Scientific Abstract
Individuals with SHANK3 mutations have severely impaired receptive and expressive language abilities. While brain responses are known to be abnormal in these individuals, the auditory cortex response to sound has remained largely understudied. In this study, we document the auditory cortex response to speech and non-speech sounds in the novel Shank3-deficient rat model. We predicted that the auditory cortex response to sounds would be impaired in Shank3-deficient rats. We found that auditory cortex responses were weaker in Shank3 heterozygous rats compared to wild-type rats. Additionally, Shank3 heterozygous responses had less spontaneous auditory cortex firing and were unable to respond well to rapid trains of noise bursts. The rat model of the auditory impairments in SHANK3 mutation could be used to test potential rehabilitation or drug therapies to improve the communication impairments observed in individuals with Phelan-McDermid syndrome.
Lay Summary
Individuals with SHANK3 mutations have severely impaired language abilities, yet the auditory cortex response to sound has remained largely understudied. In this study, we found that auditory cortex responses were weaker and were unable to respond well to rapid sounds in Shank3-deficient rats compared to control rats. The rat model of the auditory impairments in SHANK3 mutation could be used to test potential rehabilitation or drug therapies to improve the communication impairments observed in individuals with Phelan-McDermid syndrome.
Keywords: Phelan-McDermid syndrome, 22q13 deletion, Autism, SHANK3-haploinsufficiency syndromes
Introduction
Individuals with Phelan-McDermid syndrome have severely delayed or absent speech, as well as motor impairments and developmental delays (Philippe et al., 2008; Phelan and McDermid, 2012; Soorya et al., 2013; Zwanenburg et al., 2016). Phelan-McDermid syndrome is a neurodevelopmental disorder that arises from deletion or single mutation in one copy of the SHANK3 gene. Shank3 is a scaffolding protein in the postsynaptic density of glutamatergic synapses (Jiang and Ehlers, 2013). SHANK3 deletions and mutations account for up to 1.7% of individuals with autism spectrum disorder (Leblond et al., 2014). Interestingly, the neural responses evoked by sounds in individuals with Phelan-McDermid syndrome are distinct from the neural responses evoked in individuals with idiopathic autism (Wang et al., 2016). While it is well-documented that individuals with Shank3 mutations have receptive speech processing problems (Soorya et al., 2013; Sarasua et al., 2014a; Wang et al., 2016), no studies have examined basic auditory processing in these individuals compared to typically developing individuals.
Rats are an excellent model of speech processing. Rats can accurately distinguish between most English consonant and vowel sounds (Reed et al., 2003; Engineer et al., 2008; Perez et al., 2013). In addition, rats are able to correctly generalize to different talkers, and accurately discriminate speech sounds presented in background noise or in a continuous speech stream (Shetake et al., 2011; Engineer et al., 2013; Centanni et al., 2016). Speech discrimination ability in rats is well correlated with the distinctness of the neural activity patterns evoked by the speech sounds (Engineer et al., 2008; Centanni et al., 2013; Perez et al., 2013). While sounds that evoke similar neural patterns are difficult to discriminate, sounds that evoke distinct neural patterns are easy to discriminate. For example, the speech sounds ‘rad’ and ‘lad’ evoke very similar neural patterns, and are difficult for both rats and non-native English speakers to discriminate, while the speech sounds ‘dad’ and ‘bad’ evoke very distinct neural patterns, and are easy for rats to discriminate.
Because the spatiotemporal fidelity of neural processing is critical for speech processing, many genetic and environmental factors that degrade neural responses also impair speech discrimination. Many patient populations suffer from difficulties processing speech sounds due to impaired cortical responses to sounds, including individuals with autism spectrum disorders (Moore and Shannon, 2009; Lai et al., 2011; Anon, 2014; Nittrouer et al., 2014). Cortical responses to sounds are often both weaker and delayed in individuals with autism compared to typically developing individuals (Bomba and Pang, 2004; Russo et al., 2009; Gandal et al., 2010; Roberts et al., 2011). The extent of the auditory cortex impairment is correlated with language impairment (Kuhl et al., 2013). Auditory cortex responses and speech discrimination ability are also impaired in both genetic and environmental rodent models of autism (Gandal et al., 2010; Liao et al., 2012; Engineer et al., 2014a, 2014c).
A Shank3 deficient rat model has recently been developed that exhibits behavioral and neural deficits that resemble those observed in individuals with SHANK3 mutation (Harony-Nicolas et al., 2015, 2017). In an effort to identify whether sensory processing abnormalities could contribute to the receptive language problems observed in this population, we assessed the neural response characteristics to sounds in multiple auditory cortical fields in the novel Shank3 genetically modified rat model. We hypothesized that the auditory cortex response to sounds would be altered, as seen in other rodent models of disorders that include receptive language deficits (Gandal et al., 2010; Liao et al., 2012; Kim et al., 2013; Centanni et al., 2014a, 2016, Engineer et al., 2014a, 2014c, 2015a).
Methods
Shank3 rat model
Male and female Shank3 heterozygous and wild-type rats were obtained from Joseph Buxbaum at Mount Sinai. SAGE Labs (Boyertown, PA) used zinc-finger nucleases on the outbred Sprague-Dawley background to target the Shank3 ANK domain (exon 6). A 68 base-pair deletion was introduced that produced a stop codon in exon 6 (Harony-Nicolas et al., 2017). Heterozygous breeder rats were paired to generate the rats used in this study. Since no gender bias has been reported in Phelan McDermid syndrome, both male and female rats were included in the current study (Soorya et al., 2013; Sarasua et al., 2014b; Wang et al., 2016). Eight heterozygous Shank3 Sprague-Dawley rats (4 female and 4 male) and eleven control Sprague-Dawley rats (6 female and 5 male) were used in this study. Rats were housed 2 per cage, and were on a reversed 12 hour dark light cycle. All recording procedures were performed in adult rats over the age of 3 months. All procedures were approved by The University of Texas at Dallas Animal Care and Use Committee.
Sound stimuli
The speech sounds used in this study included the words: ‘bad’, ‘chad’, ‘dad’, ‘dead’, ‘deed’, ‘dood’, ‘dud’, ‘gad’, ‘sad’, ‘shad’, and ‘tad’. Each of these sounds was spoken in isolation by a female native English speaker, as in our previous studies (Engineer et al., 2008; Perez et al., 2013). Speech sounds were randomly interleaved, and each speech sound was presented 20 times with an interstimulus interval of 2 seconds. All speech sounds were frequency shifted up an octave using the STRAIGHT vocoder to shift the sounds into the rat hearing range (Kawahara, 1997). Speech sounds were approximately 500 ms in duration, and were presented so that the loudest 100 ms of the vowel was 60 dB. The noise burst trains used in this study were presented at 7, 10, 12.5, and 15 Hz. Each train consisted of six 25 ms noise bursts. The noise bursts were white noise consisting of frequencies between 1.5 – 30 kHz, and were presented at 60 dB. The noise burst trains were randomly interleaved, and each of the 4 train speeds was presented 20 times with an interstimulus interval of 2 seconds. The 1,440 tones used in this study ranged in frequency from 1 – 48 kHz in 0.0625 octave steps and intensity from 0 – 75 dB in 5 dB steps. All tones were 25 ms in duration and were randomly interleaved and presented with an interstimulus interval of 500 ms.
Auditory cortex physiology
Auditory cortex recordings were obtained from the right auditory cortex in 11 control rats and 8 heterozygous Shank3 rats. Recordings were collected from four auditory cortical fields: anterior auditory field (AAF; n = 230 naïve AAF sites and n = 158 Shank3 AAF sites), primary auditory cortex (A1; n = 298 naïve A1 sites and n = 182 Shank3 A1 sites), ventral auditory field (VAF; n = 132 naïve VAF sites and n = 113 Shank3 VAF sites), and posterior auditory field (PAF; n = 100 naïve PAF sites and n = 81 Shank3 PAF sites). There was no significant difference between the distribution of characteristic frequencies of the recorded neurons between Shank3 heterozygous rats and control rats (Table 1, Mean U = 668, z = −0.15, p = 0.88, Mann-Whitney U test; Median U = 644, z = −0.41, p = 0.68; Minimum U = 661, z = −0.23, p = 0.82; Maximum U = 678, z = −0.04, p = 0.97; Range U = 656, z = −0.28, p = 0.78). At each recording site, multi-unit responses were obtained in response to speech sounds, trains of noise bursts, and tones. Rats were initially anesthetized with sodium pentobarbital (50 mg/kg), and they received supplemental doses of dilute pentobarbital (8 mg/mL) throughout the experiment as needed. A tracheotomy was performed to ease breathing and a cisternal drain was performed to reduce swelling. A craniotomy and durotomy were performed to expose the right hemisphere auditory cortex. Four Parylene-coated microelectrodes (1.5 – 2.5 MΩ, FHC, Bowdoin, ME) were used to record auditory cortex responses at a depth of approximately 600 μm, which corresponds to layer IV/V in experimentally naïve rats. Individuals with Phelan-McDermid syndrome typically have normal brain MRIs, although some individuals exhibit cerebellar or corpus callosum abnormalities (Philippe et al., 2008; Aldinger et al., 2013). There is no evidence suggesting alterations in cortical thickness in rodent Shank3 models or individuals with Phelan-McDermid syndrome (Philippe et al., 2008; Jiang and Ehlers, 2013; Wang et al., 2016). Tucker-Davis Technologies (Alachua, FL) hardware and software were used for sound presentation and data acquisition. Sounds were presented from a free-field speaker (TDT, FF1) located 10 cm from the left ear. All recording procedures were identical to our previous studies (Centanni et al., 2014b; Engineer et al., 2014b, 2014c, 2015b).
Table 1.
The distribution of characteristic frequencies was matched between Shank3 heterozygous rats and control rats.
| Mean (kHz) | Median (kHz) | Minimum (kHz) | Maximum (kHz) | Range (kHz) | ||
|---|---|---|---|---|---|---|
| AAF | Control | 18.4±1.6 | 16.4 | 3.7±0.5 | 41.6±1.6 | 37.9±1.5 |
| Shank3 | 16.9±1.4 | 13.2 | 4.1±1.1 | 39.7±2.9 | 35.6±3.5 | |
| A1 | Control | 11.4±1.1 | 7.8 | 1.7±0.2 | 35±2.1 | 33.4±2.1 |
| Shank3 | 14.1±1.2 | 12.4 | 1.9±0.2 | 37.4±2.9 | 35.5±2.8 | |
| VAF | Control | 22.6±2.6 | 21.6 | 7.8±2.2 | 38.9±2.5 | 31.1±3.0 |
| Shank3 | 20±3.4 | 18.7 | 9.1±3.2 | 35.9±3.8 | 26.8±3.1 | |
| PAF | Control | 10.2±1.4 | 7.7 | 4±0.7 | 23.9±4.8 | 19.9±4.9 |
| Shank3 | 8.6±1.4 | 7.3 | 3.5±0.6 | 20.2±5.0 | 16.7±4.9 |
Data analysis
Each analysis technique was performed using recordings separated into individual auditory cortex fields (Polley et al., 2007; Puckett et al., 2007; Takahashi et al., 2011; Centanni et al., 2013; Engineer et al., 2014a). AAF was distinguished by short response latencies and distinct low frequency to high frequency tonotopy in an anterior to posterior direction. A1 was distinguished by short response latencies and distinct low frequency to high frequency tonotopy in a posterior to anterior direction. VAF was distinguished by longer response latencies, no distinct tonotopy, and a location between AAF and A1. PAF was distinguished by late response latencies, no distinct tonotopy, and a location posterior to A1.
All analysis was performed using MATLAB software. The response threshold was defined as the lowest tone intensity that evoked a response. The driven rate was defined as the number of driven spikes evoked. The spontaneous firing rate was the rate of firing evoked during silence. For tones, the spontaneous firing rate was calculated across the duration of the trial (400 ms) across all 90 tone frequencies, when presented at an amplitude of 0 dB. For speech sounds, in order to calculate the driven rate by subtracting the spontaneous firing rate, 100 ms of silence collected before the presentation of a sound was used to calculate the spontaneous firing rate. The bandwidth was defined as the range of frequencies that evoked a response 40 dB above the threshold response. Only sites with a bandwidth value greater than 0 were included in the bandwidth analysis in order to account for high threshold sites without data 40 dB above the threshold (the highest tone intensity was 75 dB). For the noiseburst sounds, the number of spikes evoked by the second noise burst was defined as the number of driven spikes evoked during a 25 ms window for the second noise burst. For speech sounds, the response strength was defined as the number of driven spikes evoked during the 40 ms onset of the speech sound. The peak response latency was the time point when the largest number of spikes occurred.
Neural classifier accuracy was quantified using a PSTH-based nearest-neighbor classifier, as in previous studies (Engineer et al., 2008, 2014a; Centanni et al., 2013; Perez et al., 2013). Classifier performance is highly correlated with behavioral discrimination ability across multiple auditory fields, in both anesthetized and awake rats (Engineer et al., 2008; Centanni et al., 2013). The classifier was provided the 40 ms onset response to pairs of speech sounds using 1 ms precision. Each of the 7 consonant onset sounds (‘bad’, ‘chad’, ‘dad’, ‘gad’, ‘sad’, ‘shad’, and ‘tad’) was compared to every other consonant onset, for a total of 21 consonant pairs. Each single trial response pattern was compared with the average response pattern evoked by each of the sounds. The similarity between the single trial response pattern and the average response patterns was quantified using Euclidean distance. The classifier assigned each single trial response pattern to the average response pattern that it was the most similar to. For two-group comparisons, the non-parametric Mann-Whitney U test was used to determine statistical significance. For multiple group comparisons, the non-parametric Kruskal-Wallis test was used to determine statistical significance. Bonferroni correction was used to correct for multiple comparisons.
Results
Shank3 deficiency alters the cortical response to tones
Receptive field properties were significantly impaired across auditory cortical fields in Shank3 heterozygous rats. The threshold response was unaltered in Shank3 heterozygous rats compared to control rats (U = 202143, z = −0.12, p = 0.91, Mann-Whitney U test, Table 2). There was a significant difference in threshold response across the auditory fields (H(3) = 40.7, p < 0.0001, Kruskal-Wallis test).
Table 2.
Receptive field properties were altered in Shank3 heterozygous rats compared to control rats. All property values are presented as the mean and median. Bolded numbers marked with a star are significantly different compared to control rats.
| Number of sites | Threshold(dB) | Driven rate (spikes/tone) | Spontaneous rate (Hz) | Bandwidth (octaves) | Peak latency (ms) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Mean | Median | Mean | Median | Mean | Median | Mean | Median | Mean | Median | |||
| AAF | Control | 230 | 18.3 | 12.6 | 2.5 | 2.2 | 14.5 | 12.5 | 3.2 | 3.3 | 17.6 | 17.0 |
| Shank3 | 158 | 15.8 | 12.1 | 2.0* | 1.7* | 10.0* | 8.1* | 3.2 | 3.2 | 17.0 | 17.0 | |
| A1 | Control | 298 | 11.5 | 7.0 | 3.1 | 3.0 | 16.1 | 13.9 | 2.8 | 2.8 | 19.5 | 19.0 |
| Shank3 | 182 | 14.0 | 7.2 | 2.6* | 2.5* | 15.1 | 12.2 | 2.9 | 3.0 | 19.2 | 18.0 | |
| VAF | Control | 132 | 18.7 | 12.5 | 2.6 | 2.1 | 20.0 | 17.2 | 2.6 | 2.9 | 23.8 | 21.0 |
| Shank3 | 113 | 16.9 | 11.6 | 2.5 | 2.2 | 14.6* | 14.1* | 3.6* | 3.7* | 29.1 | 21.0 | |
| PAF | Control | 100 | 20.3 | 16.5 | 2.5 | 2.1 | 15.3 | 11.0 | 3.8 | 3.7 | 30.4 | 25.5 |
| Shank3 | 81 | 13.9 | 7.2 | 1.8* | 1.3* | 8.6* | 7.8* | 3.9 | 3.9 | 40.1 | 25.0 | |
The number of driven spikes evoked by tones was significantly weaker in Shank3 heterozygous rats compared to control rats (U = 175826, z = −4.09, p < 0.0001, Mann-Whitney U test, Table 2). There was a significant difference in the number of driven spikes across the auditory fields (H(3) = 47.13, p < 0.0001, Kruskal-Wallis test). The number of driven spikes was 20% weaker in AAF in Shank3 heterozygous rats (p = 0.01, Mann-Whitney U test, Table 2), 16% weaker in A1 (p = 0.003, Mann-Whitney U test), and 28% weaker in PAF (p = 0.01, Mann-Whitney U test).
Similarly, the spontaneous firing rate was also significantly weaker in Shank3 heterozygous rats compared to control rats (U = 168073, z = −5.27, p < 0.0001, Mann-Whitney U test, Table 2). There was a significant difference in the spontaneous firing rate across the auditory fields (H(3) = 44.74, p < 0.0001, Kruskal-Wallis test). Shank3 heterozygous rats exhibited a 31% decrease in the spontaneous rate in AAF (p < 0.0001, Mann-Whitney U test, Table 2), a 27% decrease in VAF (p = 0.03, Mann-Whitney U test), and a 44% decrease in PAF (p < 0.0001, Mann-Whitney U test).
Bandwidths quantified 40 dB above the response threshold were significantly different in Shank3 heterozygous rats compared to control rats (U = 133682, z = −4.16, p < 0.0001, Mann-Whitney U test, Table 2). There was a significant difference in the bandwidth across the auditory fields (H(3) = 111.15, p < 0.0001, Kruskal-Wallis test). The bandwidth was 39% wider in VAF in Shank3 heterozygous rats compared to control rats (U = 2767, z = −5.67, p < 0.0001, Mann-Whitney U test, Table 2).
Additionally, the peak firing latency was unaltered in Shank3 heterozygous rats compared to control rats (U = 191135, z = −1.79, p = 0.07, Mann-Whitney U test, Table 2). There was a significant difference in the peak latency across the auditory fields (H(3) = 342.08, p < 0.0001, Kruskal-Wallis test).
Shank3 deficiency alters the cortical response to rapid trains of sound
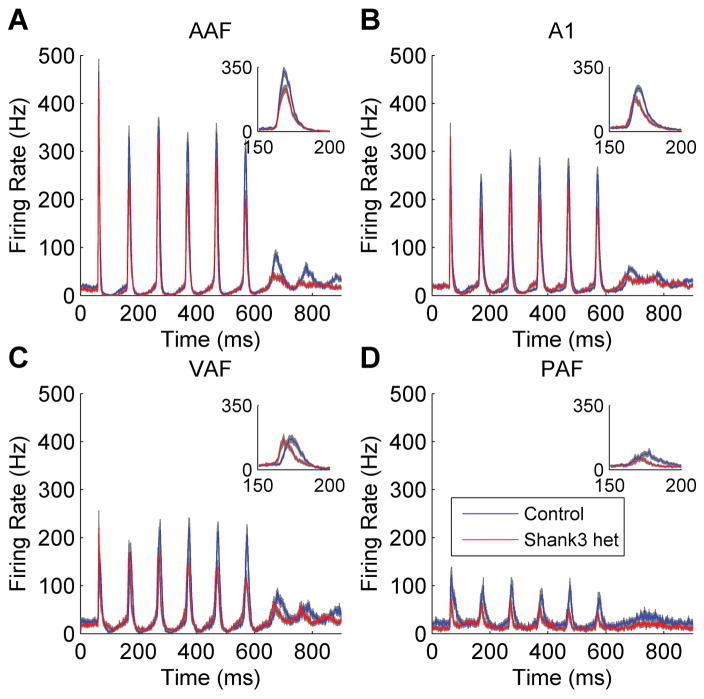
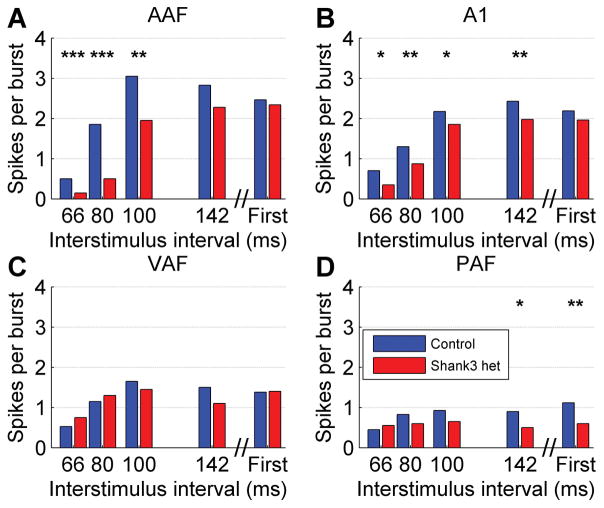
Auditory cortex responses to trains of noise bursts were recorded to assess whether there was a deficit in the neural response to rapidly presented sounds in Shank3 heterozygous rats. The auditory cortex response to noise burst trains was significantly impacted in Shank3 heterozygous rats in multiple auditory fields (Figure 1). In AAF, the number of spikes evoked in response to the second noise burst of the train was significantly weaker in Shank3 heterozygous rats compared to control rats (U = 369355, z = −7.01, p < 0.0001, Mann-Whitney U test, Figure 2a). There was a significant difference in the number of spikes evoked to the second noise burst across the presentation rates (H(4) = 313.12, p < 0.0001, Kruskal-Wallis test). The number of spikes evoked in response to the second noise burst was also significantly weaker in Shank3 heterozygous rats in A1 (U = 595054, z = −5.03, p < 0.0001, Mann-Whitney U test, Figure 2b), where there was also a significant difference in the number of spikes evoked to the second noise burst across the presentation rates (H(4) = 334.66, p < 0.0001, Kruskal-Wallis test). The number of spikes evoked in response to the second noise burst was unaltered in Shank3 heterozygous rats in VAF (U = 184206, z = −0.36, p = 0.72, Mann-Whitney U test, Figure 2c), but there was a significant difference in the number of spikes evoked to the second noise burst across the presentation rates (H(4) = 70.08, p < 0.0001, Kruskal-Wallis test). The number of spikes evoked in response to the second noise burst was also significantly weaker in Shank3 heterozygous rats in PAF (U = 88663, z = −3.22, p = 0.001, Mann-Whitney U test, Figure 2d), where there was also a significant difference in the number of spikes evoked to the second noise burst across the presentation rates (H(4) = 11.44, p = 0.02, Kruskal-Wallis test). Collectively, these findings demonstrate that the Shank3 heterozygous mutation alters auditory cortex processing.
Figure 1.
The auditory cortex response to rapidly presented noise burst trains was altered in Shank3 heterozygous rats. The post-stimulus time histogram (PSTH) response to a train of 6 noise bursts presented at 10 Hz (interstimulus interval of 100 ms) is shown in (a) AAF, (b), A1, (c) VAF, and (d) PAF. The gray shading indicates SEM across recording sites. The figure inset displays a zoomed in version of the response to the second noise burst.
Figure 2.
The median number of spikes evoked in response to the second noise burst was weaker in Shank3 heterozygous rats compared to control rats in (a) AAF, (b), A1, and (d) PAF, but not (c) VAF. The interval between the onset of the first and second noise burst is shown on the x axis. The response to the first noise burst is plotted on the right. The stars indicate statistically significant differences between Shank3 heterozygous rats and control rats (* p < 0.01, ** p < 0.001, *** p < 0.0001, Mann-Whitney U test Bonferroni corrected for multiple comparisons).
Shank3 deficiency alters the cortical response to speech sounds
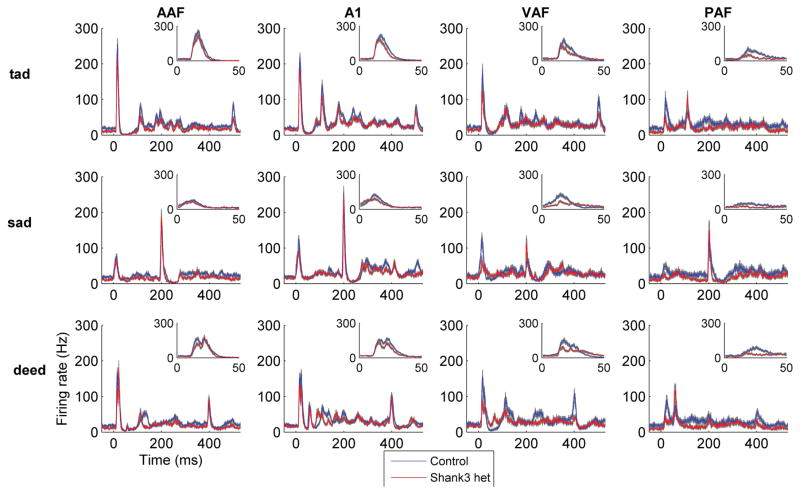
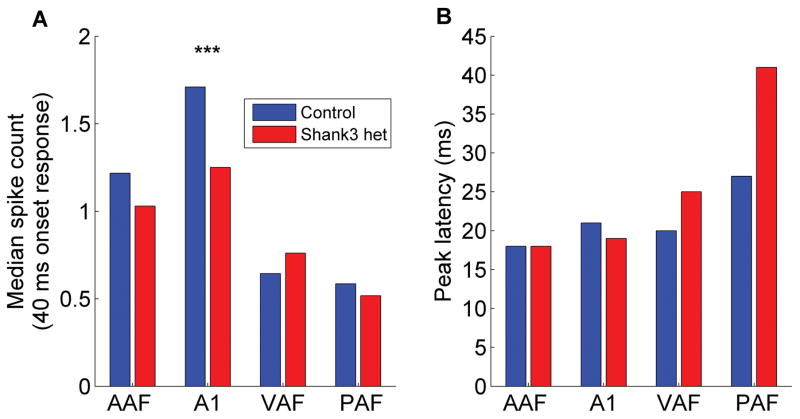
The altered auditory cortex response in Shank3 heterozygous rats was not specific to non-speech sounds. Similarly, the multi-unit auditory cortex response to speech sounds in Shank3 heterozygous rats was weaker compared to control rats (Figure 3). The onset response strength to speech sounds was significantly weaker in Shank3 heterozygous rats compared to control rats (U = 174948, z = −4.23, p < 0.0001, Mann-Whitney U test, Figure 4a). There was a significant difference in the response strength to speech sounds across the auditory fields (H(3) = 92.32, p < 0.0001, Kruskal-Wallis test). The response strength was significantly weaker in A1 in Shank3 rats compared to control rats (U = 21055, z = −4.11, p < 0.0001, Mann-Whitney U test Bonferroni corrected for multiple comparisons, Figure 4a).
Figure 3.
The auditory cortex multi-unit response to speech sounds was altered in Shank3 heterozygous rats. The post-stimulus time histogram (PSTH) response to the speech sounds ‘tad’ (top row), ‘sad’ (middle row), and ‘deed’ (bottom row) in AAF, A1, VAF, and PAF. The gray shading indicates SEM across recording sites. The figure inset displays a zoomed in version of the response to the onset of the initial consonant.
Figure 4.
The auditory cortex response to speech sounds was both (a) weaker and (b) delayed in Shank3 heterozygous rats compared to control rats. The stars indicate statistically significant differences between Shank3 heterozygous rats and control rats (p < 0.0001, Mann-Whitney U test Bonferroni corrected for multiple comparisons). The bars indicate the median spike count and median peak response latency.
The peak response latency to speech sounds was significantly delayed in Shank3 heterozygous rats compared to control rats (U = 189297.5, z = −2.06, p = 0.04, Mann-Whitney U test, Figure 4b). There was a significant difference in the response latency to speech sounds across the auditory fields (H(3) = 148.74, p < 0.0001, Kruskal-Wallis test). However, the response latency was not significantly delayed in any individual field in Shank3 rats compared to control rats (p > 0.0125, Mann-Whitney U tests Bonferroni corrected for multiple comparisons, Figure 4b). Together, these findings demonstrate that responses to sounds in multiple auditory cortex fields are disrupted in Shank3 heterozygous rats.
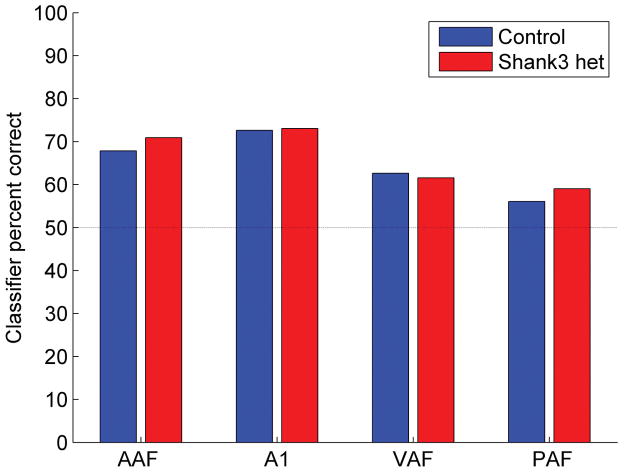
Due to the weaker auditory cortex responses to the onset of speech sounds, we tested the hypothesis that a nearest-neighbor classifier would be less able to accurately discriminate between auditory cortex responses evoked by consonant pairs in Shank3 rats compared to control rats. Using auditory cortex responses recorded from experimentally naïve rats, the nearest-neighbor classifier accurately predicts behavioral consonant discrimination accuracy (Engineer et al., 2008; Centanni et al., 2013; Perez et al., 2013). Consonant pairs that evoke similar neural patterns are difficult for naive rats to discriminate, while consonant pairs that evoke distinct neural patterns are easy for rats to discriminate. Neural classifier accuracy was unimpaired in Shank3 heterozygous rats compared to control rats (U = 199333, z = −0.54, p = 0.59, Mann-Whitney U test, Figure 5 and Supplementary Figure 1). There was a significant difference in the classifier accuracy across the auditory fields (H(3) = 150.91, p < 0.0001). The weaker response strength observed in Shank3 rats did not make the neural responses to consonant pairs less discriminable.
Figure 5.
The neural classifier accuracy of consonant pairs was unimpaired in Shank3 heterozygous rats compared to control rats. The neural classifier was provided the 40 ms onset response to pairs of consonants. The bars indicate the median percent correct. Chance discrimination performance is 50% correct, and is indicated by the dotted line.
Discussion
This study was designed to identify whether abnormalities in the auditory cortex response to sounds could contribute to the speech processing problems observed in individuals with SHANK3 happloinsufficiency. In this study, we used three distinct sound types to demonstrate that cortical responses to sound are weaker in Shank3 heterozygous rats. In addition, Shank3 heterozygous rats were less able to follow rapidly presented sounds. Despite the well-documented receptive language deficits in individuals with SHANK3 mutations (Phelan and McDermid, 2012; Soorya et al., 2013), the deficits in Shank3 heterozygous rats do not appear to be speech-specific. Instead, auditory responses in general are degraded in these rats.
Only one previous study has examined the auditory cortex response to sounds in individuals with Phelan-McDermid syndrome (Wang et al., 2016). They compared neural responses recorded using fMRI in children with Phelan-McDermid syndrome to responses in children with idiopathic ASD. Individuals with Shank3 haploinsufficiency exhibited primary auditory cortex activation in response to both communicative and non-communicative sounds, despite poor behavioral receptive language abilities in the same individuals. The current study also observed primary auditory cortex activation across a range of auditory stimuli. While the auditory cortex response to the onset of sounds was significantly weakened, there was a much weaker response to subsequently presented rapid noise bursts (Figure 2). It is possible that although individuals with Phelan-McDermid syndrome respond to communicative vocalizations, they may exhibit more severely impaired neural responses to rapidly presented sounds (Russo et al., 2010). Future work is necessary to determine whether individuals with Phelan-McDermid syndrome exhibit temporal processing problems that may explain the observed severe receptive language impairments.
Potential functional interpretation
In this study, the neural discrimination accuracy of isolated speech sounds was unaffected in Shank3 heterozygous rats compared to control rats. Neural classifier accuracy is highly correlated with behavioral discrimination ability (Engineer et al., 2008; Centanni et al., 2013; Perez et al., 2013), so this finding suggests that behavioral discrimination of isolated speech sounds is likely to be unimpaired in Shank3 heterozygous rats. Impaired neural and behavioral discrimination of speech sounds has been observed previously in other rat models of communication disorders (Centanni et al., 2014a; Engineer et al., 2014b, 2014c). Future experiments are necessary to determine the behavioral consequences of altered auditory cortex responses in these rats. While the neural responses to isolated words are largely intact in Shank3 heterozygous rats, the observed decreased ability to follow rapidly presented noise burst trains suggests that neural responses to speech sounds presented in rapid speech streams may be impaired. Wild-type rats can accurately discriminate speech sounds delivered in a speech stream at rates up to 6.7 syllables per second (Centanni et al., 2014b, 2016). Our observations indicate that Shank3 heterozygous rats exhibit relatively normal responses to isolated speech, but may have degraded neural and behavioral responses to speech sounds presented at rates that occur in conversational human speech. If future studies confirm this prediction, the Shank3 heterozygous rat model may be useful for evaluating potential therapies to improve the well-documented receptive language deficits in individuals with Phelan-McDermid Syndrome.
Relationship to other genetic disorders and autism
Individuals with autism and genetic disorders, such as Rett syndrome, commonly exhibit receptive language deficits and cortical responses that are both weaker and slower (Bader et al., 1989; Stach et al., 1994; Stauder et al., 2006; Gandal et al., 2010; Roberts et al., 2010). Severe auditory cortex deficits have also been observed in the rodent Fmr1 knockout model of fragile X syndrome, the rodent valproic acid model of autism, and the rat Mecp2 knockout model of Rett syndrome (Gandal et al., 2010; Liao et al., 2012; Kim et al., 2013; Engineer et al., 2014a, 2014c, 2015a; Anomal et al., 2015). The auditory cortex responses observed in the Shank3 heterozygous rat model are weaker, which is consistent with other rat models of ASD. These findings are observed across auditory fields, as well as across different sound types, which suggests that responses to sound would likely also be impaired in other auditory regions. Future experiments are needed to determine if the differences observed in auditory cortical responses could be due to changes earlier in the auditory pathway. While hearing is typically reported to be normal in individuals with Phelan-McDermid syndrome (Phelan and McDermid, 2012), it is possible that subcortical auditory areas, such as the inferior colliculus, could also exhibit altered responses to sounds, as seen in individuals with autism (Russo et al., 2008).
Future avenues for testing auditory processing therapies
This novel model of the auditory processing impairments observed following Shank3 mutation offers the unique opportunity to test drug or cognitive training therapies that could be used to treat patients with SHANK3 mutation. For example, it is well known that intensive cognitive intervention therapy in individuals with autism can both improve behavioral outcomes and restore typical patterns of brain activity (McEachin et al., 1993; Dawson et al., 2010, 2012; Russo et al., 2010). Similar improvements in both speech discrimination ability and the auditory cortex response to speech have been documented in the rat Mecp2 knockout model of Rett syndrome and the rat valproic acid model of autism (Engineer et al., 2014b, 2015a). A recent study documented that neural and behavioral deficits can be rescued in Shank3 mice, demonstrating that neural plasticity mechanisms can be activated in this model (Mei et al., 2016).
It would also be straightforward to evaluate the ability of potential drug therapies to improve auditory cortical responses in the Shank3 rat model. Shank3 mice have recently been used to document the reversal of both neural and behavioral deficits following IGF-1 treatment (Bozdagi et al., 2013) or by inhibiting cofilin or activating Rac1 (Duffney et al., 2015). IGF-1 treatment has also restored neural deficits in stem cells derived from individuals with Phelan-McDermid syndrome (Shcheglovitov et al., 2013) and improved social behaviors in children with Phelan-McDermid syndrome (Kolevzon et al., 2014; Costales and Kolevzon, 2015). The Shank3 rat model is ideal to quantify the improvements in auditory response strength using both these drug therapies and other promising potential therapies.
Supplementary Material
Acknowledgments
Grant sponsor National Institutes of Health; Grant number: R01DC010433.
We would like to thank Corey Lane, Nicole Moreno, and Meghan Pantalia for assistance with neural recordings. Ozlem Bozdagi and Seth Hays gave insightful suggestions on earlier versions of the manuscript. This research was supported by a grant from the National Institutes of Health to MPK (Grant # R01DC010433), the Seaver Foundation and a generous gift from William G. Gibson and Paulina Rychenkova. The funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The authors report no conflicts of interest.
This research was also supported by a HeART grant from the International Rett Syndrome Foundation to MPK (Grant # 3206). This program was supported by the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Electrical Prescriptions (ElectRx) program under the auspices of Dr. Doug Weber through the Space and Naval Warfare Systems Center, Pacific Cooperative Agreement No. HR0011-15-2-0017 and N66001-15-2-4057 and the Targeted Neuroplasticity Training (TNT) program under the auspices of Dr. Doug Weber through the Space and Naval Warfare Systems Center, Pacific Grant/Contract No. N66001-17-2-4011.
References
- Aldinger KA, Kogan J, Kimonis V, Fernandez B, Horn D, Klopocki E, Chung B, Toutain A, Weksberg R, Millen KJ, Barkovich AJ, Dobyns WB. Cerebellar and posterior fossa malformations in patients with autism-associated chromosome 22q13 terminal deletion. Am J Med Genet A. 2013;161A:131–136. doi: 10.1002/ajmg.a.35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anomal R, De Villers-sidani E, Brandão JA, Diniz R, Costa R, Romcy-pereira RN. Impaired processing in the primary auditory cortex of an animal model of autism. Front Syst Neurosci. 2015 doi: 10.3389/fnsys.2015.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- Bader GG, Witt-Engerström I, Hagberg B. Neurophysiological findings in the Rett syndrome, II: Visual and auditory brainstem, middle and late evoked responses. Brain Dev. 1989;11:110–114. doi: 10.1016/s0387-7604(89)80078-6. [DOI] [PubMed] [Google Scholar]
- Bomba MD, Pang EW. Cortical auditory evoked potentials in autism: a review. Int J Psychophysiol. 2004;53:161–169. doi: 10.1016/j.ijpsycho.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Bozdagi O, Tavassoli T, Buxbaum JD. Insulin-like growth factor-1 rescues synaptic and motor deficits in a mouse model of autism and developmental delay. Mol Autism. 2013;4:9. doi: 10.1186/2040-2392-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni TM, Booker AB, Chen F, Sloan AM, Carraway RS, Rennaker RL, LoTurco JJ, Kilgard MP. Knockdown of Dyslexia-Gene Dcdc2 Interferes with Speech Sound Discrimination in Continuous Streams. J Neurosci. 2016;36:4895–4906. doi: 10.1523/JNEUROSCI.4202-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni TM, Booker AB, Sloan AM, Chen F, Maher BJ, Carraway RS, Khodaparast N, Rennaker R, LoTurco JJ, Kilgard MP. Knockdown of the Dyslexia-Associated Gene Kiaa0319 Impairs Temporal Responses to Speech Stimuli in Rat Primary Auditory Cortex. Cereb Cortex. 2014a;24:1753–1766. doi: 10.1093/cercor/bht028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni TM, Chen F, Booker AM, Engineer CT, Sloan AM, Rennaker RL, LoTurco JJ, Kilgard MP. Speech sound processing deficits and training-induced neural plasticity in rats with dyslexia gene knockdown. In: Vicario DS, editor. PLoS One. Vol. 9. 2014b. p. e98439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni TM, Engineer CT, Kilgard MP. Cortical speech-evoked response patterns in multiple auditory fields are correlated with behavioral discrimination ability. J Neurophysiol. 2013;110:177–189. doi: 10.1152/jn.00092.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costales JL, Kolevzon A. Phelan-McDermid Syndrome and SHANK3: Implications for Treatment. Neurotherapeutics. 2015;12:620–630. doi: 10.1007/s13311-015-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Jones EJH, Merkle K, Venema K, Lowy R, Faja S, Kamara D, Murias M, Greenson J, Winter J, Smith M, Rogers SJ, Webb SJ. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51:1150–1159. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125:e17–23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffney LJ, Zhong P, Wei J, Matas E, Cheng J, Qin L, Ma K, Dietz DM, Kajiwara Y, Buxbaum JD, Yan Z. Autism-like Deficits in Shank3-Deficient Mice Are Rescued by Targeting Actin Regulators. Cell Rep. 2015:1–14. doi: 10.1016/j.celrep.2015.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Centanni TM, Im KW, Borland MS, Moreno NA, Carraway RS, Wilson LG, Kilgard MP. Degraded auditory processing in a rat model of autism limits the speech representation in non-primary auditory cortex. Dev Neurobiol. 2014a;74:972–986. doi: 10.1002/dneu.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Centanni TM, Im KW, Kilgard MP. Speech sound discrimination training improves auditory cortex responses in a rat model of autism. Front Syst Neurosci. 2014b doi: 10.3389/fnsys.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Centanni TM, Im KW, Rahebi KC, Buell EP, Kilgard MP. Degraded speech sound processing in a rat model of fragile X syndrome. Brain Res. 2014c;1564:72–84. doi: 10.1016/j.brainres.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Perez CA, Carraway RS, Chang KQ, Roland JL, Sloan AM, Kilgard MP. Similarity of cortical activity patterns predicts generalization behavior. PLoS One. 2013;8:e78607. doi: 10.1371/journal.pone.0078607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Perez CA, Chen YH, Carraway RS, Reed AC, Shetake JA, Jakkamsetti V, Chang KQ, Kilgard MP. Cortical activity patterns predict speech discrimination ability. Nat Neurosci. 2008;11:603–608. doi: 10.1038/nn.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Rahebi KC, Borland MS, Buell EP, Centanni TM, Fink MK, Im KW, Wilson LG, Kilgard MP. Degraded neural and behavioral processing of speech sounds in a rat model of Rett syndrome. Neurobiol Dis. 2015a;83:26–34. doi: 10.1016/j.nbd.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Rahebi KC, Buell EP, Fink MK, Kilgard MP. Speech training alters consonant and vowel responses in multiple auditory cortex fields. Behav Brain Res. 2015b;287:256–264. doi: 10.1016/j.bbr.2015.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TPL, Siegel SJ. Validating γ oscillations and delayed auditory responses as translational biomarkers of autism. Biol Psychiatry. 2010;68:1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harony-Nicolas H, De Rubeis S, Kolevzon A, Buxbaum JD. Phelan McDermid Syndrome: From Genetic Discoveries to Animal Models and Treatment. J Child Neurol. 2015;30:1861–1870. doi: 10.1177/0883073815600872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harony-Nicolas H, Kay M, Hoffmann J, du Klein ME, Bozdagi-Gunal O, Riad M, Daskalakis NP, Sonar S, Castillo PE, Hof PR, Shapiro ML, Baxter MG, Wagner S, Buxbaum JD. Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3-deficient rat. Elife. 2017;6:1–39. doi: 10.7554/eLife.18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y-H, Ehlers MD. Modeling autism by SHANK gene mutations in mice. Neuron. 2013;78:8–27. doi: 10.1016/j.neuron.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H. Speech representation and transformation using adaptive interpolation of weighted spectrum: Vocoder revisited. Proc ICASSP. 1997;2:1303–1306. [Google Scholar]
- Kim H, Gibboni R, Kirkhart C, Bao S. Impaired critical period plasticity in primary auditory cortex of fragile X model mice. J Neurosci. 2013;33:15686–15692. doi: 10.1523/JNEUROSCI.3246-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolevzon A, Bush L, Wang A, Halpern D, Frank Y, Grodberg D, Rapaport R, Tavassoli T, Chaplin W, Soorya L, Buxbaum JD. A pilot controlled trial of insulin-like growth factor-1 in children with Phelan-McDermid syndrome. Mol Autism. 2014;5:54. doi: 10.1186/2040-2392-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Coffey-Corina S, Padden D, Munson J, Estes A, Dawson G. Brain responses to words in 2-year-olds with autism predict developmental outcomes at age 6. PLoS One. 2013;8:e64967. doi: 10.1371/journal.pone.0064967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai G, Schneider HD, Schwarzenberger JC, Hirsch J. Speech stimulation during functional MR imaging as a potential indicator of autism. Radiology. 2011;260:521–530. doi: 10.1148/radiol.11101576. [DOI] [PubMed] [Google Scholar]
- Leblond CS, et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments. PLoS Genet. 2014;10:e1004580. doi: 10.1371/journal.pgen.1004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Gandal MJ, Ehrlichman RS, Siegel SJ, Carlson GC. MeCP2+/− mouse model of RTT reproduces auditory phenotypes associated with Rett syndrome and replicate select EEG endophenotypes of autism spectrum disorder. Neurobiol Dis. 2012;46:88–92. doi: 10.1016/j.nbd.2011.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachin JJ, Smith T, Lovaas OI. Long-term outcome for children with autism who received early intensive behavioral treatment. Am J Ment Retard. 1993;97:359-72-91. [PubMed] [Google Scholar]
- Mei Y, Monteiro P, Zhou Y, Kim J-A, Gao X, Fu Z, Feng G. Adult restoration of Shank3 expression rescues selective autistic-like phenotypes. Nature. 2016;530:481–484. doi: 10.1038/nature16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Shannon RV. Beyond cochlear implants: awakening the deafened brain. Nat Neurosci. 2009;12:686–691. doi: 10.1038/nn.2326. [DOI] [PubMed] [Google Scholar]
- Nittrouer S, Sansom E, Low K, Rice C, Caldwell-Tarr A. Language Structures Used by Kindergartners With Cochlear Implants: Relationship to Phonological Awareness, Lexical Knowledge and Hearing Loss. Ear Hear. 2014:1–13. doi: 10.1097/AUD.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CA, Engineer CT, Jakkamsetti V, Carraway RS, Perry MS, Kilgard MP. Different timescales for the neural coding of consonant and vowel sounds. Cereb Cortex. 2013;23:670–683. doi: 10.1093/cercor/bhs045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan K, McDermid HE. The 22q13.3 Deletion Syndrome (Phelan-McDermid Syndrome) Mol Syndromol. 2012;2:186–201. doi: 10.1159/000334260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe A, Boddaert N, Vaivre-Douret L, Robel L, Danon-Boileau L, Malan V, de Blois M-C, Heron D, Colleaux L, Golse B, Zilbovicius M, Munnich A. Neurobehavioral profile and brain imaging study of the 22q13.3 deletion syndrome in childhood. Pediatrics. 2008;122:e376–e382. doi: 10.1542/peds.2007-2584. [DOI] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- Puckett AC, Pandya PK, Moucha R, Dai W, Kilgard MP. Plasticity in the rat posterior auditory field following nucleus basalis stimulation. J Neurophysiol. 2007;98:253–265. doi: 10.1152/jn.01309.2006. [DOI] [PubMed] [Google Scholar]
- Reed P, Howell P, Sackin S, Pizzimenti L, Rosen S. Speech perception in rats: use of duration and rise time cues in labeling of affricate/fricative sounds. J Exp Anal Behav. 2003;80:205–215. doi: 10.1901/jeab.2003.80-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TPL, Cannon KM, Tavabi K, Blaskey L, Khan SY, Monroe JF, Qasmieh S, Levy SE, Edgar JC. Auditory magnetic mismatch field latency: a biomarker for language impairment in autism. Biol Psychiatry. 2011;70:263–269. doi: 10.1016/j.biopsych.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TPL, Khan SY, Rey M, Monroe JF, Cannon K, Blaskey L, Woldoff S, Qasmieh S, Gandal M, Schmidt GL, Zarnow DM, Levy SE, Edgar JC. MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging biomarker for autism. Autism Res. 2010;3:8–18. doi: 10.1002/aur.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo N, Zecker S, Trommer B, Chen J, Kraus N. Effects of background noise on cortical encoding of speech in autism spectrum disorders. J Autism Dev Disord. 2009;39:1185–1196. doi: 10.1007/s10803-009-0737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo NM, Hornickel J, Nicol T, Zecker S, Kraus N. Biological changes in auditory function following training in children with autism spectrum disorders. Behav Brain Funct. 2010;6:60. doi: 10.1186/1744-9081-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo NM, Skoe E, Trommer B, Nicol T, Zecker S, Bradlow A, Kraus N. Deficient brainstem encoding of pitch in children with Autism Spectrum Disorders. Clin Neurophysiol. 2008;119:1720–1731. doi: 10.1016/j.clinph.2008.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasua SM, Boccuto L, Sharp JL, Dwivedi A, Chen C-F, Rollins JD, Rogers RC, Phelan K, DuPont BR. Clinical and genomic evaluation of 201 patients with Phelan–McDermid syndrome. Hum Genet. 2014a doi: 10.1007/s00439-014-1423-7. [DOI] [PubMed] [Google Scholar]
- Sarasua SM, Boccuto L, Sharp JL, Dwivedi A, Chen CF, Rollins JD, Rogers RC, Phelan K, DuPont BR. Clinical and genomic evaluation of 201 patients with Phelan-McDermid syndrome. Hum Genet. 2014b;133:847–859. doi: 10.1007/s00439-014-1423-7. [DOI] [PubMed] [Google Scholar]
- Shcheglovitov A, Shcheglovitova O, Yazawa M, Portmann T, Shu R, Sebastiano V, Krawisz A, Froehlich W, Bernstein Ja, Hallmayer JF, Dolmetsch RE. SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients. Nature. 2013;503:267–271. doi: 10.1038/nature12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetake JA, Wolf JT, Cheung RJ, Engineer CT, Ram SK, Kilgard MP. Cortical activity patterns predict robust speech discrimination ability in noise. Eur J Neurosci. 2011;34:1823–1838. doi: 10.1111/j.1460-9568.2011.07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soorya L, Kolevzon A, Zweifach J, Lim T, Dobry Y, Schwartz L, Frank Y, Wang a T, Cai G, Parkhomenko E, Halpern D, Grodberg D, Angarita B, Willner JP, Yang A, Canitano R, Chaplin W, Betancur C, Buxbaum JD. Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol Autism. 2013;4:18. doi: 10.1186/2040-2392-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stach BA, Stoner WR, Smith SL, Jerger JF. Auditory evoked potentials in Rett syndrome. J Am Acad Audiol. 1994;5:226–230. [PubMed] [Google Scholar]
- Stauder JEa, Smeets EEJ, van Mil SGM, Curfs LGM. The development of visual- and auditory processing in Rett syndrome: an ERP study. Brain Dev. 2006;28:487–494. doi: 10.1016/j.braindev.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yokota R, Funamizu A, Kose H, Kanzaki R. Learning-stage-dependent, field-specific, map plasticity in the rat auditory cortex during appetitive operant conditioning. Neuroscience. 2011;199:243–258. doi: 10.1016/j.neuroscience.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Wang AT, Lim T, Jamison J, Bush L, Soorya LV, Tavassoli T, Siper PM, Buxbaum JD, Kolevzon A. Neural selectivity for communicative auditory signals in Phelan-McDermid syndrome. J Neurodev Disord. 2016;8:5. doi: 10.1186/s11689-016-9138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwanenburg RJ, Ruiter SAJ, van den Heuvel ER, Flapper BCT, Van Ravenswaaij-Arts CMA. Developmental phenotype in Phelan-McDermid (22q13.3 deletion) syndrome: a systematic and prospective study in 34 children. J Neurodev Disord. 2016;8:16. doi: 10.1186/s11689-016-9150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.