Abstract
Muscle satellite cells are committed myogenic progenitors capable of contributing to myogenesis to maintain adult muscle mass and function. Several experiments have demonstrated that muscle satellite cells can differentiate into adipocytes in vitro, supporting the mesenchymal differentiation potential of these cells. Moreover, muscle satellite cells may be a source of ectopic muscle adipocytes, explaining the lipid accumulation often observed in aged skeletal muscle (sarcopenia) and in muscles of patients` with diabetes. Quercetin, a polyphenol, is one of the most abundant flavonoids distributed in edible plants, such as onions and apples, and possesses antioxidant, anticancer, and anti-inflammatory properties. In this study, we examined whether quercetin inhibited the adipogenesis of muscle satellite cells in vitro with primary cells from rat limbs by culture in the presence of quercetin under adipogenic conditions. Morphological observations, Oil Red-O staining results, triglyceride content analysis, and quantitative reverse transcription polymerase chain reaction revealed that quercetin was capable of inhibiting the adipogenic induction of muscle satellite cells into adipocytes in a dose-dependent manner by suppressing the transcript levels of adipogenic markers, such as peroxisome proliferator-activated receptor-γ and fatty acid binding protein 4. Our results suggested that quercetin inhibited the adipogenesis of muscle satellite cells in vitro by suppressing the transcription of adipogenic markers.
Abbreviations: mSCs, muscle satellite cells; PPAR-γ, peroxisome proliferator-activated receptor gamma; FABP4, fatty acid binding protein 4; TG, triglyceride
Keywords: Quercetin, Muscle satellite cell, Differentiation, Intramuscular lipid
Highlights
-
•
Quercetin inhibited the adipogenesis of muscle satellite cells in vitro.
-
•
The effect of quercetin might be based on suppression at the transcriptional levels.
-
•
Quercetin could limit ectopic lipid accumulation as observed in aged muscles.
1. Introduction
Skeletal muscle has many vital functions, including movement and postural support. Healthy adult muscle is a highly plastic, dynamic tissue capable of responding to physiological stimuli for regeneration and hypertrophy. Muscle satellite cells (mSCs) are major components of resident stem cells that are involved in the regeneration and maintenance of muscle mass and function in adult muscle [1], [2]. Named for their anatomical position in muscles, mSCs are located beneath the basal lamina in a dominant quiescent state and express Pax7, which is a member of the paired box (PAX) family of transcription factors. Activated mSCs progress through the cell cycle and express MyoD, a myogenic transcriptional factor, after which they become myoblasts. Most proliferating myoblasts downregulate Pax7 and enter the final differentiation phase through myogenin upregulation, followed by fusion with each other or with existing myotubes.
mSCs are also multipotent and can form adipocyte-like cells, osteocytes, and nerve cells [3], [4]. During adipocyte formation, the expression levels of several transcription factors and their target molecules related to adipogenesis, such as peroxisome proliferator-activated receptor-γ (PPAR-γ) and fatty acid binding protein 4 (FABP4), are increased. Muscle disuse, atrophy, and aging increase, whereas muscle mass and function decrease; these features are sometimes accompanied by ectopic lipid deposition and fat mass accumulation [5], [6], [7], [8], [9]. Intramuscular lipids have also been observed in injured mutant mice with genetic depletion of Pax7 [10], indicating that defects in mSC function during muscle regeneration lead to ectopic lipid accumulation. Considering these observations, it is possible that dysfunctional mSCs may be a source of ectopic adipocytes, leading to lipid accumulation in impaired regeneration or aged muscles.
Quercetin (3,5,7,3′,4′-pentahydroxyflavone), a polyphenol, is one of the most abundant flavonoids in the human diet and is known to possess antioxidant, anti-inflammatory, and anticancer properties [11], [12]. Importantly, quercetin has been shown to have antioxidative effects in muscles or myoblasts, including upregulation of mitochondrial activity and suppression of atrophic factors, in several rodent and in vitro models [13], [14], [15], [16]. Moreover, quercetin has been reported to suppress adipocyte differentiation in 3T3-L1 pre-adipocytes [17], [18]. Thus, we hypothesized that quercetin may regulate the differentiation of multipotent mSCs into adipocytes.
In the present study, to test this hypothesis, we examined the effects of quercetin in an in vitro system. Our findings demonstrated that quercetin inhibited the adipogenesis of mSCs and affected the transcription of PPAR-γ and FABP4.
2. Material and methods
2.1. Animals
Male F344 rats (10–14 weeks of age; Japan SLC, Inc., Hamamatsu, Shizuoka, Japan) were housed at 23 ± 1 °C under a 12-h light/dark cycle and provided with standard chow and water ad libitum. This study was approved by the Juntendo University Animal Care and Use Committee (H26-05) and was performed according to the guiding principles for the care and use of laboratory animals established by the Physiological Society of Japan.
2.2. Satellite cell isolation, culture, and differentiation
Primary mSCs were isolated from the major hindlimb muscles (gastrocnemius, soleus, plantaris, tibialis anterior, extensor digitorum longus, and quadriceps) of rats, as previously described [19], and maintained at 37 °C with 5% CO2 in a humidified atmosphere. After 20–24 h, unattached cells were collected by centrifugation at 1,500 × g for 3 min, and the cell pellet was resuspended in growth medium, i.e., F-10 (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 20% fetal bovine serum and penicillin/streptomycin (Gibco). After cell counting, the cells were seeded on collagen-coated eight-well culture slides (Falcon, Corning, Bedford, MA, USA). After 3 days of incubation, the growth medium was changed to adipogenic induction medium (Lonza, Basel, Switzerland) to induce adipogenesis. After 3 more days of incubation, the adipogenic induction medium was changed to adipogenic maintenance medium (Lonza), followed by incubation for 3–4 days. Quercetin (quercetin dihydrate, Nacalai Tesque, Kyoto, Japan) was added to the cells in the adipogenic induction medium and adipogenic maintenance medium at the indicated concentrations. As negative control, dimethylsulfoxide without quercetin was used.
To confirm the myogenic potency of the isolated cells, the isolated cells were seeded at twice the typical density. After culture in growth medium for 2 days, the cells were shifted to low-serum medium (2% house serum in Dulbecco’s modified Eagle’s medium) or adipogenic maintenance medium without adipogenic induction medium for 1–6 days to induce myogenesis.
2.3. Immunostaining
To confirm the purity of the isolated cells, after 40 h of culture in growth medium, cells were fixed in methanol at 4 °C for 10 min for immunostaining. After washing, fixed cells were incubated in 10% normal goat serum, 2% bovine serum albumin (BSA), and 0.1% Triton-X100 in phosphate-buffered saline (PBS) for 30 min at room temperature and then further incubated with the following primary antibodies: anti-MyoD (M3512, Dako, Glostrup, Denmark), anti-desmin (D3, Developmental Studies Hybridoma Bank, Iowa City, IA, USA), anti-myogenin (F5D, Developmental Studies Hybridoma Bank), and anti-Pax7 (PAX7, Developmental Studies Hybridoma Bank), diluted with 2% BSA in PBS at 4 °C overnight. After washing with cold PBS four times, cells were incubated with anti-mouse biotinylated secondary antibodies or anti-mouse IgG conjugated with horseradish peroxidase (GE Healthcare, Waukesha, WI, USA) and washed with PBS. To visualize stained cells, we used the peroxidase substrate kits VIP (Vector Laboratories, Burlingame, CA, USA) combined with a VECSTAtin Elite ABC Kit (Vector Laboratories) or ImmPACT DAB (Vector Laboratories). Cells were dehydrated and mounted with Multi Mount 480 (Matsunami Glass, Osaka, Japan). Images of immunostained sections were captured as described below.
Immunofluorescence was performed with anti-myosin heavy chain antibodies (MF20, Developmental Studies Hybridoma Bank) and anti-mouse IgG Alexa Fluor 488-conjugated secondary antibodies (Thermo Fisher Scientific, Waltham, MA, USA), followed by counter staining with 4′,6-diamidino-2-phenylindole (DAPI). Images of immunofluorescence were captured using a microscope (BZ-8000, Keyence, Osaka, Japan) with a 20×/0.75 Plan Apo objective (Olympus, Tokyo, Japan).
2.4. Oil Red-O staining and imaging
Oil Red-O (Sigma-Aldrich, St. Louis, MO, USA) stock solution, at a concentration of 30% (w/v) in isopropanol, was diluted with distilled water (3:5) and used within 2 h of filtration. Cells were fixed in 10% formalin for 10 min and stained with Oil Red-O working solutions for 30 min at room temperature. Cells were then counterstained with hematoxylin (Muto Pure Chemicals, Tokyo, Japan) and mounted with an aqueous mounting medium (Aquatex, 108562, Merck, Darmstadt, Germany). Bright-field images of live and Oil Red-O-stained cells were captured using a microscope (BZ-8000, Keyence) with 2×/0.1 Plan Apo and 20×/0.75 Plan Apo objectives (Olympus).
2.5. Triglyceride (TG) and protein quantification
Cells were washed twice with PBS and harvested in 5% Triton X-100 in PBS. Whole-cell lysates were heated at 90 °C for 5 min and chilled on ice for 3 min, followed by heating and chilling again. After centrifugation at 13,000 rpm for 10 min at 4 °C, the obtained supernatants were used to determine TG and protein concentrations using a LabAssay Triglyceride kit (Wako Pure Chemical, Osaka, Japan) and BCA assay reagents (Thermo Fisher Scientific), respectively.
2.6. Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from cells using an RNeasy Mini kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions. Isolated RNA was reverse transcribed to cDNA using a High-Capacity cDNA Reverse Transcription kit (Life Technologies, Carlsbad, CA, USA). To measure mRNA levels, real-time PCR was performed with TaqMan Gene Expression Assays using an ABI 7900 real-time system (Life Technologies). All primers and probes were purchased as TaqMan Gene Expression Assays: PPAR-γ (Rn00440945_m1), FABP4 (Rn04219585_m1), MyoD (Rn01457527_g1), and Pax7 (Rn01518732_m1). The relative gene expression levels of each sample were determined using the comparative Ct method. Expression assays for each gene were normalized to 18SrRNA (Hs99999901_s1) and are expressed as fold change relative to the control level.
2.7. Statistical analysis
All values are expressed as means ± standard errors (SEs). Data were analyzed using one-way analysis of variance with Dunnett’s t-test. Differences with p values of less than 0.05 were considered statistically significant. All statistical analyses were performed using PASW v18 (IBM, Armonk, NY, USA).
3. Results
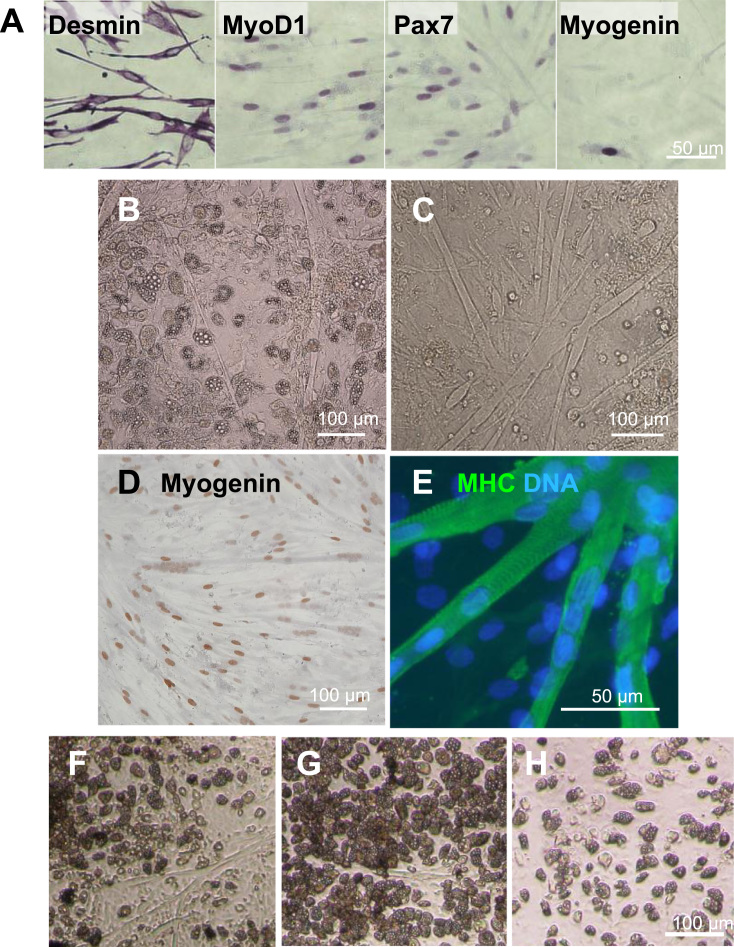
To determine the characteristics of the cellular phenotype, the myogenic markers MyoD, myogenin, and desmin and the satellite cell-specific marker Pax7 were examined by immunohistochemical analysis. After incubation in growth medium for 40 h, the percentages of myogenic/satellite cell-specific marker positive nuclei were 90% for desmin, 75% for MyoD, and 85% for Pax7 (Fig. 1A). A small percentage (~10%) of cells already expressed the late myogenesis (differentiation) marker myogenin (Fig. 1A). By shifting to the myogenic medium, myogenin-positive nuclei were increased, and the cells began to fuse to each other (1 day after shift; Fig. 1D), forming multinucleated myotubes (4 days after the shift; Fig. 1C) expressing myosin heavy chain proteins (5 days after shift; Fig. 1E). We concluded that the majority of isolated cells were myogenic progenitors, i.e., mSCs harboring myogenic differentiation potency.
Fig. 1.
Characterization of the isolated cells and conditions for adipogenic differentiation. (A) Images of cells immunostained with myogenic markers. After 40 h of culture in the growth medium, cells were fixed and stained with primary antibodies against the indicated myogenic proteins and visualized. Images of cell morphologies after adipogenic induction (B) or myogenic induction (C). Images of myogenic differentiated cells stained for myogenin (D) or fluorescent staining for myosin heavy chain (E, green) with counter staining by DAPI (E, blue). (F–H) Effects of cell density on adipogenesis induction. Images of adipogenic-differentiated cells seeded at different cell densities (F: equal cell density to myogenesis conditions, G: half density of the conditions in F, H: half density of the conditions in G).
mSCs are known to have the potential to differentiate into adipogenic lineages. Indeed, the isolated cells appeared as morphologically typical adipocytes (Fig. 1B), with Oil Red-O-stained lipid droplets in the cytoplasm (Fig. 2Aa) following adipogenic induction treatment.
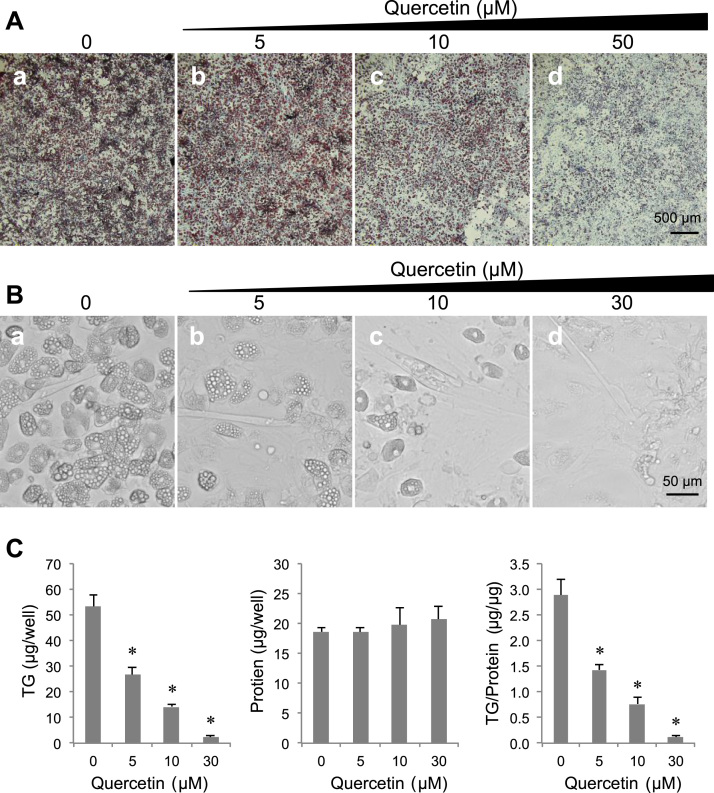
Fig. 2.
Inhibitory effects of quercetin on mSC adipogenesis. (A) Images of Oil Red-O-stained cells after 6 days of adipogenesis treatment with quercetin at the indicated concentrations. Scale bar: 500 µm. (B) Bright-field images of cells treated with quercetin as in A at the indicated concentrations. Scale bar: 50 µm. A typical result from quadruplicate experiments is shown. (C) TG contents, protein contents, and the TG/protein ratios in mSCs treated with quercetin as in B. Values are expressed as means ± SEs (n = 4). *p < 0.05 versus control (without quercetin).
To evaluate the effects of quercetin on adipogenesis, we next attempted to search for more effective conditions for induction of adipogenic differentiation with the isolated cells by seeding them at different densities. The cells were diluted twice in a step-wise manner and cultured in adipogenic induction medium. At the same cell density used for myogenesis, myotubes were formed, even under adipogenic induction conditions (Fig. 1B, F). Induction of adipogenesis was most efficient when cells were seeded at half the density used for myogenic differentiation (Fig. 1G). The conditions seemed to be better than those under lower cell densities (Fig. 1H). Accordingly, we used these effective adipogenesis conditions to evaluate the inhibitory activities of quercetin in induced adipogenesis in vitro. The results showed that the isolated cells had phenotypic characteristics of mSCs with bipotentiality for myogenesis and adipogenesis and that the cell density affected the cellular fate.
To evaluate the effects of quercetin on adipogenesis of primary mSCs, we treated the cells with quercetin during treatment for adipogenesis. In the absence of quercetin, Oil Red-O-stained lipid droplets were elevated in the cells during adipogenesis treatment (Fig. 2Aa, Ba). Following addition of quercetin, the increase in the number of morphologically typical adipocytes during adipogenic induction was repressed (Fig. 2Ab–d, Bb–d). TG content and the TG/protein ratio in the cells were also suppressed in a dose-dependent manner (Fig. 2C), and there were no changes in protein contents at all quercetin concentrations (Fig. 2C). These results clearly indicated that quercetin had the potential to inhibit differentiation of primary mSCs into adipocytes in vitro.
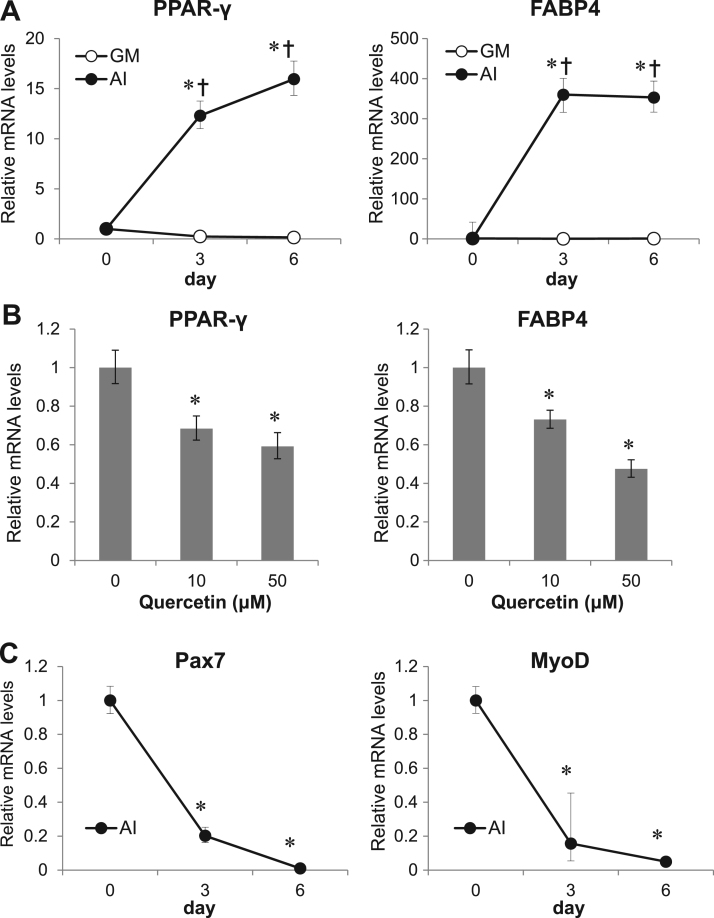
Next, we examined whether quercetin affected the expression levels of PPAR-γ, a key transcriptional regulator for adipogenesis, and FABP4, lipid transporter expressed in adipocytes, by qRT-PCR. After 6 days of adipogenic treatment, the mRNA levels of PPAR-γ and FABP4 were upregulated and reached 16- and 350-fold those in the predifferentiated state, respectively (Fig. 3A). Pax7 and MyoD mRNA levels decreased dramatically under the same adipogenic induction conditions, suggesting the repression of myogenesis and induction of adipogenesis (Fig. 3C). In the presence of quercetin, the upregulation of both adipogenic markers was suppressed in a dose-dependent manner (Fig. 3B). The mRNA levels of PPAR-γ decreased to 70% and 60% that of the control at quercetin concentrations of 10 and 50 µM, respectively. The levels of FABP4 mRNA decreased to 70% and 50% that of the control at quercetin concentrations of 10 and 50 µM, respectively. These results indicated that quercetin could suppress the expression adipogenic factors following inhibition of mSC adipogenesis.
Fig. 3.
Suppression of upregulated mRNA levels of adipogenic markers by quercetin. (A, C) Time course of adipogenic (A, PPAR-γ and FABP4) or myogenic (C, MyoD and Pax7) mRNA levels during adipogenic induction conditions. Total RNA was extracted before (0 day) and after 3 or 6 days of culture in growth medium (GM) or under adipogenic induction conditions (AI), followed by qRT-PCR. Values are expressed as means ± SEs (n = 3). *p < 0.05 versus control (without quercetin), §p < 0.05 versus GM on each day. (B) Relative mRNA levels of PPAR-γ and FABP4 versus control (without quercetin) after 6 days of adipogenesis treatment with quercetin at the indicated concentrations. Values are expressed as means ± SEs (n = 3). *p < 0.05 versus control (without quercetin).
4. Discussion
Quercetin has been widely studied and shown to exhibit several biological effects. Although quercetin inhibits the adipogenesis of 3T3-L1 pre-adipocytes [17], [18] and OP9 stromal cells [20], the effects of quercetin on the adipogenesis of mSCs are still unclear. Our results using primary mSCs were consistent with previous studies demonstrating that the biological activity of quercetin is related to suppression of adipogenic factors, including PPAR-γ. The cross-antagonistic activities of MyoD and PPAR-γ during differentiation were reported in an ectopic expression experiment with mesenchymal stem/stromal cells [21]. For adipogenesis, simultaneous shifts in expression involving not only PPAR-γ upregulation but also MyoD suppression are required. In our in vitro system, simultaneous shifts in the expression of the two master regulators were observed at the mRNA level during adipogenesis.
Skeletal muscle has the potential to adapt to its environmental conditions. Lipid accumulation within skeletal muscle is observed in obese individuals and patients with type 2 diabetes, muscular atrophy [8], [9], and sarcopenia [6] and is related to a decline in muscle function. Several cell populations in muscles are lineages with the potential to induce ectopic lipid accumulation [22], [23]. Although the precise molecular mechanisms underlying mSC adipogenesis remain unknown, dysfunctional mSCs could be one of the origins of ectopic adipocytes, leading to lipid accumulation in aged skeletal muscle during muscle regeneration after injury. Quercetin could have applications as a potential dietary supplement to suppress the ectopic lipid accumulation observed in the skeletal muscle of obese patients or patients with muscular atrophy or sarcopenia; however, further in vivo experiments are needed to test this hypothesis.
In conclusion, we demonstrated that quercetin could inhibit mSC adipogenesis in vitro by suppressing the transcription of adipogenic markers.
Acknowledgements
We thank Dr. Noriaki Kawanishi for experimental assistance.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.12.003.
Contributor Information
Tomoko Funakoshi, Email: ishii@junetndo.ac.jp.
Noriyuki Kanzaki, Email: Noriyuki_Kanzaki@suntory.co.jp.
Yuta Otsuka, Email: Yuta_Otsuka@suntory.co.jp.
Takayuki Izumo, Email: Takayuki_Izumo@suntory.co.jp.
Hiroshi Shibata, Email: Hiroshi_Shibata@suntory.co.jp.
Shuichi Machida, Email: machidas@juntendo.ac.jp.
Appendix A. Transparency document. Supplementary material
Supplementary material
References
- 1.Collins C.A., Olsen I., Zammit P.S., Heslop L., Petrie A., Partridge T.A., Morgan J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Zammit P.S. All muscle satellite cells are equal, but are some more equal than others? J. Cell Sci. 2008;121:2975–2982. doi: 10.1242/jcs.019661. [DOI] [PubMed] [Google Scholar]
- 3.Asakura A., Komaki M., Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 4.De Coppi P., Milan G., Scarda A., Boldrin L., Centobene C., Piccoli M., Pozzobon M., Pilon C., Pagano C., Gamba P., Vettor R. Rosiglitazone modifies the adipogenic potential of human muscle satellite cells. Diabetologia. 2006;49:1962–1973. doi: 10.1007/s00125-006-0304-6. [DOI] [PubMed] [Google Scholar]
- 5.Cree M.G., Newcomer B.R., Katsanos C.S., Sheffield-Moore M., Chinkes D., Aarsland A., Urban R., Wolfe R.R. Intramuscular and liver triglycerides are increased in the elderly. J. Clin. Endocrinol. Metab. 2004;89:3864–3871. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- 6.Song M.Y., Ruts E., Kim J., Janumala I., Heymusfield S., Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am. J. Clin. Nutr. 2004;79:874–880. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 7.Manini T.M., Clark B.C., Nalls M.A., Goodpaster B.H., Ploutz-Snyder L.L., Harris T.B. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am. J. Clin. Nutr. 2007;85:377–384. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 8.Grounds M.D., Terrill J.R., Radley-Crabb H.G., Robertson T., Papadimitriou J., Spuler S., Shavlakadze T. Lipid accumulation in dysferlin-deficient muscles. Am. J. Pathol. 2014;184:1668–1676. doi: 10.1016/j.ajpath.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Lott D.J., Forbes S.C., Mathur S., Germain S.A., Senesac C.R., Lee Sweeney H., Walter G.A., Vandenborne K. Assessment of intramuscular lipid and metabolites of the lower leg using magnetic resonance spectroscopy in boys with Duchenne muscular dystrophy. Neuromuscul. Disord. 2014;24:574–582. doi: 10.1016/j.nmd.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuang S., Charge S.B., Seale P., Huh M., Rudnicki M.A. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J. Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly G.S. Quercetin monograph. Altern. Med. Rev. 2011;16:172–194. [PubMed] [Google Scholar]
- 12.Kawabata K., Mukai R., Ishisaka A. Quercetin and related polyphenols: new insights and implications for their bioactivity and bioavailability. Food Funct. 2015;6:1399–1417. doi: 10.1039/c4fo01178c. [DOI] [PubMed] [Google Scholar]
- 13.Hemdan D.I., Hirasaka K., Nakao R., Kohno S., Kagawa S., Abe T., Harada-Sukeno A., Okumura Y., Nakaya Y., Terao J., Nikawa T. Polyphenols prevent clinorotation-induced expression of atrogenes in mouse C2C12 skeletal myotubes. J. Med. Investig. 2009;56:26–32. doi: 10.2152/jmi.56.26. [DOI] [PubMed] [Google Scholar]
- 14.Henagan T.M., Lenard N.R., Gettys T.W., Stewart L.K. Dietary quercetin supplementation in mice increases skeletal muscle PGC1alpha expression, improves mitochondrial function and attenuates insulin resistance in a time-specific manner. PLoS One. 2014;9:e89365. doi: 10.1371/journal.pone.0089365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le N.H., Kim C.S., Park T., Park J.H., Sung M.K., Lee D.G., Hong S.M., Choe S.Y., Goto T., Kawada T., Yu R. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediators Inflamm. 2014:834294. doi: 10.1155/2014/834294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukai R., Matsui N., Fujikura Y., Matsumoto N., Hou D.X., Kanzaki N., Shibata H., Horikawa M., Iwasa K., Hirasaka K., Nikawa T., Terao J. Preventive effect of dietary quercetin on disuse muscle atrophy by targeting mitochondria in denervated mice. J. Nutr. Biochem. 2016;31:67–76. doi: 10.1016/j.jnutbio.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Ahn J., Lee H., Kim S., Park J., Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2008;373:545–549. doi: 10.1016/j.bbrc.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 18.Eseberri I., Miranda J., Lasa A., Churruca I., Portillo M.P. Doses of quercetin in the range of serum concentrations exert delipidating effects in 3T3-L1 preadipocytes by acting on different stages of adipogenesis, but not in mature adipocytes. Oxid. Med. Cell Longev. 2015:480943. doi: 10.1155/2015/480943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machida S., Spangenburg E.E., Booth F.W. Primary rat muscle progenitor cells have decreased proliferation and myotube formation during passages. Cell Prolif. 2004;37:267–277. doi: 10.1111/j.1365-2184.2004.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo Y.S., Kang O.H., Kim S.B., Mun S.H., Kang D.H., Yang D.W., Choi J.G., Lee Y.M., Kang D.K., Lee H.S., Kwon D.Y. Quercetin prevents adipogenesis by regulation of transcriptional factors and lipases in OP9 cells. Int. J. Mol. Med. 2015;35:1779–1785. doi: 10.3892/ijmm.2015.2185. [DOI] [PubMed] [Google Scholar]
- 21.Sunadome K., Suzuki T., Usui M., Ashida Y., Nishida E. Antagonism between the master regulators of differentiation ensures the discreteness and robustness of cell fates. Mol. Cell. 2014;54:526–535. doi: 10.1016/j.molcel.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell K.J., Pannerec A., Cadot B., Parlakian A., Besson V., Gomes E.R., Marazzi G., Sassoon D.A. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat. Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 23.Uezumi A., Fukada S., Yamamoto N., Takeda S., Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material