Abstract
Objective
To identify which specific depressive symptoms predict remission to aripiprazole augmentation in late-life treatment resistant depression.
Methods
This is a secondary analysis of data from a late-life treatment resistant depression trial examining the safety and efficacy of aripiprazole augmentation. Participants aged 60 and above were randomized to aripiprazole augmentation (N = 91) versus placebo (N = 90). The main outcome was depression remission. Clinical predictors included individual MADRS item scores categorized as symptomatic (scores > 2) or non-symptomatic (scores ≤ 2).
Results
Three MADRS items predicted depression remission with aripiprazole augmentation: symptomatic scores on sleep disturbance, and non-symptomatic scores on apparent sadness and inability to feel. The 2-way and 3-way interaction terms of these MADRS items were not significant predictors of remission; therefore, the models ability to predict remission was not improved by combining the significant MADRS items.
Conclusions
The identification of specific depressive symptoms, which can be clinically assessed can be used to inform treatment decisions. Older adults with treatment resistant depression that present with sleep disturbances, lack of apparent sadness, or lack of inability to feel should be considered for aripiprazole augmentation.
Keywords: Aging, depression, aripiprazole, MADRS
More than half of older adults with major depressive disorder fail to remit with first line antidepressant treatment (e.g., selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors), making late-life treatment resistant depression (LLTRD) the rule rather than the exception (Mulsant et al., 2014, Lenze et al., 2008). Partially or untreated depression in late-life increases the risk of cognitive impairment, caregiver burden, poor quality of life, medical comorbidities, and suicide (Gallo et al., 2013, Whiteford et al., 2013, Callahan et al., 2005, Butters et al., 2008). Therefore, it is important to identify specific clinical characteristic that predict remission with second-line treatment strategies.
A recent study found that aripiprazole augmentation was both effective and safe in the treatment of LLTRD (Lenze et al., 2015). Analyses from the study examined cognitive, emotional, and medical factors of aripiprazole response. Specifically, set shifting moderates remission, anxiety is a poor prognostic factor, and medical burden neither moderates nor predicts remission to aripiprazole augmentation (Kaneriya et al., 2016). Other demographic and clinical factors associated with aripiprazole augmentation treatment effects include: white race; better physical functioning; better performance on attention, immediate, and delayed memory tests; greater psychomotor agitation and suicidality symptoms; and a history of adequate antidepressant pharmacotherapy (Smagula et al., 2016).
The identification of other clinical characteristics that predict remission can help guide treatment decisions for LLTRD. One such method of assessing clinical characteristics can be accomplished through the use of individual items of depressive symptom severity, as measured by the Montgomery-Asberg Depression Rating Scale (MADRS; (Montgomery and Asberg, 1979). The MADRS assesses 10 symptoms of depression: apparent sadness, reported sadness, inner tension, reduced sleep, reduced appetite, concentration difficulties, lassitude, inability to feel, pessimistic thoughts, and suicidal thoughts (Galinowski and Lehert, 1995). To date, no studies have looked at each item on the MADRS as a predictor of remission with aripiprazole augmentation. These depressive symptoms are routinely assessed in clinical care, even when structured measures are not used. Identifying which of these depressive symptoms predict remission has high relevance for clinical care. Therefore, we sought to explore: 1) which baseline depressive symptoms, using individual MADRS items, predict remission and 2) if combinations of the clinically significant depressive symptoms using baseline MADRS items improve the precision of predicting remission with treatment.
Methods
We used data from the Incomplete Response in Late-Life Depression: Getting to Remission (IRL-GRey) study. IRL-GRey was a 12-week, 3-site, randomized placebo-controlled, double-blind clinical trial of aripiprazole augmentation for LLTRD. The aim of IRL-GRey was to test the efficacy, safety, and tolerability of aripiprazole augmentation in older adults whose depression had not remitted with venlafaxine monotherapy for at least 12 weeks. A complete description of the methods and primary results of the IRL-GRey trial has been published previously (Lenze et al., 2015).
Participants
Participants were 60 years of age or older that met DSM-IV (American Psychiatric Association, 2000) criteria for a major depressive episode with at least moderate symptoms as defined by a MADRS total score of 15 or higher. Exclusion criteria included a diagnosis of bipolar disorder, dementia, schizophrenia, current psychotic symptoms, or alcohol or substance misuse during the last 6 months. All participants provided informed consent. The conduct of the study was overseen by a Data Safety and Monitoring Board, and institutional review boards approved the study at all three sites.
Intervention
LLTRD was established in 181 participants who did not achieve remission following open-label treatment with extended release venlafaxine, up to 300 mg a day for 12 weeks. Remission was defined as a MADRS score less than or equal to 10 for two sequential assessments. In the second phase, treatment with venlafaxine at the same dose received during the acute treatment phase was continued, and participants were randomly assigned (using permuted block randomization) to augmentation with aripiprazole or placebo for 12 weeks. Aripiprazole or placebo tablets were started at 2 mg per day and titrated as tolerated and as needed to a maximum dose of 15 mg per day. The randomized augmentation phase was conducted under double-blind conditions and outcomes were assessed by independent evaluators. The pharmacist was the only member of the research team aware of treatment assignment.
Depression Assessment
Symptoms of depression were assessed with the MADRS at each weekly or biweekly visit. The primary outcome of this analysis was remission of depression, defined as a MADRS score of 10 or less, at the last 2 consecutive visits. The MADRS includes the following items (with corresponding description): 1. Apparent sadness (the appearance of despondency, gloom or despair) 2. Reported sadness (reports of depressed mood or despondency) 3. Inner tension (edginess, inner turmoil or mental tension leading to panic or anguish) 4. Reduced sleep (reduced duration or depth of sleep) 5. Reduced appetite (a feeling of loss of appetite) 6. Concentration difficulties (difficulties in the ability to collect one’s thoughts) 7. Lassitude (difficulty getting started or being slow in initiating or performing daily activities) 8. Inability to feel (reduced interested in surroundings and activities that are normally pleasurable) 9. Pessimistic thoughts (thoughts of guilt, self-reproach, or remorse) 10. Suicidal thoughts (feeling that life is not worth living, suicidal thoughts or preparation for suicide).
Statistical analyses
The analyses were conducted separately with the 91 participants who received aripiprazole augmentation and the 90 who received placebo. To identify which of the 10 MADRS items predicted remission, participants were dichotomized into those who remitted versus those who did not remit. Baseline MADRS item scores were dichotomized into clinically symptomatic (i.e., item score 3–6) or not clinically symptomatic (score 0–2) to identify groups with high and low symptom severity as that translates to the clinical setting. Logistic regression analysis was used to estimate the relationship of predictive factors (i.e., MADRS items) with the odds of remission. Remission was the dependent variable, with individual baseline MADRS items (dichotomized as symptomatic and non-symptomatic) entered as predictive variables. All predictive factors were simultaneously entered into the model with study site as a covariate. Odds ratios and 95% confidence intervals were estimated. To explore the combination of significant predictors on odds of remission, interaction terms were subsequently entered into model. Lastly, to confirm that our findings were unique to the aripiprazole augmentation group, the above analyses were repeated in the placebo group.
Results
Please see Table 1 for the demographic and clinical characteristics of the full sample and stratified by treatment condition. Participants had a mean (SD) age of 67.4 (6.1) years; they were mostly white (57%), female (88%), and had a mean (SD) of 14.2 (2.8) years of formal education. Univariate analyses (chi-square tests, t-tests) showed there were no significant differences among the demographic characteristics between the aripiprazole and placebo group, suggesting that the randomization was successful. At baseline, participants in the aripiprazole group (3.10 ± 1.15) had higher scores on MADRS Item 3 (inner tension) than the placebo group (2.76 ± 1.18), t = −1.98, p = .05. A significantly greater proportion of the participants who received aripiprazole augmentation (44%) remitted compared to participants who received placebo (29%), χ2 = 4.43, p = .04.
Table 1.
Demographic and Clinical Characteristics of the Sample
| Full Sample (N = 181) |
Aripiprazole (N = 91) |
Placebo (N = 90) |
Test Statistic, p- value |
|
|---|---|---|---|---|
| Age (M ± SD) | 67.37 ± 6.10 | 67.67 ± 6.60 | 67.06 ± 5.59 | t = −.67, p = .50 |
| Gender (n (%)) | χ2 = .004, p = .95 | |||
| Female | 103 (56.9%) | 52 (57.1%) | 51 (56.7%) | |
| Male | 78 (43.1%) | 39 (42.9%) | 39 (43.3%) | |
| Race (n (%)) | χ2 = .001, p = .98 | |||
| White | 159 (87.8%) | 80 (87.9%) | 79 (87.8%) | |
| Black | 22 (12.2%) | 11 (12.1%) | 11 (12.2%) | |
| Education (M ± SD) | 14.17 ± 2.81 | 14.18 ± 2.95 | 14.56 ± 2.68 | t = −.05, p = .96 |
| CIRS Total (M ± SD) | 9.66 ± 4.43 | 9.98 ± 4.65 | 9.33 ± 4.19 | t = −.98, p = .33 |
| MADRS Total (M ± SD) | 28.45 ±5.45 | 28.82 ± 5.50 | 28.07 ±5.40 | t = −.93, p = .35 |
| MADRS Items (M ± SD; | ||||
| # Symptomatic (%)) | 2.75 ± .91; | 2.77 ± .97; | 2.73 ± .85; | t = −.27, p = .79 |
| 1 | 106 (58.6%) | 55 (60.4%) | 51 (56.7%) | χ2 = .27, p = .61 |
| 3.90 ± .62; | 3.90 ± .63; | 3.89 ± .61; | t = −.13, p = .90 | |
| 2 | 179 (98.9%) | 89 (97.8%) | 90 (100%) | χ2 = 2.0, p = .16 |
| 2.93 ± 1.17; | 3.10 ± 1.15 | 2.76 ± 1.18 | t = −1.98, p = .05 | |
| 3 | 129 (71.3%) | 71 (78.0%) | 58 (64.4%) | χ2 = 4.1, p = .04 |
| 3.34 ± 1.73; | 3.47 ± 1.66 | 3.20 ± 1.79 | t = −1.06, p = .29 | |
| 4 | 127 (70.2%) | 68 (74.7%) | 59 (65.6%) | χ2 = 1.8, p = .18 |
| 1.67 ± 1.38; | 1.75 ± 1.37 | 1.60 ± 1.40 | t = −.72, p = .48 | |
| 5 | 68 (37.6%) | 36 (39.6%) | 32 (35.6%) | χ2 = .31, p = .58 |
| 3.15 ± 1.17; | 3.09 ± 1.23 | 3.21 ± 1.12 | t = .71, p = .48 | |
| 6 | 137 (75.7%) | 69 (75.8%) | 68 (75.6%) | χ2 = .002, p = .97 |
| 3.53 ± .90; | 3.54 ± .96 | 3.52 ± .85 | t = −.12, p = .90 | |
| 7 | 161 (89.0%) | 79 (86.8%) | 82 (91.1%) | χ2 = .85, p = .36 |
| 3.33 ± 1.13; | 3.25 ± 1.11 | 3.41 ± 1.15 | t = .94, p = .35 | |
| 8 | 142 (78.5%) | 72 (79.1%) | 70 (77.8%) | χ2 = .05, p = .83 |
| 2.98 ± .95; | 3.08 ± .88 | 2.88 ± 1.00 | t = −1.42, p = .16 | |
| 9 | 134 (74.0%) | 71 (78.0%) | 63 (70.0%) | χ2 = 1.5, p = .22 |
| .87 ± .93; | .88 ± .87 | .87 ± 1.00 | t = −.13, p = .90 | |
| 10 | 11 (6.1%) | 4 (4.4%) | 7 (7.8%) | χ2 = .91; p = .34 |
Note. M = mean; SD = standard deviation; CIRS = Cumulative Illness Rating Scale; MADRS = Montgomery-Asberg Depression Rating Scale.
Baseline MADRS items as predictors of remission with aripiprazole augmentation
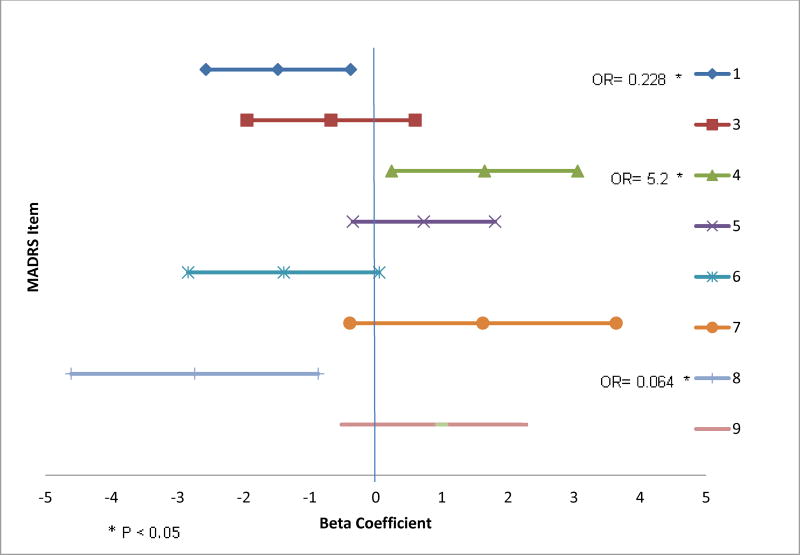
Figure 1 presents 95% confidence intervals of the beta coefficients for 8 of the 10 MADRS items. Due to limited variability in item response, Items 2 (i.e., 98% were symptomatic) and 10 (i.e., 96% were asymptomatic) were omitted from analyses. Non-symptomatic (low) scores on Items 1 (apparent sadness; OR = .23, p = .01) and 8 (inability to feel; OR =.06, p = .004), as well as symptomatic (high) scores on item 4 (sleep disturbance; OR = 5.18, p = .02) predicted depression remission with aripiprazole augmentation. On Items 1 (58% vs 35%; χ2 = 5.0, p = .03) and 8 (79% vs 35%; χ2 = 11.9, p = .001), non-symptomatic participants had a greater likelihood of remitting than symptomatic participants. While not statistically significant, for Item 4, symptomatic participants had a greater percentage of remitters than non-symptomatic participants (47% vs 35%; χ2 = 1.05, p = .31). Please see Table 2. for the distribution of participants by treatment arm, baseline symptomatic and non-symptomatic groups and treatment outcome for Items 1, 4 and 8.
Figure 1. Forest plot of the beta coefficients of MADRS items with corresponding confidence intervals and calculated odds ratio for significant items predicting remission with aripiprazole augmentation.
Items to the left of the midline (zero) represent asymptomatic scores (0,1,2) and items to the right of the midline (zero) represent symptomatic scores (3 or more). When the lines cross 0, then the corresponding items are non-significant, and when the lines do not cross 0, then they are significant. Item 1 = apparent sadness, item 3 = inner tension, item 4 = reduced sleep, item 5 = reduced appetite, item 6 = concentration difficulties, item 7 = lassitude, item 8 = inability to feel, item 9 = pessimistic thoughts. * Calculated odds ratios for the beta coefficients of significant MADRS items: Item 1 (apparent sadness): β= −1.48, CI (−2.57 −.38); Item 4 (sleep disturbances): β = 1.65, CI (0.2412, 3.0566) ; item 8 (inability to feel) : β= −2.74, CI (−4.61 −.870).
Table 2.
Distribution of participants by treatment arm, baseline symptomatic and non-symptomatic groups and treatment outcome for Items 1, 4 and 8.
| Aripiprazole
|
Placebo
|
|||||
|---|---|---|---|---|---|---|
| Item 1 | Item 4 | Item 8 | Item 1 | Item 4 | Item 8 | |
| N (%) Symptomatic (Item 4) and Non-Symptomatic (Items 1 & 8) | 36 (39.6%) | 68 (74.7%) | 19 (20.9%) | 39 (43.3%) | 59 (65.6%) | 20 (22.2%) |
| N (%) Remission of symptomatic (Item 4) and Non-Symptomatic (Items 1 & 8) | 21 (58.3%) | 32 (47.1%) | 15 (78.9%) | 12 (30.8%) | 15 (25.4%) | 6 (30.0%) |
Combinations of baseline MADRS items as predictors of remission with aripiprazole augmentation
Two-way interaction terms consisting of the different combinations among Items 1, 4, and 8 were all non-significant: Items 1 and 4 (z = .86, p = 0.4), Items 4 and 8 (z = −1.45, p = 0.1), Items 1 and 8 (z = −.99, p = 0.3). Similarly the three-way interaction term between the three significant items was not significant (z = −1.67, p = 0.1).
Baseline MADRS items as predictors of remission in those receiving placebo
As before, due to limited variability in item response, Items 2 (i.e., 100% were symptomatic) and 10 (i.e., 92% were asymptomatic) were omitted from analyses. In the placebo group, all the MADRS item scores were non-significant predictors of remission (p-values ranged from 0.14–0.95).
Discussion
Among depressed older adults who have failed to respond to first line treatment with venlafaxine, those who presented with sleep disturbances and absence of apparent sadness or inability to feel were more likely to remit with aripiprazole augmentation. However, having a combination of these three predictors did not increase the precision of predicting likelihood of remission beyond any of the single items. Furthermore, these three predictors were specific to aripiprazole treatment: they did not predict improvement with placebo.
Sleep disturbances are common in late-life, affecting approximately 50% of older adults (Ohayon, 2002). They frequently occur with other psychiatric disorders such as major depression (Ohayon, 2002). In this group, 70% of participants had symptomatic sleep disturbances. Epidemiologic studies support a bidirectional association between sleep and depression (Franzen and Buysse, 2008). As such, older adults with sleep disturbances are more likely to become depressed and those with depression are more likely to develop sleep disturbances (Perlis et al., 2006). Our finding of better response in those with sleep disturbance in this treatment-resistant group are consistent with our previous findings in the larger IRL-GRey group: with venlafaxine monotherapy, better subjective sleep was associated with a worse response trajectory (Smagula et al., 2015). These findings may be explained by sleep disturbances being a symptomatic marker of a subtype of late-life depression requiring and responding to pharmacotherapy (Buysse et al., 1996). Given the bidirectional nature of the relationship between depression and sleep, and the fact that sleep disturbances may predate a depressive episode (Baglioni et al., 2011), treating sleep disturbances in the setting of depression is clinically important.
By contrast, absence of apparent sadness and inability to feel were associated with a higher likelihood of remission with aripiprazole augmentation. This did not simply reflect a non-specific association (i.e., less symptomatic patients did better) since it was not observed with placebo. We did not expect this association since it has been hypothesized that “dopaminergic” symptoms of depression, such as psychomotor retardation, lassitude, and inability to feel are responsive to aripiprazole augmentation through potentiation of dopaminergic activity (Conway et al., 2014). It may be possible that participants without ‘apparent sadness’ (e.g., psychomotor retardation) or ‘inability to feel’ already had more activated dopaminergic circuits, which facilitated the response to aripiprazole. Alternatively, it is possible that the proposed mechanism of action of aripiprazole in LLTRD is more complex and that patients with symptoms reflecting decreased dopaminergic function (i.e., psychomotor retardation, inability to feel), do more poorly when treated with it.
There are several limitations to this study. First, our N was small. Second, the generalizability of our results are limited to older adults with similar clinical and demographic characteristics of our sample. Therefore, these finding should be replicated in other samples. Third, the MADRS sleep item may not adequately represent or measure sleep pathology in older adults, although it has been used elsewhere in the literature (Smagula et al., 2015). Lastly, while the sleep item was significant in the multivariate regression which included all the items, it only trended towards significance in the univariate analysis to predict remission. This could likely be due to the interactive effect of other symptoms and sites.
Conclusion
In conclusion, we observed that presence of sleep disturbances, and absence of apparent sadness and inability to feel predicted remission with aripiprazole augmentation of venlafaxine in LLTRD. These characteristics are typically assessed during routine clinical interviews or with routine administration of MADRS testing in the implementation of measurement-based care. Integrating this information with cognitive and anxiety comorbidity as well as information about race, physical function, memory tests, executive function, psychomotor agitation, suicidality and history of antidepressant pharmacotherapy may assist clinicians in prescribing aripiprazole augmentation for those patients most likely to respond (Kaneriya et al., 2016, Smagula et al., 2016).
Key Points.
The identification of specific depressive symptoms, which can be clinically assessed can be used to inform treatment decisions.
Older adults with treatment resistant depression that present with sleep disturbances, lack of apparent sadness, and lack of inability to feel should be considered for aripiprazole augmentation.
These three predictors are specific to remission with aripiprazole treatment as they did not predict improvement with placebo.
Acknowledgments
Financial Support. This study was supported primarily by the National Institute of Mental Health (NIMH) (R01 MH083660, P30 MH90333, and R34 MH101371 to the University of Pittsburgh; R01 MH083648 to Washington University; and R01 MH083643 and R34 MH101365 to the University of Toronto); and T32 MH019986. Additional funding was provided by the UPMC Endowment in Geriatric Psychiatry, the Taylor Family Institute for Innovative Psychiatric Research (at Washington University), the Washington University Institute of Clinical and Translational Sciences (grant UL1 TR000448) from the National Center for Advancing Translational Sciences, and the Campbell Family Mental Health Research Institute at the Centre for Addiction and Mental Health, Toronto. Bristol-Myers Squibb contributed aripiprazole and placebo tablets, and Pfizer contributed venlafaxine extended release capsules for this study.
Dr. Mulsant currently receives research funding from Brain Canada, the Centre for Addiction and Mental Health Foundation, the Canadian Institutes of Health Research, and the U.S. National Institutes of Health (NIH). During the last 5 years he also received research support from Bristol- Myers Squibb (medications for an NIH-funded clinical trial), Eli-Lilly (medications for an NIH-funded clinical trial), and Pfizer (medications for an NIH-funded clinical trial). He directly own stocks of General Electric (<$5,000).
Dr. Lenze has received research support from the NIMH, National Institute on Aging, National Center for Complementary and Integrative Health (NCCIH), Roche, Lundbeck, Janseen, Alkermes, the Sidney R. Baer, Jr. Foundation, the Taylor Family Institute for Innovative Psychiatric Research, the Barnes-Jewish Foundation, and the McKnight Brain Research Foundation.
Dr. Karp has received medication supplies for investigator-initiated trials from Pfizer and Invidior. He receives compensation for serving on the American Association for Geriatric Psychiatry editorial review board.
Dr. Blumberger receives research support from the Canadian Institutes of Health Research (CIHR), Brain Canada, Weston Brain Institute, National Institutes of Health (NIH), Temerty Family through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Family Research Institute. He receives non-salary operating funds and in-kind equipment support from Brainsway Ltd. for an investigator-initiated study. He is the site-principal investigator for several sponsor-initiated clinical trials from Brainsway Ltd. He received in-kind equipment support from Tonika/Magventure for an investigator-initiated study. He received medication supplies from Indivior for an investigator-initiated trial.
Dr. Reynolds reports receiving pharmaceutical support for NIH-sponsored research studies from Bristol-Myers Squibb, Forest, Pfizer, and Lilly; receiving grants from the National Institute of Mental Health, National Institute on Aging, National Center for Minority Health Disparities, National Heart, Lung, and Blood Institute, Centers for Medicare and Medicaid Services, Patient- Centered Outcomes Research Institute, the Commonwealth of Pennsylvania, the John A. Hartford Foundation, National Palliative Care Research Center, Clinical and Translational Science Institute, and the American Foundation for Suicide Prevention; and serving on the American Association for Geriatric Psychiatry editorial review board. He has received an honorarium as a speaker from MedScape/ WebMD. He is the co-inventor (Licensed Intellectual Property) of Psychometric analysis of the Pittsburgh Sleep Quality Index PRO10050447 (PI: Buysse).
Sponsor’s Role. The funding source had no involvement with the design, methods, subject recruitment, data collections, analysis or preparation of paper.
Footnotes
Potential conflict of interest.
The authors declare no potential conflicts of interest with respect to this research, authorship, and/or publication of this article. The attitudes expressed are those of the authors and do not necessarily reflect those of the Pittsburgh VA Healthcare System, Department of Veterans Affairs, or US government.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders 4th ed., text rev ed. Washington, DC: 2000. [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, Riemann D. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF, 3rd, Dekosky ST, Becker JT. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10:345–57. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Hoch CC, Houck PR, Kupfer DJ, Mazumdar S, Frank E. Longitudinal effects of nortriptyline on EEG sleep and the likelihood of recurrence in elderly depressed patients. Neuropsychopharmacology. 1996;14:243–52. doi: 10.1016/0893-133X(95)00114-S. [DOI] [PubMed] [Google Scholar]
- Callahan CM, Kroenke K, Counsell SR, Hendrie HC, Perkins AJ, Katon W, Noel PH, Harpole L, Hunkeler EM, Unutzer J, Investigators I. Treatment of depression improves physical functioning in older adults. J Am Geriatr Soc. 2005;53:367–73. doi: 10.1111/j.1532-5415.2005.53151.x. [DOI] [PubMed] [Google Scholar]
- Conway CR, Chibnall JT, Cumming P, Mintun MA, Gebara MA, Perantie DC, Price JL, Cornell ME, Mcconathy JE, Gangwani S, Sheline YI. Antidepressant response to aripiprazole augmentation associated with enhanced FDOPA utilization in striatum: a preliminary PET study. Psychiatry Res. 2014;221:231–9. doi: 10.1016/j.pscychresns.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10:473–81. doi: 10.31887/DCNS.2008.10.4/plfranzen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinowski A, Lehert P. Structural validity of MADRS during antidepressant treatment. Int Clin Psychopharmacol. 1995;10:157–61. doi: 10.1097/00004850-199510030-00004. [DOI] [PubMed] [Google Scholar]
- Gallo JJ, Morales KH, Bogner HR, Raue PJ, Zee J, Bruce ML, Reynolds CF., 3rd Long term effect of depression care management on mortality in older adults: follow-up of cluster randomized clinical trial in primary care. BMJ. 2013;346:f2570. doi: 10.1136/bmj.f2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneriya SH, Robbins-Welty GA, Smagula SF, Karp JF, Butters MA, Lenze EJ, Mulsant BH, Blumberger D, Anderson SJ, Dew MA, Lotrich F, Aizenstein HJ, Diniz BS, Reynolds CF., 3rd Predictors and Moderators of Remission With Aripiprazole Augmentation in Treatment-Resistant Late-Life Depression: An Analysis of the IRL-GRey Randomized Clinical Trial. JAMA Psychiatry. 2016;73:329–36. doi: 10.1001/jamapsychiatry.2015.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze EJ, Mulsant BH, Blumberger DM, Karp JF, Newcomer JW, Anderson SJ, Dew MA, Butters MA, Stack JA, Begley AE, Reynolds CF., 3rd Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:2404–12. doi: 10.1016/S0140-6736(15)00308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze EJ, Sheffrin M, Driscoll HC, Mulsant BH, Pollock BG, Dew MA, Lotrich F, Devlin B, Bies R, Reynolds CF., 3rd Incomplete response in late-life depression: getting to remission. Dialogues Clin Neurosci. 2008;10:419–30. doi: 10.31887/DCNS.2008.10.4/jlenze. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Mulsant BH, Blumberger DM, Ismail Z, Rabheru K, Rapoport MJ. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30:517–34. doi: 10.1016/j.cger.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Smith LJ, Lyness JM, Matteson SR, Pigeon WR, Jungquist CR, Tu X. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4:104–13. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- Smagula SF, Butters MA, Anderson SJ, Lenze EJ, Dew MA, Mulsant BH, Lotrich FE, Aizenstein H, Reynolds CF., 3RD Antidepressant Response Trajectories and Associated Clinical Prognostic Factors Among Older Adults. JAMA Psychiatry. 2015;72:1021–8. doi: 10.1001/jamapsychiatry.2015.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagula SF, Wallace ML, Anderson SJ, Karp JF, Lenze EJ, Mulsant BH, Butters MA, Blumberger DM, Diniz BS, Lotrich FE, Dew MA, Reynolds CF., 3rd Combining moderators to identify clinical profiles of patients who will, and will not, benefit from aripiprazole augmentation for treatment resistant late-life major depressive disorder. J Psychiatr Res. 2016;81:112–8. doi: 10.1016/j.jpsychires.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara H, Sakamoto K, Harada T, Shimizu S, Ishigooka J. A retrospective study of predictive factors for effective aripiprazole augmentation of antidepressant therapy in treatment-resistant depression. Neuropsychiatr Dis Treat. 2016;12:1151–1156. doi: 10.2147/NDT.S104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–86. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]