Abstract
Background
Biomarker assays could increase the accuracy of non-invasive detection of colorectal cancer (CRC); fecal immunochemical tests (FITs) are estimated to miss 27%–47% of CRCs and 70%–80% of advanced adenomas per round of screening. We investigated the conditions under which biomarker screens would be cost-effective compared to FIT screens of average risk individuals.
Methods
We used the MISCAN-colon microsimulation model to estimate the effects of various CRC screening test characteristics on life years gained (LYG) and costs Biomarker assays could increase the accuracy of non-invasive detection of colorectal cancer (CRC); fecal immunochemical tests (FITs) are estimated to miss 27%–47% of CRCs and 70%–80% of advanced adenomas per round of screening. We investigated the conditions under which biomarker screens would be more cost-effective than FIT screens of average-risk individuals. We modeled FIT along with hypothetical biomarker tests with different test performance levels. For each biomarker test we calculated the maximum unit cost for the test to be cost-effective compared to FIT assuming a willingness-to-pay threshold of €50,000 ($56,000) per LYG.
Results
Biennial FIT screening of subjects 55–75 years old provided 84.9 LYG, at a cost of €122,000 ($137,000) per 1000 participants. Considering a unit cost of €7 ($8) for FIT (including kit and analysis only, excluding organizational costs), a biomarker test that detects CRC with higher levels of specificity and sensitivity (100%) and advanced adenomas at a proportionally higher level of sensitivity (53%) should never exceed a cost of €51 ($57). The threshold cost could increase to more than €200 ($224) for high-performing biomarker tests in cases of limited colonoscopy capacity or higher uptake of this test.
Conclusion
Using the MISCAN-colon microsimulation model to estimate effects of CRC screening tests, we found that in order for a biomarker test with increased overall performance to be cost-effective, it should not exceed 7-fold the unit cost of FIT. This maximum would increase substantially if colonoscopy becomes more expensive or scarce, or if the new test has higher screening uptake. These values could be used to estimate the added value of new biomarkers compared to current FIT screening.
Keywords: Colon cancer, early detection, computer simulation, molecular
INTRODUCTION
In developed countries, colorectal cancer (CRC) is the third most commonly diagnosed malignancy in men and ranks second in women.1 Screening for CRC and its precursor lesions, adenomas, can detect the disease at an earlier stage when treatment is potentially more effective. Guaiac fecal occult blood tests (gFOBT) and fecal immunochemical tests (FIT) detect traces of blood in stool, and are widely used for non-invasive screening.2 However, even the newer versions have a limited sensitivity, especially for adenomas. It is estimated that FIT misses 27–47% of CRCs and 70–80% of advanced adenomas per screening round.3, 4
Improved performance of non-invasive screening could be obtained by testing for disease specific molecules like DNA in stool or blood, added to or replacing FIT.5 Molecular biomarkers have been investigated extensively, and ongoing technical innovations have improved the feasibility to use such tests for mass-screening. Exact Sciences Corp. (Madison, WI) has developed a multi-target stool DNA test, which consists of multiple DNA mutation and methylation markers, and also includes a measure of hemoglobin. Recently data were published from the first screening trial.6 Although the sensitivity for CRC of this particular test is higher than FIT, in order to be considered for implementation in population-based screening programs any new test should be both effective and cost-effective compared to current screening options.
Research and analysis methods in biomarkers for CRC are still developing, and test performance and costs are not yet settled. Therefore the aim of this study was to provide insight in the requirements for test sensitivity, specificity and unit cost in order for new technologies to be cost-effective compared to FIT screening in population-based screening programs.
MATERIALS AND METHODS
We used the MISCAN-Colon microsimulation model to estimate life years gained (LYG) and costs of various screening scenarios. We modeled a range of hypothetical biomarker tests, with varying specificity and sensitivity for adenomas and CRC and compared the outcomes with those of optimal FIT screening. For each biomarker test variant we varied the screening age range and interval, and calculated the threshold unit cost allowed to be cost-effective compared to FIT screening.
MISCAN-Colon microsimulation model
The MISCAN-colon model and the data sources that informed the quantifications of the model are described in detail in Appendix 1, and in previous publications.7–11 In brief, the MISCAN-colon model simulates the life histories of individuals from birth to death. CRC arises in the population according to the adenoma-carcinoma sequence.12, 13 More than one adenoma can occur in an individual and each adenoma can independently develop into CRC. Adenomas can progress in size from small (≤5 mm) to medium (6–9 mm) to large (≥10 mm), and some may eventually become malignant. A preclinical (i.e., not detected) cancer has a chance of progressing through stages I to IV and may be detected by symptoms at any stage. After clinical diagnosis of CRC, survival depends on the stage at diagnosis. At any time during his/her life an individual may die of other causes.
With stool- or blood-based screening, an individual with a positive test will be referred for diagnostic colonoscopy for possible removal of adenomas and detection of cancers. In this way CRC mortality can be reduced. The life years gained by screening are calculated as the difference in model-predicted life years lived in the population with and without CRC screening.
Study population
We modeled a cohort of individuals at average risk of CRC. The age-specific all-cause mortality was based on the 2010 Dutch life tables. The simulated CRC incidence rate and CRC stage distribution were calibrated to observed data in The Netherlands from 1999–2003, which was before the onset of opportunistic screening.14 Survival rates after clinical diagnosis of CRC before age 75 were based on CRC relative survival data from 1985–2004.15 The survival for individuals diagnosed at age 75 and older was based on the under 75 survival rates, and adjusted to fit the observed age-increasing mortality/incidence ratio.
Test characteristics
The test characteristics of FIT (Table 1) were fitted to the positivity and detection rates of adenomas and CRC observed in the first screening round of two Dutch randomized trials using OC-Sensor (Eiken, Japan).16–18 We modeled FIT with a cut-off level of 10 μg/g feces (50 ng/ml buffer), because this was previously found to be the most cost-effective.19
Table 1.
Overview of test characteristics used in the model.
| Screen test (reference) | Specificity (per person, %) | Sensitivity (per lesion, %)* | |||||
|---|---|---|---|---|---|---|---|
| Adenoma
|
CRC
|
||||||
| Small (≤5mm) | Medium (6–9mm) | Large (≥10mm) | Early preclinical† | Late preclinical† | Average | ||
| FIT‡ | 96 | 0 | 11 | 34 | 50 | 83 | 64 |
|
| |||||||
| Biomarker test variants | |||||||
|
| |||||||
| Sensitivity CRC 60% | 88–100§ | 0 | 11 | 32 | 45 | 80 | 60 |
| Sensitivity CRC 70% | 88–100§ | 0 | 12 | 37 | 58 | 87 | 70 |
| Sensitivity CRC 80% | 88–100§ | 0 | 14 | 42 | 71 | 92 | 80 |
| Sensitivity CRC 90% | 88–100§ | 0 | 16 | 48 | 85 | 96 | 90 |
| Sensitivity CRC 100% | 88–100§ | 0 | 18 | 53 | 100 | 100 | 100 |
| Colonoscopy|| | 90 | 75 | 85 | 95 | 95 | 95 | 95 |
CRC, colorectal cancer; FIT, fecal immunochemical test
The probability of a person to test positive (person-level sensitivity) is higher than per lesion sensitivity and depends on the number and type of lesions present as well as the probability to test positive because of other reasons, such as e.g. bleeding from a diverticulum.
It was assumed that the probability a CRC bleeds and thus the sensitivity of FIT for CRC depends on the time until clinical diagnosis, in concordance with findings for gFOBT, which were based on a prior calibration of the MISCAN-Colon model to three gFOBT trials.9 This result is to be expected when cancers that bleed do so increasingly over time, starting “occultly” and ending as clinically visible.
The test characteristics of FIT (at a cut-off of 10 μg/g feces (50 ng/ml buffer)) were fitted to the positivity and detection rates of adenomas and CRC from two Dutch randomized trials.16–18 We assumed that the sensitivity for small adenomas was 0%, and that small adenomas would only be detected because of a lack of specificity of the test.
We modeled five different sets of sensitivities for the biomarker test. All five sets of sensitivities were modeled with specificities ranging from 88% to 100%, at 2% increments, yielding a total of 35 (5×7) different sets of test characteristics for the hypothetical biomarker tests variants.
Colonoscopy was only used during follow-up and surveillance after a positive FIT or biomarker test.
We considered various biomarker test variants with sensitivities for CRC ranging from 60% to 100%, at 10% increments (Table 1). The sensitivity for adenomas varied, by assumption, by the same proportions as the sensitivity for CRC. For example, when increasing the sensitivity for CRC from 70% to 80%, the sensitivity for adenomas was also increased by a factor of 1.14 (80/70). For the biomarker test variants, the specificity was varied from 88% to 100%, at 2% increments. Varying both sensitivity and specificity resulted in 35 (5×7) different sets of test characteristics.
The sensitivity of diagnostic and surveillance colonoscopies was assumed to be 75% for adenomas ≤5 mm, 85% for adenomas 6–9 mm, and 95% for adenomas ≥10 mm and CRC.20 We assumed costs for biopsy and pathology in 10% of the population without adenomas detected for detection and removal of hyperplastic or other polyps that are not explicitly simulated in the model.21
Screening scenarios
We considered different screening schedules by varying age to start screening (45, 50, 55, or 60 years), age to stop screening (70, 75, or 80 years), and screening interval (1, 1.5, 2, 3, 5, 7, or 10 years). These screening age ranges and intervals result in 84 (4×3×7) different screening schedules, and combining them with the different biomarker test variants resulted in approximately 3000 unique screening scenarios. We compared the outcomes of these strategies with the outcomes of optimal FIT screening strategies as identified in a previous analysis.22
In the base case analysis, we simulated individuals who follow the screening, follow-up and surveillance recommendations, assuming participation and compliance would be equal between the tests. Individuals with a positive test result would be referred for diagnostic colonoscopy. If no adenomas were found during the procedure, the individual, as recommended, would return to the regular screening program after ten years. If one or more adenomas were found, they would be removed and the individual would enter surveillance according to the Dutch guidelines for follow-up after polypectomy used until recently,23 which indicates colonoscopy after six years in case of one or two adenomas and after three years in case of three or more adenomas.
Costs
An overview of cost inputs used in the model is presented in Table 2. The analysis was conducted from a modified societal perspective. This means that, next to direct medical costs, patient time costs were also included.27 Costs for FIT screening, complications after colonoscopy and treatment of CRC have been published previously.19 Using the medical cost price index from the Dutch Health Care Authority, we updated those costs to the year 2013.28 In addition, the costs for colonoscopy procedures were based on a recent internal study at the Dutch Erasmus Medical Centre (unpublished data), in the setting of a dedicated screening center. We assumed that the biomarker tests would have organizational costs (i.e. costs for the mailing of invitations, reminders and test results, gathering of address information of eligible participants, and overhead of the screening organization) equal to those of FIT screening. In the analyses preceding the calculation of threshold unit costs, the costs for the biomarker test kit and the analysis of the test was assumed to be €100 ($112) for all biomarker test variants.
Table 2.
Cost inputs used in the model, modified societal perspective.*
| Variable | Cost (€) | ||||||
|---|---|---|---|---|---|---|---|
| CRC screening, per procedure | FIT | Biomarker | |||||
|
| |||||||
| Organizational costs** | 14.61 | 14.61 | |||||
| Test | 7.29† | tbd‡ | |||||
| Patient time cost | 15.93 | 15.93 | |||||
| Total screen costs, per person invited^ | 37.83 | tbd | |||||
|
| |||||||
| Follow-up/surveillance, per procedure | |||||||
|
| |||||||
| Colonoscopy, no polypectomy | 447 | ||||||
| Colonoscopy, polypectomy | 584 | ||||||
| Colonoscopy, diagnosis clinical CRC | 688 | ||||||
| Colonoscopy, complications§ | 3,156 | ||||||
|
| |||||||
| CRC treatment, per patient per year|| | Stage I | Stage II | Stage III | Stage IV | |||
|
| |||||||
| Initial treatment | 17,219 | 22,177 | 26,585 | 30,992 | |||
| Continuous care | 685 | 685 | 685 | 685 | |||
| Terminal care, death CRC | 23,786 | 23,786 | 24,888 | 32,050 | |||
| Terminal care, death other causes | 9,352 | 8,912 | 10,234 | 19,930 | |||
CRC, colorectal cancer; FIT, fecal immunochemical test; tbd, to be determined.
For the calculation of patient time costs we assumed an average hourly wage of €15.93.24 We assumed 1, 16, and 112 hours of patient time per procedure for FIT and biomarker testing, colonoscopy (including bowel preparation), and colonoscopy complications respectively. For CRC treatment we assumed 244, 19, and 283 hours of patient time per year of care in initial treatment, continuous care, and terminal care respectively. 25, 26
Organizational costs for screening were based on the Dutch cervical cancer screening program, adjusted for differences with FIT screening, and include costs for administration, education and quality assurance.
Includes €2.48 for test kit and €4.81 for analysis.
The unit cost of the biomarker test variants (test kit and analysis of the test) was determined in the threshold analysis.
Cost per complication. We assumed a complication rate of 2.4 per 1,000 colonoscopies
CRC treatments were divided into three clinically relevant phases: initial, continuous, and terminal care. The initial phase was defined as the first 12 months following diagnosis, the terminal phase was defined as the final 12 months of life, and the continuous phase was defined as all months between the initial and terminal phase. For patients surviving less than 24 months, the final 12 months were allocated to the terminal phase. The remaining months of observation were allocated to the initial phase.
For non-attenders (sensitivity analysis) the test-analyses and patient cost were not applied
Cost-effectiveness and threshold costs
We estimated costs and LYG of each scenario compared to no screening, discounted by three percent per year.29 Subsequently, based on these results, we compared between scenarios. Scenarios that were more costly and less effective than other scenarios (simple dominance) or than a mix of other scenario’s (extended dominance) were ruled out. The remaining scenarios are not dominated and are known as “efficient”. On a plot of costs versus LYG, the line that connects the efficient scenarios is called the efficient frontier, which implies that all dominated scenarios lie below this line. The incremental cost-effectiveness ratio (ICER) of an efficient scenario was determined by comparing its additional costs and effects to those of the next less costly and less effective efficient scenario.
In the analysis of threshold unit costs, for each biomarker test variant an efficient frontier was determined from the various screening age ranges and intervals considered. Subsequently, for each scenario on the efficient frontier we calculated the cost per biomarker test that is allowed for that scenario to be on the efficient frontier of FIT. The resulting cost level may vary over the screening intensities, and we considered the highest value as the threshold unit cost for each biomarker test variant. For biomarker scenarios that were more effective than the most effective FIT scenario, the threshold cost for the biomarker scenario was calculated based on a maximal willingness-to-pay of €50,000 ($56,000) per additional LYG relative to the most effective FIT scenario.
Outcomes
The main outcomes are costs and LYG per 1,000 individuals compared to no screening for various unit costs of the new test, and threshold unit cost required for equal cost-effectiveness compared to FIT.
Sensitivity analyses
We considered several sensitivity analyses, summarized in Appendix 2, to investigate the robustness of the calculated threshold unit costs to varying model assumptions. First, we adjusted for quality of life effects of CRC screening and treatment. Second, we evaluated the effect of limited colonoscopy capacity by considering only scenarios in which colonoscopy demand did not exceed alternatively 40, 30, 20, or 10 colonoscopies per 1,000 individuals per year. Third, we considered a scenario in which screening uptake with FIT was 60%16–18 and screening uptake using the biomarker test variants would be either 20% point higher or lower than FIT. Fourth, we assumed an ICER of €100,000 ($112,000) per LYG as the upper limit for any scenario to be considered cost-effective. Fifth, we used the test characteristics for FIT (at a cut-off of 20 μg/g feces (100 ng/ml buffer)) as published by Imperiale et al. in their direct comparison of FIT with the multi-target stool DNA test.6 Sixth, we alternatively increased and decreased the costs of colonoscopy and CRC treatment. Finally, we replaced all CRC screening and treatment costs from The Netherlands, with United States (US) costs. For this analysis we used cost estimates as published by Zauber and colleagues,30 and we adjusted them to 2013 US dollars using the US Consumer Price Index for all items.31 Given the opportunistic nature of screening in the US, we did not consider organizational costs in this setting.
RESULTS
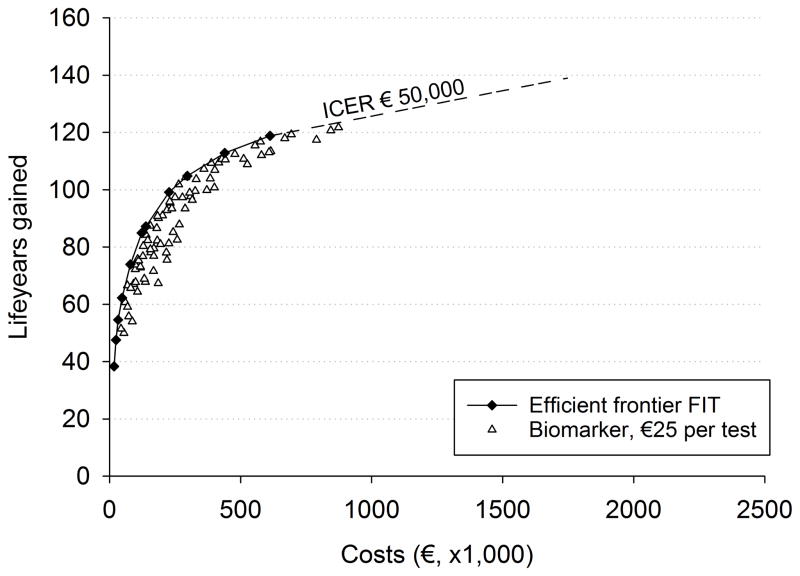
The optimal FIT screening strategies varied in LYG from 38.4 per 1,000 screening participants with 2 screens at age 60 and 72 to 118.8 with annual screening between age 45–80 (Figure 1). The costs varied from €16,600 to €611,700 ($18,600–$686,000) respectively. LYG with a biomarker test with 90% sensitivity for CRC, 48% for advanced adenomas and 88% specificity were higher than with FIT and varied between 47.0 and 121.0. For example, for biennial screening between ages 55–75 years (the schedule currently used in the Dutch program), the LYG were 84.9 with FIT, compared to 95.8 with the considered biomarker test. At unit costs of €50 ($56) per test, the biomarker test was dominated by FIT screening (Figure 1a). Most biomarker strategies saved fewer LYG than FIT for the same costs or required more costs to save the same number of LYG. Only a handful biomarker strategies were more effective than the most effective FIT strategy (e.g. annual screening from age 45 to 80). However, with unit costs of €50 ($56), the additional costs of these tests were so much greater that they were not in balance with the additional benefits (i.e. exceeded €50,000 ($56,000) per LYG).
Figure 1.
Net costs and life-years gained (3% discounted) of efficient FIT screening strategies, and of screening strategies with a hypothetical biomarker with 90% sensitivity for cancer, 48% for advanced adenomas and 88% specificity. Biomarker tests costs are equal to €50 ($56) in panel a, to €15 ($17) in panel b and €25 ($28) in panel c. Each symbol represents a strategy that differs with respect to age to begin screening, age to end screening and screening interval.
At unit costs of €15 ($17) per test (Figure 1b), several of the biomarker strategies resulted in more LYG than FIT screening for the same level of costs or lower costs for the same level of LYG. At unit costs of €25 ($28) per test (Figure 1c), there was one biomarker strategy on the efficient frontier of FIT screening, indicating that this strategy was cost-effective compared to FIT. All other strategies with the same biomarker test were dominated by FIT screening, indicating that €25 ($28) per test is the maximum cost for which this particular biomarker strategy (i.e. 90% sensitivity for CRC, 48% sensitivity for advanced adenomas and 88% specificity) could be cost-effective in comparison to FIT screening.
Threshold costs for biomarker tests
Considering the range of hypothetical biomarker test variants, the threshold costs varied considerably (Table 3). The threshold costs increased with test performance up to €50.23 ($56.34) (approximately seven times the unit cost of FIT) at the highest performance level considered. On the other hand, thresholds below the €7 ($8) for FIT were possible in instances where test specificity would be sacrificed to get improved sensitivity, resulting in increased numbers of colonoscopy.
Table 3.
Threshold unit costs of the biomarker test variants allowed for equal cost-effectiveness compared to FIT.
| Sensitivity CRC (%)* | Specificity | ||||||
|---|---|---|---|---|---|---|---|
| 88 | 90 | 92 | 94 | 96 | 98 | 100 | |
| 60 | −1 | −1 | 1 | 3 | 6 | 8 | 11 |
| 70 | 6 | 9 | 9 | 12 | 14 | 17 | 19 |
| 80 | 15 | 17 | 19 | 21 | 23 | 25 | 27 |
| 90 | 25 | 27 | 29 | 32 | 34 | 35 | 36 |
| 100 | 38 | 40 | 42 | 44 | 46 | 48 | 50 |
CRC, colorectal cancer; FIT, fecal immunochemical test; Neg., calculated threshold cost was a negative value.
Although the average sensitivity for CRC is used to label the different biomarker test variants, the sensitivity for adenomas is varied accordingly (see Table 1).
The presented unit costs include costs for the test kit and the analysis of the test.
Sensitivity analyses of threshold costs
The maximal colonoscopy demand in the base case analysis was approximately 55 per 1,000 individuals (annual screening with a low specificity test). When the analysis was limited to scenario’s with a colonoscopy demand not exceeding 10 colonoscopies per 1,000 individuals per year, the test variants with 88–92% specificity were not cost-effective compared to FIT (with 94% specificity) at any unit cost. In contrast, with higher specificity levels the threshold costs strongly increased, up to €214 – €437 ($240–$490) for perfect specificity (€11 – €50 ($12–$56) in the base case analysis).
Regarding differences in screening uptake, a 20% point greater screening uptake with biomarker screening increased maximum threshold costs from €50 ($56) to €238 ($267), while a 20% point lower screening uptake decreased maximum threshold cost to €18 ($20). Assuming US cost levels, which are approximately 25% higher for screening and more than double for treatment, approximately doubled the maximum threshold costs to $105 ($118).
The estimated threshold costs for the biomarker test variants were robust to most alternative assumptions considered, with threshold costs not exceeding €100 ($112) (Appendix 2).
DISCUSSION
This study demonstrates that, when taking FIT as a reference, the threshold unit cost of the biomarker test variants allowed for equal cost-effectiveness compared to a sensitive FIT was €50 ($56) for a test with the highest considered performance characteristics (53% and 100% sensitivity for large adenomas and CRC, respectively, and 100% specificity). The results were sensitive to differences in screening uptake between FIT and the biomarker tests (maximum €238 ($267) at 20% higher uptake), and cost assumptions (threshold costs of $105 ($118) for US cost assumptions). Also, in a situation with limited colonoscopy capacity, improving specificity would become more important, so that with a 20% capacity level (compared to the level needed for annual screening with FIT) the maximum threshold costs would become €437 ($490) for a high-specificity test. Together these results point to key determinants that need to be addressed to improve (incremental) cost-effectiveness of biomarker testing: cost, sensitivity for advanced (progressive) adenomas, specificity and compliance.
Improving the sensitivity and specificity did not greatly increase the threshold cost compared to FIT mainly because CRC is a slow growing disease, and the time for progressive adenomas to develop and progress into cancer takes on average more than 10 years,12, 13 although there will be a variation in duration, especially for certain cancer types. Although FIT has limited test sensitivity, it can be performed multiple times at relatively low costs, resulting in considerably higher program sensitivity. When FIT at short intervals is excluded from the comparison because colonoscopy capacity is insufficient for such high intensity screening, FIT screening becomes considerably less effective. This in turn has a strong positive impact on the threshold costs of biomarker tests with high specificity. Because these tests require fewer colonoscopies, they can be offered at greater frequency than the FIT test. Especially when they are also more sensitive this will result in significantly more LYG from screening than with the less intensive FIT strategies. In such a situation higher costs are warranted and under perfect sensitivity and specificity unit costs of up to €437 ($490) would still be cost-effective.
The lower sensitivity of FIT for adenomas than for CRC provides more room for improving adenoma rather than CRC detection. In fact, one could argue that it would make more sense to detect and intervene with lesions before they have become malignant, similar to e.g. cervical cancer screening. In addition, the preclinical duration of adenomas is longer than that of CRC, and earlier detection of CRC has a smaller impact on treatment costs than preventing CRC through the removal of adenomas. On the other hand, improving adenoma sensitivity, beyond the 53% we considered for large adenomas, without losing too much on specificity would also be challenging. The threshold costs of biomarker tests could also further increase if the test would be able to discriminate between progressive and non-progressive adenomas.32 With such a test fewer unnecessary colonoscopies and polypectomies would be performed for people with non-progressive adenoma reducing the burden and resources for screening, while maintaining the same benefit.
For a stool-based biomarker test a considerable difference in screening uptake seems unlikely since the method of sample collection and therefore the barriers may be very similar to FIT. A blood-based biomarker provides a different test modality, which could potentially be more acceptable for individuals who currently choose not to participate in stool-based screening. However, current blood-based biomarker tests have fairly low sensitivity.33, 34 On the other hand, people could be attracted by the novelty of a DNA-based testing methodology and the higher expected performance characteristics of new stool-based biomarkers compared to FIT. In addition, these new tests may be offered with patient navigation, as is the case for the multi-target stool DNA test, which may increase test uptake and thus comparative cost-effectiveness of the test. Still uptake increases of more than 20% are unlikely, given that FIT can also be offered with patient navigation.
Several studies have evaluated the cost-effectiveness of specific biomarker tests.35–42 The majority of the studies considered various versions of the fecal DNA test and unit costs, including laboratory analysis, varied between $51 (Taiwanese population) and $825 (US population). One study evaluated a blood-based methylated Septin 9 DNA assay at a cost of $150 per test.36 In general, DNA testing was found to be cost-effective compared to no screening, but was not cost-effective compared to other screening options, including Hemoccult II, FIT and colonoscopy. This is consistent with our findings. One study showed that the multi-target stool DNA test could be cost-effective at its current reimbursement rate of $493 if the test yielded participation rates more than 1.7-fold relative to FIT.37 This finding closely aligns with our estimate that threshold costs could be €238 ($267) at 20%-point higher participation rates (1.3 fold relative to FIT).
Two publications investigating the Exact Sciences test (version 1.1) reported threshold costs of $34–60, compared to FIT.39, 40 In our analysis, the threshold cost for corresponding sensitivity and specificity values are somewhat lower, which may be explained by us using the more cost-effective cut off of 10 instead of 20 μg/g feces (50 instead of 100 ng/ml buffer) for FIT (resulting in higher sensitivity and lower specificity for FIT), and to the Dutch costs assumptions compared to the US setting.
Our study adds to previous publications by providing threshold costs for newer test versions. For example, for the multi-target stool DNA test6, with a sensitivity for CRC of approximately 90% and a specificity of 90%, the threshold unit costs would need to be less than $56 compared to FIT in the US setting (Appendix 2). This broader range of results is important, because analysis methods, using DNA as well as other reporter molecules including proteins and miRNA, are still developing, and test performance and costs are not yet settled. Moreover, our study provides researchers and manufacturers with data to determine the requirements of their test to be cost-effective compared to current alternatives.
This study has four potential limitations to be mentioned. First, we did not explicitly model distinct pathways for traditional and sessile serrated adenomas/polyps (SSA/P). The average time it takes for an adenoma to develop into CRC was calibrated to the randomized UK flexible sigmoidoscopy screening trial43 and included both traditional and sessile serrated adenomas/polyps. Therefore both adenoma types are included in the modeled mix of slow and rapid progressing lesions. Using the data from a flexible sigmoidoscopy study to estimate the progression time off SSA/P to CRC may not be most reflective given the tendency of more significant SSA/P to be right sided and proximal CRC precursors. We would underestimate the relative effectiveness of biomarkers compared to FIT only if SSA/P would have higher malignant potential than conventional adenomas, and the biomarker sensitivity for SSA/P would be greater than FIT sensitivity. Evidence is accumulating that FIT might be less sensitive for SSA/P, possibly due to an absence of or limited number of surface vessels or because they are often flat and therefore less likely to bleed.6, 44–46 However, evidence for the malignant potential of SSA/P remains to be determined.
Second, we assumed independency of test results between screening rounds for both FIT and biomarker tests. However, systematic false negative results could negatively impact the effectiveness of screening. There is evidence indicating systematic false negative test results with FIT screening, presumably because of non-bleeding lesions.47, 48 It is unlikely that our assumption of independency of test results has influenced our results substantially. Previous analyses showed that systematic test results reduced LYG by screening by approximately 5%.47 Moreover, because of the genetic heterogeneity in carcinogenesis, and the limited number of DNA markers analyzed in DNA tests, systematic negative test results are likely to affect biomarker screening as well. Given the modest impact of systematic test results on effectiveness and the expectation that biomarker tests will be affected similarly, we expect the bias in our comparison of both tests to be limited. Combining hemoglobin markers with DNA markers in one test as with the multi-target stool DNA test, may be a tool to address the concern of subsequent systematic negative test results. However, results of multiple rounds of testing with multi-target stool DNA are needed to confirm this hypothesis.
Third, we based our stage specific CRC survival estimates on data from the south of the Netherlands (period 1985–2004), while recently data became available with national coverage and from a more recent time period (1989–2008). Compared to the current model, the five year relative survival has increased less than four percent. In a sensitivity analysis, we estimated that even a 25% increase in the relative survival for all stages would not change the calculated threshold unit costs by more than €2 ($2.25).
Finally and may be most importantly, we only considered sensitivities of the new biomarker tests for advanced adenomas of up to 53%, because of our assumption that the sensitivity for advanced adenoma increases with the same rate as the sensitivity for CRC. However, sensitivity of the current multi-target stool DNA test for advanced adenomas already exceeds 40%, approaching 70% for adenomas with high-grade dysplasia.6 Also, as only about 5% of adenomas progress to cancer49 and specific patterns of molecular alterations are associated to this progression,50 there is a rationale for an alternative approach where only a subset of (advanced) adenoma, i.e. high-risk adenomas, and early stage curable cancers are the screening target.32 In such a scenario, a test with a sensitivity of 53% for advanced adenomas might have a higher sensitivity for the actual high-risk adenomas. It is therefore not unlikely that further improvements of biomarker tests may lead to sensitivities for advanced adenoma exceeding 53%. In that case, the number of screening rounds with the biomarker test may be reduced which would positively impact threshold costs or the test. Improvement of adenoma sensitivity could therefore be an important area of future innovation. However, it is very important that this innovation would not come at the expensive of a too great loss in specificity, because our results clearly indicate how sensitive threshold costs of new biomarker tests are to its specificity.
In conclusion, in case of greatly improved overall performance the unit cost of a biomarker test should, for cost-effectiveness, not exceed approximately seven times the unit cost of FIT. This maximum would increase substantially if colonoscopy gets more expensive or scarce, or if the new test has higher screening uptake. Our findings provide a framework for researchers to estimate the potential added value of new biomarkers compared to current FIT screening.
Supplementary Material
Acknowledgments
Grant support: This work was performed within the framework of CTMM, the Centre for Translational Molecular Medicine, project DeCoDe (grant 03O-101). In addition, this publication was made possible by the National Cancer Institute at the National Institutes of Health, which supported part of the development of the MISCAN-Colon model through the Cancer Intervention and Surveillance Modeling Network (grant numbers U01-CA-097426, U01-CA-115953, and U01-CA-152959).
Abbreviations
- CRC
colorectal cancer
- FIT
fecal immunochemical test
- gFOBT
guaiac fecal occult blood test
- ICER
incremental cost-effectiveness ratio
- LYG
life years gained
- LYG
life years gained
- US
United States
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centre for Translational Molecular Medicine.
Disclosure: The authors have no conflicts to disclose.
Author contributions: MvB, MvE, and GAM conceived the idea for the study; MvB and IL-V supervised the model simulations and data analysis; SLG performed the analysis and drafted the report; IL-V, LJWB, VM, BC, MvE, GAM, HJdK and MvB interpreted the data and provided critical review of the manuscript for important intellectual content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Benson VS, Patnick J, Davies AK, et al. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. Int J Cancer. 2008;122:1357–67. doi: 10.1002/ijc.23273. [DOI] [PubMed] [Google Scholar]
- 3.van Dam L, Kuipers EJ, van Leerdam ME. Performance improvements of stool-based screening tests. Best Pract Res Clin Gastroenterol. 2010;24:479–92. doi: 10.1016/j.bpg.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer. 2013;49:3049–54. doi: 10.1016/j.ejca.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Bosch LJ, Carvalho B, Fijneman RJ, et al. Molecular tests for colorectal cancer screening. Clin Colorectal Cancer. 2011;10:8–23. doi: 10.3816/CCC.2011.n.002. [DOI] [PubMed] [Google Scholar]
- 6.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget Stool DNA Testing for Colorectal-Cancer Screening. N Engl J Med. 2014;370:1287–97. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 7.Loeve F, Boer R, van Oortmarssen GJ, et al. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Comput Biomed Res. 1999;32:13–33. doi: 10.1006/cbmr.1998.1498. [DOI] [PubMed] [Google Scholar]
- 8.Loeve F, Boer R, van Ballegooijen M, et al. Final Report MISCAN-COLON Microsimulation Model for Colorectal Cancer: Report to the National Cancer Institute Project No. NO1-CN55186. Rotterdam, The Netherlands: Department of Public Health, Erasmus University; 1998. [Google Scholar]
- 9.Lansdorp-Vogelaar I, van Ballegooijen M, Boer R, et al. A novel hypothesis on the sensitivity of the fecal occult blood test: Results of a joint analysis of 3 randomized controlled trials. Cancer. 2009;115:2410–9. doi: 10.1002/cncr.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loeve F, Boer R, Zauber AG, et al. National Polyp Study data: evidence for regression of adenomas. Int J Cancer. 2004;111:633–9. doi: 10.1002/ijc.20277. [DOI] [PubMed] [Google Scholar]
- 11.Vogelaar I, van Ballegooijen M, Schrag D, et al. How much can current interventions reduce colorectal cancer mortality in the U.S? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer. 2006;107:1624–33. doi: 10.1002/cncr.22115. [DOI] [PubMed] [Google Scholar]
- 12.Morson B. President’s address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974;67:451–7. doi: 10.1177/00359157740676P115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–70. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 14.Netherlands Comprehensive Cancer Organisation. The Netherlands Cancer Registry. [Google Scholar]
- 15.Lemmens V, van Steenbergen L, Janssen-Heijnen M, et al. Trends in colorectal cancer in the south of the Netherlands 1975–2007: rectal cancer survival levels with colon cancer survival. Acta Oncol. 2010;49:784–96. doi: 10.3109/02841861003733713. [DOI] [PubMed] [Google Scholar]
- 16.Hol L, Wilschut JA, van Ballegooijen M, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. Br J Cancer. 2009;100:1103–10. doi: 10.1038/sj.bjc.6604961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hol L, van Leerdam ME, van Ballegooijen M, et al. Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut. 2010;59:62–8. doi: 10.1136/gut.2009.177089. [DOI] [PubMed] [Google Scholar]
- 18.van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135:82–90. doi: 10.1053/j.gastro.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 19.Goede SL, van Roon AH, Reijerink JC, et al. Cost-effectiveness of one versus two sample faecal immunochemical testing for colorectal cancer screening. Gut. 2013;62:727–34. doi: 10.1136/gutjnl-2011-301917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 21.Morson BC. Precancerous lesions of the colon and rectum. Classification and controversial issues. Jama. 1962;179:316–21. doi: 10.1001/jama.1962.03050050006002. [DOI] [PubMed] [Google Scholar]
- 22.Wilschut JA, Hol L, Dekker E, et al. Cost-effectiveness analysis of a quantitative immunochemical test for colorectal cancer screening. Gastroenterology. 2011;141:1648–55. e1. doi: 10.1053/j.gastro.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Nagengast FM, Kaandorp CJ. Revised CBO guideline ‘Follow-up after polypectomy’. Ned Tijdschr Geneeskd. 2001;145:2022–5. [PubMed] [Google Scholar]
- 24.Statistics Netherlands. Statline - Average income private households. 2013. [Google Scholar]
- 25.Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99:14–23. doi: 10.1093/jnci/djk001. [DOI] [PubMed] [Google Scholar]
- 26.Yabroff KR, Warren JL, Knopf K, et al. Estimating patient time costs associated with colorectal cancer care. Med Care. 2005;43:640–8. doi: 10.1097/01.mlr.0000167177.45020.4a. [DOI] [PubMed] [Google Scholar]
- 27.Hakkaart-van Roijen L, Tan SS, Bouwmans CAM. Handleiding voor kostenonderzoek - Methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg. Vol. 2010. College voor Zorgverzekeringen; [Google Scholar]
- 28.Dutch Health Care Authority. Medical cost price index. [Google Scholar]
- 29.Siegel JE, Torrance GW, Russell LB, et al. Guidelines for pharmacoeconomic studies. Recommendations from the panel on cost effectiveness in health and medicine. Panel on cost. Effectiveness in Health and Medicine Pharmacoeconomics. 1997;11:159–68. doi: 10.2165/00019053-199711020-00005. [DOI] [PubMed] [Google Scholar]
- 30.Zauber AG, Lansdorp-Vogelaar I, Wilschut J, et al. Cost-effectiveness of DNA Stool Testing to Screen for Colorectal Cancer. Rockville, MD: Agency for Healthcare Research and Quality; 2007. [PubMed] [Google Scholar]
- 31.Bureau of Labor Statistics United States Department of Labor. Consumer Price Index. Washington D.C: 2008. [Google Scholar]
- 32.Sillars-Hardebol AH, Carvalho B, van Engeland M, et al. The adenoma hunt in colorectal cancer screening: defining the target. J Pathol. 2012;226:1–6. doi: 10.1002/path.3012. [DOI] [PubMed] [Google Scholar]
- 33.Ahlquist DA, Taylor WR, Mahoney DW, et al. The stool DNA test is more accurate than the plasma septin 9 test in detecting colorectal neoplasia. Clin Gastroenterol Hepatol. 2012;10:272–7. e1. doi: 10.1016/j.cgh.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Church TR, Wandell M, Lofton-Day C, et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63:317–25. doi: 10.1136/gutjnl-2012-304149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imperiale TF. Noninvasive screening tests for colorectal cancer. Dig Dis. 2012;30(Suppl 2):16–26. doi: 10.1159/000341884. [DOI] [PubMed] [Google Scholar]
- 36.Ladabaum U, Allen J, Wandell M, et al. Colorectal cancer screening with blood-based biomarkers: cost-effectiveness of methylated septin 9 DNA versus current strategies. Cancer Epidemiol Biomarkers Prev. 2013;22:1567–76. doi: 10.1158/1055-9965.EPI-13-0204. [DOI] [PubMed] [Google Scholar]
- 37.Ladabaum U, Mannalithara A. Comparative Effectiveness and Cost Effectiveness of a Multitarget Stool DNA Test to Screen for Colorectal Neoplasia. Gastroenterology. 2016;151:427–439. e6. doi: 10.1053/j.gastro.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev. 2011;33:88–100. doi: 10.1093/epirev/mxr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, et al. Stool DNA testing to screen for colorectal cancer in the Medicare population: a cost-effectiveness analysis. Ann Intern Med. 2010;153:368–77. doi: 10.1059/0003-4819-153-6-201009210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parekh M, Fendrick AM, Ladabaum U. As tests evolve and costs of cancer care rise: reappraising stool-based screening for colorectal neoplasia. Aliment Pharmacol Ther. 2008;27:697–712. doi: 10.1111/j.1365-2036.2008.03632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skally M, Hanly P, Sharp L. Cost effectiveness of fecal DNA screening for colorectal cancer: a systematic review and quality appraisal of the literature. Appl Health Econ Health Policy. 2013;11:181–92. doi: 10.1007/s40258-013-0010-8. [DOI] [PubMed] [Google Scholar]
- 42.Song K, Fendrick AM, Ladabaum U. Fecal DNA testing compared with conventional colorectal cancer screening methods: a decision analysis. Gastroenterology. 2004;126:1270–9. doi: 10.1053/j.gastro.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 43.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 44.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–29. doi: 10.1038/ajg.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heigh RI, Yab TC, Taylor WR, et al. Detection of colorectal serrated polyps by stool DNA testing: comparison with fecal immunochemical testing for occult blood (FIT) PLoS One. 2014;9:e85659. doi: 10.1371/journal.pone.0085659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang LC, Shun CT, Hsu WF, et al. Fecal Immunochemical Test Detects Sessile Serrated Adenomas and Polyps With a Low Level of Sensitivity. Clin Gastroenterol Hepatol. 2017;15:872–879. e1. doi: 10.1016/j.cgh.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 47.van der Meulen MP, Lansdorp-Vogelaar I, van Heijningen EM, et al. Nonbleeding adenomas: Evidence of systematic false-negative fecal immunochemical test results and their implications for screening effectiveness-A modeling study. Cancer. 2016;122:1680–8. doi: 10.1002/cncr.29952. [DOI] [PubMed] [Google Scholar]
- 48.Zorzi M, Barca A, Falcini F, et al. Screening for colorectal cancer in Italy: 2005 survey. Epidemiol Prev. 2007;31:49–60. [PubMed] [Google Scholar]
- 49.Shinya H, Wolff WI. Morphology, anatomic distribution and cancer potential of colonic polyps. Ann Surg. 1979;190:679–83. doi: 10.1097/00000658-197912000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hermsen M, Postma C, Baak J, et al. Colorectal adenoma to carcinoma progression follows multiple pathways of chromosomal instability. Gastroenterology. 2002;123:1109–19. doi: 10.1053/gast.2002.36051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.