analysis (HOMA-IR, Hb1Ac levels, and waist circumference).
Figure 1. Mediation analysis models for the association between HOMA-IR and CES-D.
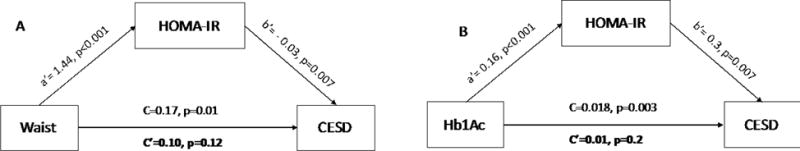
Model A: HOMA-IR level as a mediator of the association between waist circumference and CES-D scores. Model B: HOMA-IR level as a mediator of the association between glycated hemoglobin and CES-D scores.
As insulin resistance is a possible consequence of obesity and poor glycemic control in this population (Qu et al, 2011; Laing et al., 2015), HOMA-IR was considered the mediating variable between waist circumference, Hb1Ac levels (predictor variables), and CES-D scores (outcome variable). The relationship between waist circumference and glycated hemoglobin levels and depressive symptoms were fully mediated by HOMA-IR levels (Figure 1). To avoid the risk of reverse causality since HOMA-IR and the outcome variables (waist circumference and Hb1Ac) since they also may cause each other, we carried out additional mediation analysis by using waist circumference and Hb1Ac as the mediator variables and HOMA-IR as the predictor variable. In these analyses, the relationship between HOMA-IR and CES-D scores was not mediated by waist circumference or Hb1Ac levels (indirect effect c′ with p-value <0.05 in both analyses).
Discussion
To the best of our knowledge, this is the first study to investigate the association between insulin resistance, metabolic variables (i.e. obesity, cholesterol levels), and depressive symptoms in older Mexican-Americans. We found that among metabolic variables, insulin resistance, central adiposity and poor glycemic control are associated with depressive symtpoms in older Mexican-Americans. Finally, insulin resistance fully mediates the association between central adiposity, glycemic control with depressive symptoms. Our results highlights the significant association between metabolic abnormalities and depressive symptoms in older Mexican-Americans and suggest that insulin resistance plays a pivotal role in this association. Nonetheless, we cannot exclude in our analysis the possible psychological impact of the diagnosis of diabetes (Olvera et al., 2016), as well other biological factors that we did not evaluate in this current study, like inflammatory markers.
There are multiple mechanisms by which metabolic abnormalities are associated with depressive symptoms. First, metabolic abnormalities can lead to cerebrovascular disease and endothelial dysfunction which have been already linked to major depression in older adults (Taylor et al., 2013; Diniz et al., 2015; Diniz et al., 2016). Insulin resistance and metabolic abnormalities lead to the hyperactivation of GSK-3β that has been independently associated with major depression in older adults (Diniz et al., 2010), and is also a target for the action of antidepressants (Joaquim et al., 2011). Finally, mitochondrial dysfunction, increased oxidative stress, and epigenomic changes are common consequence of metabolic dysfunction and insulin resistance and they are associated with emergence of depressive symptoms in young and older adults. (Rieusset 2013). Therefore, by affecting different cellular and molecular functions, metabolic abnormalities, including insulin resistance and adverse lipid metabolism, can increase the risk of depressive symptoms in older adults.
Interventions that can improve insulin action can also lead, at least in part, to improvements in these conditions. Mild to moderate physical activity increases muscle insulin sensitivity and is a widely recommended intervention to improve glycemic control in patients with diabetes and to weight loss in obese individuals. Moreover, physical activity has also significant antidepressant effects, in particular in patients with mild depression (Rebar et al., 2015, Wu 2016). Metformin, a widely used glucose lowering agent that acts by increasing insulin sensitivity, has been shown to have mild antidepressant effect in diabetic patients (Guo et al., 2014). Therefore, interventions aiming to reduce weight, to improve insulin resistance and glycemic control can ameliorate depressive symptomatology and should be considered in all depressed subjects.
The current study reports data from a homogeneous older Mexican-American population from Texas, U.S. (Fisher-Hoch et al., 2007), and may or may not be generalizable for the general population. Moreover, there is little information about the mechanisms of depressive symptoms among older Mexican-Americans. Our results suggest that depressive symptoms are associated with central obesity and significant metabolic abnormalities in this population and help to explain the strong association of different metabolic disorders, including diabetes, and depressive disorders among them.
The current results should be also viewed in light of study’s limitations. Depressive symptoms were evaluated using self-reported depression scale (i.e. the CES-D) and there was no clinician-based interview to confirm the presence and history of depressive symptoms in this population. The CES-D is a well validated tool to identify depressive symptoms in the population; however, it does not follow strict DSM criteria. Furthermore, we could not establish chronicity, number of episodes of depression, and the psychological impact of the diagnosis of diabetes, factors that may have a significant moderator role in the metabolic factors evaluated in this study (Verhoeve et al., 2016; Revesz 2016, Olvera et al., 2016). We did not control for the effects of medications and the history of diabetes, and the insulin and fasting glucose levels. This analytical strategy was chosen to avoid circularity in the analyses since HOMA-IR values are defined by the insulin and glucose levels, and insulin resistance is a core feature of diabetes. Finally, this was a cross-sectional study and therefore cannot establish causality between insulin resistance and depressive symptoms. Longitudinal studies are necessary to evaluate whether insulin resistance and other metabolic abnormalities increase the risk of depressive symptoms and whether interventions that reduce insulin resistance have antidepressant effects.
In conclusion, our results suggest that depressive symptoms are associated with central obesity and poor glucose control and that insulin resistance may play a role in this process. Additional studies, preferably randomized clinical trials or well-designed longitudinal observational studies, should be done to evaluate whether interventions that increase insulin sensitivity can also reduce depressive symptoms in this population regardless of central obesity or metabolic conditions.
Acknowledgments
Financial support: This work was supported by NIH grant NCMHD P20MD000170
Footnotes
Conflict of interest: The authors have no conflict of interest to declare.
References
- Diniz BS, Talib LL, Joaquim HP, de Paula VR, Gattaz WF, Forlenza OV. Platelet GSK3B activity in patients with late-life depression: marker of depressive episode severity and cognitive impairment? World Journal of Biological Psychiatry. 2011;12:216–222. doi: 10.3109/15622975.2010.551408. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Teixeira AL, Campos AC, Miranda AS, Rocha NP, Talib LL, et al. Reduced serum levels of adiponectin in elderly patients with major depression. Journal of Psychiatric Research. 2012;46:1081–1085. doi: 10.1016/j.jpsychires.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Sibille E, Ding Y, Tseng G, Aizenstein HJ, Lotrich F, et al. Plasma biosignature and brain pathology related to persistent cognitive impairment in late-life depression. Molecular Psychiatry. 2015;20:594–601. doi: 10.1038/mp.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Lin CW, Sibille E, Tseng G, Lotrich F, Aizenstein HJ, et al. Circulating biosignatures of late-life depression (LLD): Towards a comprehensive, data-driven approach to understanding LLD pathophysiology. Journal of Psychiatric Research. 2016;82:1–7. doi: 10.1016/j.jpsychires.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Silva AP, Pereira KS, Tolentino-Araujo GT, Nicolau Ede S, Silva-Ferreira CM, Teixeira AL, et al. Shared Biologic Pathways Between Alzheimer Disease and Major Depression: A Systematic Review of MicroRNA Expression Studies. American Journal of Geriatric Psychiatry. 2016;24:903–912. doi: 10.1016/j.jagp.2016.07.017. [DOI] [PubMed] [Google Scholar]
- Fisher-Hoch SP, Rentfro AR, Salinas JJ, Perez A, Brown HS, Reininger BM, et al. Socioeconomic status and prevalence of obesity and diabetes in a Mexican American community, Cameron County, Texas, 2004–2007. Preventing Chronic Disease. 2010;7:A53. [PMC free article] [PubMed] [Google Scholar]
- Guo M, Mi J, Jiang QM, Xu JM, Tang YY, Tian G, et al. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clinical and Experimental Pharmacology and Physiology. 2014;41:650–656. doi: 10.1111/1440-1681.12265. [DOI] [PubMed] [Google Scholar]
- Hasan SS, Mamun AA, Clavarino AM, Kairuz T. Incidence and risk of depression associated with diabetes in adults: evidence from longitudinal studies. Community Mental Health Journal. 2015;51:204–210. doi: 10.1007/s10597-014-9744-5. [DOI] [PubMed] [Google Scholar]
- Joaquim HP, Talib LL, Forlenza OV, Diniz BS, Gattaz WF. Long-term sertraline treatment increases expression and decreases phosphorylation of glycogen synthase kinase-3B in platelets of patients with late-life major depression. Journal of Psychiatric Research. 2012;46:1053–1058. doi: 10.1016/j.jpsychires.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Laing ST, Smulevitz B, Vatcheva KP, Rahbar MH, Reininger B, McPherson DD, et al. Subclinical atherosclerosis and obesity phenotypes among Mexican Americans. Journal of the American Heart Association. 2015;4:e001540. doi: 10.1161/JAHA.114.001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochimica et Biophysica Acta. 2014;1842:446–462. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton CD, Pickup JC, Ismail K. The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol. 2015;3:461–71. doi: 10.1016/S2213-8587(15)00134-5. [DOI] [PubMed] [Google Scholar]
- Nousen EK, Franco JG, Sullivan EL. Unraveling the mechanisms responsible for the comorbidity between metabolic syndrome and mental health disorders. Neuroendocrinology. 2013;98:254–266. doi: 10.1159/000355632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvera RL, Fisher-Hoch SP, Williamson DE, Vatcheva KP, McCormick JB. Depression in Mexican Americans with diagnosed and undiagnosed diabetes. Psychological Medicine. 2016;46:637–646. doi: 10.1017/S0033291715002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvera RL, Williamson DE, Fisher-Hoch SP, Vatcheva KP, McCormick JB. Depression, obesity, and metabolic syndrome: prevalence and risks of comorbidity in a population-based representative sample of Mexican Americans. Journal of Clinical Psychiatry. 2015;76:e1300–5. doi: 10.4088/JCP.14m09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, et al. Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care. 2012;35:1171–1180. doi: 10.2337/dc11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients. 2013;5:2019–2027. doi: 10.3390/nu5062019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu HQ, Li Q, Rentfro AR, Fisher-Hoch SP, McCormick JB. The definition of insulin resistance using HOMA-IR for Americans of Mexican descent using machine learning. PLoS One. 2011;6:e21041. doi: 10.1371/journal.pone.0021041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measures. 1977;1:385. [Google Scholar]
- Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, Vandelanotte C. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychology Review. 2015;9:366–378. doi: 10.1080/17437199.2015.1022901. [DOI] [PubMed] [Google Scholar]
- Revesz D, Verhoeven JE, Milaneschi Y, Penninx BW. Depressive and anxiety disorders and short leukocyte telomere length: mediating effects of metabolic stress and lifestyle factors. Psychological Medicine. 2016;46:2337–2349. doi: 10.1017/s0033291716000891. [DOI] [PubMed] [Google Scholar]
- Rieusset J. Contribution of mitochondria and endoplasmic reticulum dysfunction in insulin resistance: Distinct or interrelated roles? Diabetes Metabolism. 2015;41:358–638. doi: 10.1016/j.diabet.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Ruas LG, Diniz BS, Firmo JO, Peixoto SV, Mambrini JV, Loyola-Filho AI, et al. Components of the metabolic syndrome and depressive symptoms in community-dwelling older people: the Bambui Cohort Aging Study. Revista Brasileira de Psiquiatria. 2016;38:183–189. doi: 10.1590/1516-4446-2015-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Molecular Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP. Vascular risk factors and depression in later life: a systematic review and meta-analysis. Biological Psychiatry. 2013;73:406–413. doi: 10.1016/j.biopsych.2012.10.028. [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, van Oppen P, Revesz D, Wolkowitz OM, Penninx BW. Depressive and Anxiety Disorders Showing Robust, but Non-Dynamic, 6-Year Longitudinal Association With Short Leukocyte Telomere Length. American Journal of Psychiatry. 2016;173:617–624. doi: 10.1176/appi.ajp.2015.15070887. [DOI] [PubMed] [Google Scholar]
- Wu S, Fisher-Hoch SP, Reininger B, McCormick JB. Recommended Levels of Physical Activity Are Associated with Reduced Risk of the Metabolic Syndrome in Mexican-Americans. PLoS One. 2016;11:e0152896. doi: 10.1371/journal.pone.0152896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgraduate Medical Journal. 2016;92:63–69. doi: 10.1136/postgradmedj-2015-133281. [DOI] [PubMed] [Google Scholar]
- Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]


