Scientific Abstract
In addition to deficits in social communication, individuals diagnosed with Autism Spectrum Disorder (ASD) frequently exhibit changes in sensory and multisensory function. Recent evidence has focused on changes in audiovisual temporal processing, and has sought to relate these sensory-based changes to weaknesses in social communication. These changes in audiovisual temporal function manifest as differences in the temporal epoch or “window” within which paired auditory and visual stimuli are integrated or bound, with those with ASD exhibiting expanded audiovisual temporal binding windows (TBWs). However, it is unknown whether this impairment is unique to audiovisual pairings, perhaps because of their relevance for speech processing, or whether it generalizes across pairings in different sensory modalities. In addition to the exteroceptive senses, there has been growing interest in ASD research in interoception (e.g., the monitoring of respiration, heartbeat, hunger, etc.), as these internally directed sensory processes appear to be altered as well in autism. In the current study, we sought to examine both exteroception and interoception in individuals with ASD and a group of typically developing (TD) matched controls, with an emphasis on temporal perception of audiovisual (exteroceptive) and cardiovisual (interoceptive to exteroceptive) cues. Results replicate prior findings showing expanded audiovisual TBWs in ASD in comparison to TD. In addition, strikingly, cardiovisual TBWs were fourfold larger in ASD than in TD, suggesting a putative complete lack of cardiovisual temporal acuity in ASD individuals. Results are discussed in light of recent evidence indicating a reduced tendency to rely on sensory priors in ASD.
Keywords: Autism, Multisensory, Interoception, Binding, Visual, Temporal
Introduction
Autism Spectrum Disorders (ASD) are defined by core deficits in social communication and interaction, as well as by the presence of repetitive patterns of behavior and restricted interests (APA, 2013). Further, clinicians and researchers are increasingly noting abnormalities in sensory processing in these patients (Le Couteur et al., 1989; Iarocci et al., 2006; Marco et al., 2011, Baum et al., 2015) – observations that have led to the incorporation of sensory and perceptual abnormalities (most often expressed as either hypo- or hyper-sensitivity to sensory stimuli; Baranek et al., 2006) as a diagnostic feature for ASD in the DSM-V (APA, 2013).
In addition to differences in responsiveness to stimuli presented within the individual senses, a number of recent reports have highlighted that individuals with ASD may also exhibit deficits in tasks requiring integration or utilization of information across the different sensory modalities (i.e., multisensory tasks; see Foxe et al., 2013; Brandwein et al., 2013; Smith & Bennetto, 2007; Woynaroski et al., 2013; Stevenson et al., 2013, 2014). Rather than combining information from the different sensory modalities in an indiscriminant manner, multisensory neurons and circuits are highly sensitive to the statistical relationships between stimuli within the environment. Among the most important of these statistical features are the spatial (e.g., Meredith et al., 1996; Noel & Wallace, 2016) and temporal (e.g., Meredith et al., 1987; Simon et al., 2017) proximity of the different stimuli to one another. Such reliance on these physical characteristics of the stimuli makes intuitive sense, as sensory energies that arrive at the peripheral receptors close together in space and time are highly likely to have emanated from the same event (Murray & Wallace, 2012). It is conceivable, therefore, that the abnormalities individuals with ASD exhibit in processing multisensory stimuli may be rooted in impairments in the spatial and/or temporal (Stevenson et al., 2014; Noel et al., 2016a, b) dimensions.
A rapidly growing body of evidence suggests that individuals with ASD have impaired multisensory temporal function, with particular emphasis on audiovisual pairings (Bebko et al., 2006; Wallace & Stevenson, 2014; Stevenson et al., 2016; Turi et al., 2016; Noel et al., 2016). A common paradigm utilized in order to test temporal acuity is the simultaneity judgment task in which stimuli from two different modalities are presented with varying degrees of temporal disparity and participants are asked to judge whether these stimuli are synchronous or asynchronous. In addition to allowing the determination of the point of temporal asynchrony in which participants are most likely to judge synchrony (i.e., the point of subjective simultaneity, PSS), this task also enables the indexing of the temporal interval over which participants are highly likely to categorize two events as occuring in synchrony (i.e., the temporal binding window, TBW). Within this context, one of the most robust findings regarding the impaired multisensory temporal function in ASD is the presence of abnormally large audiovisual TBWs (Noel et al., 2016; De Niear et al., 2017; Foss-Feig et al., 2010; Kwakye et al., 2011; Stevenson et al., 2014). The manifestation of these enlarged TBWs is that individuals with ASD are more likely to categorize an audiovisual stimulus pair presented at relatively large temporal asynchronies as co-occurring in time when compared with TD individuals. However, although studies are beginning to suggest similar temporally based impairments across other sensory modalities (see Greenfield et al., 2015 for evidence of visuo-tactile differences), it remains largely unknown whether impaired multisensory temporal acuity is a general characteristic of autism that occurs across a variety of different sensory pairings.
A sensory modality, or group of sensory modalities, that is becoming increasingly important within the study of ASD are those subserving interoception (Schauder et al., 2014; DuBois et al., 2016; Shah et al., 2016). Interoception is defined as the monitoring of sensory processes that are produced within an organism (e.g., heartbeat, respiration, hunger, salivation). Resting state functional neuroimaging studies in individuals with ASD have repeatedly demonstrated group differences in functional connectivity between brain structures thought to be involved in interoception, including the insula (which is considered to be the primary interoceptive cortex; Craig, 2003; Critchley et al., 2004) as well as for brain regions involved in exteroceptive sensory processes (Barttfeld et al., 2012; Ebisch et al., 2011; Di Martino et al., 2014). From a psychophysical standpoint, however, the evidence fails to portray a coherent picture. While influential theoretical perspectives have suggested a generalized impairment in interoceptive abilities in ASD (Quattrocki & Friston, 2014), and initial investigations corroborated this fact (Fiene & Brownlow, 2015), later studies have either demonstrated no impairment, or, in contrast, better performance in ASD at specific time-scales (Schauder et al., 2014). Further, a host of recent studies suggest that interoceptive impairments in ASD may be more closely related with alexithymia (Shah et al., 2016), a prevalent sub-clinical co-morbidity seen in ASD and in which individuals have difficulty ascribing emotions and internal state to themselves (Näring et al., 1995; Herbert et al., 2011; Longarzo et al., 2015; Brewer et al., 2015, 2016a, 2016b; Gaigg et al., 2016; Sowden et al., 2016; Murphy et al., 2017). However, one piece of evidence that seems concordant across all of the published work is that in ASD the putative interoceptive impairment is most closely related to bridging between interoception and exteroception (Tsakiris et al., 2011; Quattrocki et al., 2014; Noel et al., 2017a). For example, Quattrocki et al., 2014 specifically postulate that an early alteration in the oxytocin system could disrupt the integration of interoceptive and exteroceptive cues essential for generating the construct of the self. Further, Tsakiris et al., 2011, and Schauder et al., 2014 show in both TD and ASD individuals that there is a relationship between interoceptive function and proneness to bodily self-consciousness illusions that are reliant on the integration of exteroceptive cues (e.g., the Rubber Hand Illusion, Botvinick & Cohen, 1998). In sum, the presence of alterations in interoceptive processes and the circuitry that supports interoceptive awareness suggests that those with ASD may integrate interoceptive and exteroceptive cues differently when compared with their TD peers (see Greenfield et al., 2015 and Noel et al., 2017a, for a similar argument postulating that individuals with ASD may have difficulty in integrating between the internal and external sensory worlds).
In the current study, we were interested in determining whether individuals with ASD demonstrate impaired multisensory temporal acuity that extends beyond audiovisual pairings, and reasoned that a fruitful approach would be to index multisensory temporal acuity across the exteroceptive and interoceptive sensory modalities (and compare with indices of audiovisual temporal acuity). To address this question, we had participants with ASD and TD controls perform both a standard task indexing exteroceptive audiovisual temporal function (i.e., a simultaneity judgment) as well as a novel cardiovisual simultaneity judgment task indexing temporal function across exteroception and interoception. Furthermore, an additional group of TD and ASD individuals took part in a control visuo-tactile simultaneity judgment task (see below). The hypothetical framework for this study is that the well-established changes in audiovisual temporal acuity (i.e., temporal binding windows) seen in autism would extend to, and potentially be more apparent in, judgments that assessed integration across the extero- and interoceptive senses.
Methods
Participants
A total of 84 participants took part in this study; 54 in Experiment 1 (23 females, mean age=22.1±3.04 years; range=14–29 years old), and 30 in Experiment 2 (14 females, mean age=18.48±1.8 years; range=17–27 years old). In Experiment 1, 23 subjects were high-functioning individuals with ASD (8 females, mean age = 22.0 ± 4.24; range = 14 – 29 years old; mean TONI-4 [test of non-verbal intelligence, Brown et al., 2010] = 101.9 ± 13.5, range = 78 - 122, [mean population = 100 ± 15]) and the rest were age-matched controls (contrast of age between groups: t(53) < 1, p = 0.82, d = 0.10). No cognitive (i.e., TONI) testing was undertaken with TD participants, and thus experimental groups were not explicitly matched for IQ. Diagnosis of ASD was confirmed with research-reliable administration of the Autism Diagnostic Observation Schedule (ADOS-2; Lord et al., 2000), as well as judgment of a licensed clinical psychologist based on the DSM-5 (APA, 2013). In addition to the audio- and cardio-visual simultaneity judgment tasks a subset of ASD individuals from Experiment 1 (N = 10/23, 3 females, mean age=20.26±2.91 years) completed a control cardiac sensitivity task - the Schandry Task (see below; Schandry, 1981). Further, a subset of the ASD individuals performing the Schandry Task (N=6/10, 1 female, mean age = 19.42±2.87 years), along with a comparable subset of the TD group (N=6, 3 females, mean age =22.16±3.06 years), also performed a control visuotactile simultaneity judgment task (in addition to the audio- and cardio-visual simultaneity judgment tasks). No TD participant completed the Schandry task. Apart from confirmation of ASD-diagnosis and IQ testing in ASD subjects no other cognitive testing was performed. Participants in Experiment 2 were recruited from a sample of 234 pre-screened first-year students in psychology for having scored either below the first (N=15, mean age=18.40±1.2 years, mean AQ=11.0±2.8) or above the third (N=15, mean age=18.49±1.4 years, mean AQ=25.6±3.5) quartile in the Autism Spectrum Quotient (AQ; Baron-Cohen et al., 2001). All participants reported normal or corrected-to-normal visual acuity and normal hearing. Control participants in Experiment 1 and participants in Experiment 2 had no history of neurological or psychiatry disease. Written informed consent was obtained from all participants or their caregivers, and Vanderbilt University Institutional Review Board approved experimental protocols.
Materials and Apparatus
Visual stimuli consisted of a white ring circumscribing a fixation cross on a black background, presented for 10ms (24-inch Sony GDM-FW900 CRT, 1024 × 640 resolution, 120 Hz) and subtending 17.3° of visual angle. The auditory stimuli was presented via noise-canceling headphones (Philips SBC HN110) to both ears (72 dB peak SPL) and consisted of a 3500 Hz pure tone with a duration of 10 ms. Tactile stimuli (10 ms duration) administered during the visuotactile control task were delivered via the shaft of a vibrotactile motor (Precision MicroDrives) positioned on the right index finger. Stimulus presentation was controlled using E-Prime Prime (Psychology Software Tools Inc., Pittsburgh, PA, USA) and responses were given and recorded via foot pedal (XK-3 Xkeys, P.I. Engineering, Williamston, MI, USA). Tactile stimuli were relayed via a micro-controller (Arduino Mega2580; 16kHz sampling rate) in order to assure accurate timing. Timing of stimulus was verified by oscilloscope (Hameg 507).
Procedure
All participants engaged in both an audiovisual and cardiovisual two-alternative forced choice simultaneity judgment task in which participants were respectively asked to determine whether audiovisual or cardiovisual stimuli had occurred simultaneously or not. In addition a subset of participants equally performed a control visuotactile simultaneity judgment task. For the cardiovisual task, although each visual presentation was by definition proximate to two heartbeats (i.e., the preceding and following one) no additional instruction was given to participants apart from judging the synchrony between their heartbeat and the visual presentation. Participants were asked to fixate on a fixation cross at all times, and instructions emphasized accuracy only. In the case of the audiovisual and visuotactile tasks, auditory and visual or visual and tactile stimuli were presented with stimuli onset asynchronies (SOA) between 400ms and 0ms in steps of 50ms, with both cases of audio-leading (tactile-leading) and visual-leading trials. Twenty trials per condition were administered, for a total of 340 trials. Inter-trial interval was set to a fixed duration of 1000 ms plus a random duration between 0 and 500 ms (i.e., uniform distribution between 1000 and 1500 ms). The audio-visual temporal disparity was randomized on every trial, and every trial required a synchrony judgment. Regarding the cardiovisual task, visual stimuli were presented at an interval of 4500 ms in addition to a random duration between 0 and 1000 ms (i.e., uniform distribution between 4500 and 5500 ms). The longer inter-trial interval was chosen to permit participants to monitor their pulse at their left wrist. Participants were instructed before the experiment how to monitor their pulse (right thumb on left radial artery), a strategy that was undertaken after pilot testing demonstrated that without monitoring their pulse, participants were unable to detect cardiovisual synchrony (see Salomon et al., 2016, for a similar effect and rationale). For the cardiovisual task, a total of 385 visual presentations were administered, and categorization of these trials into appropriate SOA bins (given the timing of the most proximate heart-beat) was undertaken offline (see Analysis section below). As for the audiovisual task, since heartbeats were not experimentally generated but produced by the subject, and the inter-trial interval contained a variable portion, the stimulus onset asynchrony between cardiac and visual events was unpredictable. Audiovisual and cardiovisual tasks were counter-balanced across participants. The visuotactile task was also randomly inserted within the order of executed tasks (cardio-visual, audio-visual, or visuo-tactile simultaneity judgment) in the subset of participants who performed this control experiment.
In addition, a random subset of the ASD group (10/23, 3 females) also completed the Schandry heartbeat perception task (Schandry, 1981) following the completion of the audiovisual, cardiovisual, and visuotactile (if applicable) simultaneity judgment tasks. In this task, which is designed to determine whether individuals are introspectively able to monitor their heartbeat, they were asked to sit quietly and not monitor their pulse. They were presented with four distinct intervals of time over which they were to quietly count the number of times their heart beat (intervals; 25, 35, 45, and 100 secs), and following the termination of each interval they verbally reported the perceived number of times their heart had beat over the interval.
Analysis
Regarding the audiovisual and visuotactile simultaneity judgments, reports of synchrony were compiled and averaged for each participant and SOA. Subsequently, two statistical steps were undertaken. First, for each group separately a one-way ANOVA was conducted to determine whether reports of synchrony were SOA-dependent. That is, we examined for the ASD and TD groups whether their reports of synchrony conveyed sufficient evidence against the null hypothesis that their simultaneity judgments could be said to SOA-independent. In addition to frequency-based (“classical”) inference (Everitt & Skrondal, 2002), we supplemented these standard analyses with a Bayesian analysis (Jasp 8.0.1, Love et al., 2015). Briefly, Bayesian analysis allows for an assessment of not only whether the null hypothesis may not be discarded, but also whether the data lend support for this hypothesis (Rouder et al., 2009). The Bayes equivalent to the p-value is the Bayes Factor (BF), which is defined as the ratio of the posterior odds (i.e., once data is collected) to the prior odds (i.e., before data is collected). As a general approximation, a BF>3 indicates evidence against the null hypothesis, whereas a BF<0.3 indicates evidence in favor of the null hypothesis – a statement that may not be claimed with frequency-based statistics (Jeffrey, 1961). If Bayesian inference did not support the fact that reports of synchrony were SOA-independent, these reports were fitted to psychometric functions and multisensory temporal acuity was assessed.
As no sensory pairing or experimental group demonstrated reliable evidence in favor of their simultaneity judgments being SOA-independent, in a second step for each participant and sensory pairing we fit reports of synchrony to a Gaussian distribution (see Eq. 1, Noel et al., 2016a, 2016c, 2017b), which overall proved to adequately represent the shape of the resulting distribution (goodness-of fit, mean R2 = 0.81). The fitting procedure was conducted by optimizing the free parameters of the Gaussian function (i.e., SOA, PSS, and SD) in order to maximize the likelihood of observed responses. The maximal amplitude was not restricted to peak at or below 100% in order to optimize fitting.
| (Eq. 1) |
The amplitude, mean, and standard deviation of the Gaussian distribution were set as free parameters. The amplitude is taken as a response bias, the mean is taken to index the SOA at which judgment of synchrony is maximal (i.e., PSS; point of subjective simultaneity), and the standard deviation is taken to represent the temporal extent over which participants are highly likely to perceive asynchronous stimuli as synchronously presented (i.e., TBW; temporal binding window). Amplitude, PSS, and TBW were compared across groups via planned between-subjects t-tests.
A similar approach was undertaken for the cardiovisual task, with exception that SOA bins had to be recreated a posteriori. In order to do so, for each visual presentation the nearest peak (i.e., R) of the QRS complex was determined (be it either earlier or after in time compared to the visual stimuli, see Figure 1). Each minimal R-component/visual presentation interval was then sorted as belonging to a particular bin mimicking the bins present in the audiovisual task (namely, between −400 and 400ms of asynchrony in steps of 50ms). The QRS complex as detected via BVP is thought to occur ~200ms after the QRS complex is detected via electrocardiogram (ECG), and the period of maximum subjective perception of heartbeats occurs 200ms after each ECG’s R-wave (Brener et al. 1988, 1993; Suzuki et al. 2013; see Bessette et al., 1991, and Ring & Brener, 1992, for test-retest reliability of cardiac tasks and the temporal location of heartbeat sensations) - thus, we quantified the temporal disparity between each visual presentation and the maximum subjective perception of heartbeat. As for the audiovisual case, Gaussian distributions adequately represented the shape of the resulting distribution of reports of synchrony (goodness-of fit, mean R2=0.75).
Figure 1.
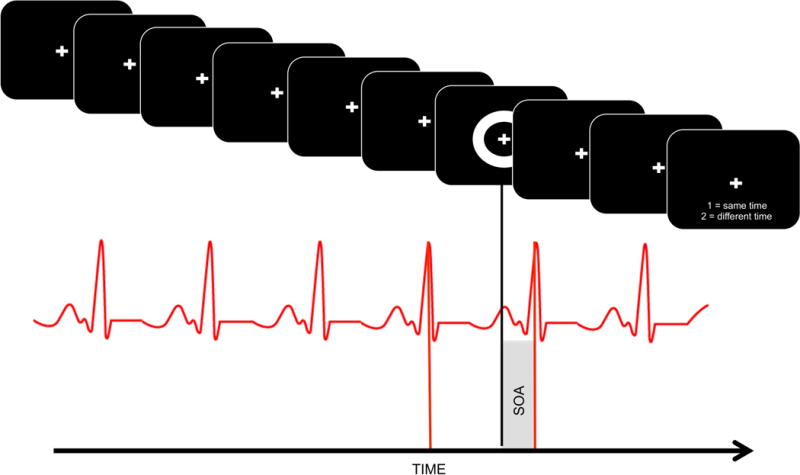
Measurement of cardiovisual temporal binding windows. Visual stimuli (upper panel; ring flash) are presented at random temporal intervals, and heartbeats are measured (lower panel). Subsequently, offline, the temporal discrepancy between each visual presentation (black trigger) and the closest QRS-complex peak (red trigger) is calculated in order to determine stimulus onset asynchrony (SOA; shaded region) for each trial (red). These SOAs are then binned and reports of synchrony within each bin are averaged. Participants are asked to judge whether the flash occurred coincident with a heartbeat.
Lastly, regarding the Schandry task, as illustrated in Eq. 2., interoceptive awareness (IA) was computed for each patient by calculating their accuracy in reported heartbeats normalized by the true number of times their heart had beat (Tsakiris et al., 2011).
| (Eq. 2) |
Correlational analyses (Spearman’s rho, r) were conducted between participant’s interoceptive awareness score and their cardiovisual and visuo-tactile TBWs in order to verify that the cardiovisual simultaneity judgment task is indexing interoceptive and not visuo-tactile ability. Similarly, within the ASD group, the severity of clinical symptoms was correlated with audio-visual, cardio-visual, and visuo-tactile TBWs. However, correlations between the remaining variables were not conducted in order to avoid inflating a putative Type I error due to multiple tests (see Noel et al., 2015 for a correlational analyses between TBWs across multiple pairings).
Results
Experiment 1. Audiovisual, cardiovisual, and visuotactile multisensory temporal function in TD and ASD
Judgments of audiovisual synchrony were SOA-dependent both in the ASD (F(16, 374)=9.43, p<0.001, partial η2=0.599, BF10>104) and TD (F(16, 510)=62.64, p<0.001, partial η2=0.785, BF10>104) groups. Further, as previously reported (Stevenson et al., 2014; Noel et al., 2016a), individuals with ASD exhibited significantly larger TBWs (M=163.44ms, SEM=5.79ms) than their TD counterparts (M=143.20ms, SEM=2.38ms; t(53)=3.55, p<0.001, d=0.97, BF10=36.8, Figure 2a). These groups also differed in the mean amplitude of the Gaussian function best-describing their reports of synchrony as a function of SOA (t(53)=2.11, p=0.039, d=0.57, BF10=1.8, figure 2a), with the TD participants more often (i.e., peak proportion of reports of synchrony of 97% or M=.97, SEM=0.01) reporting synchrony at their peak than ASD participants (M=.87, SEM=.04). Lastly, although results demonstrated a trend (t(53)=1.63, p=0.10, d=0.44, BF10=1.00, Figure 2a), the point of subjective simultaneity (i.e., PSS) did not differ among groups (ASD, M=32.01ms, SEM=11.64ms; TD, M=8.39ms, SEM=8.92ms).
Figure 2.
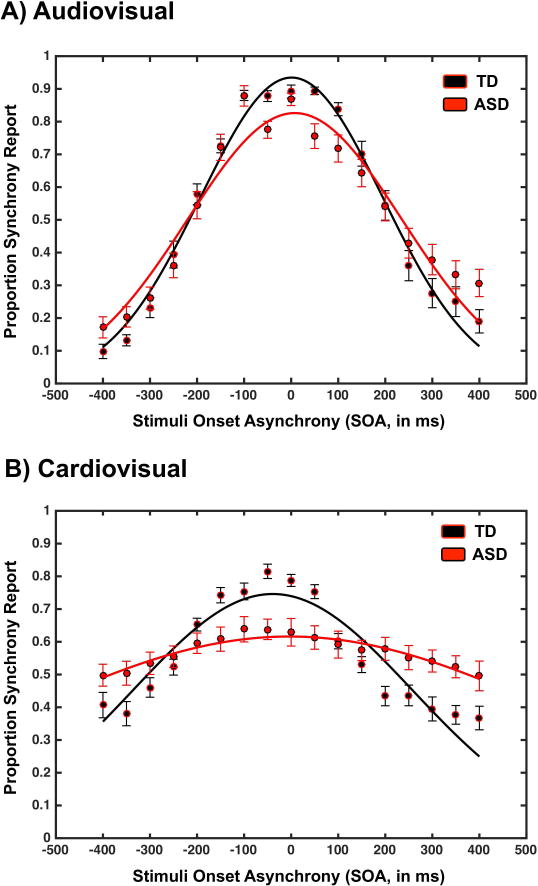
Audiovisual (upper panel) and cardiovisual (lower panel) reports of synchrony as a function of SOA and group (TD in black and ASD in red). Error bars represent ± 1 standard error of the mean (SEM).
As illustrated in Figure 2b, reports of synchrony to cardiovisual events were strikingly different between ASD and TD participants. Indeed, while the reports of synchrony were clearly SOA-dependent for the TD group (F(16,510)=21.04, p<0.001, partial η2=0.490, BF10>104), they only exhibited a trend within the ASD population (F(16,374)=3.12, p=0.09, partial η2=0.188, BF10=2.367). The mean proportion of synchrony judgments across ASD participants did not significantly differ from chance across the range of tested SOAs (one-sample t-test to 0.5, p=0.18, d=0.17, BF10=1.0), while it did for TD participants (p<0.001, d=0.89, BF10=134.0). Similarly, submitting reports of synchrony for cardiovisual events as a function of SOA and group (ASD vs. TD) to a mixed-model ANOVA revealed a clear SOA X group interaction (F(16,592)=6.730, p 0.001, partial η2=0.213, BF10>104). The main effect of SOA was significant (F(16,592)=15.75, p<0.001, partial η2=0.299, BF10>104), while the main effect of group was not (F(1,50)=0.381, p=0.441, partial η2=0.010, BF10=0.45). Thus, while frequency-based analyses seemingly indicate that cardio-visual synchrony judgments were not dependent on SOA, the supplementary Bayesian analysis (BF=2.367) did not provide strong evidence either in support of (BF<0.3) or rejecting (BF>3.0) the null hypothesis (Jarosz & Wiley, 2014). Consequently, cardiovisual TBWs were derived for both TD and ASD individuals.
Regarding the amplitude of the Gaussian function best describing the reports of synchrony (TD: mean R2=0.74; ASD: mean R2=0.78), TD participants (M=0.77, SEM=0.05) significantly more often reported synchrony (t(53)=2.80, p=0.008, d=0.76, BF10=6.24) than did their ASD counterparts (M=0.59, SEM=0.06) at their peak synchrony report. On the other hand, there was no significant difference in PSS among the groups (ASD, M=−28.75ms, SEM=72.67ms; TD, M=−49.63ms, SEM=9.41ms, t(53)=0.26, p=0.79, d 0.07, BF10=1.07). Most importantly, in terms of the duration over which participants were highly likely to judge an asynchronous presentation as synchronous (i.e., the temporal binding window or TBW), the TBW for cardiovisual stimuli was over 4 times larger in ASD (M =721.07ms, SEM=113.32ms), than in TD (M=168.18ms, SEM=39.25ms, t(53)=5.14, p 0.001, d= 1.41, BF10>104).
In order to confirm that individuals with ASD were able to monitor their heartbeat, we had a random subsample of the participants (10/23) perform the Schandry task (see methods). Overall, the IA score of this group was 0.68 (SEM=0.02), which is a score that is consistent with, if not better than, most previous reports of interoceptive awareness (~0.66 in TD in Garfinkel et al., 2013; ~0.65 in ASD in Schauder et al., 2014). Further, Schauder et al., 2014, report no difference in interoceptive awareness between ASD and TD in a larger and more heterogeneous sample (n=~20 per group as opposed to 10 here). Similarly, our analysis is restricted to a high-functioning ASD group (e.g., IQ scores are numerically larger than in the general population, see Participants Section), minimizing the possibility that the reported distinctions in cardiovisual TBWs are due to difficulties in comprehension or task-compliance. Lastly, there was a strong correlation between ASD participants’ interoceptive awareness score and the size of their cardiovisual TBW (r =- 0.90, p = 0.001, df = 9), suggesting that cardiovisual temporal acuity was best in those individuals most sensitive to their heartbeats. Audio-visual (all |r| < 0.17, all p > 0.50, df = 22) and cardio-visual (all |r| < 0.12, all p > 0.87, df = 22) TBWs did not correlate with ASD symptomatology severity as measured by the ADOS-2.
In addition to controlling for cardiac sensitivity, another important aspect of the cardiovisual simultaneity judgment task is that participants were instructed to monitor their pulse at their wrist. Hence, it may be argued that the task is a visuo-tactile one and that the impairment observed in the ASD sample is a manifestation of impaired visuo-tactile acuity (Greenfield et al., 2015). To address this possible confound, a subset of the ASD and TD subjects performed a visuo-tactile simultaneity judgment task. Results indicate that reports of synchrony on this visuo-tactile task were SOA-dependent both in the case of the TD (F(16,101)=17.86, p<0.001, partial η2=0.869, BF10>104) and ASD (F(16,101)=17.02, p<0.001, partial η2=0.867, BF10>104) individuals. The peak of the Gaussian best fitting the reports did not significantly differ between the TD (M=0.95, SEM=0.003) and ASD (M=0.97, SEM=0.012) groups (t(10)=1.67, p=0.09, d=1.05, BF10=1.35), nor did the PSS (TD: M=−30.04 ms, SEM=3.68 ms; ASD: M=−39.46 ms, SEM=4.42 ms; t(10)=1.56, p=0.12, d=0.98, BF10=1.07). On the other hand, as depicted in Figure 3, visuo-tactile TBWs were indeed larger in ASD (M=310.82ms, SEM=5.55ms) than in TD individuals (M=297.66ms, SEM=2.50ms; t(10) =2.15, p=0.05, d=1.35, BF10=2.17). However, this effect was several orders of magnitude smaller than the effect demonstrated for cardiovisual simultaneity judgments. Furthermore, in contrast to the case of cardiovisual temporal acuity, there was no correlation between interoceptive awareness score and visuo-tactile TBW size in the ASD group (r = 0.02, p = 1, df = 5). The presence of a correlation between interoceptive ability and cardio-visual but not visuo-tactile temporal acuity suggests that indeed the cardio-visual simultaneity judgment assessed cardiac sensitivity and not tactile sensitivity. There was no correlation between the size of ASD participant’s visuo-tactile TBWs and ADOS-2 calibrated severity scores (all |r| < 0.49, all p > 0.27, df = 22).
Figure 3.
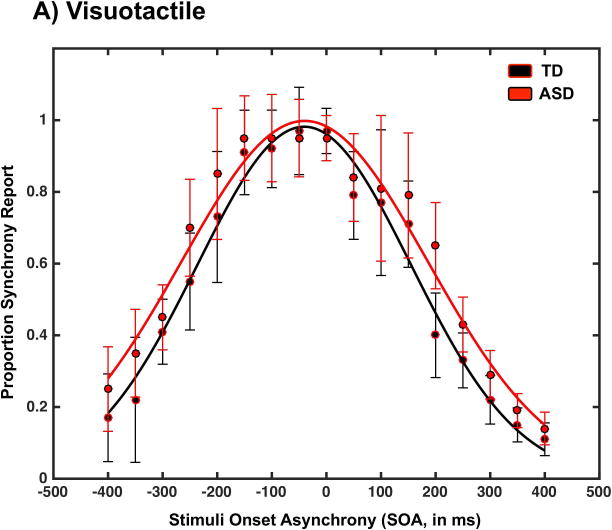
Visuotactile reports of synchrony as a function of SOA and group (TD in black and ASD in red). Error bars represent ± 1 standard error of the mean (SEM).
As further controls, we analyzed heart rate as well as inter-beat interval variability in order to determine whether these factors may play a role in the observed effects. Results revealed no difference in either beats per minute (ASD, M=76.45bpm, SEM=2.57bpm; TD, M=71.49bpm, SEM=1.83bpm; t(42)=1.54, p=0.13, d=0.47, BF10=1.11), or in inter-beat interval variability (ASD, M=0.16, SEM=0.04; TD, M=0.17, SEM=0.03; t(42)=0.354, p=0.72, d= 0.10, BF10=1.25) between the two groups.
Despite the fact that the results from the Schandry task indicate that individuals with ASD can detect their heartbeat, that cardiovisual and not visuotactile temporal function relates to the degree of interoceptive sensitivity at an individual subject level, that analyses of the heartbeats themselves did not reveal any physiological differences between the TD and ASD groups, and that a visuotactile temporal impairment may not entirely account for the reported cardiovisual deficit in ASD, to further test whether cardiovisual temporal function may be anomalous in ASD, we ran a second experiment. In this experiment we recruited not patients with ASD but healthy individuals that on a pre-test in over 230 subjects scored either in the bottom or top 25% of the Autism Spectrum Quotient (AQ).
Experiment 2. Audiovisual and cardiovisual multisensory temporal function in TD with low and high AQ scores
In contrast to the comparison between the TD and ASD groups, when comparing TD individuals with either low or high scores on the AQ, audiovisual temporal acuity did not differ between the groups (Figure 4a). Reports of audiovisual synchrony were SOA-dependent both for high-AQ group (F(16,254)=37.38, p<0.001, partial η2=0.830, BF10>104) and low-AQ group (F(16,220)=20.54, p<0.001, partial η2=0.751, BF10>104). Further, both groups exhibited a similar peak amplitude of the Gaussian function best describing their reports of audiovisual synchrony (Low AQ, M=0.95, SEM=0.04; High AQ, M=0.97, SEM =0.03, t(28)=0.22, p=0.82, d=0.08, BF10=1.02, Figure 4a), a similar PPS (Low AQ, M=25.35ms, SEM=14.96ms; High AQ, M=19.82ms, SEM=7.70ms, t(28)=0.34, p=0.73, d=0.12, BF10=1.16), and a similarly sized TBW (Low AQ, M=144.49ms, SEM=18.47ms; High AQ, M=152.37ms, SEM=15.44ms, t(28)=0.33, p=0.74, d=0.12, BF10=1.16).
Figure 4.
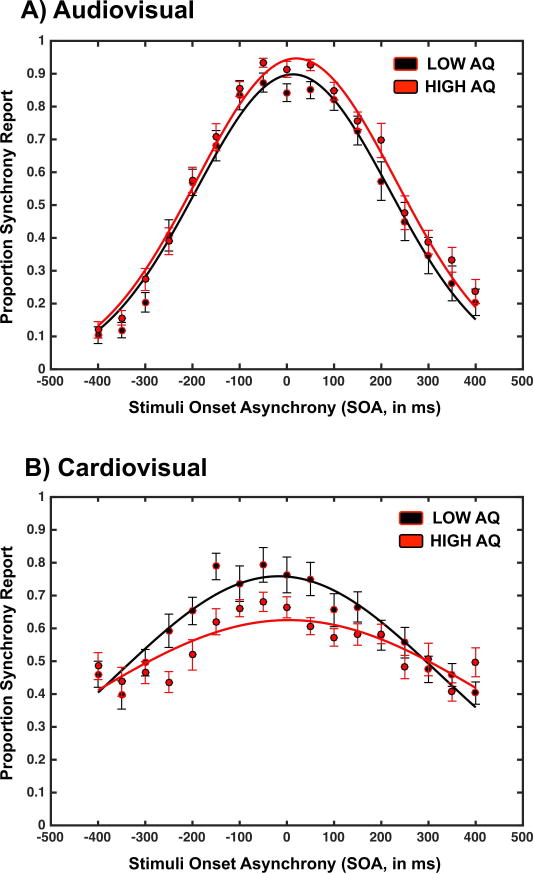
Audiovisual (upper row) and cardiovisual (lower row) reports of synchrony as a function of SOA and group (participants with low AQ scores in black and participants with high AQ scores in red). Error bars represent ± 1 standard error of the mean (SEM).
For the cardiovisual task, a one-way repeated measures ANOVA demonstrated that both for those low (F(16, 224)=9.10, p<0.001, partial η2=0.478, BF10>104) and high (F(16,224)=4.43, p<0.001, partial η2=0.241, BF10>104) on the AQ scale, there was a main effect of SOA, indicating that reports of synchrony were significantly modulated by the true physical asynchrony between cardiac and visual signals. The groups did differ in the mean maximal amplitude of the Gaussian that best described their reports of synchrony as a function of SOA, with low AQ participants (M=0.84, SEM=0.07) exhibiting a larger amplitude than high AQ counterparts (M=0.65, SEM=0.03, t(28)=2.44, p=0.023, d=0.92, BF10=4.00, Figure 4b). In contrast to the results from Experiment 1, however, in this case both the individuals scoring low (t(14)=9.66, p<0.001, d=5.16, BF10>104) and high (t(14)=2.93, p = 0.02, d=1.56, BF10=9.92) on the AQ had peak amplitudes that were significantly greater than chance, illustrating the presence of a cardiovisual TBW.
These groups did not differ in mean cardiovisual PSS (Low AQ, M=−25.25ms, SEM=31.88ms; High AQ, M=29.56ms, SEM=34.03ms, t(28)=1.16, p=0.25, d=0.43, BF10=1.36, Figure 4b). Most importantly, low and high AQ groups did not differ in the size of their cardiovisual TBW (Low AQ, M=239.40ms, SEM=45.95ms; High AQ, M=242.84ms, SEM=36.49ms, t(28)=0.05, p=0.95, d=0.01, BF10=1.01, Figure 4b).
As for TD and ASD groups in Experiment 1, low and high AQ groups did not differ in beats per minute (Low AQ, M=74.59bpm, SEM=1.87bpm; High AQ, M=75.05bpm, SEM=2.15 bpm, t(28)=0.15, p=0.87, d=0.05, BF10=1.08), nor in their inter-beat interval variability (Low AQ, M=0.15, SEM=0.02; High AQ, M=0.12, SEM=0.02, t(28)=0.82, p=0.42, d=0.30, BF10=1.45). In summarizing these results, there were less marked differences between the high and low AQ TD subjects than between low AQ subjects and individuals with ASD. However, there was a discernable difference between high and low AQ individuals in the amplitude of the cardio-visual SOA-response function, suggesting that as in the ASD cohort, high AQ subjects tend to have a flatter response profile when integrating interoceptive and visual information.
Discussion
Overall the results of the current study replicate prior findings by demonstrating larger audiovisual and visuotactile TBWs in ASD individuals when compared with TD individuals (Stevenson et al., 2014; Noel et al. 2016a; Greenfield et al., 2015). The novelty of the current work is in its extension to include temporal judgments that span the interoceptive and exteroceptive realms by specifically indexing cardiovisual processes. While cardiovisual TBWs were generally larger than audiovisual TBWs for all tested populations (TD, TD low in AQ, TD high in AQ, ASD), in individuals with ASD cardiovisual temporal acuity was so poor – cardiovisual TBW 4 times larger in ASD than TD – that reports of cardiovisual synchrony appeared nearly SOA-independent. This dramatic difference in cardiovisual performance prompted a second experiment in which we had healthy (i.e., non-ASD) individuals either low or high on the AQ scale perform the same tasks.
In this second experiment, reports of cardiovisual synchrony were modulated by SOA for both low and high AQ individuals, and these two groups did not exhibit a difference in their cardiovisual TBW size. They did, however, demonstrate a significant difference in regard to the peak amplitude of their simultaneity reports. We consider this finding particularly interesting, as it suggests that both ASD participants and healthy individuals high on the AQ scale demonstrate a reduced tendency to report cardiovisual synchrony. While the amplitude effect may represent a response bias, the TBW size effect is less easily incorporated into such a framework.
An acknowledged limitation of the current study is that we allowed participants to take their pulse over the course of the experiment, a procedure that could be construed as indexing a visuotactile TBW as opposed to a cardiovisual TBW. Although it is true that we allowed participants to take their pulse (as pilot testing demonstrated that the task was unfeasible without this), and that this act inevitably leads to a tactile sensation, we believe that for a number of reasons the task here differs from a standard visuotactile simultaneity judgment task. First, although altered temporal binding in the context of a visuotactile judgment has recently been demonstrated in ASD (Greenfield et al., 2015), the differences seen were far from the magnitude of those seen in the current study, suggesting basic differences between the cardiovisual task used here and classic measures of visuotactile temporal acuity. To address this issue, a subset of participants in Experiment 1 were administered a visuotactile simultaneity judgment task, and while results indicated larger visuotactile TBWs in ASD than TD, the magnitude of the visuotactile difference was on the order to 10 ms, as compared to a difference of approximately 500 ms for the cardiovisual task. Second, there was a strong correlation between ASD participants’ performance on a well-established interoceptive sensitivity task and their cardiovisual but not visuo-tactile temporal acuity, suggesting that the cardiovisual simultaneity task indeed indexed interoceptive ability. Third, it must be emphasized that even if the task is derivative of a visuo-tactile judgment, this judgment is based on the monitoring of an interoceptive and predictable process – the rhythmicity of one’s own heartbeat. Thus, and different from a classic visuotactile simultaneity judgment, which generally revolves around relating the timing of a visual event to an externally generated tactile event on the skin surface, participants here were making judgments based on their monitoring of an internal and rhythmic process. Lastly, it may be argued that cardiovisual, rather than visuotactile, temporal acuity is further impaired in ASD due to task difficulty. This objection remains to be tested in a future study artificially degrading visuotactile performance, for instance via the administration of sensory noise, and relating a general decrease in performance to TBW size. Furthermore, conceptually, we hypothesized that the impairment in audiovisual temporal acuity in ASD may be accentuated for an intero-exteroceptive pairing – and results indeed supported this hypothesis. Whether this phenomenon is exclusively due to the difficulty in breaching between interoception and exteroception remains to be ascertained. Beyond this first characterization of striking temporal differences across exteroceptive and interoceptive sensory modalities in ASD, future studies may seek to additionally constrain the demographic characteristics of the samples tested. For example, in the current report only high-functioning individuals were tested, ASD and TD groups were not explicitly matched for cognitive ability, co-morbidity for alexithymia (Shah et al., 2016; Brewer et al., 2016) – a sub-clinical population highly present in ASD and with documented interoceptive deficits – was not tested, and a wide age range of participants were recruited when it is well-established that multisensory temporal ability is heavily influenced by age (e.g., Hillock et al., 2011; Noel et al., 2016c).
Indeed, an initial conjecture for the current study was that due to the highly rhythmic and predictable nature of the heartbeat, once individuals are trained on the task, cardiovisual TBWs would be narrower than the TBW for externally generated audiovisual stimuli, which are less predictable. This prediction did not hold for either the TD or the ASD participants. In a related manner, it is possible that a large contributor to the difference in cardiovisual TBW size between TD and ASD is in the capacity to generate and make use of predictions. In fact, Pellicano and Burr, 2012, recently highlighted the fact that perceptual experience is influenced by both incoming sensory information (i.e., likelihoods, in Bayesian terms) and by expectations or prior knowledge of the world (i.e., priors, in Bayesian terms). They hypothesized that many of the sensory abnormalities present in ASD may be explained by attenuated priors (i.e., hypo-priors), and indeed recent evidence appears to indicate that individuals with ASD may have difficulties in predicting upcoming events (Sinha et al., 2014; van Boxtel and Lu, 2013; Van de Cruys et al., 2014), and/or in incorporating recent sensory evidence (Noel et al., 2016a; Turi et al., 2016) into their representation of the external world. The current results, thus, may be interpreted as fitting within this predictive-coding perspective on ASD. Stated in more detail, in order to categorize certain multisensory presentations as synchronous and others as asynchronous, in addition to precise and reliable sensory representations, it is necessary to have and to utilize an internal model of what constitutes synchrony. In fact, while the current results suggest that the interoceptive ability of individuals with ASD is intact (i.e., Schandry Task, see also Schauder et al., 2014), they also point to the prior belief system responsible for contextualizing this sensory evidence as awry (see Friston et al., 2013; Lawson et al., 2014, 2015, for similar arguments).
In fact, the predictive coding framework has recently been applied to interoception (Seth et al., 2012), with the postulation that the feeling of presence, which may be anomalous in ASD (Parnas et al., 2002. Uddin, 2011; Noel et al., 2017a), is at least partly accomplished by successful top-down suppression of interoceptive signals evoked by automatic control directly, and by visceral responses to afferent sensory signals indirectly. In this view, interoceptive predictive signals travel via autonomic pathways to regions of the limbic system. At this stage, information about the internal milieu converges with exteroceptive signals and imbues higher-level representations with their distinct affective valence (Seth et al., 2011; Quattrocki et al., 2014). This integration of exteroceptive and interoceptive signals must be properly weighted to appropriately balance external signals with their requisite social emotional valence; a process that the current results suggest goes awry in autism.
Lay Summary.
Studies have shown that individuals with autism have difficulty in separating auditory and visual events in time. People with autism also weight sensory evidence originating from the external world and from their body differently. We measured simultaneity judgments regarding visual and auditory events and between visual and heartbeat events. Results suggest that while individuals with autism show unusual temporal function across the senses in a general manner, this deficit is greater when pairings bridged between the external world and the internal body.
Acknowledgments
The authors acknowledge Lisa Mash, John Tracy, and Brynna Heflin for their help in participant recruitment. This project was funded by a R01 MH102272 to C.J.C., a R21 MH109225 to C.J.C. and M.T.W.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders-V-TR. American Psychological Association; Washington, DC: 2013. [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory Experiences Questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. J Child Psychol Psychiatry Allied Discip. 2006;47:591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The Autism Spectrum Quotient (AQ): Evidence from Asperger syndrome/high functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Barttfeld P, Wicker B, Cukier S, Navarta S, Lew S, Leiguarda R, Sigman M. State-dependent changes of connectivity patterns and functional brain network topology in autism spectrum disorder. Neuropsychologia. 2012;50:3653–3662. doi: 10.1016/j.neuropsychologia.2012.09.047. http://dx.doi.org/10.1016/j.neuropsychologia.2012.09.047. [DOI] [PubMed] [Google Scholar]
- Baum SH, Stevenson RA, Wallace MT. Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Progress in Neurobiology. 2015 doi: 10.1016/j.pneurobio.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessette PR, Scully BM, Jones GE. Test-retest reliability of the Brener-Kluvitse heartbeat perception paradigm. Psychophysiology. 1991;25:S12. [Google Scholar]
- Botvinick M, Cohen J. Rubber hands ‘feel’ touch that eyes see. Nature. 1998;391(6669):756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Brandwein AB, Foxe JJ, Butler JS, Russo NN, Altschuler TS, Gomes H, Molholm S. The development of multisensory integration in high functioning autism: high-density electrical mapping and psychophysical measures reveal impairments in the processing of audiovisual inputs. Cereb Cortex. 2013;23:1329–1341. doi: 10.1093/cercor/bhs109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener J, Kluvitse C. Heartbeat detection: judgments of the simultaneity of external stimuli and heartbeats. Psychophysiology. 1988;25:554–561. doi: 10.1111/j.1469-8986.1988.tb01891.x. [DOI] [PubMed] [Google Scholar]
- Brener J, Liu X, Ring C. A method of constant stimuli for examining heart-beat detection: Comparison with the Brener-Kluvitse and Whitehead methods. Psychophysiology. 1993;30:657–665. doi: 10.1111/j.1469-8986.1993.tb02091.x. [DOI] [PubMed] [Google Scholar]
- Brewer R, Cook R, Bird G. Alexithymia: a general deficit of interoception. R Soc Open Sci. 2016a;3(10):150664. doi: 10.1098/rsos.150664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer R, Cook R, Bird G. Shared interoceptive representations: the case of alexithymia. In: Obhi SS, Cross ES, editors. Shared Representations Cambridge Social Neuroscience Series. Cambridge University Press; Cambridge, UK: 2016b. [Google Scholar]
- Brewer R, Happé F, Cook R, Bird G. Commentary on Autism, oxytocin and interoception: alexithymia, not Autism Spectrum Disorders, is the consequence of interoceptive failure. Neurosci Biobehav Rev. 2015;56:348–353. doi: 10.1016/j.neubiorev.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Brown L, Sherbenou RJ, Johnsen SK. Test of nonverbal intelligence. 4th. Austin, TX: PRO-ED; 2010. [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. http://dx.doi.org/10.1016/S0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- De Niear MA, Noel JP, Wallace MT. The Impact of Feedback on the Different Time Courses of Multisensory Temporal Recalibration. Neural Plasticity. 2017;2017:12. doi: 10.1155/2017/3478742. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Yan CG, Li Q, Denio E, Castellanos FX, Alaerts K, Deen B. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry. 2014;19(6):659–667. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois D, Ameis SH, Lai MC, Casanova MF, Desakar P. Interoception in autism spectrum disorder: A review. International Journal of Developmental Neuroscience. 2016;52:104–111. doi: 10.1016/j.ijdevneu.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Ebisch SJH, Gallese V, Willems RM, Mantini D, Groen WB, Romani GL, Buitelaar JK, Bekkering H. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp. 2011;32:1013–1028. doi: 10.1002/hbm.21085. http://dx.doi.org/10.1002/hbm.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BS, Skrondal A. The Cambridge dictionary of statistics. fourth. Cambridge University Press; Cambridge, UK: 2002. [Google Scholar]
- Fiene L, Brownlow C. Investigating interoception and body awareness in adults with and without autism spectrum disorder. Autism Res. 2016;8:709–16. doi: 10.1002/aur.1486. [DOI] [PubMed] [Google Scholar]
- Foss-Feig JH, Kwakye LD, Cascio CJ, Burnette CP, Kadivar H, Stone WL, Wallace MT. An extended multisensory temporal binding window in autism spectrum disorders. Exp Brain Res Experimentelle Hirnforschung. 2010;203:381–389. doi: 10.1007/s00221-010-2240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Molholm S, Bene VAD, Frey HP, Russo NN, Blanco D, Saint-Amour D, Ross LA. Severe multisensory speech integration deficits in high-functioning school-aged children with autism spectrum disorder (ASD) and their resolution during early adolescence. Cereb Cortex. 2013:19–29. doi: 10.1093/cercor/bht213. bht213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Lawson R, Frith CD. On hyperpriors and hypopriors: comment on Pellicano and Burr. Trends Cogn Sci. 2013;17 doi: 10.1016/j.tics.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Gaigg SB, Cornell AS, Bird G. The psychophysiological mechanisms of alexithymia in autism spectrum disorder. Autism. 2016 doi: 10.1177/1362361316667062. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol Psychol. 2013;104:65–74. doi: 10.1016/j.biopsycho.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Greenfield K, Ropar D, Smith AD, Carey M, Newport R Visuo-tactile integration in autism: atypical temporal binding may underlie greater reliance on proprioceptive information. Mol Autism. 2015;6:51. doi: 10.1186/s13229-015-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert BM, Herbert C, Pollatos O. On the relationship between interoceptive awareness and alexithymia: is interoceptive awareness related to emotional awareness? J Pers. 2011;79:1149–1175. doi: 10.1111/j.1467-6494.2011.00717.x. [DOI] [PubMed] [Google Scholar]
- Hillock AR, Powers AR, Wallace MT. Binding of sights and sounds: Age-related changes in multisensory processes. Neuropsychologia. 2011;49:461–467. doi: 10.1016/j.neuropsychologia.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iarocci G, McDonald J. Sensory integration and the perceptual experience of persons with autism. J Autism Dev Disord. 2006;36:77–90. doi: 10.1007/s10803-005-0044-3. [DOI] [PubMed] [Google Scholar]
- Jarosz AF, Wiley J. What are the odds? A practical guide to computing and reporting Bayes Factors. J Probl Solving. 2014;7(1):2. [Google Scholar]
- Jeffreys H. Oxford classic texts in the physical science. 3rd. Oxford University Press; Oxford: 1961. Theory of probability. [Google Scholar]
- Kwakye LD, Foss-Feig JH, Cascio CJ, Stone WL, Wallace MT. Altered auditory and multisensory temporal processing in autism spectrum disorders. Front Integr Neurosci. 2011;4:129. doi: 10.3389/fnint.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson RP, Friston KJ, Rees G. A more precise look at context in autism. Proceedings of the National Academy of Sciences. 2015;112(38):E5226–E5226. doi: 10.1073/pnas.1514212112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson RP, Rees G, Friston KJ. An aberrant precision account of autism. Front Hum Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan J. Autism diagnostic interview: a standardized investigator-based instrument. J Autism Dev Disord. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Longarzo M, D’Olimpio F, Chiavazzo A, Santangelo G, Trojano L, Grossi D. The relationships between interoception and alexithymic trait. The Self-Awareness Questionnaire in healthy subjects. Fronti Psychol. 2015;6 doi: 10.3389/fpsyg.2015.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Love J, Selker R, Marsman M, Jamil T, Dropmann D, Verhagen AJ, Wagenmakers EJ. JASP (Version 0.8.1) 2015 [Computer software] [Google Scholar]
- Marco EJ, Hinkley LB, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res. 2011;69:48R–54R. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Spatial determinants of multisensory integration in cat superior colliculus neurons. J Neurophysiol. 1996;75:1843–1857. doi: 10.1152/jn.1996.75.5.1843. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Nemitz JW, Stein BE. Determinants of multisensory integration in superior colliculus neurons. I. Temporal factors. Journal of Neuroscience. 1987;7:3215–3229. doi: 10.1523/JNEUROSCI.07-10-03215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J, Brewer R, Catmur C, Bird G. Interoception and psychopathology: a developmental neuroscience perspective. Developmental Cognitive Neuroscience. 2017;23:45–56. doi: 10.1016/j.dcn.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näring GWB, Van der Staak CPF. Perception of heart rate and blood pressure: the role of alexithymia and anxiety. Psychother Psychosom. 1995;63:193–200. doi: 10.1159/000288959. [DOI] [PubMed] [Google Scholar]
- Noel J-P, De Niear M, Van der Burg E, Wallace MT. Audiovisual Simultaneity Judgment and Rapid Recalibration throughout the Lifespan. PLoS ONE. 2016c;11(8):e0161698. doi: 10.1371/journal.pone.0161698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel JP, Wallace M. Relative contribution of visual and auditory spatial representations to tactile localization. Neuropsychologia. 2016;82:84–90. doi: 10.1016/j.neuropsychologia.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel JP, De Niear MA, Stevenson R, Alais D, Wallace MT. Atypical rapid audiovisual temporal recalibration in autism spectrum disorders. Autism Res. 2016a doi: 10.1002/aur.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel JP, Cascio C, Wallace MT, Park S. The spatial self in schizophrenia and autism spectrum disorder. Schizophrenia Research. 2017a doi: 10.1016/j.schres.2016.09.021. http://dx.doi.org/10.1016/j.schres.2016.09.021. [DOI] [PMC free article] [PubMed]
- Noel JP, Kurela L, Baum SH, Yu H, Neimat JS, Gallagher MJ, Wallace MT. Multisensory temporal function and EEG complexity in patients with epilepsy and psychogenic nonepileptic events. Epilepsy and Behavior. 2017b;70:166–172. doi: 10.1016/j.yebeh.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel JP, Lukowska M, Wallace MT, Serino A. Multisensory simultaneity judgment and distance from the body. Journal of Vision. 2016b;16(3):21, 1–17. doi: 10.1167/16.3.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel JP, Wallace MT, Orchard-Mills E, Alais D, Van der Burg True and perceived synchrony are preferentially associated with particular sensory pairings. Sci Rep. 2015;5:17467. doi: 10.1038/srep17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas J, Bovet P, Zahavi D. Schizophrenic autism: clinical phenomenology and pathogenetic implications. World Psychiatry. 2002;1(3):131–136. [PMC free article] [PubMed] [Google Scholar]
- Pellicano E, Burr D. When the world becomes ‘too real’: a bayesian explanation of autistic perception. Trends in Cognitive Sciences. 2012;16(10):504–510. doi: 10.1016/j.tics.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Quattrocki E, Friston K. Autism, oxytocin and interoception. Neurosci Biobehav Rev. 2014;47:410–30. doi: 10.1016/j.neubiorev.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring C, Brener J. The temporal location of heartbeat sensations. Psychophysiology. 1992;29:535–545. doi: 10.1111/j.1469-8986.1992.tb02027.x. [DOI] [PubMed] [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychonomic Bulletin & Review. 2009;16(2):225–237. doi: 10.3758/PBR.16.2.225. [DOI] [PubMed] [Google Scholar]
- Salomon R, Ronchi R, Donz J, Bello-Ruiz J, Herbelin B, Martet R, Faivre N, Schaller K, Blanke O. The insula mediates access to awareness of visual stimuli presented synchronously to the heartbeat. J Neurosci. 2016;36:5115–5127. doi: 10.1523/JNEUROSCI.4262-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18:483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Schauder KB, Mash LE, Bryant LK, Cascio CJ. Interoceptive ability and body awareness in autism spectrum disorder. J Exp Child Psychol. 2014;131:193–200. doi: 10.1016/j.jecp.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK, Suzuki K, Critchley HD. An interoceptive predictive coding model of conscious presence. Front Psychol. 2012 doi: 10.3389/fpsyg.2011.00395. http://dx.doi.org/10.3389/fpsyg.2011.00395. [DOI] [PMC free article] [PubMed]
- Shah P, Hall R, Catmur C, Bird G. Alexithymia, not autism, is associated with impaired interoception. Cortex. 2016 doi: 10.1016/j.cortex.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DM, Noel JP, Wallace MT. Evoked related potentials index rapid recalibration to audiovisual temporal asynchrony. Frontiers in Integrative Neuroscience. 2017;11 doi: 10.3389/fnint.2017.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P, et al. Autism as a disorder of prediction. Proc Natl Acad Sci USA. 2014;111:15220–15225. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowden S, Brewer R, Catmur C, Bird G. The specificity of the link between alexithymia, interoception, and imitation. J Exp Psychol Hum Percept Perform. 2016;42(11):1687–1692. doi: 10.1037/xhp0000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Siemann JK, Schneider BC, Eberly HE, Woynaroski TG, Camarata SM, Wallace MT. Multisensory temporal integration in autism spectrum disorders. J Neurosci. 2014;34(3):691–697. doi: 10.1523/jneurosci.3615-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Segers M, Ferber S, Barense MD, Camarata S, Wallace MT. Keeping time in the brain: autism spectrum disorder and audiovisual temporal processing. Autism Res. 2016 doi: 10.1002/aur.1566. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, Siemann JK, Woynaroski TG, Schneider BC, Everly HE, Camarata SM, Wallace MT. Arrested Development of Audiovisual Speech Perception in Autism Spectrum Disorders. Journal of Autism and Development Disorder. 2013;44(6):1470–7. doi: 10.1007/s10803-013-1992-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Garfinkel SN, Critchley HD, Seth AK. Multisensory integration across interoceptive and exteroceptive domains modulates self-experience in the rubber hand illusion. Neuropsychologia. 2013;51:2909–2917. doi: 10.1016/j.neuropsychologia.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Tajadura-Jimenez A, Costantini M. Just a heartbeat away from one’s body: interoceptive sensitivity predicts malleability of body-representations. Proc Biol Sci. 2011;278:2470–2476. doi: 10.1098/rspb.2010.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turi M, Karaminis T, Pellicano E, Burr D. No rapid audiovisual recalibration in adults on the autism spectrum. Sci Rep. 2016;6:21756. doi: 10.1038/srep21756. (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. The self in autism: an emerging view from neuroimaging. Neurocase. 2011;17:201–208. doi: 10.1080/13554794.2010.509320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel JJA, Lu H. A predictive coding perspective on autism spectrum disorders. Frontiers in Psychology. 2013;4 doi: 10.3389/fpsyg.2013.00019. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Cruys S, et al. Precise minds in uncertain worlds: predictive coding in autism. Psychol Rev. 2014;121:649–675. doi: 10.1037/a0037665. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Stevenson RA. The construct of the multisensory temporal binding window and its dysregulation in developmental disabilities. Neuropsychologia. 2014;64:105–123. doi: 10.1016/j.neuropsychologia.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woynaroski T, Kwakye L, Foss-Feig J, Stevenson R, Stone W, Wallace M. Multisensory Speech Perception in Children with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2013;43(12):2891–902. doi: 10.1007/s10803-013-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


