Abstract
Objective
The aim of this study is to evaluate fall rates across body mass index (BMI) categories by age group, considering physical performance and comorbidities.
Method
In the Osteoporotic Fractures in Men (MrOS) study, 5,834 men aged ≥65 reported falls every 4 months over 4.8 (±0.8) years. Adjusted associations between BMI and an incident fall were tested using mixed-effects models.
Results
The fall rate (0.66/man-year overall, 95% confidence interval [CI] = [0.65, 0.67]) was lowest in the youngest, normal weight men (0.44/man-year, 95% CI = [0.41, 0.47]) and greatest in the oldest, highest BMI men (1.47 falls/man-year, 95% CI = [1.22, 1.76]). Obesity was associated with a 24% to 92% increased fall risk in men below 80 (ptrend ≤ .0001, p for interaction by age = .03). Only adjustment for dynamic balance test altered the BMI–falls association substantially.
Discussion
Obesity was independently associated with higher fall rates in men 65 to 80 years old. Narrow walk time, a measure of gait stability, may mediate the association.
Keywords: BMI, falls, obesity
Introduction
Falls are the leading source of injury and disability in adults aged 65 years and older (Stevens, Mack, Paulozzi, & Ballesteros, 2008). One in three older adults falls annually, and the likelihood of falling increases with advancing age (Tinetti, Speechley, & Ginter, 1988). In addition, performance on tests of gait and balance decline with age (Seeman et al., 1994), and in turn, poor performance is strongly associated with increased fall risk (Nevitt, Cummings, Kidd, & Black, 1989; Tinetti et al., 1988). Moreover, physical function and gait stability are lower in individuals who are obese (Capodaglio et al., 2010; Maffiuletti et al., 2005; Mignardot, Olivier, Promayon, & Nougier, 2010); therefore, obesity among older adults may be particularly problematic for falls. Individuals who are obese report a greater prevalence of fall history (Fjeldstad, Fjeldstad, Acree, Nickel, & Gardner, 2008; Mitchell, Lord, Harvey, & Close, 2014; Ren et al., 2014; Siqueira et al., 2011), but prospective studies show inconsistent associations between body mass index (BMI) and falls (Himes & Reynolds, 2012; Kelsey, Procter-Gray, Hannan, & Li, 2012). Women who have android body fat distribution or a higher center of mass, both more common in men, have an increased risk of falls (Almeida, Castro, Pedreira, Heymann, & Szejnfeld, 2011; Hita-Contreras et al., 2013), indicating that sex differences in the BMI–falls association due to body fat distribution may exist. In recent decades, the prevalence of obesity among men older than 60 years in the United States increased to an estimated 32%, one third of whom are Class II or III obese (Ogden, Carroll, Kit, & Flegal, 2014).
The concurrent aging of the U.S. population and the rise in prevalence of obesity among older adults (Mokdad et al., 1999) prompted our evaluation of the association between BMI and prospectively ascertained falls in the Osteoporotic Fractures in Men (MrOS) study, and the effects of age and physical function on this association. Although previous MrOS publications have added to our understanding of fall rates in older men, ours is the first to specifically examine the association between BMI and falls.
Method
Study Population
MrOS is a prospective cohort study of community-dwelling men in the United States. Between March 2000 and April 2002, 5,994 men were enrolled in the study at six clinical centers: Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Pittsburgh, PA; Portland, OR; and San Diego, CA. MrOS participants met the following criteria: at least 65 years of age, able to consent, able to walk without the assistance of another person, and no bilateral hip replacement. One hundred sixty participants were excluded from this analysis due to missing BMI (n = 2), underweight (BMI less than 18.5, n = 6), missing data on falls (n = 18), and missing data on physical function tests (n = 134). All participants provided informed consent, and the protocol was approved by the institutional review boards of participating institutions. MrOS study design and recruitment details have been published elsewhere (Blank et al., 2005; Orwoll et al., 2005).
BMI Measurement
During the baseline clinic visit, MrOS participants had their height (cm) measured using Harpenden stadiometers and weight (kg) on calibrated standard balance beam or digital scales. BMI was calculated from height and weight measures and classified according to the World Health Organization (WHO) guidelines as normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), Obese Class I (30–34.9 kg/m2), and Obese Classes II and III (≥35 kg/m2). Only six men were underweight (<18.5 kg/m2) at the baseline visit; therefore, this category was excluded from analysis.
Ascertainment of Falls
Study participants received a one-page follow-up questionnaire every 4 months. Those who reported falling in the previous 4 months were asked how many times (1, 2, 3, 4, or ≥5; Orwoll et al., 2005). Participants who did not initially return or who did not adequately complete the questionnaire received a telephone call. The present study included fall reports from the first through the 15th (5 years) tri-annual questionnaire cycles. A total of 5,976 men (99.7%) returned at least one questionnaire (those who did not were excluded from this analysis), of which 86.2% returned all 15 questionnaires. About 99% had at least one full year of follow-up. Participants were followed until death or termination from the study. Incident fall rates were calculated by dividing the total number of falls, including recurring falls, by corresponding person-years. The highest fall rates may be underestimated due to the questionnaire’s truncated response options, where men with five or more reported falls were categorized as having five falls.
Physical Performance
Physical performance measures included grip strength, leg power, 6 m walk speed, dynamic balance time, and repeated chair stand time. Grip strength was assessed in each hand using a handheld dynamometer, and the maximum score (kg) from two trials from both hands was analyzed. Leg extension power was measured on the Nottingham Power Rig (University of Nottingham, England) with nine trials for each leg. The maximum value (watts) from either leg was analyzed (Bassey et al., 1992; Bassey & Short, 1990). Walking speed was assessed on a standard 6-m walking course; participants were instructed to walk at their normal pace and the fastest speed (m/s) of two trials was analyzed. Time to complete a narrow walk course of 6 m × 20 cm was used as an indirect measure of dynamic balance. Participants were considered able to complete the narrow walk trial if they had no more than two deviations from the lane. Participants’ ability to rise from a chair 5 times without using their arms and the time required to complete the task were determined. Participants who could not complete a physical function measure were classified as “unable.” Those who were able were categorized into tertiles with the best scores as the first tertile, the worst as the third tertile, and “unable” as the fourth group. Participants who were missing walking speed, grip strength, chair stand time, or narrow walk time were excluded from the study (n = 134).
Physical Activity
The Physical Activity Scale for the Elderly (PASE), a score calculated from various physical activities, including sitting, walking, exercising, housework, and volunteer work, in the previous 7 days (Washburn, McAuley, Katula, Mihalko, & Boileau, 1999; Washburn, Smith, Jette, & Janney, 1993) was used to assess participants’ physical activity level at baseline. Participants who responded yes to having any difficulty with at least one of the following five activities of daily living (ADLs) were categorized as having an ADL impairment: walking two or three blocks outside on level ground, climbing 10 steps without resting, preparing their own meals, doing heavy housework, or doing their own shopping. Men who reported that they walked outside their home or yard at least 5 of the previous 7 days were categorized as “often” walking outside. PASE score, ADL impairments, and often walking outside were self-reported at baseline.
Medication Use and Medical Conditions
Participants were asked to bring all prescription medications they had taken daily or almost daily for at least 1 month to the clinic visit. If a participant did not bring one or more medications, clinic staff obtained this information by telephone or at a return visit. Each medication was verified by pill bottle examination, entered into an electronic database, and matched to its ingredient(s) using the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA; Pahor et al., 1994). Medications included antihypertensive agents, hypoglycemic agents, selective serotonin reuptake inhibitors, tricyclic antidepressants, statins, benzodiazepines, anticonvulsants, and opioid analgesics.
All medical conditions were self-reported at baseline in response to the question, “Has a doctor or other health care provider ever told you that you had or have . . . ?” Medical conditions examined in the analysis included osteoarthritis, arthritis in the hip and knee, back and lower back pain, osteoporosis, diabetes, hypertension, and stroke. Participant self-reported health was based on their response to the question “Compared with other people your own age, how would you rate your overall health?” Men who reported having one or more falls during the previous 12 months were categorized as having a history of falls.
Statistical Analyses
After exclusions, 5,834 men were included in the analytic data set. In descriptive analyses, we examined differences in baseline characteristics by BMI group, using chi-square tests for categorical variables and ANOVA for continuous variables. Sidak post hoc tests were used to compare means for each BMI group compared with the normal BMI group. Incident fall rate and confidence intervals were calculated for each BMI category. The probability of any reported fall was modeled using generalized linear mixed-effects (GLMM) models, comparing higher BMI categories with the normal BMI reference category. All models included age and clinic site as covariates. Confounding was assessed by entering covariates to the base model to determine whether the association between falls and any of the BMI categories changed by 10% or more. Potential confounders included medical conditions and medications, and potential mediators were measures of physical activity and physical function. The final multivariable model included adjustments for age, site, and narrow walk time. The interaction of age and BMI was tested in GLMM models to assess age as a potential effect modifier of the BMI–falls association. All analyses were conducted using SAS version 9.4 (SAS Institute, Inc. Cary, NC, USA).
Results
The majority of men in this study were overweight (51%) or obese (21%), and mean ages in these higher BMI groups were 2.5 to 3.5 years lower than the mean age in the normal BMI group (74.8 ± 6.3 years, Table 1). Approximately 17% of men were 80 years or older, 13% of whom were obese. In descriptive analyses, we observed that men who were overweight and obese had 13% to 26% greater leg power and 5% to 6% higher grip strength than men with normal BMI (Table 1). Men who were in the highest BMI group had 15% slower walking speed and 11% slower narrow walk time compared with men with normal BMI. These differences were significant (p < .05) in post hoc analyses. About 17% of men who were Obese Classes II and III were unable to complete the narrow walk test whereas only 8% of men with normal BMI were unable to complete the test. Incrementally higher BMI showed higher prevalence of chronic medical conditions, with hypertension nearly twofold and diabetes threefold more frequent among men with BMI in the overweight and obese categories. Likewise, medication use was more prevalent in men with higher BMI.
Table 1.
Selected Baseline Characteristics by BMI (n = 5,834).
| n | Normal (18.5 to <25) n = 1,591 | Overweight (25 to <30) n = 2,999 | Obese I (30 to <35) n = 1,012 | Obese II and III (35 and above) n = 232 | pa | |
|---|---|---|---|---|---|---|
| Age | 74.8 (±6.3) | 73.5 (±5.7)b | 72.3 (±5.2)b | 71.3 (±4.8)b | <.001 | |
| 65–69 | 1,745 | 383 (24.1) | 896 (29.9) | 368 (36.4) | 98 (42.2) | <.001 |
| 70–74 | 1,678 | 438 (27.5) | 843 (28.1) | 323 (31.9) | 74 (31.9) | |
| 75–79 | 1,402 | 387 (24.3) | 770 (25.7) | 202 (20.0) | 43 (18.5) | |
| 80 and above | 1,009 | 383 (24.1) | 490 (16.3) | 119 (11.8) | 17 (7.3) | |
| Race: White | 5,220 | 1,409 (88.6) | 2,700 (90.0) | 907 (89.6) | 204 (87.9) | .391 |
| Fair/poor health | 794 | 173 (10.9) | 376 (12.5) | 173 (17.1) | 72 (31.0) | <.001 |
| History of fallsc | 1,211 | 349 (21.9) | 583 (19.4) | 219 (21.6) | 60 (25.9) | .032 |
| PASE score | 5,831 | 150.1 (±69.8) | 148.2 (±66.6) | 145.8 (±67.3) | 129.2 (±72.6)b | .0002 |
| Physical function | ||||||
| Repeated chair stands (s) | 5,693 | 10.9 (±3.5) | 10.9 (±3.1) | 11.5 (±3.1)b | 12.5 (±3.8)b | <.001 |
| Unabled | 141 | 33 (2.1) | 64 (2.1) | 32 (3.2) | 12 (5.2) | |
| Leg power (watts) | 5,336 | 188.0 (±54.1) | 212.4 (±61.2)b | 227.1 (±68.6)b | 237.4 (±69.9)b | <.001 |
| Unablee | 83 | 18 (1.2) | 35 (1.3) | 18 (1.9) | 12 (5.7) | |
| Narrow walk time (s) | 5,352 | 5.6 (±1.7) | 5.5 (±1.9) | 5.7 (±2.2) | 6.1 (±1.5)b | <.001 |
| Unablef | 482 | 126 (7.9) | 218 (7.3) | 98 (9.7) | 40 (17.2)b | |
| Walking speed (m/s) | 5,799 | 1.3 (±0.2) | 1.3 (±0.2) | 1.2 (±0.2)b | 1.1 (±0.2)b | <.001 |
| Unableg | 35 | 8 (0.5) | 18 (0.6) | 5 (0.5) | 4 (1.7) | |
| Grip strength (kg) | 5,741 | 40.3 (±8.1) | 42.1 (±8.5)b | 42.8 (±8.7)b | 42.8 (±8.1)b | <.001 |
| Unableh | 93 | 32 (2.0) | 45 (1.5) | 12 (1.2) | 4 (1.7) | |
| ADL impairmenti | 1,145 | 253 (15.9) | 533 (17.8) | 271 (26.8)b | 88 (37.9)b | <.001 |
| Often walk outside | 2,799 | 838 (52.7) | 1,458 (48.6)b | 415 (41.0)b | 88 (37.9)b | <.001 |
| Medical conditionsj | ||||||
| Osteoarthritis | 1,131 | 283 (17.8) | 576 (19.2) | 215 (21.2) | 57 (24.6) | .031 |
| Osteoporosis | 200 | 68 (4.3) | 103 (3.4) | 27 (2.7) | 2 (0.9) | .020 |
| Diabetes | 618 | 104 (6.5) | 295 (9.8) | 162 (16.0) | 57 (24.6) | <.001 |
| Hypertension | 2,507 | 512 (32.2) | 1,332 (44.4) | 525 (51.9) | 138 (59.5) | <.001 |
| Stroke | 323 | 109 (6.9) | 150 (5.0) | 54 (5.3) | 10 (4.3) | .054 |
| Medication usej | ||||||
| Statin use | 1,531 | 356 (22.4) | 832 (27.7) | 274 (27.1) | 69 (29.7) | <.001 |
| Benzodiazepine use | 197 | 70 (4.4) | 86 (2.9) | 33 (3.3) | 8 (3.4) | .057 |
| Anticonvulsant use | 125 | 38 (2.4) | 53 (1.8) | 22 (2.2) | 12 (5.2) | .005 |
| Opioid analgesic use | 148 | 34 (2.1) | 71 (2.4) | 31 (3.1) | 12 (5.2) | .029 |
Note. BMI = body mass index; PASE = Physical Activity Scale for the Elderly; ADL = activity of daily living.
Comparisons were made by chi-square test for categorical variables or ANOVA for continuous variables.
Baseline characteristic is significantly different from the normal BMI group in post hoc pairwise comparisons.
Reported having at least one fall in the 12 months before baseline.
Unable to complete five repeated chair stands test: Unable to complete the stands without assistance, unable to complete all five stands, used their arms to stand at least once.
Unable to complete leg power test: Attempted but unable, missing value (possible physical limitations), skipped if had a hip replacement in the 6 months prior to baseline visit.
Unable to complete the narrow walk test: Used walking aids or stepped on or stepped outside tape marking 20-cm narrow walk two or more times in more than one trial.
Unable to complete the walking speed test: Used a walking aid.
Unable to complete the grip strength test: Skipped if pain in hands had gotten worse recently, or if had surgery on hands or wrists in the past 3 months.
ADL impairment indicates any difficulty with at least one of five ADLs: walking two or three blocks outside on level ground, climbing 10 steps without resting, preparing meals, doing heavy housework, or doing their own shopping.
Medical conditions and medication use were self-reported. Medication use categorized as “yes” if positive response and “no” if negative response or missing response.
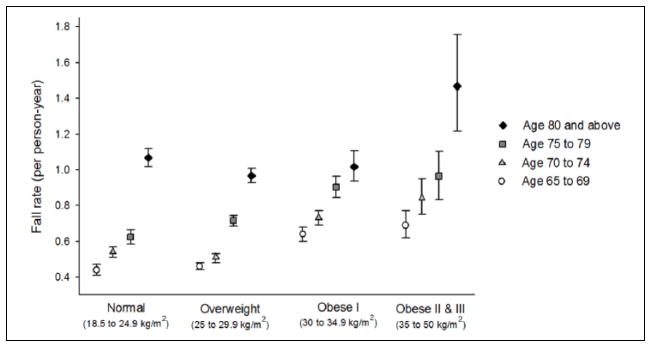
Over an average of 4.8 ± 0.8 years of follow-up, the fall rate was 0.66 per man-year. Fall rates increased with greater BMI, with men who were the most obese (≥35 kg/m2) having the highest fall rate (0.84/man-year) overall but with a wide range across ages in this BMI category (Figure 1). Rate of falls increased with greater age, with the highest fall rate in men aged 80 years and older (1.02/man-year). The strength of association between BMI and falls appeared stronger in younger age groups than in men aged 80 and older, and the interaction was statistically significant (p = .03). Among men aged 65 to 79 years, higher fall rates occurred in higher BMI categories; men aged 65 to 69 with Obesity Classes II and III had nearly twice the risk of falls of normal weight men in the same age group (Table 2). Adjustment for medical history and medication use, including history of stroke or Parkinson’s disease and use of hypoglycemic and antihypertensive agents, did not substantially alter these associations (data not shown). Adjustment for physical performance measures as potential mediators showed that narrow walk time attenuated the risk of falls for men in Obese Classes II and III by up to 26%, such that the association in men aged 70 to 79 lost significance and only the youngest age group remained significant.
Figure 1.
Fall rate by age and BMI among older men (n = 5,834).
Note. BMI = body mass index.
Table 2.
Relative Risk (95% CI) of Falls by BMI Group Among Older Men (n = 5,834).
| Age | n | Normal (18.5 to <25) n = 1,591 | Overweight (25 to <30) n = 2,999 | Obese I (30 to <35) n = 1,012 | Obese II and III (35 and above) n = 231 |
|---|---|---|---|---|---|
| Adjusted for study site | |||||
| 65–69 | 1,745 | Ref. | 1.14 [0.91, 1.44] | 1.78 [1.36, 2.33] | 1.92 [1.28, 2.89] |
| 70–74 | 1,678 | Ref. | 0.83 [0.67, 1.03] | 1.24 [0.96, 1.61] | 1.69 [1.10, 2.61] |
| 75–79 | 1,402 | Ref. | 1.17 [0.94, 1.46] | 1.43 [1.06, 1.94] | 1.88 [1.09, 3.23] |
| 80 and above | 1,009 | Ref. | 0.88 [0.70, 1.11] | 0.86 [0.60, 1.23] | 1.45 [0.65, 3.27]a |
| Adjusted for study site and narrow walk time | |||||
| 65–69 | 1,745 | Ref | 1.12 [0.89, 1.42] | 1.65 [1.26, 2.15] | 1.71 [1.14, 2.56] |
| 70–74 | 1,678 | Ref. | 0.82 [0.67, 1.02] | 1.16 [0.90, 1.51] | 1.39 [0.90, 2.13] |
| 75–79 | 1,402 | Ref. | 1.14 [0.91, 1.41] | 1.26 [0.93, 1.70] | 1.40 [0.82, 2.40] |
| 80 and above | 1,009 | Ref. | 0.88 [0.70, 1.11] | 0.83 [0.58, 1.18] | 1.24 [0.55, 2.76]a |
Note. CI = confidence interval; BMI = body mass index.
Small group (n = 17). All other cell sizes >40.
Discussion
This prospective study of older men demonstrated an increased risk of falls among men who were obese, but only among those below age 80. In fact, fall rates in men who were obese below age 80 years were equivalent to fall rates for normal weight men in age groups 5 to 10 years older (Figure 1). Being overweight carried no increased risk of falls compared with normal weight. Potential mechanistic links between obesity and fall risk include direct effects of increased body mass on postural instability and obesity-related chronic conditions and medications that affect balance and physical performance (Clark, 2015; Corbeil, Simoneau, Rancourt, Tremblay, & Teasdale, 2001; Kelsey et al., 2012; Lord, Menz, & Tiedemann, 2003; Maffiuletti et al., 2005; Mignardot et al., 2010). Greater numbers of functional impairments and physical limitations generally increase fall risk (Lord et al., 2003), and our descriptive analyses suggested that these impairments are more prevalent among individuals who are obese (Capodaglio et al., 2010). Postural control, body orienting reflexes, muscle strength and tone, and stepping height all decrease with aging and impair ability to avoid a fall after an unexpected trip or slip (Rubenstein, 2006). Therefore, in the present study, we evaluated the physical function measures as potential mediators and found that only dynamic balance performance on the narrow walk test, an indicator of gait stability, explained a portion of the observed BMI–falls association.
The Health and Retirement Study (HRS) also observed statistically significant associations between obesity and falls (minimally adjusted odds ratios of 1.34–2.20, increasing with severity of obesity; Himes & Reynolds, 2012). The magnitude of the association was attenuated after adjustment for self-reported pain, dizziness, vision problems, diabetes, stroke, and arthritis (Himes & Reynolds, 2012), suggesting these factors may be potential mediators along the obesity–falls pathway. Although the current study included repeated objective measures of >5,000 men, it has fewer total individuals than the HRS, which perhaps limited our ability to detect these chronic conditions as mediators. A cross-sectional study of community-dwelling older adults found a 25% to 31% higher risk of falls and a higher prevalence of pain and inactivity in obese participants compared with normal weight participants (Mitchell et al., 2014). Another study suggested that self-reported sedentary behavior, chronic health conditions, and medication use were mediators of the obesity–falls association (Mitchell, Lord, Harvey, & Close, 2015). In other studies, increased fall risk occurred among older adults with lower limb arthritis (Sturnieks et al., 2004), diabetes (Maurer, Burcham, & Cheng, 2005), and stroke history (Simpson, Miller, & Eng, 2011). In the MrOS cohort, adjustment for these conditions did not alter the associations between BMI and falls. However, collectively, these studies point to multiple physical function and medical management targets for prevention of falls in older adults who are obese. Our study elucidates the role of gait instability as a mediator for falls in older men who are obese.
A strength of this study is the prospective ascertainment of falls. Prospective collection of fall information is essential for evaluating risk factors for falls in older adults, as underreporting has consistently been demonstrated (Cummings, Nevitt, & Kidd, 1988; Hannan et al., 2010). In the current study, fall information was collected every 4 months using mailed questionnaires, and about 86.2% of men completed all 15 questionnaires over the follow-up period.
This study has a number of limitations. Although participation was high, fall rates may be underestimated due to imperfect recall, and falls that were reported may be biased toward injurious falls. However, we could not evaluate this possibility because information on injuries associated with falls was not collected. Nonetheless, fall incidence in the current study was similar to that reported in the MOBILIZE Boston study of community-dwelling men and women aged 70 and older (0.83 fall/person-year with M age = 78 ± 5 years; Kelsey et al., 2012), but was higher than the rate reported in the Study of Osteoporotic Fractures (0.48 fall/woman-year with M age = 76 ± 5 years), which similarly used 3-month recall reporting of falls (Faulkner et al., 2005). Although fall rates are similar, there is undoubtedly room for improvement in the self-reporting of falls in older adults. Finally, this study only included men, and it is possible that the BMI associations we observed are not generalizable to older women.
The MOBILIZE Boston Study is the most complete study of incident falls in community-dwelling Americans to date. Of 765 men and women aged 70 and older (M age = 78 years), participants who were obese had a nonsignificant increased risk of falls compared with normal weight participants (Kelsey et al., 2012), and no age interaction was reported. Those above age 80 had the highest fall risk, but few were Obese II or III. Strong independent risk factors for falls included a low Berg Balance Score and slow gait speed, indicating that factors other than obesity may contribute to fall risk at that age. Therefore, those above age 80 who are obese (13% in MrOS) may need to focus on targets other than weight reduction to prevent falls.
Obesity in older adults is accompanied by multiple comorbidities and physical limitations, and both weight loss and physical activity interventions can improve physical function in older adults who are obese (Villareal et al., 2011). Multiple observational studies suggest that weight loss is not associated with better health outcomes (Barrett-Connor, Edelstein, Corey-Bloom, & Wiederholt, 1996; Ensrud et al., 2003; Lee et al., 2011; Villareal, Shah, Banks, Sinacore, & Klein, 2008; Wallace, Schwartz, LaCroix, Uhlmann, & Pearlman, 1995); however, weight loss in these studies may be indicative of poor underlying health status rather than improved diet or exercise. Further study of safety and efficacy of weight loss therapy in older adults is necessary. Recommendations for physical activity are being tailored to older adults (Rejeski et al., 2013; Sparling, Howard, Dunstan, & Owen, 2015) and should take into account the increased fall risk in individuals who are obese in this age group. Interventions that include strength, balance training, and walking show promise in preventing both falls and mobility disability (Pahor et al., 2014). Because the higher risk of falls in older men who are obese may be mediated by dynamic balance, further studies are warranted to determine whether targeted interventions to detect and improve dynamic balance can prevent falls. Moreover, because even non-catastrophic falls can increase fear of falling and limit physical activity and life-space mobility (Lo, Brown, Sawyer, Kennedy, & Allman, 2014), additional research is needed to better understand the circumstances and risk factors for falls in middle and older age groups. This will allow for fall prevention and physical activity recommendations that minimize injury and disability due to falls in older adults.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 RR024140. C.M.N. receives support from NIAMS K01 AR062655. C.G.L. receives support from a VA Clinical Science Research and Development Career Development Award, Project number 5IK2CW000729-02.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Almeida CW, Castro CH, Pedreira PG, Heymann RE, Szejnfeld VL. Percentage height of center of mass is associated with the risk of falls among elderly women: A case-control study. Gait & Posture. 2011;34:208–212. doi: 10.1016/j.gaitpost.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling older adults. Journal of the American Geriatrics Society. 1996;44:1147–1152. doi: 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clinical Science (London) 1992;82:321–327. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: Feasibility, reliability and validity. European Journal of Applied Physiology and Occupational Physiology. 1990;60:385–390. doi: 10.1007/BF00713504. [DOI] [PubMed] [Google Scholar]
- Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the Osteoporotic Fractures in Men Study (MrOS) Contemporary Clinical Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Capodaglio P, Castelnuovo G, Brunani A, Vismara L, Villa V, Capodaglio EM. Functional limitations and occupational issues in obesity: A review. International Journal of Occupational Safety and Ergonomics. 2010;16:507–523. doi: 10.1080/10803548.2010.11076863. [DOI] [PubMed] [Google Scholar]
- Clark DJ. Automaticity of walking: Functional significance, mechanisms, measurement and rehabilitation strategies. Frontiers in Human Neuroscience. 2015;9 doi: 10.3389/fnhum.2015.00246. Article 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil P, Simoneau M, Rancourt D, Tremblay A, Teasdale N. Increased risk for falling associated with obesity: Mathematical modeling of postural control. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2001;9:126–136. doi: 10.1109/7333.928572. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Nevitt MC, Kidd S. Forgetting falls. The limited accuracy of recall of falls in the elderly. Journal of the American Geriatrics Society. 1988;36:613–616. doi: 10.1111/j.1532-5415.1988.tb06155.x. [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR of Osteoporotic Fractures Research Group Study. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. Journal of the American Geriatrics Society. 2003;51:1740–1747. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- Faulkner KA, Cauley JA, Zmuda JM, Landsittel DP, Nevitt MC, Newman AB, … Redfern MS. Ethnic differences in the frequency and circumstances of falling in older community-dwelling women. Journal of the American Geriatrics Society. 2005;53:1774–1779. doi: 10.1111/j.1532-5415.2005.53514.x. [DOI] [PubMed] [Google Scholar]
- Fjeldstad C, Fjeldstad AS, Acree LS, Nickel KJ, Gardner AW. The influence of obesity on falls and quality of life. Dynamic Medicine. 2008;7 doi: 10.1186/1476-5918-7-4. Article 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan MT, Gagnon MM, Aneja J, Jones RN, Cupples LA, Lipsitz LA, … Kiel DP. Optimizing the tracking of falls in studies of older participants: Comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston Study. American Journal of Epidemiology. 2010;171:1031–1036. doi: 10.1093/aje/kwq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes CL, Reynolds SL. Effect of obesity on falls, injury, and disability. Journal of the American Geriatrics Society. 2012;60:124–129. doi: 10.1111/j.1532-5415.2011.03767.x. [DOI] [PubMed] [Google Scholar]
- Hita-Contreras F, Martinez-Amat A, Lomas-Vega R, Alvarez P, Mendoza N, Romero-Franco N, Aranega A. Relationship of body mass index and body fat distribution with postural balance and risk of falls in Spanish postmenopausal women. Menopause. 2013;20:202–208. doi: 10.1097/gme.0b013e318261f242. [DOI] [PubMed] [Google Scholar]
- Kelsey JL, Procter-Gray E, Hannan MT, Li W. Heterogeneity of falls among older adults: Implications for public health prevention. American Journal of Public Health. 2012;102:2149–2156. doi: 10.2105/AJPH.2012.300677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Boyko EJ, Nielson CM, Stefanick ML, Bauer DC, Hoffman AR … Osteoporotic Fractures in Men Study Group. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. Journal of the American Geriatrics Society. 2011;59:233–240. doi: 10.1111/j.1532-5415.2010.03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AX, Brown CJ, Sawyer P, Kennedy RE, Allman RM. Life-space mobility declines associated with incident falls and fractures. Journal of the American Geriatrics Society. 2014;62:919–923. doi: 10.1111/jgs.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Physical Therapy. 2003;83:237–252. [PubMed] [Google Scholar]
- Maffiuletti NA, Agosti F, Proietti M, Riva D, Resnik M, Lafortuna CL, Sartorio A. Postural instability of extremely obese individuals improves after a body weight reduction program entailing specific balance training. Journal of Endocrinological Investigation. 2005;28:2–7. doi: 10.1007/BF03345521. [DOI] [PubMed] [Google Scholar]
- Maurer MS, Burcham J, Cheng H. Diabetes mellitus is associated with an increased risk of falls in elderly residents of a long-term care facility. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 2005;60:1157–1162. doi: 10.1093/gerona/60.9.1157. [DOI] [PubMed] [Google Scholar]
- Mignardot JB, Olivier I, Promayon E, Nougier V. Obesity impact on the attentional cost for controlling posture. PLoS ONE. 2010;5(12):e14387. doi: 10.1371/journal.pone.0014387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RJ, Lord SR, Harvey LA, Close JC. Associations between obesity and overweight and fall risk, health status and quality of life in older people. Australian and New Zealand Journal of Public Health. 2014;38:13–18. doi: 10.1111/1753-6405.12152. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Lord SR, Harvey LA, Close JC. Obesity and falls in older people: Mediating effects of disease, sedentary behavior, mood, pain and medication use. Archives of Gerontology and Geriatrics. 2015;60:52–58. doi: 10.1016/j.archger.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991–1998. Journal of the American Medical Association. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. Journal of the American Medical Association. 1989;261:2663–2668. [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Journal of the American Medical Association. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, … Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—A large observational study of the determinants of fracture in older men. Contemporary Clinical Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. European Journal of Epidemiology. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS … LIFE Study Investigators. Effect of structured physical activity on prevention of major mobility disability in older adults: The LIFE study randomized clinical trial. Journal of the American Medical Association. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeski WJ, Axtell R, Fielding R, Katula J, King AC, Manini TM … LIFE Study Investigator Group. Promoting physical activity for elders with compromised function: The lifestyle interventions and independence for elders (LIFE) study physical activity intervention. Clinical Interventions in Aging. 2013;8:1119–1131. doi: 10.2147/CIA.S49737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Waclawczyk A, Hartfield D, Yu S, Kuang X, Zhang H, Alamgir H. Analysis of fall injuries by body mass index. Southern Medical Journal. 2014;107:294–300. doi: 10.1097/SMJ.0000000000000097. [DOI] [PubMed] [Google Scholar]
- Rubenstein LZ. Falls in older people: Epidemiology, risk factors and strategies for prevention. Age and Ageing. 2006;35(Suppl 2):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Charpentier PA, Berkman LF, Tinetti ME, Guralnik JM, Albert M, … Rowe JW. Predicting changes in physical performance in a high-functioning elderly cohort: MacArthur studies of successful aging. Journal of Gerontology. 1994;49(3):M97–M108. doi: 10.1093/geronj/49.3.m97. [DOI] [PubMed] [Google Scholar]
- Simpson LA, Miller WC, Eng JJ. Effect of stroke on fall rate, location and predictors: A prospective comparison of older adults with and without stroke. PLoS ONE. 2011;6(4):e19431. doi: 10.1371/journal.pone.0019431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira FV, Facchini LA, Silveira DS, Piccini RX, Tomasi E, Thume E, … Dilelio A. Prevalence of falls in elderly in Brazil: A countrywide analysis. Cadernos de Saúde Pública. 2011;27:1819–1826. doi: 10.1590/s0102-311x2011000900015. [DOI] [PubMed] [Google Scholar]
- Sparling PB, Howard BJ, Dunstan DW, Owen N. Recommendations for physical activity in older adults. British Medical Journal. 2015;350 doi: 10.1136/bmj.h100. Article h100. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Mack KA, Paulozzi LJ, Ballesteros MF. Self-reported falls and fall-related injuries among persons aged ≥65 years—United States, 2006. Journal of Safety Research. 2008;39:345–349. doi: 10.1016/j.jsr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Sturnieks DL, Tiedemann A, Chapman K, Munro B, Murray SM, Lord SR. Physiological risk factors for falls in older people with lower limb arthritis. The Journal of Rheumatology. 2004;31:2272–2279. [PubMed] [Google Scholar]
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. The New England Journal of Medicine. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, … Shah K. Weight loss, exercise, or both and physical function in obese older adults. The New England Journal of Medicine. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villareal DT, Shah K, Banks MR, Sinacore DR, Klein S. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: A one-year randomized controlled trial. The Journal of Clinical Endocrinology & Metabolism. 2008;93:2181–2187. doi: 10.1210/jc.2007-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JI, Schwartz RS, LaCroix AZ, Uhlmann RF, Pearlman RA. Involuntary weight loss in older outpatients: Incidence and clinical significance. Journal of the American Geriatrics Society. 1995;43:329–337. doi: 10.1111/j.1532-5415.1995.tb05803.x. [DOI] [PubMed] [Google Scholar]
- Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The Physical Activity Scale for the Elderly (PASE): Evidence for validity. Journal of Clinical Epidemiology. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. Journal of Clinical Epidemiology. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]