Abstract
The purine nucleoside analogues clofarabine and fludarabine are active in acute myeloid leukemia (AML). We conducted a phase I/II randomized study of idarubicin and cytarabine with either clofarabine (CIA) or fludarabine (FIA) for relapsed or refractory AML. Clofarabine 15 mg/m2 was identified as the recommended phase II dose. Eighty-one patients were assigned using adaptive randomization to CIA (n = 48) or FIA (n = 33). The complete response (CR)/CR without platelet recovery rate did not differ between CIA and FIA (38% versus 30%, respectively; p = .50). In both arms, more than half of patients who had received only one prior line of therapy achieved remission. The median event-free survival for CIA and FIA was 2.0 and 1.9 months (p = .48), and the median overall survival was 6.3 and 4.7 months, respectively (p = .28). No significant differences in adverse events or early mortality rates were observed. Overall, CIA and FIA resulted in similar response rates and survival in patients with relapsed/refractory AML.
Keywords: Acute myeloid leukemia, relapsed, refractory, purine nucleoside analogues, clofarabine, fludarabine
Introduction
The outcome of patients with relapsed or refractory acute myeloid leukemia (AML) is poor, with a median overall survival (OS) after first relapse of approximately six months and only 10% of patients experiencing long-term survival [1,2]. Treatment options in the setting of relapsed/refractory disease are limited [3]. Retreatment with a high-dose cytarabine-containing regimen, followed by allogeneic stem cell transplantation (SCT) for patients with a suitable donor, is considered standard of care for patients who relapse after initial complete remission (CR) lasting ≥1 year. For patients relapsing after a shorter remission or with primary refractory disease, there is no consensus reinduction regimen.
Fludarabine and clofarabine are purine nucleoside analogues with established clinical activity in AML [4–9]. Both agents synergize with cytarabine, increasing intracellular levels of cytarabine triphosphate (Ara-CTP), which is the active antileukemic metabolite of cytarabine [10–15]. In both the frontline and relapsed/refractory setting, studies with cytarabine plus clofarabine [7–9,16–19] or fludarabine [4,20–23] have shown promising outcomes with these combination regimens. In one randomized study of clofarabine plus cytarabine versus cytarabine alone in older patients with relapsed/refractory AML, the combination arm was associated with significantly higher response rates and event-free survival (EFS) [7]. Similarly, in a randomized trial comparing FLAG-Ida (fludarabine, cytarabine, granulocyte colony-stimulating factor [G-CSF], and idarubicin) to ADE (cytarabine, daunorubicin and etoposide) in younger patients with newly diagnosed AML, FLAG-Ida resulted in significantly longer CR duration and lower rates of relapse [23].
Clofarabine possesses a number of theoretical advantages to fludarabine, including increased resistance to intracellular degradation, prolonged retention of the antileukemic triphosphate compound in leukemic blasts, and more potent inhibition of ribunucleotide reductase [24]. However, whether these theoretical advantages translate into superior clinical activity in AML is unknown. Given the limited treatment options and poor outcomes of relapsed/refractory AML, as well as the uncertainty of the optimal nucleoside analogue-based combination regimen in this setting, we evaluated the relative safety and efficacy of idarubicin and cytarabine with either clofarabine (CIA) or fludarabine (FIA) in adults with relapsed/refractory AML. We performed an initial phase I run-in study to establish the optimal dose of clofarabine for use in the CIA regimen, followed by a randomized, phase II study of CIA versus FIA.
Methods
Patients
Adults with a diagnosis of AML (other than acute pro-myelocytic leukemia) or high-risk myelodysplastic syndrome that had relapsed after prior response or was refractory to last therapy were eligible for this randomized phase I/II open-label trial. Patients ≥60 years of age were eligible if they were deemed to have a low probability of 8-week mortality with intensive chemotherapy [25]. All patients were required to have an Eastern Cooperative Oncology Group performance status ≤3 with adequate cardiac, renal and hepatic function, including a left ventricular ejection fraction ≤40%, creatinine ≤3 mg/dL, total bilirubin ≤2.5 mg/dL, alanine transaminase ≤3 times and aspartate transaminase ≤5 times the institutional upper limit of normal. Secondary AML was defined as AML that was preceded by a diagnosis of myelodysplastic syndrome or myeloproliferative neoplasm. This study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center (MDACC) and was registered at ClinicalTrials.gov (NCT01289457). All patients provided informed consent according to institutional guidelines and the Declaration of Helsinki.
Treatment and study design
In both the phase I and II portions of the study, all patients received idarubicin 10 mg/m2 intravenously (IV) daily on days 1–3 and cytarabine 1 g/m2 IV over 2 h daily on days 1–5. In the phase I study, clofarabine was given at a starting dose of 15 mg/m2 IV daily on days 1–5 with plans to escalate the dose in the standard 3 + 3 trial design until the maximum tolerated dose (MTD) was established. Dose-limiting toxicities (DLT) was defined as a clinically significant non-hematologic grade 3 or 4 adverse event or laboratory abnormality occurring within the first course of treatment and assessed as related to the study drug, or severe myelosuppression with hypoplastic marrow with <5% cellularity and no evidence of leukemia <42 days after starting therapy.
Once the phase II dose of clofarabine was established, subsequent patients were randomized to receive either CIA or FIA. An adaptive randomization algorithm was used to favor the treatment arm with a better EFS; patients with newly diagnosed AML enrolled in a separate, concurrent frontline study were also included in this algorithm. For patients assigned to receive FIA, fludarabine was given at a dose of 30 mg/m2 IV daily on days 1–5. Fludarabine and clofarabine were given 4 h before cytarabine in order to optimize ara-CTP formation [12,13,15,26].
Bone marrow examination was performed on approximately day 28 of induction to assess for remission. Patients not achieving CR or CR without platelet recovery (CRp) after one course of therapy could receive a second induction course if the treating physician determined this to be in the patient’s best interest. Patients who achieved CR or CRp could continue with up to 6 courses of consolidation. In both treatment arms, patients received idarubicin 8 mg/m2 IV daily on days 1–2 and cytarabine 1 g/m2 IV over 2 h daily on days 1–3; these consolidation doses were based on experience with the backbone IA regimen used at our institution [27]. Clofarabine was given at a dose determined by the phase I study, administered IV daily on days 1–3, and fludarabine was given at a dose of 30 mg/m2 IV daily on days 1–3. Patients with FLT3-ITD mutations (n = 5) did not receive FLT3 inhibitors. Consolidation cycles were repeated every four to six weeks, depending on the recovery of neutrophil and platelet counts and toxicity. Dose reductions of all chemotherapeutic agents during consolidation were permitted according to predetermined guidelines related to drug-related adverse events. Patients were recommended for allogeneic SCT based on availability of a suitable donor and at the discretion of the treating physician.
Response criteria and definitions
CR, CRp, CR with inadequate count recovery (CRi), and partial remission (PR) were defined according to International Working Group guidelines for AML [28]. EFS was calculated from the time of treatment initiation until treatment failure, relapse, or death. Overall survival (OS) was calculated from the time of treatment initiation until death. Neither OS nor EFS were censored for SCT in the primary analysis.
Statistical considerations
The objective of the phase I study was to determine the MTD of clofarabine, given in combination with idarubicin and cytarabine; the dose of fludarabine for use in the phase II study was already established from prior experience [23]. The primary objective of the phase II study was to compare the EFS of CIA and FIA. Secondary objectives included CR/CRp rates OS, and the safety profile of each regimen. Responses and event rates were compared with the Chi-square test. EFS and OS were calculated with Kaplan–Meier estimates, and survival estimates were compared with the log-rank test. The data cutoff for this analysis was 1 March 2016.
For the phase II study, an adaptive randomization algorithm was used to favor the treatment arm with better EFS. Initially 40 patients were randomized equally to the two treatment arms. After the completion of the equal randomization, the adaptive randomization algorithm was employed to unbalance the randomization probabilities in favor of the better performing treatment arm. A sample size of 200 patients was planned, which can provide 93% power to detect an EFS hazard ratio of 0.625 between the two arms at one-sided significance level of 0.1. Although the 200 patients were to include both frontline and refractory/relapsed patients, this report is for refractory/relapsed patients only.
Results
Phase I
Baseline characteristics of the 12 patients enrolled in the phase I study are shown in Table 1. The median age was 52 (27–68) and median number of prior therapies was 2 (1–7). Six out of 10 patients (60%) with evaluable baseline cytogenetics had complex karyotype, and three patients (25%) had undergone prior SCT.
Table 1.
Toxicities and outcomes of the phase I study of CIA.
| Patient | Age (years) | Prior lines of therapy | Cytogenetics | Prior SCT | Clofarabine dose (mg/m2) | Toxicity | DLT | Overall response | Consolidation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | 3 | Not done | No | 15 | G3 hyperbilirubinemia | Yes | CR | 1 cycle + SCT |
| 2 | 53 | 2 | IM | Yes | 15 | None | No | CR | SCT |
| 3 | 60 | 2 | Complex | Yes | 15 | G2 HFS | No | No response | N/A |
| 4 | 61 | 2 | Miscellaneous | Yes | 15 | G2 HFS | No | Early death | N/A |
| 5 | 41 | 7 | Complex | No | 15 | G2 HFS | No | No response | N/A |
| 6 | 50 | 6 | Miscellaneous | No | 15 | None | No | No response | N/A |
| 7 | 45 | 2 | Complex | No | 15 | None | No | No response | N/A |
| 8 | 43 | 2 | Complex | No | 15 | None | No | No response | N/A |
| 9 | 64 | 3 | Complex | No | 15 | G2 HFS | No | Early death | N/A |
| 10 | 55 | 1 | Complex | No | 20 | G3 HFS | Yes | No response | None |
| 11 | 27 | 2 | Diploid | No | 20 | G4 myelosuppression | Yes | CRp | 1 cycle + SCT |
| 12 | 68 | 1 | Diploid | No | 20 | G4 myelosuppression | Yes | No response | None |
SCT: stem cell transplantation; DLT: dose-limiting toxicity; CR: complete remission; IM: insufficient metaphases; HFS: hand-foot syndrome; CRp: complete remission without platelet recovery.
Nine patients received clofarabine 15 mg/m2, and three patients received clofarabine 20 mg/m2. Of the nine patients who received clofarabine at a dose of 15 mg/m2, 4 (45%) developed grade 2 hand-food syndrome (HFS); in all cases, it resolved with supportive management. Among the initial three patients treated with clofarabine 15 mg/m2, one patient developed grade 3 hyperbilirubinemia, which resolved without intervention; this patient was able to receive a subsequent course of consolidation without evidence of hepatobiliary toxicity. No other DLTs were observed at the 15 mg/m2 dose level. Two patients died before response assessment. One patient (#4) died on day 17 of pneumonia and multi-organ failure; the other patient (#9) died on day 31 from pneumonia and Pseudomonas sepsis. These were not considered DLTs, as the infectious complications were deemed to be related to the underlying disease, rather than the study regimen.
Among three patients treated with clofarabine 20 mg/m2, all developed a DLT. One patient developed grade 3 HFS which resolved with supportive care. Two other patients had prolonged myelosuppression with time to neutrophil recovery >1000/μL of 43 days and 81 days (#11 and #12, respectively). Both underwent bone marrow biopsy on day 46 which showed hypo-cellular bone marrow with blasts <5%. Due to DLTs experienced with the higher dose of clofarabine, 15 mg/m2 was determined to be the MTD and was chosen as the dose to be used in the phase II portion of the trial.
In the phase I study, three patients (25%) achieved CR or CRp, all of whom went on to receive SCT. The median EFS was 1.5 months, and the median OS was 4.9 months. One patient (#11) is still alive without evidence of relapse after 53 months of follow-up.
Phase II
Patients
Between August 2011 and April 2014, 81 patients were enrolled in this phase II, open-label study and were randomized to receive either CIA (n = 48) or FIA (n = 33). All 81 patients were included in both the efficacy and safety analyses. Table 2 shows the baseline characteristics of the study population, which were similar between both treatment arms, although slightly more patients in the CIA group had adverse risk cytogenetics as defined by complex karyotype, −5 or −7 (63% versus 45%) and more were in first relapse with a CR1 duration of ≥12 months (25% versus 6%). Ten patients (21%) in the CIA arm and 8 (24%) in the FIA arm were >60 years of age. A majority of patients in both groups were in second salvage or beyond (n = 26 [54%] for CIA and n = 20 [61%] for FIA).
Table 2.
Baseline characteristics by treatment arm.
| Characteristica | CIA (N = 48) | FIA (N = 33) |
|---|---|---|
| Age (years) | 54 (21–68) | 57 (18–69) |
| WBC (109/L) | 2.7 (0.5–59.0) | 2.3 (0.6–81.1) |
| Hemoglobin (g/dL) | 9.4 (3.7–11.4) | 9.5 (6.8–13.7) |
| Platelets (109/L) | 35 (4–320) | 25 (7–240) |
| BM blasts (%) | 34 (9–94) | 36 (9–94) |
| LDH (U/L) | 687 (300–7712) | 623 (265–8585) |
| Performance status | ||
| 0–1 | 42 (88) | 26 (79) |
| 2–3 | 5 (10) | 7 (21) |
| Unknown | 2 (4) | 0 (0) |
| Cytogenetics | ||
| Complex, −5 and/or −7 | 30 (63) | 15 (45) |
| Diploid | 11 (23) | 6 (18) |
| Others | 5 (10) | 9 (27) |
| Not done/IM | 2 (4) | 3 (9) |
| s-AML/t-AML | 15 (31) | 9 (27) |
| FLT3-ITD mutationb | 2 (6) | 3 (12) |
| Number of prior therapies | 2 (1–7) | 2 (1–5) |
| Response to last treatment | ||
| Refractory | 22 (46) | 17 (52) |
| First relapse (remission <12 months) | 6 (13) | 7 (21) |
| First relapse (remission ≥12 months) | 12 (25) | 2 (6) |
| Second or later relapse | 8 (17) | 7 (21) |
| Prior SCT | 7 (15) | 2 (6) |
Continuous variables are listed as median (range) and categorical variables as n (%).
Information on FLT3 mutation status was available in 31 and 36 patients in the CIA and FIA groups, respectively.
CIA: clofarabine, idarubicin, and cytarabine; FIA: fludarabine, idarubicin, and cytarabine; WBC:white blood cell; BM: bone marrow; LDH: lactate dehydrogenase; IM: insufficient metaphases; s-AML/t-AML: secondary/therapy-related AML; ITD: internal tandem duplication; SCT: stem cell transplantation.
Response rates
The response rates of the two treatment arms are shown in Table 3. One patient in the CIA arm and two patients in the FIA arm received two courses of induction; all other patients received only one course of induction. Of the three patients who received two courses of induction, one patient in the FIA arm achieved CR, and the others did not response. The composite CR/CRp rate did not differ between the CIA and FIA arms (38% versus 30%, respectively; p = .50). CR rates were also similar between the two treatment arms (27% versus 24%, respectively; p = .77). Response rates between the two treatment arms did not differ when patients who died prior to response assessment were excluded from the analysis.
Table 3.
Response rates by treatment arm.
| Best response | CIA n (%) | FIA n (%) | p |
|---|---|---|---|
| CR | 13 (27) | 8 (24) | .77 |
| CRp | 5 (10) | 2 (6) | |
| CR + CRp | 18 (38) | 10 (30) | .50 |
| HI | 4 (8) | 2 (6) | |
| PR | 1 (3) | 0 | |
| No response | 24 (50) | 19 (56) | |
| Early death | 5 (10) | 4 (12) |
CIA: clofarabine, idarubicin, and cytarabine; FIA: fludarabine, idarubicin, and cytarabine; CR: complete remission; CRp: complete remission without platelet recovery; PR: partial remission.
As expected, response rates in both arms were superior for patients who had only received one prior therapy. Comparing patients who had received one prior therapy to those who had received ≥2 prior therapies, the CR/CRp rates for CIA were 55% and 23%, respectively, and for FIA were 54% and 15%, respectively (p = .02 for both). Of the 12 patients in the CIA arm with a CR1 duration ≥12 months, 9 (75%) achieved CR/CRp (versus 25% for all other patients treated with CIA; p = .002). Only two patients in the FIA arm had a CR1 duration ≥12 months; one patient achieved CR and the other died prior to response assessment. Among the 9 patients who received prior SCT (CIA, n = 7; FIA, n = 2), only one patient in the CIA arm achieved CR; no patients with prior SCT in the FIA arm achieved CR or CRp. Within each treatment arm, response rates did not significantly differ based on age, white blood cell count, cytogenetics, or diagnosis of secondary- or therapy-related AML. Additionally, no differences in response were noted between treatment arms when patients were stratified by these baseline prognostic factors.
Twelve patients (25%) in the CIA group and seven patients (21%) in the FIA group received at least one additional course of consolidation. Among patients who received consolidation, the median number of consolidation courses received in two treatment arms were 1.5 (1–6) and 1 (1–3), respectively. Overall, eight patients (17%) in the CIA arm and 7 (21%) in the FIA arm proceeded to SCT. Among patients who had received only one prior therapy, 6 out 22 (27%) and 5 out of 13 (38%) underwent SCT, respectively. Among patients who achieved CR/CRp, 8 out of 18 (44%) in the CIA arm and 7 out of 10 (70%) in the FIA arm subsequently underwent SCT.
Survival
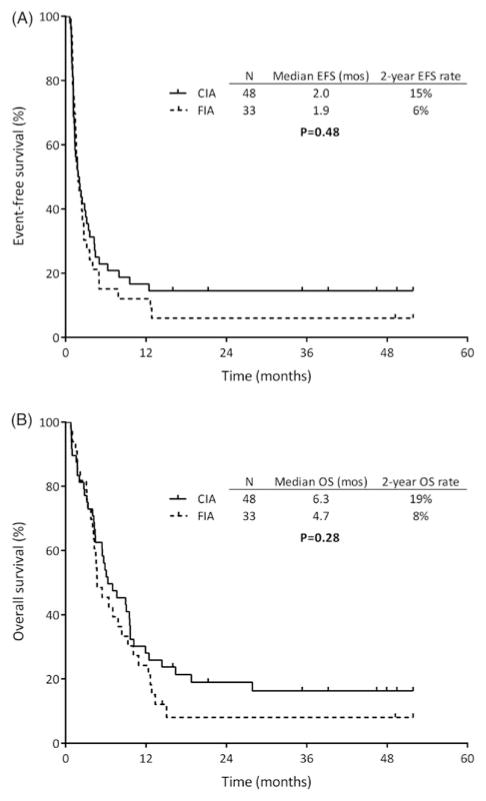
The median duration of follow-up was 43 months (5–52 months). The median EFS for patients who received CIA and FIA was 2.0 and 1.9 months, and two-year EFS rates were 15% and 6%, respectively (p = .48; Figure 1(A)). The median OS for the two groups was 6.3 and 4.7 months, and two-year OS rates were 19% and 8%, respectively. (p = .28; Figure 1(B)). Survival of the two treatment groups also did not differ when only patients who achieved CR/CRp and those who had only received one prior line of therapy were analyzed. Among patients who achieved CR/CRp, the median OS was 12.5 months for CIA and 9.9 months for FIA (p = .40). Similarly, among patients who had only received one prior therapy, the median EFS was 4.0 and 3.2 months (p = .57), and the median OS was 9.0 and 5.5 months, respectively (p = .35). In this subgroup of patients, the two-year OS rates with CIA and FIA were 27% and 15%, respectively. Patients in the CIA arm with CR1 duration ≥12 months had a median EFS and OS of 32 months and two-year EFS and OS rates of 50%. As only two patients in the FIA arm had a CR1 duration ≥12 months, survival analysis was not possible for this regimen. One patient died after one month and the other was still alive without relapse 52 months after enrollment.
Figure 1.
(A) Event-free survival (EFS) and (B) overall survival (OS) of CIA and FIA.
Safety
All randomized patients received the assigned study regimen and were evaluable for toxicity assessment. As expected, myelosuppression occurred in all patients. Among patients who achieved CR, the median time to count recovery after induction arm was 34 days (26–85 days) in the CIA arm and 36 days (26–62 days) in the FIA arm. Non-hematologic adverse events by treatment arm are shown in Table 4. The most common grade 3 or 4 adverse events observed were infection (71% in the CIA arm versus 63% in the FIA arm) and febrile neutropenia (44% versus 27%, respectively). No significant differences in individual adverse events were observed between treatments. The 30-day mortality rates in the CIA and FIA arms were 10% and 3% (p = .21), and the 60-day mortality rates were 17% and 15%, respectively (p = .86).
Table 4.
Nonhematologic toxicities possibly related to the study treatment.
| CIA (n = 48) | FIA (n = 33) | |||
|---|---|---|---|---|
|
|
|
|||
| Toxicity | All grades, n (%) | Grade 3/4, n (%) | All grades, n (%) | Grade 3/4, n (%) |
| Cardiac arrhythmia | 0 | 0 | 2 (6) | 0 |
| Colitis | 0 | 0 | 2 (6) | 2 (6) |
| Congestive heart failure | 2 (4) | 2 (4) | 0 | 0 |
| Constipation | 3 (6) | 0 | 2 (6) | 1 (3) |
| Diarrhea | 11 (23) | 1 (2) | 10 (30) | 0 |
| Dyspepsia | 1 (2) | 1 (2) | 3 (9) | 0 |
| Dyspnea | 3 (6) | 1 (2) | 1 (3) | 0 |
| Edema | 4 (8) | 1 (2) | 3 (9) | 0 |
| Elevated ALT/AST | 8 (17) | 0 | 7 (21) | 4 (12) |
| Elevated bilirubin | 16 (33) | 0 | 7 (21) | 0 |
| Elevated creatinine | 7 (15) | 1 (2) | 2 (6) | 0 |
| Febrile neutropenia | 21 (44) | 21 (44) | 9 (27) | 9 (27) |
| Hemorrhage | 7 (15) | 3 (6) | 6 (18) | 4 (12) |
| Hyperglycemia | 1 (2) | 1 (2) | 0 | 0 |
| Hypotension | 3 (6) | 3 (6) | 2 (6) | 1 (3) |
| Infection | 36 (75) | 34 (71) | 22 (66) | 21 (63) |
| Mucositis/stomatitis | 10 (21) | 1 (2) | 6 (18) | 2 (6) |
| Muscle weakness | 3 (6) | 0 | 1 (3) | 1 (3) |
| Nausea/vomiting | 18 (38) | 3 (6) | 12 (36) | 4 (12) |
| Pain | 11 (23) | 7 (15) | 12 (36) | 5 (15) |
| Pancreatitis | 1 (2) | 1 (2) | 0 | 0 |
| Pleural effusion | 0 | 0 | 4 (12) | 0 |
| Pruritus | 3 (6) | 1 (2) | 0 | 0 |
| Rash | 7 (15) | 0 | 3 (9) | 0 |
| Syncope | 1 (2) | 1 (2) | 1 (3) | 1 (3) |
| Venous thromboembolism | 3 (6) | 3 (6) | 0 | 0 |
ALT: alanine transaminase; AST: aspartate transaminase.
Discussion
The outcome of patients with relapsed or refractory AML is poor, and there is no standard-of-care induction regimen in this setting. In this study, evaluating two nucleoside analogue-containing salvage regimens in patients with relapsed/refractory AML, no differences in response rates or survival outcomes were seen in patients treated with CIA compared to those who received FIA. Adverse event rates and early mortality were also similar with these two regimens.
Despite the similar efficacy of the CIA and FIA arms, the results of the study are nevertheless notable as the response rates and survival observed with both of these regimens compare favorably to those previously reported in this population and therefore provide further support for the use of novel combination regimens for patients with relapsed/refractory AML. For example, in a large phase III study evaluating elacytarabine versus investigator choice in relapsed/refractory AML, the response rate for patients in the control arm (including patients receiving high-dose cytarabine or multiagent chemotherapy) was only 21% and the median survival was 3.3 months; no survival differences were observed between the various treatments used in the control arm [29]. Response rates for intermediate-dose cytarabine reported in other phase III trials of relapsed/refractory AML have similarly ranged between approximately 20% and 25% [7,30], which appear inferior to the response rates of 30–38% observed with CIA and FIA in the present study.
Given the poor response rates observed with single-agent cytarabine in the salvage setting, an anthracycline is often added, although the added benefit of this practice has not been confirmed in randomized, controlled trials [31]. The VALOR study showed a potential survival benefit with the addition of vosaroxin to cytarabine in patients with relapsed/refractory AML, which may be particularly useful in patients who are unable to receive additional anthracyclines [30]. Other randomized studies have investigated the addition of a third or fourth agent to standard cytarabine and anthracycline-based chemotherapy with mixed results [32].
In contrast, a number of studies in patients with AML treated with novel combination regimens incorporating nucleoside analogues have shown particularly promising results. The CLASSIC I study which evaluated cytarabine with or without clofarabine for older patients with relapsed/refractory AML resulted in significantly improved response rates and EFS with the combination regimen, although an OS benefit was not seen (median OS 6.6 months for clofarabine plus cytarabine versus 6.3 months for cytarabine alone) [7]. Similarly, FLAG-Ida has been associated with longer CR duration and lower rates of relapsed compared to ADE chemotherapy in younger patients in the frontline setting [23]. These randomized studies have provided clinical evidence in support of in vitro data that nucleoside analogues are synergistic with cytarabine [10]. Other nucleoside analogue-based regimens including BIDFA (twice-daily fludarabine and cytarabine) and cladribine, cytarabine and G-CSF with or without mitoxantrone (CLAG and CLAG-M) have also shown significant clinical activity as salvage regimens [33–35].
It is notable that in the present study CR/CRp rates of 30–38% were observed despite a particularly poor-risk patient population. A majority of enrolled patients had received ≥2 prior lines of therapy and approximately 30% had secondary- or therapy-related AML. Among patients who had received only one prior therapy, the response rates for CIA and FIA were 55% and 54%, respectively. The achievement of a remission in a majority of this subgroup of patients is important because it allowed 11 of these patients (31%) to proceed to allogeneic SCT, which is the only established curative therapy in the relapsed/refractory setting.
The results from this randomized trial have several potential therapeutic implications for the management of AML. First, given the high response rates observed with both of these regimens, as well as their similar toxicity profile, both CIA and FIA regimens appear to be reasonable treatment options in the salvage setting. The ability of these regimens to achieve remission in a majority of patients who had received only one prior line of therapy, and especially those with a CR1 duration ≥12 months, suggests that that for fit patients able to achieve intensive chemotherapy, these regimens may be particularly useful as a bridge to SCT. Notably, although these regimens combine three cytoxic agents, the chosen dosing and schedule resulted in acceptable 30- and 60-day mortality rates comparable those reported in other studies in relapsed/refractory AML [7,29,30]. Further evaluation of nucleoside analogue and cytarabine combination therapies is warranted, and a randomized trial comparing CIA and FIA in the frontline setting has completed accrual and will be reported separately.
In conclusion, we found that clofarabine at a dose of 15 mg/m2 given for five days in combination with cytarabine and idarubicin was tolerable and effective. In a phase II randomized comparison of CIA and FIA in patients with relapsed/refractory AML, response rates, survival, and toxicity were similar in the two treatment arms. The response rates achieved in patients treated with each of these regimens compare favorably to those previously reported in large AML series, especially in patients who had received only one prior line of therapy. These results suggest that CIA and FIA are safe and effective salvage treatments for patients with relapsed/refractory AML.
Acknowledgments
Funding: Supported in part by the MD Anderson Cancer Center Support Grant CA016672.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2017.1352097.
References
- 1.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23:1969–1978. doi: 10.1200/JCO.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 2.Pemmaraju N, Kantarjian H, Garcia-Manero G, et al. Improving outcomes for patients with acute myeloid leukemia in first relapse: a single center experience. Am J Hematol. 2015;90:27–30. doi: 10.1002/ajh.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thol F, Schlenk RF, Heuser M, et al. How I treat refractory and early relapsed acute myeloid leukemia. Blood. 2015;126:319–327. doi: 10.1182/blood-2014-10-551911. [DOI] [PubMed] [Google Scholar]
- 4.Estey EH, Thall PF, Pierce S, et al. Randomized phase II study of fludarabine + cytosine arabinoside + idarubicin + /− all-trans retinoic acid + /− granulocyte colony-stimulating factor in poor prognosis newly diagnosed acute myeloid leukemia and myelodysplastic syndrome. Blood. 1999;93:2478–2484. [PubMed] [Google Scholar]
- 5.Kantarjian H, Gandhi V, Cortes J, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–2386. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, Erba HP, Claxton D, et al. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. JCO. 2010;28:549–555. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- 7.Faderl S, Wetzler M, Rizzieri D, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. JCO. 2012;30:2492–2499. doi: 10.1200/JCO.2011.37.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willemze R, Suciu S, Muus P, et al. Clofarabine in combination with a standard remission induction regimen (cytosine arabinoside and idarubicin) in patients with previously untreated intermediate and bad-risk acute myelogenous leukemia (AML) or high-risk myelodys-plastic syndrome (HR-MDS): phase I results of an ongoing phase I/II study of the leukemia groups of EORTC and GIMEMA (EORTC GIMEMA 06061/AML-14A trial) Ann Hematol. 2014;93:965–975. doi: 10.1007/s00277-014-2056-6. [DOI] [PubMed] [Google Scholar]
- 9.Buckley SA, Mawad R, Gooley TA, et al. A phase I/II study of oral clofarabine plus low-dose cytarabine in previously treated acute myeloid leukaemia and high-risk myelodysplastic syndrome patients at least 60 years of age. Br J Haematol. 2015;170:349–355. doi: 10.1111/bjh.13437. [DOI] [PubMed] [Google Scholar]
- 10.Robak T. Purine nucleoside analogues in the treatment of myleoid leukemias. Leuk Lymphoma. 2003;44:391–409. doi: 10.1080/1042819021000035608. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi V, Plunkett W. Modulation of arabinosylnucleoside metabolism by arabinosylnucleotides in human leukemia cells. Cancer Res. 1988;48:329–334. [PubMed] [Google Scholar]
- 12.Seymour JF, Huang P, Plunkett W, et al. Influence of fludarabine on pharmacokinetics and pharmaco-dynamics of cytarabine: implications for a continuous infusion schedule. Clin Cancer Res. 1996;2:653–658. [PubMed] [Google Scholar]
- 13.Gandhi V, Estey E, Keating MJ, et al. Biochemical modulation of arabinosylcytosine for therapy of leukemias. Leuk Lymphoma. 1993;10:109–114. doi: 10.3109/10428199309149122. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi V, Estey E, Keating MJ, et al. Chlorodeoxyadenosine and arabinosylcytosine in patients with acute myelogenous leukemia: pharmacokinetic, pharmacodynamic, and molecular interactions. Blood. 1996;87:256–264. [PubMed] [Google Scholar]
- 15.Gandhi V, Estey E, Keating MJ, et al. Fludarabine potentiates metabolism of cytarabine in patients with acute myelogenous leukemia during therapy. J Clin Oncol. 1993;11:116–124. doi: 10.1200/JCO.1993.11.1.116. [DOI] [PubMed] [Google Scholar]
- 16.Kadia TM, Faderl S, Ravandi F, et al. Final results of a phase 2 trial of clofarabine and low-dose cytarabine alternating with decitabine in older patients with newly diagnosed acute myeloid leukemia. Cancer. 2015;121:2375–2382. doi: 10.1002/cncr.29367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nazha A, Kantarjian H, Ravandi F, et al. Clofarabine, idarubicin, and cytarabine (CIA) as frontline therapy for patients </= 60 years with newly diagnosed acute myeloid leukemia. Am J Hematol. 2013;88:961–966. doi: 10.1002/ajh.23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faderl S, Ravandi F, Huang X, et al. A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2008;112:1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowenberg B, Pabst T, Maertens J, et al. Therapeutic value of clofarabine in younger and middle-aged (18–65 years) adults with newly diagnosed AML. Blood. 2017;129:1636–1645. doi: 10.1182/blood-2016-10-740613. [DOI] [PubMed] [Google Scholar]
- 20.Koller CA, Kantarjian HM, Feldman EJ, et al. A phase I–II trial of escalating doses of mitoxantrone with fixed doses of cytarabine plus fludarabine as salvage therapy for patients with acute leukemia and the blastic phase of chronic myelogenous leukemia. Cancer. 1999;86:2246–2251. doi: 10.1002/(sici)1097-0142(19991201)86:11<2246::aid-cncr11>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. JCO. 2011;29:369–377. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- 22.Guolo F, Minetto P, Clavio M, et al. High feasibility and antileukemic efficacy of fludarabine, cytarabine and idarubicin (FLAI) induction followed by risk-oriented consolidation: a critical review of a ten-year, single-centre experience in younger, non m3 AML patients. Am J Hematol. 2016;8:755–762. doi: 10.1002/ajh.24391. [DOI] [PubMed] [Google Scholar]
- 23.Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute mye-loid leukemia: results of the medical research council AML15 trial. JCO. 2013;31:3360–3368. doi: 10.1200/JCO.2012.47.4874. [DOI] [PubMed] [Google Scholar]
- 24.Ghanem H, Kantarjian H, Ohanian M, et al. The role of clofarabine in acute myeloid leukemia. Leuk Lymphoma. 2013;54:688–698. doi: 10.3109/10428194.2012.726722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 26.Gandhi V, Huang P, Chapman AJ, et al. Incorporation of fludarabine and 1-beta-d-arabinofuranosylcytosine 5′-triphosphates by DNA polymerase alpha: affinity, interaction, and consequences. Clin Cancer Res. 1997;3:1347–1355. [PubMed] [Google Scholar]
- 27.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. JCO. 2010;28:1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. JCO. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 29.Roboz GJ, Rosenblat T, Arellano M, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. JCO. 2014;32:1919–1926. doi: 10.1200/JCO.2013.52.8562. [DOI] [PubMed] [Google Scholar]
- 30.Ravandi F, Ritchie EK, Sayar H, et al. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2015;16:1025–1036. doi: 10.1016/S1470-2045(15)00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karanes C, Kopecky KJ, Head DR, et al. A phase III comparison of high dose ARA-C (HIDAC) versus HIDAC plus mitoxantrone in the treatment of first relapsed or refractory acute myeloid leukemia Southwest Oncology Group Study. Leuk Res. 1999;23:787–794. doi: 10.1016/s0145-2126(99)00087-9. [DOI] [PubMed] [Google Scholar]
- 32.Short NJ, Ravandi F. Acute myeloid leukemia: past, present, and prospects for the future. Clin Lymphoma Myeloma Leuk. 2016;16(Suppl):S25–S29. doi: 10.1016/j.clml.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Jabbour E, Garcia-Manero G, Cortes J, et al. Twice-daily fludarabine and cytarabine combination with or without gentuzumab ozogamicin is effective in patients with relapsed/refractory acute myeloid leukemia, high-risk myelodysplastic syndrome, and blast-phase chronic myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2012;12:244–251. doi: 10.1016/j.clml.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price SL, Lancet JE, George TJ, et al. Salvage chemotherapy regimens for acute myeloid leukemia: Is one better? Efficacy comparison between CLAG and MEC regimens. Leuk Res. 2011;35:301–304. doi: 10.1016/j.leukres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Wierzbowska A, Robak T, Pluta A, et al. Cladribine combined with high doses of arabinoside cytosine, mitoxantrone, and G-CSF (CLAG-M) is a highly effective salvage regimen in patients with refractory and relapsed acute myeloid leukemia of the poor risk: a final report of the Polish Adult Leukemia Group. Eur J Haematol. 2008;80:115–126. doi: 10.1111/j.1600-0609.2007.00988.x. [DOI] [PubMed] [Google Scholar]