Abstract
The zona incerta contains GABAergic neurons that project to the superior colliculus in the cat and rat, suggesting it plays a role in gaze changes. However, whether this incertal connection represents a general mammalian pattern remains to be determined. We used neuronal tracers to examine the zona incerta connections with the midbrain tectum in the gray squirrel and macaque monkey. Collicular injections in both species revealed that most incertotectal neurons lay in the ventral layer, but anterogradely labeled tectoincertal terminals were found in both the dorsal and ventral layers. In the monkey, injections of the pretectum also produced retrograde labeling, but mainly in the dorsal layer. The dendritic fields of incertotectal and incertopretectal cells were generally contained within the layer inhabited by their somata. The macaque, but not the squirrel, zona incerta extended dorsolaterally, within the external medullary lamina. Zona incerta injections produced retrogradely labeled neurons in the superior colliculus of both species. In the squirrel, most cells inhabited the lower sublamina of the intermediate gray layer, but in the monkey, they were scattered throughout the deeper layers. Labeled cells were present among the pretectal nuclei in both species. Labeled terminals were concentrated in the lower sublamina of the intermediate gray layer of both species, but were dispersed among the pretectal nuclei. In summary, an incertal projection that is concentrated on the collicular motor output layers and that originates in the ventral layer of the ipsilateral zona incerta is a common mammalian feature, suggesting an important role in collicular function.
Keywords: Gaze, Eye Movements, GABA, Primate, Pretectum, Inhibition
INTRODUCTION
Inhibitory processes help control the bursts of activity in the superior colliculus (SC) that precede each saccade (Hikosaka and Wurtz, 1985). Unlike the widespread activity present in the superficial, visuosensory layers, in the deeper layers, activity needs to be concentrated at one location in the motor map once a target has been selected because only one saccade can be made at a time. There is evidence that the direct inputs from superficial to deep collicular layers are modulated by inhibition to arrange this pattern of activation, and that selective release of this inhibition may be important in producing express saccades (Ozen et al., 2000; Isa and Saito, 2001). Inhibition also plays a role in the relationship between the two colliculi, which have activity that codes for saccades to the left and the right, and between the hemifields within each colliculus, which code for upward and downward eye movements (Munoz and Istvan, 1998; Takahashi et al., 2005; 2007). Inhibition may even play a role in defining the duration of burst activity and the location of cells activated within the collicular motor map (Kaneda et al., 2008). Since this activity pattern results in both an orienting movement and a shift in attention by the animal (Basso and May, 2017), one may argue that inhibition is critical to the primary roles of the colliculus. Furthermore, the most salient target must be chosen from a host of possibilities, and the property of salience depends on the memory of the animal, species specific behavioral presets (e.g., what is food, what is a threat), previous reinforcements, and the internal state of the animal (Ingle, 1971; Dean et al., 1989; Westby et al., 1990; Sato and Hikosaka, 2002; Yasuda et al., 2012). All of these decisions may involve inhibiting alternative choices.
Clearly the roles of inhibition in the SC are various and numerous. In line with this, GABAergic terminals are very numerous on the surface of collicular motor output neurons (Lu et al., 1985). Indeed, the deep layers contain a prominent collection of GABAergic local circuit interneurons (Mize et al., 1988; 1991). In addition, they are targeted by a variety of extrinsic GABAergic inputs (Appell and Behan, 1990). The best studied among these is the substantia nigra pars reticulata. The nigrotectal projection provides an extensive input to the motor output layers of the SC (Jayaraman et al., 1977; May and Hall, 1984; Harting et al., 1988; Bickford and Hall, 1992; Redgrave et al., 1992). It is likely that this projection plays an important role in releasing the burst of activity in collicular cells before saccades, including those to remembered targets (Hikosaka and Wurtz, 1983B). Another major extrinsic source of GABAergic input is the central mesencephalic reticular formation (cMRF). The cMRF receives direct input from collicular gaze output neurons and then provides a GABAergic feedback projection back onto these output cells (Moshovakis et al. 1988B; Chen and May, 2000; Zhou et al., 2008; Wang et al., 2010). While it is known that the cMRF contains neurons that display long lead, saccade-related bursts, the precise role of this feedback pathway has yet to be ascertained (Waitzman et al., 1996; Cromer and Waitzman, 2007).
Yet another major extrinsic GABAergic projection to the SC comes from the zona incerta (ZI). This projection exists in both the rat and cat (Edwards et al., 1979; Watanabe and Kawana, 1982; Shammah-Lagnado et al., 1985; Ficarrola and Mize, 1989; Appell and Behan, 1990). While some ZI neurons in monkeys display saccade-related activity (Hikosaka and Wurtz, 1983A; Ma, 1996), there is evidence from non-primates that they modify movement with respect to internal state and autonomic nervous system activity levels (Mitrofanis, 2005). Nevertheless, the ZI’s precise role with respect to tectally generated gaze changes is poorly understood. Towards this end, we have endeavored to better define the pattern of connections between the ZI and the SC. Previously, we demonstrated a number of features of these connections in the cat (May et al., 1997; Perkins et al., 2006). These included the fact that the source of the incertotectal projection was largely confined to the ventral layer of ZI and that this layer extended dorsolaterally between the reticular thalamic nucleus and the external medullary lamina. Within the SC, the incertotectal terminal field was concentrated in the lower sublamina of the intermediate gray layer (SGI), and this same sublamina was the primary source of the tectoincertal projection. Finally, in the cat we noted connections between the dorsal sublamina of ZI and the pretectum, particularly the anterior pretectal nucleus. While connections between the ZI and the SC are also present in the rat, a number of the details of this projection differed from those seen in the cat (Shammah-Lagnado et al., 1985; Roger and Cadsseau, 1985). In order to determine which characteristics of the connections between the ZI and the tectum represent general mammalian features and to provide an anatomical basis for studying this system in primates, we explored the connections of the ZI in two species: the gray squirrel (Sciurus carolinensis) and cynomologous monkey (Macaca fascicularis). While both these species have evolved in a diurnal, arboreal habitat that puts a premium on vision and accurate gaze, they occupy fairly different branches of the mammalian radiation.
METHODS
The data presented here were obtained from slide collections produced during previous studies (squirrels: May and Hall, 1986; monkeys: May et al., 1990; Chen and May, 2000; Perkins et al., 2009). All procedures were undertaken in accordance with the NIH rules and regulations governing animal research, as enumerated in the Guide for Care and Use of Laboratory Animals, using protocols approved by the Institutional Animal Care and Use Committee of the University of Mississsippi Medical Center and as required by USDA regulations. Tissue from 7 squirrels (Sciurus carolinensis) and 16 monkeys (Macaca fascicularis) of both sexes was analyzed.
Squirrel procedures
Squirrels were anesthetized with sodium pentobarbital (50 mg/kg, ip) and placed in a stereotaxic head holder (Trent-Wells). Injections (0.05 μl) of horseradish peroxidase (HRP) (30 % in a 10% saponin solution) were placed in the SC (n = 3) by use of a 1.0 μl Hamilton syringe held in a micromanipulator. Inclusion of saponin was intended to increase homogeneous filling of the dendritic trees of the retrogradely labeled cells (May and Hall, 1984). The SC (n = 3) and the ZI (n = 1) were also injected iontophoretically. In this case, a 10 % solution of wheat germ agglutinin conjugated to horseradish peroxidase (WGA-HRP) was placed in a glass micropipette with a tip diameter of 25 μm that was attached to a micomanipulator. The tracer was ejected by use of 7 sec, 50 % duty cycle square wave positive pulses for 10 minutes. All injection placements utilized stereotaxic coordinates developed from an in-house atlas. These animals were deeply anesthetized with sodium pentobarbital (70 mg/kg, ip) 2–3 days later, and were perfused through the heart with a buffered saline rinse, followed by a fixative containing 0.5 % paraformaldehyde and 2.5 % glutaraldehyde in 0.1M, pH 7.2 phosphate buffer (PB). The brains were postfixed in the same solution for 2 hours and then blocked in the frontal plane.
The brains with HRP injections were cut into 100 μm frontal sections using a Vibratome. A 1 in 3 series of sections was reacted using a modification of the orthotoluidine method of Somogyi and colleagues (1979). These sections were mounted out of 0.1M, pH 6.0 PB, counterstained with cresyl violet, cleared and coverslipped. The brains with WGA-HRP injections were equilibrated in 30 % sucrose in 0.1M, pH 7.2 PB at 4° C as a cryoprotectant, before being frozen and sectioned on an AO sliding microtome at 40 μm. A 1 in 3 series of sections was reacted to reveal the presence of HRP using the tetramethylbenzidine method of Mesulam (1978). Further details of the surgical and histological procedures can be found in May and Hall (1984; 1986).
Monkey procedures
The monkeys used in these procedures were sedated with ketamine HCL (10 mg/kg, im) and anesthetized with either sodium pentobarbital (35 mg/kg, iv) or with isoflurane (2–3%). They were then placed in stereotaxic frame (Kopf). For SC injections (n = 8), the surface of the midbrain tectum was visualized by aspirating the medial surface of the parieto-occipital junction. In some cases (n = 5) a 1–2 % WGA-HRP/10 % HRP solution was used. In other cases (n = 3), a 10 % solution of biotinylated dextran amine (BDA) was injected. In both cases, the solution was contained within a 1.0 μl Hamilton syringe held by a micromanipulator at a 20–25° angle, tip up in the sagittal plane. The needle was advanced 1.5 mm into the SC and 0.01–0.02 μl of WGA-HRP or 0.1–0.2 μl of BDA was injected. One to three sites in the SC were injected. For the pretectal injections (n=6), the same approach was used to make WGA-HRP (n = 4) or BDA (n = 2) injections, but the entry point of the needle was located at the point on the surface where the tectum meets the pulvinar, and the needle was located 1–2 mm off the midline. Finally, for the injection of WGA-HRP into the ZI (n = 2), the needle was oriented vertically and the nucleus was located using stereotaxic coordinates, without aspiration (Szabo and Cowan, 1984). Butorphanol tartrate (0.3 mg/kg, im) or Buprenex (0.01 mg/kg, im) was given for postoperative analgesia. After a 1–2 day survival for the WGA-HRP injections or a 2–3 week survival for the BDA injections, the animals were sedated with ketamine HCL (10 mg/kg, im) and deeply anesthetized with sodium pentobarbital (50 mg/kg, ip). They were then perfused with a 0.1M, pH 7.2 PB saline rinse, followed by a fixative solution containing 1.0 % paraformaldehyde and 1.25 % glutaraldehyde in 0.1M, pH 7.2 PB. The brains were blocked postfixed for 1–2 hours in this solution.
Frontal sections were cut at either 100 μm using a Vibratome or 50 μm using an AO sliding microtome. In the latter case, the brains were first cryoprotected in 30 % sucrose PB before being frozen. To reveal the HRP, sections were reacted using one of two tetramethylbenzidine methods: that of Mesulam (1978) or that of Olucha and colleagues (1985). To visualize the BDA, sections were treated with avidin-HRP, and then the HRP was visualized by use of the diaminobenzidine with nickel cobalt enhancement technique of Adams (1977). Details of the surgical and histological procedures used in these experiments can be found in our previous reports (May and Porter, 1992; Chen and May, 2000; Perkins et al., 2009). With respect to nomenclature, we divided the ZI into dorsal and ventral layers, and noted the presence of a dorsolateral extension, when present, as per our previous studies (May et al, 1997; Perkins et al., 2006)
Analysis
The labeled elements were charted and drawn on a BH-2 Olympus microscope equipped with a drawing tube. Images were taken using a Nikon Eclipse 600 microscope equipped with a digital camera using Nikon Elements software. Contast and color were manipulated in Photoshop (Adobe) to best match the appearance of the material to the eye.
RESULTS
Squirrel colicular injections
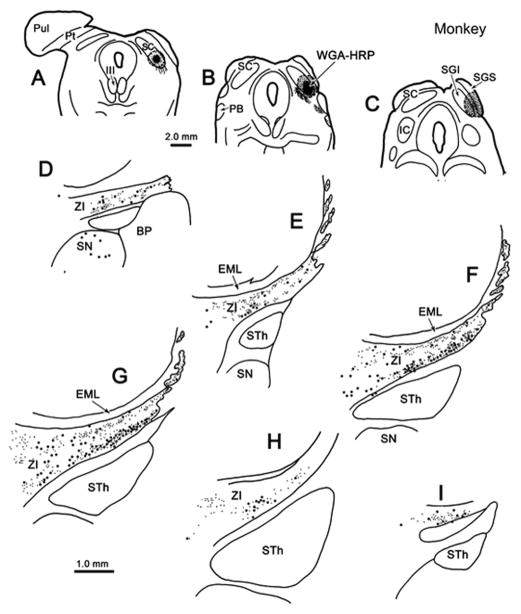
We will cover the connections seen in the gray squirrel first. Figure 1 shows some essential elements of the pattern of connections observed following injections of WGA-HRP into the SC in the context of the squirrel midbrain and thalamus. In this animal, a full thickness injection was placed in the rostral, right SC (Fig. 1A). A second, more caudal, injection that included the superficial gray layer (SGS), stratum opticum (SO) and the upper half of SGI was placed in the left SC (Fig. 1B). Both injections included the SGS, so terminal fields (stipple) were observed in the pretectal, pulvinar, dorsal lateral geniculate and parabigeminal nuclei on both sides of the brain (Fig. 1B–F). The last also contained retrogradely labeled cells (dots) (Fig. 1B). Within the ventral thalamus retrogradely labeled cells were present in both the ventral lateral geniculate and ZI from both injections (Fig. 1C–G). However, the more superficial injection on the left produced considerably fewer cells in the ventral lateral geniculate nucleus and only a couple of scattered cells in ZI, suggesting the latter projects more ventrally within SGI. Similarly, nearly all the labeled nigrotectal cells were found on the right side (Fig. 1C), in agreement with studies showing the squirrel nigrotectal projection terminates in the lower sublamina of SGI (May and Hall, 1984). Labeled terminals were evident in the ZI only on the side of the deeper injection (right).
Figure 1.

Laminar differences in squirrel incertotectal neurons. Chartings of the distribution of labeled cells (dots), axons (lines) and terminals (stipple) in the diencephalon following injections of WGA-HRP into the right (A) and left (B) SC (A&B) in a gray squirrel. Note the presence of many more labeled cells and terminals in the ventral lateral geniculate (VLG) and zona incerta (ZI) following the deeper (right) injection. Sections are arranged in rostral to caudal order in this and other figures with chartings.
Figure 2 shows a second squirrel injection case with a unilateral WGA-HRP injection site located rostrally in the SC (Fig. 2A–C). It was centered in the deep gray layer (SGP), but still included all the layers. It spread to include the lateral portion of the periaqueductal gray and portions of the pretectum, specifically the nucleus of the optic tract and posterior pretectal nucleus. The retrograde labeling (dots) within the ventral thalamus was extensive. Most of the retrogradely labeled cells were found within the ventral half of the ipsilateral ZI (Fig. 2F–H), although scattered cells were present in the dorsal half of ZI, particularly rostrally (Fig. 2D–F). A small number of labeled cells were found in contralateral ZI in this and other cases, but they were so few in number that we did not pursue this point. Labeled terminals (stipple) produced a distinct band along the top edge of the dorsal half of ZI. Scattered puncta were also present beneath this band, including some among the cell bodies in the bottom half of ZI (Figs. 2D–F). Additional labeled cells and terminals were present in the ventral lateral geniculate (Fig. 2D–G). The subdivision of ZI into two layers with different patterns of tectal connections can be further appreciated in figure 3A showing images from this case. The ventral layer of ZI (vZI) is filled with densely labeled neurons. The dorsal layer of ZI (dZI) lacks these labeled cells, but contains a prominent band of anterogradely labeled terminals in its upper half. Scattered retrogradely labeled cells are present in dZI, but these are much more lightly labeled than those in vZI (compare cells indicated by arrowheads in dZI to those in vZI in Fig. 3B) suggesting that they may be labeled by injection site spread outside the SC. Labeled puncta are present amongst the retrogradely labeled cells in vZI, but it is not clear if these represent terminals or pieces of labeled dendrite (Fig. 3B).
Figure 2.

Chartings of the distribution of labeled cells (dots), axons (lines) and terminals (stipple) in the ventral thalamus (D–I) following a unilateral injection of WGA-HRP into the SC (A–C) in a gray squirrel. Note the large numbers of cells in the ventral half of ZI and the terminal field in the dorsal half of ZI.
Figure 3.

Images of labeled tectoincertal and incertotectal elements in the gray squirrel. A. Appearance of ZI following an injection of WGA-HRP into the SC. Note the band of terminals in the upper part of the dorsal layer of ZI (dZI) and the band of labeled cells filling the ventral layer of ZI (vZI). Box indicates the region shown at higher magnification in B, where the multipolar nature of the incertotectal cells in vZI can be appreciated. In addition, the scattered, more lightly labeled, cells in dZI are indicated by arrowheads. C. Numerous scattered puncta are present in the lower sublamina of the intermediate gray layer (lSGI) of the squirrel following an injection of WGA-HRP into the zona incerta (see Fig. 6). Retrogradely labeled tectoincertal cells (arrowheads) are also present. Relatively little label is seen in either the upper sublamina of SGI (uSGI) or the intermediate white layer (SAI). D. The dendritic morphology of incertotectal cells is evident following injections of HRP in saponin into the SC. Images A–C were taken with crossed polarizers. Scale in C = A, in D = B.
The morphology of the incertotectal population is illustrated in figure 4. In this case, HRP was injected primarily in the layers of the colliculus beneath SO (Fig. 4C). The injection site spread into the adjacent periaqueductal gray and the rostral pole of the inferior colliculus. The somata of the labeled multipolar cells tended to be fusiform in shape and oriented parallel to the mediolateral axis of ZI (Fig. 4A, cells C–F, H–J). The primary and secondary dendrites of the neurons were filled with tracer, and these also tended to extend horizontally, parallel with the mediolateral axis of the nucleus. As a group (Fig. 4B), most of the incertotectal cells labeled in this and the other cases examined lay within vZI, and their labeled dendrites were constrained within this same layer. At the medial edge of ZI, the horizontal orientation became less evident and cells took on more of a multipolar character (Fig. 4A, cells A&B). At the lateral edge of ZI, the layer takes on diagonal orientation, instead of a horizontal one, and the orientation of the cells follows that change (Fig. 4, cells K&L). A second case with retrograde labeling following a more superficial WGA-HRP injection (Fig. 5B), filled even tertiary dendrites. It still demonstrated that the somata and dendrites of incertotectal cells are generally oriented in the same plane as ZI (Figs 3D & 5A, cells B–E). Even those cells that had a more multipolar organization (Fig. 5A, cell A) displayed dendrites that were largely constrained to the lower half of ZI.
Figure 4.

Dendritic distribution and somatic morphology of incertotectal neurons in the gray squirrel following a large injection of HRP and saponin into the SC. C. The injection extended into the adjacent periaqueductal gray and midbrain reticular formation. Labeled somata and dendrites were arrayed in vZI, not dZI (B). A. Most somata were fusiform and multipolar, with dendrites that were typically oriented horizontally. This orientation was less evident at the medial end of the layer, where the dendrites showed no orientation, and the lateral end of the layer where it turned in a dorsolateral direction.
Figure 5.

Detailed morphology of incertotectal neurons in the gray squirrel following a large injection of WGA-HRP into the SC. B. The injection site in this case only extended slightly into the periaqueductal gray. A. The somata of labeled incertotectal cells (Cells A–E) are typically fusiform in appearance. The dendrites branch sparsely and even the tertiary dendrites extended in the plane of the nucleus. The locations of the labeled cells in A are indicated in C.
Squirrel zona incerta injection
The pattern of labeling in the squirrel tectum following an injection of ZI is demonstrated in figure 6. The WGA-HRP injection site was quite small, and was located in the medial portion of ZI towards its caudal end (Fig. 6A–C). It spread slightly into the substantia nigra pars compacta. Within the SC, the labeled terminal field (stipple) was largely confined to the lower sublamina of SGI and to SGP (Fig. 6E–J) and extended throughout much of the rostrocaudal and mediolateral extents of these layers. The lack of terminal fields in upper SGI explains the near lack of retrograde ZI labeling from the superficial injection on the left in the case illustrated in figure 1. Scattered retrogradely labeled neurons (dots) were present in these same sublamina (Fig. 6E–J). As can be seen in figure 3C, the terminal field from this small injection was fairly sparse and the retrogradely labeled neurons (arrowheads) were relatively small. Labeled terminals were also present in the pretectal nuclei, including the anterior, posterior and medial nuclei, as well as the nucleus of the posterior commissure (Fig. 6A–D). Terminals were also observed in thalamic nuclei, in agreement with previous studies (Fig. 6A–D) (Power et al., 1999).
Figure 6.

Incertotectal termination in the squirrel SC. Chartings show the distribution of labeled cells (dots), axons (lines) and terminals (stipple) in the SC (D–J) following an injection of WGA-HRP into ZI (A–C). Most of the collicular labeling is found in the lower sublamina of the intermediate gray layer (SGI), but some is also present in the deep gray layer (SGP).
Monkey midbrain tectum injections
The connections of ZI with the midbrain tectum of the monkey will be illustrated by a series of cases. In the first, the WGA-HRP injection site was centered in the pretectum, including the anterior and posterior pretectal nuclei, portions of the nucleus of the optic tract and nucleus of the posterior commissure. This injection spread into the rostral SC and included portions of SGI (Fig. 7A–C). Retrogradely labeled neurons (dots) and anterogradely labeled terminals (stipple) were present throughout ipsilateral ZI, with no regard to lamination (Fig. 7D–I). An occasional labeled cell was observed in contralateral ZI in this and other cases, but we did not pursue this question due to their small number. Note that the band of labeled cells and terminals extends dorsolaterally to sit medial to the thalamic reticular nucleus (Fig. 7D–H). The morphology of the labeled incertotectal neurons labeled in this same case is demonstrated in figure 8. As was seen in the squirrel, the majority of the labeled cells had fusiform somata whose long axes, as well as primary and secondary dendrites, were oriented parallel to the mediolateral axis of ZI (Fig. 8A & B, cells G,H,K–M). This orientation was less evident medially (Fig 8B, cells N–Q). The cells located in dZI were generally smaller (Fig. 8B, cells F, I–K) than those located in vZI (Fig. 8B, cells G,H,L–N&Q), with a few notable exceptions (Fig. 8B, cell O). The cells located in the dorsolateral extension of ZI (dlZI) were also smaller, and their dendrites were oriented parallel to the axis of this part of the nucleus (Fig. 8B, cells A–E).
Figure 7.

Overall pattern of connections between ZI and midbrain tectum in the monkey. Chartings show the distribution of labeled cells (dots), axons (lines) and terminals (stipple) in the ZI (D–I) following an injection of WGA-HRP into pretectum (A–C) and rostral SC (C). Labeled cells and terminals were present throughout ZI and this pattern of labeling extended medial to the reticular thalamic nucleus (RT).
Figure 8.

Morphology of incertotectal neurons in a macaque monkey following a large injection of WGA-HRP into the pretectum and rostral SC. This is the same case as charted in Fig. 7. The injection shown in C. A. Labeled cells were arrayed throughout dZI and vZI. B. The cells were multipolar with somata and dendrites that were typically oriented horizontally in the main body of the nucleus (cells G–N). This orientation was less evident at the medial end of the layer (cells O–Q). At the lateral end of the layer, where it extended into dlZI, the primary orientation of the dendritic fields is also dosolateral (cells A–F). The cells in dZI (cells I–K) tended to be smaller, with a few notable exceptions (cell O).
A different pattern was observed when the WGA-HRP injection site was confined to the SC. In the illustrated example, the tracer lay primarily in lateral SGI, with some spread into more dorsal layers (Fig. 9A–C). There was no spread to the pretectum. In this case, the retrogradely labeled cells (dots) were largely confined to the ventral half of ZI (Fig. 9E–H), although this lamination was less evident at the very rostral and caudal poles of the nucleus (Fig. 9D&I, respectively). A distinct band of labeled terminals (stipple) was present in the dorsal half of ZI, along with scattered retrogradely labeled cells (Fig. 9E–G). Scattered labeled puncta were also present in the ventral half of ZI. Labeling also extended dorsolaterally in this case, but it consisted mainly of terminals found in islands of neuropil within the external medulary lamina (Fig. 9E–G). The appearance of ZI labeling following a collicular WGA-HRP injection in another case, is shown in figure 10A. Numerous labeled cells occupy vZI, but only scattered examples are seen in dZI (blue arrows). Both dZI and vZI contain many small terminal puncta. In order to determine whether these terminal puncta actually represent axon terminals, we examined cases in which BDA was injected into the SC. Figure 10C reveals that BDA labeled terminal fields were located in both dZI and vZI. The latter could be observed to have close associations (arrowheads) with retrogradely labeled incertotectal neurons (Fig. 10D).
Figure 9.
Pattern of collicular connections with ZI in the monkey. Chartings show the distribution of labeled cells (dots), axons (lines) and terminals (stipple) in the zona incerta (D–I) following an injection of WGA-HRP confined to the SC (A–C). Note the large numbers of cells in the lower half of ZI and numerous terminals in the upper half of ZI. This labeled band extended dorsolaterally, in islands of neuropil.
Figure 10.
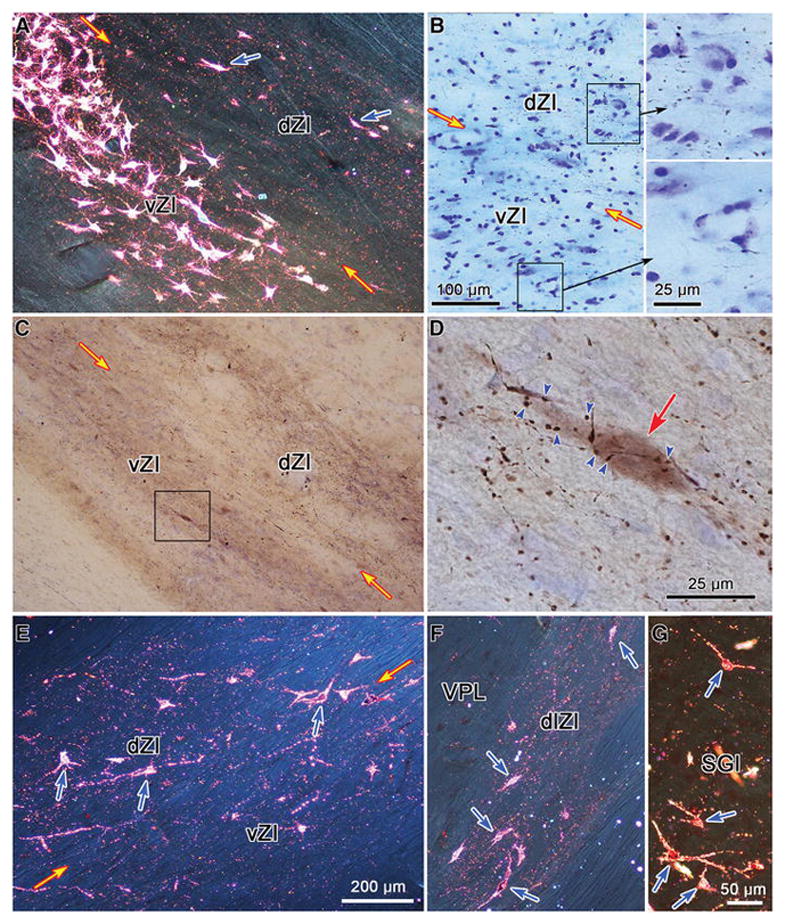
Images of labeled tectoincertal, incertotectal and pretectoincertal elements in the macaque monkey. A. Appearance of ZI following an injection of WGA-HRP into the SC. Note the band of labeled cells filling vZI and the terminal puncta in dZI. Scattered, more lightly labeled cells in dZI are indicated by blue arrows. B. Labeled axonal arbors following a BDA injection of the pretectum. Boxes indicate regions of dZI and vZI shown in the high magnification inserts. Terminations are much denser in dZI. C. Numerous labeled axonal arbors are present in both dZI and vZI following an injection of BDA into the SC. Box indicates area shown at higher magnification in D, where numerous labeled boutons are shown in close association (arrowheads) with a retrogradely labeled incertotectal neuron (red arrow). Terminal puncta are present in both dZI and vZI (E) and in the dorsolateral extension of ZI (dlZI) (F) following a WGA-HRP injection of the pretectum (shown in Fig. 12). Most of the labeled cells (blue arrows) are found in dZI (E) and dlZI (F). G. Labeled neurons (blue arrows) are present in the intermediate gray layer (SGI) following an injection of WGA-HRP into ZI (shown in Fig. 13), along with scattered terminal puncta. Images A and E–G were taken with crossed polarizers. Scale in E = A & F, in G = C.
The morphology of macaque incertotectal neurons is demonstrated in another case with a collicular injection of WGA-HRP. This was a larger injection that filled much of the SC and extended into the periaqueductal gray and adjacent mesencephalic reticular formation (Fig. 11C). The retrogradely labeled neurons were arrayed in vZI and extended into dlZI (Fig. 11B). As in the squirrel, the cells and their dendrites tended to be oriented parallel to the mediolateral axis of the nucleus, and the primary and secondary dendrites were constrained within the layer. There was some range in the size of the somata, with the smaller cells having long axes of around 20 μm and the largest cells having long axes of around 40 μm (Fig. 11A). The cells in dlZI tended to be smaller (Fig. 11A, cells H–J), although some small cells inhabited vZI (Fig 11A, cell G).
Figure 11.
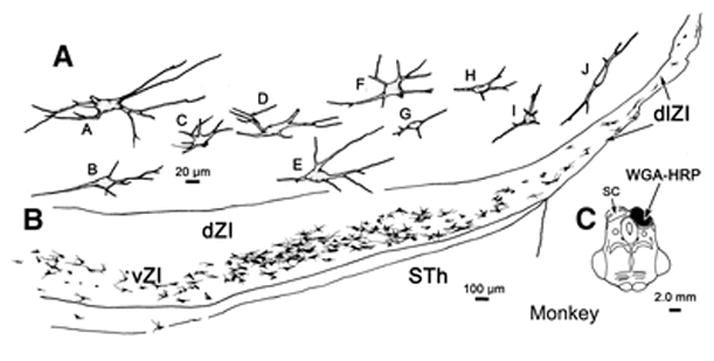
Morphology of incertotectal neurons in a macaque monkey following a large injection of WGA-HRP into the SC. The injection shown in C spreads slightly into the periaqueductal gray. B. Labeled cells were arrayed throughout vZI, but not dZI. A. The cells were multipolar with somata and dendrites that were typically oriented horizontally in the main body of the nucleus (cells B–H). This orientation was less evident at the medial end of the layer (cell A). Within dlZI, the primary orientation of the dendrites was dorsolateral (cells I–J).
A different pattern of labeling was present when the WGA-HRP injection was located solely in the pretectum, without involving the SC (Fig. 12). In this case, the injection involved the rostral pole of the posterior pretectal nucleus, the anterior pretectal nucleus and the nucleus of the posterior commissure (Fig. 12B&C). It spread rostral and dorsal to the pretectum to involve a small portion of the dorsal thalamus (Fig. 12A&B). This injection site produced considerable terminal labeling (stipple) throughout the dorsal half of ZI (Fig. 12E–H). Numerous retrogradely labeled cells (dots) were found in this same layer. The band of label extended dorsolaterally to wrap around the lateral aspect of the dorsal thalamus. At the rostral and caudal pole of ZI the layering pattern was not as discrete (Fig. 12D&I, respectively). The appearance of labeling in this case is further demonstrated in figure 10E&F. In the main body of ZI, most of the labeled cells (blue arrows) and labeled puncta were found in dZI. Both types of label were less evident in vZI (Fig. 10E). Retrograde and anterograde labeling were also evident in the dlZI (Fig. 10F). To determine whether these labeled puncta were actual axonal boutons, we examined cases with BDA injections of the pretectum. As shown in figure 10B, labeled axons with boutonal enlargements were observed throughout ZI, but these were much more evident in dZI.
Figure 12.

Pattern of pretectal connections with ZI in the monkey. Chartings show the distribution of labeled cells (dots), axons (lines) and terminals (stipple) in ZI (D–I) following an injection of WGA-HRP confined to the pretectum (A–C) in a macaque monkey. The injection site involved the anterior and posterior pretectal nuclei, nucleus of the posterior commissure and nucleus of the optic tract. Note the large numbers of cells and terminals in the dorsal half of ZI. These extended dorsolaterally, in islands of neuropil.
Monkey zona incerta injections
The pattern of incertal connections with the midbrain tectum was investigated by making WGA-HRP injections into the ZI of monkeys. In the case illustrated in figure 13, the tracer was centered in ZI, but spread into the adjacent subthalamic nucleus, the external medullary lamina and internal capsule (Fig. 13A&B). There was also some spread along the needle track through the dorsal thalamus and slight involvement of the dorsolateral corner of the substantia nigra. Within the SC, retrogradely labeled neurons (dots), were mainly located in SGI, although cells were found in SO and SGP (Fig. 13E–J). The labeled cells in this case were relatively small, multipolar neurons with roughly spherical somata having diameters in the 11–15 μm range (Fig. 10G). Labeled terminals were also present (Fig. 13E–J). These were somewhat sparse, but appeared to be more concentrated in the lower sublamina of SGI. Within the pretectum, labeled cells and terminals were observed in the anterior and posterior pretectal nuclei, nucleus of the posterior commissure and the nucleus of the optic tract (Fig. 13C–F).
Figure 13.
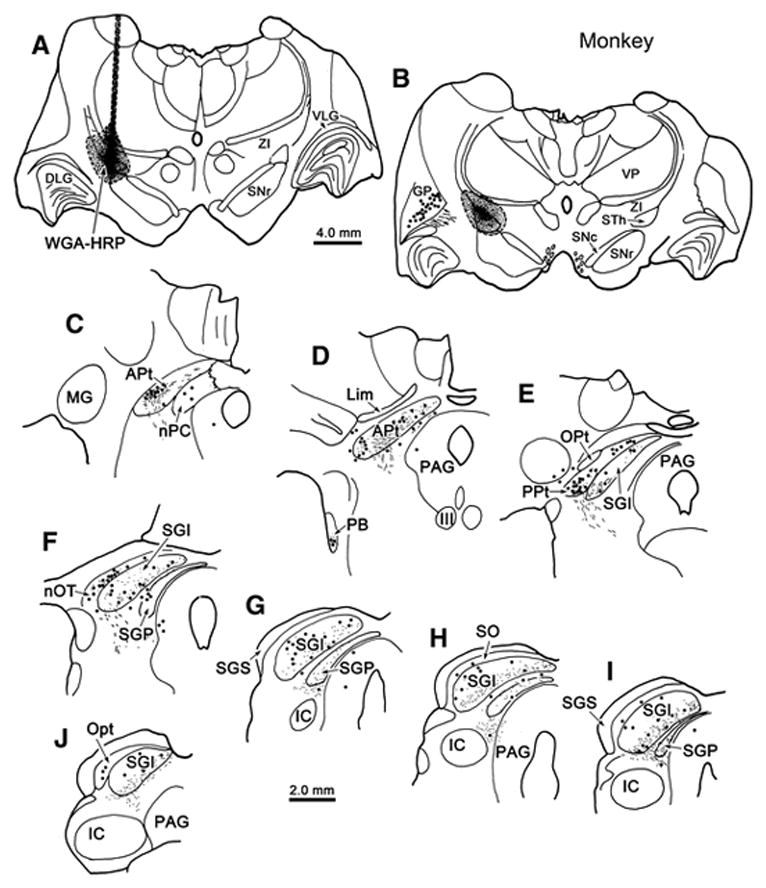
Incertotectal termination in the monkey midbrain tectum. Chartings show the distribution of labeled cells (dots), axons (lines) and terminals (stipple) in the SC (E–J) and pretectum (C–F) following an injection of WGA-HRP into ZI (A–C) in a macaque monkey. Most of the terminal labeling is found in the intermediate gray layer (SGI) and deep gray layer (SGP). Labeled cells were scattered in these layers, as well as in stratum opticum (SO). Labeled terminals and cells were present in the anterior pretectal nucleus (APt)(C&D), posterior pretectal nucleus (PPt)(E), nucleus of the posterior commissure (nPC) and the nucleus of the optic tract (nOT)(F).
DISCUSSION
The data presented here suggest that certain properties of the incertotectal circuitry are common mammalian features, and these are illustrated in figure 14. First, the incertotectal projection to the ipsilateral SC originates in vZI. While it’s laminar terminal distribution differs somewhat between species, its distribution correlates with the location of the cells of origin of the crossed descending output of the colliculus in all species tested. Second, the tectoincertal projection targets both layers of the ipsilateral ZI. It originates from a population of smaller cells whose distribution within the SC co-localizes with that of collicular saccade-related output cells. Third, the incertopretectal projection derives from cells in ipsilateral dZI. Similarly, the return projection from the pretectum preferentially targets the ipsilateral dZI. These patterns of ZI connectivity support the contention that the main body of this nucleus should be divided into dorsal and ventral layers. The presence of a dorsolateral extension of ZI in the monkey, like that seen in the cat, suggests this characteristic becomes more prominent in animals which have larger, more complex diencephalons.
Figure 14.

Circuit diagram of connections between zona incerta (ZI) and the midbrain tectum. The collicular layers that provide gaze-related signals to the brainstem and spinal cord are targeted by a largely inhibitory (red) incertotectal projection from the ventral ZI (vZI). These same SC layers provide input to ZI that is denser in the dorsal layer (dZI) than vZI. The projection to vZI terminates on incertotectal neurons. The dZI supplies a largely excitatory (green) input to the nuclei of the pretectum. This same layer is the predominant target of inputs from the pretectal nuclei. The two layers have different targets in the dorsal thalamus and receive different inputs, a few of which are indicated.
Technical considerations
Extensive labeling of cells in vZI was a feature of all animals that had retrograde tracer placed in their SC, and the fact that animals with ZI injections displayed terminals in the SC, supports this finding. The sparse nature of the terminal labeling in the colliculus following ZI injections was surprising, considering the degree of retrograde labeling following collicular injections, but this finding is consistent with data from previous studies (Ricardo, 1981; May et al., 1997). Several factors may play a role in the limited terminal labeling. First, due to its plate-like shape, the ZI injections only involved a portion of the nucleus. Second, individual incertotectal cells may project broadly, but sparsely to the SC. Third, the incertotectal projection may represent collateral projections of axons supplying other targets. Such collateral projections do not display very effective anterograde labeling with WGA-HRP. While there is evidence that many incertal cells supply a single target (Power and Mitrofanis, 1999), it appears that vZI cells project to both the thalamus and SC (Urbain and Deschênes, 2007). In the rodent, there is strong evidence for topography in the ZI projection to SC (Kim et al., 1992). No clear topography was noted in the present study, but the large injection sites employed may have masked its presence.
The tectoincertal projection originated in a set of small cells scattered within the layers beneath SGS in both species. It is possible that these cells were labeled by spread into the substantia nigra, as tectal cells with similar morphologies and distributions supply this nucleus (McHaffie et al., 2006; May et al., 2009). However, since tectal injections of both WGA-HRP and BDA produced terminals in ZI, it seems more reasonable to suspect that the same population of cells provides input to both ZI and the nigra. A pretectoincertal projection was also observed. The anterograde evidence for this projection was only available in the monkey, but it was confirmed by the monkey and squirrel ZI injection cases, which retrogradely labeled cells in the pretectal nuclei.
The incertopretectal projection was only examined in detail in monkeys. A clear pattern of increased cell labeling was observed in dZI with injections that included the pretectum. Pretectal injections are more likely to have involved dorsal thalamic structures, so the possibility exists that some of the cells labeled in dZI might actually be projecting to the thalamus (Watanabe and Kawana, 1982; Power et al., 1999). On the other hand, the fact that ZI injections produced terminal labeling among the pretectal nuclei, supports the presence of an incertopretectal projection. Incertopretectal terminal labeling was relatively sparse; possibly for the same reasons as noted above for the incertotectal projection.
Incertal subdivisions
The rat ZI has been subdivided into up to six parts (Kuzemensky, 1977; Watanabe and Kawana, 1982). Here we have adopted a simple division into two layers: dZI and vZI. This approach works well with respect to the patterns of connectivity of this structure with the midbrain tectum (rat: Kim et al., 1992; cat: May et al., 1997; Perkins et al., 2006). We also noted that the dendritic fields of the labeled cells (Figs. 4, 5, 8 & 11), which were elongated in the mediolateral dimension and similar to the cells classified as principal neurons in Golgi preparations (Ma et al., 1992), appeared to largely respect the boundary between these two layers, in agreement with previous rodent studies (Trageser et al., 2006; Urbain and Dêschenes, 2007). Furthermore, physiological studies indicate that dZI and vZI cells have different physiological characteristics (Trageser et al., 2006; Bartho et al., 2007). Likewise, the connections of these layers differ. In addition to the SC, vZI projects to the lateral dorsal, lateral posterior and posterior nuclei of the thalamus (Power and Mitrofanis, 1999). This layer receives preferential input from frontal and parietal cortex, as well as the interposed nucleus (rat: Mitrofanis and Mikuletic, 1999), and from the frontal eye fields (cat; Perkins et al., 2006). In addition to targeting the pretectum, the dorsal ZI projects to the midbrain and pontine reticular formation, periaqueductal gray, pedunculopontine nuclei and parafascicular nucleus (Kolmac et al., 1998; Power and Mitrofanis, 1999), as well as to cerebral cortex (Nicolelis et al., 1995; Lin et al., 1997). It receives input from these same brainstem nuclei, as well as from the cingulate cortex (Kolmac et al., 1998; Mitrofanis and Mikuletic, 1999). In the present study, this division into layers was not clear at the rostral and caudal poles of ZI. Consequently, others have chosen to define these poles as separate regions of ZI (rat: Watanabe and Kawana, 1982; Kolmac et al., 1998; Mitrofanis and Mikuletic, 1999; monkey: Ma et al., 1992). We did not do this since the bands of labeled cells continued into these regions.
One of the features that we observed in the ZI of the macaque is a dorsolateral extension (dlZI) that runs from the main nucleus around the lateral edge of the dorsal thalamus. In this region, ZI is arranged as islands of neuropil within the external medullary lamina. This area is commonly included in the thalamic reticular nucleus. However, in view of the fact that bands of label continue from ZI proper into dlZI after both tectal and pretectal injections, we believe that it represents a part of ZI and has the same function. Within dlZI, a division into layers is not evident, but this may be due to its narrow width. The presence of this extension was also observed in the cat (Clemence and Mitrofanis, 1992; May et al., 1997; Kolmac and Mitrofanis, 1999; Perkins et al., 2006), but not the rat (Watanabe and Kawana, 1982; Mitrofanis, 2005). We did not observe this extension in the squirrel, but did note a change in the orientation of the incertotectal cell dendrites at the lateral edge of ZI, reminiscent of that seen in the macaque dlZI. In cats and primates, there is considerable expansion and rotation of the elements of the diencephalon that parallels the expansion of the cortex. For example, the rodent ventral lateral geniculate nucleus, sitting beneath the dorsal lateral geniculate nucleus, becomes the macaque pregeniculate nucleus, sitting above the dorsal lateral geniculate. It seems reasonable to propose that this process has pulled a portion of the ZI in a dorsolateral direction in these species.
One characteristic of these layers is that the two projections to ZI shown here terminate in both of them. The presence of separate projections targeting these two layers is a feature of many ZI afferents (Mitrofanis and Mikuletic, 1999; Mitrofanis and deFoneska, 2001; Power et al., 2001; Shaw and Mitrofanis, 2002; Perkins et al., 2006; Simpson et al., 2008). Since the ventral layer provides a predominantly inhibitory output and the dorsal layer provides a predominantly excitatory output (except the GABAergic projection to cortex (Lin et al., 1990; 1997, but see Shaw et al., 2013)), the ZI may be in position to act as a multiplex switch- turning the targets of dZI on, while it turns the targets of the vZI off in response to these bilaminate inputs (Fig. 14). Perhaps it is this capability that makes deep brain stimulation of ZI effective in relief from the symptoms of a variety of motor diseases, including Parkinsons, Tourettes and multiple sclerosis (Périer et al., 2000; Babel et al., 2001; Nandi et al., 2002; Plaha et al., 2006)
This leads to the question of what information is supplied by these midbrain inputs. The SC and posterior pretectum both contain cells that display saccade-related signals (for review: Sparks and Hartwich-Young, 1989; Gamlin, 2006). In addition, tonically firing cells that pause for saccades in all directions are found within the primate nucleus of the optic tract (Mustari et al., 1997). Omnidirectional pause cells have also been described in ZI (Ma, 1996), along with cells that stop their tonic discharge for saccades made to visual targets and spontaneous saccades made in the dark (Hikosaka and Wurtz, 1983A). Since the connections between the ZI and both the pretectum and colliculus are reciprocal, we can not specify in which direction the information is passed, but it seems more likely that any saccade-related activity changes seen in the ZI would be due to inputs from the SC and pretectum. Unfortunately, the ZI layer that contains saccade-related cells has not been determined, but the fact that they are tonically active suggests that they are inhibitory neurons in vZI (Trageser et al., 2006). We will further discuss the roles that are suggested by the connections of each layer, below.
Dorsal zona incerta
Our results indicate the pretectum projects to ZI in the monkey, in agreement with previous studies (monkey: Harting et al., 1980; Benevento et al., 1977; Mustari et al., 1994; Büttner-Ennever et al., 1996; cat: May et al., 1997) and preferentially terminate in dZI (Fig. 14). A similar pretectoincertal projection has been seen in the cat, although the terminal field was densest along the border of dZI and vZI (Berman 1977; May et al., 1997). The location of the cells of origin for this projection is fairly diffuse. In the cat and rat, most of the cells labeled retrogradely from ZI injections were located in the anterior and posterior pretectal nuclei, and in the nucleus of the optic tract (rat: Roger and Cadusseau, 1985; cat: May et al., 1997). Labeled cells were found in these nuclei in the present experiments, as well as in the nucleus of the posterior commissure. In the rat and cat, the largest number of cells was found in the anterior pretectal nucleus (Roger and Cadusseau, 1985; May et al., 1997), but this was not true of the monkey and squirrel. In the rat, the anterior pretectal nucleus projects mainly to vZI (Giber et al., 2008), so it is likely that the pretectal terminals observed in the macaque dZI are from the other pretectal nuclei.
The dZI projects throughout much of the neuraxis, including the brainstem, dorsal thalamus and cerebral cortex (Watanabe and Kawana, 1982; Ricardo, 1981; Romanowska et al., 1985; Power et al., 1999). The present results indicate the dZI also provides a projection back to the pretectum. A similar projection was observed in the rat and cat (Ricardo et al., 1981; May et al., 1997). Thus, dZI is in a position to modulate activity in a wide variety of structures. Mitrofanis (2005) has suggested that the dZI plays a role in arousal, based on its projection to the intralaminar nuclei. In agreement with this, incertal neuron activity is inhibited by ascending cholinergic inputs (Trageser et al., 2006). However, Wantanabe and Kawana (1982) asserted that the intralaminar projection arises from the vZI and it is the cells in this layer that are more influenced by cholinergic inputs (Trageser et al., 2006). A related possibility is suggested by the dZI inputs from the SC and pretectum shown here. Due to the fact that attention is commonly directed with the eyes, it is believed that the ascending projections of the SC are also utilized to direct attention (for review: Basso and May, 2017). In the case of dZI, such information might be used to change activity levels in its cortical, thalamic and brainstem targets when interesting stimuli appear.
Alternatively, a sensory, as opposed to a saccade-related, function may be supported by the tectal and pretectal inputs to dZI. It has been suggested that the tectonigral projection may carry multimodal sensory information (rat: Coizet et al., 2003; cat: McHaffie et al., 2006; monkey: May et al., 2009), and, as noted above, the tectoincertal cells resemble these tectonigral cells. Similarly, the pretectum receives both retinal and somatosensory input (cat: Schweigart and Hoffman, 1992; Rees and Roberts, 1993; monkey: Hutchins and Weber, 1985; Mustari and Fuchs, 1990; Hutchins 1991), which it may pass on to dZI. The dZI in rats projects to the substantia nigra, as well as to the pedunculopontine tegmental nucleus and enteropeduncular nucleus (Heise and Mitrofanis, 2004). Another major target of dZI is the parafascicular nuclei (Power et al., 1999; Power and Mitrofanis, 2002), which in turn supplies the striatum. In view of these connections with components of the basal ganglia (Fig. 14), this multimodal excitatory pathway through ZI to the basal ganglia, may be important for directing learned and habitual behaviors (McHaffie et al., 2006; May et al., 2009; Watson et al., 2015). The fact that dZI is particularly targeted by cingulate cortex supports this idea (Mitofanis and Mikuletic, 1999).
Ventral zona incerta
We have observed a tectoincertal projection that terminates in vZI in squirrels, monkeys and cats (present results, Fig. 14; May et al., 1997). A similar projection was observed in the rat (Kim et al., 1992; Kolmac et al., 1998). So it appears that SC information is made available to the incertotectal cells in vZI. Indeed, this input appears to monosynaptically contact these cells (May et al., 1997; Fig. 10D), as well as cells that project to the posterior thalamic nucleus (Watson et al., 2015). In all animals studied to date, the source of this tectoincertal projection appears to be the deeper, motor output layers of the SC (rat: Roger and Cadusseau, 1985; Kim et al., 1992; cat: May et al., 1997). The overlap between this population and the location of the predorsal bundle output cells in the SC is particularly reinforced by the results in the squirrel, where both populations are concentrated in inner SGI (May and Hall, 1984; Fig. 6). However, the cells of origin of the tectoincertal pathway appeared to be relatively smaller and more homogeneous (Fig. 10) than the cells projecting in the predorsal bundle (squirrel: May and Hall, 1984; monkey: May and Porter, 1992), so it seems unlikely that the ZI receives an efference copy of the saccade-related long lead burst output of the tectum. Instead, as discussed above, tectoincertal cells may carry a sensory signal.
A projection from the vZI to the SC has been observed in all species investigated, suggesting this is a common mammalian feature (rat: Watanabe and Kawana, 1982; Romanowski et al., 1985; Kolmac et al., 1998; cat: Edwards et al., 1979; Ficalora and Mize, 1989; May et al., 1997; squirrel and monkey: present results). The distribution of incertotectal terminals shows some species variation. The terminal field in the monkey was denser in the lower sublamina of SGI, but sparse terminations were observed in the upper sublamina and in SGP, a pattern also seen in cats (May et al., 1997). In the squirrel, the terminal field was largely restricted to the lower sublamina of SGI and SGP, and appears similar to that described for rats (Ricardo, 1981, Kim et al., 1992). In all cases, incertotectal terminal fields are well correlated with the location of the cells of origin of the predorsal bundle (cat: Kawamura and Hashikawa, 1978; squirrel: May and Hall, 1984; monkey: May and Porter, 1992; Robinson et al., 1994; rat: Redgrave et al., 1990). Indeed, Kim and colleagues (1992) demonstrated synaptic contact by incertotectal terminals onto predorsal bundle cells. Thus, it appears that the crossed descending output pathway from the SC that initiates orienting movements of the eyes and head is targeted by the incertotectal projection (Grantyn and Grantyn, 1982; Moschovakis et al., 1988), and that vZI is specifically involved in this function. Since this layer is primarily made up of GABAergic cells (Kolmac and Mitrofanis, 1999; Mitrofanis et al., 2004), it is highly likely that the vZI exerts an inhibitory influence over collicular saccade-related activity (Ficarrola and Mize, 1989; Appell and Behan, 1991) (Fig. 14).
As noted in the introduction, inhibition plays many roles in the SC. The role played by the ZI’s inhibitory input to SC saccade initiation cells is unknown. In addition to the inputs from the SC and pretectum described here, vZI is targeted preferentially by the trigeminal sensory nuclei and somatosensory cortex (Nicolelis et al., 1992; Mitrofanis and Mikuletic, 1999; Shaw and Mitrofanis, 2002; Perkins et al., 2006; Simpson et al., 2008). These inputs produce a topographic body representation within vZI (Nicolelis et al., 1992), although the size of receptive fields is relatively large, and many cells have high tonic activity that is suppressed by stimulation. The inferior colliculus also preferentially projects to a portion of vZI (Mitrofanis, 2002B). Taken together, these projections suggest that the incertotectal projection could form a pathway whereby multisensory information modulates the saccade-related activity in the SC. However, vZI also receives additional inputs from the interposed nucleus of the cerebellum and the red nucleus (Mitrofanis and deFonseka, 2001; Mitrofanis, 2002A). While these inputs do not preferentially involve vZI, they do indicate that its role is unlikely to be purely sensory in nature.
Studies of the vZI projection to the posterior thalamic nucleus in rats may provide insight into the workings of the incertotectal pathway. This thalamic projection, like the one to the SC, is GABAergic (Bartho et al., 2002). Tonically firing inhibitory, incertothalamic projections block the capacity of ascending trigeminal projections conveying vibrissal stimulation to directly activate thalamocortical cells within the posterior nucleus (Trageser and Keller, 2004; Levallée et al., 2005). Furthermore, the activity of these inhibitory cells is actually increased by trigeminal inputs to vZI, so this incertothalamic projection represents a feed forward inhibitory pathway gating the access of somatosensory inputs to the posterior nucleus. This inhibition can be suppressed, allowing vibrissal activation of the posterior nucleus, by stimulating motor cortex. This is due to motor cortex projections that cause tonically active vZI cells to pause (Urbain and Deschênes, 2007). The proposed mechanism for this pause involves local conections between GABAergic incertal neurons (Urbain and Deschênes, 2007). Indeed, there is considerable evidence for interconnections between incertal neurons (rat: Power and Mitrofanis, 1999; cat: May et al., 1997), due to local axon collaterals emitted by ZI projection cells (Urbain and Deschênes, 2007). Based on these findings, Urbain and Deschênes (2007) have proposed that when the rat actively investigates an object with its whiskers, motor cortex projections cause their ZI target cells to inhibit incertothalamic neurons. The loss of inhibition in the posterior nucleus then permits ascending sensory information to activate thalamocortical neurons. This model may be applicable to the incertotectal projections. Connections between its GABAergic neurons may explain the pauses observed in ZI neuron activity with respect to saccadic eye movements (Hikosaka and Wurtz, 1983A, Ma, 1996). Perhaps, based on the pattern of activity coming to vZI, the incertotectal projection helps determine which of the sensory inputs competing to direct the next saccade has access to saccade-related output cells in the intermediate gray layers. In light of the role of motor cortex described above, it is worth noting that inputs to vZI come from the frontal eye fields (Fig. 14) (cat: Perkins et al., 2006; monkey: Huerta et al., 1986).
Many have suggested that the ZI is closely allied with the hypothalamus, and the borders between these structures are unclear, with some transmitter-specific populations extending between them (see Mitrofanis, 2005 for review). Of particular note are studies linking the ZI to ingestion of food, water and salt (Walsh, 1973; Huang and Mogenson, 1974; Evered and Mogenson, 1977; Kendrick and Baldwin, 1989). Studies in awake behaving sheep indicate that neurons in the ZI are activated by the sight of approaching food at levels defined by individual preference (Kendrick and Baldwin, 1986; 1989). This response extinguishes if the food is not available for consumption and it can be paired to non-food stimuli by classical conditioning. Furthermore, these stimuli produce a release of GABA in the ZI (Kendrick et al., 1991), indicating that it is highly likely that they involve vZI tectoincertal cells. Interestingly, this response is specific to the internal needs of the animal. The ZI in salt deprived animals only responds to the presence of salt, not food, while the ZI in food deprived animals responds just to food. Thus, these studies suggest that the incertotectal projection may be crucial to matching the selection of targets for orientation with the internal needs of the animal.
Conventional behavioral testing of collicular function in non-human primates has traditionally involved the presentation of individual neutral targets that have been paired with a sweetened fluid reinforcer to induce the animal to saccade to them. Given the findings described above, it seems possible that vZI could play an important role in the target selection process under such conditions. Furthermore, given its inhibitory nature, the incertotectal projection may be particularly important for suppressing activity related to less attractive targets with respect to the current needs of the individual. The high level of trigeminal input to this circuit in the rat may be due to the important role that the vibrissa play in food identification in this species (Comoli et al., 2003). When we open the refrigerator door, whether we select a food item or a drink is dependent on whether we are thirsty or hungry. Perhaps the incertotectal pathway is providing crucial information to direct our gaze towards the targets that match our internal needs and away from targets that do not match our needs within a highly complex visual environment.
Acknowledgments
This manuscript is dedicated to the memory of Dr. Rick Chia-Sheng Lin, whose infectious enthusiasm for the zona incerta and all his other interests will be sorely missed. We would also like to thank Drs. Susan Warren, Kimberly Simpson and William C. Hall for their thoughtful readings of earlier versions of this work. This work was supported by National Institutes of Health grant #EY019663 to MAB and PJM
ABBREVIATIONS
- III
oculomotor nucleus
- APt
anterior pretectal nucleus
- BDA
biotinylated dextran amine
- BP
basilar pons
- DLG
dorsal lateral geniculate nucleus
- EML
external medullary lamina
- GP
globus pallidus
- HRP
horseradish peroxidase
- IC
inferior colliculus
- ICa
internal capsule
- Lim
nucleus limitans
- MG
medial geniculate nucleus
- MPt
medial pretectal nucleus
- nPC
nucleus of the posterior commissure
- nOT
nucleus of the optic tract
- Opt
stratum opticum
- OT
optic tract
- PAG
periaqueductal gray
- PB
parabrachial nucleus
- PG
pregeniculate
- Pul
pulvinar
- Pt
pretectum
- PPt
posterior pretectal nucleus
- RT
reticular thalamic nucleus
- SAI
intermediate white layer
- SC
superior colliculus
- SGS
stratum griseum superficiale
- SGI
stratum griseum intermediale
- lSGI
SGI, lower sublamina
- uSGI
SGI, upper sublamina
- SGP
stratum griseum profundum
- SNc
substantia nigra pars compacta
- SNl
substantia nigra pars lateralis
- SNr
substantia nigra pars reticulata
- SO
stratum opticum
- STh
subthalamic nucleus
- WGA-HRP
wheat germ agglutinin conjugated HRP
- VLG
ventral lateral geniculate nucleus
- VP
ventral posterior nucleus
- ZI
zona incerta
- dZI
dorsal layer of ZI
- vZI
ventral layer of ZI
Footnotes
Conflict of interest statement
The authors have no actual or apparent conflicts of interest to report.
Statement on the welfare of animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
BIBLIOGRAPHY
- Adams JC. Technical considerations on the use of horseradish peroxidase as a neuronal marker. Neuroscience. 1977;2:141–5. doi: 10.1016/0306-4522(77)90074-4. [DOI] [PubMed] [Google Scholar]
- Appell PP, Behan M. Sources of subcortical GABAergic projections to the superior colliculus in the cat. J Comp Neurol. 1990;302:143–58. doi: 10.1002/cne.903020111. [DOI] [PubMed] [Google Scholar]
- Babel TB, Warnke PC, Ostertag CB. Immediate and long term outcome after infrathalamic and thalamic lesioning for intractable Tourette’s syndrome. J Neurol Neurosurg Psychiatry. 2001;70:666–71. doi: 10.1136/jnnp.70.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthó P, Freund TF, Acsády L. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur J Neurosci. 2002;16:999–1014. doi: 10.1046/j.1460-9568.2002.02157.x. [DOI] [PubMed] [Google Scholar]
- Barthó P, Slézia A, Varga V, Bokor H, Pinault D, Buzsáki G, Acsády L. Cortical control of zona incerta. J Neurosci. 2007;27:1670–81. doi: 10.1523/JNEUROSCI.3768-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, May PJ. Circuits for action and cognition: A view from the superior colliculus. Ann Rev Neurosci. 2017 doi: 10.1146/annurev-vision-102016-061234. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevento LA, Rezak M, Santos-Anderson An autoradiographic study of the projections of the pretectum in the rhesus monkey (Macaca mulatta): evidence for sensorimotor links to the thalamus and oculomotor nuclei. Brain Res. 1977;127:197–218. doi: 10.1016/0006-8993(77)90536-4. [DOI] [PubMed] [Google Scholar]
- Berman N. Connections of the pretectum in the cat. J Comp Neurol. 1977;174:227–54. doi: 10.1002/cne.901740204. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Cohen B, Horn AK, Reisine H. Efferent pathways of the nucleus of the optic tract in monkey and their role in eye movements. J Comp Neurol. 1996;373:90–107. doi: 10.1002/(SICI)1096-9861(19960909)373:1<90::AID-CNE8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Chen B, May PJ. The feedback circuit connecting the superior colliculus and central mesencephalic reticular formation: a direct morphological demonstration. Exp Brain Res. 2000;131:10–21. doi: 10.1007/s002219900280. [DOI] [PubMed] [Google Scholar]
- Clemence AE, Mitrofanis J. Cytoarchitectonic heterogeneities in the thalamic reticular nucleus of cats and ferrets. J Comp Neurol. 1992;322:167–180. doi: 10.1002/cne.903220203. [DOI] [PubMed] [Google Scholar]
- Comoli E, Ribeiro-Barbosa ER, Canteras NS. Predatory hunting and exposure to a live predator induce opposite patterns of Fos immunoreactivity in the PAG. Behav Brain Res. 2003;138:17–28. doi: 10.1016/s0166-4328(02)00197-3. [DOI] [PubMed] [Google Scholar]
- Coizet V, Comoli E, Westby GW, Redgrave P. Phasic activation of substantia nigra and the ventral tegmental area by chemical stimulation of the superior colliculus: an electrophysiological investigation in the rat. Eur J Neurosci. 2003;17:28–40. doi: 10.1046/j.1460-9568.2003.02415.x. [DOI] [PubMed] [Google Scholar]
- Cromer JA, Waitzman DM. Comparison of saccade-associated neuronal activity in the primate central mesencephalic and paramedian pontine reticular formations. J Neurophysiol. 2007;98:835–50. doi: 10.1152/jn.00308.2007. [DOI] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Westby GW. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 1989;12:137–47. doi: 10.1016/0166-2236(89)90052-0. [DOI] [PubMed] [Google Scholar]
- Edwards SB, Ginsburgh CL, Henkel CK, Stein BE. Sources of subcortical projections to the superior colliculus in the cat. J Comp Neurol. 1979;184:309–29. doi: 10.1002/cne.901840207. [DOI] [PubMed] [Google Scholar]
- Evered MD, Mogenson GJ. Impairment in fluid ingestion in rats with lesions of the zona incerta. Am J Physiol. 1977;233:R53–8. doi: 10.1152/ajpregu.1977.233.1.R53. [DOI] [PubMed] [Google Scholar]
- Ficalora AS, Mize RR. The neurons of the substantia nigra and zona incerta which project to the cat superior colliculus are GABA immunoreactive: a double-label study using GABA immunocytochemistry and lectin retrograde transport. Neuroscience. 1989;29:567–81. doi: 10.1016/0306-4522(89)90131-0. [DOI] [PubMed] [Google Scholar]
- Gamlin PD. The pretectum: connections and oculomotor-related roles. Prog Brain Res. 2006;151:379–405. doi: 10.1016/S0079-6123(05)51012-4. [DOI] [PubMed] [Google Scholar]
- Giber K, Slézia A, Bokor H, Bodor AL, Ludányi A, Katona I, Acsády L. Heterogeneous output pathways link the anterior pretectal nucleus with the zona incerta and the thalamus in rat. J Comp Neurol. 2008;506:122–40. doi: 10.1002/cne.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantyn A, Grantyn R. Axonal patterns and sites of termination of cat superior colliculus neurons projecting in the tecto-bulbo-spinal tract. Exp Brain Res. 1982;46:243–56. doi: 10.1007/BF00237182. [DOI] [PubMed] [Google Scholar]
- Harting JK, Huerta MF, Frankfurter AJ, Strominger NL, Royce GJ. Ascending pathways from the monkey superior colliculus: an autoradiographic analysis. J Comp Neurol. 1980;192:853–82. doi: 10.1002/cne.901920414. [DOI] [PubMed] [Google Scholar]
- Harting JK, Huerta MF, Hashikawa T, Weber JT, Van Lieshout DP. Neuroanatomical studies of the nigrotectal projection in the cat. J Comp Neurol. 1988;278:615–31. doi: 10.1002/cne.902780412. [DOI] [PubMed] [Google Scholar]
- Heise CE, Mitrofanis J. Evidence for a glutamatergic projection from the zona incerta to the basal ganglia of rats. J Comp Neurol. 2004;468:482–95. doi: 10.1002/cne.10971. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol. 1983A;49:1230–53. doi: 10.1152/jn.1983.49.5.1230. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol. 1983B;49:1285–301. doi: 10.1152/jn.1983.49.5.1285. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol. 1985;53:266–91. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- Huang YH, Mogenson GJ. Differential effects of incertal and hypothalamic lesions on food and water intake. Exp Neurol. 1974;43:276–80. doi: 10.1016/0014-4886(74)90146-0. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys: I. Subcortical connections. J Comp Neurol. 1986;253:415–39. doi: 10.1002/cne.902530402. [DOI] [PubMed] [Google Scholar]
- Hutchins B. Evidence for a direct retinal projection to the anterior pretectal nucleus in the cat. Brain Res. 1991;561:169–73. doi: 10.1016/0006-8993(91)90764-m. [DOI] [PubMed] [Google Scholar]
- Hutchins B, Weber JT. The pretectal complex of the monkey: a reinvestigation of the morphology and retinal terminations. J Comp Neurol. 1985;232:425–42. doi: 10.1002/cne.902320402. [DOI] [PubMed] [Google Scholar]
- Ingle DJ. Prey-catching behavior of anurans toward moving and stationary objects. Vision Res Suppl. 1971;3:447–56. doi: 10.1016/0042-6989(71)90057-5. [DOI] [PubMed] [Google Scholar]
- Isa T, Saito Y. The direct visuo-motor pathway in mammalian superior colliculus; novel perspective on the interlaminar connection. Neurosci Res. 2001;41:107–13. doi: 10.1016/s0168-0102(01)00278-4. [DOI] [PubMed] [Google Scholar]
- Jayaraman A, Batton RR, 3rd, Carpenter MB. Nigrotectal projections in the monkey: an autoradiographic study. Brain Res. 1977;135:147–52. doi: 10.1016/0006-8993(77)91058-7. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Phongphanphanee P, Katoh T, Isa K, Yanagawa Y, Obata K, Isa T. Regulation of burst activity through presynaptic and postsynaptic GABA(B) receptors in mouse superior colliculus. J Neurosci. 2008;28:816–27. doi: 10.1523/JNEUROSCI.4666-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Hashikawa T. Cell bodies of origin of reticular projections from the superior colliculus in the cat: an experimental study with the use of horseradish peroxidase as a tracer. J Comp Neurol. 1978;182:1–15. doi: 10.1002/cne.901820102. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Baldwin BA. The activity of neurones in the lateral hypothalamus and zona incerta of the sheep responding to the sight or approach of food is modified by learning and satiety and reflects food preference. Brain Res. 1986;375:320–8. doi: 10.1016/0006-8993(86)90752-3. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Baldwin BA. The effects of sodium appetite on the responses of cells in the zona incerta to the sight or ingestion of food, salt and water in sheep. Brain Res. 1989;492:211–8. doi: 10.1016/0006-8993(89)90903-7. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Hinton MR, Baldwin BA. GABA release in the zona incerta of the sheep in response to the sight and ingestion of food and salt. Brain Res. 1991;550:165–8. doi: 10.1016/0006-8993(91)90423-s. [DOI] [PubMed] [Google Scholar]
- Kim U, Gregory E, Hall WC. Pathway from the zona incerta to the superior colliculus in the rat. J Comp Neurol. 1992;321:555–75. doi: 10.1002/cne.903210405. [DOI] [PubMed] [Google Scholar]
- Kolmac CI, Power BD, Mitrofanis J. Patterns of connections between zona incerta and brainstem in rats. J Comp Neurol. 1998;396:544–55. doi: 10.1002/(sici)1096-9861(19980713)396:4<544::aid-cne10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kolmac C, Mitrofanis J. Distribution of various neurochemicals within the zona incerta: an immunocytochemical and histochemical study. Anat Embryol (Berl) 1999;199:265–80. doi: 10.1007/s004290050227. [DOI] [PubMed] [Google Scholar]
- Kuzemenský J. Contribution to the cytoarchitectonics of the zona incerta and Forel’s field in rodents. Folia Morphol (Praha) 1977;25:366–70. [PubMed] [Google Scholar]
- Lavallée P, Urbain N, Dufresne C, Bokor H, Acsády L, Deschênes M. Feedforward inhibitory control of sensory information in higher-order thalamic nuclei. J Neurosci. 2005;25:7489–98. doi: 10.1523/JNEUROSCI.2301-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS, Nicolelis MA, Schneider JS, Chapin JK. A major direct GABAergic pathway from zona incerta to neocortex. Science. 1990;248:1553–6. doi: 10.1126/science.2360049. [DOI] [PubMed] [Google Scholar]
- Lin RC, Nicolelis MA, Chapin JK. Topographic and laminar organizations of the incertocortical pathway in rats. Neuroscience. 1997;81:641–51. doi: 10.1016/s0306-4522(97)00094-8. [DOI] [PubMed] [Google Scholar]
- Lu SM, Lin CS, Behan M, Cant NB, Hall WC. Glutamate decarboxylase immunoreactivity in the intermediate grey layer of the superior colliculus in the cat. Neuroscience. 1985;16:123–31. doi: 10.1016/0306-4522(85)90051-x. [DOI] [PubMed] [Google Scholar]
- Ma TP. Saccade-related omnivectoral pause neurons in the primate zona incerta. Neuroreport. 1996;7:2713–6. doi: 10.1097/00001756-199611040-00061. [DOI] [PubMed] [Google Scholar]
- Ma TP, Hu XJ, Anavi Y, Rafols JA. Organization of the zona incerta in the macaque: a Nissl and Golgi study. J Comp Neurol. 1992;320:273–90. doi: 10.1002/cne.903200302. [DOI] [PubMed] [Google Scholar]
- May PJ, Hall WC. Relationships between the nigrotectal pathway and the cells of origin of the predorsal bundle. J Comp Neurol. 1984;226:357–76. doi: 10.1002/cne.902260306. [DOI] [PubMed] [Google Scholar]
- May PJ, Hall WC. The sources of the nigrotectal pathway. Neuroscience. 1986;19:159–80. doi: 10.1016/0306-4522(86)90013-8. [DOI] [PubMed] [Google Scholar]
- May PJ, Hartwich-Young R, Nelson J, Sparks DL, Porter JD. Cerebellotectal pathways in the macaque: implications for collicular generation of saccades. Neuroscience. 1990;36:305–24. doi: 10.1016/0306-4522(90)90428-7. [DOI] [PubMed] [Google Scholar]
- May PJ, McHaffie JG, Stanford TR, Jiang H, Costello MG, Coizet V, Hayes LM, Haber SN, Redgrave P. Tectonigral projections in the primate: a pathway for pre-attentive sensory input to midbrain dopaminergic neurons. Eur J Neurosci. 2009;29:575–87. doi: 10.1111/j.1460-9568.2008.06596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ, Porter JD. The laminar distribution of macaque tectobulbar and tectospinal neurons. Vis Neurosci. 1992;8:257–76. doi: 10.1017/s0952523800002911. [DOI] [PubMed] [Google Scholar]
- May PJ, Sun W, Hall WC. Reciprocal connections between the zona incerta and the pretectum and superior colliculus of the cat. Neuroscience. 1997;77:1091–114. doi: 10.1016/s0306-4522(96)00535-0. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Jiang H, May PJ, Coizet V, Overton PG, Stein BE, Redgrave P. A direct projection from superior colliculus to substantia nigra pars compacta in the cat. Neuroscience. 2006;138:221–34. doi: 10.1016/j.neuroscience.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978;26:106–17. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J. A) Distinctive patterns of connectivity between the zona incerta and the red nucleus of rats. Anat Embryol (Berl) 2002;205:283–9. doi: 10.1007/s00429-002-0259-4. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J. B) Evidence for an auditory subsector within the zona incerta of rats. Anat Embryol (Berl) 2002;205:453–62. doi: 10.1007/s00429-002-0268-3. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J. Some certainty for the “zone of uncertainty”? Exploring the function of the zona incerta. Neuroscience. 2005;130:1–15. doi: 10.1016/j.neuroscience.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J, deFonseka R. Organisation of connections between the zona incerta and the interposed nucleus. Anat Embryol (Berl) 2001;204:153–9. doi: 10.1007/s004290100187. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J, Mikuletic L. Organisation of the cortical projection to the zona incerta of the thalamus. J Comp Neurol. 1999;412:173–85. [PubMed] [Google Scholar]
- Mitrofanis J, Ashkan K, Wallace BA, Benabid AL. Chemoarchitectonic heterogeneities in the primate zona incerta: clinical and functional implications. J Neurocytol. 2004;33:429–40. doi: 10.1023/B:NEUR.0000046573.28081.dd. [DOI] [PubMed] [Google Scholar]
- Mize RR. Immunocytochemical localization of gamma-aminobutyric acid (GABA) in the cat superior colliculus. J Comp Neurol. 1988;276:169–87. doi: 10.1002/cne.902760203. [DOI] [PubMed] [Google Scholar]
- Mize RR, Jeon CJ, Hamada OL, Spencer RF. Organization of neurons labeled by antibodies to gamma-aminobutyric acid (GABA) in the superior colliculus of the rhesus monkey. Vis Neurosci. 1991;6:75–92. doi: 10.1017/s0952523800000924. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus. II. Morphological identity of presaccadic neurons. J Neurophysiol. 1988;60:263–302. doi: 10.1152/jn.1988.60.1.263. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol. 1998;79:1193–209. doi: 10.1152/jn.1998.79.3.1193. [DOI] [PubMed] [Google Scholar]
- Mustari MJ, Fuchs AF. Discharge patterns of neurons in the pretectal nucleus of the optic tract (NOT) in the behaving primate. J Neurophysiol. 1990;64:77–90. doi: 10.1152/jn.1990.64.1.77. [DOI] [PubMed] [Google Scholar]
- Mustari MJ, Fuchs AF, Kaneko CR, Robinson FR. Anatomical connections of the primate pretectal nucleus of the optic tract. J Comp Neurol. 1994;349:111–28. doi: 10.1002/cne.903490108. [DOI] [PubMed] [Google Scholar]
- Mustari MJ, Fuchs AF, Pong M. Response properties of pretectal omnidirectional pause neurons in the behaving primate. J Neurophysiol. 1997;77:116–25. doi: 10.1152/jn.1997.77.1.116. [DOI] [PubMed] [Google Scholar]
- Nandi D, Chir M, Liu X, Bain P, Parkin S, Joint C, Winter J, Stein J, Scott R, Gregory R, Aziz T. Electrophysiological confirmation of the zona incerta as a target for surgical treatment of disabling involuntary arm movements in multiple sclerosis: use of local field potentials. J Clin Neurosci. 2002;9:64–8. doi: 10.1054/jocn.2001.1012. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Chapin JK, Lin RC. Somatotopic maps within the zona incerta relay parallel GABAergic somatosensory pathways to the neocortex, superior colliculus, and brainstem. Brain Res. 1992;577:134–41. doi: 10.1016/0006-8993(92)90546-l. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Chapin JK, Lin RC. Development of direct GABAergic projections from the zona incerta to the somatosensory cortex of the rat. Neuroscience. 1995;65:609–31. doi: 10.1016/0306-4522(94)00493-o. [DOI] [PubMed] [Google Scholar]
- Olucha F, Martínez-García F, López-García C. A new stabilizing agent for the tetramethyl benzidine (TMB) reaction product in the histochemical detection of horseradish peroxidase (HRP) J Neurosci Methods. 1985;13:131–8. doi: 10.1016/0165-0270(85)90025-1. [DOI] [PubMed] [Google Scholar]
- Ozen G, Augustine GJ, Hall WC. Contribution of superficial layer neurons to premotor bursts in the superior colliculus. J Neurophysiol. 2000;84:460–71. doi: 10.1152/jn.2000.84.1.460. [DOI] [PubMed] [Google Scholar]
- Périer C, Vila M, Féger J, Agid Y, Hirsch EC. Functional activity of zona incerta neurons is altered after nigrostriatal denervation in hemiparkinsonian rats. Exp Neurol. 2000;162:215–24. doi: 10.1006/exnr.1999.7331. [DOI] [PubMed] [Google Scholar]
- Perkins E, Warren S, Lin RC, May PJ. Projections of somatosensory cortex and frontal eye fields onto incertotectal neurons in the cat. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1310–29. doi: 10.1002/ar.a.20400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins E, Warren S, May PJ. The mesencephalic reticular formation as a conduit for primate collicular gaze control: tectal inputs to neurons targeting the spinal cord and medulla. Anat Rec. 2009;292:1162–81. doi: 10.1002/ar.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain. 2006;129:1732–47. doi: 10.1093/brain/awl127. [DOI] [PubMed] [Google Scholar]
- Power BD, Kolmac CI, Mitrofanis J. Evidence for a large projection from the zona incerta to the dorsal thalamus. J Comp Neurol. 1999;404:554–65. [PubMed] [Google Scholar]
- Power BD, Mitrofanis J. Specificity of projection among cells of the zona incerta. J Neurocytol. 1999;28:481–93. doi: 10.1023/a:1007005105679. [DOI] [PubMed] [Google Scholar]
- Power BD, Leamey CA, Mitrofanis J. Evidence for a visual subsector within the zona incerta. Vis Neurosci. 2001;18:179–86. doi: 10.1017/s0952523801182027. [DOI] [PubMed] [Google Scholar]
- Power BD, Mitrofanis J. Ultrastructure of afferents from the zona incerta to the posterior and parafascicular thalamic nuclei of rats. J Comp Neurol. 2002;451:33–44. doi: 10.1002/cne.10332. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Dean P, Westby GW. Organization of the crossed tecto-reticulo-spinal projection in rat. I. Anatomical evidence for separate output channels to the periabducens area and caudal medulla. Neuroscience. 1990;37:571–84. doi: 10.1016/0306-4522(90)90092-i. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Marrow L, Dean P. Topographical organization of the nigrotectal projection in rat: evidence for segregated channels. Neuroscience. 1992;50:571–95. doi: 10.1016/0306-4522(92)90448-b. [DOI] [PubMed] [Google Scholar]
- Rees H, Roberts MH. The anterior pretectal nucleus: a proposed role in sensory processing. Pain. 1993;53:121–35. doi: 10.1016/0304-3959(93)90072-W. [DOI] [PubMed] [Google Scholar]
- Ricardo JA. Efferent connections of the subthalamic region in the rat. II. The zona incerta. Brain Res. 1981;214:43–60. doi: 10.1016/0006-8993(81)90437-6. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Phillips JO, Fuchs AF. Coordination of gaze shifts in primates: brainstem inputs to neck and extraocular motoneuron pools. J Comp Neurol. 1994;346:43–62. doi: 10.1002/cne.903460104. [DOI] [PubMed] [Google Scholar]
- Roger M, Cadusseau J. Afferents to the zona incerta in the rat: a combined retrograde and anterograde study. J Comp Neurol. 1985;241:480–92. doi: 10.1002/cne.902410407. [DOI] [PubMed] [Google Scholar]
- Romanowski CA, Mitchell IJ, Crossman AR. The organisation of the efferent projections of the zona incerta. J Anat. 1985;143:75–95. [PMC free article] [PubMed] [Google Scholar]
- Sato M, Hikosaka O. Role of primate substantia nigra pars reticulata in reward-oriented saccadic eye movement. J Neurosci. 2002;22:2363–73. doi: 10.1523/JNEUROSCI.22-06-02363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigart G, Hoffmann KP. Pretectal jerk neuron activity during saccadic eye movements and visual stimulations in the cat. Exp Brain Res. 1992;91(2):273–83. doi: 10.1007/BF00231660. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Negrão N, Ricardo JA. Afferent connections of the zona incerta: a horseradish peroxidase study in the rat. Neuroscience. 1985;15:109–34. doi: 10.1016/0306-4522(85)90127-7. [DOI] [PubMed] [Google Scholar]
- Shaw V, Mitrofanis J. Anatomical evidence for somatotopic maps in the zona incerta of rats. Anat Embryol (Berl) 2002;206:119–30. doi: 10.1007/s00429-002-0280-7. [DOI] [PubMed] [Google Scholar]
- Shaw FZ, Liao YF, Chen RF, Huang YH, Lin RC. The zona incerta modulates spontaneous spike-wave discharges in the rat. J Neurophysiol. 2013;109:2505–16. doi: 10.1152/jn.00750.2011. [DOI] [PubMed] [Google Scholar]
- Simpson K, Wang Y, Lin RC. Patterns of convergence in rat zona incerta from the trigeminal nuclear complex: light and electron microscopic study. J Comp Neurol. 2008;507:1521–41. doi: 10.1002/cne.21624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Hodgson AJ, Smith AD. An approach to tracing neuron networks in the cerebral cortex and basal ganglia. Combination of Golgi staining, retrograde transport of horseradish peroxidase and anterograde degeneration of synaptic boutons in the same material. Neuroscience. 1979;4:1805–52. doi: 10.1016/0306-4522(79)90059-9. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hartwich-Young R. The deep layers of the superior colliculus. Rev Oculomot Res. 1989;3:213–55. [PubMed] [Google Scholar]
- Szabo J, Cowan WM. A stereotaxic atlas of the brain of the cynomolgus monkey (Macaca fascicularis) J Comp Neurol. 1984;222:265–300. doi: 10.1002/cne.902220208. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Sugiuchi Y, Izawa Y, Shinoda Y. Commissural excitation and inhibition by the superior colliculus in tectoreticular neurons projecting to omnipause neuron and inhibitory burst neuron regions. J Neurophysiol. 2005;94:1707–26. doi: 10.1152/jn.00347.2005. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Sugiuchi Y, Shinoda Y. Commissural mirror-symmetric excitation and reciprocal inhibition between the two superior colliculi and their roles in vertical and horizontal eye movements. J Neurophysiol. 2007;98:2664–82. doi: 10.1152/jn.00696.2007. [DOI] [PubMed] [Google Scholar]
- Trageser JC, Keller A. Reducing the uncertainty: gating of peripheral inputs by zona incerta. J Neurosci. 2004;24:8911–5. doi: 10.1523/JNEUROSCI.3218-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trageser JC, Burke KA, Masri R, Li Y, Sellers L, Keller A. State-dependent gating of sensory inputs by zona incerta. J Neurophysiol. 2006;96:1456–63. doi: 10.1152/jn.00423.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain N, Deschênes M. Motor cortex gates vibrissal responses in a thalamocortical projection pathway. Neuron. 2007;56:714–25. doi: 10.1016/j.neuron.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Waitzman DM, Silakov VL, Cohen B. Central mesencephalic reticular formation (cMRF) neurons discharging before and during eye movements. J Neurophysiol. 1996;75:1546–72. doi: 10.1152/jn.1996.75.4.1546. [DOI] [PubMed] [Google Scholar]
- Walsh LL, Grossman SP. Zona incerta lesions: disruption of regulatory water intake. Physiol Behav. 1973;11:885–7. doi: 10.1016/0031-9384(73)90285-0. [DOI] [PubMed] [Google Scholar]
- Wang N, Warren S, May PJ. The macaque midbrain reticular formation sends side-specific feedback to the superior colliculus. Exp Brain Res. 2010;201:701–17. doi: 10.1007/s00221-009-2090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Kawana E. The cells of origin of the incertofugal projections to the tectum, thalamus, tegmentum and spinal cord in the rat: a study using the autoradiographic and horseradish peroxidase methods. Neuroscience. 1982;7:2389–2406. doi: 10.1016/0306-4522(82)90203-2. [DOI] [PubMed] [Google Scholar]
- Watson GD, Smith JB, Alloway KD. The zona incerta regulates communication between the superior colliculus and the posteromedial thalamus: Implications for thalamic interactions with the dorsolateral striatum. J Neurosci. 2015;35:9463–76. doi: 10.1523/JNEUROSCI.1606-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westby GW, Keay KA, Redgrave P, Dean P, Bannister M. Output pathways from the rat superior colliculus mediating approach and avoidance have different sensory properties. Exp Brain Res. 1990;81:626–38. doi: 10.1007/BF02423513. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Yamamoto S, Hikosaka O. Robust representation of stable object values in the oculomotor basal ganglia. J Neurosci. 2012;32:16917–32. doi: 10.1523/JNEUROSCI.3438-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Warren S, May PJ. The feedback circuit connecting the central mesencephalic reticular formation and the superior colliculus in the macaque monkey: tectal connections. Exp Brain Res. 2008;189:485–96. doi: 10.1007/s00221-008-1444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]