Abstract
Affect sharing and prosocial motivation are integral parts of empathy that are conceptually and mechanistically distinct. We used a neurodegenerative disease (NDG) lesion model to examine the neural correlates of these two aspects of real-world empathic responding. The study enrolled 275 participants, including 44 healthy older controls and 231 patients diagnosed with one of five neurodegenerative diseases (75 Alzheimer's disease, 58 behavioral variant frontotemporal dementia (bvFTD), 42 semantic variant primary progressive aphasia (svPPA), 28 progressive supranuclear palsy, and 28 non-fluent variant primary progressive aphasia (nfvPPA). Informants completed the Revised Self-Monitoring Scale's Sensitivity to the Expressive Behavior of Others (RSMS-EX) subscale and the Interpersonal Reactivity Index's Empathic Concern (IRI-EC) subscale describing the typical empathic behavior of the participants in daily life. Using regression modeling of the voxel based morphometry of T1 brain scans prepared using SPM8 DARTEL-based preprocessing, we isolated the variance independently contributed by the affect sharing and the prosocial motivation elements of empathy as differentially measured by the two scales. We found that the affect sharing component uniquely correlated with volume in right > left medial and lateral temporal lobe structures, including the amygdala and insula, that support emotion recognition, emotion generation, and emotional awareness. Prosocial motivation, in contrast, involved structures such as the nucleus accumbens (NaCC), caudate head, and inferior frontal gyrus (IFG), which suggests that an individual must maintain the capacity to experience reward, to resolve ambiguity, and to inhibit their own emotional experience in order to effectively engage in spontaneous altruism as a component of their empathic response to others.
Keywords: Empathy, Affect sharing, Prosocial motivation, Lesion model, Voxel-based morphometry, Neurodegeneration
1. Introduction
Empathy comprises a complex set of socioemotional processes that engage and redirect individuals’ emotions and interpersonal behavior. Affect sharing and prosocial motivation are two integral parts of empathy (Decety and Jackson, 2006; Zaki and Ochsner, 2012), and both may reflect mechanistically distinct dimensions of what Fan et al. (2011) termed “affective-perceptual” empathy in their meta-analysis of empathy studies. Affect sharing involves vicariously taking on and resonating with the emotional state of another, while prosocial motivation involves the desire to engage in a helping behavior. Affect sharing, sometimes referred to as emotional contagion, involves the ability to sense others’ social signals and rapidly embody their emotional experience, and may occur without explicit awareness (Zaki and Ochsner, 2012). Inherently, this occurs during an in-person interaction. Prosocial motivation or the “desire to help”, on the other hand, can be experienced in relation to another whether or not they are physically present, and may not be reflected in actual helping behavior (Aydinli et al., 2014; Batson and Powell, 2003; Perugini et al., 2011). For example, one may experience a desire to help someone while reading about them or hearing about their situation from a third party, even if one does nothing more about it.
The neuroanatomical correlates of empathy have been widely studied in functional imaging studies of healthy participants, which have been particularly helpful in dissociating the neuroanatomic substrates of these distinct aspects of empathy. In an extensive review of the empathy-neuroscience literature, Zaki and Ochsner identified several frontal, temporal, and parietal correlates of affect sharing, including the inferior parietal lobule (IPL), temporparietal junction (TPJ), posterior superior temporal sulcus (pSTS), temporal pole, anterior insula, premotor cortex (PMC), posterior cingulate cortex (PCC), anterior cingulate cortex (ACC), and medial prefrontal cortex (mPFC). Fewer studies have examined the neural underpinnings of prosocial motivation, though some have examined positive behavioral outcomes (i.e., actual helping behavior) as a proxy for motivation. One fMRI study demonstrated that the degree to which individuals more frequently engaged in daily helping behaviors with both friends and strangers was correlated with activity in mPFC, dorsal ACC, nucleus accumbens (NaCC)/ caudate, and precuneus (Rameson et al., 2012).
Despite progress in identifying the functional anatomy of empathic subprocesses, task-based neuroimaging studies with healthy individuals still have a number of limitations. First, it is difficult to elicit real-world empathic responses in an MRI scanner in a laboratory setting. Second, neuroimaging studies reveal parts of the brain that are engaged during fMRI tasks, but do less to disentangle neural correlation from causation. Lesion studies, in contrast, yield information about the necessity and sufficiency of specific neural structures for the emotional behavior in question (Hillis, 2014).
Neurodegenerative disease (NDG) provides a patient lesion model for examining the structural correlates of empathy, as marked changes in empathic behavior are characteristic of many patients with NDG (Fernandez-Duque et al., 2010; Liu et al., 2004; Rankin et al., 2005; Snowden et al., 2001), and a number of studies have examined the structural correlates of empathic concern in NDG samples (Eslinger et al., 2011; Sollberger et al., 2014; Zahn et al., 2009). Rankin and colleagues (Rankin et al., 2006) performed a brain-behavior correlation analysis across NDG patients with a diverse range of atrophy patterns and found that empathy strongly corresponded to volume in predominantly right anteromedial temporal and inferior frontal structures. Reviews of the broader empathy and lesion literature further support the relationship between right frontotemporal regions and empathy, with ventromedial PFC, inferior frontal gyrus (IFG), orbitofrontal cortex (OFC), superior temporal gyrus (STG), right temporal pole, anterior insula, amygdala, and caudate appearing across multiple lesion studies (Hillis, 2014; Olson et al., 2007) However, in contrast, Dermody and colleagues found primarily left inferior frontoinsular correlates in NDG patients with Alzheimer's disease (AD) and behavioral variant frontotemporal dementia (bvFTD) (Dermody et al., 2016). Fewer structural correlation studies have been attempted in healthy samples, likely due to methodological issues arising from lack of variance in brain volume, but one such study also found primarily left subcortical structures to correlate with empathy (Banissy et al., 2012). The diversity of structural correlates in such studies suggests that empathy is likely a composite behavior dependent on multiple neurological processes; however, none of these lesion studies provides specific insight into the differential anatomic substrates of empathy's affect sharing or prosocial motivation elements.
Most of the behavioral empathy measures used in these studies were obtained via informant report of day-to-day behavior, rather than direct laboratory measurement of empathy. Direct task-based empathy tests are more neuroscientifically precise, but due to the artificiality of the laboratory setting, are less likely to provide a true reflection of an individual's natural empathic responses and spontaneous behavior, particularly when patients have cognitive deficits that compromise laboratory task-based paradigms (Hillis, 2014). On the other hand, observational and questionnaire-based studies, while more ecologically valid, typically fail to represent empathic subcomponents with precision, because these are emotionally, behaviorally, and functionally intertwined and thus difficult to parse apart in real-life behavior. (Cliffordson, 2002; Decety et al., 2004; Rankin et al., 2005).
While observational studies of real-life empathic responding in NDG patients remain methodologically challenging and thus have typically been used to describe empathy only in very broad behavioral terms, we chose to utilize this approach for the current study because it may yet be capable of providing new information about the structural anatomy underlying specific subcomponents of empathy. We hypothesized that two aspects of empathy, affect sharing and prosocial motivation, were dissociable, and that even as measured in the overt behavior of patients with focal neurologic damage, these two aspects of empathy would correspond to discrete neurologic circuits. Specifically, we hypothesized that diminished affect sharing would correspond to volume loss predominantly in temporal lobe cortical and subcortical structures known to mediate person perception, social cue reading, and emotional experience (Olson et al., 2007; Ross and Olson, 2010; Zahn et al., 2009). Decreases in prosocial motivation or the “desire to help” were hypothesized to correspond with volume loss in medial frontal structures involved in behavioral motivation (Holroyd and Yeung, 2012; Kouneiher et al., 2009; Kringelbach, 2005) and frontal-subcortical structures involved in reward processing (Cardinal et al., 2002; Shany-Ur et al., 2011; Tekin and Cummings, 2002). For this study, our primary goal was to identify brain-behavior relationships representing fundamental neurologic mechanisms generalizable to normal human empathic functioning. While we used a neurodegenerative disease lesion model to test our hypotheses, characterization of the empathy deficits in these clinical groups with neurodegenerative disease has already been well described (Fernandez-Duque et al., 2010; Liu et al., 2004; Rankin et al., 2005; Snowden et al., 2001) and was not our main focus. In order to test our hypotheses and delineate the discrete anatomy underlying affect sharing and prosocial motivation, we used multiple overlapping but divergent measures of empathy, obtained as concurrent informant reports of neurodegenerative disease patients’ real-world empathic functioning, and employed an analytic approach designed to maximize the convergence and divergence of these empathy measures in the context of individual differences in regional brain structure on MRI across our whole group of patients.
2. Methods
2.1. Participants
Two hundred and seventy five subjects participated in the study, including 44 healthy older control subjects and 231 patients diagnosed with one of five neurodegenerative diseases: 75 patients met NINDS-ADRDA criteria for Alzheimer's disease (McKhann et al., 2011), 58 were diagnosed with behavioral variant FTD (Rascovsky et al., 2011), 42 were diagnosed with semantic variant PPA, 28 were diagnosed with PSP (Boxer et al., 2006; Litvan et al., 2006), and 28 were diagnosed with non-fluent variant PPA (Gorno-Tempini et al., 2011). Each study participant had an informant who was a family member or long-term friend who completed a series of questionnaires about the participant, thus, an additional 275 informants participated.
Patients’ diagnoses were determined by a team of neurologists, neuropsychologists, and nurses based on a thorough neurological, behavioral, neuropsychological, and neuroimaging assessments. Patients with severe language comprehension impairment or those with behavioral deficits, such as severe perseverative responses that clearly affected validity of their testing were excluded. Patients with severe cognitive impairments were excluded as determined by a Mini Mental State Examination (MMSE) score below 11 (out of 30) and a Clinical Dementia Rating (CDR) above 2 (out of 3). Patients were recruited to the research program through our memory clinic or referrals from external clinics. Control subjects were recruited through recruitment talks and local advertisements, and for inclusion were required to have an unremarkable neurological exam and MRI scan, and no functional or cognitive deficits. The study was approved by the Committee on Human Research at the University of California, San Francisco and all participants consented to participate. Demographic characteristics are presented in Table 1.
Table 1.
Participant demographic and behavioral data by diagnostic group.
| NC | bvFTD | nfvPPA | svPPA | AD | PSP | Test Statistics | p-value | Effect Size (η2) | |
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | (n=44) | (n=58) | (n=28) | (n=42) | (n=75) | (n=28) | |||
| Age | 68.7 | 60.8** | 66.1 | 63.7* | 61.3** | 66.9 | F (5, 268)=6.18 | p < 0.0001 | |
| (6.47) | (7.58) | (8.54) | (7.13) | (8.42) | (6.18) | ||||
| Male/Female | 15/29 | 39/19 | 11/17 | 21/21 | 36/33 | 12/16 | χ2=(5, N=268)=13.34 | p < 0.05 | |
| Education | 17.2 | 16.4 | 16.7 | 16.3 | 15.9 | 15.4 | F (5, 261)=1.65 | n.s. | |
| (2.30) | (2.89) | (3.56) | (2.65) | (3.04) | (2.73) | ||||
| CDR | 0.0 | 1.3** | 0.5** | 0.7** | 0.8** | 0.7** | F (5, 264)=49.66 | p < 0.0001 | |
| (0.00) | (0.68) | (0.32) | (0.37) | (0.33) | (0.29) | ||||
| CDR (SOB) | 0.0 | 7.1** | 2.1* | 4.3** | 4.6** | 4.4** | F (5, 263)=50.72 | p < 0.0001 | |
| (0.08) | (3.22) | (1.57) | (2.50) | (2.20) | (2.24) | ||||
| MMSE | 29.3 | 23.8** | 22.8** | 22.8** | 20.7** | 26.2 | F (5, 268)=16.92 | p < 0.0001 | |
| (0.86) | (4.42) | (7.25) | (7.04) | (5.7) | (2.54) | ||||
| Magnet strength (3 T/1.5 T/4 T) | 58/15/2 | 43/12/3 | 43/1/0 | 24/3/1 | 24/4/0 | 33/9/0 | χ2=(10, N=268)=13.34 | p < 0.05 | |
| GDS | 2.7 | 8.1* | 6.0 | 8.8* | 6.2 | 12.9** | F (5, 224)=9.89 | p < 0.0001 | |
| (3.30) | (5.87) | (3.93) | (5.91) | (4.77) | (6.65) | ||||
| IRI-EC | 27.3 | 18.1** | 24.6 | 20.9** | 27.0 | 24.7 | F (5, 267)=14.41 | p < 0.0001 | 0.2853 |
| (4.54) | (6.26) | (5.95) | (7.03) | (5.17) | (6.91) | ||||
| RSMS-EX | 32.9 | 20.1** | 29.0 | 22.4** | 27.3* | 25.1** | F (5, 268)=14.68 | p < 0.0001 | 0.3071 |
| (4.29) | (6.35) | (7.20) | (8.16) | (6.79) | (6.53) |
bvFTD=behavioral variant frontotemporal dementia; nfvPPA=nonfluent variant primary progressive aphasia; svPPA=semantic variant primary progressive aphasia; AD=alzheimer's disease; PSP=progressive supranuclear palsy; MMSE=Mini-Mental State Examination (out of 30 points); CDR=clinical dementia rating (0–3 range, with 0 indicating no impairment); CDR (SOB)=clinical dementia rating (some of boxes) (out of 12 points); GDS=Geriatric Depression Scale (out of 30 points); IRI-EC=Interpersonal Reactivity Scale – Empathic Concern subscale (out of 35 points); RSMS-EX=Revised Self-Monitoring Scale – Expressive Behavior subscale (out of 28 points).
Differed from controls at p < 0.05.
Differed from controls at p < 0.01.
2.2. Procedures
2.2.1. Measures
2.2.1.1. Revised Self-Monitoring Scale
The Revised Self-Monitoring Scale (RSMS) is a 13 item questionnaire, comprised of two subscales and completed by the participant's informant. The RSMS – Sensitivity to Expressive Behavior subscale (RSMS-EX) used for this study measures the ability to read and accurately interpret subtle nonverbal social cues (Cliffordson, 2002; Lennox and Wolfe, 1984). Sample items include, “The patient can usually tell when others consider a joke in bad taste, even though they may laugh convincingly,” and “The patient is often able to correctly read people's true emotions through their eyes.” Broadly speaking, the RSMS-EX is a measure of rapid “social intuition”, which can occur without deliberate reasoning, and the items are worded to refer to empathic sensitivity during in-person, face-to-face interactions. The RSMS-EX is comprised of 7 questions; each question is on a 6 point, Likert scale ranging from “certainly, always false” to “certainly, always true.”
2.2.1.2. Interpersonal Reactivity Index – empathic concern
The Interpersonal Reactivity Index (IRI) is a questionnaire completed by the participant's informant to measure their tendency to show emotional empathy (Davis, 1983). The empathic concern subscale (IRI-EC) used for this study measures the extent to which an individual experiences feelings of concern and sympathy for others. Some of the items are worded explicitly to measure the desire to help (e.g. “When the patient sees someone being taken advantage of, they feel protective towards them”), and it predicts helping behavior and prosocial tendencies (Batson et al., 1986), suggesting prosocial motivation is one aspect of the construct being measured by this scale (Hawk et al., 2013). The items on the IRI-EC do not assume that the empathic response is derived from face-to-face interactions, but are ambiguously worded such that scores on the measure might equally reflect empathic concern after indirectly hearing about another's emotional need, e.g., reading about it or hearing it described, as much as through direct in-person observation (e.g., “The patient often has tender, concerned feelings for people less fortunate than them”). Items do not assume that the individual successfully selects and engages in specific helping behaviors, but explicitly calls for ratings to be made based on the individual's expressed desire to engage with the other's perceived need. It consists of 7 items and answered using a 5-point, Likert scale ranging from ‘Does not describe him/her well’ to ‘Describes him/her very well.’.
2.2.1.3. Derived measures of affect sharing and prosocial motivation
Because the psychological constructs of affect sharing and prosocial motivation are not discretely isolated in the real-world empathic traits measured by these questionnaires, we derived measures of these constructs by conducting a regression analysis attempting to better isolate the independent contributions of each measure to dissociate the neural correlates that are unique to prosocial motivation and affect sharing. While the IRI-EC and RSMS-EX likely share overlapping elements of empathy, such as accurately determining the other's “emotional need”, the IRI-EC also contains items measuring desire to help (i.e., prosocial motivation), while the RSMS-EX does not. Thus, by removing the shared variance of RSMS-EX from IRI-EC, we are left only with variance in the IRI-EC that is associated with these higher order motivational elements that are not measured at all by the RSMS-EX. Conversely, because the RSMS-EX explicitly measures in-person awareness of subtle social cues, while the IRI-EC does not directly measure this (Davis, 1983; Cliffordson, 2002). By removing the shared variance of IRI-EC from RSMS-EX, higher order aspects of empathic cognition are removed. Thus, we are left primarily with variance associated with direct affect sharing as a result of social cue reading, which is not explicitly measured by the IRI-EC. These statistical dissociation analyses were performed via VBM analysis, with behavior measure as primary predictor, and age, gender, MMSE and TIV as confounds.
2.2.1.4. Other cognitive/behavioral tests
An extensive neuropsychological battery was administered to all participants to support their diagnostic characterization, details for this battery are described elsewhere (Rosen et al., 2002). For this study we included MMSE, CDR, Clinical Dementia Rating – Sum of Boxes (CDR-SOB), and Geriatric Depression Scale (GDS) for patient characterization and control of confounding.
2.2.1.5. Neuroimaging acquisition and VBM preprocessing
Participants underwent a 3-T, 1.5-T, or 4-T research quality structural MRI within 90 days of completing the IRI-EC and RSMS-EX. Details of the MRI acquisition are described elsewhere (Shany-Ur et al., 2014; Sturm et al., 2013). Preprocessing involved segmenting the T1-structural images into gray matter, white matter, and cerebrospinal fluid and spatially normalizing them into MNI space using statistical parametric mapping (SPM8) (Friston et al., 2007). To preprocess these images, the SPM8 software package and the VBM8 toolbox running on MATLAB was used. To optimize intersubject registration, each participant's image was warped to a template created from 150 healthy older control participants using the Diffeomorphic Anatomical Registration through Exponentiated Lie algebra (DARTEL) toolbox (Ashburner, 2007). Spatially normalized, segmented, and modulated gray matter images were smoothed using an 8-mm FWHM isotropic Gausian kernel. Otherwise, the standard preprocessing SPM8 approach was used, as described elsewhere (Shany-Ur et al., 2014; Sturm et al., 2013).
2.2.2. Statistical analysis
2.2.2.1. Behavioral data
Potential covariates such as age, gender, education, depression (GDS), and disease severity (MMSE, CDR, and CDR-SOB) were assessed using SAS proc glm. Diagnostic group differences for IRI-EC and RSMS-EX scores were analyzed using SAS proc glm controlling for age, gender, and MMSE. Post-hoc Dunnett and Dunnett-Hsu tests were used to identify pairwise diagnostic group differences in each variable compared to controls (Table 1).
2.2.2.2. VBM analyses
All VBM analyses included age, gender, MMSE, magnet strength, and total intracranial volume (TIV) as covariates in each design matrix, correlating behavioral score with smoothed gray matter volume using a one-tailed, t-contrast. First, the main effects of RSMS-EX and IRI-EC were derived using separate design matrixes, then each of these variables was analyzed as a predictor of interest controlling for the other test. To identify the neural correlates of affect sharing, the IRI-EC score was added as a confound to the design matrix when RSMS-EX score was the primary predictor. To identify the neural correlates of prosocial motivation, the RSMS-EX score was added as a confound when IRI-EC score was the predictor. Only results surviving a family-wise-error threshold of p < 0.05 with a cluster size > 100 mm3 were considered significant.
Because different dementias included in this sample are associated with diverse patterns of brain atrophy, we conducted a set of secondary error checks to determine whether any brain-behavior relationships seen in these analyses were only seen in a single diagnostic group and did not generalize across the sample. First, we examined scatterplots of patients’ covariates-adjusted RSMS_EX and IRI_EC scores versus their gray matter volume at the peak voxel for the 3 regions with the highest T-values, grouping the plot by diagnosis, in order to confirm adequate variance on the measures within each diagnostic group. Second, we performed an additional VBM analysis (coatrophy error check) in which each diagnostic group was parameterized and added to the model (+5 predictors for 6 levels of diagnosis), essentially factoring out any brain-behavior relationship that only appears within one diagnostic group. A detailed rationale for this procedure is described elsewhere (Sollberger et al., 2012).
3. Results
3.1. Behavioral results
An analysis of variance, using SAS proc glm and a Dunnett-Hsu post hoc test revealed significant group differences in age, CDR, CDR-SOB, MMSE, and GDS (p <0.0001) when diagnostic group means were compared to controls (Table 1). The mean CDR was less than 1.3 across all patient groups, indicating that patients were at an early point in disease progression. Gender also significantly differed between patients and the control group (p < 0.05) and thus was included in subsequent models. Education did not significantly differ between patient and control groups and thus was not included in the models.
A general linear model controlling for age, gender, and MMSE revealed diagnostic group differences in both IRI-EC and RSMS-EX. IRI-EC score was significantly lower in patients with bvFTD (p < 0.0001) and svPPA (p < 0.0001) compared to controls. RSMS-EX was significantly worse in all patient groups, except the svPPA group, as compared to controls (bvFTD, nfvPPA, and PSP p < 0.0001, AD p < 0.005) (Table 1).
3.2. Neuroimaging results
3.2.1. RSMS-EX: sensitivity to expressive behavior of others
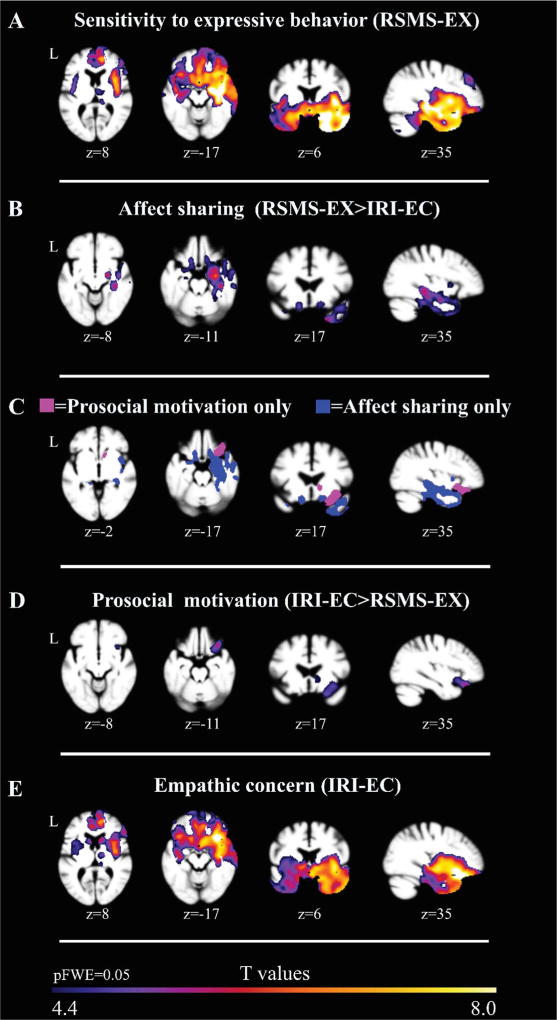
RSMS-EX was significantly correlated (pFWE < 0.05) with right greater than left gray matter volumes in the medial and lateral temporal lobes as well as in subcortical, frontal, and cerebellar regions. Subcortical structures that most strongly correlated with RSMS-EX included the amygdala, thalamus, and basal ganglia structures (caudate, putamen, and globus pallidus.) Structures throughout the medial and lateral temporal lobes were significant, including the temporal pole, entorhinal/ parahippocampal areas, as well as the superior, medial and inferior temporal gyri. Frontal regions that most strongly correlated with RSMS-EX included the mPFC and orbitofrontal gyri, medial cingulate structures as well as lateral frontal gyri such as the superior, middle, opercular, and triangular parts of the IFG (Fig. 1A, Table 2, Supplementary Table 1).
Fig. 1.
Patterns of gray matter volume in neurodegenerative disease patients and healthy older controls (N=275) corresponding to measures of empathy, adjusting for age, gender, MMSE (as a proxy for disease severity), total intracranial volume, and magnet strength. Images were overlaid on an average brain based on a template used for DARTEL warping derived from gray matter images of N=150 healthy older controls. All results shown in A–E are significant at pFWE < 0.05, corresponding to a critical T-threshold of 4.4. A: Regions where RSMS-EX scale score corresponds with brain volume. B: Regions where RSMS-EX scale score corresponds with brain volume after removing effects related to the IRI-EC scale score. C: Regions corresponding to B and D represented in direct contrast. D. Regions where IRI-EC scale score corresponds with brain volume after removing effects related to the RSMS-EX scale score. E. Regions where IRI-EC scale score corresponds with brain volume.
Table 2.
Differential anatomical correlates of affect sharing and prosocial motivation.
| Anatomic region and structure
|
Right Lateralized
|
Left Lateralized
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Specific to Affect Sharing (AS) | Evident in both (AS+PM) | Specific to Prosocial Motivation (PM) | x | y | z | MaxT | x | y | z | MaxT |
| Subcortical/striatal | ||||||||||
| Caudate | 10 | 13 | −3 | 4.68 | −6 | 10 | 1 | 4.43 | ||
| Amygdala | 21 | −10 | −12 | 6.47* | −25 | −2 | −18 | 4.53 | ||
| Hippocampus | 28 | −8 | −19 | 6.17* | −12 | −37 | 1 | 4.71 | ||
| Pallidum | 24 | −8 | −7 | 5.88* | NS | |||||
| Putamen (AS) | 30 | −10 | −10 | 5.29* | NS | |||||
| Putamen (PM) | 30 | −8 | −3 | 4.51* | NS | |||||
| Thalamus Proper | 25 | −29 | −3 | 4.70* | −13 | −35 | 0 | 4.82 | ||
| Nucleus accumbens | 9 | 15 | −4 | 4.6 | NS | |||||
| Temporal | ||||||||||
| Temporal pole | 21 | 10 | −34 | 6.19* | −27 | 9 | −28 | 4.6 | ||
| Entorhinal area | 28 | 1 | −18 | 5.98* | −27 | 3 | −21 | 4.94 | ||
| Inferior temporal gyrus | 27 | 3 | −40 | 5.77* | NS | |||||
| Fusiform gyrus | 39 | −23 | −21 | 5.72* | −27 | −31 | −24 | 4.46 | ||
| Middle temporal gyrus | 57 | 6 | −22 | 5.64* | NS | |||||
| Planum polare | 42 | −10 | −9 | 5.48* | NS | |||||
| Posterior insula | 40 | −10 | −7 | 5.36* | NS | |||||
| Parahippocampal gyrus | 27 | −31 | −19 | 5.33* | −15 | −35 | −1 | 4.75 | ||
| Superior temporal gyrus | 52 | 4 | −21 | 5.12 | NS | |||||
| Anterior insula (AS) | 30 | 7 | −16 | 5.00* | NS | |||||
| Anterior insula (PM) | 30 | 19 | −13 | 5.08 | NS | |||||
| Parietal | ||||||||||
| Posterior cingulate cortex | NS | −13 | −37 | 0 | 4.7 | |||||
| Frontal | ||||||||||
| Basal Forebrain | 28 | 4 | −16 | 5.70* | −12 | 6 | −18 | 5.01 | ||
| Lateral orbital gyrus | 39 | 30 | −18 | 5.14* | NS | |||||
| Medial orbital gyrus (AS) | 18 | 7 | −21 | 5.33 | −13 | 16 | −18 | 5.07 | ||
| Medial orbital gyrus (PM) | 19 | 21 | −21 | 4.88 | NS | |||||
| Posterior orbital gyrus (AS) | 22 | 7 | −18 | 5.18 | −25 | 9 | −25 | 4.97 | ||
| Posterior orbital gyrus (PM) | 33 | 27 | −18 | 5.73 | NS | |||||
| Subcallosal area (AM) | 10 | 18 | −16 | 4.7 | −12 | 15 | −18 | 5.07 | ||
| Subcallosal area (PS) | 6 | 10 | −18 | 4.46 | NS | |||||
| Orbital part of the inferior frontal gyrus | 37 | 22 | −10 | 4.5 | NS | |||||
| Gyrus rectus (AM) | 10 | 21 | −16 | 4.625 | −13 | 19 | −16 | 4.91 | ||
| Gyrus rectus (PS) | 4 | 22 | −24 | 4.72 | NS | |||||
| Cerebellum | ||||||||||
| Cerebellum | 34 | −34 | −31 | 4.84* | −27 | −31 | −28 | 5.00* | ||
Table Legend: Specific to affect sharing: regions where affect sharing correlated significantly with gray matter volumes, adjusting for prosocial motivation, age, gender, MMSE, total intracranial volume, and magnet strength. Evident in both: regions where both affect sharing and prosocial motivation correlated with gray matter volumes, adjusting for age, gender, MMSE, total intracranial volume, and magnet strength. Specific to prosocial motivation: regions where prosocial motivation correlated significantly with gray matter volumes, adjusting for affect sharing, age, gender, MMSE, total intracranial volume, and magnet strength. All results shown in this table are corrected for family-wise error [FWE] across the whole brain at a significance level of p < 0.05. Locations of peaks are reported in MNI reference space.
Regions remaining significant after the coatrophy error check including parameterized diagnostic group in the model. NS=Not significant at pFWE < 0.05.
3.2.2. IRI-EC: empathic concern
IRI-EC was significantly correlated with bilateral gray matter volumes in the medial and lateral temporal lobes as well as in subcortical, frontal, and cerebellar regions (pFWE < 0.05). Subcortical structures that most strongly correlated with IRI-EC included the caudate, putamen and NaCC. Temporal structures that most strongly correlated with IRI-EC included the anterior insula, posterior insula, and middle temporal gyrus. Frontal structures that most strongly correlated with IRI-EC included the orbital gyrii including the posterior, medial, and lateral orbital gyrus (Fig. 1E, Table 2, Supplementary Table 2).
3.2.3. Affect sharing – RSMS-EX removing contributions from IRI-EC
Regions uniquely mediating affect sharing after family-wise error correction (pFWE < 0.05) included the left medial and lateral temporal lobes (entorhinal area, fusiform gyrus, and planum polare, temporal pole, inferior temporal gyrus, middle temporal gyrus), bilateral basal forebrain, right posterior cingulate, bilateral cerebellar cortex as well as subcortical regions (amygdala, hippocampus, pallidum, putamen, and thalamus) (Fig. 1B, Table 2, Supplementary Table 1). When error check analyses were performed to identify whether these brain-behavior relationships generalized across more than one diagnostic group, left-sided regions dropped out (i.e., were contributed predominantly by a single diagnostic group) but almost all right temporal and subcortical regions remained significant (i.e., the same brain-behavior relationship was found in multiple diagnostic groups).
3.2.4. Prosocial motivation – IRI-EC removing contributions from RSMS-EX
Prosocial motivation was correlated with gray matter volume in the right medial temporal lobe, the right greater than left subcortical structures, and the right frontal regions, including the right anterior insula, right greater than left caudate, right NaCC, right putamen, lateral and medial orbital gyri, and lateral and medial parts of the medial prefrontal cortex (Fig. 1D, Table 2, Supplementary Table 2). All results survived family-wise error correction (pFWE < 0.05). The right lateral orbital gyrus remained significant in coatrophy error check when removing results appearing predominantly within a single diagnostic group.
4. Discussion
Our voxel-based morphometry analysis showed that affect sharing was associated with predominantly right temporal regions involved in emotion recognition, emotion generation, and awareness of one's own emotional state. Prosocial motivation, in contrast, involved frontal and subcortical structures associated with social reward, decision making, emotion regulation, and behavioral inhibition. This dissociation in sub-elements of emotional empathy was identifiable despite substantial overlap of regions associated with the original empathy measures.
4.1. Structural anatomy underlying affect sharing
Affect sharing, a key element of empathy, involves recognizing and interpreting another's emotion, followed by the visceral generation of an emotional response in reaction to the other's emotional state, and awareness of the newly generated emotion at a basic neurological level (De Vignemont and Singer, 2006; Singer and Lamm, 2009). These elements were reflected in the structural anatomic correlates in our study.
4.1.1. Emotion recognition
We found a linear relationship between affect sharing and gray matter volume in many medial and lateral temporal regions (medial: bilateral temporal poles and fusiform gyrus, lateral: predominately right lateral temporal gyri [STG, MTG, and ITG]) as well as in nearby subcortical and limbic structures (parahippocampal gyrus and entorhinal area). These structures are important for a variety of processes involved in socioemotional perception and low-level interpretation of social cues, such as reading emotional and non-emotional facial expressions (Kumfor and Piguet, 2012; Rosen et al., 2006; Snowden et al., 2001; Snowden et al., 2004) comprehending vocal prosody (Ethofer et al., 2006), interpreting emotion and intent from body position and gestures (Olson et al., 2007), theory of mind (Irish et al., 2014; Shamay-Tsoory and Aharon-Peretz, 2007), and reading paralinguistic cues conveying insincere communication such as sarcasm and deception (Ethofer et al., 2006; Olson et al., 2007; Rankin et al., 2009; Winston et al., 2002). Furthermore, these regions are critical for social concepts, socioemotional semantic knowledge and memory (Olson et al., 2007; Shamay-Tsoory, 2011; Zahn et al., 2009). Accurately decoding subtle social stimuli likely contributes to affect sharing by providing a more precise and detailed representation of another's emotional experience. This more complete representation facilitates and enhances the internal reproduction of that experience. These findings support the role of these temporal emotion-reading regions in mediating the degree to which participants engage in accurate affect sharing. Because a correlational lesion model was used in this study, the causal directionality between emotion reading and affect sharing remains ambiguous; in an alternate interpretation, better attunement may also facilitate detection and accurate reading of emotions in our patients by increasing attention and engagement (Sened et al., 2016).
4.1.2. Emotion generation
In addition to recognizing another's emotional state, affect sharing, by definition, involves internally generating the other's emotion. The association we found between the amygdala and affect sharing is consistent with studies that demonstrate the role of the amygdala in emotion generation. Emotions are generated in both top-down and bottom-up processes, and the amygdala participates in both (Ochsner et al., 2009). In the top-down mechanism, emotions are generated after interpreting socioemotional stimuli such as speech or facial expressions. In the bottom-up mechanism, emotions are elicited in response to internal physiological states of which the individual may or may not be conscious (McRae et al., 2010). The relationship between amygdala volume and affect sharing may result from the specific role of the amygdala in generating emotional states, either on the basis of external (top-down) or internal (bottom-up) stimuli. This association with amygdala volume was not seen with prosocial motivation, in contrast, which suggests that while generating emotion may be an essential part of affect sharing, it may not be critical for engendering a desire to help.
4.1.3. Subjective awareness of emotional experience
In addition to regions associated with emotion recognition and generation, affect sharing involves structures associated with awareness of one's own emotional state (Singer et al., 2004), which was reflected in our finding that capacity for affect sharing correlated with insula volume. The insula integrates somatosensory information, environmental stimuli, hedonic conditions, and motivational, social and cognitive conditions, which allows it to play an important role in maintaining subjective awareness of emotional state (Craig, 2002, 2003). Greater capacity for emotional self-awareness, in turn, may enhance affective resonance. The insula is anatomically organized in such a way that the posterior insula receives primary interorceptive and somatic information, while increasingly complex, top-down information about motivational, social and cognitive conditions is progressively integrated over a posterior to anterior gradient (Craig, 2009). Therefore, our finding that affect sharing correlates with preserved volume both in the right posterior and anterior insula suggests that effective affect sharing involves the integration of basic somatosensory awareness with complex cognitive information derived from higher-order executive, semantic, and other social contextual processes.
The degree to which awareness of basic and higher order emotional information is integrated may lend greater accuracy to shared affective resonance, leading to more appropriate downstream empathic behaviors. These findings align well with results from previous studies which report the role of the insula in affect sharing (Hadjikhani et al., 2014; Lerner et al., 2016). For instance, bilateral anterior insula activation occurs when healthy participants view others drinking a disgust inducing beverage (Jabbi et al., 2007) suggesting that insula plays a role in sharing the experience of another's bodily and emotional state. Studies of intrinsic functional connectivity in healthy individuals also show that those with higher self-reported affective empathy have stronger functional connectivity across socioemotional regions including the anterior insula (Cox et al., 2012). Other lesion studies, including NDG models, have also shown that the insula is a key structure for affect sharing (Leigh et al., 2013). Gu and colleagues found that patients with anterior insula damage had impaired affect sharing in the context of implicit and explicit perception of others pain (Gu et al., 2012). The insula may also contribute to affect sharing via its role in self-awareness (Craig, 2009). Emotional self-awareness can also up-regulate emotions through a resonance loop in which the insula mediates awareness of one's emotional state, and can promote and amplify the generation of that emotional state by the anterior cingulate (Critchley, 2005). Thus, by reducing emotional self-awareness, insular atrophy may also decrease this synergistic cingulate-insula resonance and limit emotional contagion.
4.2. Structural anatomy underlying prosocial motivation
Prosocial motivation is the desire to engage in an altruistic helping behavior, a precursor to empathic behavior (Eisenberg and Miller, 1987; Penner et al., 2005; Weinstein and Ryan, 2010). In our study, this empathic desire to help was related to structures involved in reward, social decisionmaking, and emotion regulation.
4.2.1. Social reward
We found that prosocial motivation had a linear relationship with volume in striatal structures that are involved in processing rewards (caudate and NaCC). Functional imaging studies show that engaging in prosocial interpersonal behaviors activates reward circuitry, suggesting that these striatal structures are also sensitive to social rewards. For instance, learning that one has a good reputation among others, results significantly increased activity in the caudate and putamen (Izuma et al., 2008). Furthermore, fMRI studies demonstrate that the ventral striatum activates when participants decide to engage in prosocial behaviors, such as donating money to charity to help others (Báez-Mendoza and Schultz, 2013; Harbaugh et al., 2007; Moll et al., 2006). These studies reveal a causal relationship in which the social reward was experienced as a result of being liked or simply of producing a helping behavior, and this mechanistic understanding of the role of reward in prosocial behaviors is widely held (Fehr and Fischbacher, 2003).
However, our lesion data suggests that preservation of volume in the NaCC and caudate may also be a prerequisite to being characteristically motivated to help another. If individuals only experience reward in response to their prosocial acts, then we would expect some of the patients in our study with early neurodegeneration of the caudate and NaCC to still be habitually engaging in prosocial behaviors in an attempt to derive previously experienced social rewards based on learned behavioral associations, even if their neural damage had recently reduced or eliminated their experience of that reward. Yet we found a strong linear relationship between prosocial motivation and these reward structures, meaning that it was uncommon for patients with atrophy of these reward structures to continue to demonstrate prosocial motivation in their daily lives. This suggests that being capable of experiencing a reward state may predispose one towards engaging in helping behaviors, while conversely the reduced capacity for reward may decrease the tendency towards prosocial behavior, as has been shown in animal and human models (Heshmati and Russo, 2015; Riga et al., 2015). There is evidence from other NDG lesion studies that some patient groups are less likely to seek out social reward than others (Perry et al., 2014; Perry and Kramer, 2015), and that patients with damage to reward circuitry are more likely to be socially and emotionally detached (Bickart et al., 2014). Taken with our findings, this suggests that reward circuitry may be necessary to experience prosocial motivation. An alternative explanation that cannot be resolved due to the cross-sectional nature of this study is that this linear brain-behavior relationship may have resulted from a selective invulnerability to neurodegeneration, in which individuals engaging in higher frequency and intensity of prosocial behavior retained volume in these reward structures relative to other patients.
4.2.2. Social decision making and ambiguity
The relationship observed between caudate volume and prosocial motivation may also reflect a second mechanism. In addition to functioning in the reward system, the caudate has a well-established role in social decision making (Rilling, 2008). When there is uncertainty or a lack of clarity in a social context, resolving this ambiguity is an important aspect of deciding whether or not to engage in a helping behavior. Ambiguity resolution is a process associated with the caudate as evidenced by fMRI paradigms with healthy individuals (O’Doherty, 2011; Ketteler et al., 2008). fMRI studies demonstrate that the caudate is recruited when an individual is deciding whether to perform an altruistic helping behavior or a non-helping behavior (FeldmanHall et al., 2015). Also, resolving ambiguity and making a decision to act or refuse to act may in itself be rewarding (Aupperle et al., 2015; Soares et al., 2012).
4.2.3. Emotion regulation
In our study, prosocial motivation had unique associations with volume in the IFG and the lateral orbital gyrus. The IFG is involved in inhibition of one's own behavior (Aron et al., 2004) and is active when individuals engage emotion regulation strategies such as cognitive reappraisal (Goldin et al., 2008; Ochsner et al., 2004). Unlike affect sharing, prosocial motivation often requires an individual to inhibit their own emotional response or self-protective behavior in order to behave in a way that helps the other. For instance, empathy might motivate one to confront a bully who is attacking another person, even though drawing that bully's attention would be directly contrary to one's self-preservation interests. Other lesion studies have shown that some NDG patients have a decreased ability to inhibit their own mental perspective during a false belief task (Le Bouc et al., 2012), suggesting that they can lose this capacity for self-regulation.
4.3. Limitations
The observational nature of this study is a major limitation, as informant reports of empathic functioning by necessity only tap into broader observable behaviors, and thus may be insensitive to more precise elements of cognitive-affective processing. Also, by focusing on structures that were in disjunction between the two empathy measures (RSMS-EX and IRI), we likely removed areas relevant to empathy that are important for both affect sharing and prosocial motivation. Though analyses of these overall measures can be found elsewhere (Rankin et al., 2006), other studies employing alternative approaches will be needed to discover any putatively shared regions. Additionally, while there are many advantages to using a lesion-model, such as increased variability in both brain volume and behavior, this approach makes it difficult to determine if a structure directly mediates the behavior, or if damage to that structure results in disconnection from other regions that are necessary for the behavior. Additional studies prospectively delineating these brain-behavior relationships, or looking at the development of deficits within individuals whose premorbid socioemotional functioning is well-characterized, are certainly warranted.
4.4. Conclusions
In summary, the fact that many measures of empathy are conceptually and anatomically overlapping can be used advantageously in patient lesion studies, if care is taken to delineate the subprocesses involved in empathy rather than simply looking at the entire map of regions involved. We found that affect sharing uniquely draws upon diverse structures in temporal lobe, including the amygdala and insula, that support emotion recognition, emotion generation, and emotional awareness. Overall, this provides neurologic support for models of affect sharing predicated on intact ability to recognize, internally generate, and viscerally sense their own emotional state in direct response to the other. We also found that prosocial motivation, in contrast, involves structures such as the NaCC, caudate, and IFG, which suggests individuals must maintain the capacity to experience reward, to resolve ambiguity, and to inhibit one's own emotional experience in order to effectively engage in spontaneous altruism as a component of their empathic response to others.
Supplementary Material
Acknowledgments
This research was supported by NIH [National Institute on Aging 5 K23 AG021606 (PI: Rankin), R01 AG029577 (PI: Rankin), PPG P01 AG1972403 (PI: Miller)], P50 AG02350 (PI: Miller), and the Larry L. Hillblom Foundation #2002/2J (PI: Rankin); #2007/2I (PI: Miller).
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.neuropsychologia.2017.02.010.
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Francisco A, Paulus MP, Stein MB. Neural substrates of approach-avoidance conflict decision-making. Hum. Brain Mapp. 2015;36(2):449–462. doi: 10.1002/hbm.22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydinli A, Bender M, Chasiotis A, Cemalcilar Z, van de Vijver FJR. When does self-reported prosocial motivation predict helping? The moderating role of implicit prosocial motivation. Motiv. Emot. 2014;38(5):645–658. doi: 10.1007/s11031-014-9411-8. [DOI] [Google Scholar]
- Báez-Mendoza R, Schultz W. The role of the striatum in social behavior. Front. Neurosci. 2003;7:233. doi: 10.3389/fnins.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banissy MJ, Kanai R, Walsh V, Rees G. Inter-individual differences in empathy are reflected in human brain structure. NeuroImage. 2012;62(3):2034–2039. doi: 10.1016/j.neuroimage.2012.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson CD, Bolen MH, Cross JA, Neuringer-Benefiel HE. Where is the altruism in the altruistic personality? J. Personal. Soc. Psychol. 1986;50(1):212. [Google Scholar]
- Batson CD, Powell AA. Altruism and prosocial behavior. Handb. Psychol 2003 [Google Scholar]
- Bickart KC, Dickerson BC, Barrett LF. The amygdala as a hub in brain networks that support social life. Neuropsychologia. 2014;63:235–248. doi: 10.1016/j.neuropsychologia.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller BL, Rosen HJ. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch. Neurol. 2006;63(1):81–86. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Cliffordson C. The hierarchical structure of empathy: dimensional organization and relations to social functioning. Scand. J. Psychol. 2002;43:49–59. doi: 10.1111/1467-9450.00268. [DOI] [PubMed] [Google Scholar]
- Cox CL, Uddin LQ, Di Martino A, Castellanos FX, Milham MP, Kelly C. The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain's intrinsic functional dynamics. Soc. Cogn. Affect. Neurosci. 2012;7(6):727–737. doi: 10.1093/scan/nsr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel – now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol. 2005;493(1):154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J. Personal. Soc. Psychol. 1983;44(1):113. [Google Scholar]
- De Vignemont F, Singer T. The empathic brain: how, when and why? Trends Cogn. Sci. 2006;10(10):435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. A social-neuroscience perspective on empathy. Curr. Dir. Psychol. Sci. 2006;15(2):54–58. [Google Scholar]
- Decety J, Jackson PL, Sommerville JA, Chaminade T, Meltzoff AN. The neural bases of cooperation and competition: an fMRI investigation. NeuroImage. 2004;23(2):744–751. doi: 10.1016/j.neuroimage.2004.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermody N, Wong S, Ahmed R, Piguet O, Hodges JR, Irish M. Uncovering the neural bases of cognitive and affective empathy deficits in alzheimer's disease and the behavioral-variant of frontotemporal dementia. J. Alzheimer's Dis. 2016:1–16. doi: 10.3233/JAD-160175. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Miller PA. The relation of empathy to prosocial and related behaviors. Psychol. Bull. 1987;101(1):91. [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Anderson C, Grossman M. Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. J. Neuropsychiatry Clin. Neurosci. 2011;23(1):74–82. doi: 10.1176/appi.neuropsych.23.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethofer T, Anders S, Wiethoff S, Erb M, Herbert C, Saur R, Wildgruber D. Effects of prosodic emotional intensity on activation of associative auditory cortex. Neuroreport. 2006;17(3):249–253. doi: 10.1097/01.wnr.0000199466.32036.5d. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci. Biobehav. Rev. 2011;35(3):903–911. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Fehr E, Fischbacher U. The nature of human altruism. Nature. 2003;425(6960):785–791. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- FeldmanHall O, Dalgleish T, Evans D, Mobbs D. Empathic concern drives costly altruism. NeuroImage. 2015;105:347–356. doi: 10.1016/j.neuroimage.2014.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque D, Hodges SD, Baird JA, Black SE. Empathy in frontotemporal dementia and alzheimer's disease. J. Clin. Exp. Neuropsychol. 2010;32(3):289–298. doi: 10.1080/13803390903002191. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Kiebel SJ, Nichols TE, Penny WD, editors. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Academic Press; San Diego, CA: 2007. [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Gao Z, Wang X, Liu X, Knight RT, Hof PR, Fan J. Anterior insular cortex is necessary for empathetic pain perception. Brain: A J. Neurol. 2012;135(Pt9):2726–2735. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Zürcher N, Rogier O, Hippolyte L, Lemonnier E, Ruest T, Billstedt E. Emotional contagion for pain is intact in autism spectrum disorders. Transl. Psychiatry. 2014;4(1):e343. doi: 10.1038/tp.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316(5831):1622–1625. doi: 10.1126/science.1140738. (doi: 316/5831/1622) (pii) [DOI] [PubMed] [Google Scholar]
- Hawk ST, Keijsers L, Branje SJ, Graaff JVd, Wied Md, Meeus W. Examining the interpersonal reactivity index (IRI) among early and late adolescents and their mothers. J. Personal. Assess. 2013;95(1):96–106. doi: 10.1080/00223891.2012.696080. [DOI] [PubMed] [Google Scholar]
- Heshmati M, Russo SJ. Anhedonia and the brain reward circuitry in depression. Curr. Behav. Neurosci. Rep. 2015;2(3):146–153. doi: 10.1007/s40473-015-0044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE. Inability to empathize: brain lesions that disrupt sharing and understanding another’s emotions. Brain: A J. Neurol. 2014;137(Pt 4):981–997. doi: 10.1093/brain/awt317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn. Sci. 2012;16(2):122–128. doi: 10.1016/j.tics.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Irish M, Hodges JR, Piguet O. Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia. Brain: A J. Neurol. 2014;137(Pt4):1241–1253. doi: 10.1093/brain/awu003. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58(2):284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. NeuroImage. 2007;34(4):1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Ketteler D, Kastrau F, Vohn R, Huber W. The subcortical role of language processing. High level linguistic features such as ambiguity-resolution and the human brain; an fMRI study. NeuroImage. 2008;39(4):2002–2009. doi: 10.1016/j.neuroimage.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat. Neurosci. 2009;12(7):939–945. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kumfor F, Piguet O. Disturbance of emotion processing in frontotemporal dementia: a synthesis of cognitive and neuroimaging findings. Neuropsychol. Rev. 2012;22(3):280–297. doi: 10.1007/s11065-012-9201-6. [DOI] [PubMed] [Google Scholar]
- Le Bouc R, Lenfant P, Delbeuck X, Ravasi L, Lebert F, Semah F, Pasquier F. My belief or yours? Differential theory of mind deficits in frontotemporal dementia and alzheimer's disease. Brain: A J. Neurol. 2012;135(Pt 10):3026–3038. doi: 10.1093/brain/aws237. [DOI] [PubMed] [Google Scholar]
- Leigh R, Oishi K, Hsu J, Lindquist M, Gottesman RF, Jarso S, Hillis AE. Acute lesions that impair affective empathy. Brain. 2013;136(8):2539–2549. doi: 10.1093/brain/awt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox RD, Wolfe RN. Revision of the Self-monitoring Scale. 1984 doi: 10.1037//0022-3514.46.6.1349. [DOI] [PubMed] [Google Scholar]
- Lerner Y, Hendler T, Levit-Binnun N, Golland Y. Shared feelings: investigating neural attunement to the emotions of others. Eur. Psychiatry. 2016;33:S457–S458. [Google Scholar]
- Litvan J, Briva A, Wilson MS, Budinger GR, Sznajder JI, Ridge KM. Beta-adrenergic receptor stimulation and adenoviral overexpression of superoxide dismutase prevent the hypoxia-mediated decrease in na, K-ATPase and alveolar fluid reabsorption. J. Biol. Chem. 2006;281(29):19892–19898. doi: 10.1074/jbc.M602064200. [DOI] [PubMed] [Google Scholar]
- Liu W, Miller BL, Kramer JH, Rankin K, Wyss-Coray C, Gearhart R, Rosen HJ. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62(5):742–748. doi: 10.1212/01.wnl.0000113729.77161.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Mayeux R. The diagnosis of dementia due to alzheimer's disease: recommendations from the national institute on aging-alzheimer's association workgroups on diagnostic guidelines for alzheimer's disease. alzheimer's Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. J. Cogn. Neurosci. 2010;22(2):248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc. Natl. Acad. Sci. USA. 2006;103(42):15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J. Cogn. Neurosci. 2004;16(10):1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J, Gross JJ. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol. Sci. 2009;20(11):1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP. Contributions of the ventromedial prefrontal cortex to goal-directed action selection. Ann. N.Y. Acad. Sci. 2011;1239(1):118–129. doi: 10.1111/j.1749-6632.2011.06290.x. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130(7):1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Penner LA, Dovidio JF, Piliavin JA, Schroeder DA. Prosocial behavior: multilevel perspectives. Annu. Rev. Psychol. 2005;56:365–392. doi: 10.1146/annurev.psych.56.091103.070141. [DOI] [PubMed] [Google Scholar]
- Perry DC, Kramer JH. Reward processing in neurodegenerative disease. Neurocase. 2015;21(1):120–133. doi: 10.1080/13554794.2013.873063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Sturm VE, Seeley WW, Miller BL, Kramer JH, Rosen HJ. Anatomical correlates of reward-seeking behaviours in behavioural variant frontotemporal dementia. Brain: A J. Neurol. 2014;137(Pt6):1621–1626. doi: 10.1093/brain/awu075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perugini M, Conner M, O’Gorman R. Automatic activation of individual differences: a test of the gatekeeper model in the domain of spontaneous helping. Eur. J. Personal. 2011;25(6):465–476. doi: 10.1002/per.826. [DOI] [Google Scholar]
- Rameson LT, Morelli SA, Lieberman MD. The neural correlates of empathy: experience, automaticity, and prosocial behavior. J. Cogn. Neurosci. 2012;24(1):235–245. doi: 10.1162/jocn_a_00130. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Salazar A, Gorno-Tempini ML, Sollberger M, Wilson SM, Pavlic D, Miller BL. Detecting sarcasm from paralinguistic cues: anatomic and cognitive correlates in neurodegenerative disease. NeuroImage. 2009;47(4):2005–2015. doi: 10.1016/j.neuroimage.2009.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, Miller BL. Structural anatomy of empathy in neurodegenerative disease. Brain: A J. Neurol. 2006;129(Pt11):2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn. Behav. Neurol.: Off. J. Soc. Behav. Cogn. Neurol. 2005;18(1):28–36. doi: 10.1097/01.wnn.0000152225.05377.ab. (doi: 00146965-200503000-00004) (pii) [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain: A J. Neurol. 2011;134(Pt9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riga D, Theijs JT, De Vries TJ, Smit AB, Spijker S. Social defeat-induced anhedonia: effects on operant sucrose-seeking behavior. Front. Behav. Neurosci. 2015;9:195. doi: 10.3389/fnbeh.2015.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK. Neuroscientific approaches and applications within anthropology. Am. J. Phys. Anthropol. 2008;(Suppl. 47):S2–S32. doi: 10.1002/ajpa.20947. [DOI] [PubMed]
- Rosen HJ, Gorno-Tempini M, Goldman WP, Perry RJ, Schuff N, Weiner M, Miller BL. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(20):198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Wilson MR, Schauer GF, Allison S, Gorno-Tempini M, Pace-Savitsky C, Miller BL. Neuroanatomical correlates of impaired recognition of emotion in dementia. Neuropsychologia. 2006;44(3):365–373. doi: 10.1016/j.neuropsychologia.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Ross LA, Olson IR. Social cognition and the anterior temporal lobes. NeuroImage. 2010;49(4):3452–3462. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sened H, Yovel I, Bar-Kalifa E, Gadassi R, Rafaeli E. Now you have my attention: empathic accuracy pathways in couples and the role of conflict. Emotion. 2016 doi: 10.1037/emo0000220. (doi: 2016-41152-001) (pii) [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG. The neural bases for empathy. Neurosci.: A Rev. J. Bringing Neurobiol. Neurol. Psychiatry. 2011;17(1):18–24. doi: 10.1177/1073858410379268. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J. Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia. 2007;45(13):3054–3067. doi: 10.1016/j.neuropsychologia.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Shany-Ur T, Poorzand P, Grossman SN, Wilson SM, Miller BL, Rankin KP. Divergent Neuroanatomic Correlates of Sarcasm and Lie Comprehension in Neurodegenerative Disease (Abstract) 2011 [Google Scholar]
- Shany-Ur T, Lin N, Rosen HJ, Sollberger M, Miller BL, Rankin KP. Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain: A J. Neurol. 2014;137(Pt8):2368–2381. doi: 10.1093/brain/awu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Lamm C. The social neuroscience of empathy. Ann. N. Y. Acad. Sci. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J. Neurol. Neurosurg. Psychiatry. 2001;70(30):323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Thompson JC, Neary D. Knowledge of famous faces and names in semantic dementia. Brain: A J. Neurol. 2004;127(Pt4):860–872. doi: 10.1093/brain/awh099. [DOI] [PubMed] [Google Scholar]
- Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques F, Palha JA, Sousa N. Stress-induced changes in human decision-making are reversible. Transl. Psychiatry. 2012;2:e131. doi: 10.1038/tp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberger M, Rosen HJ, Shany-Ur T, Ullah J, Stanley CM, Laluz V, Rankin KP. Neural substrates of socioemotional self-awareness in neurodegenerative disease. Brain Behav. 2014;4(2):201–214. doi: 10.1002/brb3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberger M, Stanley CM, Ketelle R, Beckman V, Growdon M, Jang J, Rankin KP. Neuropsychological correlates of dominance, warmth, and extraversion in neurodegenerative disease. Cortex; A J. Devoted Study Nerv. Syst. Behav. 2012;48(6):674–682. doi: 10.1016/j.cortex.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Yokoyama JS, Seeley WW, Kramer JH, Miller BL, Rankin KP. Heightened emotional contagion in mild cognitive impairment and alzheimer's disease is associated with temporal lobe degeneration. Proc. Natl. Acad. Sci. USA. 2013;110(24):9944–9949. doi: 10.1073/pnas.1301119110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J. Psychosom. Res. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Weinstein N, Ryan RM. When helping helps: autonomous motivation for prosocial behavior and its influence on well-being for the helper and recipient. J. Personal. Soc. Psychol. 2010;98(2):222–244. doi: 10.1037/a0016984. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat. Neurosci. 2002;5(3):277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Iyengar V, Huey ED, Tierney M, Krueger F, Grafman J. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain: A J. Neurol. 2009;132(Pt3):604–616. doi: 10.1093/brain/awn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Ochsner KN. The neuroscience of empathy: progress, pitfalls and promise. Nat. Neurosci. 2012;15(5):675–680. doi: 10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.