Abstract
A new biomimetic nanoreactor design, MaBiDz, is presented based on a copolymer brush in combination with superparamagnetic nanoparticles. This cellular nanoreactor features two species of magnetic particles, each functionalized with two components of a binary deoxyribozyme system. In the presence of a target mRNA analyte and a magnetic field, the nanoreactor is assembled to form a biocompartment enclosed by the polymeric brush that enables catalytic function of the binary deoxyribozyme with enhanced kinetics. MaBiDz was demonstrated here as a cellular sensor for rapid detection and imaging of a target mRNA biomarker for metastatic breast cancer, and its function shows potential to be expanded as a biomimetic organelle that can downregulate the activity of a target mRNA biomarker.
Introduction
Detection of cellular processes for the purposes of diagnostics and biosensing relies on well-controlled, selective methods,1,2 particularly benefiting from nanotechnology advances,3 which are highly robust in a complex biological environment. In nature, cellular pathways rely on enzymes to constantly monitor cellular conditions and control cellular operations.4,5 These complex metabolic pathways take place in a concerted fashion to catalyze multiple chemical reactions that are crucial for metabolism and cell functions. As crucial as enzymes are to the metabolic machinery of the cell, they must be sequestered to prevent unwanted reactions and degradation of the enzymes, as well as to provide very specific environments required for optimal function. To perform such parallel multistep reactions, cells adapt a hierarchical design called biocompartmentalization, in which enzymes are spatially confined in cellular compartments.6,7
Based on this hierarchical design, nanotechnological advances have been made in designing biomimetic reaction spaces which simultaneously protect catalysts from their environment, while preserving their activity inside the confined space.8-10 Such miniature reaction vessels, termed nanoreactors,11 provide a platform for enzymatic reactions to occur in a protected environment which prevents entry of extraneous species and thus side reactions, and protects reacting species from degradation. In order to function as required, nanoreactors must possess the following features: (i) an inner cavity to allow the reaction to proceed in situ and (ii) a semipermeable membrane in order to allow transport of the substrates required for the reaction and export of the generated products.12 The membrane is usually composed of a block copolymer with tunable mechanical properties and thickness.12 Nanoreactors have been developed for a variety of applications, such as the production and release of desired compounds13 (e.g., antibiotics14, other drugs15), detoxification of free radicals involved in oxidative stress,16 degradation of unwanted species,17 and use in enzyme replacement therapy.18
One direction that has been overlooked in nanoreactor design is leveraging this architecture for accelerating kinetics of reactions. Since the inner cavity of a nanoreactor incurs confinement of the reacting species, this facet of cellular compartments can be leveraged in nanoreactors to improve kinetics of enzymatic reactions. Time-consuming reactions or processes can thus benefit from compartmentalization. Confinement of the reacting species causes a high local substrate concentration and thus facilitates rates of catalytic reactions in the artificial compartments.19 Of course, certain considerations must be made. First, in designing a nanoreactor which can function with accelerated kinetics, the substrates must be chosen carefully as to preserve the integrity of the nanoreactor. In addition, the substrate should not inhibit the enzymatic reaction itself, which could be the case in reactions involving free radicals or peroxides. Biomolecules, such as DNA, bound to nanoparticle-based scaffolds have been shown to exhibit greater stability in molecular crowding conditions;20 such a scaffold could be employed to impart greater stability and faster reaction kinetics for gene therapy or real time mRNA detection applications, which normally require a 24-hour period.21 However, as of yet there are no reported studies utilizing DNA-modified nanoparticles formulated as nanoreactors.22
In contexts which necessitate accelerated kinetics, such as diagnostics or translational medicine,23 the nanoreactor must be delivered promptly to the intended site. Thus, a nanoreactor should be propelled by a nanomotor or manipulated by an external, remotely applied signal.24,25 Indeed, magnetic field-based transport enables accelerated delivery of a biomaterial to a target site by overcoming Brownian diffusion.26 As applied to nanoreactors, this would require that the reacting species are immobilized onto magnetic field-responsive species such as superparamagnetic nanoparticles and manipulated using a remotely applied magnetic field. Further, once the nanoreactor arrives at the site, its enzymatic/biocatalytic activity should be activated at that point; it should not be delayed or be initiated prematurely. Thus, the nanoreactor should be stimuli-responsive, which would allow the catalysis to proceed “on demand” in a temporally controlled manner. Stimuli such as pH,27 temperature28 and light29 have been employed in recent studies to cause dissolution of the nanoreactor, allowing its cargo to be released. However, the external signal should not cause the nanoreactor to disintegrate; instead it must activate the desired catalytic reaction within the inner cavity of the nanoreactor while preserving the nanoreactor architecture for sustained nanoreactor function. As it has been shown previously that magnetic field application is not damaging to mammalian tissue30 biomolecule-functionalized magnetic nanoparticles, which can be manipulated in a magnetic field, represent a promising basis for a stimuli-responsive, stable nanoreactor design.22,31
Nanoreactors reported in recent literature32–34 include polymersomes,35–39 liposomes,40,41 hollow nanoparticles,42 viruses and protein cages,43,44 as well as other nano-hollow species entrapping (bio)molecular reacting species inside nano-compartments. In a different approach, the reacting species can be associated with external surfaces of modified nanoparticles, usually being entrapped in polymeric shells.45–48 However, magnetic nanoparticles have not yet been explored in the context of nanoreactor design, except a few recent preliminary reports.22,31 Therefore, in the interest of developing new biomimetic designs, we have exploited a polymer brush-modified magnetic nanoparticle to construct a new nanoreactor design.
Here, we report a magnetic field-responsive catalytic system, magnetic field-responsive binary deoxyribozyme (MaBiDZ), which enables sensing of intracellular target mRNA, Fig. 1. The biocatalytic process is activated upon the application of an external magnetic field, which causes assembly of two components of the biocatalytic system to yield the catalytically active site. The said components are covalently bound to a polymeric brush on two distinct species of superparamagnetic nanoparticles, which aggregate in a magnetic field and create a compartment allowing the biocatalytic system to react. The compartment is gated by the polymeric brush which is grafted onto the nanoparticles; this functions to prevent interactions of both the precursors with other competitive molecules and with each other in the absence of a magnetic field. The polymeric brush also imparts stability to the immobilized biocatalytic components, preventing the penetration of degrading enzymes and leaching of the loaded substances.
Fig. 1.

Principle of magnetic field-activated deoxyribozyme (MaBiDZ) nanoreactor. (A) Binary deoxyribozyme sensor (BiDZ) as reported earlier.49 DNA strands DZa and DZb hybridize to adjacent position of analyte and form deoxyribozyme catalytic core, which cleaves fluorogenic F-sub and increases sample fluorescence. Note that the BiDZ operates in a solution and it is not responsive to the magnetic field. (B) MaBiDZ developed in this study. Magnetic bead (MaB1)-bound DZa forms a catalytic core with DZb in the presence of analyte. The activated nanoreactor produces signal only when: (i) second species of magnetic beads, MaB2 carrying F-sub is present and (ii) magnetic field that aggregates MaB1 and MaB2 is applied. See the DNA sequences in Table 1.
Experimental Section
The major experimental steps and conditions have been detailed in the preliminary short publication where they appeared in the Supporting Information.22 Now they are collected in the Supporting Information (SI) to the present paper; some important technical details are given below for the readers’ convenience.
Chemicals and Reagents
Custom made DNA were purchased from Integrated DNA technologies (IDT, Coralville, IA USA); see the sequences in Table 1 and applied concentrations are specified in the Supporting Information. MCF-7 cell line, Eagle’s Minimum Essential Medium (EMEM) cell culture medium, PBS were purchased from American Type Culture Collection (ATCC), VA, USA. Trypsin (E.C. 3.4.21.4), fetal bovine serum (FBS), fetal calf serum (FCS), Trypan Blue (stain dye), (1-ethyl-3[3-(dimethylamino)-propyl] carbodiimide (EDC), N-hydroxysuccimide (NHS), Tween-20, 2-(N-morpholino)ethanesulfonic acid (MES-buffer), (4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (HEPES-buffer), silicon tetraethoxide (TEOS), (3-aminopropyl)triethoxysilane (APS), α-bromoisobutyryl bromide (BIB), ethyl α-bromoisobutyrate (EBIB), N,N,N′,N″,N″-pentamethyldiethylenetriamine (PMDTA), and other standard organic and inorganic materials and reactants were obtained from Sigma-Aldrich or J.T. Baker and used without further purification. tert-Butyl acrylate (TBA) and poly(ethylene glycol) methyl ether acrylate (PEGMA) (average molecular mass 480 g/mol) were purchased from Sigma-Aldrich and purified using a flash-chromatography column containing inhibitor removers (Sigma #311340 and Sigma #311332). All solutions for the experiments were prepared using ultrapure water (18.2 MΩ∙cm; Barnstead NANOpure Diamond).
Table 1.
Oligonucleotides used in the study.
| Namea | Sequences |
|---|---|
| F-sub | 5′-CGGT ACA TTG TAG AAG TT AAG GTTFAM TCC TCg uCC CTG GGC A-BHQ1 |
| Twist | 5′-TAGT GGG ACG CGG ACA TGG ACC AGG CCC CCT CCA TCC TCC AGA CCG AGA AGG CGT AGC TGA GCC GCT CGT GAG CCA CAT AGC TGC A |
| DZa | 5′- NH2/AAA AAA AAA AAA AAA AAA AAC GAG CGG CTC AGC TAC GCC T AC AAC CGA GAG AGG AAA C |
| DZb | 5′-CCA GGG A GG CTA GCT TCT CGG TCT GGA GGA TGG AG |
| Hook | 5′-NH2/AAA AAA AAA AAA AAA AAA AA/iSp9/AAC TTC TAC AAT GTA CCG |
iSp9 - triethylene glycol linker; FAM attached to the DNA is a fluorescein derivative; BHQ1—“Black Hole Quencher” is a fluorescence quencher; ribonucleotides are in low case.
Control experiment in the presence of a non-complimentary analyte
The MaBiDZ specificity to the Twist analyte was studied in the presence of a non-complimentary analyte (see Fig. 2B, e). A probe complementary to the M.smegmatis (abbreviated M.smeg) analyte (an mRNA not found in MCF-7 cells; see the Supporting Information for preparation of the MCF-7 cells) was incubated in MCF-7 lysates. Binding arm sequences were as follows: DZa(M.smeg): 5′- CCA TCC CAC ACC GCA AAA GCT TTCC A CAA CGA GAGGAAACCTT and DZb(M.smeg) 5′-TGC CCA GGG A GG CTA GCT CCT ACC AGG CCA TGC GAC CAG CAG G. The responses of the M.smeg and Twist sensors were studied respectively in MCF-7 lysate over the course of 12 hours. MCF-7 lysates consist of 600,000 cells each. After 6 hours, the Twist BiDZ probe produced a 54-fold response compared to the M.smeg probe in MCF-7 lysates. This confirms that the fluorescent intensity produced by the sensor is indeed in response to hybridization with Twist analyte present in the cell. Increase in fluorescence observed over the 12 h period in response to the M.smeg probe can be attributed to nuclease-induced non-specific cleavage of F-sub produced by active nucleases.
Fig. 2.

In vitro characterization. (A) Fluorescent output signal generated by the MaBiDZ nanoreactor systems upon application of various signals. Fluorescence output normalized to the background fluorescence measured after 30 min of the reaction: (a) without F-sub, (b) without synthetic Twist analyte (see Table 1), (c) response of MaBiDz in the presence of the Twist analyte without magnet applied, (d) MaBiDZ activated with magnetic field in the presence of 1 nM synthetic Twist analyte (see Experimental section for concentrations of all other components of the MaBiDZ probe.), (e) shows the MaBiDZ response to a non-complimentary M.smeg analyte (see details in the Experimental section). (B) Response of (a) free F-Sub and (b) F-Sub immobilized on magnetic particles modified with our polymeric brush to treatment with 4 U of DNase I. The F-Sub protected by the polymeric brush exhibited slow generation of fluorescence, and required c.a 1.5 days to be completely consumed. The free F-Sub was easily consumed within 40 minutes of treatment.
Analysis of possible cell membrane damage by magnetic nanoparticles
Cell membrane damage test is examined by using Trypan Blue staining method. MCF-7 cells were incubated with 1 pM of MaBiDZ for 4 h. Cells only (without MaBiDZ), cells containing MaBiDZ with and without applying magnetic field were detached from the surface by using trypsin/EDTA (Gibco) and resuspended with complete EMEM medium. Cell suspension was added to an equal volume of 0.4% Trypan Blue. Then, cells were counted by using a hemocytometer. Under optical microscope, Trypan Blue-stained cells, which lost the integrity of cell membrane, are counted. Finally, normal cell membrane percentage of each sample is calculated.
Results and Discussion
We demonstrated the efficacy of this nanoreactor design using a binary DNAzyme (BiDZ) probe developed earlier49–52 in order to detect an intracellular mRNA substrate. BiDZ consists of three components (Fig. 1A): the analyte binding arms (DZa and DZb) and a fluorogenic reporter substrate (F-sub). F-sub is an oligonucleotide strand composed of a fluorophore and quencher conjugated to the opposite sides of the cleavage site. DZa and DZb can hybridize to a specific DNA or RNA analyte and form the DNAzyme (DZ) catalytic core, which cleaves F-sub, thus resulting in separation of the fluorophore and quencher followed by fluorescent signaling.
The magnetic bead (MaB) architecture is composed of a 15 nm iron oxide (Fe3O4) superparamagnetic core encased in a silica shell. The shell is modified with a grafted polymeric brush of a block copolymer PAA-b-PEG composed of poly(acrylic acid) (PAA) and poly(ethylene glycol) (PEG). The cores have a saturation magnetization value (47 emu/g),53 which is sufficient for effective utilization of magnetic force. The DNA strands are conjugated to the polymeric brush using a flexible linker. The brush permits passage of the nanoparticles through cell membranes, and prevents nanoparticle aggregation in the absence of a magnetic field. This allows the gated compartments to form only in the presence of a magnetic field (ca. 0.4 T), creating a stimuli-responsive nanoreactor. The magnetic bead characterization was performed in the preliminary study and the data are collected in the Supporting Information of that short communication.22
MaBiDZ consists of the DZb strand, MaB1 conjugated with DZa and MaB2 conjugated with DNA hook strand complementary to F-sub, Fig. 1B. F-sub is incubated with the Hook-MaB2 conjugate, which is then rinsed to remove unbound F-sub. The polymer brush serves as a permeable membrane facilitating the entry of a DNA or RNA analyte into the reaction compartment to hybridize to DZa and DZb strands, enabling formation of the DZ catalytic core. The catalytic core does not produce the fluorescent signal unless hybridized with F-sub. Application of an external magnetic field induces aggregation of the MaB1 and MaB2, thus bringing the activated BiDZ sensor in close proximity to F-sub, which is followed by F-sub cleavage and amplification of fluorescent signal in the presence of analyte. Though the 3D-motion of MaBiDZ may be restricted in the reaction compartment created under a magnetic field, both the flexible linker and large particle size allow a greater degree of contact points between the two DZ species.
For the proof-of-concept study, we chose to target Twist mRNA. Twist is a helix−loop−helix transcription factor. Its overexpression has been shown to contribute to metastasis by promoting an epithelial-mesenchymal transition.54 Thus, an intracellular sensor that can fluorescently report Twist mRNA levels would be useful to assess metastatic potential of cells in clinical applications. We first optimized the performance of the sensor in in vitro experiments using a synthetic DNA analyte with the sequence of Twist mRNA (see Twist sequence in Table 1). Fig. 2A shows typical fluorescence spectra produced by the MaBiDZ nanoreactor in the absence or presence of the magnetic field (OFF and ON states, respectively) measured in vitro. The results of the in vitro studies demonstrated a dramatic enhancement of the fluorescent signal when MaBiDZ is switched ON in the presence of the magnetic field (Fig. 2A, d) compared to the OFF state, Fig. 2A, c, for which the signal does not change over time. Importantly, the signal remained at near-background level in the absence of an applied magnetic field, Fig. 2A, c, and in the absence of analyte, Fig. 2B, b. This shows that the polymeric brush does indeed prevent formation of the reaction compartments in the absence of a magnetic field, confirming the stimuli-responsiveness of MaBiDz. In support of selective reactivity, signal response to a non-complementary target remains at the background level, Fig. 2A, e.
The MaBiDZ response to the Twist analyte in the presence of the magnetic field reaches saturation in 30 min after applying the magnetic field to the system, while the BiDZ catalytic species, which are not bound to magnetic nanoparticles require more than 72 hours to reach saturation (See SI, Fig. SI1). The accelerated reaction of MaBiDZ in the presence of the magnetic field originates from the high local concentration of the reacting species. Indeed, it has been reported elsewhere that dipole-dipole interactions between superparamagnetic nanoparticles in a magnetic field result in the formation of chain-like structures.31,55 When the magnetic field is ON, the chain structure grows in length and diameter, consuming eventually all the magnetic NPs in the solution. In the growing 3D-structure, the number of the catalytic species contacts progressively increases so that virtually all the loaded substrate is involved in the biocatalytic reaction.31 This leads to fast cleavage of all available F-sub, leading to saturation within a short amount of time.
To determine whether the polymeric brush conferred a protective functionality to the immobilized species, we treated both particle-bound and free F-Sub with 4 U of DNase, a hydrolytic endonuclease which cleaves DNA molecules. Fluorescence from cleavage of equal amounts of F-Sub, one set immobilized to magnetic particles and one set with free F-Sub was monitored; if the polymeric brush is indeed capable of compartmentalizing reacting species while preventing penetration of foreign entities, we could expect to see minimal fluorescent signal from the separation of the fluorophore and quencher via DNase-induced cleavage. Indeed, the particle-bound F-Sub took 70 times longer to be hydrolyzed by DNase, which confirms the protective functionality of the polymeric brush, Fig. 2B.
To determine whether accelerated kinetics could be achieved in a compartmentalized microenvironment, we compared the enzymatic activity of the free BiDz system with that of MaBiDz. From the kinetic studies of F-sub cleavage by free BiDz, and MaBiDz both with and without a magnetic field applied (see Fig. SI2 in the SI), the apparent kinetic constants (Km and Vmax) were calculated by fitting the Michaelis–Menten equation to plot the initial reaction rates versus the substrate concentrations (Table 2). For Twist as the analyte, a lower Km value was observed for MaBiDz (0.178 ± 0.062 × 10−9 M) with a magnetic field applied, with a 40-fold decrease, compared to the Km value of free BiDz (7.42 ± 0.66 × 10−9 M). This indicated the higher Twist affinity to the MaBiDz nanoreactors, which can be explained in terms of confinement effects,10 which cause high local analyte concentration. Accordingly, a threefold increase in the maximum reaction rate (Vmax) was observed for the MaBiDz nanoreactors (112.77 ± 13.39 × 10−9 F min−1; F is the fluorescence in arbitrary units), compared to free BiDz (33.77 ± 1.10 × 10−9 F min−1). This was due to the fast reaction rate, suggesting high catalytic activity of the confined Twist within the membrane-enclosed compartment. The permeability of Twist through the polymer membrane allowed a continuous flow of analyte, which led to a high local concentration of Twist and contributed to a high substrate affinity and fast catalytic reactions of confined BiDz in the nanoreactor. In addition, the MaBiDz system without a magnetic field applied proceeded, as expected, with limited reactivity, producing a Km of 0.115±0.078 × 10−9 M and a Vmax of 0.004 ± 0.001 F min−1.
Table 2.
The kinetics parameters, Michaelis–Menten constant (Km) and maximum reaction rate (Vmax) values of free BiDz operating in a solution and MaBiDz with and without a magnetic field with Twist as the analyte.
| System | Vmax (F min−1) | Km (× 10−9 M) |
|---|---|---|
| BiDZ operating in solution | 33.8 ± 1.1 | 7.42 ± 0.66 |
| MaBiDZ without magnetic field | 0.004 ± 0.001 | 0.116 ± 0.078 |
| MaBiDZ with magnetic field | 112.8 ± 13.4 | 0.178 ± 0.063 |
After establishing the enhanced kinetics, substrate selectivity, and stimuli-responsiveness of MaBiDz in vitro, we wanted to demonstrate its potential to function as a nanoreactor in a complex cellular environment to selectively detect Twist analyte and produce a fluorescent signal in response. We chose the MCF-7 breast cancer cell line, which expresses a high level of Twist mRNA.54,56
Our first aim was to demonstrate the advantage of a remotely applied magnetic field for cellular entry. To do so, we incubated magnetic particles tagged with Quasar 670 fluorescent dye into two sets of mammalian cell culture dishes, one with a magnet placed underneath and one with no magnetic field applied. Confocal images confirm cellular integration of the particles in both sets of cells as evidenced by the localization of the Quasar 670-dye with the nuclear Hoechst stain, Fig. 3, but demonstrated a 95% uptake for the sample exposed to an applied magnetic field (see Fig. SI3 in the SI for calculations). While we could duplicate the advantage of the magnetic field for cellular uptake demonstrated elsewhere57 it is important to note that cellular uptake of nanoparticles on its own is not a measure of availability for target mRNA sensing. Previous studies58 show that nanoparticles are sequestered into endosomal compartments upon cellular entry, after which they may undergo endosomal escape. If these intracellular nanoparticles cannot escape from the endosomal compartments, they are not available for intracellular sensing. Their ability to undergo endosomal escape depends on their design, and previous studies suggest that magnetic beads controlled by a remotely applied magnetic field demonstrate enhanced cellular entry and intracellular transport kinetics.57 To investigate this, we monitored the distribution and co-localization of fluorescently-tagged, oligo-modified MaBs and endosomes by confocal laser scanning microscopy (CLSM) at various time points. Results indicate that, at the peak of endosomal internalization of MaB, the ON state demonstrated about 50% less co-localization of MaB and endosomes compared to the OFF state (Fig. 4A).
Fig. 3.

Confocal laser scanning microscopy (CLSM) showing co-localization between magnetic nanoparticles and endosomes with and without the presence of a magnetic field. MCF-7 cells were incubated for specified time periods. Nuclei are stained with Hoechst nuclear stain and visualized with 408 nm laser, endosomes with Lysotracker Green and 488 nm laser, and Q670-labelled oligo-modified magnetic nanoparticles with 635 nm laser. Arrows indicated sites of co-localization (yellow) between magnetic nanoparticles and endosomes. Scale bar is 20 μm.
Fig. 4.
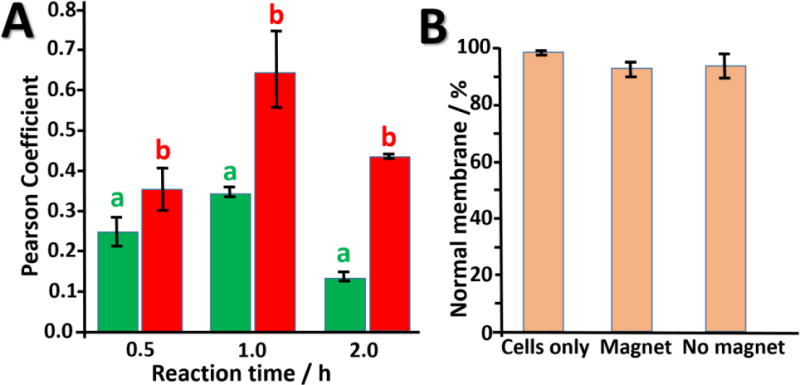
(A) Quantification of confocal microscopy results using ImageJ co-localization plugin: in the presence (a) and absence (b) of the magnetic field. (B) Analysis of possible cell membrane damage by magnetic nanoparticles (see details in the Experimental Section). According to the figure, a magnetic field does not induce membrane damage.
Next, we tested cytocompatibility of MaBiDz in the presence of a magnetic field. It is possible that the magnetic field, transduced into a force mobilizing the magnetic particles through the endosomal and cell membranes, could induce damage to the respective membranes. We, therefore, stained cells with Trypan Blue dye to see damaged membranes; no significant damage was observed for cells incubated with oligo-modified particles, with or without a magnetic field, Fig. 4B.
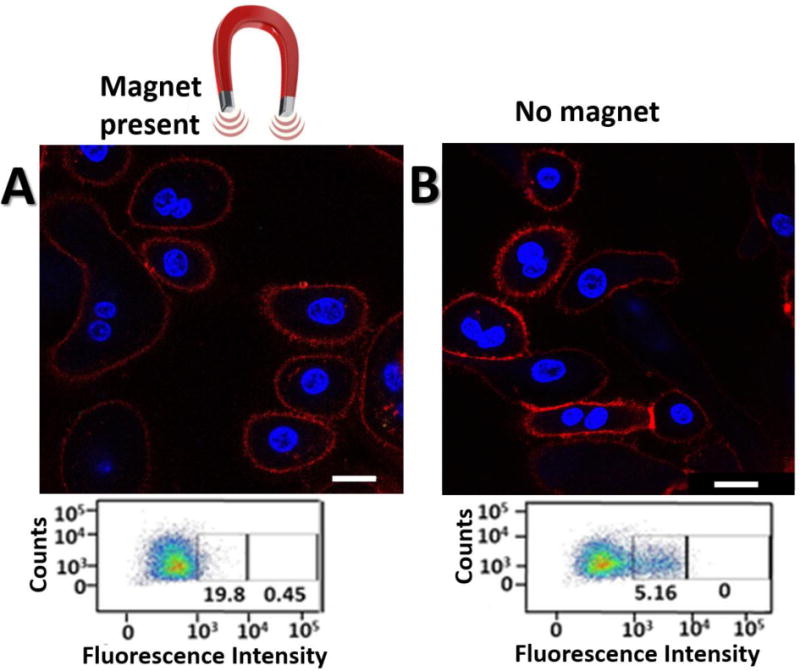
Our next aim was to reproduce in cell culture the magnetic field-responsiveness of MaBiDz observed in vitro. To do so, we incubated MaBiDz with MCF-7 cells both with and without an applied magnetic field and measured the fluorescent response of MaBiDz using confocal microscopy, Fig. 5. To quantify the intracellular signaling of MaBiDZ, we examined a large population of cells treated with MaBiDz using flow cytometry to eliminate variations inherent for confocal microscopy. Flow cytometry results, Figure 5, insets, show that MCF-7 cells treated with MaBiDZ and a magnetic field (ON state) exhibited 4 times greater fluorescence than MaBiDZ-treated MCF-7 cells without a magnetic field (OFF state), thus reproducing ex vivo the magnetic field-dependence of the MaBiDz nanoreactor seen in vitro.
Figure 5.

Intracellular testing of MaBiDZ sensor. CLSM images of (A) Twist-over-expressing MCF-7 cancer cells treated with MaBiDZ sensor with magnetic field applied and (B) no magnetic field applied. Images were taken after 2.5 hours of incubation time. Nuclei are stained with Hoechst nuclear stain and visualized with 408 nm laser. Cell surfaces are stained with epithelial cell adhesion molecule (EpCAM) antibody and visualized with a 635 nm laser. Fluorescence from the MaBiDZ probe is visualized with the 488 nm laser. Corresponding flow cytometry data are shown as insets below each image. The gates in the flow cytometry plots indicate percent of EpCAM cell population with low and high MaBiDZ fluorescence. Scale bar is 20 μm.
Next, we demonstrated nanoreactor stability and semi-permeability intracellularly, Fig. 6, reproducing results observed in in vitro experiments, Fig. 2B. When DNA is introduced to a cell, it must contain protective modifications in its sequence in order to prevent hydrolytic cleavage by cellular DNases.59 As with in vitro experiments, fluorescence from cleavage of F-Sub-modified magnetic particles was monitored in the cell; if DNases penetrated through the polymer brush, we would see a growing fluorescent signal as the fluorophore and quencher were separated via DNase-induced cleavage. However, while the brush permitted entry of the Twist mRNA strand as demonstrated in vitro, a lack of fluorescent signal from the F-Sub bound particles (9-fold lower than the MaBiDz response to Twist mRNA in Fig. 5A) indicates that the DNase molecules did not access F-sub through the brush. This could be because of size-selectivity of the brush, or because of the high salt concentration surrounding DNA-modified nanoparticles as reported elsewhere,60 which denatures or otherwise inhibit approaching enzymes.
Fig. 6.

Confocal laser scanning microscopy (CLSM) images and flow cytometry data for a series of negative controls. White channels correspond to Hoechst nuclear stain; blue channels correspond to EpCAM antibody cell surface stain; and green to F-Sub fluorescence. Images, taken after 2 hours, correspond to the following: (A) cells incubated with only the Hook-F-sub- MaBiDZ species, and (B) cell only control. Minimal fluorescence is observed for the Hook-F-sub only control, indicating a lack of DNase penetration through the polymer brush. This underlines a level of substrate-specificity required for entry through the polymer brush, as well as its role as a protective nanoreactor gate.
Having confirmed stimuli-responsive nanoreactor function, as well as the rapid cellular entry, biocompatibility, stability and semi-permeability of MaBiDz, we aimed to further confirm specificity of the nanoreactor that was demonstrated in vitro. To demonstrate that the fluorescent response was a result of the detection of Twist alone, we tested the response of MaBiDz in human cervical epithelial cells, which have low reported Twist levels,61 Fig 7. The nanoreactor produced 20-fold lower fluorescent signal consistent with the lower levels of Twist. Not only does this result demonstrate the specificity of nanoreactor response, it also demonstrates the ability of MaBiDz to serve as a diagnostic tool for identifying cancerous or metastatic cells based on bioimaging of target biomarkers.
Fig. 7.
Intracellular testing of MaBiDZ sensor. CLSM images of (A) Twist-under-expressing cervical epithelial cells treated with MaBiDZ sensor with magnetic field applied and (B) no magnetic field applied. Images were taken after 2.5 hours of incubation. Nuclei are stained with Hoechst nuclear stain and visualized with 408 nm laser. Cell surfaces are stained with EpCAM antibody and visualized with a 635 nm laser. Fluorescence from the MaBiDZ probe is visualized with the 488 nm laser. Corresponding flow cytometry data are shown as insets below each image. The numbers to the right of the flow cytometry histograms are the total mean fluorescence of the cell populations). Scale bar is 20 μm.
Conclusions
In summary, we have presented a novel nanoreactor design and demonstrated diverse applications, namely bioimaging, and biocatalysis within biomimetic organelles. MaBiDz demonstrates stability, specificity and semi-permeability owing to its polymeric brush, which provides a suitable microenvironment for intracellular biocatalysis to take place within a compartmentalized space. MaBiDz kinetics were markedly enhanced compared to free BiDz. The in vitro cell experiments demonstrated that the MaBiDz are cytocompatible, readily integrated with cells, and demonstrate intracellular stability. The culmination of these features enabled rapid detection of a biomarker for breast adenocarcinoma, which was employed to distinguish between cancer and non-cancerous cells. We believe the unique architecture of MaBiDz provides a promising platform for nanoreactor design, and can be further explored for additional nanoreactor functionalities.
Supplementary Material
Acknowledgments
This work at Clarkson University (EK) and at the University of Georgia (SM) was supported by the NSF awards CBET-1403208 and DMR-1309469. The work at University of Central Florida (DMK) and Clarkson (CDW) was supported by NIH awards R15AI10388001A1 and 1R15CA173703-01. D.M.K. was partially supported by the ITMO University Fellowship and Professorship Program.
References
- 1.Sadana A, Sadana N. Handbook of Biosensors and Biosensor Kinetics. Elsevier Science; Amsterdam: 2011. [Google Scholar]
- 2.Cooper J, Cass T, editors. Biosensors. Oxford University Press; Oxford: 2004. [Google Scholar]
- 3.Tiwari A, Turner APF, editors. Biosensors Nanotechnology. Wiley; Hoboken: 2014. [Google Scholar]
- 4.Choi S, editor. Systems Biology for Signaling Networks. Springer-Verlag; New York: 2010. [Google Scholar]
- 5.Hancock J. Cell Signalling. 3rd. Oxford University Press; Oxford: 2010. [Google Scholar]
- 6.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. Garland Science; New York: 2007. [Google Scholar]
- 7.Strauss AW, Boime I, Kreil G, editors. Protein Compartmentalization. Springer-Verlag; New York: 1986. [Google Scholar]
- 8.Marguet M, Bonduelle C, Lecommandoux S. Chem Soc Rev. 2013;42:512–529. doi: 10.1039/c2cs35312a. [DOI] [PubMed] [Google Scholar]
- 9.Schoonen L, van Hest JCM. Adv Mater. 2016;28:1109–1128. doi: 10.1002/adma.201502389. [DOI] [PubMed] [Google Scholar]
- 10.Küchler A, Yoshimoto M, Luginbühl S, Mavelli F, Walde P. Nat Nanotechnol. 2016;11:409–420. doi: 10.1038/nnano.2016.54. [DOI] [PubMed] [Google Scholar]
- 11.Gunkel-Grabole G, Sigg S, Lomora M, Lörcher S, Palivan CG, Meier WP. Biomater Sci. 2015;3:25–40. doi: 10.1039/c4bm00230j. [DOI] [PubMed] [Google Scholar]
- 12.Einfalt T, Goers R, Dinu IA, Najer A, Spulber M, Onaca-Fischer O, Palivan CG. Nano Lett. 2015;15:7596–7603. doi: 10.1021/acs.nanolett.5b03386. [DOI] [PubMed] [Google Scholar]
- 13.Lomora M, Gunkel-Grabole G, Mantrib S, Palivan CG. Chem Commun. 2017;53:10148–10151. doi: 10.1039/c7cc04739h. [DOI] [PubMed] [Google Scholar]
- 14.Langowska K, Palivan CG, Meier W. Chem Commun. 2013;49:128–130. doi: 10.1039/c2cc36345c. [DOI] [PubMed] [Google Scholar]
- 15.Ranquin A, Versées W, Meier W, Steyaert J, Van Gelder P. Nano Lett. 2005;5:2220–2224. doi: 10.1021/nl051523d. [DOI] [PubMed] [Google Scholar]
- 16.Axthelm F, Casse O, Koppenol WH, Nauser T, Meier W, Palivan CG. J Phys Chem B. 2008;112:8211–8217. doi: 10.1021/jp803032w. [DOI] [PubMed] [Google Scholar]
- 17.Dobrunz D, Toma AC, Tanner P, Pfohl T, Palivan CG. Langmuir. 2012;28:15889–15899. doi: 10.1021/la302724m. [DOI] [PubMed] [Google Scholar]
- 18.De Vocht C, Ranquin A, Willaert R, Van Ginderachter RJ, Vanhaecke T, Rogiers V, Versées VW, Van Gelder P, Steyaert J. J Controlled Release. 2009;137:246–254. doi: 10.1016/j.jconrel.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Balasubramanian V, Correia A, Zhang H, Fontana F, Mäkilä E, Salonen J, Hirvonen J, Santos HA. Adv Mater. 2017;29 doi: 10.1002/adma.201605375. art. No. 1605375. [DOI] [PubMed] [Google Scholar]
- 20.Zaki A, Dave N, Liu J. J Am Chem Soc. 2012;134:35–38. doi: 10.1021/ja207661z. [DOI] [PubMed] [Google Scholar]
- 21.Seferos DS, Giljohann DA, Hill HD, Prigodich AE, Mirkin CA. J Am Chem Soc. 2007;129:15477–15479. doi: 10.1021/ja0776529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakshi SF, Guz N, Zakharchenko A, Deng H, Tumanov A, Woodworth CD, Minko S, Kolpashchikov DM, Katz E. J Am Chem Soc. 2017;139:12117–12120. doi: 10.1021/jacs.7b06022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahzad A, editor. Translational Medicine: Tools and Techniques. Academic Press; San Diego: 2015. [Google Scholar]
- 24.Diez P, Esteban-Fernández de Ávila B, Ramirez-Herrera DE, Villalonga R, Wang J. Nanoscale. 2017;9:14307–14311. doi: 10.1039/c7nr05535h. [DOI] [PubMed] [Google Scholar]
- 25.Soto F, Wagner GL, Garcia-Gradilla V, Gillespie KT, Lakshmipathy DR, Karshalev E, Angell C, Chen Y, Wang J. Nanoscale. 2016;8:17788–17793. doi: 10.1039/c6nr06603h. [DOI] [PubMed] [Google Scholar]
- 26.Li T, Li J, Morozov KI, Wu Z, Xu T, Rozen I, Leshansky AM, Li L, Wang J. Nano Lett. 2017;17:5092–5098. doi: 10.1021/acs.nanolett.7b02383. [DOI] [PubMed] [Google Scholar]
- 27.Lane DD, Su FY, Chiu DY, Srinivasan S, Wilson JT, Ratner DM, Stayton PS, Convertine AJ. Polym Chem. 2015;6:1255–1266. doi: 10.1039/C4PY01249F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agut W, Brûlet A, Schatz C, Taton D, Lecommandoux S. Langmuir. 2010;26:10546–10554. doi: 10.1021/la1005693. [DOI] [PubMed] [Google Scholar]
- 29.Cabane E, Malinova V, Menon S, Palivan CG, Meier W. Soft Matter. 2011;7:9167–9176. [Google Scholar]
- 30.Funk RHW, Monsees T, Ӧzkucur N. Prog Histochem Cytochem. 2009;43:177–264. doi: 10.1016/j.proghi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Zacharchenko A, Guz N, Laradji AM, Katz E, Minko S. Nature Catalysis. 2017 doi: 10.1038/s41929-017-0003-3. in press. [DOI] [Google Scholar]
- 32.Renggli K, Baumann P, Langowska K, Onaca O, Bruns N, Meier W. Adv Funct Mater. 2011;21:1241–1259. [Google Scholar]
- 33.Deraedt C, Astruc D. Coord Chem Rev. 2016;324:106–122. [Google Scholar]
- 34.Palivan CG, Fischer-Onaca O, Delcea M, Itel F, Meier W. Chem Soc Rev. 2012;41:2800–2823. doi: 10.1039/c1cs15240h. [DOI] [PubMed] [Google Scholar]
- 35.Larrañaga A, Lomora N, Sarasua JR, Palivan CG, Pandit A. Prog Mater Sci. 2017;90:325–357. [Google Scholar]
- 36.Che H, van Hest JCM. J Mater Chem B. 2016;4:4632–4647. doi: 10.1039/c6tb01163b. [DOI] [PubMed] [Google Scholar]
- 37.Peters RJRW, Louzao I, van Hest JCM. Chem Sci. 2012;3:335–342. [Google Scholar]
- 38.Tanner P, Baumann P, Enea R, Onaca O, Palivan C, Meier W. Acc Chem Res. 2011;44:1039–1049. doi: 10.1021/ar200036k. [DOI] [PubMed] [Google Scholar]
- 39.Tanner P, Balasubramanian V, Palivan CG. Nano Lett. 2013;13:2875–2883. doi: 10.1021/nl401215n. [DOI] [PubMed] [Google Scholar]
- 40.Antonietti M, Förster S. Adv Mater. 2003;15:1323–1333. [Google Scholar]
- 41.Municoy S, Bellino MG. RSC Adv. 2017;7:67–70. [Google Scholar]
- 42.Lee J, Kim SM, Lee IS. Nano Today. 2014;9:631–667. [Google Scholar]
- 43.Maity B, Fujita K, Ueno T. Curr Opin Chem Biol. 2015;25:88–97. doi: 10.1016/j.cbpa.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 44.de la Escosura A, Nolte RJM, Cornelissen JJLM. J Mater Chem. 2009;19:2274–2278. [Google Scholar]
- 45.Lu Y, Ballauff M. Prog Polym Sci. 2016;59:86–104. [Google Scholar]
- 46.Zuo C, Wei W, Zhou Q, Wu S, Li S. ChemistrySelect. 2017;2:6149–6153. [Google Scholar]
- 47.Lu Y, Welsch N, Dzubiella J, Ballauff M. Intelligent Hydrogels. In: Sadowski G, Richtering W, editors. Book Series: Progress in Colloid and Polymer Science. Vol. 140. Springer Int Publ; Heidelberg: 2013. pp. 113–130. [Google Scholar]
- 48.Roa R, Kim WK, Kanduc M, Dzubiella J, Angioletti-Uberti S. ACS Catalysis. 2017;7:5604–5611. doi: 10.1021/acscatal.7b01701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolpashchikov DM. ChemBioChem. 2007;8:2039–2042. doi: 10.1002/cbic.200700384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mokany E, Bone SM, Young PE, Doan TB, Todd AV. J Am Chem Soc. 2010;132:1051–1059. doi: 10.1021/ja9076777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerasimova YV, Cornett E, Kolpashchikov DM. ChemBioChem. 2010;11:811–817. doi: 10.1002/cbic.201000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerasimova YV, Kolpashchikov DM. Angew Chem, Int Ed. 2013;52:10586–10588. doi: 10.1002/anie.201303919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bumb A, Brechbiel MW, Choyke PL, Fugger L, Eggeman A, Prabhakaran D, Hutchinson J, Dobson PJ. Nanotechnology. 2008;19 doi: 10.1088/0957-4484/19/33/335601. art. No. 335601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 55.Jimenez J, Sheparovych R, Pita M, Narvaez Garcia A, Dominguez E, Minko S, Katz E. J Phys Chem C. 2008;112:7337–7344. [Google Scholar]
- 56.Watanabe O, Imamura H, Shimizu T, Kinoshita J, Okabe T, Hirano A, Yoshimatsu K, Konno S, Aiba M, Ogawa K. Anticancer Res. 2004;24:3851–3856. [PubMed] [Google Scholar]
- 57.Plank C, Schillinger U, Scherer F, Bergemann C, Rémy JS, Krötz F, Anton M, Lausier J, Rosenecker J. Biol Chem. 2003;384:737–747. doi: 10.1515/BC.2003.082. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen J, Szoka F. Acc Chem Res. 2012;45:1153–1162. doi: 10.1021/ar3000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takeshita H, Yasuda T, Nakajima T, Hosomi O, Nakashima Y, Kishi K. Biochem Mol Biol Int. 1997;42:65–75. doi: 10.1080/15216549700202441. [DOI] [PubMed] [Google Scholar]
- 60.Zwanikken JW, Guo P, Mirkin CA, Olvera de la Cruz M. J Phys Chem C. 2011;115:16368–16373. [Google Scholar]
- 61.Li Y, Wang W, Wang W, Yang R, Wang T, Su T, Weng D, Tao T, Li W, Ma D, Wang S. Gynecol Oncol. 2012;124:112–118. doi: 10.1016/j.ygyno.2011.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


