Abstract
Background
US Health care disparities persist despite repeated countermeasures. Research identified race, ethnicity, gender, and socio-economic status as factors, mediated through individual provider and/or systemic biases; little research exists in anesthesiology. We investigated anti-emetic prophylaxis as a surrogate marker for anesthesia quality by individual providers because anti-emetics are universally available, indicated contingent on patient characteristics (gender, age, etc.), but independent of co-morbidities and not yet impacted by regulatory or financial constraints. We hypothesized that socioeconomic indicators (measured as insurance status or median income in the patients’ home zip code area) are associated with the utilization of anti-emetic prophylaxis (as a marker of anesthesia quality).
Methods
We tested our hypothesis in several subsets of electronic anesthesia records from the National Anesthesia Clinical Outcomes Registry (NACOR), fitting frequentist and novel Bayesian multi-level logistic regression models.
Results
NACOR contained 12 million cases in 2013. Six institutions reported on anti-emetic prophylaxis for 441,645 anesthesia cases. Only 173,133 cases included details on insurance information. Even fewer (n=92683) contained complete data on procedure codes and provider identifiers. Bivariate analysis, multivariable logistic regression and our Bayesian hierarchical model all showed a large and statistically significant association between socioeconomic markers and anti-emetic prophylaxis (ondansetron and dexamethasone). For Medicaid versus commercially insured patients, the odds ratio of receiving the anti-emetic ondansetron is 0.85 in our Bayesian hierarchical mixed regression model, with a 95% Bayesian credible interval of [0.81, 0.89] with similar inferences in classical (frequentist) regression models.
Discussion
Our analyses of NACOR anesthesia records raise concerns that patients with lower socioeconomic status may receive inferior anesthesia care provided by individual anesthesiologists, as indicated by less anti-emetics administered. Effects persisted after we controlled for important patient characteristics and for procedure and provider influences. Findings were robust to sensitivity analyses. Our results challenge the notion that anesthesia providers do not contribute to health care disparities.
Introduction
The healthcare disparities in the United States of America described decades ago by Gornick1, persist and are linked to social determinants of health and equality2–4, among them poverty, poor education, differences in medical insurance coverage, geographic location, legal or social status, race or gender, patient and community attitudes & perceptions5. A systematic review by Haider suggested that insurance status, median income, race, ethnicity, and socioeconomic status are associated with trauma outcomes, independent of injury type6. LaPar, analyzing the National Inpatient Database7, showed that Medicaid and uninsured payer status conferred increased risk-adjusted mortality for major surgery. We will focus on provider bias leading to healthcare disparities8.
Significance
Do anesthesiologists contribute to healthcare disparities? A systematic review and meta-analysis by Meghani raises alarm about the persistent racial and ethnic disparities in the treatment of pain, clearly a domain of anesthesiologists9. We described language as an access barrier to chronic pain services10,11. Jimenez found disparities in pain treatment in children12. Unfortunately, apart from labor analgesia13–16 and pain medicine17–20, the literature on anesthesia-related health disparities seems sparse5,21. Spencer et al. expressed concern that “differences in payment between public and private payers may result in inferior care”, and more patient safety events22.
Objective
We sought to explore if healthcare disparities are also prevalent in anesthesiology and examined the contribution of individual providers. Our objective was to investigate if antiemetic prophylaxis as a marker of quality anesthesia care was independently associated with socioeconomic status, indicating health care disparity attributable to anesthesiologists. Previous research showed remarkable variability between providers in antiemetic utilization, possibly due to gaps in knowledge, or provider perceptions of importance of PONV as an outcome for the patient at hand, leading to underutilization of proven therapies23. Several arguments support antiemetic prophylaxis as a suitable marker of anesthesia quality.
Antiemetic Prophylaxis is relatively independent of patient co-morbidities.
It is indicated contingent on specific measurable risk factors for PONV.
A standard of care with explicit guidelines is widely accepted24,25,
Antiemetic administration is the sole responsibility of anesthesia providers23.
Regulatory or insurance constraints have not yet impacted treatment choices.
Antiemetic prophylaxis clearly improves outcomes24.
PONV prevention is a patient-centered outcome26.
Approach
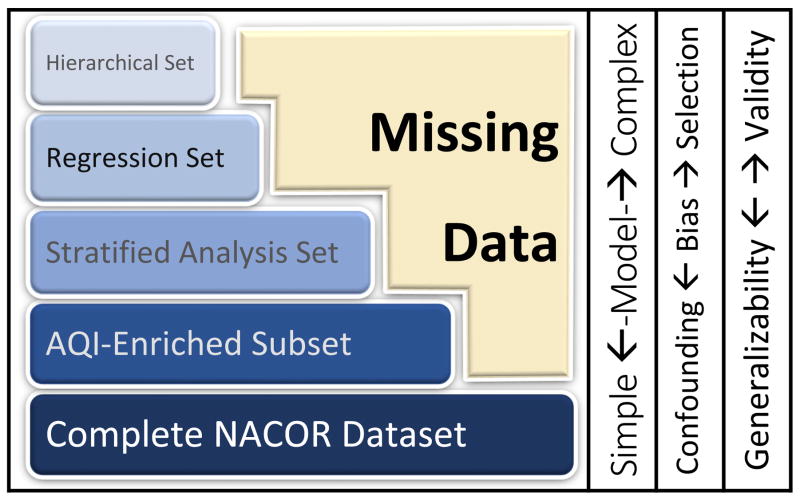
We encountered two contradicting dilemmas, inherent in any electronic medical records-based health disparities research (Figure 1):
Figure 1.
Missing data in electronic anesthesia records lead to a trade-off between selection bias and confounding bias in research using large databases like NACOR. Inferences based on the analysis of the complete dataset or the larger enriched datasets will more likely be generalizable, but lack of control for confounders (age and gender) may lead to bias. As we control for confounding with increasingly complex models, adding more variables, the dataset becomes smaller due to missing data: We can only include records with complete data in the analysis. Any increase in validity with advanced modelling may come at the expense of generalizability due to selection bias: The few institutions uploading all variables of interest may not represent typical anesthesia practice.
Investigating the association between antiemetics and SES, we may want to control for confounding by including known risk factors for PONV, e.g. age or gender. Including more potential confounders may reduce spurious associations.
Electronic anesthesia records uploaded to NACOR are missing data, partly because participating institutions differ in the data they upload. In a regression analysis, we cannot include any case missing confounder variables. Attempting to control for more confounders therefore makes the dataset smaller and less representative.
On one hand, a crude analysis may fail to adjust for potential confounders leading to biased results. On the other hand, concerns about generalizability make limiting our regression to only complete cases (with all data on all confounders) problematic; complete cases may not be representative of overall anesthesia practice, introducing selection bias. Complicating the analysis further, anesthesia and healthcare delivery is clustered in hospitals/services/providers27; this hierarchical structure needs to be considered in statistical modeling for correct inferences28.
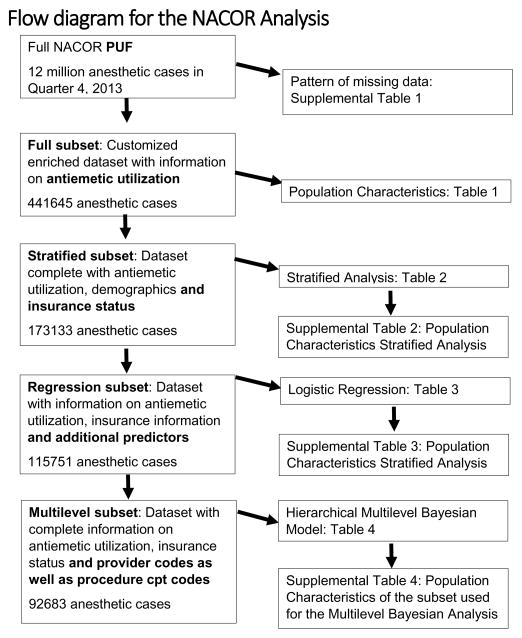
Any statistical modeling approach is open to critique for being too crude or over-complicated. Worse, model misspecification, for example, controlling for too many confounders, can lead to incorrect inferences. To compellingly attribute the disparities to the individual anesthesia provider, we wanted to convince the reader of the robustness of the association between antiemetic utilization and SES. Hence, we investigated the association between antiemetics and SES in a spectrum of models and datasets. We considered crude models (including all data), as well as several increasingly sophisticated regression analyses applied to progressively smaller ‘complete cases’ datasets. This is outlined in the Flow Diagram (Figure 2). We effectively conducted sensitivity analyses to cover the range between no adjustment versus potential over adjustment, complete data versus complete cases, frequentist versus Bayesian statistical approaches, across different subsets of NACOR (Figure 1).
Figure 2.
Hypothesis
Our hypothesis is that socioeconomic patient characteristics are consistently associated with antiemetic prophylaxis as a marker of anesthesia quality in NACOR.
Methods
We pre-specified our hypothesis and our analysis methods (Appendix 1). We obtained the NACOR Public User File (PUF), from Quarter 4 of 2013, enriched with additional information on anti-emetic usage and insurance status by the Anesthesia Quality Institute (AQI). The Albert Einstein College of Medicine Institutional Review Board determined that our study does not meet the definition of human subject research as defined by 45 CFR 46.102(f), as AQI removed all identifiers.
NACOR receives information on anesthesia cases from participating institutions and anesthesia providers29. The data had been uploaded by participating provider institutions. Participating provider upload a minimum dataset to NACOR, containing mostly demographics. Only few providers additionally uploaded the complete electronic anesthesia record including intra-operative physiologic data and administered medications.
Our unit of analysis is the anesthesia case. Without patient identifiers, repeated anesthetics provided to the same individual could not be identified and therefore were analyzed independently. In Quarter 4 of 2013, NACOR contained about one million complete electronic anesthesia records. Our AQI created, customized data subset contained 441645 cases (full subset), where intra-operative anti-emetic utilization was electronically accessible; anti-emetics were utilized in 234453 cases.
Our customized data set specified administration of the antiemetics dexamethasone, droperidol, ondansetron and/or phenergan, patient demographics and American Society of Anesthesiologists Physical Status Classification, provider identifier, institution and location, procedure codes and some other case characteristics. Hence, we were limited to the most widely used anti-emetics ondansetron and dexamethasone, with the strongest supporting evidence base. We omitted droperidol with its boxed warning by the FDA. Unfortunately, the timing of anti-emetic administration intra- versus postoperatively was not specified. The NACOR set contained the median income-based on patient’s zip rounded to 1000, generic and detailed insurance information, but with missing data for many cases as detailed in Supplemental Table 1.
We described the population characteristic of the NACOR data sets forming the bases of our analysis, i.e. anesthesia records with complete information on the administration of anti-emetic prophylaxis and/or insurance information, procedure code, median income, etc. We explored the bivariate associations between anti-emetic utilization and independent variables describing patients, procedures & providers. We defined our dichotomous outcome as the administration of ondansetron and/or dexamethasone. Patient characteristics included medical insurance status, (as our primary predictor/independent variable of interest), patient age, gender, and American Society of Anesthesiologists Physical Status Classification. Neither race nor ethnicity was recorded in NACOR. We reported procedure types and indications (Billing code, modifiers, indication ICD code). Provider characteristics included information on the anesthetist (nurse anesthetist versus resident versus attending alone) and institutional data (geographic location, academic versus private versus government institution).
Statistical analysis
We explained in the introduction why we preferred a priori to utilize several concurrent statistical approaches to analyze the data. We wanted to demonstrate the consistency of the statistical association regardless of modelling choices. A complete case analysis of a limited subset with complete data on arbitrarily selected independent variables would have raised concerns about selection bias (Figure 1). Also, we wanted to preempt critique of our model specification. We employed
bivariate analysis,
stratified analysis,
logistic regression models and
mixed effects hierarchical Bayesian models.
We compared the different approaches provided above in sensitivity and subgroup analyses. We investigated if any potential association between socioeconomic status and anti-emetic prophylaxis depended on the mode of analysis and/or on the inclusion or exclusion of potential confounders. We present several of our analyses with the code used to generate them (Appendix 2). We rarely if ever reported p-values. The strength of the statistical association (AKA p-value) is less relevant for our inferences, likely inflated, and possibly spurious, given the sheer size of our data30. AQI had removed all patient identifiers, which made it impossible to fit models to account for possible within-subject correlation. However, within-subject correlation tends to affect the confidence interval, but less the point estimate of effect. Therefore, correction for within-subject correlation would likely not have affected our inferences, given the very low p-values observed in our Big Data analysis.
Bivariate and stratified analysis
We used classical (frequentist) parametric tests where the assumptions of normality did not seem violated. We used non-parametric tests where graphical or statistical tests suggested possible violations of the underlying assumptions. In the table of characteristic of patients, we reported frequency (%), mean (± standard deviation) or the median (with the interquartile range) as appropriate for the distribution of values observed for each parameter and indicated the statistical test used. We calculated odds ratios for the association between insurance status and anti-emetic administration and with the data stratified by gender, age and other demographics and case characteristics. We described the population characteristics in the Supplemental Table 2.
Classical frequentist logistic regression
We fit classical (frequentist) logistic regression models in the subsets of anesthesia cases in the NACOR with information on intra-operative anti-emetic administration and medical insurance status. We described the population characteristics in the Supplemental Table 3. We investigated the association of medical insurance with the administration of anti-emetic medication as primary outcome variable, controlling for potential confounders including patient characteristics, provider characteristics and procedure type and indication. Insurance status can be seen as a categorical variable; possible values are (unordered) private commercial insurance, Health Maintenance Organization, Medicare, Medicaid, Selfpay and including no medical insurance reported. We collapsed them to four unordered categories, Self, Medicaid, Medicare and Commercial. Our outcome is dichotomous, anti-emetic prophylaxis administered or not. Our unit of analysis is the anesthesia case, not the patient. We focused our analysis on the most frequent procedures performed. We considered findings statistically significant if the p-value was less than the type I error rate of 0.01.
A priori, we included gender and age as likely confounders, because they are known risk factors for PONV. Given the increased power of our analysis in such a big data set, the exploration of confounding and interaction should be limited and preordained a priori to prevent the detection of spurious associations. For the initial model, we chose those independent variables which showed a statistically significant association in the bivariate analysis. We used a stepwise backward elimination model selection technique. We eliminated independent variables from the model based on the likelihood ratio test with a cut off at 0.05. For each elimination, we confirmed that the given variable was not a confounder for the present model. A change in the beta coefficient of larger than 20% was used as our cutoff to determine if a variable was considered a confounder. This is admittedly an arbitrary cutoff31, deliberately conservative to prevent overfitting32. We determined the correct functional form and explored potential violations of the assumptions of linearity. We graphically assessed for potential violations of the assumption of linearity by running locally weighted regression and examined the graph for all independent variables in our final model. We tested for the correct functional form, fitting fractional polynomials as part of our final logistic regression model. We examined if the addition of a polynomial improves the model significantly. We explored the potential interaction between the independent variables age and gender in a logistic regression model; a cut off for our likelihood ratios test was at a type I error rate level of 0.05. We did not consider the goodness of fit with the Hosmer-Lemeshow goodness of fit test, because it will erroneously detect small departures from the proposed model as significant in large data sets33,34. Instead, we calculated the concordance statistic35.
Bayesian hierarchical model
We build hierarchical Bayesian models for the subset with data on medical insurance (short: insurance), median income in patient home zip code (short: income), respectively. We described the population characteristics in the Supplemental Table 4. We used Bayesian approaches after classical mixed effects models failed to converge due to the large size of the population studied. Bayesian and classical inference will give similar inferences, but, for large datasets, frequentist models often fail to converge. We overcame this hindrance with Bayesian estimation of probability distributions using Hamiltonian Markov chain Monte Carlo simulation, a faster and novel more robust algorithm36. We studied the administration of only ondansetron or of ondansetron and dexamethasone as primary outcomes. We included either insurance or income [divided in quantiles] as categorical independent variables in our models. We controlled for patient characteristics gender and age [Table 2 Stratified Analysis of Ondansetron Utilization by Insurance] and other patient, procedure or anesthetic-related confounders [Table 3 Logistic Regression and Table 4. Bayesian Hierarchical Model]. In some models, we included mixed (random) effects to control for the potential confounding influence of procedure type or provider behavior, by allowing each procedure and each provider to have an individual intercept. We present more formal details on the Bayesian modeling in Appendix 3.
Table 2.
Stratified Analysis of Ondansetron Utilization by Insurance
| Stratified by gender/female | ||||||||
|---|---|---|---|---|---|---|---|---|
| Insurance | Cases | % | Ondansetron | % | Odds | OR | [low | up] |
| Commercial | 57653 | 0.33 | 33127 | 0.57 | 1.35 | 1.00 | NA | NA |
| MEDICAID | 16689 | 0.10 | 7405 | 0.44 | 0.80 | 0.59 | 0.57 | 0.61 |
| Medicare | 25634 | 0.15 | 12795 | 0.50 | 1.00 | 0.74 | 0.72 | 0.76 |
| SELF | 683 | 0.00 | 398 | 0.58 | 1.40 | 1.03 | 0.89 | 1.20 |
| Stratified by gender/male | ||||||||
| Insurance | Cases | % | Ondansetron | % | Odds | OR | [low | up] |
|
| ||||||||
| Commercial | 37971 | 0.22 | 21736 | 0.57 | 1.34 | 1.00 | NA | NA |
| MEDICAID | 8816 | 0.05 | 3729 | 0.42 | 0.73 | 0.55 | 0.52 | 0.57 |
| Medicare | 24836 | 0.14 | 10758 | 0.43 | 0.76 | 0.57 | 0.55 | 0.59 |
| SELF | 851 | 0.00 | 503 | 0.59 | 1.45 | 1.08 | 0.94 | 1.24 |
| Stratified by anes_type/general | ||||||||
| Insurance | Cases | % | Ondansetron | % | Odds | OR | [low | up] |
|
| ||||||||
| Commercial | 67193 | 0.39 | 48450 | 0.72 | 2.58 | 1.00 | NA | NA |
| MEDICAID | 16101 | 0.09 | 9277 | 0.58 | 1.36 | 0.53 | 0.51 | 0.54 |
| Medicare | 37071 | 0.21 | 21984 | 0.59 | 1.46 | 0.56 | 0.55 | 0.58 |
| SELF | 1201 | 0.01 | 847 | 0.71 | 2.39 | 0.93 | 0.82 | 1.05 |
| Stratified by anes_type/neuroaxial | ||||||||
| Insurance | Cases | % | Ondansetron | % | Odds | OR | [low | up] |
|
| ||||||||
| Commercial | 16634 | 0.10 | 4569 | 0.27 | 0.38 | 1.00 | NA | NA |
| MEDICAID | 6307 | 0.04 | 1491 | 0.24 | 0.31 | 0.82 | 0.76 | 0.87 |
| Medicare | 2374 | 0.01 | 451 | 0.19 | 0.23 | 0.62 | 0.56 | 0.69 |
| SELF | 112 | 0.00 | 27 | 0.24 | 0.32 | 0.84 | 0.54 | 1.30 |
| Stratified by anes_type/regional | ||||||||
| Insurance | Cases | % | Ondansetron | % | Odds | OR | [low | up] |
|
| ||||||||
| Commercial | 964 | 0.01 | 244 | 0.25 | 0.34 | 1.00 | NA | NA |
| MEDICAID | 305 | 0.00 | 53 | 0.17 | 0.21 | 0.62 | 0.45 | 0.86 |
| Medicare | 1144 | 0.01 | 190 | 0.17 | 0.20 | 0.59 | 0.47 | 0.73 |
| SELF | 18 | 0.00 | 6 | 0.33 | 0.50 | 1.48 | 0.55 | 3.97 |
| Stratified by anes_type/MAC | ||||||||
| Insurance | Cases | % | Ondansetron | % | Odds | OR | [low | up] |
|
| ||||||||
| Commercial | 10827 | 0.06 | 1600 | 0.15 | 0.17 | 1.00 | NA | NA |
| MEDICAID | 2788 | 0.02 | 312 | 0.11 | 0.13 | 0.73 | 0.64 | 0.83 |
| Medicare | 9874 | 0.06 | 927 | 0.09 | 0.10 | 0.60 | 0.55 | 0.65 |
| SELF | 202 | 0.00 | 21 | 0.10 | 0.12 | 0.67 | 0.42 | 1.05 |
Table 2: The stratified analysis of ondansetron utilization is exemplified by gender and anesthesia type and shows the odds of antiemetic administration by insurance status for each stratum. The odds ratio (OR) compares the odds to the reference population with commercial insurance (with the upper and lower 95% confidence interval in the following two columns) and favors commercial insurance over medicaid and medicare in all strata in this NACOR dataset (n = 173133) with complete information on insurance and antiemetic utilization (p<0.01), reported by 4 institutions.
Table 3.
Logistic Regression
| OR | 2.5 % | 97.5 % | |
|---|---|---|---|
| (Intercept) | 3.724 | 3.478 | 3.988 |
| Age | 1.005 | 1.004 | 1.006 |
| Female | 0.979 | 0.953 | 1.005 |
| Medicare | 0.639 | 0.616 | 0.662 |
| Medicaid | 0.628 | 0.604 | 0.652 |
| Self-insured | 1.073 | 0.955 | 1.205 |
| Median income | 1.000 | 1.000 | 1.000 |
| Population | 1.000 | 1.000 | 1.000 |
| Dexamethasone | 3.418 | 3.348 | 3.490 |
| Droperidol | 4.653 | 4.095 | 5.303 |
| Phenergan | 2.060 | 0.547 | 8.522 |
| Case duration | 0.999 | 0.999 | 0.999 |
Table 3 presents the results of our classical (frequentist) logistic regression, with OR (odds ratios with the coresponding 95% confidence intervals), indicating that medicaid or medicare (compared to commercially insured) patients, are less likely to receive ondansetron as antiemetic prophylaxis after controlling for potential confounders (gender, age, median income in patients’ home zip code, case duration) in this NACOR data Regression subset with complete information on antiemetic use, insurance status and all additional predictors (n = 115750, p<0.01), reported. Income is in $1000, population in 1000 souls and case duration in 10 min.
Table 4.
Bayesian Hierarchical Model
| odds.ratios | 2.5% | 97.5% | |
|---|---|---|---|
| (Intercept) | 3.05 | 1.14 | 7.58 |
| payMEDICAID | 0.85 | 0.81 | 0.89 |
| payMedicare | 0.85 | 0.80 | 0.90 |
| paySELF | 0.85 | 0.72 | 1.01 |
| age_groupUnder 1 | 0.06 | 0.05 | 0.08 |
| age_group1–18 | 0.91 | 0.82 | 1.01 |
| age_group50 – 64 | 0.86 | 0.81 | 0.91 |
| age_group65 – 79 | 0.85 | 0.80 | 0.91 |
| age_group80+ | 0.68 | 0.62 | 0.75 |
| sexmale | 0.75 | 0.72 | 0.78 |
| ASA2 | 0.88 | 0.80 | 0.97 |
| ASA3 | 0.67 | 0.61 | 0.74 |
| ASA4 | 0.25 | 0.22 | 0.28 |
| ASA5 | 0.01 | 0.01 | 0.02 |
| anes_typeNeuroaxial | 0.09 | 0.08 | 0.10 |
| anes_typeRegional | 0.09 | 0.08 | 0.11 |
| anes_typeMAC | 0.09 | 0.08 | 0.10 |
| practiceD | 1.58 | 0.63 | 4.27 |
| practiceE | 4.19 | 1.61 | 11.28 |
| practiceF | 1.83 | 0.71 | 4.74 |
Table 4 lists the regression coefficients of our Bayesian hierarchical mixed effects model in the limited NACOR subset with complete data on insurance status, antiemetic administration and procedure code (n= 92683, two institutions). Compared to commercial insurance, Medicaid patients were less likely to receive antiemetic prophylaxis with ondansetron (OR 0.85, with Bayesian Credible 95% Intervals 0.8, 0.9) after controlling for age, gender, ASA risk classification, anesthesia type, and practice as fixed effects, allowing providers and procedures a random intercept, with very similar results for Medicare patients. As we would expect given the known riks for PONY, woman and younger (reference age group 19–49 years) patients were more likely to recieve prophylaxis (as indicated by older patients’ OR below 1); more prophylaxis was administered in cases using general anesthesia. Increasing ASA risk classification was associatated with lower odds of prophylaxis. Differences in odds ratios between institutions and procedure codes were large.
We relied on the Gelman-Rubin diagnostic as a convergence diagnostic, after exploring the Monte Carlo Markov Chain output graphically36,37. Gelman-Rubin diagnostic, develop by Rubin and Gelman, is comparing between and within variances of multiple chains in Marcov chain Monte Carlo simulations, to assess convergence of multiple chains run in parallel. Individual chains are initialized with different starting values. After discarding the burn in, convergence is assumed, when the output from all chains is indistinguishable. In simple terms, chains “forgot” their starting values, when individual chains have become independent of initialization. Values below 1.1 indicate convergence38.
Bayesian Prior Distributions
In Bayesian parlance36, the use of informative Bayesian prior distributions refers to incorporating existing knowledge about parameters into the model. For example, the choice of prior could force the estimated treatment effect in a clinical trial to tend to fall within reasonable bounds. Such a prior would express our disbelief in a miracle drug39. Informative Bayesian prior distributions are possibly more relevant for Bayesian meta-analysis40,41. In contrast, uninformative Bayesian prior distributions relax any such constraints, leading Bayesian and classical statistical approaches to converge to similar inferences36.
We used the default uninformative Bayesian prior distributions, as described in the software package42. These uninformative priors for the main effect of insurance status on anti-emetic prophylaxis are spelled out both formally and as function call to rstanarm in Appendix 3.
We performed a sensitivity analysis investigating prior distributions and our model specifications. Representative examples of various models, with fixed and/or random intercepts and slopes, are presented in Appendix 2.
Contrasting different modeling approaches and sensitivity analyses
We contrasted the results of our three models (bivariate, logistic regression and Bayesian analysis) to confirm the robustness of our findings regardless of the model choices or statistical approach chosen (Figure 1). We performed a sensitivity analysis of our model assumptions and choices with various data subsets. In particular, we fit the stratified and regression analyses to the multi-level subset, respectively. Detailed code and selected results are presented in the online supplement Appendix 2. However, the different model specifications (bivariate versus standard linear regression versus hierarchical/mixed effects) and different fixed and random effects (procedure versus provider random effects), meant that models were not always nested. While equivalent for inferences, one should expect to see somewhat different estimates for the regression coefficients in the different models.
Software used
We used the statistical software Stata for the logistic regression and bivariate analysis43. The public domain statistical software package R/Rstudio and the probabilistic programming software Stan were used in conjunction with the R software packages rstan and rstanarm42,44,45 to implement the hierarchical mixed Bayesian models with Stan’s Hamiltonian Monte Carlo algorithms. These are available under the General Public License of the Free Software Foundation46 at no cost. For graphical exploration of model convergence and the Monte Carlo Markov Chain output, to generate the contrasts to compare commercial versus Medicaid and for posterior predictive checking, we used the software package shinyStan47.
Results
Description of the data set
The flow diagram in Figure 2 details the NACOR subset used for each of our statistical analyses. Our AQI created customized data set contained 441645 cases (full subset) where intra-operative utilization of the anti-emetic dexamethasone or ondansetron was electronically accessible. Dexamethasone and/or ondansetron were utilized in 233498 cases. Dexamethasone only was administered in 86280 and ondansetron only in 223472 cases. Both anti-emetics were used together in 76254 cases. The reporting institutions were mostly Northeastern university hospitals or medium to large Southern community hospitals. Our data set contained no cases from the Midwest or the West of the Unites States. Anesthesia was provided between January 1st 2010 and December 31st 2013.
Unfortunately, the Public User File (PUF) (4th quarter 2013) only contained 441645 cases with detailed information on medications administered during anesthesia. Six unique institutions reported anti-emetic utilization, complete demographics and insurance status for 173133 anesthesia cases, out of the 12 million cases in NACOR. The data set shrank further, when we limited this set further to cases with information on additional independent variables for our regression analysis (n = 115751) and our Bayesian hierarchical model (n = 92683).
Population characteristics and bivariate analysis of demographic characteristics
The demographics of the population in the NACOR database with information on anti-emetic administration are described in Table 1. Forty-three percent of anesthetics were administered to male patients. Patients’ age ranged from newborn to 90 years of age with a median of 52 (Interquartile range (IQR) between 35 and 67 years). Most patients were classified as ASA status 2, 3 (35 and 30 percent, respectively) with few cases in class 5. Sixty-two percent were outpatients among the 64% of cases where this information was available. For 25865 cases (5.9%) insurance status was reported as Medicaid, for another 51441 cases (12%) as Medicare for the remaining 97443 cases (22%) as commercial insurance with 1585 cases (0.36%) self-insured, but insurance status was not available in 265311 cases. “Self-pay” may reflect charity care in some and high socioeconomic status of the patient in other settings. “Self-pay” may therefore be a poor indicator of socioeconomic status. Insurance was reported as “Self-pay” in only a small fraction of cases (less than 0.5 percent of cases in Table 1). “Self-pay” was the only predictor with inconsistent results, likely due to the small numbers. At least one anti-emetic (either ondansetron or dexamethasone) was administered in 53 percent of the NACOR full subset case.
Table 1.
Population Characteristics
| Gender | Cases | % Cases | Ondansetron | % | Odds for Ondansetron |
|---|---|---|---|---|---|
| female | 249909 | 56.6 | 131667 | 52.7 | 1.1 |
| male | 191654 | 43.4 | 91782 | 47.9 | 0.9 |
| NA | 82 | 0.0 | 23 | 28.0 | 0.4 |
| Age | Cases | % Cases | Ondansetron | % | Odds for Ondansetron |
|
| |||||
| Under 1 | 6357 | 1.4 | 1354 | 21.3 | 0.3 |
| 1–18 | 35881 | 8.1 | 20573 | 57.3 | 1.3 |
| 19 – 49 | 159002 | 36.0 | 90636 | 57.0 | 1.3 |
| 50 – 64 | 116869 | 26.5 | 58614 | 50.2 | 1.0 |
| 65 – 79 | 96274 | 21.8 | 42501 | 44.1 | 0.8 |
| 80+ | 27252 | 6.2 | 9792 | 35.9 | 0.6 |
| NA | 10 | 0.0 | 2 | 20.0 | 0.2 |
| Anesthetic | Cases | % Cases | Ondansetron | % | Odds for Ondansetron |
|
| |||||
| General | 277112 | 62.7 | 191822 | 69.2 | 2.2 |
| Neuroaxial | 31208 | 7.1 | 8962 | 28.7 | 0.4 |
| Regional | 10710 | 2.4 | 2046 | 19.1 | 0.2 |
| MAC | 89241 | 20.2 | 15943 | 17.9 | 0.2 |
| NA | 33374 | 7.6 | 4699 | 14.1 | 0.2 |
| ASA class | Cases | % Cases | Ondansetron | % | Odds for Ondansetron |
|
| |||||
| 1 | 42143 | 9.5 | 27192 | 64.5 | 1.8 |
| 2 | 153117 | 34.7 | 88204 | 57.6 | 1.4 |
| 3 | 133965 | 30.3 | 68478 | 51.1 | 1.0 |
| 4 | 39382 | 8.9 | 10244 | 26.0 | 0.4 |
| 5 | 1407 | 0.3 | 29 | 2.1 | 0.0 |
| NA | 71631 | 16.2 | 29325 | 40.9 | 0.7 |
| Insurance | Cases | % Cases | Ondansetron | % utilized | Odds for Ondansetron |
|
| |||||
| Commercial | 97443 | 22.1 | 55654 | 57.1 | 1.3 |
| MEDICAID | 25865 | 5.9 | 11245 | 43.5 | 0.8 |
| Medicare | 51441 | 11.6 | 23798 | 46.3 | 0.9 |
| SELF | 1585 | 0.4 | 922 | 58.2 | 1.4 |
| NA | 265311 | 60.1 | 131853 | 49.7 | 1.0 |
Table 1 details the population characteristcs and ondansetron utilization in the NACOR dataset (n = 441645) reported by 6 unique institutions in the Public Use File of the Anesthesia Quality Institute, enriched with antiemetic utilization and insurance provider information. The first column tabulates patient or case characteristics. The second column list the number of cases with that characteristic (and the third column in brackets the corresponding case frequency in percent). The fourth column tabulates the number of cases with antiemetic utilized for that characteristic (and the fifth presents the corresponding antiemetic utilization in percent).
We stratified the NACOR data set with complete information on insurance status and anti-emetic administration into levels by potential confounders as a crude but robust approach to correct for potential confounding. We calculated the odds ratios for receiving anti-emetic. We explored the preponderance for anti-emetic prophylaxis using ondansetron and/or dexamethasone. In Table 2, we present a stratified analysis by gender and anesthetic choice showing ondansetron utilization contingent on patient insurance status. For example, patients on Medicaid are less likely to receive anti-emetic prophylaxis than those with commercial insurance, regardless of gender. The OR was similar for women (0.59, 95%CI [0.57, 0.61]) as for men (0.55, 95%CI [0.52, 0.57]). The results were consistent regardless of antiemetic (data not shown).
Stratification sometimes changed the odds ratios, for example contingent on anesthesia type, as we would expect for confounders. In Table 2, comparing Medicaid versus commercial insurance, the OR ranged from 0.53, 95%CI [0.51, 0.54] under general anesthesia to 0.82, 95%CI [0.76, 0.87] under neuraxial anesthesia. However, regardless of anesthesia type, Medicaid status was associated with significantly reduced odds of receiving anti-emetic prophylaxis. The strong and consistent association between insurance status and anti-emetic prophylaxis held also when we fit stratified analyses to the multi-level subset (data not shown).
Ondansetron was more often used in longer cases, and in outpatients, and less during emergency surgery, and more frequently if the patient lived in a zip code with higher mean income and smaller population size. Results suggested that patients who received ondansetron were, on average, younger. Ondansetron was more frequently given to women (p<0.001).
Regression analysis
Classical frequentist logistic regression model
Being on Medicaid or Medicare, compared to having commercial insurance, drastically reduces the odds of receiving ondansetron during anesthesia. For the average patient on Medicare (or similarly Medicaid), the odds of receiving ondansetron for anti-emetic prophylaxis are 0.64, with a 95% confidence interval of [0.62, 0.66] compared to a patient with commercial insurance with otherwise similar characteristics. This reflects the association after controlling for age and gender, case duration, median income and population in the patient’s home zip code. We present the results of our final logistic regression model in Table 3, modeling ondansetron use only by patient insurance status as socioeconomic indicator and controlling for age and gender. The concordance statistic for the classical logistic regression was calculated as 0.73, which indicates good model fit35. We found similar results when we fit the regression analyses to the multi-level subset, [data not shown].
Hierarchical Bayesian generalized linear models
We also fitted more complex hierarchical Bayesian mixed effects models to control for procedure and provider influences in the propensity to administer anti-emetics. In all contrasts, we consistently found strongly and significantly reduced odds ratio for receiving anti-emetic prophylaxis (using ondansetron alone or either ondansetron and/or dexamethasone as outcomes) for patients with lower socio-economic status, after we fitted several hierarchical mixed effects Bayesian models (including random intercepts for anesthesia provider, institution, or procedure). In Table 4, we present the contrast of the reference category commercial insurance versus Medicaid (and versus Medicare insurance, respectively). In Supplemental Table 5, we present the association of median income in the patients’ home zip code with antiemetic prophylaxis. We present the detailed results of several modes in the supplemental online Appendix 2 for transparency. The convergence of our Bayesian models was confirmed by looking at trace plots and the Gelman Rubin statistics48, shown for selected parameters of our Bayesian models.
We show the odds ratios (with 95% Bayesian credible intervals) of two representative Bayesian hierarchical mixed effects model in Table 4 and in Supplemental Table 5. They reflect the effect of insurance and median income in the patients’ home zip code as independent variables of interest, respectively. The association between socioeconomic indicator and anti-emetic prophylaxis was consistent regardless of what variable we used as measure of socioeconomic status.
We modelled ondansetron administration in the NACOR subset of anesthesia cases with complete data on insurance status, anti-emetic administration, provider and procedure code (n = 92683). Results are reported in Table 4, Compared to commercial insurance, Medicaid and Medicare patients were less likely to receive anti-emetic prophylaxis with ondansetron (OR 0.85, with a 95% Bayesian credible interval of [0.81, 0.89]), after controlling for age, gender, ASA status, anesthesia type, and type of practice as fixed effects, allowing providers and procedures a random intercept. The concordance statistic for this Bayesian hierarchical model was calculated as 0.91, which indicated an excellent model fit35. This concordance statistic of the Bayesian hierarchical model was larger than the concordance statistic for the classical linear regression model, which should not surprise given that we fitted individual intercepts for the individual anesthesia providers and procedure.
Modeling median income as quantiles, patients were more likely to receive anti-emetic prophylaxis with any anti-emetic if they lived in neighborhoods (zip codes) with high median income (OR 1.16 with Bayesian Credible 95% Intervals 1.09 to 1.25) or middle median income (OR 1.10 with Bayesian Credible 95% Intervals 1.05 to 1.17) compared to neighborhoods with very low median income. Detailed results are presented in Supplemental Table 5,
As we would expect given the known risks for PONV, women and the younger (reference age group 19–49 years) patients were more likely to receive prophylaxis (as indicated by older patients’ OR below 1). More prophylaxis was administered in cases using general anesthesia. Increasing ASA status was associated with lower odds of prophylaxis. Differences between institutions were large suggesting that healthcare disparities may be endemic, i.e., locally more or less pronounced.
Sensitivity analysis
The strong and statistically significant association between indicators of patients’ socioeconomic status (insurance status or median income in the patients’ home zip code) and the odds of receiving anti-emetic prophylaxis remained unchanged after stratification to control for the patient characteristics gender and age [Table 2. Stratified Analysis of Ondansetron Utilization by Insurance], in our logistic regressions [Table 3. Logistic Regression] and hierarchical modeling [Table 4. Bayesian Hierarchical Model]. Our inferences were invariant to the statistical approach (Bayesian versus classical frequentist analysis) used and bore out in the full subset and any subset used for multivariable logistic regression. In the online supplement Appendix 2, we presented several additional analysis to corroborate the consistency of our findings.
Discussion
Summary of the findings
In our enriched NACOR data set, we found a clinically meaningful and statistically strong association between socioeconomic status (insurance status or median income in home zip code) and the utilization of anti-emetic medication (ondansetron and/or dexamethasone), regardless of the modeling approach used. The magnitude of the difference is large: 40% of patients on Medicaid receiving anti-emetics versus 60% of patients with commercial insurance would correspond to a number needed to treat of 5. In our most refined Bayesian hierarchical mixed regression model, the odds ratio of receiving the anti-emetic ondansetron is 0.85 for Medicaid versus commercially insured patients, with a 95% Bayesian credible interval of [0.81, 0.89]. Inferences were robust to sensitivity analyses.
The authors believe that socioeconomic status or payer should NOT influence PONV prophylaxis49. Given that antiemetic administration is the sole domain of the individual anesthesia providers, our results point to possible, unappreciated and worrisome healthcare disparities in anesthesia50. The size of the NACOR dataset we studied likely makes this the largest study of healthcare disparities in anesthesia undertaken to date. Controlling for likely confounders decreases the chance that the association is spurious. Demonstrating health care disparities for which only anesthesiologists are accountable, in such a large data set, is novel. Disparity in antiemetic utilization (as a marker of anesthesia quality and exclusively in the domain of the anesthesiologist) will likely make a greater impression on the anesthesia community than differences in which anesthesiologists are only marginally involved (e.g., procedure time5) or where anesthesiologists are not the sole decision maker13.
Generalizability
The comprehensive records uploaded to NACOR mostly by academic institutions in the North East, are likely not random or representative samples of anesthesia practice in the US. For example, private practice may cater better to PONV, as an outcome perceived as important to patients51. Differences in demographic characteristics between NACOR subsets neither prove nor disprove generalizability. More importantly, the rich heterogeneity of anesthesia practice in the US (apparent in the variability of the case mix between institutions, and the diversity of its providers) defeats a single simplified modeling approach; a description of the typical anesthetic is as useful as the description of the typical American (voter, consumer, etc.)52–54. The observed disparities seem pervasive, even though we concede (and hope) that they may not be ubiquitous. Our findings compel because they are consistent across different data sets and are indifferent to modeling approaches55.
Critique of the modeling approach
Which of our models is the best? George Box reminded us that “all models are wrong, some are useful”, and argued for robustness56 (which, however, is no guarantee for correct inferences). We discuss below confounding, missing data, model misspecification and overfitting as potential limitations of our bivariate, multi-variable, Bayesian model specifications and illustrate the trade-off in Figure 1. All our models provided evidence against our Null-hypothesis of no anesthesia related health care disparities57. Our final random effects modeling by individual provider and procedure, in conjunction with the redundancy and multiplicity of our sensitivity analyses of different subsets together, might serve as a model to address this challenging complexity of Big Data health disparities research58.
Confounding, missing data and model misspecification
Age, gender, history of PONV and smoking are the four most important commonly accepted risk factors for PONV. It is a limitation of our work, that we controlled only for the first two. In our anecdotal experience, however, only gender and age are routinely elicited pre-operatively by anesthesia providers and used for decision making regarding anti-emetic prophylaxis24. We attempted to partially, (but admittedly incompletely), control for postoperative opioid administration with
fixed effects for anesthesia type (regional versus general anesthesia) and with
random effects for (a) procedure (related need for opioids) and (b) provider (individuals’ propensity to administer opioids versus NSAIDs).
Stratified analysis only controls for the specific variable by which the stratification is done and substratification quickly becomes overwhelming. Logistic regression does not control well for confounding when the probability of effect is around 0.5. Hierarchical models lead to shrinkage, which makes for a better fit of the model to the data, but this may lead, among other issues, to overfitting.
However, our results were invariant to our statistical approach. Bivariate analysis, stratification, hierarchical Bayesian or classical frequentist logistic regression, all led to the same inferences. We controlled for many patient characteristics including ASA status, age and gender [Table 3 Logistic Regression and Table 4 Bayesian Hierarchical Model]. We controlled for the choice of anesthetic given. We considered surgical procedure and provider as confounding factors by allowing individual random intercepts, implemented in a Bayesian hierarchical model. Likewise, while there was considerable missing data as detailed in the flow diagram in Figure 2, the healthcare disparities were apparent across all data subsets analyzed.
In our enriched NACOR dataset, reporting of anti-emetic utilization did not differentiate intra-operative, (when anti-emetics would have been administered for prophylaxis) versus postoperative use, (when anti-emetics might have been given as treatment for active nausea and vomiting). While consistent under-treatment of patients of lower socioeconomic status would be equally concerning as inferior prophylaxis, treatment in the post anesthesia period is no longer under the sole domain of the anesthesia providers. We concede that this limits our inferences. Not all providers and institutions use electronic anesthesia records. Some upload only selected data to NACOR, e.g., for regulatory or privacy reasons. All the foregoing may limit the generalizability of our findings. Hence, our analysis should be repeated in other larger electronic anesthesia databases. We should investigate the effect of additional markers of socioeconomic status (e.g. scholastic attainment, social networks). Also, providers may have forgotten to record the administration of anti-emetic medication, but still have administered the prophylaxis. Such misclassification could lead to an over- or underestimation of anesthesia healthcare disparities.
We did not control for smoking, an important PONV risk factor. Smoking arguably is associated with socio-economic status. Beyond lamenting this limitation, we conceptualize this as a potential mechanism to explain the observed disparity59. Smoking status is rarely accessible in the preoperative anesthesia evaluation or transmitted in handovers. We hypothesize that when providers decide on antiemetic prophylaxis, they infer smoking status from patients’ aspect and appearance, which may lead to bias. Looking at the author of this article, anesthesia providers may think “this old bearded sailor does not need prophylaxis”, when based on history and smoking status, the opposite is true.
Those who do not accept anti-emetic prophylaxis as a valid indicator of anesthesia quality, will have to concede that disparities in anti-emetic prophylaxis due to insurance or socioeconomic status are worrisome. On the other hand, while insurance status and median income in patients’ home zip code are closely linked to race and socioeconomic status, they may not be the best predictors of healthcare disparities. We furthermore concede that our cross-sectional analysis neither discerns causal pathways, nor proves causation. We concede that we did not investigate other accepted approaches to PONV prophylaxis, for example other antiemetic medications or non-pharmacological interventions including regional anesthesia, total intravenous anesthesia, avoidance of nitrous oxide, not least because this information was not provided in our enhanced NACOR dataset. Demonstrating the disparity for any anti-emetic intervention should be concerning enough, although it would have been even more compelling to make the point for every single one. We focused instead on the simple choice of the anesthesia provider to administer a cheap, ubiquitously available, proven effective prophylactic agent, or not. We hope other investigators will join us in a more comprehensive analysis of how practice patterns are influenced by socioeconomic status.
Difference, disparity, and bias
In the process of quality improvement, we typically go through the four stages described succinctly by Don Berwick60:
Stage I: “The data are wrong…”
Stage II: “The data are right, but it’s not a problem…”
Stage III: “The data are right, it’s a problem, but it’s not my problem…”
Stage IV: “The data are right, it’s a problem, it’s my problem…”
We hope to convince anesthesiologists with the presented strong, robust and consistent association between insurance status and anti-emetic prophylaxis with ondansetron and/or dexamethasone, that the findings are solid (Stage I). Disparity in anesthesia quality is a problem, even if it concerned not anesthesia quality in general, but only anti-emetic prophylaxis (Stage II). We think there is a clear argument that the described association describes a healthcare disparity for which anesthesia providers are accountable (Stage III). In the framework for interpreting socio-economic and racial differences in health care, we demonstrated difference, disparity, and bias61. Anesthesiologists have a tradition as the leaders in perioperative quality improvement addressing individual performance as well as systems to improve care for all patients18. The next step would be to investigate what interventions (e.g., electronically triggered reminders) might improve anesthesia quality regardless of patient characteristics.
Conclusion
Our analyses of the association between insurance status (as a marker of patient socioeconomic status) and anti-emetic administration (as a marker of anesthesia quality provided by the individual anesthesiologist) in the NACOR database admittedly fall short of proving the existence of bias among anesthesia providers, but still provide substantial evidence against our Null-hypothesis of no anesthesia related health care disparities57. Further observational studies and possibly randomized trials62 may be needed, to convince the anesthesia community that, where there’s smoke, there’s fire63. Our novel modeling approach may be a first step towards addressing the rich multilevel heterogeneity inherent in electronic (anesthesia) records and characteristic of contemporary anesthesia practice in the US52,64.
Supplementary Material
Key Points.
Question: Anesthesia providers might contribute to healthcare disparity by administering antiemetic prophylaxis contingent on patients’ social status.
Findings: We investigated the association of antiemetic prophylaxis with social determinants of health in the National Anesthesia Clinical Outcomes Registry (NACOR).
Meaning: status or median income in the patient’s home zip code predict the utilization of antiemetics dexamethasone and ondansetron, pointing to potential healthcare disparity mediated by anesthesia providers.
Acknowledgments
This research is supported in part by the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), through CTSA grant numbers 5KL2TR001071-03 and UL1 TR001073. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We would like to acknowledge Dr. Dutton and the team of the Anesthesia Quality Institute for advice in the conception and development of the project and the provision of the enriched NACOR datafile. We would like to acknowledge Drs. Sonia Vaida, Thomas Verbeek, and Diane McCloskey for their suggestions in the editorial process.
Footnotes
Conflicts of Interest: None
Author contributions
Michael Andreae conceived the idea for the project, wrote the protocol, obtained the enriched NACOR datafile after obtaining institutional review board clearance, cleaned the data, executed and verified the analysis, provided the figures and tables, wrote the draft of the manuscript and lead the submission and editorial revision process. Michael Andreae approved the final manuscript and attests to having reviewed the original data reported in the manuscript. He is the archival author and the corresponding author.
Jonah Gabry guided the advanced Bayesian modelling implementation in the probabilistic programming software package Rstan. In particular he assisted with the parallel processing on the multicore cluster. He advised on convergence diagnostics and implemented graphical visualization of chain output and analysis results in rstanarm and shinystan, software packages he designed with and for the Stan team. He contributed to the discussion of the results and the defense of the manuscript in the editorial process, and approved the final version to be submitted.
Ben Goodrich is a core developer for the STAN team. The STAN developed the Hamiltonian MCMC algorithms and probabilistic programming software underlying the R packages used to fit the models. He is the lead developer of the software packages Rstan and rstanarm used to render the statistical models. He supervised and advised Michael Andreae and Jonah Gabry in the implementation of the multilevel Bayesian models and the interpretation of the results and revisions of the manuscript. He approved the final manuscript.
Robert White contributed in the development of the project idea, assisted in the cleaning of the data, performed the initial regression analysis in Stata, wrote the draft for the poster, and contributed to drafting of the manuscript draft. He approved the final manuscript and attests to having reviewed the original data reported in the manuscript.
Charles Hall mentored Michael Andreae during the conception and execution of the project, discussed and develop the various statistical approaches with him, provided advice during the protocol stage, the institutional review board submission, and the data acquisition from the Anesthesia Quality Institute. He supervised the analysis and visualization of the data and contributed to the drafting of the manuscript and the responses to the reviewers during the editorial process. He approved the final manuscript.
Contributor Information
Michael H. Andreae, Department of Anesthesiology, Penn State Health Milton S. Hershey Medical Center, Penn State College of Medicine, Hershey. PA
Jonah S. Gabry, Department of Statistics, Columbia University, New York, NY
Ben Goodrich, Department of Political Science, Columbia University, New York, NY
Robert S White, Department of Anesthesiology, Weill Cornell Medical Center, New York, NY
Charles Hall, Department of Epidemiology & Population Health, Albert Einstein College of Medicine, Bronx, NY.
References
- 1.Gornick ME, Eggers PW, Reilly TW, Mentnech RM, Fitterman LK, Kucken LE, Vladeck BC. Effects of race and income on mortality and use of services among medicare beneficiaries. N Engl J Med. 1996;335:791–9. doi: 10.1056/NEJM199609123351106. Available at: http://dx.doi.org/10.1056/NEJM199609123351106. [DOI] [PubMed] [Google Scholar]
- 2.Smedley BD, Stith AY, Nelson AR, editors. IOM. Unequal treatment: Confronting racial and ethnic disparities in health care (full printed version) The National Academies Press; 2003. Available at: http://www.nap.edu/openbook.php?record_id=10260. [PubMed] [Google Scholar]
- 3.Cooper RA, Cooper MA, McGinley EL, Fan X, Rosenthal JT. Poverty, wealth, and health care utilization: A geographic assessment. J Urban Health. 2012;89:828–47. doi: 10.1007/s11524-012-9689-3. Available at: http://dx.doi.org/10.1007/s11524-012-9689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoen C, Osborn R, Squires D, Doty MM. Access, affordability, and insurance complexity are often worse in the united states compared to ten other countries. Health Aff (Millwood) 2013;32:2205–15. doi: 10.1377/hlthaff.2013.0879. Available at: http://dx.doi.org/10.1377/hlthaff.2013.0879. [DOI] [PubMed] [Google Scholar]
- 5.Silber JH, Rosenbaum PR, Ross RN, Even-Shoshan O, Kelz RR, Neuman MD, Reinke CE, Ludwig JM, Kyle FA, Bratzler DW, Fleisher LA. Racial disparities in operative procedure time: The influence of obesity. Anesthesiology. 2013;119:43–51. doi: 10.1097/ALN.0b013e31829101de. Available at: http://dx.doi.org/10.1097/ALN.0b013e31829101de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haider AH, Weygandt PL, Bentley JM, Monn MF, Rehman KA, Zarzaur BL, Crandall ML, Cornwell EE, Cooper LA. Disparities in trauma care and outcomes in the united states: A systematic review and meta-analysis. J Trauma Acute Care Surg. 2013;74:1195–205. doi: 10.1097/TA.0b013e31828c331d. Available at: http://dx.doi.org/10.1097/TA.0b013e31828c331d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaPar DJ, Bhamidipati CM, Mery CM, Stukenborg GJ, Jones DR, Schirmer BD, Kron IL, Ailawadi G. Primary payer status affects mortality for major surgical operations. Annals of surgery. 2010;252:544–0. doi: 10.1097/SLA.0b013e3181e8fd75. discussion 550–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer KL, Grace M. Social foundations of health care inequality and treatment bias. Annual Review of Sociology. 2016;42:101–20. Available at: http://www.annualreviews.org/doi/10.1146/annurev-soc-081715-074226. [Google Scholar]
- 9.Meghani SH, Byun E, Gallagher RM. Time to take stock: A meta-analysis and systematic review of analgesic treatment disparities for pain in the united states. Pain Med. 2012;13:150–74. doi: 10.1111/j.1526-4637.2011.01310.x. Available at: http://dx.doi.org/10.1111/j.1526-4637.2011.01310.x. [DOI] [PubMed] [Google Scholar]
- 10.Shaparin N, White R, Andreae M, Hall C, Kaufman A. A longitudinal linear model of patient characteristics to predict failure to attend an inner-city chronic pain clinic. J Pain. 2014;15:704–11. doi: 10.1016/j.jpain.2014.03.004. Available at: http://dx.doi.org/10.1016/j.jpain.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreae MH, White R, Chen K, Nair S, Hall C, Shaparin ND. The effect of initiatives to overcome language barriers and improve attendance: A cross-sectional analysis of adherence in an inner city chronic pain clinic. Pain Management. 2016 doi: 10.1093/pm/pnw161. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jimenez N, Seidel K, Martin LD, Rivara FP, Lynn AM. Perioperative analgesic treatment in latino and non-latino pediatric patients. J Health Care Poor Underserved. 2010;21:229–36. doi: 10.1353/hpu.0.0236. Available at: http://dx.doi.org/10.1353/hpu.0.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toledo P, Sun J, Grobman WA, Wong CA, Feinglass J, Hasnain-Wynia R. Racial and ethnic disparities in neuraxial labor analgesia. Anesth Analg. 2012;114:172–8. doi: 10.1213/ANE.0b013e318239dc7c. Available at: http://dx.doi.org/10.1213/ANE.0b013e318239dc7c. [DOI] [PubMed] [Google Scholar]
- 14.Glance LG, Wissler R, Glantz C, Osler TM, Mukamel DB, Dick AW. Racial differences in the use of epidural analgesia for labor. Anesthesiology. 2007;106:19–25. doi: 10.1097/00000542-200701000-00008. discussion 6–8. [DOI] [PubMed] [Google Scholar]
- 15.Rust G, Nembhard WN, Nichols M, Omole F, Minor P, Barosso G, Mayberry R. Racial and ethnic disparities in the provision of epidural analgesia to georgia medicaid beneficiaries during labor and delivery. Am J Obstet Gynecol. 2004;191:456–62. doi: 10.1016/j.ajog.2004.03.005. Available at: http://dx.doi.org/10.1016/j.ajog.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Elisha S, Rutledge DN. Clinical education experiences: Perceptions of student registered nurse anesthetists. AANA J. 2011;79:S35–42. [PubMed] [Google Scholar]
- 17.Glance LG, Kellermann AL, Hannan EL, Fleisher LA, Eaton MP, Dutton RP, Lustik SJ, Li Y, Dick AW. The impact of anesthesiologists on coronary artery bypass graft surgery outcomes. Anesth Analg. 2015;120:526–33. doi: 10.1213/ANE.0000000000000522. Available at: http://dx.doi.org/10.1213/ANE.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 18.Gaba DM. Anaesthesiology as a model for patient safety in health care. BMJ. 2000;320:785–8. doi: 10.1136/bmj.320.7237.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leape LL. Error in medicine. JAMA. 1994;272:1851–7. [PubMed] [Google Scholar]
- 20.Silber JH, Kennedy SK, Even-Shoshan O, Chen W, Koziol LF, Showan AM, Longnecker DE. Anesthesiologist direction and patient outcomes. Anesthesiology. 2000;93:152–63. doi: 10.1097/00000542-200007000-00026. [DOI] [PubMed] [Google Scholar]
- 21.Andreae MH, Nair S, Gabry JS, Goodrich B, Hall C, Shaparin N. A pragmatic trial to improve adherence with scheduled appointments in an inner-city pain clinic by human phone calls in the patient’s preferred language. J Clin Anesth. 2017;42:77–83. doi: 10.1016/j.jclinane.2017.08.014. Available at: http://dx.doi.org/10.1016/j.jclinane.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer CS, Roberts ET, Gaskin DJ. Differences in the rates of patient safety events by payer: Implications for providers and policymakers. Med Care. 2015;53:524–9. doi: 10.1097/MLR.0000000000000363. Available at: http://dx.doi.org/10.1097/MLR.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macario A, Chung A, Weinger MB. Variation in practice patterns of anesthesiologists in california for prophylaxis of postoperative nausea and vomiting. J Clin Anesth. 2001;13:353–60. doi: 10.1016/s0952-8180(01)00283-5. [DOI] [PubMed] [Google Scholar]
- 24.Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, Zernak C, Danner K, Jokela R, Pocock SJ, Trenkler S, Kredel M, Biedler A, Sessler DI, Roewer N I M. P. A. C. T Investigators. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–51. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, Watcha M, Chung F, Angus S, Apfel CC, Bergese SD, Candiotti KA, Chan MT, Davis PJ, Hooper VD, Lagoo-Deenadayalan S, Myles P, Nezat G, Philip BK, Tramèr MR Society for Ambulatory Anesthesia. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118:85–113. doi: 10.1213/ANE.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 26.Wender RH. Do current antiemetic practices result in positive patient outcomes? Results of a new study. Am J Health Syst Pharm. 2009;66:S3–10. doi: 10.2146/ajhp080465. Available at: http://dx.doi.org/10.2146/ajhp080465. [DOI] [PubMed] [Google Scholar]
- 27.Witt WP, Coffey RM, Lopez-Gonzalez L, Barrett ML, Moore BJ, Andrews RM, Washington RE. Understanding Racial and Ethnic Disparities in Postsurgical Complications Occurring in U.S. Hospitals. Health Services Research. 2016 doi: 10.1111/1475-6773.12475. n/a–a. Available at: http://doi.wiley.com/10.1111/1475-6773.12475. [DOI] [PMC free article] [PubMed]
- 28.Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR. Methods in health service research. An introduction to bayesian methods in health technology assessment. BMJ (Clinical research ed) 1999;319:508–12. doi: 10.1136/bmj.319.7208.508. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10454409 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1116393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dutton RP, Dukatz A. Quality improvement using automated data sources: The anesthesia quality institute. Anesthesiol Clin. 2011;29:439–54. doi: 10.1016/j.anclin.2011.05.002. Available at: http://dx.doi.org/10.1016/j.anclin.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Lin M, Lucas HC, Shmueli G. Research commentary—too big to fail: Large samples and the p-value problem. Information Systems Research. 2013;24:906–17. Available at: http://pubsonline.informs.org/doi/abs/10.1287/isre.2013.0480. [Google Scholar]
- 31.Adams J. A computer experiment to evaluate regression strategies. Proc american statistical association section on statistical computing. 1991:55–62. [Google Scholar]
- 32.Babyak MA. What you see may not be what you get: A brief, nontechnical introduction to overfitting in regression-type models. Psychosomatic medicine. 2004;66:411–21. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 33.Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: The hosmer-lemeshow test revisited. Critical care medicine. 2007;35:2052–6. doi: 10.1097/01.CCM.0000275267.64078.B0. [DOI] [PubMed] [Google Scholar]
- 34.Onitilo AA, Engel JM, Stankowski RV, Doi SA. Survival comparisons for breast conserving surgery and mastectomy revisited: Community experience and the role of radiation therapy. Clinical medicine & research. 2015;13:65–73. doi: 10.3121/cmr.2014.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology. 2010;21:128–38. doi: 10.1097/EDE.0b013e3181c30fb2. Available at: http://dx.doi.org/10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelman A, Carlin JB, Stern HS. Bayesian data analysis. Taylor & Francis; 2014. [Google Scholar]
- 37.Cowles MK, Carlin BP. Markov chain monte carlo convergence diagnostics: A comparative review. Journal of the American Statistical Association. 1996;91:883–904. [Google Scholar]
- 38.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. Journal of computational and graphical statistics. 1998;7:434–55. [Google Scholar]
- 39.Johnson SR, Tomlinson GA, Hawker GA, Granton JT, Feldman BM. Methods to elicit beliefs for bayesian priors: A systematic review. Journal of Clinical Epidemiology. 2010;63:355–69. doi: 10.1016/j.jclinepi.2009.06.003. Available at: // www.sciencedirect.com/science/article/pii/S0895435609001759. [DOI] [PubMed] [Google Scholar]
- 40.Andreae MH, Carter GM, Shaparin N, Suslov K, Ellis RJ, Ware MA, Abrams DI, Prasad H, Wilsey B, Indyk D, Johnson M, Sacks HS. Inhaled Cannabis for Chronic Neuropathic Pain: A Meta-analysis of Individual Patient Data. The journal of pain : official journal of the American Pain Society. 2015;16:1221–32. doi: 10.1016/j.jpain.2015.07.009. Available at: http://linkinghub.elsevier.com/retrieve/pii/S1526590015008123 http://www.ncbi.nlm.nih.gov/pubmed/26362106 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4666747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carter GM, Indyk D, Johnson M, Andreae M, Suslov K, Busani S, Esmaeili A, Sacks HS. Micronutrients in HIV: a Bayesian meta-analysis. Shang H, editor. PloS one. 2015;10:e0120113. doi: 10.1371/journal.pone.0120113. Available at: http://dx.plos.org/10.1371/journal.pone.0120113 http://www.ncbi.nlm.nih.gov/pubmed/25830916 http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4382132. [DOI] [PMC free article] [PubMed]
- 42.Gabry J, Goodrich B. Rstanarm: Bayesian applied regression modeling via stan. 2016 Available at: http://CRAN.R-project.org/package=rstanarm.
- 43.StataCorp. Stata statistical software: Release 14. 2014 Available at: http://www.stata.com/
- 44.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. Available at: http://www.R-project.org. [Google Scholar]
- 45.Stan Development Team. Stan: A c++ library for probability and sampling, version 2.5.0. 2014 Available at: http://mc-stan.org/
- 46.License GGP. Free software foundation. License used by the Free Software Foundation for the GNU Project. 1991 See http://www fsf org/copyleft/gpl html ( http://www fsf org/copyleft/gpl html)
- 47.shinyStan Team. ShinyStan: R package for interactive exploration of markov chain monte carlo output, version 0.1. 2015 Available at: https://github.com/stan-dev/shinystan.
- 48.Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Statistical science. 1992:457–72. [Google Scholar]
- 49.Remembering the right to health. Lancet. 2015;386:2366. doi: 10.1016/S0140-6736(15)01230-1. Available at: http://dx.doi.org/10.1016/S0140-6736(15)01230-1. [DOI] [PubMed] [Google Scholar]
- 50.Cook BL, McGuire TG, Zaslavsky AM. Measuring racial/ethnic disparities in health care: Methods and practical issues. Health Serv Res. 2012;47:1232–54. doi: 10.1111/j.1475-6773.2012.01387.x. Available at: http://dx.doi.org/10.1111/j.1475-6773.2012.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anaesthesia and surgery: Results of a prospective survey of 10,811 patients. Br J Anaesth. 2000;84:6–10. doi: 10.1093/oxfordjournals.bja.a013383. [DOI] [PubMed] [Google Scholar]
- 52.Wang W, Rothschild D, Goel S, Gelman A. Forecasting elections with non-representative polls. International Journal of Forecasting. 2015;31:980–91. [Google Scholar]
- 53.Silber JH, Kennedy SK, Even-Shoshan O, Chen W, Mosher RE, Showan AM, Longnecker DE. Anesthesiologist board certification and patient outcomes. Anesthesiology. 2002;96:1044–52. doi: 10.1097/00000542-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Schubert A, Eckhout GV, Ngo AL, Tremper KK, Peterson MD. Status of the anesthesia workforce in 2011: Evolution during the last decade and future outlook. Anesth Analg. 2012;115:407–27. doi: 10.1213/ANE.0b013e3182575b4e. Available at: http://dx.doi.org/10.1213/ANE.0b013e3182575b4e. [DOI] [PubMed] [Google Scholar]
- 55.Draper D. Assessment and propagation of model uncertainty. Journal of the Royal Statistical Society Series B (Methodological) 1995:45–97. [Google Scholar]
- 56.Box GE. Robustness in the strategy of scientific model building. Robustness in statistics. 1979;1:201–36. [Google Scholar]
- 57.Kaufman JS. Epidemiologic analysis of racial/ethnic disparities: Some fundamental issues and a cautionary example. Social Science & Medicine. 2008;66:1659–69. doi: 10.1016/j.socscimed.2007.11.046. Available at: http://dx.doi.org/10.1016/j.socscimed.2007.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fleisher LA, Anderson GF. Perioperative risk: How can we study the influence of provider characteristics? Anesthesiology. 2002;96:1039–41. doi: 10.1097/00000542-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Kaufman JS, Cooper RS. In search of the hypothesis. Public Health Reports. 1995;110:662. [PMC free article] [PubMed] [Google Scholar]
- 60.Berwick DM. Continuous improvement as an ideal in health care. N Engl J Med. 1989;320:53–6. doi: 10.1056/NEJM198901053200110. Available at: http://dx.doi.org/10.1056/NEJM198901053200110. [DOI] [PubMed] [Google Scholar]
- 61.Rathore SS, Krumholz HM. Differences, disparities, and biases: Clarifying racial variations in health care use. Ann Intern Med. 2004;141:635–8. doi: 10.7326/0003-4819-141-8-200410190-00011. [DOI] [PubMed] [Google Scholar]
- 62.Loring M, Powell B. Gender, race, and dsm-iii: A study of the objectivity of psychiatric diagnostic behavior. Journal of health and social behavior. 1988:1–22. [PubMed] [Google Scholar]
- 63.Schulman KA, Berlin JA, Harless W, Kerner JF, Sistrunk S, Gersh BJ, Dube R, Taleghani CK, Burke JE, Williams S, et al. The effect of race and sex on physicians’ recommendations for cardiac catheterization. New England Journal of Medicine. 1999;340:618–26. doi: 10.1056/NEJM199902253400806. [DOI] [PubMed] [Google Scholar]
- 64.Gelman A. Tracking public opinion with biased polls. Available at: https://www.washingtonpost.com/news/monkey-cage/wp/2014/04/09/tracking-public-opinion-with-biased-polls/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.