Abstract
Objective
Depressive and anxiety symptoms are common in older adults, significantly affect quality of life and are risk factors for Alzheimer’s Disease. We sought to identify the determinants of predominant trajectories of depressive and anxiety symptoms in cognitively normal older adults.
Method
423 older adults recruited from the general community underwent Aβ PET imaging, APOE and BDNF genotyping and cognitive testing at baseline and had follow-up assessments. All participants were cognitively normal and free of clinical depression at baseline. Latent growth mixture modeling (LGMM) was used to identify predominant trajectories of subthreshold depressive and anxiety symptoms over 6 years. Binary logistic regression analysis was used to identify baseline predictors of symptomatic depressive and anxiety trajectories.
Results
LGMM revealed two predominant trajectories of depressive and anxiety symptoms: a chronically elevated trajectory and a low, stable symptom trajectory, with almost 1 in 5 participants falling into the elevated trajectory groups. Male sex (relative risk ratio [RRR]=3.23), lower attentional function (RRR=1.90) and carriage of the BDNF Val66Met allele in women (RRR=2.70) were associated with increased risk for chronically elevated depressive symptom trajectory. Carriage of the APOE ε4 allele (RRR=1.92) and lower executive function in women (RRR=1.74) were associated with chronically elevated anxiety symptom trajectory.
Conclusion
Our results indicate distinct and sex-specific risk factors linked to depressive and anxiety trajectories, which may help inform risk stratification and management of these symptoms in older adults at risk for Alzheimer’s Disease.
Introduction
Subthreshold depressive and anxiety symptoms are defined as those that have a meaningful impact on quality of life and/or functioning but remain below the threshold necessary for formal diagnosis of a mood disorder1, 2. Subthreshold depressive and anxiety symptoms affect 15–25%3–5 and 24–43%2, 3, 5 of older adults in the general community, respectively. These symptoms can be chronic in nature6, 7, and are associated with reduced functioning and quality of life,3, 7, 8 as well as increased risk for Alzheimer’s disease (AD)9, 10,11. Thus, such symptoms are important to identify and treat, and could be instrumental in ascertaining early manifestations of age-related neurodegenerative disease.
Support for the relationship between depressive and anxiety symptoms and AD comes from studies showing that these symptoms are associated with increased amyloid load12, 13, 14, carriage of the apolipoprotein epsilon 4 (APOE ε4) allele15–17 and the val66Met polymorphism of the brain derived neurotrophic factor (BDNF) gene18, as well as with higher salivary cortisol levels 19, 20 and faster cognitive decline21–23. However, to date, no study has sought to understand how markers of the AD prodrome, such as demographic factors (e.g., sex, education), amyloid burden, genetic risk factors (e.g., APOE ε4) and cognition, may relate to the longitudinal trajectory of depressive and anxiety symptoms.
Aims of the study
To address these gaps in understanding, the first aim of the current study was to identify the nature and magnitude of changes in depressive and anxiety symptoms over six years in a large cohort of cognitively normal older adults. The second aim was to evaluate AD-related biological risk factors such as amyloid level, demographic risk factors such as female sex and low education level, genetic factors such as carriage of an APOE ε4 or BDNFval66met allele24, and cognitive factors such as reduced executive function and attention25–29, as possible determinants of symptomatic trajectories of depressive and anxiety symptoms.
Method
Participants
Participants were 423 cognitively normal older adults who had undergone Aβ PET imaging and genotyping as part of the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging 30 and completed clinical assessments over 6 years. Table 1 shows demographic and clinical characteristics of the sample. Exclusion criteria at entry were: a diagnosis of schizophrenia, presence of clinical depression as assessed by ≥6 on the Geriatric Depression Scale [GDS-15], a diagnosis of Parkinson’s disease, previous head injury with >1 hour of posttraumatic amnesia, cancer within the last 2 years, stroke, diabetes, obstructive sleep apnea where disclosed, and heavy regular alcohol intake. Medical, psychiatric and neuropsychological data were used to confirm the cognitive health and clinical classification of each participant. The institutional research and ethics committees of Austin Health, St Vincent’s Health, Hollywood Private Hospital and Edith Cowan University approved the study. All participants gave written informed consent.
Table 1.
Demographic and clinical characteristics of the sample (n=423)
| Mean (SD) or n (%) | Range | |
|---|---|---|
| Age | 69.4 (6.6) | 60–89 |
| Sex | ||
| Male | 192 (45.4%) | - |
| Female | 231 (54.6%) | - |
| Education | ||
| <15 yrs | 264 (62.4%) | - |
| >15 yrs | 157 (37.1%) | - |
| HADS depression score | 2.6 (2.3) | 0–14 |
| HADS anxiety score | 4.3 (2.9) | 0–15 |
| Aβ+ | 97 (22.9%) | - |
| APOE ε4 carrier | 115 (27.2%) | - |
| BDNFMet carrier | 156 (36.9%) | |
| Cortisol (ng/mL) | 143.9 (62.7) | 19.6–522.7 |
| Executive function composite score | 0.01 (0.8) | −2.16–2.63 |
| Attention composite score | 0.02 (0.8) | −1.67–2.41 |
Note. HADS, Hospital Anxiety and Depression Scale; Aβ+, PET scans with above threshold levels of β-amyloid; APOE ε4 carrier, individuals with 1 or 2 copies of the apoliprotein epsilon 4 allele; BDNFMet carrier, brain-derived neurotrophic factor val66Met allele carrier; SD, standard deviation
PET imaging and genotyping
Aβ imaging with PET was conducted using [11C]PiB (PiB), [18F]florbetapir (FBP) or [18F]flutemetamol (FLUTE). Given different pharmacokinetic characteristics, a different acquisition protocol was adopted for each tracer. Thirty minute acquisitions were started 40 minutes after injection of PiB, and 20-minute acquisitions were performed 50 minutes after injection of FBP and 90 minutes after injection of FLUTE. The outcome measure was standardized uptake value ratio (SUVR). Cerebellar cortex, whole cerebellum and pons were used as reference regions for PiB, FBP or FLUTE, respectively. The SUVR was classified dichotomously as low or high (Aβ− or Aβ+), with thresholds set at 1.5, 1.10 or 0.62 for PiB, FBP or FLUTE, respectively 30. Blood samples were collected for APOE and BDNF genotyping, as previously described31. Morning fasted plasma cortisol levels were analyzed using a cortisol enzyme-linked immunosorbent assay (IBL International GmbH, Hamburg, Germany). PET imaging, genotyping and measurement of cortisol was performed at baseline.
Anxiety and depressive symptoms, and neuropsychological assessment
Depressive and anxiety symptoms were assessed at baseline, and at 1.5-, 3-, 4.5-, and 6-year follow-up assessments using the Hospital Anxiety and Depression Scale (HADS)32. Cut-off scores of ≥8 on the depression and anxiety subscales were used as indicative of clinically significant depression and anxiety symptoms, as recommended by the developers of the HADS32 and consistent with a review concluding that 8 is an optimal cut-off score for the HADS subscales33. Comprehensive neuropsychological assessment was also conducted at these time points, with composite scores of cognitive functioning derived from standardized scores in relation to a large normative database30. Composite scores were computed for tests of executive function (Cogstate One-Back, Letter Fluency, Category Fluency Switching [Fruit/Furniture]); episodic memory (Logical Memory delayed recall, California Verbal Learning Test, Rey Complex Figure Test [RCFT] 3-min delayed recall, RCFT 30-min delayed recall, Cogstate One-Card Learning); language (Category Fluency [Animals/Boys’ Names], Boston Naming Test); and attention (Digit Symbol, Cogstate Detection, Cogstate Identification). The calculation and validation of these composite scores have been described previously30.
Data analysis
To identify predominant trajectories of depressive and anxiety symptoms, latent growth mixture modeling (LGMM) was applied to HADS-assessed depressive and anxiety symptoms over the 6-year study period using robust full-information maximum likelihood estimation in Mplus, version 7.11. To determine the best fitting trajectories, 1- to 5-class unconditional LGMMs were compared for relative fit using conventional fit indices34. Best fitting models were identified based on smaller Bayesian Information Criterion and Akaike Information Criterion values, higher entropy values, and from results of the Lo-Mendell-Rubin likelihood test and bootstrap likelihood ratio test, which quantify the likelihood that the data can be described by a model with one less trajectory. Class sizes, parsimony, and theoretical interpretability were also considered when identifying optimal solutions. To enhance generalizability, the final model selected contained a meaningful proportion of the sample (>10%) in the smallest class. Each participant was assigned the class having the greatest posterior probability. Bivariate analyses were then used to evaluate relationship between each predictor variable and symptomatic trajectories of depressive and anxiety symptoms. Variables associated significantly (p<0.05) with symptomatic trajectories were then entered into binary logistic regression analyses using Forward Wald estimation to identify independent baseline predictors of symptomatic depressive and anxiety trajectories. Moderating effects of sex24 were evaluated by incorporating interaction terms (e.g. Aβ × sex) into these models.
Results
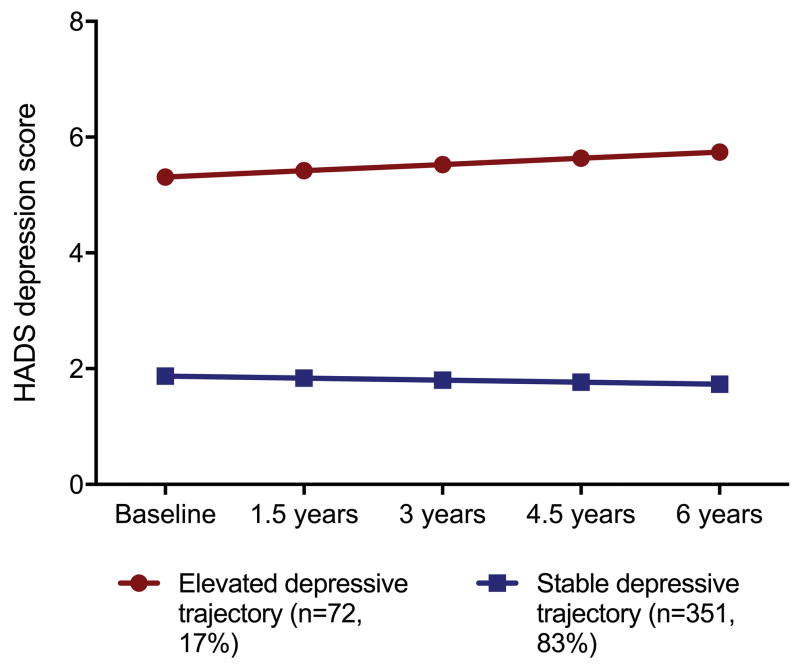
As shown in Table 2, a 2-class solution was determined to be the optimal model for both depression and anxiety symptoms, fitting the data better than the 1- 3- and 4- and 5-class solutions. For depressive symptoms, the 2-class model was characterized by a low/stable trajectory group, in which scores on the depressive subscale of the HADS were low at baseline and followed a stable trajectory over time (n=346, 81.8%, intercept=1.9 (standard error[SE]=0.1, slope= −0.002 [SE=0.002]) and an elevated depressive symptom trajectory group, characterized by persistently elevated depressive symptoms across the study period (n=77, 18.2%, intercept=5.3 (SE=0.5), slope=0.006 [SE=0.007]) (Figure 1). In this group, 16.7% (n=9), 9.3% (n=5), 22.2% (n=12), 20.4% (n=11), and 24.1% (n=13) screened positive for clinically significant depressive symptoms (score ≥ 8) at the baseline, and 1.5-, 3-, 4.5-, and 6-year assessments, respectively.
Table 2.
Fit indices for 1- to 5-class unconditional latent growth mixture models of depressive and anxiety symptoms over the 6-year study period
| Depressive symptoms | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Class | Log-likelihood | AIC | BIC | SSABIC | Entropy | LMR adjusted LRT p | % in smallest class |
| 1 | −3611.39 | 7242.77 | 7283.25 | 7251.51 | 100 | ||
| 2 | −3567.91 | 7161.82 | 7214.44 | 7173.18 | 0.83 | 0.0042 | 18.9 |
| 3 | −3554.23 | 7140.47 | 7205.23 | 7154.45 | 0.88 | 0.0656 | 0.4 |
| 4 | −3531.35 | 7100.71 | 7177.61 | 7117.31 | 0.874 | 0.0863 | 0.5 |
| 5 | −3523.16 | 7090.33 | 7179.37 | 7109.56 | 0.879 | 0.1974 | 0.5 |
|
| |||||||
| Anxiety symptoms | |||||||
|
| |||||||
| Class | Log-likelihood | AIC | BIC | SSABIC | Entropy | LMR adjusted LRT p | % in smallest class |
|
| |||||||
| 1 | −4046.79 | 8113.59 | 8154.06 | 8122.33 | 100 | ||
| 2 | −4028.65 | 8083.3 | 8135.92 | 8094.67 | 0.688 | 0.0127 | 17 |
| 3 | −4021.32 | 8074.64 | 8139.4 | 8088.62 | 0.731 | 0.57 | 3.4 |
| 4 | −4017.15 | 8072.29 | 8149.19 | 8088.9 | 0.786 | 0.19 | 0.7 |
| 5 | Failure of model convergence | ||||||
Note. AIC=Akaike Information Criterion; BIC=Bayesian Information Criterion; SSABIC=Sample size-adjusted Bayesian Information Criterion; LMR LRT=Lo-Mendell-Rubin likelihood ratio test.
Figure 1.
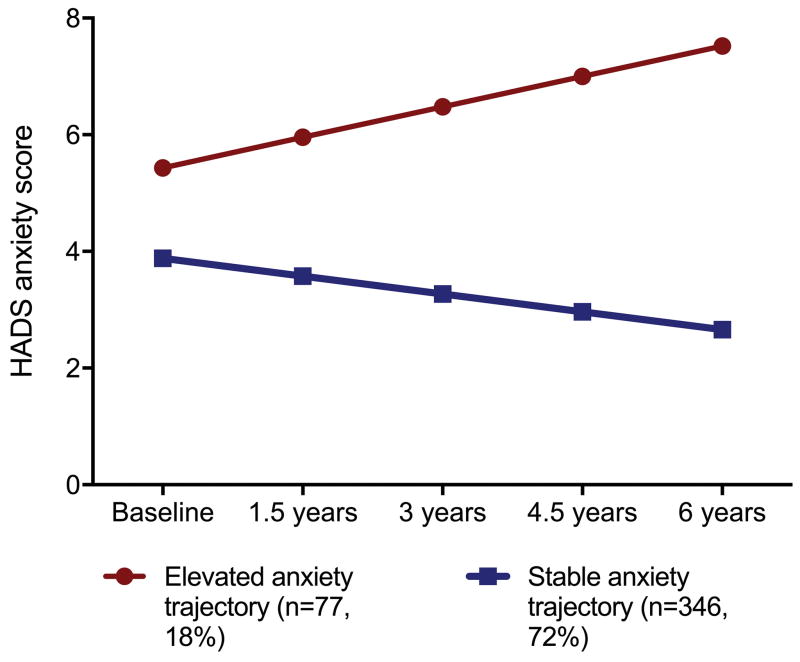
For anxiety symptoms, the 2-class model was characterized by a stable trajectory group, characterized by lower than average anxiety HADS scores at baseline and stable trajectory of scores over time (n=351, 83.0%, intercept=3.9 (SE=0.2, slope= −0.02 [SE=0.002]) and an elevated anxiety symptom trajectory group, characterized by higher anxiety HADS scores at baseline and a slight increase in scores over time (n=72, 17.0%, intercept=5.4 (SE=0.4), slope=0.029 [SE=0.007]) (Figure 2). In this group, 33.3% (n=24), 30.6% (n=22), 31.9% (n=23), 41.7% (n=30), and 31.9% (n=23) screened positive for clinically significant anxiety symptoms at the baseline, and 1.5-, 3-, 4.5-, and 6-year assessments, respectively
Figure 2.
Examination of overlap of the 2-class anxiety and depressive symptom trajectory solutions revealed that, of the elevated anxiety trajectory group, only 27 (37.5%) were in the elevated depression trajectory group. Given this low overlap of elevated depressive and anxiety trajectories, determinants of symptomatic trajectories were examined separately.
Table 3 shows results of bivariate analyses that examined how baseline demographic, biological, and cognitive variables were associated with depressive and anxiety symptom trajectories. Compared with the stable/low depressive symptom trajectory group, the elevated depressive symptom trajectory group was older, more likely to be male, and scored lower on baseline measures of executive function and attention; and for women, more likely to carry the BDNF Met allele. Compared with the stable/low anxiety symptom trajectory, the elevated anxiety symptom trajectory group was more likely to be comprised of APOE ε4 allele carriers (a finding driven by men) and, for women, lower executive function scores were associated with an elevated anxiety symptom trajectory.
Table 3.
Results of bivariate analyses evaluating baseline correlates of predominant depression and anxiety trajectories over the 6-year study period
| Stable depressive trajectory | Increasing depressive trajectory | F or χ2, p | Stable anxiety trajectory | Increasing anxiety trajectory | F or χ2, p | |
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SE) or n (%) | Mean (SE) or n (%) | Mean (SE) or n (%) | ||||
| Age | 69.0 (0.34) | 71.8 (0.95) | 8.53, 0.004 | 69.5 (0.4) | 68.9 (0.8) | 0.47, 0.494 |
| Male sex | 160 (43.4%) | 32 (59.3%) | 4.80, 0.028 | 163 (46.4%) | 29 (40.3%) | 0.92, 0.339 |
| Education < 15 yrs | 116 (41.6%) | 19 (47.5%) | 0.50, 0.478 | 153 (57.3%) | 31 (59.6%) | 0.10, 0.440 |
| APOE ε4 carrier (total) | 102 (27.6%) | 13 (24.1%) | 0.30, 0.582 | 87 (24.8%) | 28 (38.9%) | 6.00, 0.014 |
| APOE ε4 male | 39 (24.4%) | 10 (31.3%) | 0.66, 0.272 | 34 (20.9%) | 15 (51.7%) | 12.34, 0.001 |
| APOE ε4 female | 63 (30.1%) | 3 (13.6%) | 2.66, 0.103 | 53 (28.2%) | 13 (20.2%) | 0.07, 0.789 |
| BDNFMet carrier (total) | 132 (38.5%) | 30 (55.6%) | 0.70, 0.405 | 125 (38.5%) | 31 (43.1%) | 0.52, 0.470 |
| BDNFMet male | 60 (40.3%) | 10 (31.3%) | 0.90, 0.342 | 56 (36.8%) | 14 (48.3%) | 1.34, 0.247 |
| BDNFMet female | 72 (37.1%) | 14 (63.6%) | 5.80, 0.016 | 69 (39.9%) | 17 (39.5%) | 0.00, 0.967 |
| Aβ+ (total) | 85 (23.0%) | 12 (22.2%) | 0.02, 0.894 | 80 (22.8%) | 17 (23.6%) | 0.02, 0.880 |
| Aβ+ male | 37 (23.1%) | 8 (25.0%) | 0.05, 0.819 | 39 (23.9%) | 6 (20.7%) | 0.14, 0.705 |
| Aβ+ female | 48 (23.0%) | 4 (18.2%) | 0.26, 0.609 | 41 (21.8%) | 11 (25.6%) | 0.29, 0.593 |
| Cortisol (total) | 144.1 (3.3) | 142.4 (8.3) | 0.04, 0.849 | 145.4 (3.4) | 136.2 (6.7) | 1.28, 0.258 |
| Cortisol male | 139.4 (5.1) | 139.8 (9.1) | 0.00, 0.974 | 141.2 (5.0) | 130.0 (9.9) | 0.78, 0.378 |
| Cortisol female | 147.6 (4.3) | 146.1 (15.8) | 0.10, 0.919 | 149.1 (4.7) | 140.3 (8.9) | 0.68, 0.411 |
| Executive function (total) | 0.05 (0.04) | −0.25 (0.09) | 6.40, 0.012 | 0.04 (0.04) | −0.14 (0.08) | 2.78, 0.096 |
| Executive function male | −0.07 (0.06) | −0.34 (0.13) | 3.48, 0.064 | −0.13 (0.06) | −0.06 (0.11) | 0.19, 0.664 |
| Executive function female | 0.14 (0.06) | −0.12 (0.14) | 1.92, 0.167 | 0.18 (0.06) | −0.18 (0.11) | 6.66, 0.011 |
| Attention (total) | 0.06 (0.04) | −0.27 (0.10) | 8.30, 0.004 | 0.05 (0.04) | −0.14 (0.08) | 3.28, 0.071 |
| Attention male | 0.12 (0.06) | −0.29 (0.14) | 7.56, 0.007 | 0.08 (0.06) | −0.04 (0.12) | 0.594, 0.442 |
| Attention female | 0.007 (0.5) | −0.24 (0.15) | 2.05, 0.153 | 0.02 (0.06) | −0.20 (0.10) | 2.77, 0.097 |
Note. Bolded F and p values indicate statistically significant differences between trajectory groups (p<0.05)
Binary logistic regression analysis predicting an elevated depressive symptom trajectory revealed a main effect of sex, with men more likely to be in the elevated depressive symptom trajectory group than women (Wald χ2=7.48, p=0.005; relative risk ratio [RRR]=3.23, 95% confidence interval (CI)=1.41–7.35). Lower attention composite scores were also associated with greater likelihood of being in the elevated depressive symptom trajectory group (Wald χ2=9.22, p=0.002; RRR=1.90, 95%CI=1.25–2.87). Further, there was a significant BDNF × sex interaction, with female Met allele carriers being more likely than male Met allele carriers to be in the elevated depressive symptom trajectory group (Wald χ2=4.41, p=0.036; RRR=2.70, 95%CI=1.07–6.82). Specifically, of female Met carriers, 63.6% (n=14/22) were in the elevated depressive symptom trajectory group compared to 37.1% (n=72/194) in the stable/low trajectory group. Main and interactive effects of Aβ, cortisol, APOE, education level and executive function were unrelated to the elevated depressive symptom trajectory (all Wald χ2<1.41, all p’s>0.235)
Binary logistic regression analysis predicting an elevated anxiety symptom trajectory revealed a main effect of APOE ε4 allele carriage in predicting this trajectory (Wald χ2=5.41, p=0.020; RRR=1.92, 95%CI=1.11–3.33). Of ε4 carriers, 38.9% (n=28/72) were in the elevated anxiety symptom trajectory group vs. 24.8% (n=87/351) in the stable/low anxiety trajectory group. There was also a significant interaction between executive function and sex (Wald χ2=5.98, p=0.014; RRR=1.74, 95%CI=1.12–2.71), with lower executive function scores in women, but not men, being linked to the elevated anxiety symptom trajectory. Main and interactive effects of Aβ, cortisol, BDNF, education level, and attention scores were unrelated to an elevated anxiety symptom trajectory (all Wald χ2<1.55, all p’s>0.213).
Discussion
This study is among the first to evaluate the nature and determinants of predominant longitudinal trajectories of depressive and anxiety symptoms in older adults. Results revealed that a significant proportion of older adults have a trajectory of elevated depressive (18%) and anxiety (17%) symptoms, which have distinct and sex-specific risk factors. Specifically, male sex, lower attentional function, and carriage of the BDNF Val66Met allele in women were linked to an elevated depressive symptom trajectory; while carriage of the APOE ε4 allele and lower executive function in women were linked to an elevated anxiety symptom trajectory. Of note, APOE status was not disclosed, so knowledge of increased AD risk was not driving symptoms.
In a previous cross-sectional study, we found that female BDNF Val66 Met carriers reported greater severity of depressive symptoms24. The current data show that this increase remains over time. BDNF is important for neuronal proliferation and survival, and regulation of synaptic plasticity35. Lower CNS BDNF has been proposed to be involved in the pathophysiology of depression due to a resultant reduction in neurogenic cell survival and decline in hippocampal function36. The BDNF Val66Met variant is associated with lower levels of BDNF37, and there is some evidence for an association between the BDNF val66Met allele and vulnerability for depression38. For example, one study found a significantly greater proportion of Met allele carriers in depressed elderly individuals compared to controls39, supporting a role for the BDNF Met allele in depressive symptoms in older adults. Our finding that this polymorphism was linked to risk for an elevated depressive symptom trajectory in women but not men is consistent with preclinical data indicating that female but not male BDNF knockout mice display a striking increase in depressive-like behavior,40 as well as human data that women with the BDNF Val66Met genotype are particularly vulnerable to social stress mediated by HPA axis activity41. This sex-specific finding also aligns with evidence that many genes act differentially in males and females42, possibly due to hormonal influences. Indeed, estrogen has a regulatory effect on BDNF expression43 and may thus underlie sex-specific associations between the BDNF Val66Met allele and an elevated depressive symptom trajectory.
Lower attentional function at baseline was also associated with a chronically elevated trajectory of depressive symptoms. This finding is consistent with research showing that lower attentional function is associated with higher levels of anxiety and depression symptoms 44. For example, a large prospective study in the Netherlands found that lower attentional function in older adults at their baseline assessment was associated with an accelerated increase in depressive symptoms45. It is thought that individuals with lower attentional function may have greater difficulties with emotion regulation46. Indeed, mounting evidence from neuroimaging studies suggests that diminished top-down control from prefrontal brain regions crucial for attentional function is associated with reduced modulation of brain regions implicated in emotion, such as the amygdala47, 48. It is also possible that older adults with depressive symptoms have limited processing efficiency due to ruminative thinking, which can preoccupy attentional resources49. Taken together, these findings suggest that interventions designed to improve attentional function, for example through the use of ‘cognitive-enhancing’ drugs such as modafinil50 and cognitive rehabilitation51, may help mitigate risk for chronic depressive symptoms in cognitively normal older men and women.
APOE ε4 allele carriage was associated with a chronic trajectory of anxiety symptoms, independently of Aβ load. In our previous cross-sectional study, we found that APOE ε4 allele carriage was associated with greater severity of anxiety symptoms assessed at baseline, both independently and in conjunction with Aβ load. Here, we did not observe an interaction between Aβ and APOE ε4 allele carriage in predicting a chronically elevated anxiety symptom trajectory, but did observe an independent association between APOE ε4 and this outcome. A link between APOE ε4 carriage and anxiety has been demonstrated previously53 and there is evidence that probable AD patients who are APOE ε4 allele carriers report greater severity of anxiety symptoms than non-ε4 carriers52. APOE ε4 allele carriage is also associated with cognitive decline and increased susceptibility to AD53. Taking this together with mounting evidence indicating that anxiety can increase risk for cognitive decline28, 54, 55 and dementia10, 56, it is plausible that APOE ε4 allele carriage is associated with manifestation of anxiety symptoms in the first instance, thus representing an upstream risk factor for AD. Given that APOE ε4 allele carriage is linked to an increase in the rate and extent of amyloid deposition in the brain57, increased amyloid load is likely to be involved in—and possibly mediates—the association between APOE ε4 allele carriage and the development and maintenance of anxiety symptoms. While our cross-sectional findings24 revealed that an interaction between Aβ and APOE ε4 was strongly associated with severity of anxiety symptoms in older women, this interaction was unrelated to a chronically elevated anxiety symptom trajectory. One explanation for this is that in the current study, we examined determinants of predominant trajectories of anxiety and depressive symptoms, not variance in overall symptom levels. Consequently, a reduction in statistical power (i.e., 18% of the sample showing a chronically elevated anxiety symptom trajectory) may, at least in part, account for this discrepancy between the cross-sectional and longitudinal findings.
Reduced executive function was associated with a chronically elevated anxiety symptom trajectory in women. This finding is consistent with research showing that reduced executive control over subcortical threat-related processing may be linked to anxiety58, 59,60. For example, fMRI studies have shown that increased anxiety levels are associated with both increased amygdala response to threat distractors and with reduced activation of the PFC in response to threat-processing58 and symptom provocation61. The finding that reduced executive function was linked to a chronically elevated anxiety symptom trajectory in women but not men may be attributable, at least in part, to ruminative thinking, which is more common in women than men62. Rumination plays a major role in anxiety-related thinking, and is thought to deplete executive resources that are necessary for cognitive regulation of emotion and behavior. Specifically, as ruminative thinking is verbal in nature, it is thought to preoccupy prefrontal circuits underlying executive function, and to divert attentional resources to threat-related information, which may in turn perpetuate symptoms of anxiety28, 63. Women with lower executive function might therefore be at a greater risk for experiencing higher and increasing levels of anxiety over time. Interventions aimed at reducing ruminative thinking and improving executive functioning, for example through cognitive behavioral therapy64 or ‘brain training’65, may minimize or prevent anxiety symptoms, which may in turn reduce the risk for cognitive decline and AD in older adults.
Methodological limitations of this study must be noted. First, the AIBL sample included a larger number of APOE ε4 carriers than is observed in the general population, as well as high levels of social class and education, and thus the results may not be generalizable to population-based samples. Second, although all of the older adults studied were cognitively normal at baseline, the sample included older adults who went on to develop MCI and AD. Additional research is needed to examine the nature and predictors of depressive and anxiety symptoms in those who went on to develop MCI or AD specifically. Third, given that the majority of older adults in our sample had subthreshold depressive and anxiety symptoms, more subtle pathological changes in these symptoms that are linked to Aβ and cortisol may not have been detectable. Furthermore, the generalisability of results is limited to older adults who did not meet screening criteria for depression on the GDS at baseline, and further research is needed to examine the nature and determinants of depressive and anxiety trajectories in more psychiatrically heterogeneous samples of cognitively normal older adults. Fourth, it is possible that older adults with high levels of both depressive and anxiety symptoms are associated with distinct risk factors. A lack of statistical power prevented examination of this group separately, but it would be important for future research to examine the trajectories and factors associated with comorbid depressive and anxiety in older adults. Finally, other biological and psychological risk factors for depressive and anxiety symptoms, which were not assessed in the AIBL study (e.g. inflammatory markers, childhood adversity and trauma history), may also contribute to and moderate the assessed variables in predicting symptomatic trajectories of depressive and anxiety symptoms in older adults.
Notwithstanding these limitations, to our knowledge, this study is among the first to evaluate biological, demographic and cognitive factors associated with longitudinal trajectories of depressive and anxiety symptoms in older adults. Given that depressive and anxiety symptoms may be an early manifestation of neurodegenerative disease12, 13, 66, results of the current study may help to inform prevention and treatment efforts aimed at mitigating risk for cognitive decline and AD in at-risk older adults. Further research is needed to replicate these results; evaluate additional biopsychosocial factors that may additionally inform prediction of chronic depressive and anxiety symptom trajectories; and examine whether personalized therapies that target specific risk factors for chronic depressive and anxiety symptoms may help reduce these symptoms, preserve cognitive function, and mitigate risk for AD.
Acknowledgments
Alzheimer’s Australia (Victoria and Western Australia) assisted with promotion of the study and the screening of telephone calls from volunteers. We thank all the participants who underwent assessments and the staff who collected the data. Funding for the study was provided in part by the study partners (Commonwealth Scientific Industrial and Research Organization (CSIRO), Edith Cowan University (ECU), Mental Health Research Institute (MHRI), National Ageing Research Institute (NARI), Austin Health, CogState Ltd]. The study received support from the National Health and Medical Research Council (NHMRC) Australia via a project grant (APP1009292) awarded to SML and RNM, as well as funding from the Science and Industry Endowment Fund (SIEF) and the Cooperative Research Centre for Mental Health (CRCMH), an Australian Government Initiative. Drs. Holmes and Esterlis are supported by National Institutes of Health grants 5R01MH104459 and 1K01MH092681. Dr. Pietrzak is supported in part by the U.S. Department of Veterans Affairs National Center for Posttraumatic Stress Disorder.
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
References
- 1.Rowe SK, Rapaport MH. Classification and treatment of sub-threshold depression. Current opinion in psychiatry. 2006;19:9–13. doi: 10.1097/01.yco.0000194148.26766.ba. [DOI] [PubMed] [Google Scholar]
- 2.Grenier S, Préville M, Boyer R, O’Connor K, Béland S-G, Potvin O, et al. The impact of DSM-IV symptom and clinical significance criteria on the prevalence estimates of subthreshold and threshold anxiety in the older adult population. The American Journal of Geriatric Psychiatry. 2011;19:316–326. doi: 10.1097/JGP.0b013e3181ff416c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braam AW, Copeland JR, Delespaul PA, Beekman AT, Como A, Dewey M, et al. Depression, subthreshold depression and comorbid anxiety symptoms in older Europeans: results from the EURODEP concerted action. Journal of affective disorders. 2014;155:266–272. doi: 10.1016/j.jad.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Schoevers RA, Smit F, Deeg DJ, Cuijpers P, Dekker J, van Tilburg W, et al. Prevention of late-life depression in primary care: do we know where to begin? American Journal of Psychiatry. 2006;163:1611–1621. doi: 10.1176/ajp.2006.163.9.1611. [DOI] [PubMed] [Google Scholar]
- 5.Kvaal K, McDougall FA, Brayne C, Matthews FE, Dewey ME. Co-occurrence of anxiety and depressive disorders in a community sample of older people: results from the MRC CFAS (Medical Research Council Cognitive Function and Ageing Study) International journal of geriatric psychiatry. 2008;23:229–237. doi: 10.1002/gps.1867. [DOI] [PubMed] [Google Scholar]
- 6.Beekman AT, Geerlings SW, Deeg DJ, Smit JH, Schoevers RS, de Beurs E, et al. The natural history of late-life depression: a 6-year prospective study in the community. Archives of general psychiatry. 2002;59:605–611. doi: 10.1001/archpsyc.59.7.605. [DOI] [PubMed] [Google Scholar]
- 7.Meeks T, Vahia I, Lavretsky H, Kulkarni G, Jeste D. A Tune in “A Minor” Can “B Major”: A Review of Epidemiology, Illness Course, and Public Health Implications of Subthreshold Depression in Older Adults. Journal of affective disorders. 2011;129:126–142. doi: 10.1016/j.jad.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilms HU, Kanowski S, Baltes MM. Limitations in activities of daily living: towards a better understanding of subthreshold mental disorders in old age. Comprehensive psychiatry. 2000;41:19–25. doi: 10.1016/s0010-440x(00)80004-8. [DOI] [PubMed] [Google Scholar]
- 9.Byers AL, Yaffe K. Depression and Risk of Developing Dementia. Nature Reviews Neurology. 2011;7:323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer K, Berger AK, Monastero R, Winblad B, Backman L, Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68:1596–1602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- 11.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Archives of general psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marano C, Workman C, Lyman C, Narrow T, Zhou Y, Munro C, et al. Cortical Beta-Amyloid Deposition in Late-Life Depression. The American Journal of Geriatric Psychiatry. 21:S127. [Google Scholar]
- 13.Lavretsky H, Siddarth P, Kepe V, Ercoli LM, Miller KJ, Burggren AC, et al. Depression and Anxiety Symptoms Are Associated with Cerebral FDDNP-PET Binding in Middle-Aged and Older Non-Demented Adults. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2009;17:493–502. doi: 10.1097/jgp.0b013e3181953b82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington KD, Lim YY, Gould E, Maruff P. Amyloid-beta and depression in healthy older adults: a systematic review. The Australian and New Zealand journal of psychiatry. 2015;49:36–46. doi: 10.1177/0004867414557161. [DOI] [PubMed] [Google Scholar]
- 15.Yen YC, Rebok GW, Gallo JJ, Yang MJ, Lung FW, Shih CH. ApoE4 allele is associated with late-life depression: a population-based study. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2007;15:858–868. doi: 10.1097/JGP.0b013e3180f63373. [DOI] [PubMed] [Google Scholar]
- 16.Caselli RJ, Reiman EM, Hentz JG, Osborne D, Alexander GE. A distinctive interaction between chronic anxiety and problem solving in asymptomatic APOE e4 homozygotes. J Neuropsychiatry Clin Neurosci. 2004;16:320–329. doi: 10.1176/jnp.16.3.320. [DOI] [PubMed] [Google Scholar]
- 17.Altmann A, Tian L, Henderson VW, Greicius MD. Sex modifies the APOE-related risk of developing Alzheimer disease. Annals of neurology. 2014;75:563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang JP, Tsai SJ, Hong CJ, Yang CH, Lirng JF, Yang YM. The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiology of aging. 2006;27:1834–1837. doi: 10.1016/j.neurobiolaging.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A Longitudinal Study of Hippocampal Volume, Cortisol Levels, and Cognition in Older Depressed Subjects. American Journal of Psychiatry. 2004;161:2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 20.Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE, et al. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008;33:773–781. doi: 10.1016/j.psyneuen.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jorm AF. Is depression a risk factor for dementia or cognitive decline? Gerontology. 2000;46:219–227. doi: 10.1159/000022163. [DOI] [PubMed] [Google Scholar]
- 22.Geerlings MI, BOUTER LM, SCHOEVERS R, BEEKMAN AT, JONKER C, DEEG DJ, et al. Depression and risk of cognitive decline and Alzheimer’s disease. The British Journal of Psychiatry. 2000;176:568–575. doi: 10.1192/bjp.176.6.568. [DOI] [PubMed] [Google Scholar]
- 23.Sinoff G, Werner P. Anxiety disorder and accompanying subjective memory loss in the elderly as a predictor of future cognitive decline. International journal of geriatric psychiatry. 2003;18:951–959. doi: 10.1002/gps.1004. [DOI] [PubMed] [Google Scholar]
- 24.Holmes SE, Esterlis I, Mazure CM, Lim YY, Ames D, Rainey-Smith S, et al. Amyloid-B, APOE and BDNF Genotype, and Depressive and Anxiety Symptoms in Cognitively Normal Older Women and Men. The American Journal of Geriatric Psychiatry. 2016 doi: 10.1016/j.jagp.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Lockwood KA, Alexopoulos GS, van Gorp WG. Executive Dysfunction in Geriatric Depression. American Journal of Psychiatry. 2002;159:1119–1126. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- 26.Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, et al. Cognitive Function in Late Life Depression: Relationships to Depression Severity, Cerebrovascular Risk Factors and Processing Speed. Biological psychiatry. 2006;60:58–65. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Beaudreau SA, O’Hara R. The Association of Anxiety and Depressive Symptoms with Cognitive Performance in Community-Dwelling Older Adults. Psychology and aging. 2009;24:507–512. doi: 10.1037/a0016035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beaudreau SA, O’Hara R. Late-Life Anxiety and Cognitive Impairment: A Review. The American Journal of Geriatric Psychiatry. 2008;16:790–803. doi: 10.1097/JGP.0b013e31817945c3. [DOI] [PubMed] [Google Scholar]
- 29.Vink D, Aartsen MJ, Schoevers RA. Risk factors for anxiety and depression in the elderly: A review. Journal of affective disorders. 2008;106:29–44. doi: 10.1016/j.jad.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K, et al. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain : a journal of neurology. 2014;137:221–231. doi: 10.1093/brain/awt286. [DOI] [PubMed] [Google Scholar]
- 31.Pietrzak RH, Lim YY, Ames D, Harrington K, Restrepo C, Martins RN, et al. Trajectories of memory decline in preclinical Alzheimer’s disease: results from the Australian Imaging, Biomarkers and Lifestyle Flagship Study of ageing. Neurobiology of aging. 2015;36:1231–1238. doi: 10.1016/j.neurobiolaging.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 33.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. Journal of psychosomatic research. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 34.Nylund KL, Asparouhov T, Muthén BO. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Structural Equation Modelling. 2007;14:535–569. [Google Scholar]
- 35.McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annual review of neuroscience. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 36.Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular medicine. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- 37.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 38.Groves J. Is it time to reassess the BDNF hypothesis of depression? Molecular psychiatry. 2007;12:1079–1088. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- 39.Hwang J-P, Tsai S-J, Hong C-J, Yang C-H, Lirng J-F, Yang Y-M. The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiology of aging. 2006;27:1834–1837. doi: 10.1016/j.neurobiolaging.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, et al. Brain-Derived Neurotrophic Factor Conditional Knockouts Show Gender Differences in Depression-Related Behaviors. Biological psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 41.Shalev I, Lerer E, Israel S, Uzefovsky F, Gritsenko I, Mankuta D, et al. BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrinology. 2009;34:382–388. doi: 10.1016/j.psyneuen.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Weiss LA, Pan L, Abney M, Ober C. The sex-specific genetic architecture of quantitative traits in humans. Nature genetics. 2006;38:218–222. doi: 10.1038/ng1726. [DOI] [PubMed] [Google Scholar]
- 43.Sohrabji F, Lewis DK. Estrogen–BDNF interactions: implications for neurodegenerative diseases. Frontiers in neuroendocrinology. 2006;27:404–414. doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reinholdt-Dunne ML, Mogg K, Bradley BP. Attention control: Relationships between self-report and behavioural measures, and symptoms of anxiety and depression. Cognition & emotion. 2013;27:430–440. doi: 10.1080/02699931.2012.715081. [DOI] [PubMed] [Google Scholar]
- 45.Vinkers DJ, Gussekloo J, Stek ML, Westendorp RG, Van der Mast RC. Temporal relation between depression and cognitive impairment in old age: prospective population based study. Bmj. 2004;329:881. doi: 10.1136/bmj.38216.604664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothbart MK, Ellis LK, Posner MI. Temperament and self-regulation. Handbook of self-regulation: Research, theory, and applications. 2004;2:441–460. [Google Scholar]
- 47.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to Regulate: Counterproductive Recruitment of Top-Down Prefrontal-Subcortical Circuitry in Major Depression. The Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 49.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion (Washington, DC) 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 50.Husain M, Mehta MA. Cognitive enhancement by drugs in health and disease. Trends in cognitive sciences. 2011;15:28–36. doi: 10.1016/j.tics.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Priyamvada R, Ranjan R, Chaudhury S. Cognitive rehabilitation of attention and memory in depression. Industrial psychiatry journal. 2015;24:48. doi: 10.4103/0972-6748.160932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robertson J, Curley J, Kaye J, Quinn J, Pfankuch T, Raber J. apoE isoforms and measures of anxiety in probable AD patients and Apoe−/− mice. Neurobiology of aging. 2005;26:637–643. doi: 10.1016/j.neurobiolaging.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. Jama. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 54.Pietrzak RH, Maruff P, Woodward M, Fredrickson J, Fredrickson A, Krystal JH, et al. Mild worry symptoms predict decline in learning and memory in healthy older adults: a 2-year prospective cohort study. The American Journal of Geriatric Psychiatry. 2012;20:266–275. doi: 10.1097/JGP.0b013e3182107e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wetherell JL, Reynolds CA, Gatz M, Pedersen NL. Anxiety, cognitive performance, and cognitive decline in normal aging. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2002;57:P246–P255. doi: 10.1093/geronb/57.3.p246. [DOI] [PubMed] [Google Scholar]
- 56.Gallacher J, Bayer A, Fish M, Pickering J, Pedro S, Dunstan F, et al. Does anxiety affect risk of dementia? Findings from the Caerphilly Prospective Study. Psychosomatic Medicine. 2009;71:659–666. doi: 10.1097/PSY.0b013e3181a6177c. [DOI] [PubMed] [Google Scholar]
- 57.Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiology of aging. 2004;25:641–650. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 58.Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature neuroscience. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 59.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends in cognitive sciences. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 60.Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature neuroscience. 2009;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- 61.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 62.Johnson DP, Whisman MA. Gender differences in rumination: A meta-analysis. Personality and individual differences. 2013;55:367–374. doi: 10.1016/j.paid.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion (Washington, DC) 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 64.Abbott MJ, Rapee RM. Post-event rumination and negative self-appraisal in social phobia before and after treatment. Journal of Abnormal Psychology. 2004;113:136. doi: 10.1037/0021-843X.113.1.136. [DOI] [PubMed] [Google Scholar]
- 65.Nouchi R, Taki Y, Takeuchi H, Hashizume H, Akitsuki Y, Shigemune Y, et al. Brain training game improves executive functions and processing speed in the elderly: a randomized controlled trial. PloS one. 2012;7:29676. doi: 10.1371/journal.pone.0029676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Korczyn AD, Halperin I. Depression and dementia. Journal of the neurological sciences. 2009;283:139–142. doi: 10.1016/j.jns.2009.02.346. [DOI] [PubMed] [Google Scholar]