Abstract
Previous studies of autism spectrum disorder (ASD) and birth spacing had limitations; few examined phenotypic case subtypes or explored underlying mechanisms for associations and none assessed whether other (non-ASD) developmental disabilities (DDs) were associated with birth spacing. We assessed associations between inter-pregnancy interval (IPI) and both ASD and other DDs using data from the Study to Explore Early Development, a multi-site case-control study with rigorous case-finding and case-classification methods and detailed data collection on maternal reproductive history. Our sample included 356 ASD cases, 627 DD cases, and 524 population (POP) controls born in second or later births. ASD and DD cases were further sub-divided according to whether the child had intellectual disability (ID). ASD cases were also sub-divided by ASD symptom severity, and DD cases were subdivided by presence of some ASD symptoms (indicated on an autism screener). Odds ratios, adjusted for maternal-child sociodemographic factors, (aORs) and 95% confidence intervals were derived from logistic regression models. Among term births, ASD was associated with both IPI <18 months (aOR 1.5 [1.1–2.2]) and ≥60 months (1.5 [0.99–2.4]). Both short and long IPI associations were stronger among ASD cases with high severity scores (aORs 2.0 [1.3–3.3] and 1.8 [0.99–3.2], respectively). Associations were unchanged after adding several factors potentially related to the causal pathway to regression models. DD was not associated with either short or long IPI—overall, among term births, or in any subgroup examined. These findings extend those from previous studies and further inform recommendations on optimal pregnancy spacing.
Lay Summary
We investigated whether the amount of time between pregnancies was associated autism spectrum disorder (ASD) or other developmental disabilities (DD) in children. ASD was increased in second and later-born children who were conceived less than 18 months or 60 or more months after the mother's previous birth. Other DDs were not associated with birth spacing.
Keywords: autism spectrum disorder, developmental disabilities, birth spacing, epidemiology, risk factor
Introduction
Autism spectrum disorder (ASD) is estimated to occur in 1–2% of U.S. children [Christensen et al., 2016; Blumberg et al., 2013]. While genetic factors are implicated in the etiology of ASD [Miles, 2011], the composite evidence supports gene–environment interactions [Kim & Leventhal, 2015]. ASD risk factors identified from epidemiologic studies include maternal-fetal health conditions, such as diabetes, preeclampsia, infection, and preterm birth, and maternal exposures such as medication use, environmental pollutants, and folic acid deficiency before and during pregnancy [Ornoy, Weinstein-Fudim, & Ergaz, 2016]. Neurobiological studies also suggest that embryogenesis is a critical period for ASD exposures [Arndt, Stodgell, & Rodier, 2005]. Thus, investigation of factors related to early pregnancy might provide clues about the causes of ASD. Short birth spacing or inter-pregnancy-interval (IPI), an established risk factor for adverse perinatal outcomes including preterm delivery, intrauterine-growth-restriction, and neonatal intensive care admission [Conde-Agudelo, Rosas-Bermúdez, & Kafury-Goeta, 2006; Conde-Agudelo, Rosas-Bermúdez, Castaño, & Norton, 2012; Appareddy Pryor, & Bailey, 2016], is thus also of concern for potential longer-term child health effects, such as ASD.
Several studies report associations between ASD and short IPI [Cheslack-Postava, Liu, & Bearman, 2011; Dodds et al., 2011; Gunnes et al., 2013; Cheslack-Postava et al., 2014; Coo et al., 2015; Durkin, DuBois, & Maenner, 2015; Zerbo, Yoshida, Gunderson, Dorward, & Croen, 2015]. Some also report associations with long IPI [Cheslack-Postava et al., 2014; Coo et al., 2015; Durkin et al., 2015; Zerbo et al., 2015]. However, short IPI was variously defined as <9 to <24 months and several studies did not separate births after long IPI from the referent category. Few studies present data on IPI as a continuous variable to further inform the binary or ordinal IPI categories presented and only one study assessed a continuous IPI variable in a model that allowed for non-linear prediction effects [Gunnes et al., 2013]. However, that study was nonetheless limited in that estimates from the continuous IPI assessment were imprecise, especially for long IPIs.
Because previous studies were based on data from existing medical or administrative records, they were further limited in the data available for both pregnancy history and child developmental outcomes. In most studies, cases were limited to those who had accessed services and received an ASD diagnosis; in several studies, ASD case definitions were based on non-standardized administrative diagnostic coding, with the possibility of varying diagnostic rigor and possible mis-classification and selective ascertainment of the most severe cases. Data on maternal reproductive history from existing data sources is also often incomplete. For instance, many population-based registries do not include fetal losses at all or do not include timing of fetal losses; thus, IPI was necessarily defined based only on previous livebirths. However, evidence supports treating stillbirths similar to livebirths in IPI calculations; short IPI after stillbirth has been linked to increased risks for preeclampsia, intrauterine-growth-restriction, preterm delivery, and perinatal and neonatal death [Bigelow & Bryant, 2015]. Previous studies were also limited in their ability to assess potential biologic mechanisms and phenotypic case subtypes. Moreover, the few studies that included assessments of DSM-IV ASD subtypes report inconsistent findings. Gunnes et al. [2013] reported that short IPI was associated with autistic disorder but not other disorders (Asperger's Disorder and pervasive developmental disorder–not otherwise specified [PDD-NOS] combined); Zerbo et al. [2015] reported that both short and long IPI were associated with ASD regardless of subtype; and Cheslack-Postava et al. [2014] reported that short IPI was associated with autistic disorder and PDD-NOS but not Asperger's disorder and long IPI was associated only with Asperger's disorder. Nonetheless, Cheslack-Postava et al. [2014] also reported that IPI associations were similar for ASD cases with and without co-occurring intellectual disability (ID).
A few studies have reported associations between short IPI and developmental delay [reviewed in Conde-Agudelo, Rosas-Bermudez, & Norton, 2016]; however, various methodological limitations hamper interpretation of the findings. To our knowledge, no study has examined associations between IPI and ASD and non-ASD developmental disabilities (DDs) in the same study population. Thus, it has not been possible to assess whether associations reported between IPI and ASD might reflect a more general neurodevelopmental effect.
The Study to Explore Early Development (SEED), a large multi-site case-control study specifically designed to assess pre-conception and prenatal ASD risk factors, has several advantages over prior studies. Case finding included identification of young children with a range of developmental delays from numerous sources serving diverse population subgroups, thus casting a “wide net” to identify possible ASD cases (including yet undiagnosed cases) and a second non-ASD DD case group. Final case classification was based on in-depth standardized in-person developmental assessments administered by research-reliable clinical study staff, rather than reports of past diagnoses. Developmental assessment data also allowed us to systematically define various case subtypes. Exposure ascertainment was based on detailed maternal reproductive histories taken as part of a comprehensive maternal interview, which also included data on conditions possibly in the causal pathway of an IPI–ASD association.
Using SEED data, we conducted a comprehensive analysis of the association between IPI and ASD. We examined both short and long IPI, including assessments of an ordinal IPI variable that aligns with clinical guidelines and a continuous IPI variable evaluated using cubic spline analyses. We examined associations with non-ASD DDs in addition to ASD and also examined associations for several ASD and DD phenotypic subtypes. Finally, we assessed several factors possibly related to the underlying causal pathway.
Methods
SEED Methodology
SEED was implemented in 2007 in six sites located in California, Colorado, Georgia, Maryland, North Carolina, and Pennsylvania. Institutional review boards at CDC and each site approved the SEED protocol.
Details of the SEED methodology were previously published [Schendel et al., 2012]. Each site followed a common protocol, including enrollment of three study groups: children with ASD, children with other (non-ASD) DDs, and children from the general population (POP). Children for the ASD and DD groups were identified from multiple special education and clinical sources that provide services to children with disabilities. Children recruited from each source were those with select special education or International Classification of Disease codes indicative of autism/ASD or other DDs often seen as precursor or co-occurring diagnoses in children eventually diagnosed with ASD. POP children were selected from random samples of the birth certificates within a given site's defined study area.
Eligible children for the first phase of SEED were born between 2003 and 2006, lived in the respective site's study area both at birth and at study enrollment, and lived with a caregiver since 6 months of age who could provide legal consent and was capable of communicating in English (all sites) or Spanish (two sites). For 98% of children, the caregiver was the biological mother. Children were enrolled at 2–5 years of age.
Although children were initially identified as potentially being eligible for a given group—ASD, DD, or POP—final study group classification was determined from standardized research developmental assessments [Wiggins et al., 2015]. Upon enrollment, all children were screened for possible autism characteristics through their caregivers' completion of the Social Communication Questionnaire (SCQ). Children with SCQ scores at or above the predetermined threshold of 11 were designated as potential ASD cases regardless of how they were initially identified. Additionally, all children who had a previous ASD diagnosis or autism special education classification were designated as potential ASD cases regardless of their SCQ scores. Children in all study groups were seen in person and administered a general developmental assessment, the Mullen Scales of Early Learning (MSEL). Children in the potential ASD group were additionally administered the Autism Diagnostic Observation Schedule (ADOS) and their caregivers were administered the Autism Diagnostic Interview - Revised (ADI-R). Final ASD case classification was based on ADOS and ADI-R scores. Children who had been designated as potential ASD cases, who did not meet the criteria for classification as an ASD case after the ADOS and ADI-R, received a final classification of either DD or POP depending on their original identification source (education/clinical source vs. birth certificate sample, respectively).
A wealth of data were collected from all study groups, including an extensive interview with the care-giver about family socio-demographics and, if the care-giver was the biological mother, her reproductive history and information about her pregnancy with the child.
Sample Selection
Altogether, 3,200 children enrolled in SEED and completed sufficient assessments to receive a final classification of ASD, DD, or POP. Of these, 2,728 children had data on maternal pregnancy history; 2,725 also had data on potential confounders of interest. The sample for the current analysis was necessarily limited to second or later births (n = 1,507).
Birth Spacing
During the computer-assisted-telephone-interview, the biological mother was asked how many times she had been pregnant, and for each pregnancy, she was asked the outcome and date (exact date or month and year of outcome, year of outcome, or her age at outcome if she could not recall the date). Birth spacing or IPI was calculated as time in months from most recent date of birth (stillbirth or livebirth) preceding the index pregnancy to estimated date of conception of index pregnancy. Date of conception was calculated as date of index birth minus gestational age, obtained from birth certificate files. Our choice to calculate IPI as interval from most recent stillbirth or livebirth, but not most recent miscarriage, was based on previous studies that document adverse perinatal and infant consequences for pregnancies conceived within a short interval of a stillbirth, but few risks for pregnancies conceived within a short interval of a previous miscarriage [Bigelow & Bryant, 2015].
Statistical Analysis
We examined distributions of maternal reproductive history variables and potential confounders—maternal age and education at birth, maternal race-ethnicity, gestational age, and child sex—by case-control study group. Potential confounders were factors previously found to be associated with ASD and IPI [Bilder, Pinborough-Zimmerman, Miller, & McMahon, 2009; Schieve, Clayton, Durkin, Wingate, & Drews-Botsch, 2015; Thoma, Copen, & Kirmeyer, 2016; Appareddy et al., 2016]. We tested the significance of differences between ASD and POP groups and DD and POP groups with chi-square tests.
We assessed associations between ASD and DD and IPI in several sets of analyses.
Assessment of ordinal IPI variable
We initially computed unadjusted odds ratios (OR) and 95% confidence intervals (CI) for associations between both ASD and DD (each compared to POPs) and IPI (<18 months or ≥60 months vs. 18–59 months). The IPI cut points were chosen based on review of previous IPI-ASD studies [Dodds et al., 2011; Gunnes et al., 2013; Cheslack-Postava et al., 2014; Coo et al., 2015; Durkin et al., 2015; Zerbo et al., 2015], published clinical recommendations [Bigelow & Bryant, 2015], and sample sizes of our study groups—overall and within subgroups of interest. We also subdivided the lowest IPI category as <12 months and 12 to <18 months and examined findings separately for these groups; as expected estimates were less precise for these smaller categories.
We conducted stratified analyses examining each potential confounder individually; the findings informed our final multivariable adjustment models. Because we observed notable differences in IPI associations within gestational age strata, we conducted adjusted analyses for the total sample and for the sample restricted to term births. Adjusted ORs (aORs) were derived from ordinary logistic regression models that included IPI, child sex, maternal race/ethnicity, maternal education at birth, and maternal age at birth as independent variables. While we also examined household income as a potential confounding factor, we did not include income in our final models to avoid reducing our sample size; 3–5% of mothers did not respond to the income question on the maternal interview. The aOR estimates derived from models with and without income were very similar; we thus present only the latter here.
We also assessed several factors possibly related to the underlying causal path between IPI and adverse neuro-development (i.e., potential mediator variables): (1) pregnancy planning; (2) maternal infertility disorder diagnoses; and (3) maternal hypertension or diabetes during pregnancy. We re-ran our logistic regression models among term births with each of these factors added. Data on pregnancy planning and infertility disorders were collected as part of the maternal telephone interview. The interview included a question on general pregnancy planning—whether before her pregnancy with the index child the mother was trying to get pregnant. Infertility was ascertained from a series of questions on whether before the index pregnancy a doctor or other healthcare provider ever told the mother it would be impossible or difficult to get pregnant—over-all and because of specific infertility-related disorders, including blocked or damaged fallopian tubes; PCOS or multiple ovarian cysts; diminished ovarian reserve because of advanced age, premature ovarian failure or a medical condition; endometriosis; uterine problem, such as fibroids; or a diagnosis of unexplained infertility. Women could respond affirmatively to more than one disorder. For this analysis, we created a single, “any maternal infertility disorder” variable. Multiple SEED instruments included information on hypertension and diabetes during pregnancy: the maternal telephone interview; prenatal care record abstractions (available for ∼70% of study participants); and a self-administered maternal medical history checklist form. A previous SEED analysis reported high agreement for medical record and self-reported data for both hypertension and diabetes (currently unpublished data). Therefore, we classified the mother as having each condition if it was reported in any of the three study instruments.
Assessment of ASD and DD subgroups
We assessed both ASD and DD within subgroups defined according to whether or not the child scored in the intellectual disability (ID) range (<70) on the MSEL, which was administered to all children during the in-person developmental assessment. We additionally examined ASD subgroups defined according to the severity of ASD symptoms. A calibrated ADOS severity score was calculated for all ASD cases based on the ADOS total score, ADOS language level, and child age at developmental assessment. The ten-point scale was dichotomized into ‘less severe’ (scores of 4–7) and ‘more severe’ (scores of 8–10). We also subdivided the DD group by whether the child had an indication of some autism symptoms, even though s/he had not met the ADOS and ADI-R criteria for classification as an ASD case. Autism symptoms were defined as SCQ score=>11. Separate regression models were run for each phenotypic subgroup. In each of these models, we compared IPI among children in a given subgroup (ASD with ID, ASD without ID, DD with ID, DD without ID, ASD with more severe symptoms, ASD with less severe symptoms, DD with autism symptoms, DD without autism symptoms) to the total POP group.
Assessment of continuous IPI variable
IPI was also analyzed as a continuous variable using logistic regression with restricted cubic splines to allow for nonlinear (log scale) risk relationships between IPI length and outcome odds. We specified 3 knots at 12, 18, and 59 months, and specified 36-month IPI as the reference value. The covariates included in the multivariable logistic regression with restricted cubic splines were the same as those included in the multivariable categorical approach. The models with restricted cubic splines were compared to the models without splines using Likelihood ratio tests for testing non-linearity.
Results
In our sample, children with ASD and DD were more likely than children in the POP group to be male and to have been delivered preterm (Table 1). Their mothers were less likely than POP mothers to be Non-Hispanic white or to have completed college at the time of their births and were more likely to have had hypertension during pregnancy. Mothers of ASD children were also more likely than mothers of POP children to have had an infertility disorder diagnosis and were less likely to report trying to get pregnant. DD mothers were more likely than POP mothers to have had diabetes during pregnancy. Over two-thirds of ASD children and about one-fourth of DD children had ID. Close to 40% of ASD children had a high ASD symptom severity score and nearly 30% of DD children were classified as having some autism symptoms.
Table 1. Distribution of Socio-Demographic, Perinatal, and Child Factors by Study Group, Study to Explore Early Development (SEED), United States.
| ASD (N=356) | DD (N=627) | POP (N=524) | P ASD vs POP | P DD vs POP | |
|---|---|---|---|---|---|
| % | % | % | |||
| Child sex | |||||
| Male | 81.7 | 64.9 | 53.6 | <0.001 | <0.001 |
| Female | 18.3 | 35.1 | 46.4 | ||
| Maternal race/ethnicity | |||||
| Non-Hispanic-white | 51.7 | 58.5 | 71.4 | <0.001 | <0.001 |
| Non-Hispanic-black | 23.9 | 17.7 | 13.2 | ||
| Hispanic | 13.5 | 14.8 | 8.2 | ||
| Non-Hispanic –Asian/Pacific Islander/American Indian | 7.6 | 4.2 | 4.0 | ||
| Non-Hispanic -multiracial | 3.4 | 4.8 | 3.2 | ||
| Maternal age at birth (years) | |||||
| <25 | 9.8 | 8.1 | 7.1 | 0.270 | 0.728 |
| 25–34 | 56.7 | 56.8 | 56.3 | ||
| ≥35 | 33.4 | 35.1 | 36.7 | ||
| Maternal education at birth (years) | |||||
| <12 | 25.3 | 25.8 | 13.7 | <0.001 | <0.001 |
| 13–15 | 30.9 | 23.6 | 27.9 | ||
| ≥16 | 43.8 | 50.6 | 58.4 | ||
| Gestational age at birth (weeks) | |||||
| <37 | 14.3 | 21.9 | 8.2 | 0.004 | <0.001 |
| ≥37 | 85.7 | 78.2 | 91.8 | ||
| Mother trying to get pregnant just before index pregnancy | |||||
| Yes | 52.3 | 59.0 | 62.4 | 0.004 | 0.249 |
| No | 47.7 | 41.1 | 37.6 | ||
| Maternal diagnosis of infertility disorder prior to index pregnancy | |||||
| Yes | 18.9 | 16.3 | 12.3 | 0.010 | 0.068 |
| No | 81.1 | 83.7 | 87.7 | ||
| Maternal hypertension during index pregnancy | |||||
| Yes | 13.8 | 14.6 | 9.1 | 0.030 | 0.006 |
| No | 86.2 | 85.4 | 90.9 | ||
| Maternal diabetes during index pregnancy | |||||
| Yes | 9.0 | 12.1 | 8.1 | 0.640 | 0.032 |
| No | 91.0 | 87.9 | 91.9 | ||
| Intellectual disability | |||||
| Yes | 66.8 | 24.0 | 2.8 | <0.001 | <0.001 |
| No | 33.2 | 76.0 | 97.2 | ||
| ASD severity | |||||
| More severe (ADOS score 8–10) | 41.1 | NA | NA | NA | NA |
| Less severe (ADOS score 4–7) | 58.9 | ||||
| DD group with autism symptoms? | |||||
| Yes | NA | 27.3 | NA | NA | NA |
| No | 72.7 | ||||
ASD is autism spectrum disorder case group; DD is other (non-ASD) developmental delay; POP is general population control group: ADOS is Autism Diagnostic Interview Schedule.
The ASD group had higher frequencies of both short and long IPI than the POP group (Fig. 1) and the difference between the ASD and POP distributions was statistically significant (P = 0.005). The distribution of IPI in the DD group was not significantly different from the POP group distribution.
Figure 1.
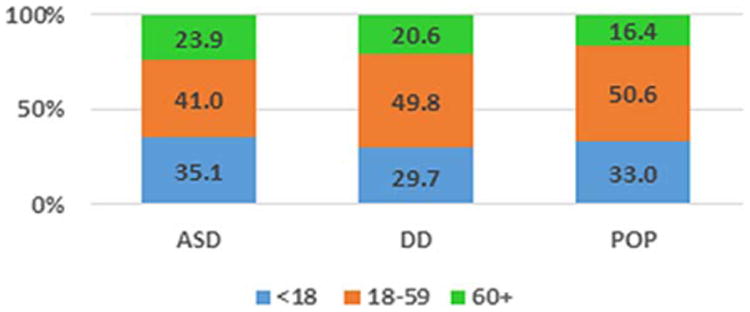
Distribution of Inter-pregnancy-interval (months) by Study Group, Study to Explore Early Development (SEED), United States. Chi-Square test for differences in the IPI distribution was statistically significant for ASD vs POP comparison (P = 0.005) but not DD vs POP comparison (P = 0.156).
Both before and after adjustment, we observed positive associations between ASD and both short (<18 months) and long (≥60 months) IPI. Associations were more pronounced among term births (aORs 1.5 [95% CI: 1.1–2.2] and 1.5 [95% CI: 0.99–2.4] for short and long IPI, respectively) (Table 2). We could not assess adjusted associations between ASD and IPI among preterm births due to small sample sizes and instability of the regression model; however, unadjusted stratified analyses were suggestive of an effect modification between IPI and gestational age (P = 0.075 with positive associations between ASD and both short and long IPI among term, but not preterm births) (data not shown). Analyses of finer gradations of short IPI revealed that the magnitude of effects for associations between ASD and IPIs of <12 months and 12–18 months were similar. Addition of various mediator factors to regression models had minimal impact on effect estimates for either short or long IPI.
Table 2. Associations between Birth Spacing and Autism Spectrum Disorder, Study to Explore Early Development (SEED), United States.
| Inter-pregnancy Interval (m) | Total Sample Unadjusted OR (95% CI) | Total Sample, Adjustment for sociodemographics aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics + maternal report of trying to get pregnant aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics + maternal infertility disorder aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics + prenatal hypertension, diabetes aOR† (95% CI) |
|---|---|---|---|---|---|---|
| ASD (all) | ||||||
| <12 | 1.4 (0.97–2.1) | 1.3 (0.9–2.0) | 1.6 (1.00–2.4) | 1.6 (0.99–2.5) | 1.6 (1.03–2.6) | 1.5 (0.97–2.4) |
| 12–<18 | 1.2 (0.8–1.8) | 1.4 (0.9–2.0) | 1.5 (0.98–2.4) | 1.4 (0.9–2.2) | 1.4 (0.9–2.1) | 1.5 (0.95–2.3) |
| All <18 | 1.3 (0.97–1.8) | 1.3 (0.96–1.9) | 1.5 (1.1–2.2) | 1.5 (1.03–2.2) | 1.5 (1.03–2.2) | 1.5 (1.04–2.1) |
| 18–59 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥60 | 1.8 (1.3–2.6) | 1.4 (0.9–2.03) | 1.5 (0.99–2.4) | 1.7 (1.1–2.6) | 1.7 (1.1–2.7) | 1.5 (0.97–2.3) |
ASD is autism spectrum disorder case group.
aOR is adjusted odds ratio, derived from logistic regression models in which the ASD case group is compared to the POP (general population) control group. All models include adjustment for child sex, maternal age at birth, maternal education at birth, and maternal race/ethnicity. Supplemental models additionally adjusted for various potential mediator factors as indicated.
Associations were similar for ASD with and without ID (Table 3). Associations for “more severe ASD” were stronger than those observed for ASD overall. Conversely, associations between “less severe ASD” and either short or long IPI were attenuated and no longer statistically significant.
Table 3. Associations between Birth Spacing and Autism Spectrum Disorder Subtypes, Study to Explore Early Development (SEED), United States.
| Inter-pregnancy Interval (m) | Total Sample Unadjusted OR (95% CI) | Total Sample, Adjustment for sociodemographics aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics + maternal report of trying to get pregnant aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics + maternal infertility disorder aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics + prenatal hypertension, diabetes aOR† (95% CI) |
|---|---|---|---|---|---|---|
| ASD with ID | ||||||
| <18 | 1.4 (0.99–2.0) | 1.4 (0.97–2.1) | 1.6 (1.1–2.4) | 1.6 (1.01–2.4) | 1.5 (0.99–2.4) | 1.6 (1.03–2.3) |
| 18–59 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥60 | 1.9 (1.3–2.8) | 1.3 (0.8–2.0) | 1.4 (0.9–2.4) | 1.6 (0.97–2.7) | 1.6 (0.97–2.7) | 1.4 (0.9–2.3) |
| ASD w/out ID | ||||||
| <18 | 1.2 (0.7–1.9) | 1.3 (0.8–2.0) | 1.4 (0.9–2.4) | 1.4 (0.8–2.3) | 1.4 (0.8–2.4) | 1.4 (0.8–2.3) |
| 18–59 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥60 | 1.6 (0.96–2.8) | 1.4 (0.8–2.6) | 1.5 (0.8–2.9) | 1.4 (0.7–2.8) | 1.5 (0.7–2.8) | 1.5 (0.8–2.8) |
| ASD with high severity score | ||||||
| <18 | 1.7 (1.1–2.5) | 1.8 (1.2–2.8) | 2.0 (1.3–3.3) | 2.1 (1.3–3.5) | 1.9 (1.2–3.2) | 2.0 (1.2–3.3) |
| 18–59 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥60 | 2.1 (1.3–3.4) | 1.5 (0.9–2.6) | 1.8 (0.99–3.2) | 1.9 (1.04–3.6) | 1.9 (1.03–3.5) | 1.8 (0.98–3.2) |
| ASD with lower severity score | ||||||
| <18 | 1.1 (0.8–1.6) | 1.1 (0.7–1.6) | 1.3 (0.8–1.9) | 1.2 (0.8–1.8) | 1.2 (0.8–1.9) | 1.2 (0.8–1.9) |
| 18–59 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥60 | 1.6 (1.1–2.5) | 1.2 (0.7–1.9) | 1.3 (0.8–2.2) | 1.4 (0.8–2.4) | 1.4 (0.8–2.4) | 1.2 (0.7–2.1) |
ASD is autism spectrum disorder case group; ID is intellectual disability.
aOR is adjusted odds ratio, derived from logistic regression models in which the each ASD case sub-group is compared to the POP (general population) control group. All models include adjustment for child sex, maternal age at birth, maternal education at birth, and maternal race/ethnicity. Supplemental models additionally adjusted for various potential mediator factors as indicated.
DD was not significantly associated with either short or long IPI in the total sample or among term births (Table 4). Although sample sizes were small and estimates were imprecise, there was also no association evident among preterm births (data not shown). Addition of mediator factors to regression models did not impact the findings. Additionally, there was no association between IPI and any of the DD case subgroups examined (Table 5).
Table 4. Associations between Birth Spacing and Non-ASD Developmental Disabilities, Study to Explore Early Development (SEED), United States.
| Inter-pregnancy Interval (m) | Total Sample Unadjusted OR (95% CI) | Total Sample, Adjustment for sociodemographics aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics + maternal report of trying to get pregnant aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics + maternal infertility disorder aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics + prenatal hypertension, diabetes aOR† (95% CI) |
|---|---|---|---|---|---|---|
| Non-ASD DD (all) | ||||||
| <12 | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) | 0.9 (0.6 –1.4) | 0.9 (0.6–1.4) | 0.9 (0.6–1.4) | 0.8 (0.5–1.2) |
| 12–<18 | 0.9 (0.6–1.2) | 1.0 (0.7–1.4) | 1.1 (0.8–1.6) | 1.1 (0.7–1.5) | 1.1 (0.7–1.5) | 1.1 (0.7–1.6) |
| All <18 | 0.9 (0.7–1.2) | 1.0 (0.7–1.3) | 1.0 (0.8–1.4) | 1.0 (0.7–1.3) | 1.0 (0.7–1.3) | 0.9 (0.7–1.3) |
| 18–59 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥60 | 1.3 (0.9–1.8) | 1.1 (0.8–1.6) | 1.2 (0.8–1.8) | 1.3 (0.8–1.9) | 1.2 (0.8–1.8) | 1.1 (0.8–1.7) |
DD is other (non-ASD) developmental delay.
aOR is adjusted odds ratio, derived from logistic regression models in which the DD case group is compared to the POP (general population) control group. All models include adjustment for child sex, maternal age at birth, maternal education at birth, and maternal race/ethnicity. Supplemental models additionally adjusted for various potential mediator factors as indicated.
Table 5. Associations between Birth Spacing and Non-ASD Developmental Disabilities Subtypes, Study to Explore Early Development (SEED), United States.
| Inter-pregnancy Interval (m) | Total Sample Unadjusted OR (95% CI) | Total Sample, Adjustment for sociodemographics aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics + maternal report of trying to get pregnant aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics + maternal infertility disorder aOR† (95% CI) | Term Birth Sample, Adjustment for sociodemographics + prenatal hypertension, diabetes aOR† (95% CI) |
|---|---|---|---|---|---|---|
| DD with ID | ||||||
| <18 | 0.7 (0.4–1.1) | 0.7 (0.4–1.2) | 0.7 (0.4–1.3) | 0.7 (0.4–1.3) | 0.7 (0.4–1.3) | 0.6 (0.3–1.2) |
| 18–59 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥60 | 1.6 (0.98–2.5) | 1.1 (0.7–1.9) | 1.3 (0.7–2.4) | 1.4 (0.8–2.8) | 1.5 (0.8–2.8) | 1.2 (0.6–2.3) |
| DD w/outID | ||||||
| <18 | 1.0 (0.7–1.3) | 1.0 (0.8–1.4) | 1.1 (0.8–1.4) | 1.0 (0.8–1.5) | 1.0 (0.8–1.4) | 1.0 (0.7–1.3) |
| 18–59 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥60 | 1.1 (0.8–1.6) | 1.1 (0.7–1.5) | 1.2 (0.8–1.8) | 1.2 (0.8–1.9) | 1.2 (0.8–1.9) | 1.1 (0.7–1.7) |
| DD with autism symptoms | ||||||
| <18 | 1.0 (0.7–1.5) | 1.0 (0.6–1.5) | 1.0 (0.6–1.6) | 0.9 (0.5–1.4) | 0.9 (0.6–1.5) | 0.6 (0.3–1.2) |
| 18–59 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥60 | 1.5 (0.98–2.4) | 1.2 (0.7–2.0) | 1.3 (0.7–2.3) | 1.4 (0.7–2.6) | 1.4 (0.7–2.6) | 0.9 (0.4–2.1) |
| DD w/out autism symptoms | ||||||
| <18 | 0.9 (0.7–1.2) | 0.9 (0.7–1.3) | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) | 1.0 (0.70–1.3) |
| 18–59 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥60 | 1.2 (0.8–1.7) | 1.1 (0.8–1.6) | 1.2 (0.8–1.8) | 1.2 (0.8–1.9) | 1.2 (0.8–1.9) | 1.2 (0.8–1.8) |
DD is other (non-ASD) developmental delay; ID is intellectual disability.
aOR is adjusted odds ratio, derived from logistic regression models in which the each DD case sub-group is compared to the POP (general population) control group. All models include adjustment for child sex, maternal age at birth, maternal education at birth, and maternal race/ethnicity. Supplemental models additionally adjusted for various potential mediator factors as indicated.
The U-shaped association between ASD and IPI is more fully illustrated in the findings from the cubic spline analyses (Fig. 2). Likelihood ratio tests indicated that a restricted cubic spline model fit the data significantly better than the model without the spline for the total sample (P = 0.044, Fig. 2A), term birth sample (P = 0.005, Fig. 2B) and term birth sample with assessment of more severe ASD only (P = 0.011 Fig. 2C). The findings from the cubic spline model also suggest that the magnitude of the association between ASD and long IPI increases gradually after 59 months. Cubic spline models for the DD group did not fit the data better than models without cubic splines (P = 0.777 for total sample and P = 0.888 for term birth sample). The plots, which showed flat straight lines, indicated no association between DD and any level of IPI (Fig. 3).
Figure 2.
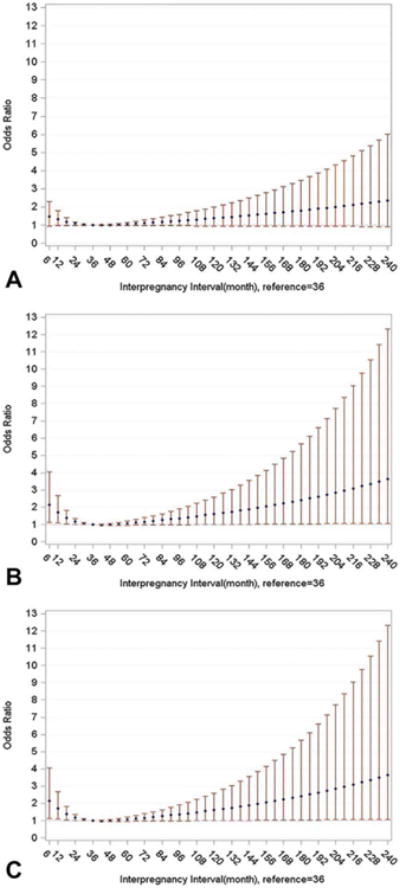
Adjusted odds ratios and 95% confidence intervals for association between autism spectrum disorder and inter-pregnancy-interval fitted with logistic regression with restricted cubic splines, knots = 3 (12, 18, 59), Study to Explore Early Development (SEED), United States. (A) Total sample, (B) Term births, (C) Term births and ASD cases limited to those with high ASD severity scores.
Figure 3.
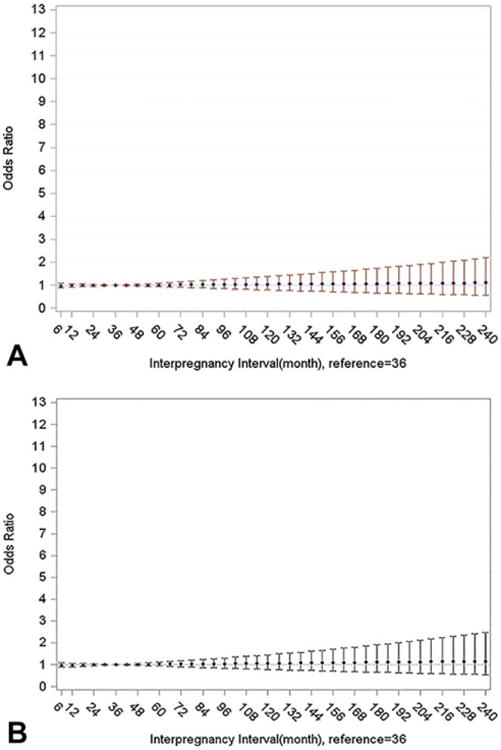
Adjusted odds ratios and 95% confidence intervals for association between non-ASD developmental disabilities and inter-pregnancy-interval fitted with logistic regression with restricted cubic splines, knots = 3 (12, 18, 59), Study to Explore Early Development (SEED), United States. (A) Total sample, (B) Term births.
Discussion
We found that in children born in 2nd or later term births, ASD was associated with pregnancy conception either <18 months or ≥60 months after the mother's preceding birth. Our cubic spline analyses confirmed this pattern of results and also suggested associations become stronger with IPI's beyond 60 months. We did not observe an association between ASD and IPI among preterm births, a finding we could not explore fully due to sample size constraints. However, one possible reason for this finding is that preterm birth posed a competing risk that masked associations with IPI.
While we observed similar findings for children with ASD plus ID and ASD without ID, we found that associations with both short and long IPI were primarily limited to ASD cases with a higher calibrated ADOS severity score, a measure of core ASD symptoms designed to limit influences from child demographics or non-ASD developmental outcomes, including ID [Wiggins et al., 2017]. Moreover, we found that non-ASD DD was not associated with either short or long IPI - overall, in term or preterm births, among subgroups with or without ID, or among subgroups with or without ASD symptoms reported. Altogether, these findings support the hypothesis that the ASD–IPI associations are unique to ASD rather than being reflective of a more general neurodevelopmental effect.
Our findings are consistent with several previous studies showing associations between ASD and short IPI [Cheslack-Postava et al., 2011; Dodds et al., 2011; Gunnes et al., 2013; Cheslack-Postava et al., 2014; Coo et al., 2015; Durkin et al., 2015; Zerbo et al., 2015] and the few studies that additionally demonstrated a U-shaped relationship between ASD and IPI [Cheslack-Postava et al., 2014; Coo et al., 2015; Durkin et al., 2015; Zerbo et al., 2015]. The findings from the few previous studies that assessed ASD diagnostic subtypes [Gunnes et al., 2013; Cheslack-Postava et al., 2014; Zerbo et al., 2015] and ASD with and without ID [Cheslack-Postava et al., 2014] were inconsistent. In the current study, we were able to examine phenotypic subtypes more fully. The developmental assessment data we collected allowed us to subdivide ASD cases based on the child's core ASD symptoms, rather than relying on a previous subtype diagnosis. As noted, this particular subdivision was more informative than that based on cognitive functioning.
There has been little study of other DDs and IPI overall and the few previous studies available must be considered cautiously, given methodological limitations. Thompson et al. [2003] examined a general developmental delay outcome, defined based on education eligibility data that encompassed a broad array of delay types and also included children deemed at risk for delay. They reported modest associations (most effect estimates <1.3) with low and high IPI. De Moura et al. [2010] reported an association between IPI <24 months and suspected developmental delay at two years in a study of an urban Brazilian population; however, the outcome was defined based on Battelle Screening Developmental Inventory which has not been validated in a Brazilian population and given the young age of the cohort, might have selectively identified the most severe cases of DD. Pinto-Martin, Cnaan, & Zhao, [1998] reported an association between very short IPI (<6 months) and disabling cerebral palsy. Of note, none of the aforementioned studies defined cases of disability in a way that specifically excluded ASD; thus, it is not possible to determine if associations observed were related to ASD, which often co-occurs with other disabilities [Boulet, Boyle, & Schieve, 2009].
To our knowledge, no previous study of IPI has examined ASD and non-ASD DD associations in the same study population. SEED provided an opportunity to compare ASD and non-ASD DDs in a systematic manner with an in-depth case classification. While our DD group was a heterogeneous mix of disabilities with a range of severity levels, the SEED protocol included screening of all children enrolled in the study for autism symptoms with the SCQ and follow-up of those who screened positive, such that even children without a previous ASD diagnosis, were classified as an ASD case if they met study criteria on the ADOS and ADI-R. Thus, our DD group is unique in that we intentionally excluded children meeting ASD criteria from it; moreover, data from various developmental assessments allowed us to sub-divide the DD group based on both ID and autism symptomatology.
Possible hypotheses that have been put forth to explain associations between ASD and short IPI include nutrition depletion from the previous pregnancy, particularly folic acid depletion, which plays a key role in neuronal development and DNA methylation and is vulnerable to depletion by the end of a pregnancy [Shachar & Lyell, 2012; Conde-Agudelo et al., 2012], lingering maternal inflammation from the previous pregnancy [Shachar & Lyell, 2012; Conde-Agudelo et al., 2016; Cheslack-Postava et al., 2014], maternal stress that might be more common in pregnancies after short IPI [Shachar & Lyell, 2012; Conde-Agudelo et al., 2016], an increased risk for maternal complications during pregnancies conceived after a short IPI [Cheslack-Postava et al., 2014], and unintended pregnancy and the consequent delays in various health behavior changes due to later recognition of pregnancy (e.g., smoking cessation, changes in medication use, initiation of folic acid supplementation) [Cheslack-Postava et al., 2014]. The trend toward delayed childbearing among U.S. women (i.e., first birth at age 35 or older) has also been associated with shorter IPI [Nabukera et al., 2009]; this phenomenon might represent an intentional desire for short pregnancy spacing and it might also occur among women who have difficulty conceiving their first birth due to an infertility disorder. The association between ASD and long IPI has been hypothesized to reflect an association between ASD and underlying sub-fertility [Conde-Agudelo et al., 2016]; additionally some of the adverse impact associated with long IPI might be due to unintended pregnancy [Cheslack-Postava et al., 2014].
Previous studies of ASD and IPI were limited in their examination of potential causal pathways. Only a few studies included some analyses of potential intermediary variables beyond preterm delivery, and those studies were themselves limited. Dodd et al. [2011] included adjustment of a binary maternal condition variable in their analyses that was broadly defined to include pulmonary disease, heart disease, renal disease, or anemia. Durkin et al. [2015] included adjustment for gestational diabetes, but their data were from birth certificates, a source with only moderate sensitivity for diabetes [Devlin, Desai, & Walaszek, 2009]. Zerbo et al. [2015] adjusted for maternal use of antidepressants and maternal body mass index. We had data linked to several potential causal pathways—pregnancy planning, infertility, and maternal pregnancy complications. Moreover, our data on pregnancy complications were more comprehensive than past studies and our data collection protocol, which included abstraction of prenatal care records for a subset of our sample, allowed us to validate maternal self-report data. Our findings from analyses that included potential mediator variables indicate that the associations we observed between ASD and both short and long IPI were not explained by unplanned pregnancy, maternal infertility disorders, or two common maternal antenatal complications, hypertension and diabetes.
Nonetheless, we lacked data to examine several other factors possibly related to the causal pathway, such as folic acid supplementation, folic acid levels early in pregnancy, and inflammatory markers in pregnancy. Given evidence linking ASD to variations in genetic pathways for folate metabolism [Frye, Slattery, & Quadros, 2017] and also immune function [Ansel, Rosenzweig, Zisman, Melamed, & Gesundheit, 2017], these two areas might be particularly important to examine in future studies. It is possible that certain pregnancies associated with ASD risk specifically are particularly vulnerable to the impacts of short pregnancy spacing on maternal nutritional stores and inflammation.
Limitations
This study must be interpreted in the context of limitations. Because SEED sought to include mother–child pairs from diverse population subgroups, children were recruited from multiple clinical and education sources and population-based controls were sampled from birth certificates. One drawback to this approach is that a number of families initially targeted for potential recruitment could not be located. It is possible many of these families were ineligible for inclusion since our a priori eligibility criteria required both current and birth residence in the study catchment area. Still, we cannot rule out some selection bias in our sample. However, we conducted a detailed analysis of characteristics of responders and non-responders in the one site with data on all non-responders and found that while certain demographic factors were associated with nonresponse—younger maternal age and lower maternal education—other perinatal factors, including birth order, were not (unpublished data). We adjusted analyses for maternal age and education and we initially assessed all associations within demographic strata. Additionally, we note that the proportions of short and long IPI among mother–child pairs in the SEED POP group are in line with expectations for US births with similar race-ethnicity and maternal education distributions [Gemmill & Lindberg, 2013; Copen, Thoma, & Kirmeyer, 2015].
Because we did not exclude children from the general population control group if they had evidence of disabilities, with the exception of those who met our ASD case definition, our associations could have been slightly attenuated, particularly for ASD and DD subgroups defined based on ID. However, we conducted sensitivity analyses in which we repeated ID subgroup analyses after excluding from the POP group children who had evidence of ID (2.8%) and the findings were very similar to those presented here (data not shown).
Strengths
Despite limitations, this study has a number of important strengths including more rigorous case-classification than previous studies, which relied on existing medical and administrative records. Indeed, a prior SEED analysis suggests that reliance on existing clinical diagnoses data for ASD case classification in young children is subject to misclassification as a result of both under- and over-ascertainment; 17% of children enrolled in SEED who had a previous clinical ASD diagnosis did not meet SEED ASD-case criteria based on the ADOS and ADI-R and 15% of children who met SEED ASD-case criteria did not have a previous diagnosis reported [Wiggins et al., 2015]. Also, as discussed, the inclusion of a separate DD group and the wealth of developmental data collected using research reliable methods allowed us to examine associations with ASD alongside associations with non-ASD DDs and to subdivide both case groups into more homogenous subgroups. The in-depth SEED data collection protocol, which encompassed maternal reproductive history and pregnancy complications, allowed us to assess several important potential mediator factors that other studies could not and allowed us to define IPI more appropriately than past studies by including time since last stillbirth. We note however, that this was not a driving factor for the associations observed in this study; we conducted a sensitivity analysis in which mother–child pairs with a previous stillbirth were excluded from our sample and observed comparable ASD–IPI associations as reported here (data not shown).
Conclusion
Our findings suggest ASD is increased in children born in second or later births after a short or long IPI. Additionally, the disparate findings between the ASD and DD groups and between ASD subgroups with high severity score versus lower severity score suggest that the underlying mechanism for the associations between ASD and birth spacing might be unique to autism rather than reflecting neurodevelopment effects more generally. Despite unanswered questions, these findings in concert with those from other studies can inform public health and clinical guidelines about optimal pregnancy spacing. Since pregnancy spacing is potentially modifiable, it is particularly important to more fully understand the underlying explanation for the ASD–IPI associations such that women can be fully informed of the potential risks associated with short and long IPIs.
Acknowledgments
The investigators gratefully acknowledge the families and children who participated in SEED and thank the project staff across sites for their hard work. We additionally thank Dr. Gnakub Norbert Soke for assistance with data analysis. This study was supported by six cooperative agreements from the Centers for Disease Control and Prevention: Cooperative Agreement Number U10DD000180, Colorado Department of Public Health; Cooperative Agreement Number U10DD000181, Kaiser Foundation Research Institute (CA); Cooperative Agreement Number U10DD000182, University of Pennsylvania; Cooperative Agreement Number U10DD000183, Johns Hopkins University; Cooperative Agreement Number U10DD000184, University of North Carolina at Chapel Hill; and Cooperative Agreement Number U10DD000498, Michigan State University.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of interest: The authors have no competing interests to declare.
References
- Ansel A, Rosenzweig JP, Zisman PD, Melamed M, Gesundheit B. Variation in gene expression in autism spectrum disorders: An extensive review of transcriptomic studies. Frontiers in Neuroscience. 2017;10:601. doi: 10.3389/fnins.2016.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appareddy S, Pryor J, Bailey B. Inter-pregnancy interval and adverse outcomes: Evidence for an additional risk in health disparate populations. Journal of Maternal-Fetal & Neonatal Medicine. 2016;30:2640–2644. doi: 10.1080/14767058.2016.1260115. https://doi.org/10.1080/14767058.2016.1260115. [DOI] [PubMed] [Google Scholar]
- Arndt TL, Stodgell CJ, Rodier PM. The teratology of autism. International Journal of Developmental Neuroscience. 2005;23:189–199. doi: 10.1016/j.ijdevneu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Bigelow CA, Bryant AS. Short interpregnancy intervals: An evidence-based guide for clinicians. Obstetrical Gynecological Survey. 2015;70:458–464. doi: 10.1097/OGX.0000000000000195. [DOI] [PubMed] [Google Scholar]
- Bilder D, Pinborough-Zimmerman J, Miller J, McMahon W. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics. 2009;123:1293–1300. doi: 10.1542/peds.2008-0927. [DOI] [PubMed] [Google Scholar]
- Blumberg SJ, Bramlett MD, Kogan MD, Schieve LA, Jones JR, Lu MC. Changes in prevalence of parent-reported autism spectrum disorder in school-aged U.S. children: 2007 to 2011-2012. National Health Statistics Report. 2013 Mar 20;65:1–11. [PubMed] [Google Scholar]
- Boulet SL, Boyle CA, Schieve LA. Health care use and health and functional impact of developmental disabilities among US children, 1997-2005. Archives of Pediatric & Adolescent Medicine. 2009;163:19–26. doi: 10.1001/archpediatrics.2008.506. [DOI] [PubMed] [Google Scholar]
- Cheslack-Postava K, Liu K, Bearman PS. Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics. 2011;127:246–253. doi: 10.1542/peds.2010-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheslack-Postava K, Suominen A, Jokiranta E, Lehti V, McKeague IW, Sourander A, Brown AS. Increased risk of autism spectrum disorders at short and long interpregnancy intervals in Finland. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53:1074–1081.e4. doi: 10.1016/j.jaac.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Braun KV, Bilder D, Charles J, Constantino JN, et al. Yeargin-Allsopp M. Prevalence and characteristics of autism spectrum disorder among children aged 8 years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. Morbidity and Mortality Weekly Report Surveillance Summary. 2016;65(No. SS-3):1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: A meta-analysis. JAMA. 2006;295:1809–1823. doi: 10.1001/jama.295.15.1809. [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A, Rosas-Bermúdez A, Castaño F, Norton MH. Effects of birth spacing on maternal, perinatal, infant, and child health: A systematic review of causal mechanisms. Studies in Family Planning. 2012;43:93–114. doi: 10.1111/j.1728-4465.2012.00308.x. [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A, Rosas-Bermudez A, Norton MH. Birth spacing and risk of autism and other neurodevelopmental disabilities: A systematic review. Pediatrics. 2016:137. doi: 10.1542/peds.2015-3482. [DOI] [PubMed] [Google Scholar]
- Coo H, Ouellette-Kuntz H, Lam YM, Brownell M, Flavin MP, Roos LL. The association between the interpregnancy interval and autism spectrum disorder in a Canadian cohort. Canadian Journal of Public Health. 2015;106:e36–e42. doi: 10.17269/CJPH.106.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copen CE, Thoma ME, Kirmeyer S. Interpregnancy intervals in the United States: Data from the birth certificate and the National Survey of Family Growth. National Vital Statistics Reports. 2015;64:1–11. [PubMed] [Google Scholar]
- de Moura DR, Costa JC, Santos IS, Barros AJ, Matijasevich A, Halpern R, et al. Barros FC. Risk factors for suspected developmental delay at age 2 years in a Brazilian birth cohort. Paediatric & Perinatal Epidemiology. 2010;24:211–221. doi: 10.1111/j.1365-3016.2010.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin HM, Desai J, Walaszek A. Reviewing performance of birth certificate and hospital discharge data to identify births complicated by maternal diabetes. Maternal & Child Health Journal. 2009;13:660–666. doi: 10.1007/s10995-008-0390-9. [DOI] [PubMed] [Google Scholar]
- Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. Journal of Autism & Developmental Disorders. 2011;41:891–902. doi: 10.1007/s10803-010-1114-8. [DOI] [PubMed] [Google Scholar]
- Durkin MS, DuBois LA, Maenner MJ. Inter-pregnancy intervals and the risk of autism spectrum disorder: Results of a population-based study. Journal of Autism & Developmental Disorders. 2015;45:2056–2066. doi: 10.1007/s10803-015-2368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Slattery JC, Quadros EV. Folate metabolism abnormalities in autism: Potential biomarkers. Biomarkers in Medicine. 2017 doi: 10.2217/bmm-2017-0109. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Gemmill A, Lindberg LD. Short interpregnancy intervals in the United States. Obstetrics & Gynecology. 2013;122:64–71. doi: 10.1097/AOG.0b013e3182955e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnes N, Surén P, Bresnahan M, Hornig M, Lie KK, Lipkin WI, et al. Susser ES. Interpregnancy interval and risk of autistic disorder. Epidemiology. 2013;24:906–912. doi: 10.1097/01.ede.0000434435.52506.f5. [DOI] [PubMed] [Google Scholar]
- Kim YS, Leventhal BL. Genetic epidemiology and insights into interactive genetic and environmental effects in autism spectrum disorders. Biological Psychiatry. 2015;77:66–74. doi: 10.1016/j.biopsych.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles JH. Autism spectrum disorders-A genetics review. Genetics in Medicine. 2011;13:278–294. doi: 10.1097/GIM.0b013e3181ff67ba. [DOI] [PubMed] [Google Scholar]
- Nabukera SK, Wingate MS, Salihu HM, Owen J, Swaminathan S, Alexander GR, Kirby RS. Pregnancy spacing among women delaying initiation of childbearing. Archives of Gynecology and Obstetrics. 2009;279:677–684. doi: 10.1007/s00404-008-0793-2. [DOI] [PubMed] [Google Scholar]
- Ornoy A, Weinstein-Fudim L, Ergaz Z. Genetic syndromes, maternal diseases and antenatal factors associated with autism spectrum disorders (ASD) Frontiers in Neuroscience. 2016;10:316. doi: 10.3389/fnins.2016.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Martin JA, Cnaan A, Zhao H. Short interpregnancy interval and the risk of disabling cerebral palsy in a low birth weight population. Journal of Pediatrics. 1998;132:818–821. doi: 10.1016/s0022-3476(98)70310-5. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Clayton HB, Durkin MS, Wingate MS, Drews-Botsch C. Comparison of perinatal risk factors associated with autism spectrum disorder (ASD), intellectual disability (ID), and co-occurring ASD and ID. Journal of Autism & Developmental Disorders. 2015;45:2361–2372. doi: 10.1007/s10803-015-2402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shachar BZ, Lyell DJ. Interpregnancy interval and obstetrical complications. Obstetrical & Gynecological Survey. 2012;67:584–596. doi: 10.1097/OGX.0b013e31826b2c3e. [DOI] [PubMed] [Google Scholar]
- Schendel DE, Diguiseppi C, Croen LA, Fallin MD, Reed PL, Schieve LA, et al. Miller L. The Study to Explore Early Development (SEED): A multisite epidemiologic study of autism by the Centers for Autism and Developmental Disabilities Research and Epidemiology (CADDRE) network. Journal of Autism & Developmental Disorders. 2012;42:2121–2140. doi: 10.1007/s10803-012-1461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma ME, Copen CE, Kirmeyer SE. Short inter-pregnancy intervals in 2014: Differences by maternal demographic characteristics. NCHS Data Brief. 2016;240:1–8. [PubMed] [Google Scholar]
- Thompson JR, Carter RL, Edwards AR, Roth J, Ariet M, Ross NL, Resnick MB. A population-based study of the effects of birth weight on early developmental delay or disability in children. American Journal Perinatology. 2003;20:321–332. doi: 10.1055/s-2003-42773. [DOI] [PubMed] [Google Scholar]
- Wiggins LD, Reynolds A, Rice CE, Moody EJ, Bernal P, Blaskey L, et al. Levy SE. Using standardized diagnostic instruments to classify children with autism in the study to explore early development. Journal of Autism & Developmental Disorders. 2015;45:1271–1280. doi: 10.1007/s10803-014-2287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins LD, Barger B, Moody E, Soke G, Pandey J, Levy S. Brief Report: The ADOS calibrated severity score best measures autism diagnostic symptom severity in pre-school children. Journal of Autism & Developmental Disorders. 2017 doi: 10.1007/s10803-017-3072-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O, Yoshida C, Gunderson EP, Dorward K, Croen LA. Interpregnancy interval and risk of autism spectrum disorders. Pediatrics. 2015;136:651–657. doi: 10.1542/peds.2015-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]


