Abstract
Emerging evidence shows that oligodendrogenesis and myelination are highly responsive to behavioral experience, including physical activity and social experience. This form of myelin plasticity is being increasingly appreciated and examined in the prefrontal cortex (PFC), a critical brain region involved in complex emotional and cognitive behavior. However, it remains unclear whether myelination in other brain regions is affected by behavioral experience. Here we report that exposure to four weeks of chronic variable stress induced anxiety- and depressive-like behavior in male adult mice. In concert with these behavioral responses, transcriptional analysis of PFC and nucleus accumbens (NAc), a brain region critical for reward response, revealed downregulation of transcripts encoding for myelin genes and oligodendrocyte-specific genes. In contrast, upregulation of myelin-related transcripts was observed in the corpus callosum (CC), whereas the amygdala (AMG) did not show significant changes. Shorter exposure to the same stressors induced behavioral changes to a less extent and was followed by a stress habituation period. However, reduced myelin and oligodendrocyte-specific gene transcripts were detected as early as one week following stress exposure in the PFC and NAc. These data indicate that oligodendrocyte and their progenitors in multiple brain regions are responsive to stressful experiences and show distinctive and region-specific patterns of gene expression.
Keywords: Depression, Chronic Stress, Myelin Plasticity, Prefrontal Cortex, Nucleus Accumbens
INTRODUCTION
It has been increasingly appreciated that myelination and oligodendrocyte development are sensitive to stressful experience, even during adult life (Liu et al., 2012; Makinodan et al., 2012; Birey et al., 2015; Liu et al., 2016; Yang et al., 2016; Lehmann et al., 2017). Neuroimaging studies have demonstrated abnormal myelin structure and reduced oligodendrocyte function that nicely correlated with evidence of decreased oligodendrocyte-related gene expression and reduced oligodendrocyte numbers and density in post-mortem human brains from subjects with psychiatric disorders, including major depressive disorder, schizophrenia and bipolar disorder (Tkachev et al., 2003; Aston et al., 2005; Katsel et al., 2005; Takahashi et al., 2011; Tham et al., 2011; Haroutunian et al., 2014; Birey et al., 2015). Recent studies using animal models have shown a clear alteration of myelination and oligodendrocyte function in response to stressful stimuli. For instance, social isolation in mice either during the critical period of adolescence or in the adulthood resulted in reduced social interaction, a sign of depressive-like behavior, which are associated with changes in oligodendrocyte gene expression, morphology and myelin thickness in the medial PFC (Liu et al., 2012; Makinodan et al., 2012; Liu et al., 2016). Mice exposed to chronic unpredictable stress displayed reduced myelination, oligodendrocyte progenitor cell (OPC) proliferation and density (NG2+ cells) in the PFC (Banasr et al., 2007; Yang et al., 2016). Similarly, rodents subjected to chronic social stress, for instance, daily social defeat by an aggressive conspecific, showed reduced myelin gene transcripts, OPC proliferation and total numbers of NG2+ cells in the medial PFC (Czeh et al., 2007; Birey et al., 2015; Lehmann et al., 2017), the same region as identified in other types of stress models. Collectively, these data suggest a strong association of oligodendrocyte dysfunction in the medial PFC with behavioral deficits in psychiatric disorders.
Despite the increasing documentation of myelin plasticity in the PFC following various types of stress, reports on altered myelination and oligodendrocyte function in other brain regions have been sparse. Mice following social isolation did not show any structural or transcriptional impairments in myelin in the NAc, CC or cerebellum (Liu et al., 2012), whereas these brain regions were not examined in chronic unpredictable stress or chronic social stress (Banasr et al., 2007; Czeh et al., 2007; Birey et al., 2015; Yang et al., 2016; Lehmann et al., 2017). PFC is a critical brain region involved in complex emotional and cognitive function. The medial PFC connects to multiple cortical and subcortical areas (Del Arco and Mora, 2008; Riga et al., 2014), and may act as a hub to integrate information from numerous input structures and converge to the updated information to output structures. Therefore, it is not surprising that the medial PFC is highly sensitive and detrimental to stress (Arnsten, 2009). However, it still remains an open question that whether oligodendrocyte and OPCs residing outside the PFC, particularly regions which receive projections from the medial PFC, are capable to response to stress stimuli and generate molecular changes.
In this study we asked whether transcriptional activity in oligodendrocytes and OPCs in regions outside of PFC are also responsive to chronic stress. We measured the behavioral consequence and the myelin-related transcriptional changes in multiple brain regions receiving inputs from the medial PFC, including NAc and amygdala; and the major white matter track (i.e. CC), following four weeks of chronic stress induced by a combination of three stressors: footshock, tail suspension and restraint. We also determined the molecular changes occurring prior to the appearance of anxiety- and depression-associated behavior. Our data suggests a wide-spread effect of chronic stressful experience on cells of the oligodendrocyte lineage.
METHODS
Animals
All experimental C57Bl/6 male mice (7–8 weeks old) were obtained from the Jackson Laboratory (Bar Harbor, Maine) and allowed for one week acclimation prior to the onset of each experiment. Mice were group housed and maintained on a 12 h light/dark cycle with ad libitum access to food and water. Mouse procedures were performed in accordance with the Institutional Animal Care and Use Committee guidelines of the Icahn School of Medicine at Mount Sinai.
Chronic variable stress (CVS)
Chronic variable stress was performed as described previously (LaPlant et al., 2009), which consists of three different stressors over 7, 14, 21 or 28 days depending on each experiment setup. Stressors alternated during the entire period. Mice were exposed to only one stressor each day at various time of the day. Stressors were administered in the following order: 100 random mild foot shocks at 0.45 mA for one hour (10 mice per footshock chamber), a tail suspension stress for one hour and a restraint stress, placed inside a 50mL falcon tube in home cage, for one hour. The three stressors were then repeated for the following days in the same order. The control mice were handled daily by the same experimenters.
Behavioral assessments
Splash test
Splash test was performed according to previously published protocols with adaptation (Isingrini et al., 2010). Test was performed under red light (230V, 15W). Mice were habituated to the testing room for 1 hour prior to testing. Mice were sprayed on the back with a 10% sucrose solution 3 times. Mice were then placed into an empty housing cage and behavior for 5 minutes was recorded via videotape. The total amount of time grooming over the 5-minute period was recorded by an observer who is blind to the condition.
Novelty Suppressed Feeding (NSF)
NSF was performed according to previously published protocols with adaptation (Santarelli et al., 2003). Mice were food restricted overnight prior to testing. On day of testing mice habituated to the testing room for 1 hour under red light conditions. Mice were then placed into a plastic box 50 × 50 × 20 cm with bedding. A single pellet of food is placed in the center of the box. Mice were placed in the corner of the box and the latency to eat was scored up to 10 minutes. Mice were then immediately transferred to their home cage in standard lighting conditions with a single pellet of food placed in the center of the home cage and the latency to eat was recorded.
Forced Swim Test (FST)
FST was conducted according to previously published protocols (Golden et al., 2016). Animals were placed in the test room for 1 hour before behavioral testing for habituation. Mice were tested in a 4L Pyrex glass beaker, containing 2L of water at 25±1°C for 6 minutes. Behavioral was videotaped and hand scored for time spent immobile by an observer blind to experimental conditions.
RNA extraction from tissue and quantitative PCR analysis
One day after the last behavioral test was performed, tissue punches were taken from the medial prefrontal cortex, nucleus accumbens, corpus callosum and amygdala and flash frozen for subsequent processing. RNA was extracted using Trizol (ThermoFisher) and purified with the RNeasy Micro kit (QIAGEN) following the manufacturer’s protocol. RNA was reverse transcribed with qScript cDNA Supermix (Quanta) and qRT-PCR was performed using Perfecta Sybr Fast Mix Rox 1250 (Quanta). For a list of the primers used for amplification, please refer to (Liu et al., 2012). Each transcript value was calculated as the average of triplicate samples from several mice per experimental condition (typically 6–12). After normalization to a reference gene (i.e. Gapdh), the average values for each transcript was calculated based on the values obtained in all the samples included for each experimental condition. Two tailed Student’s t-tests were performed to assess statistical differences between the average values in each condition, and a false discovery rate (FDR) correction was applied for multiple testing within gene groups, with a significance threshold of 0.05. All statistical analysis was performed using GraphPad Prism software.
RESULTS
Chronic variable stress induced anxiety- and depressive-like behavior in adult male mice
The current study was designed to test whether oligodendrocytes and oligodendrocyte progenitor cells (OPC) in multiple brain regions are responsive to behavioral experience. We and others have previously shown that social isolation of adult mice resulted in reduced oligodendroglial transcripts and myelin thickness in the PFC, but not other brain regions, including NAc, CC or cerebellum (Liu et al., 2012; Makinodan et al., 2012; Liu et al., 2016). These results raised the question of whether these results could be explained by the mild severity of the stress related to lack of social experience. In this study we adopted a modified chronic unpredictable stress paradigm, chronic variable stress (CVS) (LaPlant et al., 2009), in which mice were exposed to one type of stressors, including footshock, tail suspension and restraint each day in a sequence that was repeated for a total of 28 days. Twenty-four hours after exposure to the last stressor, mice were subjected to a battery of anxiety- and depression-associated behavioral tests, including splash test, novelty suppressed feeding (NSF) test, and forced swim test (FST) (Fig.1A). For the splash test, mice were sprayed with 10% sucrose solution, which induced grooming behavior and the amount of grooming time was measured. Reduced grooming time is considered a measurable outcome of stressed behavior (Hodes et al., 2015). For the NSF test, mice were placed into a novel environment with a food pellet and the latency to start consuming the food pellet was recorded. Immediately after the test, mice were transferred back to their home cages. A food pellet was placed in the home cage and the latency to eat was recorded again. This test has been associated with both anxiety and depression-like behavior, in which stressed mice display longer latency to consume the food pellet (Santarelli et al., 2003). FST is an established measure of behavioral ‘despair’ (Porsolt et al., 1978). For this task, mice were placed in an inescapable tank that is filled with water and the time they spent immobile was measured (Golden et al., 2016). Mice that underwent 28 days of CVS, displayed a series of anxiety-associated and depressive-like behavior, including reduced grooming time (87.0 ± 7.0 sec in control vs. 69.4 ± 3.8 sec in CVS, p=0.0456) (Fig.1B), longer latency in NSF test in both novel environment (224.8 ± 24.2 sec in control vs. 412.3 ± 32.3 sec in CVS, p< 0.0001) and home cage (61.6 ±8.7 sec in control vs. 157.3 ± 7.1 sec in CVS, p=0.0024) (Fig.1C), and increased immobility time in FST (92.2 ± 15.0 sec in control vs. 140.0 ± 14.3 sec in CVS, p=0.035) (Fig.1D). These data clearly indicated that four weeks of CVS exposure induced anxiety- and depressive-like behavior in adult mice.
Figure 1. Chronic variable stress (CVS) induces depressive-like behavior in adult mice.
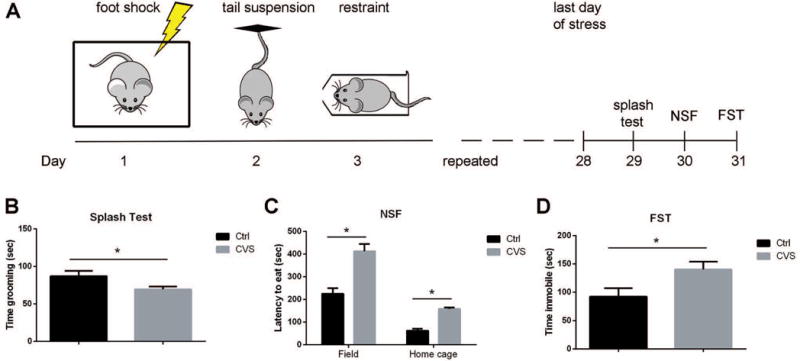
(A) Schematic of CVS and behavioral testing. Mice were exposed to one type of stress per day for 28 days. Stressors were repeated in the order of foot shock, tail suspension, and restraint. (B) Mice undergoing CVS spent less time grooming in splash test compared to controls. * p<0.05 by two-tailed unpaired t-test, n=10–12 mice per group. (C) Mice undergoing CVS demonstrated longer latencies to eat in a novel environment and in their home cages in the novelty suppressed feeding (NSF) test. * p<0.05 by two-tailed unpaired t-test corrected for multiple comparison using Holm-Sidak method, n=10–12 mice per group. (D) Mice undergoing CVS displayed longer time immobile in the forced swim test (FST). * p<0.05 by two-tailed unpaired t-test, n=10–12 mice per group. Data represented as mean ± S.E.M.
Chronic variable stress induced wide-spread oligodendrocyte-specific transcriptional changes in multiple brain regions
In order to determine whether CVS induced wide-spread molecular changes in oligodendrocytes, we sought to define transcriptional changes in multiple brain regions. Considering the capability of PFC in controlling other cortical and subcortical brain regions through its dense projections, we examined not only the medial PFC, but also areas receiving glutamatergic inputs from the PFC, including the NAc and amygdala (AMG), and the major trans-hemispheric white matter track, CC (Fig.2). Indeed, downregulated myelin gene transcripts were detected not only in the PFC (Fig.2A), but also in the NAc, a critical brain region involved in reward response (Fig.2B). However, other brain regions, such as the CC, showed an opposite transcriptional response (i.e. upregulation of myelin gene transcription) (Fig.2C), while the basolateral amygdala (AMG) was not significantly affected (Fig.2D).
Figure 2. Chronic variable stress (CVS) induces transcriptional changes in myelin genes in the adult mouse brain.
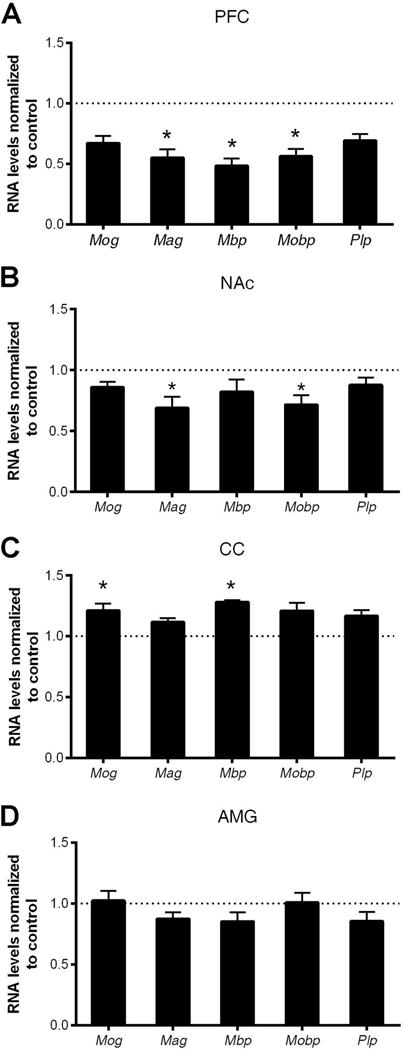
Quantitative RT-PCR of myelin gene transcripts in (A) prefrontal cortex (PFC), (B) nucleus accumbens (NAc), (C) corpus callosum (CC) and (D) amygdala (AMG) in mice following 28 days of CVS. Bar graphs indicate average values in stressed mice after Gapdh normalization relative to average control levels (dashed line) (n=5–6 mice per group, * two-tailed unpaired t-test, FDR < 0.05). Note the decreased myelin gene transcripts in the PFC and NAc, while increased myelin gene transcripts in the corpus callosum (CC). Data represented as mean ± S.E.M.
To associate the wide-spread myelin gene transcript changes in multiple brain regions with specific stages of the oligodendrocyte lineage, we further analyzed the transcript levels of genes expressed throughout the differentiation (e.g. Sox10, Olig2, and Olig1) as well as those specific for the progenitor stage (e.g. Cspg4 and Pdgfra) (Fig. 3). Consistent with recent reports on NG2+ cell loss in the PFC following chronic stress (Birey et al., 2015; Yang et al., 2016), we observed reduced levels of Cspg4 and Pdgfra as well as Olig2 in the PFC (Fig. 3A), thereby suggesting an overall reduction in total number of OPC after four weeks of chronic stress. It is worth mentioning that in the NAc, reduced myelin gene expression was also associated with decreased levels of genes specific for OPC (Fig. 3B), suggesting a similar type of oligodendrocyte progenitor response in the NAc and PFC. In contrast, the CC revealed only an upregulation of gene transcripts specific for the progenitor (i.e. Cspg4) stage (Fig.3C), thereby suggesting an opposite type of regulation or possibly a compensatory mechanism occurring in the major white matter tract connecting the two hemispheres. The levels of oligodendrocyte transcripts were not significantly altered in the amygdala (Fig.3D). Together, these results suggest that the transcriptional program in oligodendrocyte-lineage cells is highly plastic, region-specific and responsive to external stressors.
Figure 3. Chronic variable stress induces transcriptional changes in oligodendrocyte-specific genes in the adult mouse brain.
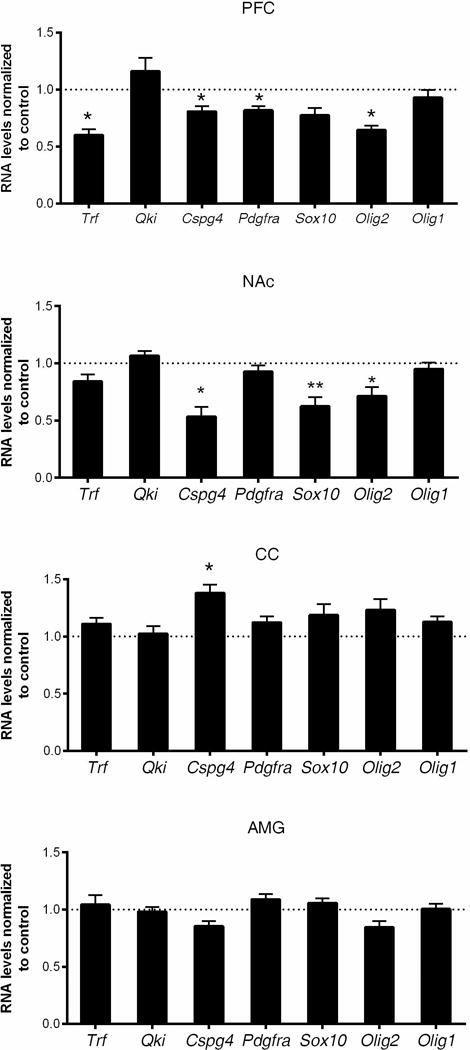
Quantitative RT-PCR of oligodendrocyte-specific gene transcripts in (A) prefrontal cortex (PFC), (B) nucleus accumbens (NAc), (C) corpus callosum (CC) and (D) amygdala (AMG). Bar graphs indicate average values in stressed mice after Gapdh normalization relative to average control levels (dashed line) (n=5–6 mice per group, two-tailed unpaired t-test, * FDR < 0.05, ** FDR < 0.01). Note the decreased oligodendrocyte-specific gene transcripts in the PFC and NAc, while increased oligodendrocyte progenitor gene transcript (i.e., Cspg4) in the corpus callosum (CC). Data represented as mean ± S.E.M.
Shorter period of stress induced minor behavioral changes and transcriptional alterations in adult mice
We have previously demonstrated that shorter period of social isolation in adult mice induced ultrastructural changes in myelin and alterations in chromatin condensation in oligodendrocyte in the PFC, preceding the appearance of any behavioral consequences (Liu et al., 2012). Given the extent of behavioral and transcriptional changes following four weeks of CVS, we then asked whether briefer period of stress exposure would result in any behavioral and transcriptional changes.
Mice were exposed to same type of stressors as described previously for 7, 14 or 21 days, respectively. Anxiety- and depression-associated behaviors were assessed by a battery of behavioral tests, including splash test, NSF test, and FST (Fig. 4). Interestingly, the greatest behavioral changes were observed after only one week of stress, although these mice never displayed behavioral changes to the same extent as those exposed to four weeks of CVS. After one week of CVS, we detected reduced grooming time in splash test (102.2 ± 5.7 sec in control vs. 75.0 ± 4.5 sec in CVS, p=0.0012), and increased latency to consume food pellet only when placed in novel environment (249.4 ± 18.2 sec in control vs. 355.5 ± 42.3 sec in CVS, p=0.0056), but not in their home cages. No behavioral despair was detected in FST at any time point (Fig.4A–C). As the exposure period of CVS increased, behavioral changes started to normalize to control levels (Fig.4B–C), with the exception of NSF test after two weeks of CVS, where increased latency to eat was observed in the stressed mice (220.9 ± 25.4 sec in control vs. 303.7 ± 32.4 sec in CVS, p=0.016), although the difference became progressively smaller (Fig.4B). These results suggest a minor behavioral change with shorter period of CVS exposure, with a possible induction of stress habituation after one week of CVS.
Figure 4. Time kinetics of behavioral assessments on mice undergoing chronic variable stress (CVS).
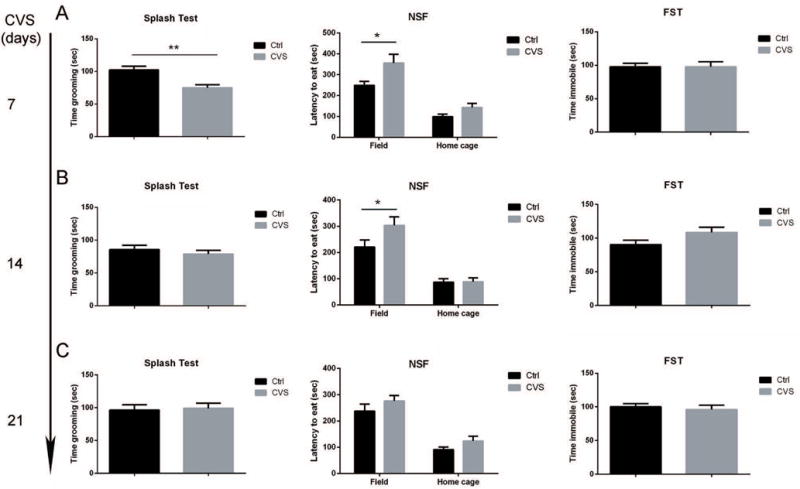
Mice were exposed to 7 days (A), 14 days (B), or 21 days (C) of stress followed by behavioral assessments on each group. Only mice exposed to 7 days of stress displayed minor depressive-like behavior in splash test and novelty suppressed feeding (NSF) test, but not in forced swim test (FST). Mice exposed to 14 days or 21 days of stress displayed a stress habituation phenotype. For splash test and FST, * p<0.05, ** p<0.01 by two-tailed unpaired t-test. For NSF test, statistical significance was determined by two-tailed unpaired t-test corrected for multiple comparisons using Holm-Sidak method. * p<0.05, n=10–12 mice per group. Data represented as mean ± S.E.M.
Next, we asked whether any transcriptional changes would occur even with shorter exposure of stress, when no or minor behavioral consequence. We focused our analysis in the PFC, NAc and CC, which showed transcriptional changes after four weeks of CVS. Interestingly, the transcriptional changes in myelin preceded changes in behavior. One week of CVS exposure, for instance, was sufficient to decrease the levels of myelin gene transcripts, but did not result in prominent changes in depressive-like or anxiety-like behavior as extensively as the four-week exposure to CVS. The most extensive early transcriptional changes were detected in the NAc (Fig.5A), and to a lower extent also in PFC (i.e. Mag), but not in the CC. The myelin gene transcriptional profiles correlated with the downregulation of oligodendrocyte-specific genes, including progenitor-specific genes (i.e. Cspg4 and Pdgfra) (Fig.6A). Interestingly, after two weeks of CVS, a subset of myelin gene transcripts (i.e. Mag and Mobp) remained down-regulated in the NAc, whereas others and those in the PFC recovered to control levels (Fig.5B). By three weeks of CVS exposure, all myelin gene transcripts normalized to control levels with no significant changes detected in the PFC and NAc, possibly revealing an underlying adaptive response to stress involving regulation of myelin genes (Fig.5C). Transcripts of oligodendrocyte-specific genes returned to comparable levels to control group by two weeks of CVS and remained indistinguishable until three weeks of CVS (Fig.6B–C). These data indicate that the extensive transcriptional changes of oligodendrocyte lineage gene preceded measurable behavioral consequences and suggested plastic changes in oligodendrocyte and OPC in multiple brain regions during the early phase of the response to stress.
Figure 5. Dynamic transcriptional changes of myelin genes in the prefrontal cortex (PFC), nucleus accumbens (NAc) and corpus callosum (CC) following increasing days of stress exposure.
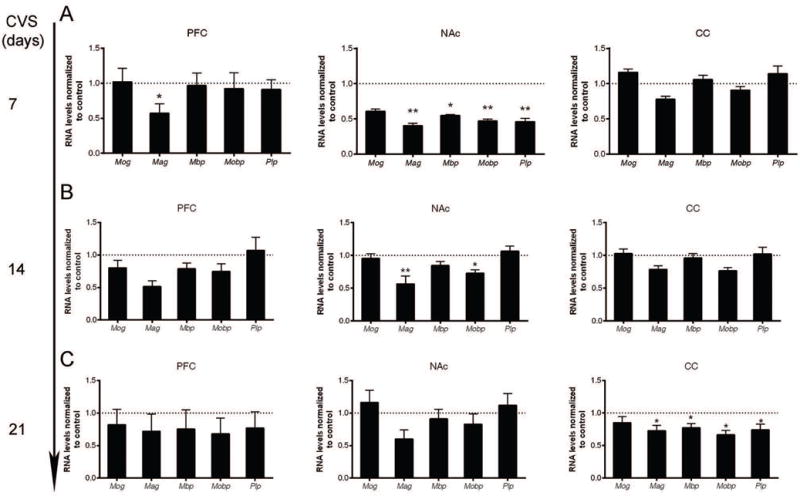
Quantitative RT-PCR of myelin gene transcripts in the PFC, NAc, and CC following 7 days (A), 14 days (B), or 21 days (C) of chronic variable stress (CVS). Bar graphs indicate average values in stressed mice after Gapdh normalization relative to average control levels (dashed line) (n=5–6 mice per group, statistical significance was determined by two-tailed unpaired t-test, * FDR < 0.05, ** FDR < 0.01). Note the decreased myelin gene transcripts in the PFC and NAc in the early period of CVS. The downregulation of myelin gene transcripts was compensated later in the PFC and NAc, followed by downregulation of myelin gene transcripts in the CC. Data represented as mean ± S.E.M.
Figure 6. Dynamic transcriptional changes of oligodendrocyte-specific genes in the prefrontal cortex (PFC), nucleus accumbens (NAc) and corpus callosum (CC) following increasing days of stress exposure.
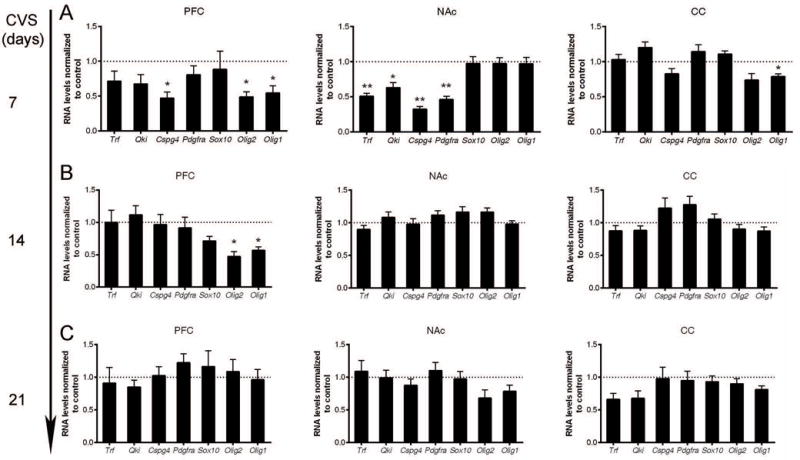
Quantitative RT-PCR of oligodendrocyte-specific gene transcripts in PFC, NAc, and CC following 7 days (A), 14 days (B), or 21 days (C) of chronic variable stress (CVS). Bar graphs indicate average values in stressed mice after Gapdh normalization relative to average control levels (dashed line) (n=5–6 mice per group, statistical significance was determined by two-tailed unpaired t-test, * FDR < 0.05, ** FDR < 0.01). Note the decreased oligodendrocyte-specific gene transcripts in the PFC and NAc in the early period of CVS. Data represented as mean ± S.E.M.
DISCUSSION
Widespread alterations in oligodendrocyte and myelin following chronic stress
It is increasingly accepted that myelination and oligodendrocyte, the cell forms myelin in the central nervous system, are dynamic and responsive to experience. We and others have demonstrated recently that social experience drives oligodendrocyte lineage dynamics and myelin plasticity in the medial prefrontal cortex (PFC), a highly evolved brain region involved in higher cognitive function (Liu et al., 2012; Makinodan et al., 2012; Birey et al., 2015; Liu et al., 2016; Yang et al., 2016; Lehmann et al., 2017). Here, we expand our findings by demonstrating transcriptional regulations in myelin and oligodendrocyte lineage in brain regions outside the PFC, including NAc and CC, following chronic stress. We reported here wide-spread transcriptional alterations that are associated with oligodendrocyte and OPC following exposure to chronic stress in adult mice. Furthermore, such adaptive changes occurred in the early phase of stress exposure, preceding the appearance of a wide extent of anxiety- and depressive-associated behavior. Data presented here suggests that the presence of myelin plasticity is not a unique feature of the PFC, but also extends to areas receiving projections from the PFC and the white matter track.
One caveat of our study is that we cannot exclude the possibility that the detected gene transcriptional changes in glial cells following chronic stress exposure could be the consequence of changes in population dynamics and network function. Although not examined here, recent studies have demonstrated loss of NG2+ cell in the PFC in mice subject to chronic stress (Birey et al., 2015; Yang et al., 2016). These results are consistent with our findings of reduced oligodendrocyte- and OPC-related genes (e.g. Cspg4, Pdgfra and Olig2) in the PFC (Fig. 3A) after exposure to strong stressors. Importantly, milder forms of chronic stress for prolonged periods of time, such as 8 week social isolation in adult mice, have milder effects and did not induce death in oligodendrocyte or OPC in the PFC, as only moderate alterations in transcripts associated with the maturation of OPC could be detected (Liu et al., 2012; Liu et al., 2016). The exact cellular mechanisms underlying the transcriptional changes remain to be further investigated.
It is intriguing to notice that CVS induced a biphasic response in transcription alterations in the PFC and NAc. Significant downregulation of myelin- and oligodendrocyte-specific transcripts was detected as early as one week following CVS and after four weeks of CVS exposure, but returned to control level in between. The underlying mechanism on the biphasic response in oligodendrocyte is completely unknown. One possibility is that an immediate adaptive response is generated after an acute (i.e. one week) stress. This adaptive response may be compensated by enhanced transcription or by establishing a new homeostasis (e.g. stress habituation), in the second and third week of CVS exposure, as suggested by the behavioral tests. If the stress exposure is prolonged (i.e. four weeks), however, the new homeostasis is eventually terminated, due to the accumulation of signaling molecules that induce cell death, such as the upregulation of genes like the death receptor 6 (Yang et al., 2016), or the lack of signaling molecules such as, FGF2 (Birey et al., 2015). To date, the exact underlying mechanism remains unclear.
Region-specific transcriptional changes in response to chronic stress
Chronic exposure to stress has been well documented to alter the activity of neurons in various brain regions, including, but not limited to, medial PFC (Radley et al., 2006; Vialou et al., 2014), NAc (Vialou et al., 2010; Christoffel et al., 2011), amygdala (Akirav and Maroun, 2007) and hippocampus (Surget et al., 2011). As an interplay between experience and molecular changes in oligodendrocyte, neuronal activity has been shown to affect oligodendrogenesis and myelin plasticity using rodent and zebrafish models (Gibson et al., 2014; McKenzie et al., 2014; Hines et al., 2015; Mensch et al., 2015; Koudelka et al., 2016; Xiao et al., 2016). Functioning as a control board with numerous connections to cortical and subcortical areas, the PFC has been demonstrated to be the most sensitive and susceptible region to changes in myelin structure and oligodendrocyte function in various mouse models of depression (Liu et al., 2012; Makinodan et al., 2012; Birey et al., 2015; Liu et al., 2016; Yang et al., 2016; Lehmann et al., 2017). Restoring myelination in the PFC has been shown to exert an anti-depressant function in socially isolated mice (Liu et al., 2016). Consistent with previous findings, we show here that PFC displayed downregulation of myelin genes and oligodendrocyte-specific transcripts, thereby suggesting an impairment in oligodendrocyte lineage function, including myelination.
The glutamatergic projection from the medial PFC to the NAc is well established (Britt et al., 2012; Riga et al., 2014). We show here that NAc displayed downregulated myelin and oligodendroglial transcripts to the same extent as the PFC, and that PFC and NAc showed the highest level of transcriptional co-regulation of myelin and oligodendrocyte-specific genes, either at the early or late stages of stress exposure. These data suggest that excitatory input, or the weakening of an excitatory input, may be the driving force of the transcriptional regulation occurring in oligodendrocyte lineage cells in response to chronic stress. However, not all regions receiving glutamatergic inputs displayed similar transcriptional response. Glutamatergic projections from the medial PFC to the basolateral AMG, for instance, did not result in a significant impairment of myelin or oligodendroglial transcription. The exact mechanism underlying the differential response is not clear. One possibility is that the interaction between glia and neurons following chronic stress may be region-specific, with neurons (or oligodendrocytes) in the AMG displaying different properties than those cell populations in the NAc or PFC. For instance, since changes in neuronal arborization in the AMY have been reported in rodents following chronic stress (Wang et al., 2012), it is conceivable that oligodendrocytes may not need to further change their transcriptional program because the changes occurring in the local neuronal function may provide sufficient adaptation to stress. Another possibility for the lack of transcriptional myelin response in AMG could be the intensity of the stressful experience, as longer exposure (for instance seven weeks) and increasing types of stressors are capable of to inducing transcriptional myelin changes in the AMG (Sibille et al., 2009). What underlies the intensity of stress experience is not clear, but it is reasonable to speculate that sustained circuitry change may be one of the underlying mechanisms.
Different transcriptional response in various animal models of stress
Stress exposure is tightly associated with the onset and development of depression, where proper PFC function is required (Riga et al., 2014). However, despite the consistent impairment in structure and molecular function of myelin and oligodendrocytes in the PFC in different models of stress, reports on changes occurring in other brain regions have been very limited. Intriguingly, when examined in the social isolation model of stress, myelination was not impaired in the NAc or CC, even when adult mice isolated for a prolonged period of time (Liu et al., 2012). This suggests the differential response in the NAc or CC could be due to the intensity of the stress, in which case CVS appears to be a more aggressive form than social isolation. Underlying the intensity of stress might be the extent in neuronal circuitry change, although a direct comparison is lacking and may be difficult to accurately quantify. A distinct model could posit that the neuronal circuitry change may need to exceed a certain threshold in order to induce myelin plasticity, in which case, only stronger stimuli such as CVS, would reach the level needed to overcome the threshold. Overall our data suggest a distinctive and stressor-dependent regulation of gene expression in which the type of stressors, the duration and intensity of the stress could affect the response d by oligodendrocyte, possibly resulting in various degrees of adaptive myelin plasticity.
In conclusion, our data indicate that oligodendrocyte in multiple brain regions are responsive to stress stimuli and suggest a widespread responsiveness of the oligodendrocyte transcriptional program following behavioral experience.
Acknowledgments
We thank Ms. Nicole Chavez and Ms. Stephanie Ciraula for technical help. This work is supported by National Institute of Neurological Disorders and Stroke (NIH-R37 NS042925 to P.C).
Footnotes
Conflict of Interest: None
References
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007;2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston C, Jiang L, Sokolov BP. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry. 2005;10:309–322. doi: 10.1038/sj.mp.4001565. [DOI] [PubMed] [Google Scholar]
- Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Birey F, Kloc M, Chavali M, Hussein I, Wilson M, Christoffel DJ, Chen T, Frohman MA, Robinson JK, Russo SJ, Maffei A, Aguirre A. Genetic and Stress-Induced Loss of NG2 Glia Triggers Emergence of Depressive-like Behaviors through Reduced Secretion of FGF2. Neuron. 2015;88:941–956. doi: 10.1016/j.neuron.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav. 2008;90:226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, Aleyasin H, Menard C, Zhang H, Hodes GE, Bregman D, Khibnik L, Tai J, Rebusi N, Krawitz B, Chaudhury D, Walsh JJ, Han MH, Shapiro ML, Russo SJ. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016;534:688–692. doi: 10.1038/nature18601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutunian V, Katsel P, Roussos P, Davis KL, Altshuler LL, Bartzokis G. Myelination, oligodendrocytes, and serious mental illness. Glia. 2014;62:1856–1877. doi: 10.1002/glia.22716. [DOI] [PubMed] [Google Scholar]
- Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci. 2015;18:683–689. doi: 10.1038/nn.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, Menard C, Aleyasin H, Koo JW, Lorsch ZS, Feng J, Heshmati M, Wang M, Turecki G, Neve R, Zhang B, Shen L, Nestler EJ, Russo SJ. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci. 2015;35:16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isingrini E, Camus V, Le Guisquet AM, Pingaud M, Devers S, Belzung C. Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PLoS One. 2010;5:e10404. doi: 10.1371/journal.pone.0010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsel P, Davis KL, Haroutunian V. Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: a gene ontology study. Schizophr Res. 2005;79:157–173. doi: 10.1016/j.schres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Koudelka S, Voas MG, Almeida RG, Baraban M, Soetaert J, Meyer MP, Talbot WS, Lyons DA. Individual Neuronal Subtypes Exhibit Diversity in CNS Myelination Mediated by Synaptic Vesicle Release. Curr Biol. 2016;26:1447–1455. doi: 10.1016/j.cub.2016.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, Kalahasti G, Bradbury KR, Taylor SV, Maze I, Kumar A, Graham A, Birnbaum SG, Krishnan V, Truong HT, Neve RL, Nestler EJ, Russo SJ. Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol Psychiatry. 2009;65:874–880. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Weigel TK, Elkahloun AG, Herkenham M. Chronic social defeat reduces myelination in the mouse medial prefrontal cortex. Sci Rep. 2017;7:46548. doi: 10.1038/srep46548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dupree JL, Gacias M, Frawley R, Sikder T, Naik P, Casaccia P. Clemastine Enhances Myelination in the Prefrontal Cortex and Rescues Behavioral Changes in Socially Isolated Mice. J Neurosci. 2016;36:957–962. doi: 10.1523/JNEUROSCI.3608-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci. 2015;18:628–630. doi: 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Riga D, Matos MR, Glas A, Smit AB, Spijker S, Van den Oever MC. Optogenetic dissection of medial prefrontal cortex circuitry. Front Syst Neurosci. 2014;8:230. doi: 10.3389/fnsys.2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, Belzung C, Tseng GC, Lewis DA. A molecular signature of depression in the amygdala. Am J Psychiatry. 2009;166:1011–1024. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011;93:13–24. doi: 10.1016/j.pneurobio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham MW, Woon PS, Sum MY, Lee TS, Sim K. White matter abnormalities in major depression: evidence from post-mortem, neuroimaging and genetic studies. J Affect Disord. 2011;132:26–36. doi: 10.1016/j.jad.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, Fallon B, Mazei-Robison M, Ku SM, Harrigan E, Winstanley CA, Joshi T, Feng J, Berton O, Nestler EJ. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of DeltaFosB. J Neurosci. 2014;34:3878–3887. doi: 10.1523/JNEUROSCI.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, Iniguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolanos CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Ho UC, Ko MC, Liao CC, Lee LJ. Differential neuronal changes in medial prefrontal cortex, basolateral amygdala and nucleus accumbens after postweaning social isolation. Brain Struct Funct. 2012;217:337–351. doi: 10.1007/s00429-011-0355-4. [DOI] [PubMed] [Google Scholar]
- Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, Emery B, Li H, Richardson WD. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci. 2016;19:1210–1217. doi: 10.1038/nn.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang Y, Luo F, Li B. Chronic stress regulates NG2(+) cell maturation and myelination in the prefrontal cortex through induction of death receptor 6. Exp Neurol. 2016;277:202–214. doi: 10.1016/j.expneurol.2016.01.003. [DOI] [PubMed] [Google Scholar]


